Abstract
Hippocampal damage contributes to cognitive dysfunction after traumatic brain injury (TBI). We previously showed that Fluoro-Jade, a fluorescent stain that labels injured, degenerating brain neurons, allows us to estimate the extent of hippocampal injury after experimental fluid-percussion TBI in rats. Coincidentally, we observed that injured neurons in the rat hippocampus also stained with Newport Green, a fluorescent dye specific for free ionic zinc. Here, we show that, regardless of injury severity or drug treatment, the post-TBI population of injured neurons in rat hippocampal subfields CA1, CA3 and dentate gyrus is indistinguishable, both in numbers and anatomical distribution, from the population of neurons containing high levels of zinc. Treatment with lamotrigine, which inhibits presynaptic release of glutamate and, by inference, zinc that is co-localized with glutamate, reduced numbers of Fluoro-Jade-positive and Newport Green-positive neurons equally as did treatment with nicardipine, which blocks voltage-gated calcium channels through which zinc enters neurons. To confirm using molecular techniques that Fluoro-Jade and Newport Green-positive neurons are equivalent populations, we isolated total RNA from 25 Fluoro-Jade-positive and 25 Newport Green-positive pyramidal neurons obtained by laser capture microdissection (LCM) from the CA3 subfield, linearly amplified the mRNA and used quantitative ribonuclease protection analysis to demonstrate similar expression of mRNA for selected TBI-induced genes. Our data suggest a strong association between reduced neurotoxic zinc levels after TBI and reduced hippocampal neuronal injury.
Keywords: Zinc neurotoxicity, Fluoro-Jade, Newport Green, traumatic brain injury, hippocampal injury, gene expression
Traumatic brain injury (TBI) injures approximately 1.5 million patients per year in the United States, causes 50,000 deaths [7,25,37] and generates an enormous economic burden in long-term disability [27,42]. The long-term disabilities suffered by survivors very often include cognitive deficits, which are attributable in part to damage to the hippocampus, a central locus of learning and memory [3,5].
Hippocampal vulnerability to TBI is striking histologically; within anatomically homogeneous populations of hippocampal neurons, injured neurons are scattered among apparently uninjured neurons. In rats subjected to TBI, we observed that injured neurons, identified by Fluoro-Jade (FJ) staining, expressed significantly lower levels of neuroprotective genes than adjacent uninjured neurons [18].
The hippocampus contains substantial amounts of sequestered ionic zinc [14]. Intraneuronal cytosolic levels of ionic zinc, while normally low, increase dramatically after excitotoxic injury, presumably in part because zinc is co-released with glutamate from presynaptic glutamatergic nerve terminals [9] and also because zinc is released from intraneuronal depots as a consequence of intracellular nitric oxide signaling [6]. Zinc neurotoxicity contributes to neurodegeneration and cell death after ischemic brain injury and TBI [10,21,22,34,35]. In rat hippocampal neurons, chelation of zinc with Ca-EDTA significantly reduced both neuronal zinc accumulation and neuronal death [8,36]. We showed that intracerebroventricularly injected CaEDTA significantly increased mRNA levels of neuroprotective genes in the rat brain after TBI [17].
Cell-impermeant Newport Green (NG), a di-2-picolylamine derivative bound to dichlorofluorescein, is a selective zinc indicator [32,39] that is considered to be specific for zinc but less sensitive than other zinc indicators [38,39]. We chose NG to detect neurotoxic zinc accumulation in hippocampal neurons because staining with an ultra-sensitive or cell-permeant indicator could obscure injury-induced neuronal accumulation due to high levels of zinc in pre-synaptic mossy fibers.
Fluoro-Jade, an anionic fluorochrome considered to be a specific indicator of lethal neuronal injury [2,13,28], has been used to quantify acute neuronal degeneration after TBI [2]. In previous studies, we determined that the number of FJ-positive neurons correlated with the severity of TBI [16]. We also obtained preliminary evidence that adjacent sections of rat hippocampus showed nearly identical distribution and numbers of FJ-positive and NG-positive neurons, as Suh et al. had previously suggested with TSQ staining and eosinophilic counterstaining [34]. We therefore hypothesized that acute hippocampal neuronal degeneration after TBI was closely associated with intracellular zinc accumulation. Here, we provide evidence supporting our hypothesis that zinc neurotoxicity and hippocampal neuronal injury after TBI are closely associated. Therefore, we speculate that zinc neurotoxicity is a potential mechanism of hippocampal neuronal injury.
Material and Methods
Surgical Procedures
Adult, male, Sprague-Dawley rats, 400–500g (n = 6 per experimental group, 54 rats total for the first study and the subsequent study with lamotrigine and nicardipine) were anesthetized with 4% isoflurane, endotracheally intubated and mechanically ventilated with 1.0–1.5% isoflurane throughout the surgical procedure and subsequent experiments. We performed a right-sided craniotomy lateral to the sagittal suture, midway between bregma and lambda and subjected the animals to sham, mild (1.2 atmospheres) or moderate (2.0 atm) TBI using a fluid percussion device. Fluid percussion TBI is described in detail in several articles [12,23,24]. For the first study examining the effects of injury severity on numbers and gene expression of Fluoro-Jade positive and Newport Green positive neurons, rats were sacrificed 4 and 24 hour post-TBI.
In the second study, to examine the effects of lamotrigine and nicardipine, male Sprague Dawley rats (n=6 per group) were anesthetized (4% isoflurane), intubated, mechanically ventilated (1.5% isoflurane in O2:air (30:70)), prepared for moderate (2.0 atm) or sham parasagittal fluid percussion injury and randomly assigned to receive saline, nicardipine (5mg/kg) or lamotrigine (20mg/kg) 30 minutes pre-TBI. In each experiment, the right common jugular vein was cannulated for intravenous fluid administration and the tail artery was cannulated for arterial blood gas sampling and mean arterial pressure (MAP) monitoring. Baseline measurements of each animal’s MAP, blood gasses, blood glucose and body (rectal) and temporalis temperatures were taken immediately before drug administration to ensure each rat is within a normal range. Using blood removed from the tail artery, blood gasses and pH measurements were taken using an iStat cartridge and read on an iStat machine (Abbott laboratories). Adjustments of tidal volume and breath rate were made to correct for abnormal PCO2 values and a table lamp and heating pad were used to regulate the temperatures. All drug treatments were infused through the right jugular vein over a 15 minute period. Twenty-four hours later, rats were anesthetized, brains were harvested and frozen sectioned. Adjacent sections were stained with FJ or NG as described below. Numbers of FJ- or NG-positive neurons in CA1 and CA3 were counted by an investigator who was blinded to group assignment.
