Abstract
In order to determine whether dietary berries and ellagic acid prevent 17β estradiol (E2) -induced mammary tumors by altering estrogen metabolism, we randomized ACI rats (n=6/group) into 5 groups − sham implant + control diet (SH-CD), E2 − implant + control diet (E2-CD), E2+2.5% black raspberry (E2-BRB); E2+2.5% blueberry (E2-BB) and E2+ 400ppm ellagic acid (E2-EA). Animals were euthanized at early (6wk), intermediate (18wk) and late (24wk) phases of E2-carcinogenesis and the mammary tissue analyzed for gene-expression changes using quantitative real-time PCR. At 6 weeks, E2-treatment caused 48-fold increase in cytochrome P4501A1(CYP1A1) (p<0.0001), which was attenuated by both BRB and BB diets to 12- and 21-fold, respectively (p<0.001). E2 did not alter CYP1B1 levels, but both berry and EA diets significantly suppressed it by 11- and 3.5-fold, respectively from baseline (p<0.05). There was a 5-fold increase in 17β-Hydroxysteroid dehyrdogenase(17βHSD7) and this was moderately abrogated to about 2-fold by all supplementation (p<0.05). At 18 weeks, CYP1A1 was elevated by 15-fold in E2-CD and only E2-BB reduced this increase to 7-fold (p<0.05). Catechol-O-methyl transferase(COMT) expression was elevated 2-fold by E2-treatment (p<0.05) and all supplementation reversed this. At 24 weeks, CYP1A1 expression was less pronounced, but still high (8-fold) in E2-treated rats. This increase was reduced to 3.2 and 4.6-fold, by E2-BRB and E2-EA, respectively (p<0.05), but not by E2-BB. Supplementation did not alter the effect of E2 on steroid receptors. The diets also significantly suppressed mammary tumor incidence (10–30%), volume (41–67%) and multiplicity (38 to 51%) (p<0.05). Berries may prevent mammary tumors by suppressing the levels of E2-metabolizing enzymes during the early phase of E2-carcinogenesis.
Keywords: Estrogen, ACI rats, berries, polyphenols, breast cancer prevention, CYP450
INTRODUCTION
Breast cancer is the most diagnosed cancer among women in the United States and currently costs the American economy $85 billion in terms of value of life lost due to cancer mortality (1). The primary factors that determine mortality rate such as age, stage at diagnosis and race/ethnicity are interlinked with another integral risk factor for breast cancer development – female reproductive hormone - 17β-estradiol (E2) (2). Understanding the mechanism and prevention of E2-induced breast cancer can lead to considerable long-term gains in both value of life for women as well as reduced health care costs in the US. E2 is a known, yet unavoidable risk factor for breast cancer. Women chronically exposed to even physiological levels of this hormone can be at an increased risk to develop breast cancer (3).
Research suggests that in-situ synthesis of estradiol may play a major role in the development of breast cancer, especially in post-menopausal women (4). Primary enzymes involved in de-novo estradiol synthesis are aromatase, which converts androgen precursors to estrone, and 17β-hydroxysteroid dehydrogenase (17βHSD), which converts estrone (E1) to estradiol (E2) (5–6). Eight isozymes of 17βHSD have been identified so far (7). The type 1 isozyme of 17βHSD, which converts estrone to estradiol, is found in both normal and malignant breast (8). The rodent homologue of this enzyme is 17βHSD, type 7 (17HSD7), also known as the prolactin receptor associated protein (PRAP) (8–9). This enzyme has high specificity for the conversion of E1 to E2 and is controlled by both prolactin and estrogen signaling pathways (9).
