Abstract
Cytokines are soluble proteins that regulate immune responses. A current paradigm is that cytokine production in lymphoid tissues is tightly localized and signaling occurs between conjugate cells. Here we assess cytokine signaling during infection by measuring in vivo phosphorylation of intracellular signal transducers and activators of transcription (STATs). We show that interferon γ (IFN-γ) and interleukin 4 (IL-4) signal to the majority of lymphocytes throughout the reactive lymph node, and that IL-4 conditioning of naïve, bystander cells is sufficient to override opposing Th1 instruction. Our results demonstrate that, despite localized production, cytokines can permeate a lymph node and modify the majority of cells therein. Cytokine conditioning of bystander cells could provide a mechanism by which chronic worm infections subvert the host response to subsequent infections or vaccination attempts.
INTRODUCTION
Cytokines are soluble proteins that direct the initiation, differentiation and termination of immune responses. Different microbial pathogens elicit distinct patterns of cytokine expression in hosts, and these responses are often classified according to the dominant cytokines secreted by CD4+ T helper (Th) cells1. Intracellular protozoa typically induce the differentiation of Th1 cells, which make interferon-γ (IFN-γ) and drive cytolytic immunity, while helminth parasites elicit Th2 cells that are characterized by production of interleukin (IL) -4, IL-13 and IL-52, 3. IL-4 is central to type 2 responses, promoting Th2 differentiation and B cell class switch recombination to IgG1 and IgE isotypes4–6.
Despite their importance, surprisingly little is known about the spatiotemporal range over which cytokines exert their influence in vivo. Recent studies in helminth disease models have demonstrated that the production of IL-4 in the reactive lymph node is restricted to CD4+ T cells within the B cell follicles7–9. During Leishmania major infection, which elicits a mixed Th1/Th2 immune response in BALB/c mice, the expression of both IFN-γ and IL-4 is limited to B cell areas7. These data have been interpreted to imply that IFN-γ and IL-4 function in the lymph node in a paracrine manner between B and T cell conjugates7. This is consistent with a wider consensus that cytokine signals are tightly localized, affecting only cells in close proximity to the source10–12.
Cytokine signaling is mediated via the engagement of specific cell surface receptors and subsequent phosphorylation of intracellular signal transducers and activators of transcription (STATs)13. The linearity of the IL-4- IL-4 receptor- STAT6 pathway means that in lymphocytes, which do not express the IL-13 receptor, STAT6 phosphorylation is a specific indicator of stimulation with IL-414, 15. IFN-γ binds a heterodimeric IFN-γ receptor and activates STAT1, a signal transducer also used by other cytokines such as IFN-α, IFN-β and IL-2713. To investigate the spatial and temporal range over which canonical T helper cytokines operate in vivo, we infected mice with parasitic pathogens and examined the consequent phosphorylation of STAT proteins directly ex vivo. We found that the striking majority of lymphocytes in a Th2 reactive lymph node, including naïve, bystander cells, received IL-4 signals and consequently changed their phenotype and their functional bias. This IL-4 conditioning was restricted to the draining lymph node and was sufficient to override opposing Th1 instruction by dendritic cells (DC). During Th1-polarized infection, IFN-γ signaling was similarly pervasive throughout the draining lymph node and altered the majority of cells therein. Our data suggest that signaling by T helper effector cytokines is widespread throughout the lymph node, is independent of direct cell contact and is not a limiting factor in B cell maturation or Th differentiation.
RESULTS
IL-4 signals permeate the Th2 reactive lymph node
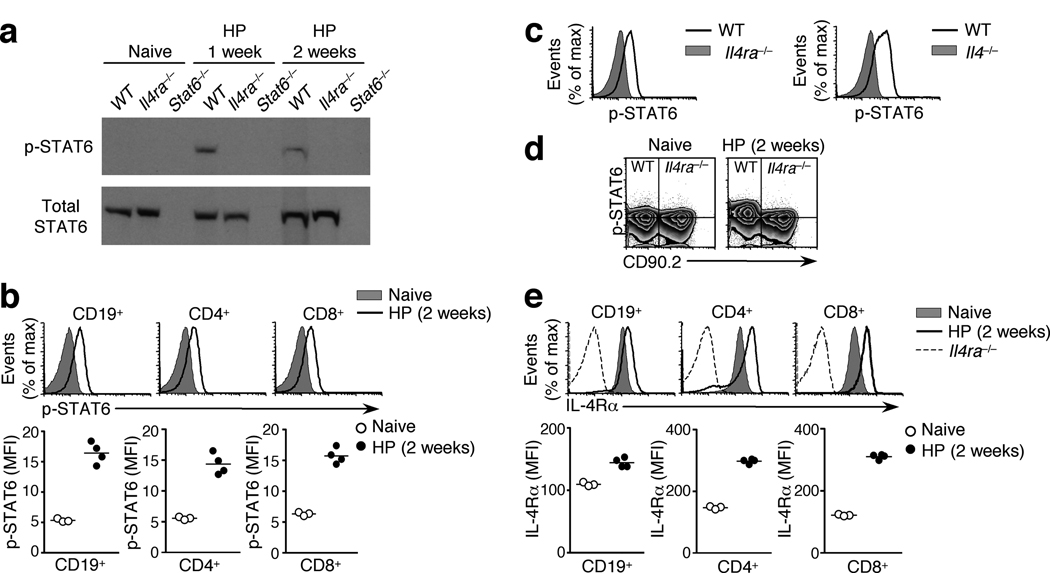
To examine the receipt of IL-4 signals during helminth infection, we infected mice with the strictly enteric murine pathogen Heligmosomoides polygyrus8, 16 and collected the draining mesenteric lymph nodes (mesLN). Immunoblot analysis revealed STAT6 phosphorylation (p-STAT6) in the mesLN of mice infected with H. polygyrus one or two weeks earlier, but not in naïve animals or infected Il4ra−/− or Stat6−/− controls (Fig. 1a). We used flow cytometry to assess p-STAT6 at the single cell level. While cells from naive mice showed no STAT6 phosphorylation, similar to Stat6−/− or Il4ra−/− controls (Supplementary Fig. 1), p-STAT6 was apparent in essentially all CD19+ B cells from the mesLN of H. polygyrus -infected animals (Fig. 1b). Moreover, p-STAT6 was also detected in the majority of CD4+ and CD8+ T cells, which predominantly reside outside the B cell follicle (Fig. 1b). STAT6 phosphorylation in the bulk population of lymphocytes in the reactive lymph node was also elicited by subcutaneous injection of Schistosoma mansoni eggs, demonstrating that it is a common response to multiple Th2-inducing pathogens (Supplementary Fig. 2). During H. polygyrus infection, STAT6 phosphorylation was more extensive in bystander CD4+ cells (CD44lo GFP−) than in the antigen-experienced CD4+ population (defined as CD44hi IL-4-GFP+ in 4get IL-4 reporter animals17), consistent with the downregulation of IL-4Rα on highly activated Th2 cells16, 18 (Supplementary Fig. 3). In all lymphocyte subsets the phosphorylation of STAT6 required both IL-4 and the IL-4Rα chain, since p-STAT6 was not detected in H. polygyrus -infected Il4−/− or Il4ra−/− mice (Fig. 1a, c, Supplementary Fig. 1). The dependence of STAT6 phosphorylation on IL-4Rα was a cell intrinsic effect, because in mixed bone marrow (BM) chimeras reconstituted with equal parts of wild-type (CD90.2−) and Il4ra−/− (CD90.2+) BM, the Il4ra−/− population failed to phosphorylate STAT6 in response to H. polygyrus infection (Fig. 1d). These data suggest that direct IL-4 signals are received by the majority of lymphocytes throughout a Th2 reactive lymph node.
Figure 1. IL-4 signals elicit STAT6 phosphorylation and IL-4R upregulation throughout a Th2 reactive lymph node.
