Abstract
Agents that are safe, affordable and efficacious are urgently needed for prevention of chronic diseases such as cancer. Sesamin, a lipid-soluble lignan is one such agent that belongs to a class of phytoestrogens, isolated from sesame (Sesamum indicum), and has been linked with prevention of hyperlipidemia, hypertension, and carcinogenesis through an unknown mechanism. Because the transcription factor NF-κB has been associated with inflammation, carcinogenesis, tumor cell survival, proliferation, invasion and angiogenesis of cancer, we postulated that sesamin might mediate its effect through the modulation of NF-κB pathway. We found that sesamin inhibited the proliferation of wide variety of tumor cells including leukemia, multiple myeloma, and cancers of the colon, prostate, breast, pancreas and lung. Sesamin also potentiated TNF-α induced apoptosis and this correlated with suppression of gene products linked to cell survival (e.g.; Bcl-2 and survivin), proliferation (e.g.; cyclin D1), inflammation (e.g.; COX-2), invasion (e.g.; MMP-9, ICAM-1) and angiogenesis (e.g.; VEGF). Sesamin downregulated constitutive and inducible NF-κB activation induced by various inflammatory stimuli and carcinogens; and inhibited degradation of IκBα, the inhibitor of NF-κB through the suppression of phosphorylation of IκBα and inhibition of activation of IκBα protein kinase (IKK); thus resulting in the suppression of p65 phosphorylation and nuclear translocation, and NF-κB-mediated reporter gene transcription. The inhibition of IKK activation was found to be mediated through the inhibition of TAK1 kinase. Overall our results demonstrated that sesamin may have potential against cancer and other chronic diseases through the suppression of pathway linked to the NF-κB signaling.
Introduction
Most modern drugs, commonly referred to as targeted therapies, designed within last two decades for cancer are not so safe, are highly ineffective and are unaffordable. Thus agents, which can overcome these problems, are needed not only for treatment but also for the prevention of cancer. “Let food be thy medicine or medicine be thy food” proclaimed by Hippocrates almost 25 centuries ago; or twelve serving of fruits and vegetables daily, proclaimed recently by National cancer Institute; suggests to look for agents in the diet that may have potential for cancer. Sesamin, a class of phytoestrogens, is one such agent isolated from the oil of sesame seeds (Sesamum indicum), that has been shown to exhibit variety of activities (1). Sesame contains water-soluble lignan glycosides (sesaminol triglucoside and sesaminol diglucoside) and lipid soluble lignans (sesamin and sesamolin). Sesamin, a furofuran class of lignin, has been found to reduce hypertension (2), lowers serum and hepatic cholesterol (3–5) and decreases serum triglycerides (6), inhibits endotoxin-mediated shock (7) and suppress 7,12-dimethylbenz[a]anthracene (DMBA)-induced mammary carcinogenesis (8). These effects of sesamin were shown to be due to its ability to inhibit delta 5 desaturase involved in polyunsaturated fatty acid synthesis (9); decrease arachidonic acid and prostaglandin synthesis (8, 10); increase serum levels of gamma tocopherol (11); diminish the endotoxin-induced interleukin (IL)-1β, prostaglandin E2 (PGE2) and thromboxane B2 production (7); and abrogate the production of IL-6 (10).
In cell culture, sesamin has been shown to inhibit the growth of variety of tumor cells including leukemia (12, 13) and gastric cancer (14). Mechanism by which sesamin mediates anticancer effects are not fully understood. However, its role in suppression of reactive oxygen species (ROS) and mitogen-activated protein kinase (MAPK) activation (15), inhibition of nitric oxide (NO) production (16), and inflammatory cytokine production (17), and inhibition of expression of cyclin D1 in human cancer cells (18), have been reported.
Since inflammation, survival, proliferation, chemosensitization, carcinogenesis, invasion, and angiogenesis; all have been linked to the transcription factor NF-κB-regulated gene products, we postulated that sesamin must mediate its effects through modulation of the NF-κB activation pathway. This factor belongs to Rel family and has been shown to be activated by a wide array of stimuli, which include various types of carcinogens, inflammatory stimuli, and growth factors (19). Aberrant activation of NF-κB cascade has been linked to the expression of genes that linked with survival, proliferation, invasion, and metastasis of tumors (20).
Thus in the present report we investigated how sesamin modulates NF-κB-mediated cellular responses, gene products and signaling pathway. We demonstrate that sesamin suppresses the proliferation of a wide variety of tumor cells, downregulates the expression of gene products that mediate inflammation, tumor cell survival, cell proliferation, cell invasion and angiogenesis, abrogates both constitutive and inducible NF-κB activation pathway stimulated by various carcinogens and inflammatory stimuli through the inhibition of activation of IκBα protein kinase.
Materials and methods
Reagents
A 50 mM solution of sesamin was prepared initially in dimethyl sulfoxide, stored as small aliquots at −20°C, and then thawedand diluted in a cell culture medium as required. Bacteria-derivedhuman recombinant tumor necrosis factor (TNF), purified to homogeneity with a specific activity of 5 × 107 U/mg, was provided by Genentech (South San Francisco, CA). Penicillin, streptomycin, RPMI1640, Iscove’s modified Dulbecco’s medium, and Dulbecco’s modified Eagle’s medium were obtained from Invitrogen (Grand Island, NY). Fetal bovine serum (FBS) was supplied by Atlanta Biologicals (Lawrenceville, GA). Antibodies against p65, p50, IκBα, cyclin D1, cyclooxygenase-2, Bcl-2, and intercellular adhesion molecule (ICAM)-1 were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). For immunocytochemistry, an antibody against p65 was obtained from Abcam (Cambridge, MA). An anti-vascular endothelial growth factor (VEGF) antibody was purchased from Thermo Scientific (Fremont, CA). Phosphospecific anti-IκBα (Ser32/36) and phosphospecific anti-p65 (Ser536) antibodies were purchased from Cell Signaling Technology (Beverly, MA). Anti-IKK-α and anti-IKK-β antibodies were provided by Imgenex (San Diego, CA).
Cell lines
The cell lines KBM-5 (human chronic myeloid leukemia), A293 (human embryonic kidney carcinoma), H1299 (human lung adenocarcinoma), HCT116 (human epithelial colon cancer) and RPMI-8226 (human multiple myeloma) were obtained from the American Type Culture Collection. KBM-5 cells were culturedin Iscove’s modified Dulbecco’s medium with 15% FBS; H1299 and RPMI-8226 cells were cultured in RPMI1640; and A293 and HCT116 cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% FBS. Culture media were supplemented with 100 U/mL penicillin and 100 μg/mL streptomycin.
Electrophoretic mobility shift assay
To assess NF-κB activation, nuclear extracts were prepared and electrophoretic mobility shift assay (EMSA) was performed as described previously (21). Briefly, nuclear extracts prepared from treated cells and untreated cells (1×106/mL) were incubated with 32P-end-labeled 45-mer double-stranded oligonucleotide (15μg protein with 16 fmol DNA) from the HIV long terminal repeat, 5 -TTGTTACAAGGGACTTTCCGCTGGGGACTTTCCAGGGAGGCGTGG-3 (boldface indicates NF-κB binding sites), for 30 min at 37°C. The DNA-protein complex formed was separated from free oligonucleotide on 6.6% native polyacrylamide gels. The dried gels were visualized with a Storm 820, and radioactive bands were quantified using ImageQuant software (GE Healthcare, Piscataway, NJ).