There are several phase I and phase II enzymes involved in the metabolism of E2, of particular importance in the breast are cytochrome P450 1A1 (CYP1A1), CYP1B1, catechol-O-methyl transeferase (COMT), UDP-glucuronosyl transferase (UGT) and glutathione-S-transferase (GST). The phase I enzyme, CYP1B1 has received wide attention due to its function in converting E2 to 4-hyrdroxy estradiol (4E2), a postulated potentially carcinogenic metabolite (10). Also, breast tumors show high levels of both CYP1B1 and 4E2 (11–12). Nevertheless, metabolites of CYP1A1 action such as 2-hydroxy estrone can produce stable DNA adducts and inhibition of CYP1A1 metabolism reduces the formation of estrogen-induced kidney tumors in hamsters, suggesting that this pathway may also play a definitive role in estrogen carcinogenesis (13). The hydroxy-metabolites of estradiol and estrone are conjugated for removal by several enzymes, including COMT, GST and UGT (14–15). The 2-hydroxy metabolites are better substrates for COMT (10), suggesting that CYP1A1 and COMT expression may be coupled. Polymorphisms in both phase I and II genes have been associated with a risk of breast cancer, indicating the importance of these enzymes in the production and removal of estradiol metabolites (4, 16). The estrogen metabolism pathway interacts with the estrogen-signaling pathway. Hydroxy-metabolites of estradiol such as 4E2 and 2E2 bind to ERs with varying affinities (17). Progesterone receptor (PGR) is known to be up-regulated by estrogen via ER signaling, hence PGR expression is a downstream effect of ER activation (18). Thus, studying the expression of these genes provides some idea about control of estrogen metabolism in the mammary tissue.
Berries are an integral part of the Western cuisine and are also used in several other cuisines around the world. Blueberries and black raspberries have been commercially cultivated in the United States since the late 19th and early 20th century. They are also excellent sources of many vitamins, minerals and cancer-preventing phytochemicals such as anthocyanins and ellagic acid (19). These berries vary significantly in both their phytochemical content and composition. Typically, black raspberry, which contains cyanidin as the primary anthocyanin component is a richer source of ellagic acid than blueberry, while a poor source of ellagic acid contains 5 different anthocyanin pigments (20–21). Both berries are high in antioxidant capacity and have shown cancer preventative effects (22–23). We have previously shown that dietary berries (2.5% w/w) and ellagic acid (400 ppm) can significantly inhibit the growth of E2-induced mammary tumors in ACI rats (24). Berries also prevented the pituitary associated mortality (24) and reduced E2-induced hepatic DNA adducts (25). However, the exact mechanisms by which they provide protection are not known. Berry phytochemicals such as anthocyanins and ellagic acid (and its metabolites urolithins A and B) show selective estrogen receptor modulating (SERM) activity in some studies (26–27). These phytochemicals are can be absorbed into the systemic circulation in both humans and rodents and can be detected after ingestion at various levels (28–30). Thus, they may play a role in modulating estrogen metabolism in organs other than the gut, which was previously thought to be the primary target organ.
In order to determine whether berries and ellagic acid affect estrogen metabolism, we examined the regulation of gene expression of key enzymes involved in estrogen metabolism and signaling, in the mammary tissue during the course of mammary tumorigenesis. Three time points – early (6 weeks), intermediate (18 weeks) and late (24 weeks) were chosen and the expression of 9 selective genes, 3 each involved in the phase I , phase II metabolism and estrogen signaling were selected and their relative gene expression changes analyzed using quantitative real-time polymerase chain reaction (qRTPCR). The genes tested were: phase I metabolism- 17βHSD7, CYP1A1, CYP1B1; phase II- COMT, GSTA1, GSTM1; steroid signaling – ERα, ERβ, PGR.
In a separate study we also studied the effects of 2 doses- 1% w/w and 2.5% w/w, of blueberry and black raspberry- in an ACI rat model in which mammary tumors are induced by a lower dose of E2 (9 mg) that significantly eliminates pituitary hyperplasia-induced mortality. Ellagic acid dose was maintained at 400 ppm similar to the previous study to provide a reference point (24). We measured the effect of dietary berries and ellagic acid on tumor incidence, latency, volume and multiplicity to prove that berries are consistently effective in prevention of estrogen-induced mammary tumors.