Sectioning & Staining
In order to perform counts of FJ- and NG-positive neurons, we collected ten sets of 10 µm adjacent hippocampal sections at every 15th section throughout the injury site (10 pairs of adjacent sections per brain). All sections were fixed in 75% ethanol, dehydrated in graded alcohols and counterstained for 15 seconds with 1% cresyl violet [18]. We stained one section per pair with .001% FJ for 4 minutes; the other was stained with 5 µM NG (cell-impermeable) for 4 minutes.
Neuronal Counting Procedure
Standard methods for stereological counting of neurons require thick sections (30 to 50 µm). Since adjacent sections were used for laser capture (for which we require thin 10 µm sections) and since our goal was to determine concordance of the two types of labeled neurons in adjacent sections, we employed a systematic neuronal counting procedure that we used previously to estimate numbers of injured neurons in TBI rats [16]. Two investigators who were blinded to treatment groups counted simultaneously and independently using the PixCell IIe imaging system monitor (Arcturus, Mountain View, CA). The fluorescence signal from both FJ and NG is best viewed using the FITC filter. For data presentation, the hippocampus was divided into the CA1/2, CA3 and dentate gyrus subfields. The investigators recorded the number of FJ- or NG-positive neurons for each region and the numbers for each indicator were added to obtain a total count for that region. The two investigators’ counts were averaged to get a mean count for each animal for CA1/2, CA3 and DG.
Statistical Analysis of neuronal counts
We completed statistical analysis using Statview 5.0. We performed one-way analyses of variance (ANOVA) and Fisher’s post-hoc tests.
Laser capture microdissection (LCM)
LCM was performed on sections from brains of rats in the first study subjected to moderate TBI and sacrificed 24 hours after injury. We fixed 10 µm frozen hippocampal sections in 75% ethanol (1 min), stained with FJ or NG, counterstained with 1% cresyl violet (15 sec) and dehydrated in graded alcohols and xylene as previously described [18]. We performed LCM with a PixCell IIe LCM system (Arcturus, Mountain View, CA). 25 FJ-positive or 25 NG-positive neurons were captured from each rat hippocampus on separate Capsure HS LCM Caps (Arcturus). We isolated total RNA with the RNAaqueous Micro kit (Ambion, Austin, TX) and linearly amplified the mRNA once with T7 polymerase (MessageAmp II kit, Ambion) as previously described [18].
Ribonuclease Protection Assay (RPA)
We performed RPA analysis with 100 ng of linearly amplified antisense mRNA using the Hybspeed RPA kit (Ambion) as previously described [16,33]. This is equivalent to using approximately 5 µg total RNA per sample (assuming mRNA is 2% of total RNA in the brain). Sequences of primers used for RT-PCR cloning of four TBI-induced genes (Bcl-2, Caspase 3, Caspase 9 and Hsp70) and primers for nested amplification of RPA templates from the cloned fragments are shown in Table 1. Following exposure of RPA gels to phosphoimaging screens, we analyzed gene expression data with OptiQuant software (Perkin Elmer Life Sciences, Downers Grove, IL) and imported the data into Microsoft Excel for further analysis.
Table 1.
Primer sequences and size of amplimers and protected fragments
| Gene | Primer Sequence (Forward and Reverse) | Product (bp) | GenBank no. |
|---|---|---|---|
| Heat Shock Protein 70 | 5'-CGGCTGGTGAGCCACTTCGT-3' (Forward) | 589 | Z27118 |
| 5'-GAGTAGGTGGTGAAGGTCTG-3' (Reverse) | |||
| Caspase-3 | 5'-CAGCTCGCAATGGTACCGAT-3' (Forward) | 620 | NM_012922.1 |
| 5'-GCATTGACACAATACACGGG-3' (Reverse) | |||
| BCL2 | 5'-GGATACTGGAGATGAAGACT-3' (Forward) | 567 | L14680 |
| 5'-GCTGAGCAGCGTCTTCAGAG-3' (Reverse) | |||
| Caspase-9 | 5'-GCTTCGTGGTGGTCATCCTC-3' (Forward) | 471 | NM_031632 |
| 5'-CTGAGAAGGAGGGACTGCAG-3' (Reverse) | |||
| Glyceraldehyde-3-phosphate dehydrogenase |
5'-ACCACAGTCCATGCCATCAC-3' (Forward) | 451 | NM_017008 |
| 5'-TCCACCACCCTGTTGCTGTA-3' (Reverse) | |||
| Gene | Primer Sequence (T3 T7) | Product (bp) | GenBank no. |
| Heat Shock Protein 70 | 5'-GCCTAATTAACCCTCACTAAAGGGAGCTGTGCTCGGACCTGTTCCGCG-3' (T3) | 304 | Z27118 |
| 5'-CTCGGTAATACGACTCACTATAGGGCCGTCTCCAGACCCAGCGACACG-3' (T7) | |||
| Caspase-3 | 5'-GCCTAATTAACCCTCACTAAAGGGAGGTACAGAGCTGGACTGCGGTAT-3' (T3) | 271 | NM_012922.1 |
| 5'-CTCGGTAATACGACTCACTATAGGGGTGGCGTCCAGGGAGAAGGACTC-3' (T7) | |||
| BCL2 | 5'-GCCTAATTAACCCTCACTAAAGGGACGCTGTGCACCGAGACACGGCTG-3' (T3) | 236 | L14680 |
| 5'-CTCGGTAATACGACTCACTATAGGGCCATCCCTGAAGAGTTCCTCCAC-3' (T7) | |||
| Caspase-9 | 5'-GCCTAATTAACCCTCACTAAAGGGAGCTGTTCTTCATCCAGGCCTGTG-3' (T3) | 171 | NM_031632 |
| 5'-CTCGGTAATACGACTCACTATAGGGGTGGGCAAACTTGACACTGCGTC-3' (T7) | |||
| Glyceraldehyde-3-phosphate dehydrogenase |
5'-GCCTAATTAACCCTCACTAAAGGGAGCTGCCAAGGCTGTGGGCAAGGT-3' (T3) | 140 | NM_017008 |
| 5'-CTCGGTAATACGACTCACTATAGGGCTTGATGTCATCATACTTGGCAG-3' (T7) | |||
Statistical Analysis of gene expression levels
We analyzed the levels of mRNA separately for each gene (Bcl-2, Caspase 3, Caspase 9 and Hsp70) using a two-way mixed model. The two factors were rat (random effect) and stain (FJ and NG). To account for multiple testing, differences in gene expression of the four genes between the two stained populations of neurons were considered significant at the 0.025 level. We conducted data analysis using SAS® 9.1 [26]
Results
Concordance of injured and zinc-positive neurons in rat hippocampus
To identify molecular mechanisms associated with neuronal survival following TBI, we previously reported differences in gene expression between injured and uninjured neurons [18]. To confirm our impression that injured neurons always appeared to stain with a zinc-positive dye, we designed a series of experiments (Figure 1) to compare the distribution and numbers of FJ-positive and NGreen-positive neurons in the injured rat hippocampus and expression of selected TBI-induced genes in these two neuronal populations. As we reported previously [18], we found virtually no injured or zinc-positive cells in the contralateral hippocampus of injured rats or in the hippocampus of sham-operated rats. In injured rats, the anatomical distribution of FJ-positive and NG-positive neurons was virtually indistinguishable in the CA1 and CA3 subfields (Figure 2).