Western blot analysis
Western blot analysis was performed as described previously (22). Briefly 30μg protein was resolved on SDS-PAGE and probed with specific antibodies according to the manufacturer’s recommended protocol. The blots were washed, exposed to HRP-conjugated secondary antibodies for 2 hr, and finally detected by enhanced chemiluminescence (ECL) reagent (GE Healthcare). The bands were quantified with a Personal Densitometer Scan v1.30 using ImageQuant software version 3.3 (GE Healthcare).
IKK assay
IKK assay was performed as described previously (22). Briefly, the IKK complex from whole-cell extracts was precipitated with IKK-α and then pulled down with protein A/G-agarose beads (Pierce, Rockford, IL). After 2 hr, the beads were washed with lysis buffer and resuspended in a kinase assay mixture containing 50 mM HEPES (pH 7.4), 20 mM MgCl2, 2 mM DTT, 20 mCi [γ-32P] ATP, 10mM unlabeled ATP, and 2 μg substrate GST-IκBα (amino acids 1–54) and incubated at 30°C for 30 min. Boiling with SDS sample buffer for 5 min terminated the reaction. Finally, the protein was resolved on 10% SDS-PAGE, the gel was dried, and the radioactive bands were visualized with a Storm 820. To determine the total amounts of IKK-α and IKK-β in each sample, 30 μg of whole-cell proteins was resolved on 10% SDS-PAGE, electrotransferred to a nitrocellulose membrane, and then blotted with either anti–IKK-α or anti–IKK-β antibodies.
Immunocytochemical analysis for NF-κB p65 localization
To determine the effect of sesamin on TNF-induced nuclear translocation of p65 we used an immunocytochemical method. Slides were analyzed under a fluorescence microscope (Labophot-2; Nikon), and images were captured using a Photometrics Coolsnap CF color camera (Nikon) as described previously (22).
NF-κB-dependent reporter gene expression assay
An NF-κB-dependent reporter gene expression assay was performed as described previously (23). The effect of sesamin on TNF-, TNF receptor (TNFR)-, TNFR-associated death domain (TRADD)-, NF-κB-inducing kinase (NIK)-, and transforming growth factor β-activated kinase 1(TAK1)/TAK-1 binding protein-1 (TAB1)-dependent reporter gene expression was analyzed using a secretory alkaline phosphatase (SEAP) assay.
Cell proliferation assay
The tumor cell proliferation was evaluated using a modified tetrazolium salt 3-(4-5-dimethylthiozol-2-yl) 2-5-diphenyl-tetrazolium bromide (MTT) assay. Briefly, cells (2000 cells/well) were incubated in the presence or absence of the indicated concentration of sesamin for 0, 2, 4, and 6 days. Thereafter, 20 μL of MTT solution (5 mg/mL in PBS) was added to each well. After 2 hr of incubation at 37°C, 0.1 mL of the extraction buffer (20% SDS, 50% dimethyl formamide) was added. After incubation overnight at 37°C, the optical densities at 570 nm were measured using a 96-well plate reader.
Live/Dead assay
The Live/Dead assay (Invitrogen), which assesses plasma membrane integrity, was used to measure the intracellular esterase activity. This assay was performed as described previously (24).
Results
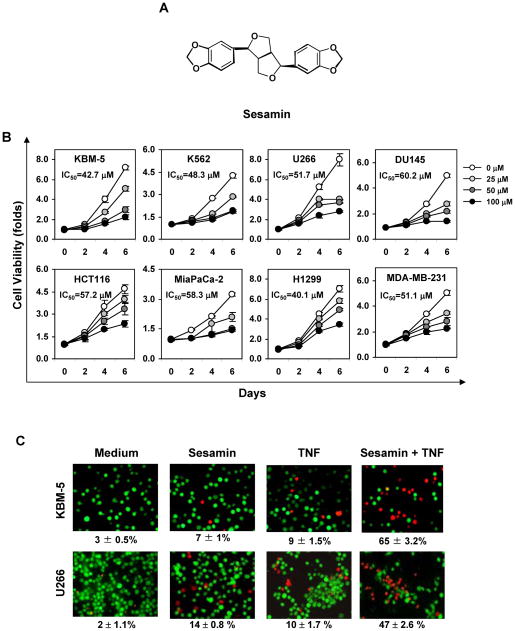
Sesamin is a lignan with a structure as shown (Fig. 1A). The aim of the present study is to determine the effect of this lignan on NF-κB-mediated cellular responses, NF-κB-regulated gene products and on the signaling pathway leading to NF-κB activation. Most of our studies were done on KBM -5 cells as these cells express both types of TNF receptors. TNF was used for most studies because of a variety of reasons; first, TNF is a primary mediator of inflammation; second, TNF activates both apoptosis and NF-κB; third, TNF induced NF-κB activation cascade is well characterized; and fourth, TNF-induced NF-κB can downregulate apoptosis. Under the conditions we examined the effect of sesamin on NF-κB pathway, it had no effect on cell viability; thus downregulation of NF-κB was not due to decrease in viability.
FIGURE 1.
(A) Chemical structure of sesamin. (B) Sesamin suppresses tumor cell proliferation. Cells were incubated with 0, 25, 50, and 100 μM sesamin for different days. Cell proliferation was then analyzed by the MTT method as described under “Materials and Methods.” (C) Sesamin potentiates TNF-induced cytotoxicity. KBM-5 (1 × 106) or U266 (1 × 106) cells were incubated with 100 μM sesamin for 12 h and then incubated with 1 nM TNF for 24 h. The cells were stained with a Live/Dead assay reagent for 30 min and then analyzed under a fluorescence microscope as described under “Materials and Methods.”
Sesamin suppresses cell proliferation in various tumor cells
We examined whether sesamin can modulate the proliferationof various tumor cell types. As shown in Fig 1B, sesamin by itself suppressed the proliferation of human leukemic cells (such as KBM-5 and K562), and multiple myeloma cells (U266). The suppression was both dose- and time-dependent. Besides these cells, sesamin also inhibited the proliferation of solid tumor cells, such as human pancreatic cancer MiaPaCa-2 cells, human colon cancer HCT-116 cells, human prostate cancer DU145, human lung adenocarcinoma H1299 and human breast cancer MDA-MB-231 cells (Fig. 1B). The fifty percent inhibitory sesamin dose was found to be 42.7, 48.3, 51.7, 60.2, 57.2, 58.3, 40.1, and 51.1 μM for KBM-5, K562, U266, DU145, HCT116, MiaPaCa-2, H1299 and MDA-MB-231 cells, respectively.
Sesamin potentiated TNF-induced apoptosis
We sought to determine whether sesamin affects TNF-induced apoptosis in human chronic myeloid leukemia KBM-5 cells. Using a Live/Dead assay, which measures intracellular esterase activity and assesses plasma membrane integrity, we found that sesamin increased the TNF-induced apoptosis from 9% to 65% in KBM-5 cells (Fig. 1C, top panel).