MATERIALS AND METHODS
Animals and treatment
Female ACI rats (7–8 weeks old) were purchased from Harlan-Sprague Dawley (Indianapolis, IN), housed under ambient conditions and fed AIN-93M diet and water ad libitum. After a week of acclimation, 18 animals each were randomized into 5 groups. Two of the 5 groups received control diet and the other 3 received diets supplemented with 2.5% (w/w) dehydrated powdered blueberry; 2.5% (w/w) freeze-dried black raspberry or 400 ppm ellagic acid. The sources, preparation and caloric contents of these diets have been previously described in detail (24). After 2 weeks of pre-feeding, each group received either sham implants or implants containing 27 mg E2 as described (24). The animals were maintained on their respective diets throughout the study period and 6 animals from each group were euthanized at 6, 18 and 24 weeks after E2 treatment by carbon dioxide asphyxiation, and mammary tissue was collected and frozen for further analysis.
In a separate second study, female ACI rats (5–6 weeks old) were randomized into different groups (Table 2) and fed experimental diets for 2 weeks. Animals then received either a 1.2-cm silastic implant containing 9 mg 17β-estradiol as described (31) or sham implants and maintained on respective diets throughout the study. All diets were ordered from Harlan-Teklad, Inc. (Madison, WI). The AIN-93M diet was supplemented with powdered berries (1% or 2.5% w/w) or ellagic acid (400 ppm) and prepared as described earlier (24). Animals were weighed biweekly to track weight changes and disease progression. Mammary gland from this study was not used for RNA analysis.
Table 2.
Comparison of organ weights and tumor indices between ACI rats fed control diet or diet supplemented with different doses of blueberry, black raspberry or 400 ppm ellagic acid ¶
| Group |
Animal Weight (g) |
Liver (g) |
Mammary§ (g) |
Pituitary (mg) |
Tumor Incidence# (at 26 weeks) |
Tumor Volume# (mm3) |
Tumor Multiplicity# |
|---|---|---|---|---|---|---|---|
| ( % reduction) | |||||||
|
Sham + Control diet |
182 ± 4 | 4.6 ± 0.2 | 3.4 ± 0.2 | 9.6 ± 0.8 | NA | NA | NA |
| (n=6) | p<0.005 | p<0.005 | p<0.005 | p<0.005 | |||
| E2 + Control diet | 204 ± 2 | 6.8 ± 0.2 | 4.8 ± 0.3 | 70 ± 4.5 | 100% | 2804 ± 547 | 11.7 ± 1.4 |
| (n=15) | |||||||
|
E2 + 1 % Blueberry diet |
207 ± 3 | 6.9 ± 0.2 | 5.4 ± 0.2 | 60 ± 7 | 100% |
1641 ± 405 | 11.4 ± 2.2 |
| (n=11) | −41% | ||||||
|
E2 + 2.5% Blueberry diet |
203 ± 6 | 6.6 ± 0.3 | 5.7 ± 0.4 | 69 ± 16 |
69% |
1146 ± 276 |
7.2 ± 0.8 |
| (n=13) |
p< 0.05 |
−59% |
p< 0.05 −38% |
||||
|
E2 + 1 % Black raspberry diet |
201 ± 4 | 6.5 ± 0.2 | 6.0 ± 0.4 | 55 ± 9 |
81% |
1573 ± 403 |
6.6 ± 0.8 |
| (n=14) |
p< 0.05 |
−44% |
p< 0.008 −43.50% |
||||
|
E2 + 2.5% Black raspberry diet |
210 ± 6 | 6.7 ± 0.3 | 7.0 ± 0.6 | 39 ± 3 | 87% | 915 ± 250 | 5.7 ± 1.0 |
| (n=11) |
p <0.05 |
p< 0.05 |
p< 0.05 |
p< 0.05 −67% |
p< 0.007 −51% |
||
|
E2 + Ellagic acid diet |
205 ± 6 | 6.5 ± 0.2 | 5.5 ± 0.3 | 57 ± 6 | 81% | 983 ± 331 | 6.9 ± 1.2 |
| (n=11) |
p< 0.05 |
p< 0.05 −65% |
p< 0.05 −41% |
||||
The data presented in this table are from a separate independent study carried out with lower dose of the carcinogen E2 than what was previously published (Aiyer et al., 2008b). The key differences between the studies are decribed in detail in Materials and Methods as well as Results sections. It was seen that berry/ellagic acid supplementation at the same dietary dose (2.5%/400 ppm) as before had a much higher preventive effect in animals where mamamry tumors were induced with lower dose E2. A lower dietary dose (1%) of black raspberries also had a significant effect.
