Abstract
Stem cell biology has increasingly gained scientific and public interest in recent years. In particular, the use of stem cells for treatment of heart disease has been strongly pursued within the scientific and medical communities. Significant effort has gone into the use of adult tissue-derived stem cells for cardiac repair including bone marrow, blood, and cardiac-derived cell populations. Significant interest in this area has been balanced by the difficulties of understanding stem cells, cardiac injury, and the amalgamation of these areas of investigation in translational medicine. Recent studies have emerged on adipose-derived stem cells which show the potential for cardiac lineage development in vitro and may have application in cell-mediated in vivo therapy for the diseased heart. This review provides a summary of current findings within the field of adipose-derived stem cell biology regarding their cardiac differentiation potential.
Keywords: Adipose tissue, cardiac, heart failure, cell regeneration
ADIPOSE TISSUE: THE MESODERMAL NICHE AND CORRELATION WITH METABOLIC SYNDROME
According to the American Society for Aesthetic Plastic Surgery, nearly half million elective liposuction surgeries are performed each year in the United States [1]. Adipose tissue is of mesodermal and neuroectodermal derivation and contains adipocytes in addition to the stromal-vascular fraction (SVF). The SVF is composed of microvascular endothelial cells, smooth muscle cells, and stem cells [2]. The dynamic changes of adipose tissue in response to metabolic demands is mediated by stem cells thought to reside in the perivascular niche where they support other cell types [3, 4]. However, the multilineage differentiation potential of adipose derived stem cells has only recently emerged [2, 4–10]. Similar to related mesenchymal stem cells, reports have shown the ability for adipose tissue derived stem cells to undergo adipogenesis, chondrogenesis, osteogenesis, myogenesis, and vasculogenesis [4, 7, 8, 11–13]. These cell populations have been referred to as processed lipoaspirate cells (PLA) [14], adipose-derived stem cells (ADSC) [15], brown adipose tissue derived cardiomyocytes [16], adipose-derived mesenchymal cells [16, 17] and adipose derived stromal vascular cells (SVC) [18, 19].
In recent years, the prevalence of conditions such as metabolic syndrome have brought to light the critical relationship between adipose tissue and another organ derived from the mesodermal germ layer, the heart [20–22]. Aspects of metabolic syndrome associated with cardiovascular disease include abdominal obesity, atherogenic dyslipidemia, elevated blood pressure, insulin resistance with or without glucose intolerance, a pro-inflamatory state, and a prothrombotic state [23]. Studies in the U.S. have shown that nearly a quarter of the population (an estimated 47 million people) have metabolic syndrome [24]. The strong correlation between metabolic dysregulation and risk factors for cardiovascular disease brings to the fore the importance of investigating mechanisms that may ameliorate numerous pathologies observed in these patients.
CARDIOVASCULAR DISEASE: ETIOLOGY OF ACUTE ISCHEMIC EVENTS AND LIMITATIONS TO CURRENT THERAPIES
Cardiovascular disease (CVD), the leading cause of morbidity and mortality world-wide, accounts for one third of all deaths globally [25]. Nearly half of all deaths associated with CVD are the result of heart failure due to ischemic events such as myocardial infarction (MI). The prevalence of CVD is expected to increase to three fourths of all deaths by 2020 [25], in part due to an increasing prevalence of risk factors related to coronary disease, such as obesity and sedentary lifestyle (metabolic syndrome), making CVD an even greater public health concern.
Acute myocardial infarction, a pathology associated with ischemic cardiomyopathy, is characterized by clot formation within a coronary artery, which prevents blood flow to the region of the heart distal to the site of occlusion. This acutely compromises regional cardiac contractility and increases the risk for arrhythmias and heart failure. Unless blood flow is restored, this event typically results in severe damage to the myocardium and subsequent acute and chronic tissue pathologies associated with compromised cardiovascular function. Medical strategies to treat myocardial infarction (MI) include thrombolytic drugs to dissolve the obstructive clot as well as urgent mechanical revascularization procedures such as percutaneous coronary artery stenting or coronary artery bypass grafting [26]. While medical therapies may improve function, none of these strategies provide long-term regeneration of damaged heart tissue [27]. Furthermore, the window of opportunity to save heart muscle by revascularization is brief and the therapeutic benefit incomplete.
Restoration of cardiac function after an MI is also limited because the heart is a terminally differentiated organ and consequently limited in its capacity to regenerate injured tissue. Reports have provided evidence that, in the context of injury, the heart contains stem cells thought to have proliferative capacity [28, 29]. Furthermore, chimerism in transplanted hearts (female heart into male recipient) reveal that circulating stem cells target the heart [30–32]. These cell based responses are not sufficient to provide therapeutic regeneration of the injured heart. The most dramatic and potentially deleterious responses of the heart to ischemic injury include compensatory cardiac hypertrophy of healthy tissue together with necrosis and apoptosis of ischemic regions of the myocardium [33]. Given the limited reparative capacity of the heart, current revascularization interventions provide modest regeneration of damaged heart tissue caused by ischemic injury. In the most severe case, global cardiac dysfunction leads to dilated cardiomyopathy and congestive heart failure. These critical consequences drive exploration of alternative approaches that provide regenerative treatment for myocardium damaged by ischemic injury.
STEM CELLS FOR CARDIAC REPAIR
During development, cells undergo a process of hierarchically restricted differentiation into specified tissues. The degree of specification, however, is variable due to the presence of niches of stem or progenitor cells capable of multi-lineage differentiation in most somatic tissues. These stem cells, originally described nearly five decades ago by Becker et al. [34], are characterized by their capacity for clonogenicity, self-renewal, and multipotentiality. Many studies have investigated the potential of stem cells for treatment of heart failure [35–38]. Specifically, in the last decade, interest in adult-derived stem cells has increased due to their value in studying cell lineage as well as their intrinsic therapeutic potential. Published reports describe a vast array of stem cell populations found in all primary germ layers [39]. Currently, investigations are underway in both basic research [40, 41] and clinical trials [42–44] exploring the potential for different stem cell populations to regenerate damaged cardiac tissue and subsequently improve cardiac function. Cell populations being studied include hematopoetic stem cells [45], mesenchymal stem cells [46, 47], endothelial progenitor cells [48], skeletal myoblasts [49, 50], cardiac progenitor cells [28, 29, 51], embryonic stem cells [52–55], and adipose-derived SVCs [18, 19, 56, 57].
Cell autonomous mechanisms of tissue regeneration are thought to contribute some of the therapeutic effects of stem cells based on observations that they can undergo non-autologous transdifferentation into multiple cell types [5, 58–61]. Studies have suggested that stem cell treatment in the heart can improve cardiac function by a paracrine mechanism to reduce cell death, stimulate neovascularization [62, 63], recruit stem cells [51, 64], and attenuate the inflammatory response [65]. Prima facie, these observations suggest that stem cells may offer an ideal therapy for ischemic heart disease. However, contradicting and negative results [16, 66–69], as well as limited success in clinical trials [70], have brought a healthy dose of reality to the challenge of understanding stem cells, heart disease, and the prospects of these entities in translational medicine.
In principle, the goals of stem cell research for the heart seek a cell type capable of providing a source of forcegenerating myocytes as well as supporting vascular development (angiogenesis) in the injured heart [3]. With varying degrees of success, stem or progenitor cell populations have shown the capacity to influence heart structure and function in this way including bone marrow cells, mesenchymal stem cells, endothelial progenitor cells, skeletal myoblasts, and cardiac progenitor cells [71]. This review provides an update on studies of adipose derived stem cells and their cardiogenic potential in vitro and in vivo (Fig. (1)). Furthermore, we will suggest additional methodologies that may be helpful in clarifying our understanding of this unique cell population.