To determine whether the effect is cell type specific, we also examine the effect of sesamin on TNF-induced apoptosis in human multiple myeloma U266 cells. Like KBM-5 cells, sesamin also increased the TNF-induced apoptosis from 10 % to 47% in U266 cells (Fig 1C, bottom panel).
Sesamin inhibited TNF-induced cell survival gene products
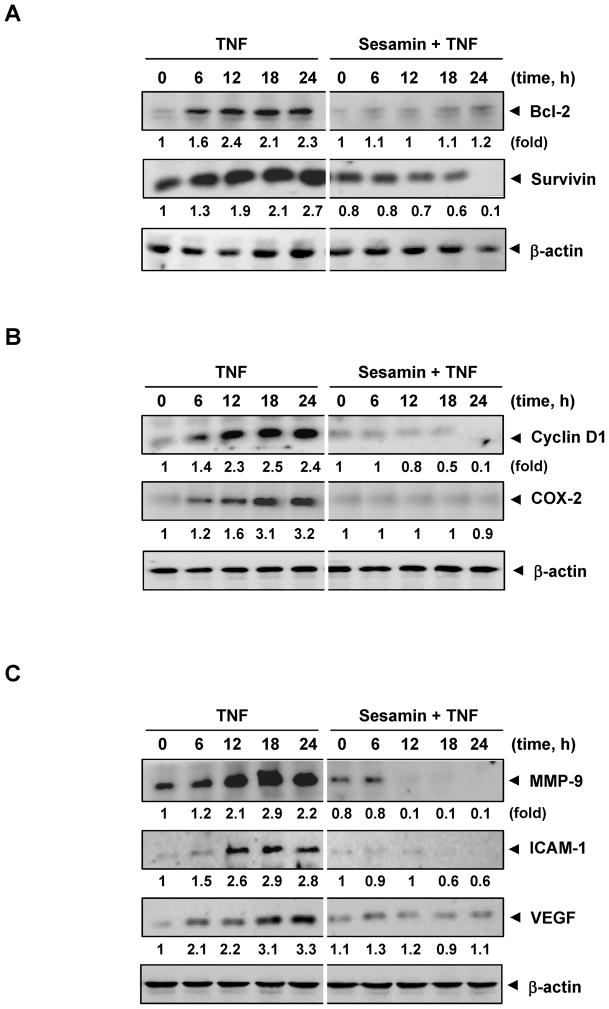
How sesamin potentiates the apoptotic effects of TNF was investigated. One of the possible mechanisms is through downregulation of cell survival gene products, such as Bcl-2 and survivin. We found that TNF-induced the expression of Bcl-2 and survivin and sesamin inhibited this expression (Fig. 2A).
FIGURE 2.
(A) Sesamin suppresses the expression of TNF-induced tumor cell survival proteins. KBM-5 cells were incubated with 100 μM sesamin for 12 h and then treated with 1 nM TNF for the indicated times. Whole cell extracts were prepared and analyzed by Western blotting using the indicated antibodies. (B and C) Sesamin inhibits the expression of tumor cell proliferative and metastatic proteins. KBM-5 cells were incubated with 100 μM sesamin for 12 h and then treated with 1 nM TNF for the indicated times. Whole cell extracts were prepared and analyzed by Western blotting using the indicated antibodies. Anti-β-actin antibody was used as a loading control. Densitometric values of bands were corrected based on β-actin and were expressed relative to that of untreated cells, which was set as 1.0.
Sesamin inhibited the TNF-induced expression of cell-proliferative gene products
Both cyclin D1 and COX-2 have been linked with the proliferation of different types of tumor cells. Thus, we investigated the effect of sesamin on the expression of cyclin D1 and COX-2 induced by TNF treatment. We found that TNF induced the expression of these gene products and that pre-treatment with sesamin inhibited this expression (Fig. 2B).
Sesamin inhibited the expression of gene products involved in invasion and angiogenesis
TNF also induces the expression of genes involved in adhesion (e.g. ICAM-1) and invasion (MMP-9). Whether sesamin affects the expression of ICAM-1 and MMP-9, was examined. As shown in Fig 2C, TNF-induced the expression ICAM-1 and MMP-9; and sesamin suppressed the expression.
VEGF plays a major role in the process of tumor angiogenesis (25). We found that TNF-induced expression of VEGF and sesamin inhibited the expression (Fig. 2C).
Sesamin inhibited the NF-κB activation induced by TNF in a dose- and time-dependent manner
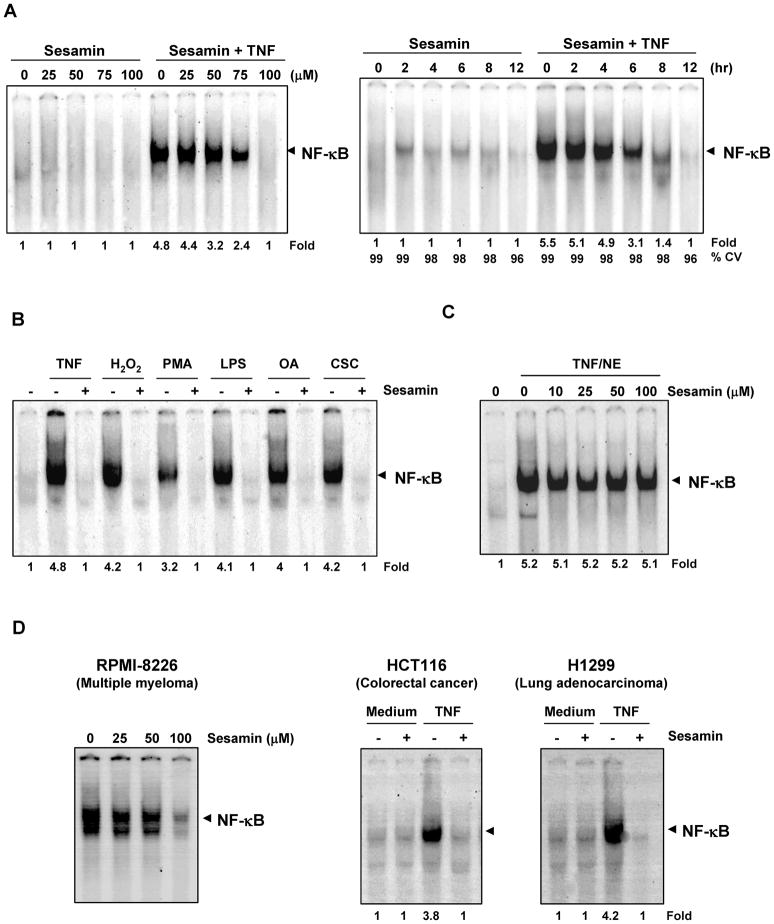
All the cellular response and gene products modulated by sesamin as described above are regulated by NF-κB activation. Whether sesamin can suppress NF-κB activation, was examined directly. For this we exposed KBM-5 cells to sesamin at different concentrations and then exposed them to TNF and examined for the activation of NF-κB. We found that sesamin by itself had no effect on the activation of NF-κB. However, it suppressed the TNF-induced NF-κB activation in a dose-dependent manner, with maximum inhibition occurring at a concentration of 100 μM (Fig. 3A, left panel). Moreover when we accessed the cells viability under these conditions it was greater than 90%.
FIGURE 3.