(a) Immunoblots for total STAT6 and phosphorylated STAT6 in mesLN cells from naïve or H. polygyrus (HP) -infected wild-type, Il4ra−/− and Stat6−/− mice, assessed directly ex vivo. (b) STAT6 phosphorylation in lymphocyte subsets of the mesLN, in the presence or absence of infection. (c) STAT6 phosphorylation in wild-type, Il4ra−/− and Il4−/− mesLN 2 weeks after H. polygyrus infection, gated on total lymphocytes. (d) STAT6 phosphorylation in CD4+ lymphocytes in mesLN of mixed BM chimeras (1:1 ratio of wild-type (CD90.2−) and Il4ra−/− (CD90.2+) cells) infected with H. polygyrus. Horizontal lines indicate the mode fluorescence intensity of p-STAT6 staining in cells from naïve animals. (e) IL-4Rα expression in lymphocyte subsets of wild-type mesLN, in the presence or absence of H. polygyrus infection. Il4ra−/− cells were used as a negative staining control. In all panels, graphed data are shown as the geometric mean fluorescence intensity (MFI) and are gated on GFP− cells to exclude activated Th2 cells. Each data point represents an individual mouse. All data are representative of two or more independent experiments, using 3–4 mice per group.
Because IL-4 stimulation increases IL-4Rα expression on lymphocytes by a STAT6-dependent mechanism18, 19 (Supplementary Fig. 4), we predicted that the ubiquitous STAT6 phosphorylation in the mesLN of H. polygyrus -infected animals would result in widespread IL-4Rα upregulation. Indeed, infection with H. polygyrus enhanced IL-4Rα expression on the majority of CD19+ B cells and CD4+ and CD8+ T cells in the mesLN, compared to naïve controls (Fig. 1e). The subset of CD4+ T cells that displayed little or no IL-4Rα were highly activated Th2 cells, which have been previously shown to downregulate IL-4Rα16, 18. These data are consistent with previous work that the bulk population of B cells in the mesLN increase expression of CD23, major histocompatibility complex (MHC) class II, and CD86 upon H. polygyrus infection in an IL-4 dependent manner8, and together demonstrate a physiological consequence of IL-4 signaling in the majority of lymphocytes in a Th2 reactive lymph node.
IFN-γ signaling is ubiquitous in a Th1 reactive lymph node
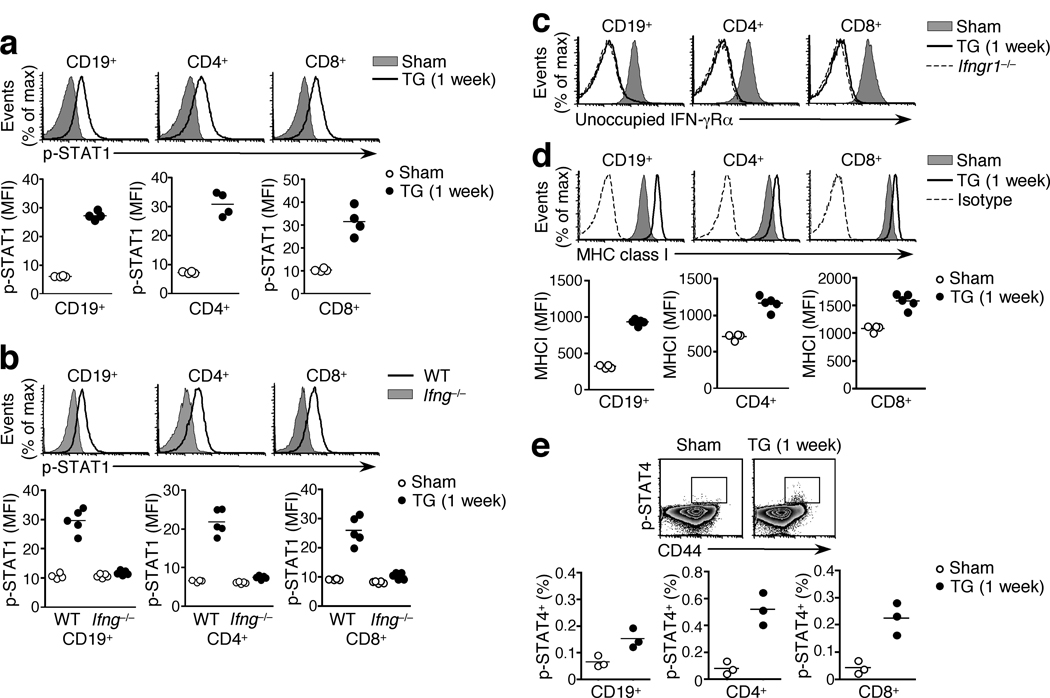
To investigate whether signature Th1 cytokines also show widespread signaling within a reactive lymph node, we infected mice with the protozoan parasite Toxoplasma gondii. The canonical Th1 effector cytokine, IFN-γ, is critical for B cell class switching to IgG2a and its expression in the Th1 reactive lymph node is restricted to T follicular helper cells (Tfh) in the B cell follicle7. IFN-γ signaling is mediated by phosphorylation of STAT113, 20, 21 and following oral T. gondii infection, p-STAT1 was evident in the striking majority of CD19+ B cells and CD4+ and CD8+ T cells in the reactive mesLN (Fig. 2a). p-STAT1 reflected IFN-γ signaling rather than activation by other cytokines since it was absent in T. gondii -infected Ifng−/− or Ifngr1−/− mice (Fig. 2b and data not shown). Consistent with these data, essentially all lymphocytes had bound IFN-γ to surface IFN-γ receptors, as revealed by the lack of staining with the GR-20 mAb which is specific for the unoccupied IFN-γ receptor α chain but whose epitope is masked by the presence of IFN-γ22 (Fig. 2c, Supplementary Fig. 5). GR-20 staining was not reduced in T. gondii-infected Ifng−/− animals where the epitope cannot be blocked (Supplementary Fig. 5). To test for functional evidence of IFN-γ signaling throughout the lymph node, we measured MHC class I expression in the mesLN following T. gondii infection. We found a marked, IFN-γ-dependent MHC class I upregulation on the bulk population of all lymphocyte subsets relative to sham-infected controls (Fig. 2d, Supplementary Fig. 6)23. Thus IFN-γ, like IL-4, can permeate a reactive lymph node and modify the majority of cells therein.
Figure 2. IFN-γ signals permeate a Th1 reactive lymph node.
(a) STAT1 phosphorylation assessed ex vivo in the mesLN of wild-type mice 1 week post T. gondii (TG) or sham infection, gated on the indicated lymphocyte subsets. (b) STAT1 phosphorylation in wild-type and Ifng−/− mesLN 1 week post T. gondii or sham infection, gated on the indicated lymphocyte subsets. (c) IFN-γ receptor occupancy on indicated lymphocyte subsets from mesLN of T. gondii- or sham-infected wild-type or Ifngr1−/− mice, as assessed by staining with GR-20 mAb. (d) MHC class I (H-2Kb) expression, or isotype antibody staining, in lymphocyte subsets of wild-type mesLN, with or without T. gondii infection. (e) STAT4 phosphorylation ex vivo in mesLN from T. gondii- or sham-infected wild-type mice. Plots are shown gated on CD4+ cells. In all panels, graphs depict the geometric mean fluorescence intensity and each data point represents an individual mouse. Data are representative at least three separate experiments, using 3–5 mice per group.
During T. gondii infection Th1 differentiation is dependent on the polarizing cytokine IL-1224, 25, which signals through STAT4 phosphorylation. Previous reports have described the absence of a functional IL-12 receptor on naïve T cells and its rapid upregulation in response to antigenic stimulation26. Consistent with this, p-STAT4 was detected only in a minority of T cells, all contained within the activated CD44hi subset, in the mesLN of T. gondii-infected animals (Fig. 2e). Of note, IL-12 stimulation of NK cells during T.gondii infection resulted in clear p-STAT4 staining, measured by the same technique27. These data demonstrate that STAT4 phosphorylation, in contrast to that of STAT1, is tightly restricted in a Th1 reactive lymph node.