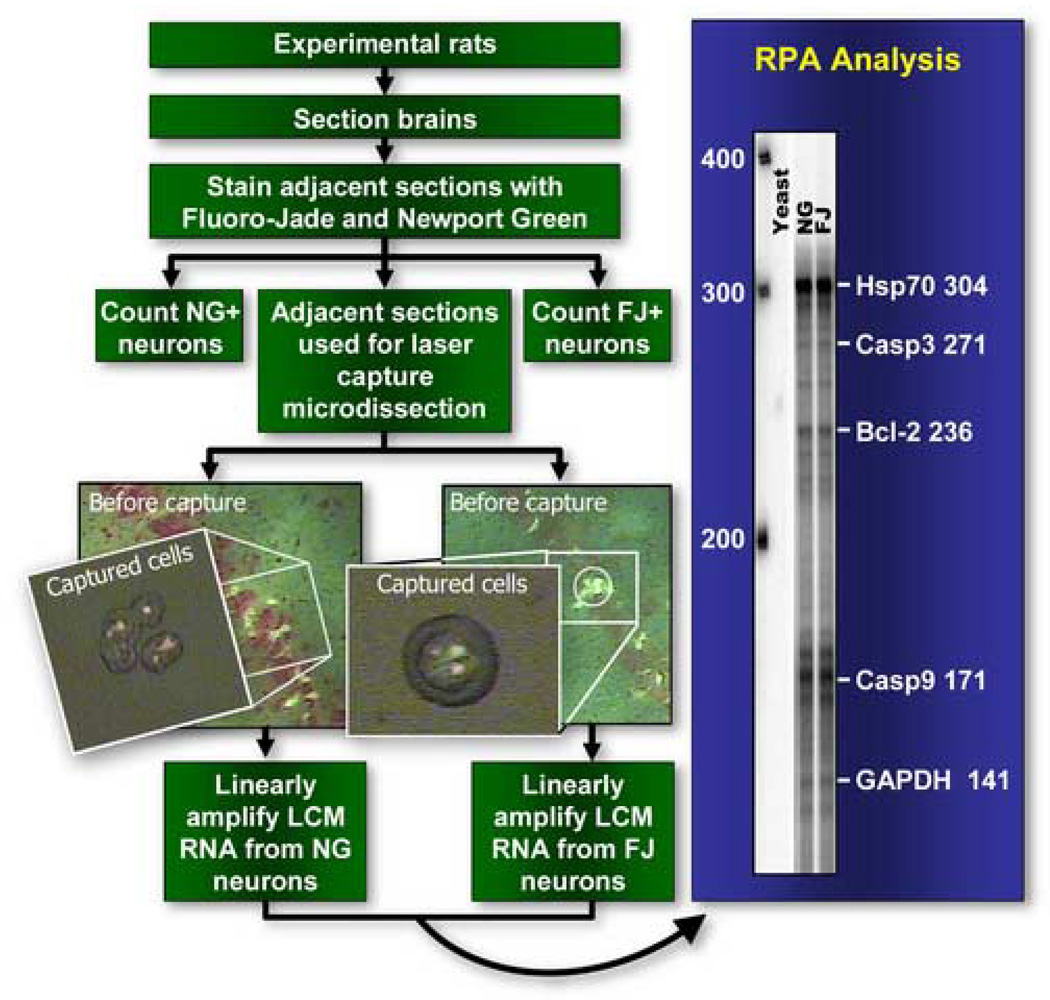
Figure 1.
Schematic overview of the experimental design for the dose response and gene expression study. We performed neuronal counts of fluorescent cells on 10 representative sections of all experimental rat brains (subjected to sham, mild or moderate injury) that were serially sectioned through the hippocampus and stained for Fluoro-Jade (FJ) or Newport Green (NG). Quantitative ribonuclease protection assay (RPA) analysis was performed using linearly amplified mRNA (from 25 FJ positive neurons in one section and 25 NG positive neurons from the adjacent section in each rat) from a subset of traumatic brain injured rats (n=6).
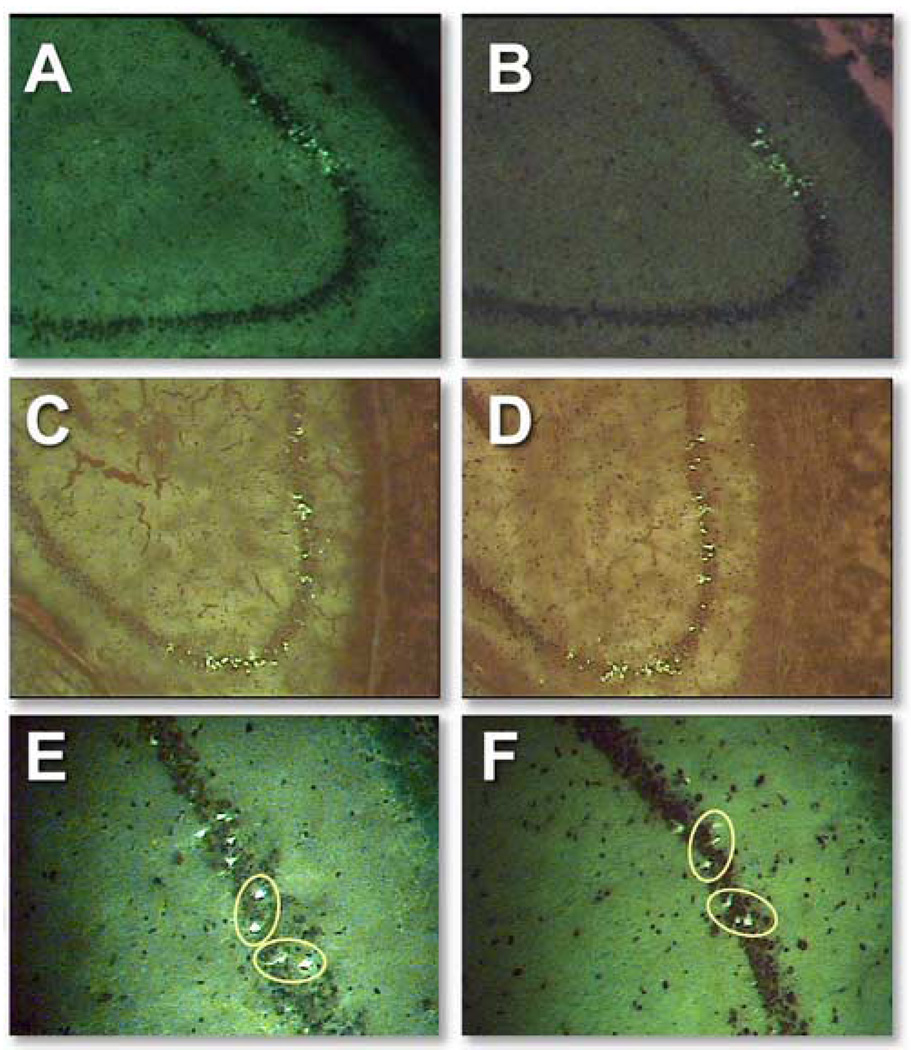
Figure 2.