Fig. (1).
Adipose derived stem cells may provide a mechanism of treating heart disease. Aesthetic cardiology provides a platform for reducing adiposity in obese patients while extracting stem cells useful for autologous cell transplantation in treatment of ischemic heart disease. Adapted with kind permission of Springer Science Business Media [120].
IN VITRO ANALYSIS OF ADIPOSE DERIVED STEM CELLS AS CARDIAC PROGENITORS
Several studies have been performed analyzing the in vitro differentiation potential of adipose derived stem cells along the cardiac lineage [6, 7, 17–19]. Perhaps the most convincing evidence for cardiac lineage development of SVCs was published by Planat-Benard et al. [18]. Consistent with this and other studies, we have recently published a report showing molecular and functional characteristics of SVCs capable of differentiating into beating myocytes [19]. Evidence to date, outlined below, provides evidence for the capacity for stem cells derived from adipose-tissue to differentiate along the cardiac lineage.
Numerous methodologies have been used to investigate the cardiogenic potential of SVCs. These include culturing in methylcellulose media [18, 19], culturing with nuclear and cytoplasmic extracts from rat cardiomyocytes [72], co-culturing with neonatal rat cardiomyocytes [15] use of 5-azacytidine [73] or other culturing methods [74, 75]. Furthermore, different sources of adipose-derived stem cells have been used including human subcutaneous adipose tissue [6, 15, 72, 75], mouse inguinal adipose tissue [18], and interscapular brown adipose tissue [18, 19, 56]. Unless otherwise specified, the findings outlined below do not delineate between specific methods but, rather, outline general observations regarding cardiac differentiation of adipose-tissue derived stem cells.
Studies have shown that adipose tissue stem cells are more proliferative than related marrow stromal cells [4]. Morphometric analysis of SVCs that have differentiated along the cardiac lineage pathway has shown the development of an intercalated syncitium of cells capable of spontaneous contractions [18, 19, 72] (Fig. (2a)). By transmission electron microscopy, differentiated SVC-derived myocytes have been shown to contain morphologic characteristics consistent with the cardiac phenotype including the presence of a sarcomeric ultrastructure [18]. Connexin, needed for coordinated electrical conductance throughout the cellular syncytium of the heart, has also been detected throughout colonies of spontaneously beating SVC-derived cardiomyocytes [18].
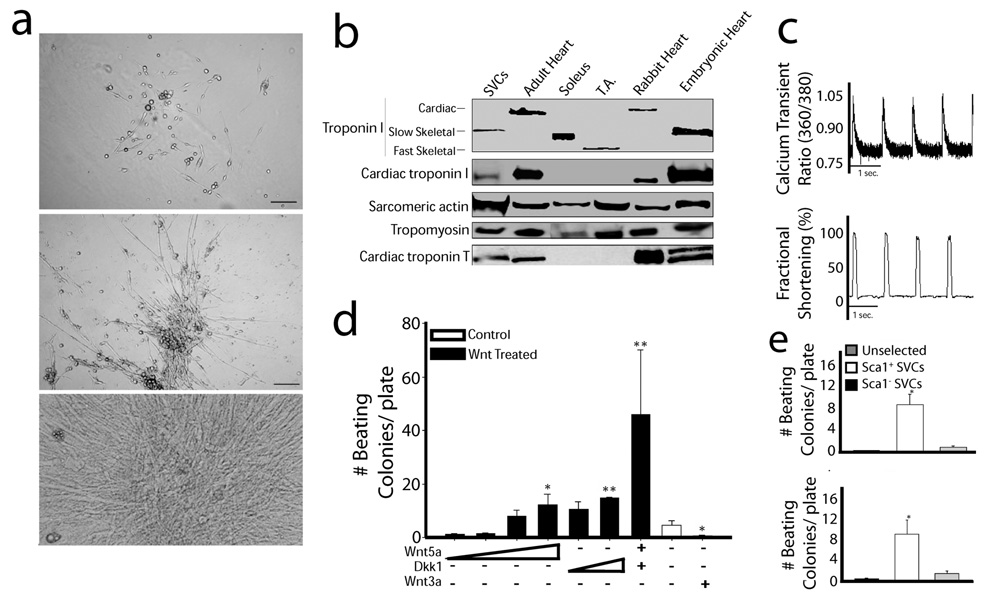
Fig. (2).
In vitro differentiation of adipose derived SVCs. (a) Phase-contrast microscope image of cultured SVCs after 5 (top), 10 (middle), and 15 (bottom) days of in vitro differentiation. (b) Expression profile of proteins identified in differentiated SVCs showing members of the cardiac thin filament. (c) Calcium transient measurements and contractile performance in differentiated SVCs during field stimulation. (d) Differentiation potential assessed during manipulation with recombinant Wnt proteins. (e) Identification of Sca1+ and c-kit+ stem cell markers associated with adipose-derived SVCs. Figure has been adapted with permission from the Journal of Molecular and Cellular Cardiology [19]. Abbreviations: SVCs: stromal vascular cells; T.A.: tibialis anterior; Dkk1: dickkopf homolog 1.
Studies have shown that SVCs express a spectrum of lineage specific markers (proteins and transcription factors) that are present during the course of embryonic cardiac development as well as the adult heart. Detected by real time polymerase chain reaction and immunohistochemistry, these markers included MEF2c, GATA4, Nkx2.5, ANP, β-MHC, MLC2v, MLC2a, and the L-type calcium channel [6, 15, 18, 72]. We found that the protein expression profile of these differentiated SVCs revealed the presence of proteins required for formation of the critical regulatory complex of the thin filament (Fig. (2b)) [19]. These proteins include troponin I (TnI), troponin T, tropomyosin, and actin. The expression of the slow skeletal isoform of TnI, present during the early developmental stages of the heart in mammals, reveals an embryonic heart phenotype of these cells. Interestingly, expression of the cardiac isoform of TnI confirms that these SVCs are capable of differentiating into the adult cardiac phenotype [19]. Importantly, this cell population does not express the major skeletal muscle marker MyoD or the smooth muscle isoform of actin [18]. Zhu et al. have suggested that co-culturing with neonatal cardiomyocytes enhances the expression of these cardiogenic markers beyond expression achieved by culturing with DMEM [15].
We performed functional analysis of differentiated SVCs [19]. Cells were loaded with the calcium indicator dye FURA2-AM and calcium handling and contractile kinetics were analyzed simultaneously during field stimulation and within spontaneously beating SVCs (Fig. (2c)). These data show that differentiated SVCs have functional properties of myocytes and calcium handling kinetics indicative of a developing sarcoplasmic reticulum. These observations have also been replicated by others [6]. Furthermore, we found that the intrinsic pacemaker phenotype observed in spontaneously beating SVCs could be ablated by acute administration of muscarine chloride [19]. The elegant study by Planat-Benard et al. showed that synchronized beating patterns observed in SVC-derived cardiomyocytes respond to chronotropic stimulation using adrenergic and cholinergic agents [18]. Lastly, Bai and colleagues used whole-cell patch-clamp recordings to show functional voltage-dependent calcium and potassium channels [6]. Taken together, these data indicate significant maturation of excitation contraction coupling involving key proteins associated with sarcollemal membrane depolarization, the sarcoplasmic reticulum calcium cycling, and myofilament-based contractile responses.