IL-4 signaling is restricted to the draining lymph node
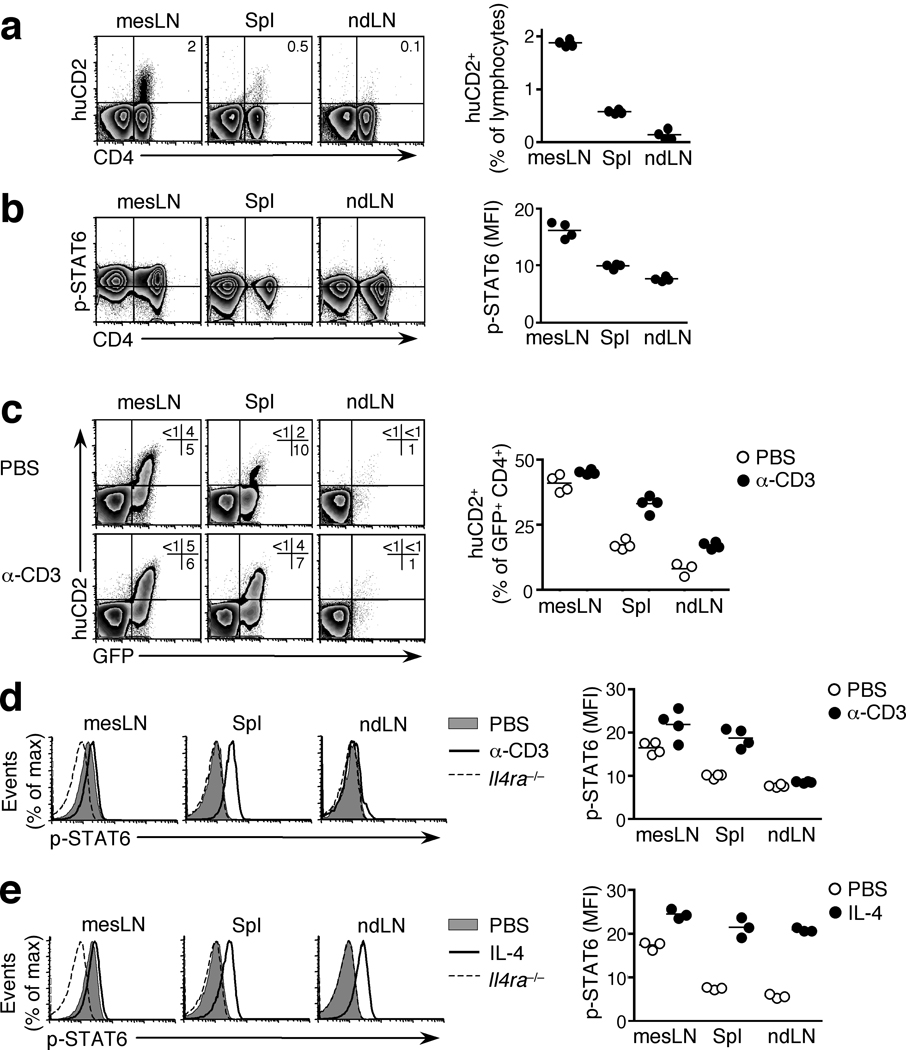
To test whether cytokine signaling in localized infections extends beyond the reactive lymph node, we infected mice with H. polygyrus and analyzed various lymphoid tissues. We used 4get-KN2 IL-4 dual reporter mice, in which IL-4 competent cells are GFP+ and IL-4 producing cells additionally express huCD216. Like IL-4 production (Fig. 3a), STAT6 phosphorylation was observed only in the draining mesLN and not in the spleen or non-draining lymph nodes (Fig. 3b). Phosphorylated STAT6 reflected the IL-4-rich Th2 environment of the reactive lymph node rather than an inherent property of the mesLN, since subcutaneous injection of H. polygyrus larvae into one footpad, which generates a local Th2 response in the absence of infection28, elicited STAT6 phosphorylation in the draining popliteal lymph node but not in the contralateral popliteal lymph node, the mesLN or the spleen (Supplementary Fig. 7 and data not shown).
Figure 3. STAT6 phosphorylation occurs in draining but not non-draining lymphoid tissues.
(a) huCD2 expression in 4get/KN2 IL-4 dual reporter mice infected with H. polygyrus. Graphs indicate percentage of huCD2+ cells within the total lymphocyte population. (b) STAT6 phosphorylation in 4get mice 2 weeks post H. polygyrus infection. Horizontal lines indicate mode fluorescence intensity of p-STAT6 staining in the negative control (mesLN cells of Il4ra−/− mice). Graphs depict geometric mean fluorescence intensity of p-STAT6 in CD4+ GFP− cells. (c) huCD2 expression (gated on CD4+ cells) and (d) STAT6 phosphorylation (gated on CD4+ GFP− cells) in wild-type or Il4ra−/− 4get/KN2 mice infected with H. polygyrus and injected i.v. with either PBS or an anti-CD3ε antibody 2 weeks later. Graphs depict percentage of huCD2+ cells within the GFP+ CD4+ population. (e) STAT6 phosphorylation in 4get mice infected with H. polygyrus and injected i.v. with recombinant murine IL-4 on day 14. Data shown are gated on CD4+ GFP− cells and Il4ra−/− mice given IL-4 were included as a baseline staining control. In all panels, each data point represents an individual mouse and data are representative two or more independent experiments with 3–4 mice per group.
These data suggested that the local presence of IL-4 is sufficient to induce the phosphorylation of STAT6 and, conversely, that the absence of p-STAT6 in non-draining lymphoid tissues is due to the lack of IL-4 and not any inability of these cells to respond. To test this we infected 4get/KN2 IL-4 dual reporter mice with H. polygyrus, which elicited both IL-4 competent GFP+ and IL-4 producing huCD2+ cells in the mesLN while the spleen contained IL-4 competent but few IL-4 producing cells and the non-draining lymph nodes were devoid of both populations (Fig. 3c, PBS controls)16. Systemic administration of anti-CD3 (ref 16) into H. polygyrus -infected mice induced production of IL-4, denoted by huCD2 expression, in resident GFP+ IL-4-competent T cells in the spleen (Fig. 3c). The non-draining, inguinal lymph node contained very few GFP+ cells and consequently the frequency of huCD2+ cells within the CD4+ population remained low (Fig. 3c). Importantly, the induction of IL-4 production in the spleen resulted in the phosphorylation of STAT6 in splenocytes but not in cells from the non-draining lymph nodes (Fig. 3d). Moreover, when exogenous IL-4 was delivered systemically into either H. polygyrus -infected (Fig. 3e) or naïve mice (Supplementary Fig. 8), STAT6 was phosphorylated in the mesLN, spleen and peripheral lymph nodes of all animals. These data demonstrate that, during H. polygyrus infection, the absence of STAT6 phosphorylation in non-draining lymphoid tissues reflects a local deficit in IL-4 production and not in IL-4 responsiveness.