Representative sets of adjacent cells stained for Fluoro-Jade (A and C) and Newport Green (B and D) in the CA1/2 and CA3 subfields of the rat hippocampus. We counterstained slides for 15 seconds with 1% cresyl violet. Different background levels that varied from experiment to experiment were not adjusted. In adjacent serial sections of injured rat hippocampus, circles indicate identified neurons that appear to be stained by both Fluoro-Jade (E) and Newport Green (F).
Because both NG and FJ are fluorescent stains with overlapping emission spectra, we could not study concordance using a dual-staining technique on the same slides. We performed preliminary experiments to determine whether counting FJ-stained neurons in sections adjacent to those stained with NG would permit precise correlation. In CA-1, 21 neurons were stained in both slides, while 11 were visible only on the FJ-stained slide and 7 were visible only on the NG-stained slide. In CA-3, 19 neurons were stained in both slides, while 17 were visible only on the FJ-stained slide and 14 were visible only on the NG-stained slide. We then compared adjacent slides, both stained with FJ: in CA-1, 22 neurons were stained in both slides, while 16 were visible only on slide A and 23 were visible only on slide B. In CA-3, 22 neurons were stained in both slides, while 16 were visible only on slide A and 23 were visible only on slide B. In those slides in which a neuron was stained that did not appear on the adjacent section, no corresponding unstained neuron was ever apparent. Consequently, we concluded 100% correlation between NG-positive and FJ-positive neurons in adjacent 10 µm sections was not attainable. We reasoned that one 10 µm section could contain one end of a 20–30 µm neuron that would not be present in the adjacent section. Given this limitation, we found a remarkable concordance between the two populations of FJ-positive and NG-positive neurons after TBI.
Numbers of FJ-stained and NG-stained neurons after TBI
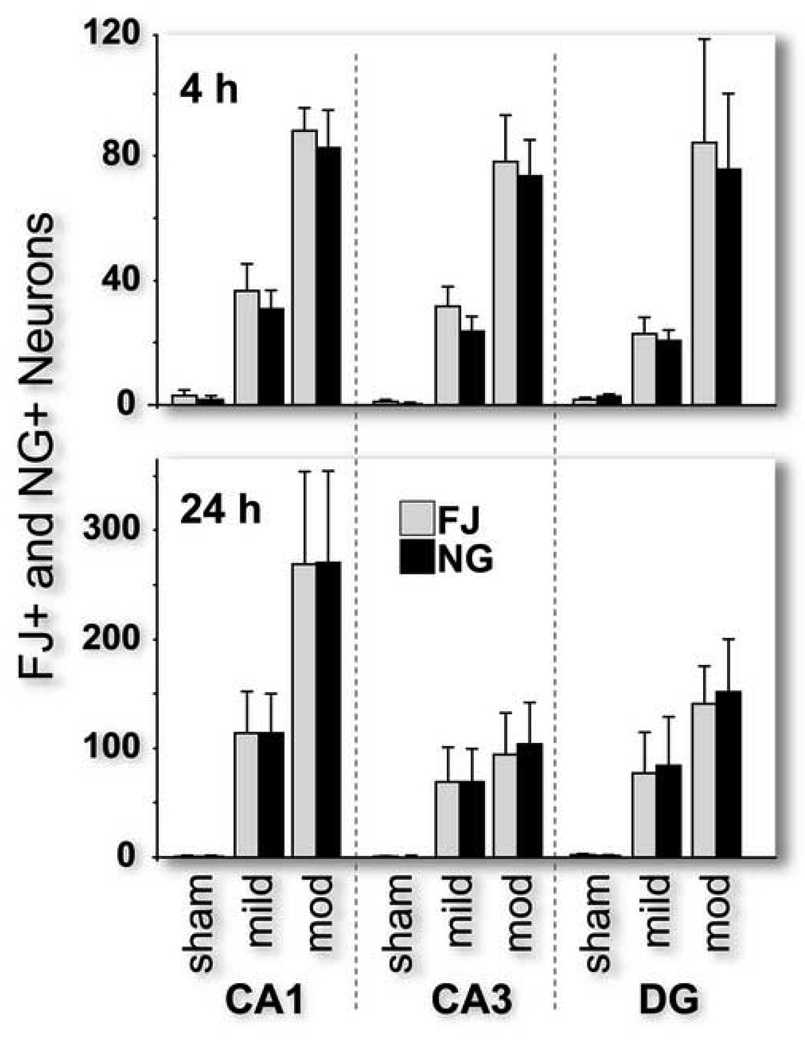
We found that adjacent sections of the rat hippocampus have similar numbers of FJ-and NG-positive neurons in a similar distribution 4 and 24 hour after sham, mild and moderate fluid percussion TBI (Figure 3). We found no statistical difference between the numbers of FJ- and NG-positive neurons regardless of time-point (4 or 24 hour post-TBI), level of injury or hippocampal region. Four hours after moderate TBI, there was a significant and equal increase in the numbers of FJ-and NG-labeled neurons in all three hippocampal subfields (p<.05). Twenty-four hours post-TBI there was also an injury-severity-dependent increase in the numbers of injured and zinc-positive neurons in the CA1 and CA3 subfields (p<.05). In the dentate gyrus, the numbers of positive neurons significantly increased after 24 hours with moderate injury only (p<.05).
Figure 3.
Numbers of Fluoro-Jade-positive (injured) and Newport Green-positive (zinc) neurons increased with increasing levels of injury in the CA1, CA3 and dentate gyrus subfields of the rat hippocampus at both 4 and 24 hours post-injury (n=6 per group). The numbers of injured and zinc-positive neurons were not statistically different from each other.