It has been well established that Wnt signaling is critically involved in cardiogenic differentiation of various stem cell populations [76–81]. We tested the hypothesis that SVC differentiation along the cardiac lineage could be manipulated by alterations in Wnt signaling pathways. We observed that stimulation of the canonical Wnt signaling pathway by administration of recombinant Wnt3a significantly attenuated the cardiogenic potential of differentiating SVCs (Fig. (2d)). In contrast, these data indicate that suppression of canonical Wnt signaling by administration with Dkk1 and activation of non-canonical Wnt signaling by administration of recombinant Wnt5a independently and synergistically enhances the differentiation potential of SVCs.
Screening for cell surface markers has revealed that adipose-derived stem cells express markers very similar to its mesodermal stem cell cousin, bone marrow derived mesenchymal stem cells [4]. Evidence that SVCs are of mesenchymal origin stem from its consistent expression of CD29. Surface antigens found on various stem cells capable of multilineage differentiation are also expressed by SVCs including CD105, STRO-1, CD166, and CD117 (c-kit) [4]. However, markers of hematopoietic lineage are largely absent in SVCs including lin, CD31, and CD45 [4]. We tested the hypothesis that isolation of Sca1+ and c-kit+ stem cells would yield a high concentration of SVCs with cardiogenic potential. Isolation of these cell populations by antibody-labeled magnetic nanoparticles resulted in approximately 8 fold increase in the number of spontaneously beating colonies compared to unselected cells (Fig. (2e)). Further studies are required to determine whether SVCs fall into the category of mesoangioblasts which appear to exist within most mature mesodermally-derived tissues [82, 83].
A recent study by Madonna et al. [74] analyzed mesenchymal stem cells from brown adipose tissue for the presence of telomere reverse transcriptase (TERT), which is required for maintenance of nuclear telomere length and replication potential. They found that TERT was expressed in cells that also expressed myocardin A, a regulator of cardiovascular myogenic development. Furthermore, co-immunoprecipitation assays showed that TERT and myocardin A formed a protein complex. These observations imply that myocardin A enhances TERT activation and promotes myogenic differentiation in adipose tissue stem cells similar to what was been observed during cardiogenic differentiation of embryoid bodies.
Lastly, secretion of paracrine factors released by adipose-derived stem cells that may be therapeutically beneficial in the context of cell transplantation has also been analyzed with in vitro methodologies. Several groups have shown secretion of MMP-9, MMP-3, HGF, TGF-β, VEGF and IGFI from adipose-derived stem cells [75, 84–87]. Similarly, Yamada et al. found that culturing either cord blood cells or bone marrow non-hematopoietic cells together with brown adipose tissue-derived stem cells can induce cardiac differentiation in these alternate cell types [56, 88]. Consistent with findings in related cell populations such as marrow-derived mesenchymal stem cells, these data suggest that adipose tissue stem cells may enhance the differentiation potential of other stem cells through a paracrine mechanism.
IN VIVO CELL THERAPY FOR ISCHEMIC CARDIOMYOPATHY
The comparative value of using adipose-derived stem cells for cardiac repair can be argued from a number of vantage points including efficiency of isolation, in vivo regenerative capacity, and functional implications on performance of the injured heart. Compared to other cell types of mesenchymal origin such as bone marrow derived MSCs, adipose tissue stem cells are reported to result in greater yield where 2% of nucleated cells in processed lipoaspirate result in at least five times more CFU-F than bone marrow MSCs [4]. On behalf of patient wellness, isolation procedures for adipose tissue stem cells requires only local anesthesia with little donor site morbidity.
Miyahara and colleagues [17] have provided evidence that mesenchymal stem cells derived from adipose tissue could be grown into a cellular monolayer including cardiomyocytes and endothelial cells using cell sheet technology and temperature-responsive cell culture. Consistent with the MSC phenotype, cells derived from adipose tissue were found to differentiate into adipocytes, osteoblasts, and vascular endothelial cells. In vivo, adipose-derived stem cell monolayers were engrafted over the infarcted region of the heart and adhered to the injured tissue. Interestingly, this engrafted tissue developed new blood vessels and incorporated host cells based on GFP expression. Functional data at eight weeks post-infarction showed that hearts containing engrafted adipose-derived stem cells had decreased end-diastolic pressure, and improved derivatives of contractility. The geometry of the heart was also improved based on increased wall thickness of the infarcted anterior wall during diastole and decreased dilation of the left ventricle. The survival rate of mice with stem cell grafts was also improved.
In addition to this report, other studies have tested the therapeutic role of adipose-derived stem cells for repair of the infarcted heart [16, 89–93]. All of these studies have used a model of acute myocardial infarction in the context of various animal models including pig [92], and rat [16, 89–91, 93]. Stem cell delivery methods have included direct intramyocardial injection [16, 90, 93], as well as intracoronary [92], and intravenous [89, 91] routes. Furthermore, each has shown evidence that adipose-derived stem cells are capable of repairing the infarcted myocardium through addition of cardiomyocytes [16, 93], vascular angiogenesis [89–93], increased nerve sprouting [90], decreased apoptosis [16], and/or reduction in fibrotic depositions within the necrotic myocardium [16, 90, 91, 93]. In all cases, functional analysis at varying time points showed that hearts receiving cell transplantation had improved cardiac performance compared to non-treated hearts.
In support of in vitro observations[75, 84–87], paracrine factors released by injected stem cells in vivo may also contribute to the beneficial effects of this therapeutic approach. This area of investigation has been extensively studied using various stem cell populations such as marrow derived stromal cells [94, 95]. Data show that trophic factors (e.g. growth factors, SDF-1, Ang-1, TB4) activate various signaling pathways (e.g. JAK-STAT, ERK, and PI3K-AKT) resulting in therapeutic effects including neovascularization and tissue regeneration that ultimately improve contractile performance of the injured heart. More research is required to know how these paracrine effects are involved in adipose-tissue stem cell regeneration.
Recently a study by van der Bogt et al. has provided negative results regarding the effects of cell transplantation into the injured heart [69]. In this study, adipose derived stem cells were injected directly into the peri-infarct zone in a mouse model of myocardial infarction. Longitudinal analysis using in vivo luminometry confirmed localization of cells in the heart after transplantation. However, marked donor cell death within 4 to 5 weeks was seen by luminometry and supported by histological results. Furthermore no functional improvement was reported based on in vivo cardiac hemodynamic analysis.
Numerous studies have also shown that various disease states can markedly impact the viability, functionality, or abundance of stem cell populations [96–101]. Thus, the degree of morbidity of a patient may have significant impact on the potential for effective cell therapy for given pathologies including adipose tissue stem cell repair of the ischemic heart. The relevance of existing disease states in patients intended for cell transplantation provides a very relevant line of investigation to determine how adipose tissue stem cells are affected by disease pathologies such as cardiac ischemic events or diabetes.
In light of this, clinical trials are also required to know if adipose tissue stem cells are efficacious in human patients. Currently on-going, the two first-in-man trials with these cells in acute MI and chronic ischemic patients are the APOLLO [102] and the PRECISE trials being conducted in Madrid, Spain. The APOLLO trial investigated adipose-derived stem cells in patients with acute MI and LV ejection fraction impairment. Cells were delivered by intracoronary infusion after appropriate infarct related artery repair with stent implantation. The PRECISE trial included patients with end-stage coronary artery disease not amenable for revascularization and with moderate to severe left ventricular dysfunction. In this case, cells were delivered via transendocardial injections after LV electromechanical mapping. The results of these trials are yet to be published. However, these clinical trials will be the first effort to elucidate the role of adipose tissue stem cells for cardiac repair in humans.