Sustained STAT6 phosphorylation reflects continuous IL-4 signaling
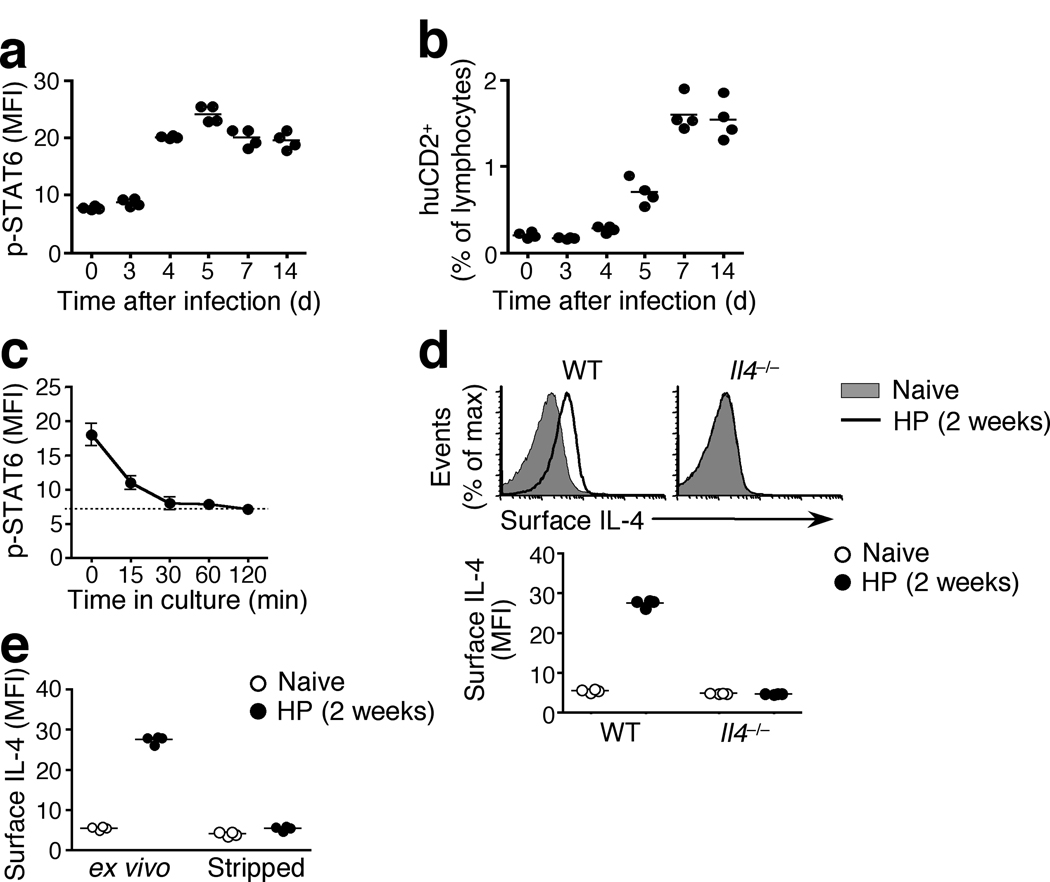
To investigate the temporal dimension of the IL-4 response during H. polygyrus infection, we performed kinetic studies of STAT6 phosphorylation. STAT6 phophorylation was detected in the draining mesLN as early as 4 days after infection and was sustained at similar amounts throughout the 2 week experiment (Fig. 4a). The onset of STAT6 phosphorylation corresponded with the earliest significant IL-4 production in response to H. polygyrus infection, as determined both by huCD2 expression (naive vs d3, p = 0.11; d3 vs d4, p = 0.0021) (Fig. 4b)8, 16 and by consequent IL-4Rα upregulation18. However, it was possible that the sustained presence of p-STAT6 did not reflect continued IL-4 signaling but was due to an unusually long half-life of the phosphorylated form of STAT6. To test this, we isolated mesLN cells from H. polygyrus -infected animals, quenched any persistent IL-4 signaling by culturing in the presence of an IL-4-neutralizing antibody (clone 11B11), and assessed the decay of p-STAT6 over time by flow cytometry. Changes in the fluorescence intensity of anti-phospho staining correlate closely with corresponding immunoblot analysis29. We determined the half-life of p-STAT6 to be in the order of 15 min in CD4+, CD8+ and CD19+ cells (Fig. 4c and data not shown), an observation consistent with earlier demonstrations that, like many signaling cascades, the phosphorylation of STAT6 is inherently transient30. This suggests that the sustained detection of p-STAT6 in the draining mesLN throughout the course of H. polygyrus infection reflects the continuous presence of IL-4 and its sustained triggering of the IL-4 receptor. Indeed, we detected IL-4 on the surface of the vast majority of CD4+, CD8+ and CD19+ cells in the mesLN of wild-type but not Il4−/− or Il4ra−/− mice during H. polygyrus infection (Fig. 4d, Supplementary Fig. 9 and data not shown). When mesLN cells from H. polygyrus - infected wild-type mice were incubated with an IL-4-neutralizing antibody, their rapid loss of STAT6 phosphorylation corresponded with the stripping of detectable IL-4 from their surface (Fig. 4e, Supplementary Fig. 9). These data suggest that while intracellular signaling events are inherently short-lived following the cessation of receptor stimulation, the persistent presence of cytokines in vivo results in sustained signaling.
Figure 4. STAT6 phosphorylation in the Th2 reactive lymph node is sustained and reflects continuous IL-4R triggering.
(a) STAT6 phosphorylation in mesLN cells from 4get/Kn2 IL-4 dual reporter mice at the indicated times after H. polygyrus infection. Data shown are gated on CD4+ GFP− cells. (b) huCD2 expression in mesLN cells from 4get/Kn2 mice at the indicated times after H. polygyrus infection. (c) STAT6 phosphorylation in cells isolated from the mesLN of 4get mice 2 weeks post H. polygyrus infection and incubated with an IL-4 neutralizing antibody (clone 11B11) for the indicated times. Data shown are gated on CD4+ GFP− cells and the dotted line indicates geometric mean fluorescence of H. polygyrus -infected Il4ra−/− controls. Error bars indicate SD of four individuals per group. (d). Detection of IL-4 bound to the cell surface in mesLN cells from naïve or H. polygyrus -infected wild-type 4get or Il4−/− mice using an anti-IL-4 antibody (clone BVD6). Data shown are gated on CD4+ GFP− cells. (e) Surface IL-4 expression in mesLN cells harvested from naïve or 2 weeks H. polygyrus -infected wild-type 4get mice and either stained immediately (ex vivo) or incubated with an IL-4 neutralizing antibody (clone 11B11) for 60min (stripped). Data shown are gated on CD4+ GFP− cells. Each data point represents an individual mouse. All panels are representative of two to four independent experiments using 4 mice per group.
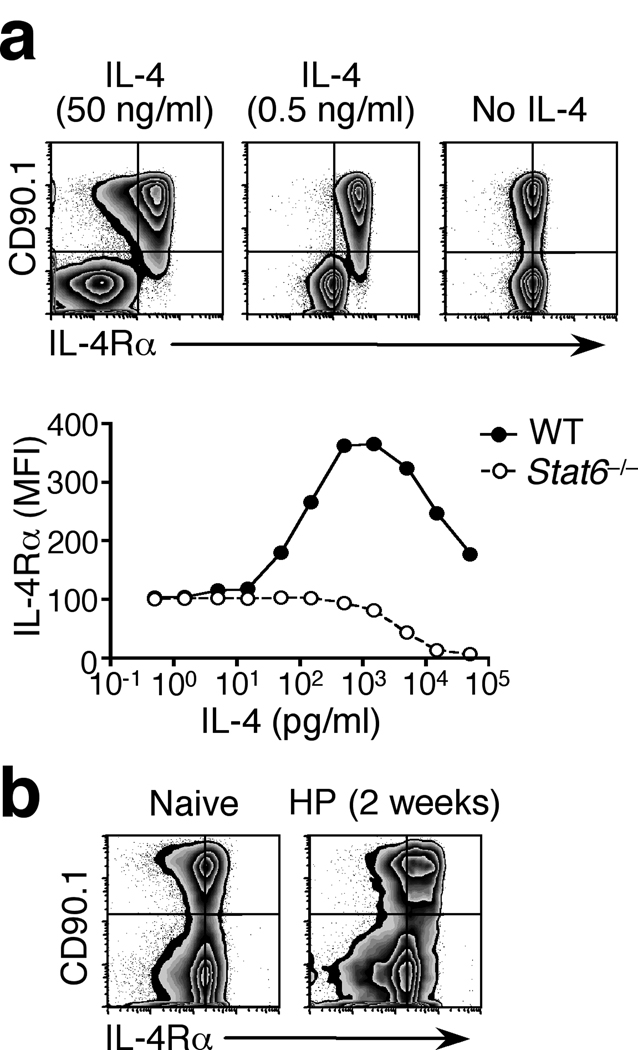
Although the STAT6 phosphorylation and IL-4Rα upregulation seen in the draining mesLN during H. polygyrus infection are evidence of IL-4 signaling19 (Fig. 1), the effective concentration of IL-4 within the lymph node is currently unknown. To address this, we exploited the IL-4-dependent changes in IL-4Rα surface expression. Increased IL-4Rα expression in response to IL-4 stimulation of lymphocytes is preceded by rapid internalization of the IL-4-IL-4R complex, followed by replenishment of IL-4Rα on the cell surface31. The second step is dependent on the transduction of IL-4 signals31 and, as such, STAT6-deficient cells are predicted to progressively lose surface IL-4Rα expression when exposed to increasing concentrations of IL-4. We co-cultured CD90.1+ wild-type and CD90.1− Stat6−/− CD4+ T cells in the presence of recombinant IL-4. Both populations expressed equivalent levels of IL-4Rα in the absence of IL-4 (Fig. 5a). In wild-type cells IL-4Rα upregulation was elicited by as little as 15 pg/ml of IL-4, while Stat6−/− cells lost surface IL-4Rα at concentrations exceeding 500 pg/ml (Fig. 5a). These changes in cell surface phenotype provided a traceable functional response that was calibrated to bench mark concentrations of IL-4. To use this system in vivo, we generated mixed BM chimeras reconstituted with equal parts of wild-type (CD90.1+) and Stat6−/− (CD90.1−) BM cells. Wild-type and Stat6−/− cells expressed identical levels of surface IL-4Rα in naïve mice and, upon H. polygyrus infection, wild-type cells displayed clear IL-4Rα upregulation (Fig. 5b). These data revealed a functionally effective in vivo IL-4 concentration equivalent to at least 15 pg/ml. The Stat6−/− cells present in the same lymph node showed no substantial loss of IL-4Rα expression (Fig. 5b), indicating that the local concentration of IL-4 in vivo is functionally equivalent to less than 500 pg/ml of recombinant cytokine. Thus, we estimate the effective concentration of IL-4 in vivo to be equivalent to 15–500 pg/ml.