Mammary glands were weighed after the removal of all grossly visible tumors. Typically it was seen that the higher the number of tumors, the lower the mammary wet weight. Hence there was a strong inverse correlation between tumor volume/multiplicity and mammary gland wet weight.
Tumor incidence was compared using log-rant test; volume using one-way ANOVA and multiplicity using poisson regression as described in Materials and Methods. A p-value ≤ 0.05 was considered significant.
RNA isolation, reverse transcription and quantitative real time polymerase chain reaction (qRTPCR)
RNA from whole mammary tissue was isolated using the Trizol® method (Invitrogen, Carlsbad, CA), with modifications. Briefly, mammary tissue was suspended in Trizol® at 4°C and homogenized with a hand-held polytron at maximum speed. This homogenate was then passed through a syringe with a 22.5 gauge needle to ensure complete dissociation of the mammary tissue. The resultant tissue homogenate was sequentially extracted with chloroform and the aqueous phase was precipitated using ice-cold iso-propanol. The quality of the RNA was ascertained by gel electrophoresis and quantitated using NanoDrop® (NanoDrop Technologies, Wilmington, DE). One hundred ng of RNA was reverse transcribed using the high capacity cDNA archive kit (Applied Biosystems, CA) and 3 ng of cDNA equivalent was used for PCR. These conditions were standardized to achieve consistent and reproducible results.
Primers for qRTPCR were designed across exon boundary to avoid amplification of genomic DNA, using Primer express® 3.0 software (Applied Biosystems, Foster City, CA) and synthesized by Integrated DNA Technologies, Inc., (Coralville, IA). The sequences of the forward and reverse primers for each gene tested are listed in Table 1. The PCR amplification was done using Power SYBR® Green PCR master mix (Applied Biosystems, CA) and 500nM of forward and reverse primers for each gene except CYP1A1 for which the final primer concentration was 125 nM each. Quantitative PCR was performed using a 7500 Fast-Real Time PCR system (Applied biosystems, Foster City, CA). The PCR conditions were: 50°C for 2 min; DNA polymerase activation at 95°C for 10 min; followed by 40 cycles at 95°C for 15 sec and 60°C for 1 min. All gene analyses were performed at least three times.
Table 1.
Primer sequences used for quantitative real-time PCR.