(A) Dose- and time-dependent effect of sesamin on TNF-induced NF-κB activation. (Left) KBM-5 cells were incubated with the indicated concentrations of sesamin for 12 h and treated with 0.1 nM TNF for 30 min. (Right) KBM-5 cells were pre-incubated with 100 μM sesamin for the indicated time points and then treated with 0.1 nM TNF for 30 min. The nuclear extracts were assayed for NF-κB activation by EMSA. (B) Sesamin inhibits NF-κB activation induced by CSC, H2O2, PMA, LPS, okadaic acid and TNF. KBM-5 cells were preincubated with 100 μM sesamin for 12 h and then treated with 0.1 nM TNF for 30 min, 250 μM H2O2 for 2 hr, 25 ng/mL PMA for 2 hr, 10 μg/mL LPS for 1 hr, 500 nM okadaic acid for 4 hr, and 10 μg/mL CSC for 2 hr. Nuclear extracts were analyzed for NF-κB activation by EMSA. (C) Sesamin did not inhibit direct binding of NF-κB to DNA. Nuclear extracts were prepared from untreated KBM-5 cells or cells treated with 0.1 nM TNF for 30 min, incubated for 30 min with indicated concentrations of sesamin for 30 min and EMSA was performed. (D) Effect of sesamin on constitutive and inducible NF-κB activation. (Left) Human multiple myeloma cells (RPMI-8226) were incubated with the indicated concentrations of sesamin for 12 hr, the nuclear extracts were prepared and analyzed for NF-κB activation by EMSA. (Right) Human colorectal cancer cells (HCT116) and human lung adenocarcinoma cells (H1299) were pretreated with 100 μM sesamin for 12 hr, treated with 0.1 nM TNF for 30 min, and then EMSA was performed.
Whether suppression of NF-κB by sesamin was time-dependent, was also examined. For this KBM-5 cells were treated with sesamin for different time intervals followed by 30 minute exposure to TNF. We observed that sesamin inhibited the activation of NF-κB triggered by TNF in a time-dependent manner, with optimum inhibition occurring at 12 h (Fig. 3A, right panel).
Sesamin inhibited NF-κB activation induced by carcinogens and other inflammatory stimuli
Earlier studies reported from our laboratory and by others clearly showed that a wide variety of agents which include cigarette smoke condensate (CSC), tumor promoters such as okadaic acid (OA), phorbol myristate acetate (PMA), inflammatory agents such as hydrogen peroxide and lipopolysaccharide (LPS), can activate NF-κB but the mechanisms by which these agents induce activation of NF-κB vary significantly (22, 26). Whether sesamin affects NF-κB activation induced by all these agents, was examined. As shown in Fig 3B, sesamin suppressed the activation NF-κB induced by all these agents. So it can be concluded that this lignan acts at a step in the NF-κB activation pathway that is common to all of these agents.
Sesamin does not have any effect on the binding of NF-κB p65 subunit to the DNA
We also investigated whether this lignan can directly interact with the p65 subunit of NF-κB and inhibit its binding to DNA. Nuclear extracts isolated from TNF-treated KBM-5 cells were exposed to sesamin at different concentrations and then examined for binding to DNA. As given in Fig. 3C we found that sesamin did not modulate the p65 binding to DNA even at the highest dose.
Sesamin inhibited constitutive NF-κB expression
A wide variety of tumor cell types are known to harbor constitutively active form NF-κB which often results in chemoresistance and treatment failure (19). Multiple myeloma cell lines (e.g., RPMI-8226) are known to express constitutively active NF-κB (27). Whether sesamin affects NF-κB expression in these cells, was examined. For this we exposed cells to sesamin at different concentrations for 12 hr and then analyzed them for DNA binding by EMSA. We found that this lignan completely suppressed constitutive NF-κB activation in RPMI-8226 cells (Fig. 3D, left panel), indicating that this sesamin can suppress both inducible as well as constitutive NF-κB activation.
Inhibition of NF-κB activation by sesamin is not cell type-specific
Our next aim was to examine whether the inhibition of activation of NF-κB by sesamin is specific to a particular cell type. We treated human lung adenocarcinoma H1299 cell and human colon cancer cell line HCT116 to sesamin and then exposed to TNF. EMSA showed that this lignan inhibited the activation of NF-κB indicating that the effect is not cell type specific (Fig. 3D, right panel).
Sesamin inhibits TNF-dependent phosphorylation and degradation of IκBα
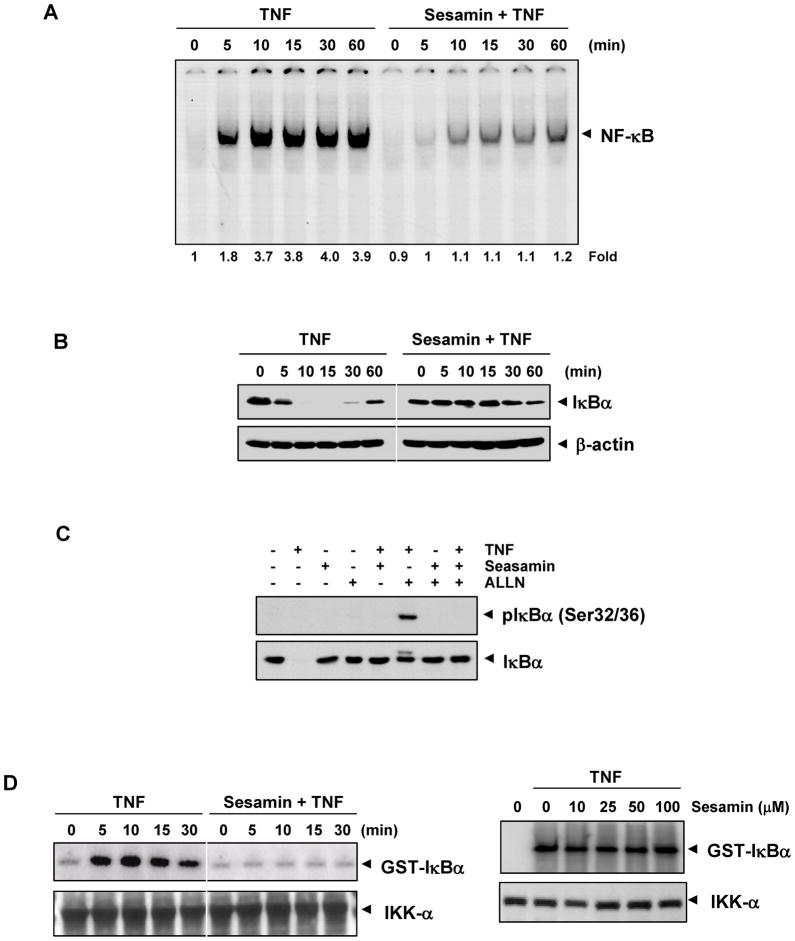
Translocation of NF-κB to the nucleus is accompanied by phosphorylation, ubiquitination, and degradation of IκBα, the inhibitory subunit associated with the NF-κB complex (28). To determine whether inhibition of TNF-induced NF-κB activation is associated with degradation of IκBα, we pretreated KBM-5 cells with sesamin and then exposed them to TNF at various time intervals. We analyzed nuclear extracts for NF-κB activation using EMSA and cytoplasmic extracts for IκBα degradation using Western blotting. TNF activated NF-κB in a time-dependent manner; however, in sesamin-pretreated cells we found that there is no activation of NF-κB (Fig. 4A).