Figure 5. Estimation of the effective interstitial IL-4 concentration in the Th2 reactive lymph node.
(a) IL-4Rα expression in naïve (CD44lo GFP−) CD4+ cells sorted from wild-type 4get (CD90.1+) and Stat6−/− 4get (CD90.1−) lymph nodes and co-cultured in the presence of graded concentrations of IL-4 for 24 h. No additional stimuli or antibodies were included. Vertical lines indicate the mode fluorescence intensity of IL-4Rα staining on cells cultured in medium alone. Graph illustrates geometric mean fluorescence intensity of IL-4Rα staining on each gated population. (b) IL-4Rα expression in CD4+ GFP− cells from the mesLN of mixed BM chimeras (1:1 ratio of wild-type CD90.1+ 4get and CD90.1− Stat6−/− 4get cells) infected with H. polygyrus (HP) and analyzed 2 weeks later. Data shown are representative of 4 mice per group. Both panels are representative of two to four independent experiments.
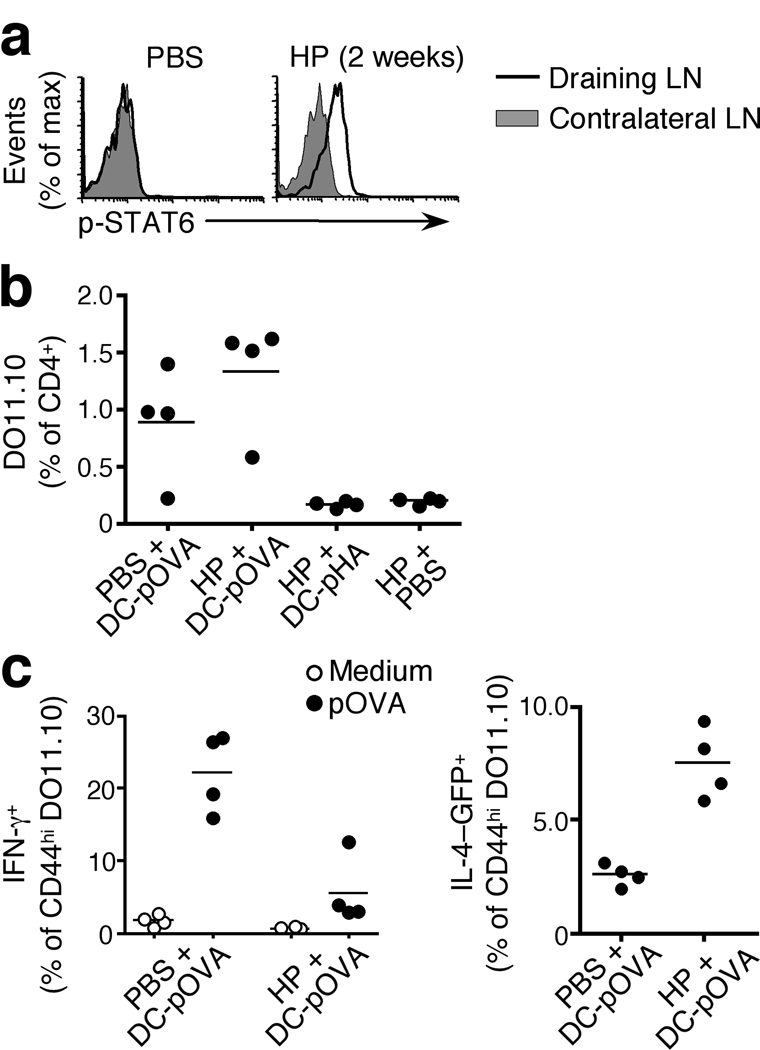
IL-4 conditioning overrides Th1 instruction
The ubiquitous and sustained phosphorylation of STAT6 in the Th2 reactive lymph node (Fig. 1, 3) and the potent ability of IL-4 to promote Th2 differentiation15, 32 suggest that an IL-4-conditioned lymph node is likely to favor Th2 polarization in naïve bystander CD4+ T cells upon encounter of cognate antigen. To test this hypothesis, we established a Th2 reactive lymph node by immunizing mice with H. polygyrus larvae into the footpad (Supplementary Fig. 7) and, by adoptive transfer, added a cohort of CD4+ DO11.10 TCR transgenic 4get cells that are specific for an OVA peptide not present in H. polygyrus. The DO11.10 cells in the draining lymph node were STAT6 phosphorylated within 18 h of transfer (Fig. 6a). These animals were then immunized with dendritic cells (DC) co-pulsed with heat-killed Y. pestis, which stimulates IL-12 release and directs Th1 polarization33, and either OVA peptide or an irrelevant control peptide (pHA). All OVA-pulsed DC resulted in a marked expansion of the DO11.10 population irrespective of previous conditioning of the lymph node (Fig. 6b). As expected33, DC pulsed with Y. pestis elicited a robust Th1 response in naïve lymph nodes, revealed by the production of IFN-γ upon antigen-specific stimulation and the low frequency of the IL-4+ (GFP+ in reporter mice) in the activated 4get DO11.10 population (Fig. 6c). In contrast, when the same Y. pestis-pulsed DC were injected into a footpad draining into a Th2 reactive, IL-4-conditioned lymph node, the frequency of IFN-γ-producing cells was markedly decreased and that of GFP+ DO11.10 cells correspondingly increased (Fig. 6c). These data demonstrate that the conditioning of a Th2 reactive lymph node is sufficient to override Th1 instruction by DC.
Figure 6. IL-4 conditioning of naïve lymphocytes alters subsequent T helper cell polarization.
(a) STAT6 phosphorylation in 4get DO11.10 TCR Tg CD4+ cells 18 h post-transfer into 4get mice that had been immunized with H. polygyrus larvae in the footpad 6 d before adoptive transfer. Draining and contralateral popliteal lymph nodes were analyzed. (b) Frequency of DO11.10 cells in draining popliteal lymph nodes of 4get mice injected with H. polygyrus and adoptively transferred with DO11.10 cells, as in (a), and immunized 7 days later into the same footpad with Y. pestis -pulsed DC loaded with OVA peptide (pOVA) or an irrelevant peptide control (pHA). (c) Cytokine polarization of transferred DO11.10 cells as assessed 7 d after DC immunization. IFN-γ was measured by intracellular staining following stimulation of lymph node cells for 18h in the presence or absence of OVA peptide. IL-4 expression was quantified using the 4get reporter and is shown as the frequency of GFP+ cells within the activated (CD44hi) DO11.10 population. Each data point represents an individual mouse and all panels are representative of two to four independent experiments with 4 mice per group.
DISCUSSION
We show here that during infection the canonical Th1 and Th2 cytokines, IFN-γ and IL-4, permeate the reactive lymph node and signal to the majority of cells therein. We demonstrate that infection with a gastrointestinal helminth parasite induces sustained and ubiquitous STAT6 phosphorylation reflective of direct IL-4 signaling in the Th2 reactive mesLN. The majority of B and T cells display surface-bound IL-4 and have increased IL-4Rα expression. Similarly, infection with a protozoan parasite elicits widespread STAT1 phosphorylation in the Th1 reactive lymph node that is associated with surface-bound IFN-γ and consequent MHC class I upregulation. Together our data reveal extensive signaling by T helper cytokines throughout a reactive lymph node.