| Gene | Forward (5’-3’) | Reverse (5’-3’) |
|---|---|---|
| 17β HSD | CTTTATCCTGATTCGGGAACTG | GTCCTCAAGACTGAAGTTAGA C |
| CYP1A1 | TGGAGACCTTCCGACATTCAT | GGGATATAGAAG CCATTCAGACTT G |
| CYP1B1 | AACCCAGAGGACTTTGATCCG | CGTCGTTTGCCCACTGAAAA |
| COMT | GGATGCAGTGATTCGGGAGTA | GCAGCGTAGTCAGGGTTCATCT |
| GSTA1 | CCAGCCTTCTGACCTCTTTCC | TCTTCGATTTGTTTTGCATCCA |
| GSTM1 | TCTTGACCAGTACCACATTTTTGAG | TCGAAAATATAGGTGTTGAGAGGTAGTG |
| ERα | GGCACATGAGTAACAAAGGCA | GGCATGAAGACGATGAGCAT |
| ERβ | CTCCTTTAGCGACCCATTGC | CTCCCACTAAGCTTCCTCTTCAGT |
| PGR | TCACAACGCTTCTATCAACTTACAAA | GGCAGCAATAACTTCAGACATCA |
| β-Actin | GCCAACCGTGAAAAGATGAC | ACCCTCATAGATGGGCACAG |
Primers were designed using Primer Express® software across exon boundary for the following genes: 17βHSD7- 17β hydroxyl steroid dehydrogenase 7;CYP1A1-Cytochrome P450 1A1; CYP1B1-Cytochrome P450 1B1; COMT- Catechol-O-methyl transferase; GSTA1- Glutathione-S-transferase A1; GSTM1-Glutathione-S-transferase M1; ERα-Estrogen receptor α; ERβ-Estogen receptor β; PGR- Progesterone receptor.
Analysis of gene expression
Gene expression analysis was done using the relative-quantification (ΔΔcT) method as described (32). Each sample (refers to cDNA from individual animals) was analyzed in triplicate for each gene tested. ΔcT was calculated as the difference between cT of gene of interest (GOI) and the house-keeping gene (β-actin) (ΔcT = cTGOI – cTβ-actin). One sample (sham treated) was chosen as the calibrator and ΔΔcT of all other samples was calculated using the formula ΔcT = ΔcTsample - ΔcTcalibrator. A different calibrator sample (typically a sample from the sham treated group) was chosen for the different time points (6, 18 and 24 wk) and fold change (2−ΔΔcT) in gene expression was calculated for all genes. The results are represented as relative fold change, which is the average fold change among the biological replicates (n=6/group) and represents the biological variation within a specific group.
Assessment of tumor indices for tumor study
In the second study, starting at 12 weeks after estrogen implantation, animals were palpated weekly for tumor appearance. The frequency of palpation was increased to twice a week, upon appearance of the first tumor, to record tumor latency and incidence. Tumor incidence was calculated using the formula: Percentage tumor incidence = (number of tumor bearing animals/ total number of animals per group) X100. The tumor incidence was considered 100% when all animals in a group had palpable tumors. The time between E2-implants and the appearance of the first palpable tumor in any animal in a particular group was considered as the tumor latency for that group.
After 32 weeks of estrogen treatment, animals were euthanized and each animal was examined grossly for the presence of mammary tumors. Tumor volume was calculated as described (24). Liver, pituitary and mammary glands were harvested and weighed. Representative tumors from each animal were analyzed by histopathology to confirm that they were mammary adenocarcinomas. All animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Louisville.
Statistical analysis
Relative fold changes of gene expression in each group and tumor volumes were compared using one-way analysis of variance (ANOVA), followed by a Tukey’s multiple comparison post test. The difference in tumor incidence was assessed using the non-parametric log-rank test. All statistical analyses were performed using the Graphpad Prism ® software (Graphpad Software, San Diego, CA) except for tumor multiplicity, which was compared in SAS version 8, using the Poisson Regression Model (SAS procedure PROC GENMOD). A p-value <0.05 was considered significant in all cases. The data are presented as mean ± SE.