FIGURE 4.
(A) Sesamin inhibits TNF-induced activation of NF-κB. KBM-5 cells were incubated with 100 μM sesamin for 12 hr, treated with 0.1 nM TNF for the indicated time intervals, and then analyzed by EMSA for NF-κB activation. (B) Effect of sesamin on TNF-induced degradation of IκBα. KBM-5 cells were incubated with 100 μM sesamin for 12 hr and treated with 0.1 nM TNF for the indicated times. Cytoplasmic extracts were prepared and analyzed by Western blotting using antibodies against anti-IκBα. Equal protein loading was evaluated by β-actin. (C) Effect of sesamin on phosphorylation of IκBα induced by TNF. Cells were preincubated with 100 μM sesamin for 12 hr, incubated with 50μg/ml N-acetyl-leucyl-leucyl-norleucinal (ALLN) for 30 min, and then treated with 0.1 nM TNF for 10 min. Cytoplasmic extracts were fractionated and then subjected to Western blot analysis using phospho-specific IκBα antibody. (D) Effect of sesamin on the activation of IKK by TNF. (Left) KBM-5 cells were preincubated with 100 μM sesamin for 12 hr, and then treated with 1 nM TNF for the indicated time points. Whole cell extracts were immunoprecipitated with antibody against IKK-α and analyzed by an immune complex kinase assay. To examine the effect of sesamin on the level of expression of IKK proteins, whole cell extracts were fractionated on SDS-PAGE and examined by Western blot analysis using anti-IKK-α antibody. (Right) Direct effect of sesamin on IKK activation induced by TNF. Whole cell extracts were prepared from KBM-5 cells treated with 1 nM TNF and immunoprecipitated with anti-IKK-α antibody. The immunocomplex kinase assay was performed in the absence or presence of the indicated concentration of sesamin.
Western blot analysis showed that TNF-induced IκBα degradation started at 5 min after TNF treatment and reached maximum level at 10 min. The resynthesis of IκBα started at 60 min after TNF exposure (Fig. 4B; left panel). However, we noticed that no degradation of IκBα in sesamin-pretreated cells (Fig. 4B; right panel). These results indicated that sesamin mediates its effect via suppression of TNF-induced IκBα degradation, which in turn leads to suppression of the activation of NF-κB.
We also decided to determine whether inhibition of TNF-induced degradation of IκBα was caused by inhibition of phosphorylation of IκBα. For this purpose we used the proteasome inhibitor N-acetyl-leucyl-leucyl-norleucinal (ALLN) to block this degradation. We performed a western blot analysis using an antibody that specifically recognized the IκBα phosphorylated at serine 32. The results of this analysis showed that TNF induced IκBα phosphorylation at serine 32 and that sesamin suppressed this phosphorylation (Fig. 4C).
Sesamin inhibits IκBα kinase (IKK) activation induced by TNF
TNF-induced activation of the enzyme IKK which triggers the phosphorylation of IκBα (28). We sought to determine whether in KBM-5 cells sesamin inhibits TNF-induced activation of IKK using immune complex assays. As given in Fig. 4D, TNF activated IKK in a time-dependent manner and sesamin suppressed TNF-induced activation of IKK. Neither TNF nor sesamin affected the expression of IKK-α (Fig. 4D, left panel).
We then decided to examine whether sesamin suppresses IKK activity directly by binding to IKK or indirectly by suppressing its activation. For this we incubated the immune complexes with various concentrations of sesamin and then examined the IKK activity in vitro. The results showed that sesamin did not directly inhibit activity of IKK (Fig. 4D, right panel).
TNF-induced translocation of NF-κB p65 subunit to nucleus is inhibited by sesamin
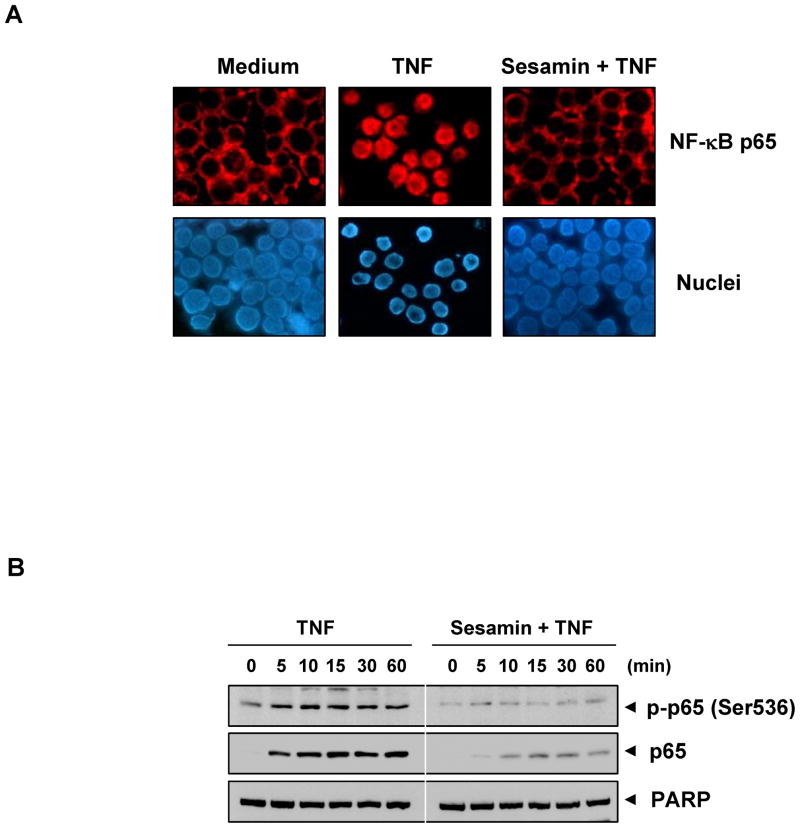
Once the degradation of IκBα started, it triggers the nuclear translocation of p65 subunit. We decided to determine whether sesamin has any effect on TNF-induced nuclear translocation of p65 subunit. Immunocytochemical analysis showed that sesamin suppressed the TNF-induced translocation of p65 subunit to the nucleus in KBM-5 cells (Fig. 5A). However in both untreated cells and cells pre-treated with sesamin, p65 was localized in the cytoplasm, whereas in cells treated with TNF alone, p65 subunit was translocated to the nucleus. These results support the conclusion that sesamin inhibits nuclear translocation of p65.
FIGURE 5.
(A) Sesamin inhibits TNF-induced nuclear translocation of p65 assayed by immunocytochemical analysis. KBM-5 cells were initially treated with 100 μM sesamin for 12 hr and then exposed to 0.1 nM TNF for 15 min. After cytospin, immunocytochemical analysis was done as described under “Materials and Methods.” (B) Sesamin inhibits TNF-induced phosphorylation of p65. KBM-5 cells were either untreated or pretreated with 100 μM sesamin for 12 hr and then treated with 0.1 nM TNF for the indicated times. Nuclear extracts were prepared and analyzed by Western blotting using p65 and phospho-specific p65 antibodies. For loading control of nuclear extracts, the membrane was reblotted with anti-PARP antibody, respectively.