The prevailing model of T helper cytokine function in the lymph node is one in which target cells are in intimate proximity to the source, often forming a focal point of direct cell contact7, 10–12, 34. Limited access to cytokines has been proposed as a key determinant in T helper polarization and B cell class switching7, 21. In contrast to this model, we report in vivo evidence for widespread cytokine signaling. During helminth infection IL-4 production in the Th2 reactive lymph node is restricted to Tfh cells in B cell areas7–9. Naive lymphocytes are highly motile, capable of speeds greater than 25 µm/min35, and one explanation for IL-4 signaling in the majority of B cells is that their movement within the lymph node temporarily places each one close to the cytokine source. However, several factors argue against this. First, the Tfh cells that provide IL-4 in vivo are engaged in B:T conjugates7 that represent a more stable interaction than the rapid scanning of APC by naïve lymphocytes35. Second, the phosphorylation of STAT6 persists only minutes after the cessation of IL-4 stimulation30 yet H. polygyrus elicits a sustained p-STAT6 signal in the bulk lymphocyte population for several days. Third, and perhaps most convincingly, IL-4 signaling is also evident in the majority of CD4+ and CD8+ T cells, most of which are restricted to the T zones and will exit the lymph node without entering B cell follicles36, 37. We propose that despite their punctate secretion, canonical T helper cytokines diffuse throughout the reactive lymph node. Microscopy studies have demonstrated that immobilized T helper lymphocytes release IL-4 multidirectionally but target IFN-γ secretion to the immunological synapse38. Our data suggests that, despite striking differences in the spatial patterns of their release on glass slides, both cytokines elicit ubiquitous signaling in vivo. Such pervasive cytokine signaling in the lymph node indicates that IFN-γ secretion by activated T cells during infection is less directional than that previously measured in static cultures in vitro.
Because STAT signaling is inherently transient, the continuous phosphorylation of STAT6 during H. polygyrus infection indicates a sustained presence of IL-4 in the mesLN30. In vitro, persistent stimulation with IL-4 was proposed to curtail STAT6 phosphorylation due to the concurrent induction of protein tyrosine phosphatases39, 40, although these data remain controversial30, 41. In vivo, the bulk population of lymphocytes in the Th2 reactive lymph node shows stable STAT6 phosphorylation. The in vitro observation of rapidly self-terminating IL-4 signaling could in part be explained by the use of supraphysiological concentrations of IL-4. Here we estimate the concentration of IL-4 in a Th2 reactive lymph node to be functionally equivalent to 15–500 pg/ml, one to two orders of magnitude lower than that which elicited phosphatase activity in culture39, 40.
A model of unrestricted access to cytokines emphasizes a critical role of receptor expression in regulating cytokine signaling. During Th2 differentiation, highly activated cells lose IL-4Rα expression18 in a process believed to prevent excessive immune responses42. Similarly, the IFN-γRα chain is expressed on naïve lymphocytes and downregulated on activated Th1 cells43. It has been proposed that cytokine signaling is controlled by the inclusion or exclusion of the relevant cytokine receptors from the immunological synapse21, 34. This would be consistent with the ability of effector cytokines to condition naive lymphocytes and influence their reaction to subsequent antigen encounter.
During H. polygyrus infection, IL-4 coordinates the commitment and expansion of the Th2 response6; in T. gondii infection, both IL-12 and IFN-γ are essential for appropriate Th1 polarization25. We show that IL-12 signaling is rare in the Th1 reactive lymph node and is evident only in a subset of antigen-experienced, CD44hi T cells, consistent with induction of the IL-12 receptor only as a consequence of specific TCR engagement26. Together these data illustrate a discrete logic in Th1 and Th2 development in vivo: while naïve cells remain ignorant of Th1-driving IL-12 signals until TCR ligation enables IL-12 receptor expression, Th2-polarizing IL-4 signals are promiscuous and affect bystander cells of all antigen specificities prior to antigen encounter. This might suggest that naïve CD4+ cells exposed to IL-4 will be biased towards Th2 differentiation, but that IL-12-driven Th1 development is less likely to be promoted by established bystander activity. However, this dominance in T cell polarization may differ if the Th1- and Th2- driving stimuli are delivered simultaneously. Immunization with mixed adjuvants elicits concurrent, independent Th1 and Th2 responses in vivo44 and it remains possible, particularly in response to coincident challenge, that local concentrations of cytokine activity can supersede the predisposition imposed by ambient signals.
We show that, during H. polygyrus infection, sustained IL-4 conditioning of the Th2 reactive lymph node alters the response of naïve, bystander cells to subsequent antigen encounter. This may contribute to the ability of helminth infections to modify the host response to concomitant pathogens or other antigens draining into the same lymph node45, 46. An estimated 1 billion people are chronically infected with helminth parasites47. Our data are therefore pertinent to vaccine design, as they suggest that oral and parenteral immunization strategies may be differently affected by endemic intestinal helminths.
METHODS
Mice
We used 4get (C.129-Il4tm1Lky/J)17 and KN216 IL-4 reporter mice, Il4−/− (KN2 homozygotes), Il4ra−/− 48, Stat6−/− (C.129S2-Stat6tm1Gru/J), Ifng−/− (B6.129S7-Ifngtm1Ts/J), Ifngr1−/−(B6.129S7-Ifngrtm1Agt/J), BALB/c CD90.2 or CD90.1 and C57BL/6 mice. TCR transgenic mice specific for the I-Ad–restricted ovalbumin323–339 epitope (DO11.10, C.Cg-Tg(DO11.10)10Dlo/J) were used on a BALB/c, CD90.2 background. Animals were kept under specific pathogen-free conditions in filter top cages at the animal facility of the Trudeau Institute and were used at 8–12 weeks of age. All experimental procedures involving mice were approved by the Institutional Animal Care and Use Committee.
Mixed bone marrow chimeras
4get CD90.1+ were lethally irradiated (950 rad) and reconstituted with a total of 1 × 107 donor BM cells from wild-type 4get CD90.1+ mice mixed in equal number with either Il4ra−/− 4get or Stat6−/− 4get CD90.2+ BM cells. Mice were allowed to reconstitute for 6–8 weeks before use.
Infections and immunizations
Mice were orally infected with either 200 H. polygyrus bakeri infective third-stage larvae, or 10 cysts of ME49 T. gondii, as described16, 49. 200 H. polygyrus larvae28 or 2500 S. mansoni eggs9 were injected into the footpad for s.c. immunization.
Immunoblot analyses
Unfractionated mesLN cells were washed in PBS and lysed in RIPA buffer (Sigma), for 10 min on ice, in the presence of Phosphatase Inhibitor Cocktails 1 and 2 (Sigma) and Protease Inhibitor Cocktail (Sigma). The active ingredients are canthardin, bromotetramisole, microstatin LR, sodium orthovanadate, sodium molybdate, sodium tartrate, imidazole, AEBSF, aprotinin, bestatin hydrochloride, E-64, leupeptin hemisulfate salt and pepstatin A. Lysates were spun to remove membrane fractions and protein concentration was estimated using the BCA Protein Assay kit (Pierce) and a BSA standard curve. Samples were separated on 4–12% polyacrylamide gels (NuPAGE, Invitrogen) run under reducing conditions, loading 15µg protein per lane, before transfer to a nitrocellulose membrane. Membranes were then probed with an anti-phosphoSTAT6 mAb (clone J71-773.58.11; BD). Blots were stripped and re-probed with an anti-STAT6 mAb (clone 23, BD) to confirm equal presence of the STAT protein. Bound Abs were detected using ECL (GE Healthcare).