Influence of neuroprotective drug interventions
To test our hypothesis that that zinc neurotoxicity and hippocampal neuronal injury after TBI are closely associated, we chose two interventions that have been shown to be neuroprotective and that also exert pharmacologic effects that reduce intraneuronal accumulation of zinc. All rats used for this study exhibited normal ranges for physiological measurements: PCO2: 35–41 mmHg, pH: 7.40–7.44, MAP: above 80mmHg, rectal temperature: 37.0–37.5 °C, temporalis temperature: 36.5–37.5 °C
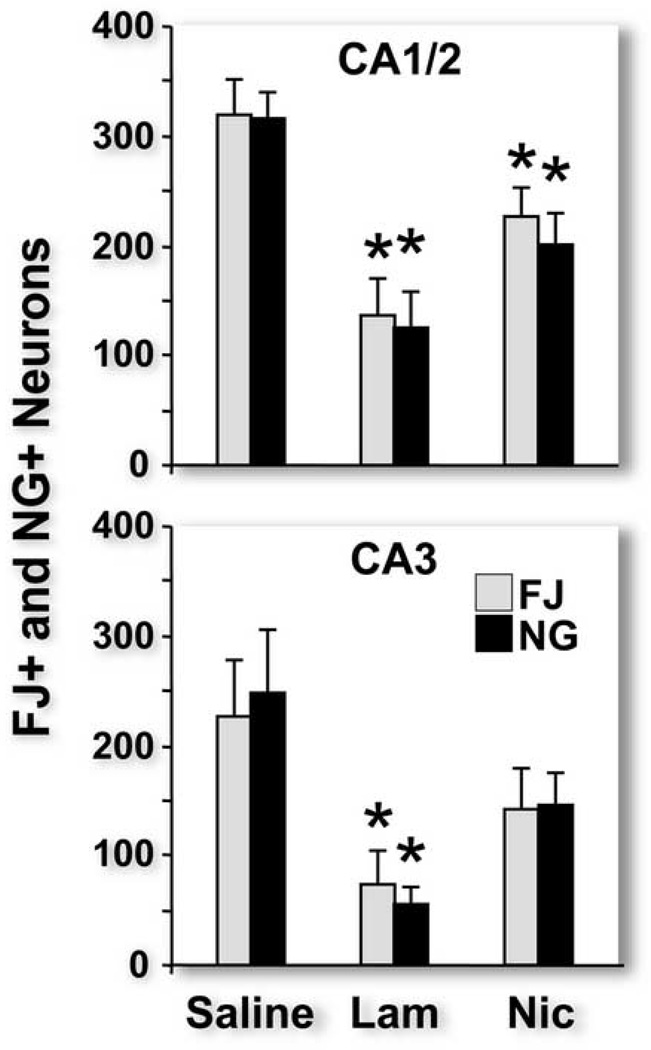
We examined the effects of lamotrigine (a sodium channel blocker that inhibits presynaptic release of glutamate and, by inference, the release of zinc that is co-located in presynaptic vesicles with glutamate) and nicardipine (an L-type calcium channel antagonist) on FJ and NG staining after TBI. We found that treatment of TBI rats with either drug significantly and equally reduced numbers of FJ-positive and NG-positive neurons in the CA1 subfield and that lamotrigine significantly reduced neuronal injury in the CA3 subfield (Figure 4).
Figure 4.
Pretreatment with lamotrigine (lam) or nicardipine (nic) 30 minutes before traumatic brain injury (TBI) significantly reduced numbers of Fluoro-Jade positive and Newport Green positive neurons in both the CA1 (for both lam and nic) and CA3 (lam only) when compared to saline injected TBI rats. * p<0.05
Gene expression in injured and zinc-positive neurons
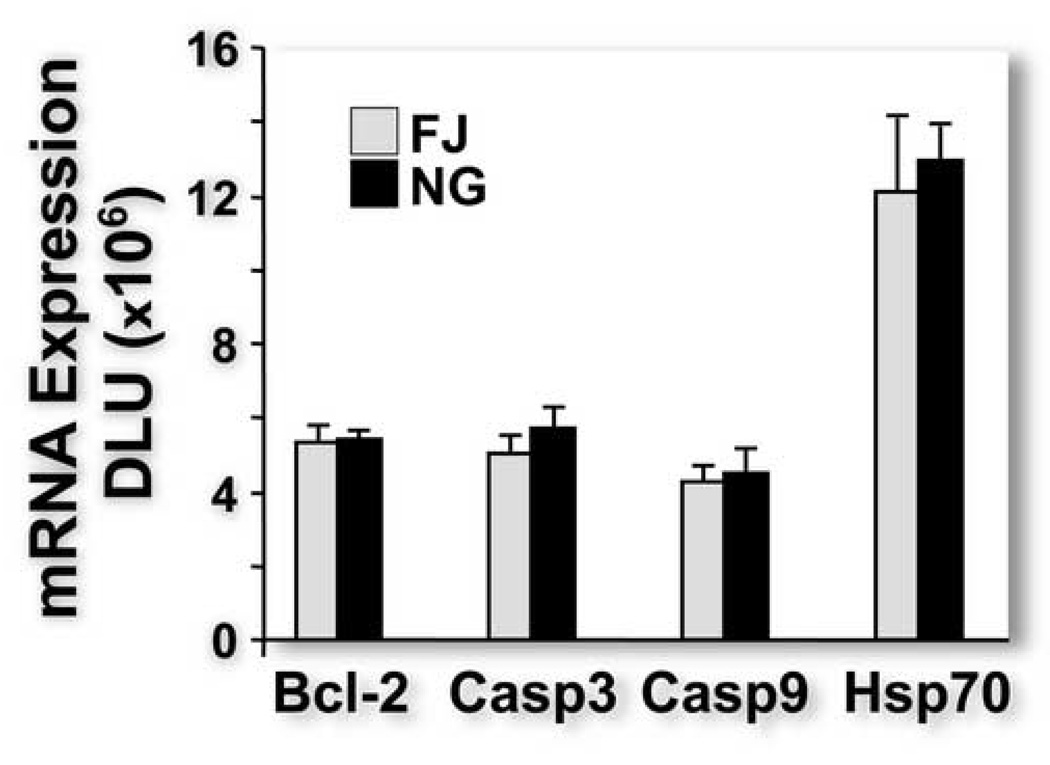
Because we found previously that TBI-induced gene expression was significantly different in injured and immediately adjacent uninjured neurons [18], we reasoned that, if FJ and NG were staining the same group of neurons, TBI-induced gene expression would be similar in neurons identified with the two stains. Using LCM, we obtained equal numbers of FJ- and NG-positive neurons in adjacent sections and compared injury-induced gene expression in the two sets of neurons by quantitative RPA analysis. Expression of Bcl-2, Caspase 3 and Caspase 9 and Hsp 70 was not different between the two groups of stained neurons (Figure 5). Between-reading variability (within-rat variability) of FJ was smaller than NG for Bcl-2, Caspase 3 and Hsp70, but not Caspase 9. These data suggest that FJ staining is easier to visualize and quantify than NG. Between rat variability and between-reading variability were similar in magnitude regardless of gene expression.