Sesamin suppressed TNF-induced phosphorylation of NF-κB p65 subunit
It is now established that transcriptional activation of p65 requires phosphorylation at the serine 536 residues (29). Thus, we also sought to investigate the effect of sesamin on TNF-induced phosphorylation of p65. We found that in a time-dependent manner phosphorylation of p65 occurred in TNF-treated KBM-5 cells. We also observed that in sesamin pre-treated cells there was no phosphorylation of p65 (Fig. 5B).
Sesamin suppressed TNF-induced NF-κB-dependent reporter gene expression
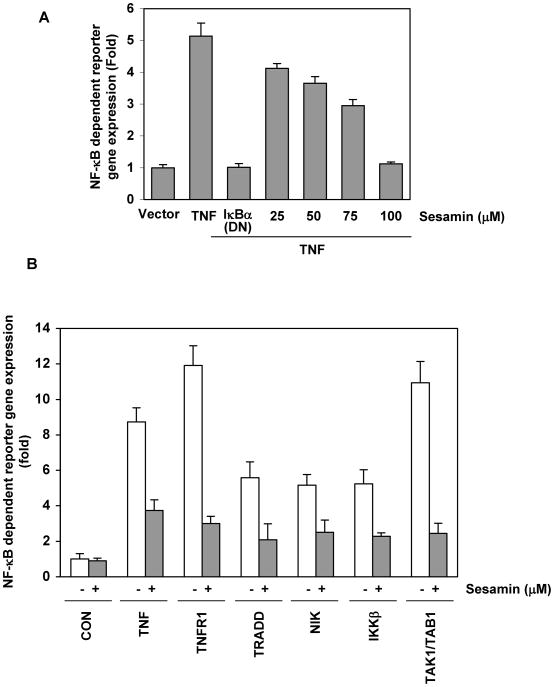
Although we showed using EMSA, that sesamin inhibited TNF-induced NF-κB expression, DNA binding alone does not always correlate with NF-κB-dependent gene transcription, suggesting that additional regulatory steps are involved. Thus, we decided to examine whether sesamin affects TNF-induced reporter gene transcription. For this, cells were transiently transfected with an NF-κB-regulated SEAP reporter construct, followed by treatment with sesamin, and then exposed to TNF. We found that TNF induced NF-κB reporter gene activity, and in a dose-dependent manner sesamin inhibited the TNF-induced NF-κB reporter activity (Fig. 6A).
FIGURE 6.
(A) Sesamin inhibits TNF-induced NF-κB-dependent reporter gene (SEAP) expression. A293 cells treated were transiently transfected with a NF-κB-containing plasmid linked to the SEAP gene. After 24 hr of transfection cells were treated with the indicated concentrations of sesamin for 12 hr and with 1 nM TNF for 24 hr, cell supernatants were collected and assayed for SEAP activity as described under “Materials and Methods.” Results are expressed as -fold activity over the activity of the vector control. (B) Sesamin inhibited NF-κB-dependent reporter gene expression induced by TNF, TNFR1, TRADD, NIK, IKK and TAK1/TAB1. A293 cells were transiently transfected with the indicated plasmids along with a NF-κB-containing plasmid linked to the SEAP gene and after 24 hr cells were either untreated or treated with 100 μM sesamin for 12 hr. Where indicated, cells were exposed to 1 nM TNF for 24 hr. Cell supernatants were assayed for SEAP activity as described under “Materials and Methods.” Results are expressed as fold activity over the activity of the vector control. DN, dominant negative.
Sesamin inhibits NF-κB activation induced by TNFR1, TRADD, NIK, TAK1/TAB1 and IKK
TNF-induced NF-κB activation requires a sequential recruitment of TNFR1, TRADD, TAK1, and IKK (30, 31). To determine where in the pathway sesamin blocks the TNF-induced NF-κB activation, we decided to examine the effect of sesamin on TNFR1, TRADD, NIK, TAK1/TAB1 and IKK-induced NF-κB–dependent reporter gene transcription. We observed that all of these plasmids induced NF-κB reporter activity and sesamin was found to inhibit the activation (Fig 6B).
Discussion
Although numerous studies have indicated that sesamin exhibits activity against hypertension, hyperlipidimia, septic shock and carcinogenesis, its precise mechanism of action is not understood. Antihypertensive effects have been documented even in human clinical trials (32). Since inflammation has been linked with most chronic diseases including cancer, it is possible that modulation of inflammatory pathway is one of the major sites of action of sesamin. Over the last decade NF-κB pathway has emerged as a major mediator of inflammation (19, 20). The major aim of current study was to determine the effects of sesamin on NF-κB mediated cellular responses linked to prevention of cancer. We found that sesamin inhibited the NF-κB pathway induced by various carcinogens, inflammatory stimuli and cytokines. It also inhibited constitutive expression of NF-κB activation.
We found sesamin suppressed the proliferation of wide variety of tumor cells including leukemia and solid tumor cells of the prostate, colon, pancreas, lung and breast. Suppression of proliferation of these cells is most likely linked to inhibition of gene products linked with survival and proliferation of cells such as Bcl-2, survivin, cyclin D1 and COX-2.
By using DNA binding assay, we showed NF-κB activated by highly diverse stimuli was blocked by sesamin suggesting that sesamin acts at a step common to all. Our results are in agreement with a recent report about suppression of LPS-induced NF-κB monitored by nuclear pool of p65 in microglia cells (17). How sesamin inhibits LPS-induced NF-κB activation was not examined by these investigators. We found that sesamin inhibited the activation of IKK thereby prevents the phosphorylation as well as degradation of IκBα. When we examined the effects of sesamin on IKK in details we found that this lignan did not directly modulate the activity of IKK. However it blocked the activation of the kinase. Numerous kinases have been linked with activation of IKK. TAK1 is one of the kinase that has been shown to mediate TNF-induced NF-κB (31). We found that sesamin blocked TNF-induced TAK1 mediated NF-κB activation. IKK has also been shown to mediate the phosphorylation of p65, the DNA binding subunit (28). We found that sesamin also inhibited the phosphorylation of p65. NF-κB reporter gene expression induced by TNF and TNF signaling components was also suppressed by this lignan.
When examined for the expression of antiapoptotic gene products survivin and Bcl-2, both regulated by NF-κB, were suppressed by sesamin. In addition, sesamin inhibited the expression of protein COX-2 closely linked with inflammation. Although sesamin has been shown to exhibit anti-inflammatory activity and downregulate prostaglandin production (7, 8, 10); ours is the first report to show that this agent can downregulate COX-2 expression. Reports about the anti-inflammatory activity due to downregulation of IL-1, IL-6 (10), NO (16) and thromboxane B2 could also be due to its ability to downregulate NF-κB as described here, as inducible NO synthase and lipooxygenase are also regulated by NF-κB. Lee et al., (33) showed that sesamin inhibited the expression of phospholipase C (PLC)-γ1 but the mechanism was not shown. Because PLC-γ1 expression is also regulated by NF-κB (34), downregulation of NF-κB signaling sesamin may decrease the expression of PLC-γ.
Our results also indicate that sesamin inhibition of NF-κB led to the downregulation of the expression cyclin D1 closely associated with proliferation of cells. This is in agreement with observations of Yokota et al (18). It is possible that reports which indicated the suppression of proliferation of various tumor cells including leukemia (12, 13), breast cancer (18) and gastric cancer (14), is due to the suppression of cyclin D1 expression.