Flow cytometry and cell sorting
Phosphorylation of STAT6 at tyrosine 641 (pY641), pY701 of STAT1 and pY693 of STAT4 was detected by intracellular staining with AlexaFluor488 or PE -conjugated, Phosflow mAb (clones J71-773.58.11, 4a and 38/p-Stat4 respectively; all BD), using PhosFlow Fix Buffer I and Perm Buffer III reagents (BD) according to the manufacturer’s instructions. Surface staining with mAb was performed as described16, using the following mAbs as biotin, FITC, PE, APC, APC-eFluor780, Pacific Blue, AlexaFluor405 or V500 conjugates: CD4 (RM4-5), CD8α (53-6.7), CD19 (ID3), CD44 (IM7), CD90.1 (OX-7), CD119 (IFN-γRα, GR-20), CD124 (IL-4Rα, M1), MHC class I (34-1-2S) and IL-4 (BVD6). Staining for surface IL-4 was amplified using biotinylated anti-PE (clone eBIO PE-DLF). Additional reagents included anti-human CD2 (RPA-2.10), streptavidin-PE and streptavidin-APC. All antibodies for surface staining were purchased from BD or eBioscience. Samples were acquired on a FACSCalibur or Canto II flow cytometer (BD) and analyzed using FlowJo (TreeStar) software. Cell sorting was performed on a FACSVantage flow cytometer (BD) equipped with DiVa electronics.
In vivo treatments
To activate endogenous production of IL-4 in vivo, 2µg of purified anti-CD3ε mAb (145-2C11; BD) was injected i.v. into mice infected with H. polygyrus 14 d earlier, as previously described16. To supply exogenous IL-4, 5µg of recombinant murine IL-4 (R&D systems) was injected i.v. into similarly infected or naïve mice.
In vitro cultures
To estimate the half-life of STAT6 phosphorylation, lymphocytes were harvested from the mesLN mice infected with H. polygyrus 2 weeks earlier, and cultured at 37°C in the presence of anti-IL-4 mAb (11B11; 20 µg/ml) for various periods of time. p-STAT6 was then measured by flow cytometry, as detailed above. To quantify changes in IL-4Rα expression in response to stimulation with IL-4, naïve (CD44lo) CD4+ T cells were sorted from lymph nodes of wild-type CD90.1+ and Stat6−/− CD90.2+ BALB/c mice and co-cultured at 1 × 106 each/ml in medium alone or in the presence of graded concentrations of recombinant IL-4. Flow cytometric analysis was performed 24 h later.
Cell transfer and DC immunization
2.5 × 106 lymph node cells from 4get DO11.10 CD90.2+ TCR transgenic donors were transferred i.v. into 4get CD90.1+ mice immunized with H. polygyrus into one footpad 6d earlier. In some experiments, popliteal lymph node cells were harvested from the recipient mice 18 h later and analyzed by flow cytometry. In others, recipient mice were immunized into the same footpad with 1 × 106 dendritic cells that had been pulsed with 2µM ovalbumin323–339 peptide or an irrelevant peptide control (influenza haemagluttinin126–138). Dendritic cells were grown from bone marrow precursors using recombinant GM-CSF (R&D systems) as described50, harvested and stimulated for 6 h in the presence of peptide Ag (2µM) and heat killed Yersinia pestis (strain Kim5, grown at 26°C)33 at a ratio of 10 bacteria to 1 DC. Mice were sacrificed 7 d after DC immunization and lymphocytes from the draining popliteal lymph nodes either analyzed directly for IL-4-GFP expression by flow cytometry, or restimulated with ovalbumin323–339 peptide (2µM) for 18h and their IFN-γ production measured by intracellular cytokine staining with a PE-conjugated mAb (XMG1.2) and the BD Fix/Perm kit, used according to the manufacturer’s instructions.
Statistical analysis
The differences between two data sets were assessed using a two-tailed, unpaired Student’s t-test.
Supplementary Material
ACKNOWLEDGEMENTS
We thank L.L. Johnson for T. gondii infections, J.-S. Lin and S.T. Smiley for provision of heat killed Yersinia pestis, E.J. Pearce for S. mansoni eggs, R.T. Robinson and A.M. Cooper for Ifng−/− mice and E.J. Pearce and D. Gray for discussion and comments on the manuscript. This work was supported by funds from the Trudeau Institute and the National Institutes of Health (grants AI072296 and AI076479).
Footnotes
AUTHOR CONTRIBUTIONS
G.P.-W. and M.M. designed the research and prepared the manuscript; G.P.-W. and K.M. performed the experiments; G.P-W. analyzed and interpreted the results; M.M. guided the study.
STATEMENT OF COMPETING INTERESTS
The authors have no competing financial interests.
REFERENCES
- 1.O'Garra A, Murphy K. Role of cytokines in determining T-lymphocyte function. Curr Opin Immunol. 1994;6:458–466. doi: 10.1016/0952-7915(94)90128-7. [DOI] [PubMed] [Google Scholar]
- 2.Anthony RM, Rutitzky LI, Urban JF, Jr, Stadecker MJ, Gause WC. Protective immune mechanisms in helminth infection. Nat Rev Immunol. 2007;7:975–987. doi: 10.1038/nri2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood. 2008;112:1557–1569. doi: 10.1182/blood-2008-05-078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shimoda K, et al. Lack of IL-4-induced Th2 response and IgE class switching in mice with disrupted Stat6 gene. Nature. 1996;380:630–633. doi: 10.1038/380630a0. [DOI] [PubMed] [Google Scholar]
- 5.Kaplan MH, Schindler U, Smiley ST, Grusby MJ. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity. 1996;4:313–319. doi: 10.1016/s1074-7613(00)80439-2. [DOI] [PubMed] [Google Scholar]
- 6.Finkelman FD, et al. Cytokine regulation of host defense against parasitic gastrointestinal nematodes: lessons from studies with rodent models. Annu Rev Immunol. 1997;15:505–533. doi: 10.1146/annurev.immunol.15.1.505. [DOI] [PubMed] [Google Scholar]
- 7.Reinhardt RL, Liang HE, Locksley RM. Cytokine-secreting follicular T cells shape the antibody repertoire. Nat Immunol. 2009;10:385–393. doi: 10.1038/ni.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.King IL, Mohrs M. IL-4-producing CD4+ T cells in reactive lymph nodes during helminth infection are T follicular helper cells. J Exp Med. 2009;206:1001–1007. doi: 10.1084/jem.20090313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zaretsky AG, et al. T follicular helper cells differentiate from Th2 cells in response to helminth antigens. J Exp Med. 2009;206:991–999. doi: 10.1084/jem.20090303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poo WJ, Conrad L, Janeway CA., Jr Receptor-directed focusing of lymphokine release by helper T cells. Nature. 1988;332:378–380. doi: 10.1038/332378a0. [DOI] [PubMed] [Google Scholar]
- 11.Kupfer A, Mosmann TR, Kupfer H. Polarized expression of cytokines in cell conjugates of helper T cells and splenic B cells. Proc Natl Acad Sci U S A. 1991;88:775–779. doi: 10.1073/pnas.88.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paul WE, Seder RA. Lymphocyte responses and cytokines. Cell. 1994;76:241–251. doi: 10.1016/0092-8674(94)90332-8. [DOI] [PubMed] [Google Scholar]
- 13.O'Shea JJ. Jaks, STATs, cytokine signal transduction, and immunoregulation: are we there yet? Immunity. 1997;7:1–11. doi: 10.1016/s1074-7613(00)80505-1. [DOI] [PubMed] [Google Scholar]
- 14.Takeda K, et al. Essential role of Stat6 in IL-4 signalling. Nature. 1996;380:627–630. doi: 10.1038/380627a0. [DOI] [PubMed] [Google Scholar]
- 15.Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE. The IL-4 receptor: signaling mechanisms and biologic functions. Annu Rev Immunol. 1999;17:701–738. doi: 10.1146/annurev.immunol.17.1.701. [DOI] [PubMed] [Google Scholar]
- 16.Mohrs K, Wakil AE, Killeen N, Locksley RM, Mohrs MA. Two-Step Process for Cytokine Production Revealed by IL-4 Dual-Reporter Mice. Immunity. 2005;23:419–429. doi: 10.1016/j.immuni.2005.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohrs M, Shinkai K, Mohrs K, Locksley RM. Analysis of type 2 immunity in vivo with a bicistronic IL-4 reporter. Immunity. 2001;15:303–311. doi: 10.1016/s1074-7613(01)00186-8. [DOI] [PubMed] [Google Scholar]