RECOMMENDED METHODOLOGIES FOR IN VIVO CELL THERAPY
There has been a significant effort toward understanding the therapeutic potential of adipose-tissue derived stem cells for cardiac repair. However, the lack of proper controls and/or potential misinterpretation of results make the conclusions from some of these studies uncertain. As a result, the following section is designed to provide additional methodologies used with success in other stem cell studies that may clarify issues regarding the therapeutic role of adipose tissue stem cells.
A brief analysis of reporters used to identify donor cells in recipient hearts showed that the vast majority of studies using adipose tissue derived stem cells track cell identity by green fluorescence protein as the marker of choice [16, 17, 69, 89, 91–93]. Other methodologies included identification of human adipose stem cells in recipient rodent hearts by labeling with human HLA [90] or the use of luciferase expression in donor cells [69]. Use of GFP as a reporter is advantageous because of the clear ease of use. However, the use of GFP or other fluorescent markers for identification of donor stem cells in the myocardium can be misleading [103–105]. Fundamentally, donor cell identity should be validated by other means regardless of the reporter used. Some studies have provided dual labeling with cardiac specific markers (as described above) which provide additional evidence to support the cardiac differentiation of donor stem cells in the host myocardium [16, 17]. However, even secondary markers can be found in extracardiac tissues and thus may not be bonafide indicators of cardiac lineage specification [103].
Other methodologies that could be used to track stem cells in vivo include membrane dyes such as PKH26 and DiI which intercalate into cell membrane lipids through hydrophobic interactions [106]. The nucleoside analog bromodeoxyuridine (BrdU) incorporates into genomic DNA and can thus be identified in transplanted stem cells by antibody labeling against BrdU [107]. Additional DNA labeling dyes include DAPI (4',6-diamidino-2-phenylindole) and Hoechst which are advantageous in doing live cell detection because they are membrane permeable [108, 109].
Methodologies using genetic markers could also be used in the investigation of the therapeutic potential for adipose-derived stem cells in vivo (Fig. (3)). Previous studies have shown that genetic modification of SVCs is feasible and could be a technique useful for tracking cells in vivo as well as for overexpresson of therapeutic proteins. Reports have used adenovirus mediated gene transfer to track adipose stem cells (CMV-Luciferase) delivered into an animal model of spinal injury [110], over-express bone morphogenic protein (BMP-2) to enhance the osteogenic differentiation of adipose stem cells in vitro and in vivo [111], and to over-express brain derived neurotrophic factor (BDNF) in SVCs delivered to brains that had undergone ischemia reperfusion injury [112] (Fig. (3a)).
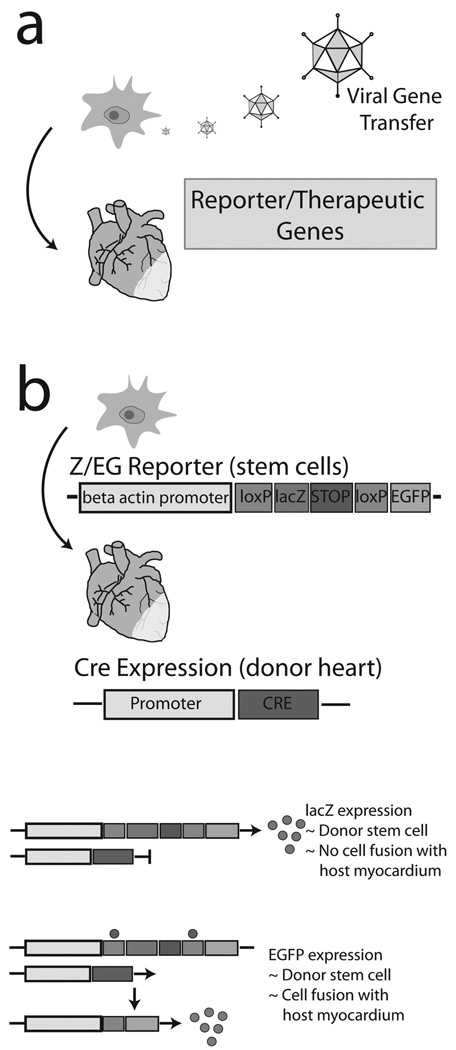
Fig. (3).
Advanced genetic engineering methods for assessing stem cell transplantation potential of SVCs in vivo. (a) Gene transfer of reporter or therapeutic genes into SVCs prior to transplantation into the injured heart. (b) Use of the conditional dual reporter mouse, Z/EG, for detection of donor stem cells in the host myocardium and further determination of intrinsic differentiation or cell fusion events. In the absence of Cre enzyme (host cardiomyocytes), LacZ is expressed in donor stem cells indicating no cell fusion. If cell fusion occurs, Cre (host cardiac myocytes) will recombine the Z/EG construct (donor stem cells) resulting in expression of EGFP. Adapted with kind permission of Springer Science Business Media [120].
These reports using viral mediated gene transfer as well as other known methodologies may provide concepts for future assays to clarify the potential for adipose tissue stem cells to regenerate host myocardium. To begin, adenoviral gene transfer could be useful for transduction of donor adipose stem cells with a unique reporter expression vector such as tissue restricted expression (α-MHC promoter or CMV promoter with floxed gene activation) of luciferase or lacZ. Alternatively, use of these regulatory genetic elements could also direct expression of therapeutic proteins. Previous stem cells studies have shown that over-expression of the pro-survival signaling molecule Akt [113] or the cell adhesion molecule stem cell derived factor (SDF-1) [114] can increase the potential for therapeutic regeneration of the heart by stem cells (Fig. (3a)).
Another technology includes the use of the Z/EG reporter mouse (strain name: B6.Cg-Tg(ACTB-Bgeo/GFP)21Lbe/J, stock no: 004178, Jackson Laboratories) [115] which has been used in other stem cell studies to delineate between cell fusion and intrinsic differentiation of donor cells in the recipient heart [116, 117] (Fig. (3b)). The Z/EG mouse contains a transgene with the β-actin promoter (ubiquitous) driving expression of a floxed lacZ cassette. In the presence of Cre (a recombining enzyme), loxP sites recombine resulting in expression of EGFP instead of lacZ. Due to the numerous Cre expressing mice available [118], various permutations of Cre-Z/EG chimeric mice could be engineered. For example, delivery of adipose tissue stem cells from Z/EG mice into infarcted mice expressing CMV-Cre would indicate donor cell incorporation into the host myocardium by lacZ expression and cell fusion by expression of EGFP (Fig. (3b)). Intrinsic differentiation of donor cells in the host myocardium would require further characterization by secondary cardiac markers.
CONCLUSION
Although the field of adipose-derived stem cells is still in its infancy, studies are accumulating showing their cardiac differentiation potential in vitro and some potential to therapeutically improve the infarcted heart in vivo. Clinical trials using adipose-derived stem cells for treatment of various tissue pathologies are underway [57, 102, 119]. Considering the complicated nature of clinical applications using stem cells, what is the rationale for such enthusiasm? From the perspective of cardiac biology, first, adipose tissue is easily accessible and liposuction-acquired stem cells for heart repair has secondary value in reducing cardiovascular risks induced by excessive adiposity. Second, issues of histocompatability and immune rejection, problematic in non-autologous or xeno stem cell transplantation, are avoided. As further studies appear, particularly with application to human cardiac physiology, interest and therapeutic application of adipose tissue derived cardiomyocytes may gain momentum in a new branch of clinical and research medicine, namely aesthetic cardiology. On-going basic science studies will be essential to fully understand the developmental potential of SVCs and their possible use in cardiac repair.