Figure 5.
Quantitative ribonuclease protection assay analysis of Bcl-2, Caspase 3 (casp 3), Caspase 9 (casp 9), and heat shock protein 70 (Hsp 70) mRNA (linearly amplified with T7 polymerase) expression in identified Fluoro-Jade positive and Newport Green positive neurons in immediately adjacent sections. We found no statistical difference in gene expression between the two groups of stained neurons. DLU, digital light units.
Discussion
In our study, we sought to corroborate and confirm the strong association between neuronal injury and zinc neurotoxicity in the rat hippocampus after TBI. Regardless of level of injury or type or dose of experimental neuroprotective treatment, we found that, in adjacent hippocampal sections, the population of FJ-positive (injured) and NG-positive (zinc-positive) neurons had similar anatomic distributions and statistically similar numbers. In addition, injury-induced gene expression, previously shown to be significantly different in injured and uninjured hippocampal neurons after TBI [18], was similar in FJ-positive and NG-positive neurons. Since our ultimate goal is to understand the molecular mechanisms of hippocampal injury and then design effective therapeutic interventions that target the identified molecular mechanisms, the results of this study suggest that reducing zinc neurotoxicity may be helpful in limiting TBI-induced neuronal injury and post-TBI cognitive dysfunction.
These data suggest, but do not prove, a cause-and-effect relationship between hippocampal neuronal zinc accumulation and neuronal injury. There are several potential explanations for the strong association between zinc accumulation and hippocampal neuronal injury. First, zinc accumulation could be an essential mechanism of acute hippocampal neuronal injury after TBI; i.e., as zinc accumulates, neurons die. Second, zinc accumulation could be an epiphenomenon; that is, as hippocampal neurons die, zinc accumulates. Third, NG staining could represent a histologic artifact; perhaps after TBI, NG nonspecifically stains non-zinc products of injured, degenerating neurons.
We consider the first explanation to be the most likely. Both lamotrigine and nicardipine significantly reduced numbers of zinc-positive neurons in the hippocampus. Furthermore, there was an equal and consistent reduction in the distribution and numbers of injured, Fluoro-Jade positive neurons in every experimental rat in our study. However, neither lamotrigine nor nicardipine is specific for zinc and our experimental design does not distinguish among the possible mechanisms of neuroprotection provided by lamotrigine and nicardipine. We chose lamotrigine because of previous work demonstrating that the anti-epileptic, Na+channel blocker lamotrigine blocked glutamate release from glutamatergic presynaptic terminals [4,11]. Although lamotrigine treatment apparently does not reduce basal levels of GABA, aspartate, glutamate and taurine (as measured by hippocampal microdialysis in rats), it did decrease the release of these amino acids after 50µM veratridine stimulation [1]. We inferred that lamotrigine would also decrease zinc release from presynaptic glutamatergic vesicles in which glutamate and zinc were colocated [15]. For these in vivo TBI experiments, we chose nicardipine because previous in vitro studies in neocortical cultures from embryonic mice demonstrated that other voltage-gated calcium channel blockers (verapamil and nimodipine) reduced post-synaptic zinc accumulation [30][20]. As expected, we found that both lamotrigine and nicardipine treatment were associated with decreased numbers of zinc-positive neurons and proportionately decreased numbers of injured, FJ-positive neurons. We have thus confirmed a strong association between hippocampal zinc accumulation and neuronal injury but have not proved that the primary mechanism of neuronal protection by either lamotrigine or nicardipine is zinc-specific.
While none of these data specifically disprove the possibility that zinc accumulation is an epiphenomenom, they are more consistent with the probability that zinc is a primary mediator of hippocampal neuronal death after TBI. We cannot disprove the possibility that NG nonspecifically stains non-zinc molecules; however, multiple authors have demonstrated the specificity of NG staining for zinc [9,29,31,39,41]. The molecular binding sites of FJ are not known, although the binding site has been assumed to be cationic products of neurodegeneration such as spermidine or cadaverine [29].
Further evidence for the central role of zinc in hippocampal neuronal degeneration is the virtually identical gene expression data from FJ-positive and NG-positive neurons. In a prior study using LCM to capture homogeneous populations of hippocampal neurons, we demonstrated distinctive differences in TBI-related gene expression in FJ-positive injured neurons and immediately adjacent FJ-negative uninjured neurons; specifically, uninjured neurons expressed significantly higher mRNA levels of neuroprotective genes than injured neurons [18]. We realized that the differences or similarities in neuroprotective gene expression profiles could be used to distinguish injured from uninjured hippocampal neurons or to potentially identify neurons with similar functions. In experiments in which we examined the effects of chelating zinc after TBI, we found that injured neurons had a similar anatomic distribution to zinc-positive neurons. In the present study, to confirm that injured, FJ-positive neurons and zinc-loaded, NG-positive neurons were similar populations of cells, we obtained these two sets of labeled neurons by LCM, linearly amplified the mRNA [19,40] and compared gene expression levels by quantitative RPA analysis. In contrast to our previous results comparing injured and immediately adjacent uninjured neurons [18], we found that mRNA levels of selected injury-induced genes were statistically not different between the two microdissected populations of neurons. In light of reported intrinsic differences in gene expression between pyramidal neurons in CA1 and CA3 subfields [33] and differences between injured and uninjured neurons, we believe that the similar levels of TBI-induced gene expression in these two populations of neurons suggests a causal role for zinc neurotoxicity in hippocampal neurodegeneration after TBI.
In summary, we have provided evidence that zinc neurotoxicity may contribute significantly to hippocampal injury after TBI. The clinical implication is that pharmacological interventions designed to reduce or ameliorate the effects of zinc toxicity immediately after TBI may have significant therapeutic benefits for human TBI patients and may alleviate the cognitive problems that often disable TBI survivors.