We also found for the first time that the expression of protein linked with adhesion (ICAM-1), invasion (MMP-9) and angiogenesis (VEGF), was also abrogated by sesamin. Although sesamin has been shown to inhibit DMBA-induced breast carcinogenesis in rats, it is possible that these effects of sesamin involve suppression of NF-κB pathway. DMBA indeed has been shown to activate NF-κB (35).
Additionally we also found that downregulation of expression of antiapoptotic gene products led to the enhancements of apoptosis induced by cytokines as well as by chemotherapeutic agents. That NF-κB activation can inhibit apoptosis and induce chemoresistance is well established (19, 36). These studies suggest that sesamin can be used not only alone but also in combination with existing therapies both to potentiate their effect and to overcome chemoresistance.
One of the earliest activity reported of sesamin is it ability to lower cholesterol (3). Like sesamin, well known cholesterol-lowering drugs such as statin, have also been shown to suppress NF-κB pathway and sensitize the cells to chemotherapeutic agents (37, 38). Besides numerous animal studies (39), several clinical trials have been done with sesamin, demonstrating its safety and bioavailability (1, 32, 40–42). In conclusion, our results demonstrate the mechanism by which sesamin could mediate antiinflamatory, antiproliferative, and antiangiogenic effects against cancer through the inhibition of NF-κB and NF-κB-regulated gene products. A further investigation in animals and humans with sesamin is needed to fully realize its potential.
Acknowledgments
Dr. Aggarwal is the Ransom Horne, Jr., Professor of Cancer Research. This work was supported by a grant from the Clayton Foundation for Research (B.B.A.), a core grant from the National Institutes of Health (CA-16 672), a program project grant from National Institutes of Health (NIH CA-124787-01A2), and grant from Center for Targeted Therapy of M.D. Anderson Cancer Center.
References
- 1.Lampe JW, Atkinson C, Hullar MA. Assessing exposure to lignans and their metabolites in humans. J AOAC Int. 2006;89:1174–81. [PubMed] [Google Scholar]
- 2.Matsumura Y, Kita S, Morimoto S, et al. Antihypertensive effect of sesamin. I. Protection against deoxycorticosterone acetate-salt-induced hypertension and cardiovascular hypertrophy. Biol Pharm Bull. 1995;18:1016–9. doi: 10.1248/bpb.18.1016. [DOI] [PubMed] [Google Scholar]
- 3.Hirose N, Inoue T, Nishihara K, et al. Inhibition of cholesterol absorption and synthesis in rats by sesamin. J Lipid Res. 1991;32:629–38. [PubMed] [Google Scholar]
- 4.Ogawa H, Sasagawa S, Murakami T, Yoshizumi H. Sesame lignans modulate cholesterol metabolism in the stroke-prone spontaneously hypertensive rat. Clin Exp Pharmacol Physiol Suppl. 1995;22:S310–2. doi: 10.1111/j.1440-1681.1995.tb02932.x. [DOI] [PubMed] [Google Scholar]
- 5.Hirata F, Fujita K, Ishikura Y, Hosoda K, Ishikawa T, Nakamura H. Hypocholesterolemic effect of sesame lignan in humans. Atherosclerosis. 1996;122:135–36. doi: 10.1016/0021-9150(95)05769-2. [DOI] [PubMed] [Google Scholar]
- 6.Fukuda N, Miyagi C, Zhang L, et al. Reciprocal effects of dietary sesamin on ketogenesis and triacylglycerol secretion by the rat liver. J Nutr Sci Vitaminol (Tokyo) 1998;44:715–22. doi: 10.3177/jnsv.44.715. [DOI] [PubMed] [Google Scholar]
- 7.Chavali SR, Zhong WW, Utsunomiya T, Forse RA. Decreased production of interleukin-1-beta, prostaglandin-E2 and thromboxane-B2, and elevated levels of interleukin-6 and -10 are associated with increased survival during endotoxic shock in mice consuming diets enriched with sesame seed oil supplemented with Quil-A saponin. Int Arch Allergy Immunol. 1997;114:153–60. doi: 10.1159/000237661. [DOI] [PubMed] [Google Scholar]
- 8.Hirose N, Doi F, Ueki T, et al. Suppressive effect of sesamin against 7,12-dimethylbenz[a]-anthracene induced rat mammary carcinogenesis. Anticancer Res. 1992;12:1259–65. [PubMed] [Google Scholar]
- 9.Shimizu S, Akimoto K, Shinmen Y, Kawashima H, Sugano M, Yamada H. Sesamin is a potent and specific inhibitor of delta 5 desaturase in polyunsaturated fatty acid biosynthesis. Lipids. 1991;26:512–6. doi: 10.1007/BF02536595. [DOI] [PubMed] [Google Scholar]
- 10.Chavali SR, Forse RA. Decreased production of interleukin-6 and prostaglandin E2 associated with inhibition of delta-5 desaturation of omega6 fatty acids in mice fed safflower oil diets supplemented with sesamol. Prostaglandins Leukot Essent Fatty Acids. 1999;61:347–52. doi: 10.1054/plef.1999.0112. [DOI] [PubMed] [Google Scholar]
- 11.Yamashita K, Nohara Y, Katayama K, Namiki M. Sesame seed lignans and gamma-tocopherol act synergistically to produce vitamin E activity in rats. J Nutr. 1992;122:2440–6. doi: 10.1093/jn/122.12.2440. [DOI] [PubMed] [Google Scholar]
- 12.Miyahara Y, Komiya T, Katsuzaki H, et al. Sesamin and episesamin induce apoptosis in human lymphoid leukemia Molt 4B cells. Int J Mol Med. 2000;6:43–6. [PubMed] [Google Scholar]
- 13.Ju Y, Still CC, Sacalis JN, Li J, Ho CT. Cytotoxic coumarins and lignans from extracts of the northern prickly ash (Zanthoxylum americanum) Phytother Res. 2001;15:441–3. doi: 10.1002/ptr.686. [DOI] [PubMed] [Google Scholar]
- 14.Hibasami H, Fujikawa T, Takeda H, et al. Induction of apoptosis by Acanthopanax senticosus HARMS and its component, sesamin in human stomach cancer KATO III cells. Oncol Rep. 2000;7:1213–6. doi: 10.3892/or.7.6.1213. [DOI] [PubMed] [Google Scholar]
- 15.Hou RC, Huang HM, Tzen JT, Jeng KC. Protective effects of sesamin and sesamolin on hypoxic neuronal and PC12 cells. J Neurosci Res. 2003;74:123–33. doi: 10.1002/jnr.10749. [DOI] [PubMed] [Google Scholar]
- 16.Hou RC, Chen HL, Tzen JT, Jeng KC. Effect of sesame antioxidants on LPS-induced NO production by BV2 microglial cells. Neuroreport. 2003;14:1815–9. doi: 10.1097/00001756-200310060-00011. [DOI] [PubMed] [Google Scholar]