REFERENCES
- 1.Housman TS, Lawrence N, Mellen BG, et al. The safety of liposuction: results of a national survey. Dermatol Surg. 2002;28:971–978. doi: 10.1046/j.1524-4725.2002.02081.x. [DOI] [PubMed] [Google Scholar]
- 2.Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 3.Psaltis PJ, Zannettino AC, Worthley SG, Gronthos S. Concise review: mesenchymal stromal cells: potential for cardiovascular repair. Stem Cells. 2008;26:2201–2210. doi: 10.1634/stemcells.2008-0428. [DOI] [PubMed] [Google Scholar]
- 4.Strem BM, Hicok KC, Zhu M, et al. Multipotential differentiation of adipose tissue-derived stem cells. Keio J Med. 2005;54:132–141. doi: 10.2302/kjm.54.132. [DOI] [PubMed] [Google Scholar]
- 5.Safford KA, Rice HE. Stem cell therapy for neurologic disorders: Therapeutic potential of adipose-derived stem cells. Curr Drug Targets. 2005;6:57–62. doi: 10.2174/1389450053345028. [DOI] [PubMed] [Google Scholar]
- 6.Bai X, Pinkernell K, Song AH, Nabzdyk C, Reiser J, Alt E. Genetically selected stem cells from human adipose tissue express cardiac markers. Biochem Biophys Res Commun. 2007;353:665–671. doi: 10.1016/j.bbrc.2006.12.103. [DOI] [PubMed] [Google Scholar]
- 7.Fraser JK, Schreiber R, Strem B, et al. Plasticity of human adipose stem cells toward endothelial cells and cardiomyocytes. Nat Clin-Pract Cardiovasc Med. 2006;3 Suppl 1:S33–S37. doi: 10.1038/ncpcardio0444. [DOI] [PubMed] [Google Scholar]
- 8.Fraser JK, Wulur I, Alfonso Z, Hedrick MH. Fat tissue: an underappreciated source of stem cells for biotechnology. Trends Biotechnol. 2006;24:150–154. doi: 10.1016/j.tibtech.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Strem BM, Zhu M, Alfonso Z, et al. Expression of cardiomyocytic markers on adipose tissue-derived cells in a murine model of acute myocardial injury. Cytotherapy. 2005;7:282–291. doi: 10.1080/14653240510027226. [DOI] [PubMed] [Google Scholar]
- 10.Strem BM, Jordan MC, Kim JK, et al. Adipose tissue-derived stem cells enhance cardiac function following surgically-induced myocardial infarction. Circulation. 2005;112:U330–U330. [Google Scholar]
- 11.Miranville A, Heeschen C, Sengenes C, Curat CA, Busse R, Bouloumie A. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004;110:349–355. doi: 10.1161/01.CIR.0000135466.16823.D0. [DOI] [PubMed] [Google Scholar]
- 12.Morizono K, De Ugarte DA, Zhu M, et al. Multilineage cells from adipose tissue as gene delivery vehicles. Hum Gene Ther. 2003;14:59–66. doi: 10.1089/10430340360464714. [DOI] [PubMed] [Google Scholar]
- 13.Peterson B, Zhang J, Iglesias R, et al. Healing of critically sized femoral defects, using genetically modified mesenchymal stem cells from human adipose tissue. Tissue Eng. 2005;11:120–129. doi: 10.1089/ten.2005.11.120. [DOI] [PubMed] [Google Scholar]
- 14.Fraser JK, Schreiber RE, Zuk PA, Hedrick MH. Adult stem cell therapy for the heart. Int J Biochem Cell Biol. 2004;36:658–666. doi: 10.1016/j.biocel.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 15.Zhu Y, Liu T, Song K, Ning R, Ma X, Cui Z. ADSCs differentiated into cardiomyocytes in cardiac microenvironment. Mol Cell Biochem. 2009;324:117–129. doi: 10.1007/s11010-008-9990-3. [DOI] [PubMed] [Google Scholar]
- 16.Yamada Y, Wang XD, Yokoyama S, Fukuda N, Takakura N. Cardiac progenitor cells in brown adipose tissue repaired damaged myocardium. Biochem Biophys Res Commun. 2006;342:662–670. doi: 10.1016/j.bbrc.2006.01.181. [DOI] [PubMed] [Google Scholar]
- 17.Miyahara Y, Nagaya N, Kataoka M, et al. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat Med. 2006;12:459–465. doi: 10.1038/nm1391. [DOI] [PubMed] [Google Scholar]
- 18.Planat-Benard V, Menard C, Andre M, et al. Spontaneous cardiomyocyte differentiation from adipose tissue stroma cells. Circ Res. 2004;94:223–229. doi: 10.1161/01.RES.0000109792.43271.47. [DOI] [PubMed] [Google Scholar]
- 19.Palpant NJ, Yasuda S-i, MacDougald O, Metzger JM. Non-canonical Wnt signaling enhances differentiation of Sca1+/c-kit+ adipose-derived murine stromal vascular cells into spontaneously beating cardiac myocytes. J Mol Cell Cardiol. 2007;43:362–370. doi: 10.1016/j.yjmcc.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ford ES. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence. Diabetes Care. 2005;28:1769–1778. doi: 10.2337/diacare.28.7.1769. [DOI] [PubMed] [Google Scholar]
- 21.Ford ES. The metabolic syndrome and mortality from cardiovascular disease and all-causes: findings from the National Health and Nutrition Examination Survey II Mortality Study. Atherosclerosis. 2004;173:309–314. doi: 10.1016/j.atherosclerosis.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 22.Ford ES, Li C. Metabolic syndrome and health-related quality of life among U.S. adults. Ann Epidemiol. 2008;18:165–171. doi: 10.1016/j.annepidem.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 23.Grundy SM, Brewer HB, Jr., Cleeman JI, Smith SC, Jr., Lenfant C for the Conference P. Definition of Metabolic Syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association Conference on Scientific Issues Related to Definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 24.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 25.Thom T, Haase N, Rosamond W, et al. Heart disease and stroke statistics–2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113:e85–e151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- 26.Fonarow GC. Heart failure: recent advances in prevention and treatment. Rev Cardiovasc Med. 2000;1:25–33. 54. [PubMed] [Google Scholar]
- 27.Schinkel AFL, Poldermans D, Rizzello V, et al. Why do patients with ischemic cardiomyopathy and a substantial amount of viable myocardium not always recover in function after revascularization? J Thorac Cardiovasc Surg. 2004;127:385–390. doi: 10.1016/j.jtcvs.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 28.Oh H, Bradfute SB, Gallardo TD, et al. Cardiac progenitor cells from adult myocardium: Homing, differentiation, and fusion after infarction. Proc Natl Acad Sci USA. 2003;100:12313–12318. doi: 10.1073/pnas.2132126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laugwitz KL, Moretti A, Lam J, et al. Postnatal isl1+cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433:647–653. doi: 10.1038/nature03215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muller P, Pfeiffer P, Koglin J, et al. Cardiomyocytes of noncardiac origin in myocardial biopsies of human transplanted hearts. Circulation. 2002;106:31–35. doi: 10.1161/01.cir.0000022405.68464.ca. [DOI] [PubMed] [Google Scholar]
- 31.Quaini F, Urbanek K, Beltrami AP, et al. Chimerism of the transplanted heart. N Engl J Med. 2002;346:5–15. doi: 10.1056/NEJMoa012081. [DOI] [PubMed] [Google Scholar]