Acknowlegements
This work was funded by National Institutes of Health grant R01 NS042849-01A1 (D.S.P.). Bridget E. Hawkins’ work was supported by a predoctoral fellowship under NIEHS grant no. T32 ES007254, “Molecular Mechanisms for Environmental Injury.” We thank Andrew Hall and Molly Lynch for excellent editorial assistance and Christy Perry for excellent preparation of figures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ahmad S, Fowler LJ, Whitton PS. Effects of combined lamotrigine and valproate on basal and stimulated extracellular amino acids and monoamines in the hippocampus of freely moving rats. Naunyn Schmiedebergs Arch Pharmacol. 2005;371:1–8. doi: 10.1007/s00210-004-1008-4. [DOI] [PubMed] [Google Scholar]
- 2.Anderson KJ, Miller KM, Fugaccia I, Scheff SW. Regional distribution of Fluoro-Jade B staining in the hippocampus following traumatic brain injury. Exp Neurol. 2005;193:125–130. doi: 10.1016/j.expneurol.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 3.Arciniegas D, Adler L, Topkoff J, Cawthra E, Filley CM, Reite M. Attention and memory dysfunction after traumatic brain injury: cholinergic mechanisms, sensory gating, and a hypothesis for further investigation. Brain Inj. 1999;13:1–13. doi: 10.1080/026990599121827. [DOI] [PubMed] [Google Scholar]
- 4.Bacher A, Zornow MH. Lamotrigine inhibits extracellular glutamate accumulation during transient global cerebral ischemia in rabbits. Anesthesiology. 1997;86:459–463. doi: 10.1097/00000542-199702000-00021. [DOI] [PubMed] [Google Scholar]
- 5.Bigler ED, Blatter DD, Anderson CV, Johnson SC, Gale SD, Hopkins RO, Burnett B. Hippocampal volume in normal aging and traumatic brain injury. AJNR Am J Neuroradiol. 1997;18:11–23. [PMC free article] [PubMed] [Google Scholar]
- 6.Bossy-Wetzel E, Talantova MV, Lee WD, Scholzke MN, Harrop A, Mathews E, Gotz T, Han J, Ellisman MH, Perkins GA, Lipton SA. Crosstalk between nitric oxide and zinc pathways to neuronal cell death involving mitochondrial dysfunction and p38-activated K+ channels. Neuron. 2004;41:351–365. doi: 10.1016/s0896-6273(04)00015-7. [DOI] [PubMed] [Google Scholar]
- 7.Bruns J, Hauser WA., Jr The epidemiology of traumatic brain injury: a review. Epilepsia. 2003;44 Suppl 10:2–10. doi: 10.1046/j.1528-1157.44.s10.3.x. [DOI] [PubMed] [Google Scholar]
- 8.Calderone A, Jover T, Mashiko T, Noh KM, Tanaka H, Bennett MV, Zukin RS. Late calcium EDTA rescues hippocampal CA1 neurons from global ischemia-induced death. J Neurosci. 2004;24:9903–9913. doi: 10.1523/JNEUROSCI.1713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Canzoniero LMT, Turetsky DM, Choi DW. Measurement of intracellular free zinc concentrations accompanying zinc-induced neuronal death. J. Neurosci. 1999;19:1–6. doi: 10.1523/JNEUROSCI.19-19-j0005.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi DW, Koh JY. Zinc and brain injury. Annu. Rev Neurosci. 1998;21:347–375. doi: 10.1146/annurev.neuro.21.1.347. [DOI] [PubMed] [Google Scholar]
- 11.Crumrine RC, Bergstrand K, Cooper AT, Faison WL, Cooper BR. Lamotrigine protects hippocampal CA1 neurons from ischemic damage after cardia arrest. Stroke. 1997;28:2230–2237. doi: 10.1161/01.str.28.11.2230. [DOI] [PubMed] [Google Scholar]
- 12.Dixon CE, Lyeth BG, Povlishock JT, Findling RL, Hamm RJ, Marmarou A, Young HF, Hayes RL. A fluid percussion model of experimental brain injury in the rat. J Neurosurg. 1987;67:110–119. doi: 10.3171/jns.1987.67.1.0110. [DOI] [PubMed] [Google Scholar]
- 13.Duckworth EA, Butler TL, De Mesquita D, Collier SN, Collier L, Pennypacker KR. Temporary focal ischemia in the mouse: Technical aspects and patterns of Fluoro-Jade evident neurodegeneration. Brain Res. 2005;1042:29–36. doi: 10.1016/j.brainres.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 14.Frederickson CJ, Danscher G. Hippocamal zinc, the storage granule pool: localization, physiochemistry, and possible functions. In: Morley JE, Sterman MB, Walsh JH, editors. Nutritional Modulation of Neural Function. San Diego, CA: Academic Press, Inc.; 1988. pp. 289–306. [Google Scholar]
- 15.Frederickson CJ, Suh SW, Silva D, Thompson RB. Importance of zinc in the central nervous system: the zinc-containing neuron. J. Nutr. 2000;130:1471S–1483S. doi: 10.1093/jn/130.5.1471S. [DOI] [PubMed] [Google Scholar]
- 16.Hellmich HL, Capra B, Eidson K, Garcia J, Kennedy D, Uchida T, Parsley M, Cowart J, DeWitt DS, Prough DS. Dose-dependent neuronal injury after traumatic brain injury. Brain Res. 2005;1044:144–154. doi: 10.1016/j.brainres.2005.02.054. [DOI] [PubMed] [Google Scholar]
- 17.Hellmich HL, Frederickson CJ, DeWitt DS, Saban R, Parsley MO, Stephenson R, Velasco M, Uchida T, Shimamura M, Prough DS. Protective effects of zinc chelation in traumatic brain injury correlate with upregulation of neuroprotective genes in rat brain. Neurosci Lett. 2004;355:221–225. doi: 10.1016/j.neulet.2003.10.074. [DOI] [PubMed] [Google Scholar]
- 18.Hellmich HL, Garcia JM, Shimamura M, Shah SA, Avila MA, Uchida T, Parsley MA, Capra BA, Eidson KA, Kennedy DR, Winston JH, DeWitt DS, Prough DS. Traumatic brain injury and hemorrhagic hypotension suppress neuroprotective gene expression in injured hippocampal neurons. Anesthesiology. 2005;102:806–814. doi: 10.1097/00000542-200504000-00017. [DOI] [PubMed] [Google Scholar]
- 19.Hinkle DA, Eberwine JH. Single-cell molecular biology: implications for diagnosis and treatment of neurologic disease. Biol Psychiatry. 2003;54:413–417. doi: 10.1016/s0006-3223(03)00322-6. [DOI] [PubMed] [Google Scholar]