RESULTS
Two animal studies with slightly varying protocols are presented in this section. The first, ACI rats implanted with 27 mg E2-implants and euthanized at various time points (early, intermediate and late) during the course of carcinogenesis. In this model, as published, all animals develop 100% mammary gland tumors by 24 weeks after implantation. Dietary berries and ellagic acid were very effective in reducing the tumor volume and multiplicity in the order 2.5 % black raspberry > 400 ppm ellagic acid > 2.5% blueberry (24). However, a considerable shortcoming of this model has been its tendency to develop debilitating pituitary hyperplasia, as a response to the high E2 dose, leading to significant morbidity and mortality (24, 31, 33). Several steps have been taken to correct this. Other investigators have taken a genomic approach and have developed a new strain of inbred rats that respond with reduced pituitary lactotroph hyperplasia upon treatment with E2 (34–36). Prior to this, we took a pharmacological approach by reducing the E2 dosage and showed that mammary tumors can be induced with 9 mg E2, instead of 27 mg previously used, at a longer duration (32 instead of 24 weeks) with essentially no mortality (31). This model with the reduced E2 dose allows us to study the effects of different chemopreventive agents without the confounding mortality present in the previous model. The effect of dietary berries, even at doses lower (1%) than previously used (2.5%), on mammary tumor indices are presented here. Although 2 different protocols are presented, the assumption is that the basic mechanism of E2-carcinogenesis is similar in both.
Dietary berries but not ellagic acid significantly reverse the E2-induced increase in CYP1A1 expression
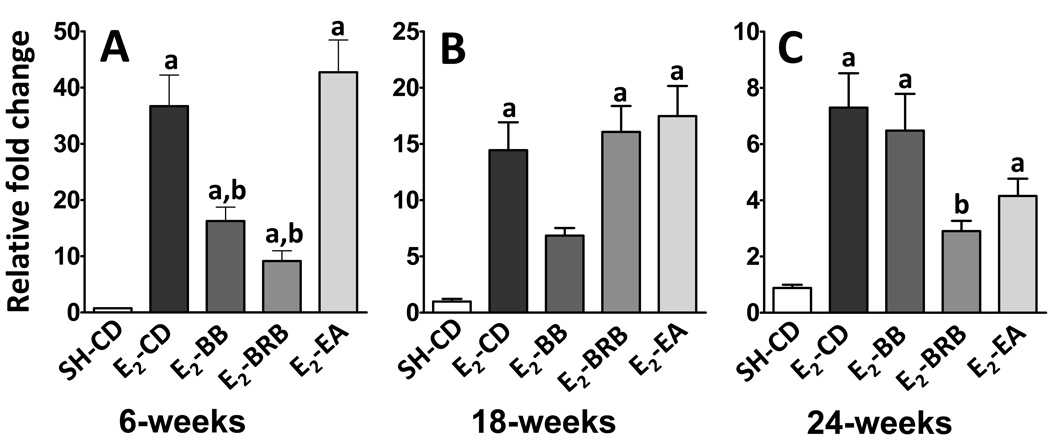
In the early phases of treatment (6 weeks), the strongest increase in expression after E2-treatment occurred for CYP1A1. Compared to sham, E2-treatment caused a 48-fold induction in CYP1A1 expression (0.75 ± 0.09 vs 36.7 ± 5.5; p< 0.0001; Figure 1A). This increase was significantly countered by both BB (16.3 ± 2.5; p<0.001) and BRB (9.2 ±1.8; p<0.001), but not by ellagic acid (42.7 ± 5.7). The effect of E2 on CYP1A1 induction was somewhat blunted at 18 weeks to only 15-fold of sham levels (0.98 ± 0.2 vs 14.4 ± 2.5; p< 0.01; Figure 1B). BB diet continued to counter this increase during the intermediate phase (6.8 ±0.7), whereas BRB (16.1 ± 2.3) and ellagic acid (17.5 ± 2.7) did not show any effect. However, this trend was reversed during the late phase (24 weeks). Although E2 increased CYP1A1 by only 8-fold compared to sham (0.9 ± 0.1 vs 7.3 ± 1.2; P <0.05; Figure 1C), both BRB (2.9 ± 0.4; p <0.05) and EA (4.2 ± 0.6) reduced this increase. BB diet showed only slight but insignificant reduction (Figure 1C). In summary, E2 significantly boosts the levels of CYP1A1 mRNA during the early, middle and late phases of E2-carcinogenesis with the effect plateauing over the time course. Blueberry is highly effective during the early and intermediate phase, black raspberry during early and late phase and ellagic acid only during the late phase in countering this E2-induced increase.