- 32.Thiele J, Varus E, Wickenhauser C, et al. Mixed chimerism of cardiomyocytes and vessels after allogeneic bone marrow and stem-cell transplantation in comparison with cardiac allografts. Transplantation. 2004;77:1902–1905. doi: 10.1097/01.tp.0000127591.34203.8e. [DOI] [PubMed] [Google Scholar]
- 33.MacLellan WR, Schneider MD. Genetic dissection of cardiac growth control pathways. Annu Rev Physiol. 2000;62:289–319. doi: 10.1146/annurev.physiol.62.1.289. [DOI] [PubMed] [Google Scholar]
- 34.Becker AJ, Mc CE, Till JE. Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature. 1963;197:452–454. doi: 10.1038/197452a0. [DOI] [PubMed] [Google Scholar]
- 35.Dimmeler S, Zeiher AM. Wanted! The best cell for cardiac regeneration. J Am Coll Cardiol. 2004;44:464–466. doi: 10.1016/j.jacc.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 36.Dimmeler S, Zeiher AM, Schneider MD. Unchain my heart: the scientific foundations of cardiac repair. J Clin Invest. 2005;115:572–583. doi: 10.1172/JCI24283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Orlic D. The strength of plasticity: stem cells for cardiac repair. Int J Cardiol. 2004;95:S16–S19. doi: 10.1016/s0167-5273(04)90005-8. [DOI] [PubMed] [Google Scholar]
- 38.Orlic D, Hill JM, Arai AE. Stem cells for myocardial regeneration. Circ Res. 2002;91:1092–1102. doi: 10.1161/01.res.0000046045.00846.b0. [DOI] [PubMed] [Google Scholar]
- 39.Nardi NB. All the adult stem cells, where do they all come from? An external source for organ-specific stem cell pools. Med Hypotheses. 2005;64:811–817. doi: 10.1016/j.mehy.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 40.Bick-Forrester J, Lee MS, Makkar RR, Forrester JS. Partial restoration of myocardial function and perfusion by cell therapy following myocardial infarction. Curr Opin Cardiol. 2004;19:631–637. doi: 10.1097/01.hco.0000142061.84471.a7. [DOI] [PubMed] [Google Scholar]
- 41.Makino S, Fukuda K, Miyoshi S, et al. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest. 1999;103:697–705. doi: 10.1172/JCI5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schachinger V, Assmus B, Britten MB, et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction - Final one-year results of the TOPCARE-AMI trial. J Am Coll Cardiol. 2004;44:1690–1699. doi: 10.1016/j.jacc.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 43.Schachinger V, Tonn T, Dimmeler S, Zeiher AM. Bone-marrow-derived progenitor cell therapy in need of proof of concept: design of the REPAIR-AMI trial. Nat Clin Pract Cardiovasc Med. 2006;3 Suppl 1:S23–S28. doi: 10.1038/ncpcardio0441. [DOI] [PubMed] [Google Scholar]
- 44.Wollert KC, Meyer GP, Lotz J, Ringes-Lichtenberg S, Lippolt P, Breidenbach C, et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364:141–148. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- 45.Nygren JM, Jovinge S, Breitbach M, et al. Bone marrow-derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nat Med. 2004;10:494–501. doi: 10.1038/nm1040. [DOI] [PubMed] [Google Scholar]
- 46.Kawada H, Fujita J, Kinjo K, et al. Nonhematopoietic mesenchymal stem cells can be mobilized and differentiate into cardiomyocytes after myocardial infarction. Blood. 2004;104:3581–3587. doi: 10.1182/blood-2004-04-1488. [DOI] [PubMed] [Google Scholar]
- 47.Pittenger MF, Martin BJ. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res. 2004;95:9–20. doi: 10.1161/01.RES.0000135902.99383.6f. [DOI] [PubMed] [Google Scholar]
- 48.Yoshioka T, Ageyama N, Shibata H, et al. Repair of infarcted myocardium mediated by transplanted bone marrow-derived CD34+ stem cells in a nonhuman primate model. Stem Cells. 2005;23:355–364. doi: 10.1634/stemcells.2004-0200. [DOI] [PubMed] [Google Scholar]
- 49.Menasche P. Skeletal myoblast for cell therapy. Coron Artery Dis. 2005;16:105–110. doi: 10.1097/00019501-200503000-00005. [DOI] [PubMed] [Google Scholar]
- 50.Menasche P, Hagege AA, Vilquin JT, et al. Autologous skeletal myoblast transplantation for severe postinfarction left ventricular dysfunction. J Am Coll Cardiol. 2003;41:1078–1083. doi: 10.1016/s0735-1097(03)00092-5. [DOI] [PubMed] [Google Scholar]
- 51.Beltrami AP, Barlucchi L, Torella D, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 52.Kehat I, Gepstein L. Human embryonic stem cells for myocardial regeneration. Heart Fail Rev. 2003;8:229–236. doi: 10.1023/a:1024709332039. [DOI] [PubMed] [Google Scholar]
- 53.Kehat I, Kenyagin-Karsenti D, Snir M, et al. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J Clin Invest. 2001;108:407–414. doi: 10.1172/JCI12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kehat I, Khimovich L, Caspi O, et al. Electromechanical integration of cardiomyocytes derived from human embryonic stem cells. Nat Biotechnol. 2004;22:1282–1289. doi: 10.1038/nbt1014. [DOI] [PubMed] [Google Scholar]
- 55.Min JY, Yang YK, Sullivan MF, et al. Long-term improvement of cardiac function in rats after infarction by transplantation of embryonic stem cells. J Thorac Cardiovasc Surg. 2003;125:361–369. doi: 10.1067/mtc.2003.101. [DOI] [PubMed] [Google Scholar]
- 56.Yamada Y, Yokoyama Si, Wang XD, Fukuda N, Takakura N. Cardiac stem cells in brown adipose tissue express CD133 and induce bone marrow nonhematopoietic cells to differentiate into cardiomyocytes. Stem Cells. 2007;25:1326–1333. doi: 10.1634/stemcells.2006-0588. [DOI] [PubMed] [Google Scholar]
- 57.Nakagami H, Morishita R, Maeda K, Kikuchi Y, Ogihara T, Kaneda Y. Adipose tissue-derived stromal cells as a novel option for regenerative cell therapy. J Atheroscler Thromb. 2006;13:77–81. doi: 10.5551/jat.13.77. [DOI] [PubMed] [Google Scholar]
- 58.Chu K, Kim M, Chae SH, et al. Distribution and in situ proliferation patterns of intravenously injected immortalized human neural stem-like cells in rats with focal cerebral ischemia. Neurosci Res. 2004;50:459–465. doi: 10.1016/j.neures.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 59.Yannaki E, Athanasiou E, Xagorari A, et al. G-CSF-primed hematopoietic stem cells or G-CSF per se accelerate recovery and improve survival after liver injury, predominantly by promoting endogenous repair programs. Exp Hematol. 2005;33:108–119. doi: 10.1016/j.exphem.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 60.Cannon RO. Cardiovascular potential of BM-derived stem and progenitor cells. Cytotherapy. 2004;6:602–607. doi: 10.1080/14653240410005294. [DOI] [PubMed] [Google Scholar]
- 61.Hakelien AM, Collas P. Novel approaches to transdifferentiation. Cloning Stem Cells. 2002;4:379–387. doi: 10.1089/153623002321025050. [DOI] [PubMed] [Google Scholar]