- 20.Kerchner GA, Canzoniero LMT, Yu SP, Ling C, Choi DW. Zn2+ current is mediated by voltage-gated Ca2+ channels and enhanced by extracellular acidity in mouse cortical neurons. J. Physiol. 2000;528:39–52. doi: 10.1111/j.1469-7793.2000.00039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koh JY. Zinc and disease of the brain. Mol. Neurobiol. 2001;24:99–106. doi: 10.1385/MN:24:1-3:099. [DOI] [PubMed] [Google Scholar]
- 22.Lee JM, Grabb MC, Zipfel GJ, Choi DW. Brain tissue responses to ischemia. J. Clin. Invest. 2000;106:723–731. doi: 10.1172/JCI11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mathew BP, DeWitt DS, Bryan RM, Bukoski RD, Jr, Prough DS. Traumatic brain injury reduces myogenic responses in pressurized rodent middle cerebral arteries. J. Neurotrauma. 1999;16:1177–1186. doi: 10.1089/neu.1999.16.1177. [DOI] [PubMed] [Google Scholar]
- 24.McIntosh TK, Vink R, Noble L, Yamakami I, Fernyak S, Soares H, Faden AL. Traumatic brain injury in the rat: characterization of a lateral fluid-percussion model. Neuroscience. 1989;28:233–244. doi: 10.1016/0306-4522(89)90247-9. [DOI] [PubMed] [Google Scholar]
- 25.Narayan RK, Michel ME, Ansell B, Baethmann A, Biegon A, Bracken MB, Bullock MR, Choi SC, Clifton GL, Contant CF, Coplin WM, Dietrich WD, Ghajar J, Grady SM, Grossman RG, Hall ED, Heetderks W, Hovda DA, Jallo J, Katz RL, Knoller N, Kochanek PM, Maas AI, Majde J, Marion DW, Marmarou A, Marshall LF, McIntosh TK, Miller E, Mohberg N, Muizelaar JP, Pitts LH, Quinn P, Riesenfeld G, Robertson CS, Strauss KI, Teasdale G, Temkin N, Tuma R, Wade C, Walker MD, Weinrich M, Whyte J, Wilberger J, Young AB, Yurkewicz L. Clinical trials in head injury. J Neurotrauma. 2002;19:503–557. doi: 10.1089/089771502753754037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.SAS Institute I. SAS/STAT User's Guide. Version 8. Cary, NC: SAS Institute, Inc.; 1999. [Google Scholar]
- 27.Schiff ND, Plum F, Rezai AR. Developing prosthetics to treat cognitive disabilities resulting from acquired brain injuries. Neurol Res. 2002;24:116–124. doi: 10.1179/016164102101199576. [DOI] [PubMed] [Google Scholar]
- 28.Schmued LC, Albertson C, Slikker W., Jr Fluoro-Jade: a novel fluorochrome for the sensitive and reliable histochemical localization of neuronal degeneration. Brain. Res. 1997;751:37–46. doi: 10.1016/s0006-8993(96)01387-x. [DOI] [PubMed] [Google Scholar]
- 29.Schmued LC, Hopkins KJ. Fluoro-Jade: novel fluorochromes for detecting toxicant-induced neuronal degeneration. Toxicol Pathol. 2000;28:91–99. doi: 10.1177/019262330002800111. [DOI] [PubMed] [Google Scholar]
- 30.Sensi SL, Canzoniero LMT, Yu SP, Ying HS, Koh JY, Kerchner GA, Choi DW. Measurement of intracellular free zinc in living cortical neurons: routes of entry. J. Neurosci. 1997;17:9554–9584. doi: 10.1523/JNEUROSCI.17-24-09554.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sensi SL, Ton-That D, Weiss JH. Mitochondrial sequestration and Ca(2+)-dependent release of cytosolic Zn(2+) loads in cortical neurons. Neurobiol. Dis. 2002;10:100–108. doi: 10.1006/nbdi.2002.0493. [DOI] [PubMed] [Google Scholar]
- 32.Sensi SL, Yin HZ, Carriedo SG, Rao SS, Weiss JH. Preferential Zn2+ influx through Ca2+-permeable AMPA/kainate channels triggers prolonged mitochondrial superoxide production. Proc. Natl. Acad. Sci. U. S. A. 1999;96:2414–2419. doi: 10.1073/pnas.96.5.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shimamura M, Garcia JM, Prough DS, Hellmich HL. Laser capture microdissection and analysis of amplified antisense RNA from distinct cell populations of the young and aged rat brain: effect of traumatic brain injury on hippocampal gene expression. Mol Brain Res. 2004;17:47–61. doi: 10.1016/j.molbrainres.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 34.Suh SW, Chen JW, Motamedi M, Bell B, Listiak K, Pons NF, Danscher G, Frederickson CJ. Evidence that synaptically-released zinc contributes to neuronal injury after traumatic brain injury. Brain Res. 2000;852:268–273. doi: 10.1016/s0006-8993(99)02095-8. [DOI] [PubMed] [Google Scholar]
- 35.Suh SW, Frederickson CJ, Danscher G. Neurotoxic zinc translocation into hippocampal neurons is inhibited by hypothermia and is aggravated by hyperthermia after traumatic brain injury in rats. J Cereb Blood Flow Metab. 2005 doi: 10.1038/sj.jcbfm.9600176. [DOI] [PubMed] [Google Scholar]
- 36.Suh SW, Garnier P, Aoyama K, Chen Y, Swanson RA. Zinc release contributes to hypoglycemia-induced neuronal death. Neurobiol. Dis. 2004;16:538–545. doi: 10.1016/j.nbd.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 37.Thompson K, Antony A, Holtzman A. The costs of traumatic brain injury. NCMJ. 2001;62:376–379. [PubMed] [Google Scholar]
- 38.Thompson RB, Cramer ML, Bozym R. Excitation ratiometric fluorescent biosensor for zinc ion at picomolar levels. J Biomed Opt. 2002;7:555–560. doi: 10.1117/1.1501886. [DOI] [PubMed] [Google Scholar]
- 39.Thompson RB, Peterson D, Mahoney W, Cramer M, Maliwal BP, Suh SW, Frederickson C, Fierke C, Herman P. Fluorescent zinc indicators for neurobiology. J Neurosci Methods. 2002;118:63–75. doi: 10.1016/s0165-0270(02)00144-9. [DOI] [PubMed] [Google Scholar]
- 40.Van Gelder RN, von Zastrow ME, Yool A, Dement WC, Barchas JD, Eberwine JH. Amplified RNA synthesized from limited quantities of heterogeneous cDNA. Proc. Natl. Acad. Sci. U. S. A. 1990;87:1663–1667. doi: 10.1073/pnas.87.5.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wei G, Hough CJ, Sarvey JM. The mitochondrial toxin, 3-nitropropionic acid, induces extracellular Zn2+ accumulation in rat hippocampus slices. Neurosci Lett. 2004;370:118–122. doi: 10.1016/j.neulet.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 42.Ylvisaker M, Jacobs HE, Feeney T. Positive supports for people who experience behavioral and cognitive disability after brain injury: a review. J Head Trauma Rehabil. 2003;18:7–32. doi: 10.1097/00001199-200301000-00005. [DOI] [PubMed] [Google Scholar]