- 17.Jeng KC, Hou RC, Wang JC, Ping LI. Sesamin inhibits lipopolysaccharide-induced cytokine production by suppression of p38 mitogen-activated protein kinase and nuclear factor-kappaB. Immunol Lett. 2005;97:101–6. doi: 10.1016/j.imlet.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Yokota T, Matsuzaki Y, Koyama M, et al. Sesamin, a lignan of sesame, down-regulates cyclin D1 protein expression in human tumor cells. Cancer Sci. 2007;98:1447–53. doi: 10.1111/j.1349-7006.2007.00560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aggarwal BB, Vijayalekshmi RV, Sung B. Targeting inflammatory pathways for prevention and therapy of cancer: short-term friend, long-term foe. Clin Cancer Res. 2009;15:425–30. doi: 10.1158/1078-0432.CCR-08-0149. [DOI] [PubMed] [Google Scholar]
- 20.Aggarwal BB. Nuclear factor-kappaB: the enemy within. Cancer Cell. 2004;6:203–8. doi: 10.1016/j.ccr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Chaturvedi MM, Mukhopadhyay A, Aggarwal BB. Assay for redox-sensitive transcription factor. Methods Enzymol. 2000;319:585–602. doi: 10.1016/s0076-6879(00)19055-x. [DOI] [PubMed] [Google Scholar]
- 22.Sung B, Pandey MK, Aggarwal BB. Fisetin, an inhibitor of cyclin-dependent kinase 6, down-regulates nuclear factor-kappaB-regulated cell proliferation, antiapoptotic and metastatic gene products through the suppression of TAK-1 and receptor-interacting protein-regulated IkappaBalpha kinase activation. Mol Pharmacol. 2007;71:1703–14. doi: 10.1124/mol.107.034512. [DOI] [PubMed] [Google Scholar]
- 23.Darnay BG, Ni J, Moore PA, Aggarwal BB. Activation of NF-kappaB by RANK requires tumor necrosis factor receptor-associated factor (TRAF) 6 and NF-kappaB-inducing kinase. Identification of a novel TRAF6 interaction motif. J Biol Chem. 1999;274:7724–31. doi: 10.1074/jbc.274.12.7724. [DOI] [PubMed] [Google Scholar]
- 24.Takada Y, Aggarwal BB. Flavopiridol inhibits NF-kappaB activation induced by various carcinogens and inflammatory agents through inhibition of IkappaBalpha kinase and p65 phosphorylation: abrogation of cyclin D1, cyclooxygenase-2, and matrix metalloprotease-9. J Biol Chem. 2004;279:4750–9. doi: 10.1074/jbc.M304546200. [DOI] [PubMed] [Google Scholar]
- 25.Dvorak HF, Sioussat TM, Brown LF, et al. Distribution of vascular permeability factor (vascular endothelial growth factor) in tumors: concentration in tumor blood vessels. J Exp Med. 1991;174:1275–8. doi: 10.1084/jem.174.5.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schreck R, Rieber P, Baeuerle PA. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. Embo J. 1991;10:2247–58. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bharti AC, Donato N, Singh S, Aggarwal BB. Curcumin (diferuloylmethane) down-regulates the constitutive activation of nuclear factor-kappa B and IkappaBalpha kinase in human multiple myeloma cells, leading to suppression of proliferation and induction of apoptosis. Blood. 2003;101:1053–62. doi: 10.1182/blood-2002-05-1320. [DOI] [PubMed] [Google Scholar]
- 28.Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109 (Suppl):S81–96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 29.Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–60. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 30.Hsu H, Shu HB, Pan MG, Goeddel DV. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell. 1996;84:299–308. doi: 10.1016/s0092-8674(00)80984-8. [DOI] [PubMed] [Google Scholar]
- 31.Blonska M, Shambharkar PB, Kobayashi M, et al. TAK1 is recruited to the tumor necrosis factor-alpha (TNF-alpha) receptor 1 complex in a receptor-interacting protein (RIP)-dependent manner and cooperates with MEKK3 leading to NF-kappaB activation. J Biol Chem. 2005;280:43056–63. doi: 10.1074/jbc.M507807200. [DOI] [PubMed] [Google Scholar]
- 32.Miyawaki T, Aono H, Toyoda-Ono Y, Maeda H, Kiso Y, Moriyama K. Antihypertensive effects of sesamin in humans. J Nutr Sci Vitaminol (Tokyo) 2009;55:87–91. doi: 10.3177/jnsv.55.87. [DOI] [PubMed] [Google Scholar]
- 33.Lee JS, Kim J, Yu YU, Kim YC. Inhibition of phospholipase Cgamma1 and cancer cell proliferation by lignans and flavans from Machilus thunbergii. Arch Pharm Res. 2004;27:1043–7. doi: 10.1007/BF02975429. [DOI] [PubMed] [Google Scholar]
- 34.Li X, Hua L, Deng F, et al. NF-kappaB and Hsp70 are involved in the phospholipase Cgamma1 signaling pathway in colorectal cancer cells. Life Sci. 2005;77:2794–803. doi: 10.1016/j.lfs.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 35.Kim DW, Sovak MA, Zanieski G, et al. Activation of NF-kappaB/Rel occurs early during neoplastic transformation of mammary cells. Carcinogenesis. 2000;21:871–9. doi: 10.1093/carcin/21.5.871. [DOI] [PubMed] [Google Scholar]
- 36.Shishodia S, Aggarwal BB. Nuclear factor-kappaB: a friend or a foe in cancer? Biochem Pharmacol. 2004;68:1071–80. doi: 10.1016/j.bcp.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 37.Ahn KS, Sethi G, Aggarwal BB. Simvastatin potentiates TNF-alpha-induced apoptosis through the down-regulation of NF-kappaB-dependent antiapoptotic gene products: role of IkappaBalpha kinase and TGF-beta-activated kinase-1. J Immunol. 2007;178:2507–16. doi: 10.4049/jimmunol.178.4.2507. [DOI] [PubMed] [Google Scholar]
- 38.Ahn KS, Sethi G, Aggarwal BB. Reversal of chemoresistance and enhancement of apoptosis by statins through down-regulation of the NF-kappaB pathway. Biochem Pharmacol. 2008;75:907–13. doi: 10.1016/j.bcp.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Z, Saarinen NM, Thompson LU. Sesamin is one of the major precursors of mammalian lignans in sesame seed (Sesamum indicum) as observed in vitro and in rats. J Nutr. 2006;136:906–12. doi: 10.1093/jn/136.4.906. [DOI] [PubMed] [Google Scholar]
- 40.Frank J, Lee S, Leonard SW, Atkinson JK, Kamal-Eldin A, Traber MG. Sex differences in the inhibition of gamma-tocopherol metabolism by a single dose of dietary sesame oil in healthy subjects. Am J Clin Nutr. 2008;87:1723–9. doi: 10.1093/ajcn/87.6.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu WH, Kang YP, Wang NH, Jou HJ, Wang TA. Sesame ingestion affects sex hormones, antioxidant status, and blood lipids in postmenopausal women. J Nutr. 2006;136:1270–5. doi: 10.1093/jn/136.5.1270. [DOI] [PubMed] [Google Scholar]
- 42.Penalvo JL, Heinonen SM, Aura AM, Adlercreutz H. Dietary sesamin is converted to enterolactone in humans. J Nutr. 2005;135:1056–62. doi: 10.1093/jn/135.5.1056. [DOI] [PubMed] [Google Scholar]