- 62.Dimmeler S, Tjwa M. Better regenerative output after cellular input: healing hearts by combining basic fibroblast factor and cell-based therapy. J Am Coll Cardiol. 2008;52:1866–1868. doi: 10.1016/j.jacc.2008.07.065. [DOI] [PubMed] [Google Scholar]
- 63.Kamihata H, Matsubara H, Nishiue T, et al. Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of an-gioblasts, angiogenic ligands, and cytokines. Circulation. 2001;104:1046–1052. doi: 10.1161/hc3501.093817. [DOI] [PubMed] [Google Scholar]
- 64.Askari AT, Unzek S, Popovic ZB, et al. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003;362:697–703. doi: 10.1016/S0140-6736(03)14232-8. [DOI] [PubMed] [Google Scholar]
- 65.Guo J, Lin GS, Bao CY, Hu ZM, Hu MY. Anti-inflammation role for mesenchymal stem cells transplantation in myocardial infarction. Inflammation. 2007;30:97–104. doi: 10.1007/s10753-007-9025-3. [DOI] [PubMed] [Google Scholar]
- 66.Murry CE, Soonpaa MH, Reinecke H, et al. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–668. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- 67.Orlic D, Kajstura J, Chimenti S, et al. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci USA. 2001;98:10344–10349. doi: 10.1073/pnas.181177898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Orlic D, Kajstura J, Chimenti S, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 69.van der Bogt KE, Schrepfer S, Yu J, et al. Comparison of transplantation of adipose tissue- and bone marrow-derived mesenchymal stem cells in the infarcted heart. Transplantation. 2009;87:642–652. doi: 10.1097/TP.0b013e31819609d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lunde K, Solheim S, Aakhus S, et al. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006;355:1199–1209. doi: 10.1056/NEJMoa055706. [DOI] [PubMed] [Google Scholar]
- 71.Laflamme MA, Murry CE. Regenerating the heart. Nat Biotechnol. 2005;23:845–856. doi: 10.1038/nbt1117. [DOI] [PubMed] [Google Scholar]
- 72.Gaustad KG, Boquest AC, Anderson BE, Gerdes AM, Collas P. Differentiation of human adipose tissue stem cells using extracts of rat cardiomyocytes. Biochem Biophys Res Commun. 2004;314:420–427. doi: 10.1016/j.bbrc.2003.12.109. [DOI] [PubMed] [Google Scholar]
- 73.van Dijk A, Niessen HW, Zandieh Doulabi B, Visser FC, van Milligen FJ. Differentiation of human adipose-derived stem cells towards cardiomyocytes is facilitated by laminin. Cell Tissue Res. 2008;334:457–467. doi: 10.1007/s00441-008-0713-6. [DOI] [PubMed] [Google Scholar]
- 74.Madonna R, Willerson JT, Geng YJ. Myocardin a enhances telomerase activities in adipose tissue mesenchymal cells and embryonic stem cells undergoing cardiovascular myogenic differentiation. Stem Cells. 2008;26:202–211. doi: 10.1634/stemcells.2007-0490. [DOI] [PubMed] [Google Scholar]
- 75.Song YH, Gehmert S, Sadat S, et al. VEGF is critical for spontaneous differentiation of stem cells into cardiomyocytes. Biochem Biophys Res Commun. 2007;354:999–1003. doi: 10.1016/j.bbrc.2007.01.095. [DOI] [PubMed] [Google Scholar]
- 76.Nakamura T, Sano M, Songyang Z, Schneider MD. A Wnt- and beta-catenin-dependent pathway for mammalian cardiac myogenesis. Proc Natl Acad Sci USA. 2003;100:5834–5839. doi: 10.1073/pnas.0935626100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pandur P, Lasche M, Eisenberg LM, Kuhl M. Wnt-11 activation of a non-canonical Wnt signalling pathway is required for cardiogenesis. Nature. 2002;418:636–641. doi: 10.1038/nature00921. [DOI] [PubMed] [Google Scholar]
- 78.Terami H, Hidaka K, Katsumata T, Iio A, Morisaki T. Wnt11 facilitates embryonic stem cell differentiation to Nkx2.5-positive cardiomyocytes. Biochem Biophys Res Commun. 2004;325:968–975. doi: 10.1016/j.bbrc.2004.10.103. [DOI] [PubMed] [Google Scholar]
- 79.Koyanagi M, Haendeler J, Badorff C, et al. Non-canonical Wnt signaling enhances differentiation of human circulating progenitor cells to cardiomyogenic cells. J Biol Chem. 2005;280:16838–16842. doi: 10.1074/jbc.M500323200. [DOI] [PubMed] [Google Scholar]
- 80.Foley AC, Mercola M. Heart induction by Wnt antagonists depends on the homeodomain transcription factor Hex. Genes Dev. 2005;19:387–396. doi: 10.1101/gad.1279405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brand T. Heart development: molecular insights into cardiac specification and early morphogenesis. Dev Biol. 2003;258:1–19. doi: 10.1016/s0012-1606(03)00112-x. [DOI] [PubMed] [Google Scholar]
- 82.Minasi MG, Riminucci M, De Angelis L, et al. The mesoangioblast: a multipotent, self-renewing cell that originates from the dorsal aorta and differentiates into most mesodermal tissues. Development. 2002;129:2773–2784. doi: 10.1242/dev.129.11.2773. [DOI] [PubMed] [Google Scholar]
- 83.Galli D, Innocenzi A, Staszewsky L, et al. Mesoangioblasts, vessel-associated multipotent stem cells, repair the infarcted heart by multiple cellular mechanisms - A comparison with bone marrow progenitors, fibroblasts, and endothelial cells. Arterioscler Thromb Vasc Biol. 2005;25:692–697. doi: 10.1161/01.ATV.0000156402.52029.ce. [DOI] [PubMed] [Google Scholar]
- 84.Urbanek K, Sheikh F, Silvestri F, et al. Cardiac stem cells in ischemic heart failure. J Mol Cell Cardiol. 2005;38:851. [Google Scholar]
- 85.Kim Y, Kim H, Cho H, Bae Y, Suh K, Jung J. Direct comparison of human mesenchymal stem cells derived from adipose tissues and bone marrow in mediating neovascularization in response to vascular ischemia. Cell Physiol Biochem. 2007;20:867–876. doi: 10.1159/000110447. [DOI] [PubMed] [Google Scholar]
- 86.Rehman J, Traktuev D, Li JL, et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:1292–1298. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 87.Sadat S, Gehmert S, Song YH, et al. The cardioprotective effect of mesenchymal stem cells is mediated by IGF-I and VEGF. Biochem Biophys Res Commun. 2007;363:674–679. doi: 10.1016/j.bbrc.2007.09.058. [DOI] [PubMed] [Google Scholar]
- 88.Yamada Y, Yokoyama S, Fukuda N, et al. A novel approach for myocardial regeneration with educated cord blood cells cocultured with cells from brown adipose tissue. Biochem Biophys Res Commun. 2007;353:182–188. doi: 10.1016/j.bbrc.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 89.Schenke-Layland K, Strem BM, Jordan MC, et al. Adipose tissue-derived cells improve cardiac function following myocardial infarction. J Surg Res. 2009;153(2):217–223. doi: 10.1016/j.jss.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cai L, Johnstone BH, Cook TG, et al. IFATS series: human adipose tissue-derived stem cells induce angiogenesis and nerve sprouting following myocardial infarction, in conjunction with potent preservation of cardiac function. Stem Cells. 2009;27(1):230–237. doi: 10.1634/stemcells.2008-0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhu XY, Zhang XZ, Xu L, Zhong XY, Ding Q, Chen YX. Transplantation of adipose-derived stem cells overexpressing hHGF into cardiac tissue. Biochem Biophys Res Commun. 2009;379:1084–1090. doi: 10.1016/j.bbrc.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 92.Valina C, Pinkernell K, Song YH, et al. Intracoronary administration of autologous adipose tissue-derived stem cells improves left ventricular function, perfusion, and remodelling after acute myocardial infarction. Eur Heart J. 2007;28:2667–2677. doi: 10.1093/eurheartj/ehm426. [DOI] [PubMed] [Google Scholar]