- 18.Perona-Wright G, Mohrs K, Mayer KD, Mohrs M. Differential regulation of IL-4Ralpha expression by antigen versus cytokine stimulation characterizes Th2 progression in vivo. J Immunol. 2010;184:615–623. doi: 10.4049/jimmunol.0902408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohara J, Paul WE. Up-regulation of interleukin 4/B-cell stimulatory factor 1 receptor expression. Proc Natl Acad Sci U S A. 1988;85:8221–8225. doi: 10.1073/pnas.85.21.8221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schindler C, Shuai K, Prezioso VR, Darnell JE., Jr Interferon-dependent tyrosine phosphorylation of a latent cytoplasmic transcription factor. Science. 1992;257:809–813. doi: 10.1126/science.1496401. [DOI] [PubMed] [Google Scholar]
- 21.Maldonado RA, et al. Control of T helper cell differentiation through cytokine receptor inclusion in the immunological synapse. J Exp Med. 2009;206:877–892. doi: 10.1084/jem.20082900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bach EA, et al. Ligand-induced autoregulation of IFN-gamma receptor beta chain expression in T helper cell subsets. Science. 1995;270:1215–1218. doi: 10.1126/science.270.5239.1215. [DOI] [PubMed] [Google Scholar]
- 23.Zhou F. Molecular mechanisms of IFN-gamma to up-regulate MHC class I antigen processing and presentation. Int Rev Immunol. 2009;28:239–260. doi: 10.1080/08830180902978120. [DOI] [PubMed] [Google Scholar]
- 24.Manetti R, et al. Natural killer cell stimulatory factor (interleukin 12 [IL-12]) induces T helper type 1 (Th1)-specific immune responses and inhibits the development of IL-4-producing Th cells. J Exp Med. 1993;177:1199–1204. doi: 10.1084/jem.177.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 26.Szabo SJ, Dighe AS, Gubler U, Murphy KM. Regulation of the interleukin (IL)-12R beta 2 subunit expression in developing T helper 1 (Th1) and Th2 cells. J Exp Med. 1997;185:817–824. doi: 10.1084/jem.185.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perona-Wright G, et al. Systemic but not local infections elicit immunosuppressive IL-10 production by natural killer cells. Cell Host Microbe. 2009;6:503–512. doi: 10.1016/j.chom.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ekkens MJ, et al. The role of OX40 ligand interactions in the development of the Th2 response to the gastrointestinal nematode parasite Heligmosomoides polygyrus. J Immunol. 2003;170:384–393. doi: 10.4049/jimmunol.170.1.384. [DOI] [PubMed] [Google Scholar]
- 29.Hotson AN, Hardy JW, Hale MB, Contag CH, Nolan GP. The T cell STAT signaling network is reprogrammed within hours of bacteremia via secondary signals. J Immunol. 2009;182:7558–7568. doi: 10.4049/jimmunol.0803666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andrews RP, Ericksen MB, Cunningham CM, Daines MO, Hershey GK. Analysis of the life cycle of stat6. Continuous cycling of STAT6 is required for IL-4 signaling. J Biol Chem. 2002;277:36563–36569. doi: 10.1074/jbc.M200986200. [DOI] [PubMed] [Google Scholar]
- 31.Galizzi JP, Zuber CE, Cabrillat H, Djossou O, Banchereau J. Internalization of human interleukin 4 and transient down-regulation of its receptor in the CD23-inducible Jijoye cells. J Biol Chem. 1989;264:6984. [PubMed] [Google Scholar]
- 32.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 33.Robinson RT, et al. Yersinia pestis evades TLR4-dependent induction of IL-12(p40)2 by dendritic cells and subsequent cell migration. J Immunol. 2008;181:5560–5567. doi: 10.4049/jimmunol.181.8.5560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maldonado RA, Irvine DJ, Schreiber R, Glimcher LH. A role for the immunological synapse in lineage commitment of CD4 lymphocytes. Nature. 2004;431:527–532. doi: 10.1038/nature02916. [DOI] [PubMed] [Google Scholar]
- 35.Miller MJ, Wei SH, Parker I, Cahalan MD. Two-photon imaging of lymphocyte motility and antigen response in intact lymph node. Science. 2002;296:1869–1873. doi: 10.1126/science.1070051. [DOI] [PubMed] [Google Scholar]
- 36.Campbell DJ, Butcher EC. Rapid acquisition of tissue-specific homing phenotypes by CD4(+) T cells activated in cutaneous or mucosal lymphoid tissues. J Exp Med. 2002;195:135–141. doi: 10.1084/jem.20011502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weninger W, Crowley MA, Manjunath N, von Andrian UH. Migratory properties of naive, effector, and memory CD8(+) T cells. J Exp Med. 2001;194:953–966. doi: 10.1084/jem.194.7.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huse M, Lillemeier BF, Kuhns MS, Chen DS, Davis MM. T cells use two directionally distinct pathways for cytokine secretion. Nat Immunol. 2006;7:247–255. doi: 10.1038/ni1304. [DOI] [PubMed] [Google Scholar]
- 39.Haque SJ, et al. Receptor-associated constitutive protein tyrosine phosphatase activity controls the kinase function of JAK1. Proc Natl Acad Sci U S A. 1997;94:8563–8568. doi: 10.1073/pnas.94.16.8563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haque SJ, Harbor P, Tabrizi M, Yi T, Williams BR. Protein-tyrosine phosphatase Shp-1 is a negative regulator of IL-4- and IL-13-dependent signal transduction. J Biol Chem. 1998;273:33893–33896. doi: 10.1074/jbc.273.51.33893. [DOI] [PubMed] [Google Scholar]
- 41.Hanson EM, Dickensheets H, Qu CK, Donnelly RP, Keegan AD. Regulation of the dephosphorylation of Stat6. Participation of Tyr-713 in the interleukin-4 receptor alpha, the tyrosine phosphatase SHP-1, and the proteasome. J Biol Chem. 2003;278:3903–3911. doi: 10.1074/jbc.M211747200. [DOI] [PubMed] [Google Scholar]
- 42.Tanaka Y, et al. T helper type 2 differentiation and intracellular trafficking of the interleukin 4 receptor-alpha subunit controlled by the Rac activator Dock2. Nat Immunol. 2007;8:1067–1075. doi: 10.1038/ni1506. [DOI] [PubMed] [Google Scholar]
- 43.Skrenta H, Yang Y, Pestka S, Fathman CG. Ligand-independent down-regulation of IFN-gamma receptor 1 following TCR engagement. J Immunol. 2000;164:3506–3511. doi: 10.4049/jimmunol.164.7.3506. [DOI] [PubMed] [Google Scholar]
- 44.Toellner KM, et al. T helper 1 (Th1) and Th2 characteristics start to develop during T cell priming and are associated with an immediate ability to induce immunoglobulin class switching. J Exp Med. 1998;187:1193–1204. doi: 10.1084/jem.187.8.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sutton TL, et al. Anti-Inflammatory mechanisms of enteric Heligmosomoides polygyrus infection against trinitrobenzene sulfonic acid-induced colitis in a murine model. Infect Immun. 2008;76:4772–4782. doi: 10.1128/IAI.00744-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elliott DE, et al. Colonization with Heligmosomoides polygyrus suppresses mucosal IL-17 production. J Immunol. 2008;181:2414–2419. doi: 10.4049/jimmunol.181.4.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bethony J, et al. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet. 2006;367:1521–1532. doi: 10.1016/S0140-6736(06)68653-4. [DOI] [PubMed] [Google Scholar]
- 48.Mohrs M, et al. Differences between IL-4- and IL-4 receptor alpha-deficient mice in chronic leishmaniasis reveal a protective role for IL-13 receptor signaling. Journal of Immunology. 1999;162:7302–7308. [PubMed] [Google Scholar]
- 49.Mayer KD, et al. The functional heterogeneity of type 1 effector T cells in response to infection is related to the potential for IFN-gamma production. J Immunol. 2005;174:7732–7739. doi: 10.4049/jimmunol.174.12.7732. [DOI] [PubMed] [Google Scholar]
- 50.MacDonald AS, Straw AD, Bauman B, Pearce EJ. CD8- dendritic cell activation status plays an integral role in influencing Th2 response development. J Immunol. 2001;167:1982–1988. doi: 10.4049/jimmunol.167.4.1982. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.