- 93.Mazo M, Planat-Benard V, Abizanda G, et al. Transplantation of adipose derived stromal cells is associated with functional improvement in a rat model of chronic myocardial infarction. Eur J Heart Fail. 2008;10:454–462. doi: 10.1016/j.ejheart.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 94.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 96.Makino H, Okada S, Nagumo A, et al. Decreased circulating CD34+ cells are associated with progression of diabetic nephropathy. Diabet Med. 2009;26:171–173. doi: 10.1111/j.1464-5491.2008.02638.x. [DOI] [PubMed] [Google Scholar]
- 97.Chen MC, Sheu JJ, Wang PW, et al. Complications impaired endothelial progenitor cell function in Type 2 diabetic patients with or without critical leg ischaemia: implication for impaired neovascularization in diabetes. Diabet Med. 2009;26:134–141. doi: 10.1111/j.1464-5491.2008.02649.x. [DOI] [PubMed] [Google Scholar]
- 98.Zhang H, Park Y, Wu J, et al. Role of TNF-alpha in vascular dysfunction. Clin Sci (Lond) 2009;116:219–230. doi: 10.1042/CS20080196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ohshima M, Li TS, Kubo M, Qin SL, Hamano K. Antioxidant therapy attenuates diabetes-related impairment of bone marrow stem cells. Circ J. 2009;73:162–166. doi: 10.1253/circj.cj-08-0123. [DOI] [PubMed] [Google Scholar]
- 100.Urbich C, Dernbach E, Rossig L, Zeiher AM, Dimmeler S. High glucose reduces cathepsin L activity and impairs invasion of circulating progenitor cells. J Mol Cell Cardiol. 2008;45:429–436. doi: 10.1016/j.yjmcc.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 101.Voo S, Dunaeva M, Eggermann J, Stadler N, Waltenberger J. Diabetes mellitus impairs CD133+ progenitor cell function after myocardial infarction. J Intern Med. 2009;265:238–249. doi: 10.1111/j.1365-2796.2008.02011.x. [DOI] [PubMed] [Google Scholar]
- 102.Meliga E, Strem BM, Duckers HJ, Serruys PW. Adipose-derived cells. Cell Transplant. 2007;16:963–970. doi: 10.3727/096368907783338190. [DOI] [PubMed] [Google Scholar]
- 103.Reinecke H, Minami E, Zhu WZ, Laflamme MA. Cardiogenic differentiation and transdifferentiation of progenitor cells. Circ Res. 2008;103:1058–1071. doi: 10.1161/CIRCRESAHA.108.180588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668–673. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- 105.Chien KR. Stem cells: lost in translation. Nature. 2004;428:607–608. doi: 10.1038/nature02500. [DOI] [PubMed] [Google Scholar]
- 106.Dai W, Hale SL, Martin BJ, et al. Allogeneic mesenchymal stem cell transplantation in postinfarcted rat myocardium: short- and long-term effects. Circulation. 2005;112:214–223. doi: 10.1161/CIRCULATIONAHA.104.527937. [DOI] [PubMed] [Google Scholar]
- 107.Kicic A, Shen WY, Wilson AS, Constable IJ, Robertson T, Rakoczy PE. Differentiation of marrow stromal cells into photoreceptors in the rat eye. J Neurosci. 2003;23:7742–7749. doi: 10.1523/JNEUROSCI.23-21-07742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Davani S, Marandin A, Mersin N, et al. Mesenchymal progenitor cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a rat cellular cardiomyoplasty model. Circulation. 2003;108 Suppl 1:II253–II258. doi: 10.1161/01.cir.0000089186.09692.fa. [DOI] [PubMed] [Google Scholar]
- 109.Leiker M, Suzuki G, Iyer VS, Canty JM, Jr., Lee T. Assessment of a nuclear affinity labeling method for tracking implanted mesenchymal stem cells. Cell Transplant. 2008;17:911–922. doi: 10.3727/096368908786576444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Leo BM, Li X, Balian G, Anderson DG. In vivo bioluminescent imaging of virus-mediated gene transfer and transduced cell transplantation in the intervertebral disc. Spine. 2004;29:838–844. doi: 10.1097/00007632-200404150-00004. [DOI] [PubMed] [Google Scholar]
- 111.Dragoo JL, Choi JY, Lieberman JR, et al. Bone induction by BMP-2 transduced stem cells derived from human fat. J Orthop Res. 2003;21:622–629. doi: 10.1016/S0736-0266(02)00238-3. [DOI] [PubMed] [Google Scholar]
- 112.Kang SK, Lee DH, Bae YC, Kim HK, Baik SY, Jung JS. Improvement of neurological deficits by intracerebral transplantation of human adipose tissue-derived stromal cells after cerebral ischemia in rats. Exp Neurol. 2003;183:355–366. doi: 10.1016/s0014-4886(03)00089-x. [DOI] [PubMed] [Google Scholar]
- 113.Gnecchi M, He H, Noiseux N, et al. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006;20:661–669. doi: 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- 114.Elmadbouh I, Haider H, Jiang S, Idris NM, Lu G, Ashraf M. Ex vivo delivered stromal cell-derived factor-1alpha promotes stem cell homing and induces angiomyogenesis in the infarcted myocardium. J Mol Cell Cardiol. 2007;42:792–803. doi: 10.1016/j.yjmcc.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Novak A, Guo CY, Yang WY, Nagy A, Lobe CG. Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis. 2000;28:147–155. [PubMed] [Google Scholar]
- 116.Hsieh PC, Segers VF, Davis ME, et al. Evidence from a genetic fate-mapping study that stem cells refresh adult mammalian cardiomyocytes after injury. Nat Med. 2007;13:970–974. doi: 10.1038/nm1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Andrade J, Lam JT, Zamora M, et al. Predominant fusion of bone marrow-derived cardiomyocytes. Cardiovasc Res. 2005;68:387–393. doi: 10.1016/j.cardiores.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 118.Ruiz-Lozano P, Chien KR. Cre-constructing the heart. Nat Genet. 2003;33:8–9. doi: 10.1038/ng0103-8. [DOI] [PubMed] [Google Scholar]
- 119.Garcia-Olmo D, Garcia-Arranz M, Herreros D, Pascual I, Peiro C, Rodriguez-Montes JA. A phase I clinical trial of the treatment of Crohn’s fistula by adipose mesenchymal stem cell transplantation. Dis Colon Rectum. 2005;48:1416–1423. doi: 10.1007/s10350-005-0052-6. [DOI] [PubMed] [Google Scholar]
- 120.Turner I, Belema-Bedada F, Martindale J, et al. Molecular Cardiology in Translation: Gene, Cell and Chemical-Based Experimental Therapeutics for the Failing Heart. J Cardiovasc Transl Res. 2008;1:317–327. doi: 10.1007/s12265-008-9065-6. [DOI] [PMC free article] [PubMed] [Google Scholar]