Figure 1. (A–C). Effect of berry and ellagic acid diets on E2-induced elevation in CYP1A1 expression.
ACI rats (n=6) were treated with E2 (27 mg) for 6 (A), 18 (B) or 24 (C) weeks and fed either a control diet (E2-CD) or diet supplemented with 2.5% w/w black raspberry (E2-BRB), blueberry (E2-BB) or 400 ppm ellagic acid (E2-EA). The whole mammary mRNA was analyzed for CYP1A1 expression using qRTPCR as described in Materials and Methods. The relative fold change was calculated using the 2−ddcT method with a sample from SH-CD as the calibrator for each particular time point. a- significantly different from SH-CD, p<0.0001; b- significantly different from E2-CD, p<0.005.
Both dietary berries and ellagic acid significantly reduce the levels of CYP1B1 during the early phase of carcinogenesis
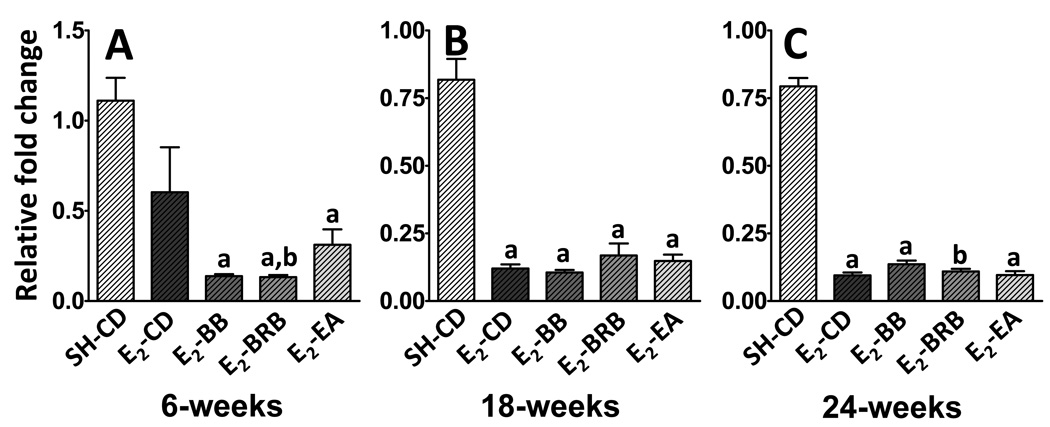
The level of CYP1B1 mRNA in the whole mammary gland was essentially unaltered after 6 weeks of E2-treatment (SH-CD: 1.1 ± 0.1 vs E2-CD: 0.6 ± 0.2; not significant; Figure 2A). However, all supplemented diets significantly reduced the levels of CYP1B1 both from baseline and E2-treatment. Both BB and BRB had similar effects and lowered CYP1B1 levels by up to 11-fold from SH-CD (E2-BB: 0.1 ±0.01 and E2-BRB: 0.1 ± 0.01; p <0.01) and 6-fold from E2-CD. Ellagic acid was less effective and caused a 4-fold decrease compared to baseline (0.3 ± 0.1; p <0.05). During the intermediate and late phases, E2-treatment itself caused a significant reduction in CYP1B1 levels (Figure 2B, C) and consequently, supplementation did not have any additional effect on this decrease. All E2-treated groups regardless of the supplementation had similar CYP1B1 expressions at 18 and 24 weeks (Figure 2B and C).
Figure 2. (A–C). Effect of berry and ellagic acid diets on E2-induced elevation in CYP1B1 expression.
ACI rats (n=6) were treated with E2 (27 mg) for 6 (A), 18 (B) or 24 (C) weeks and fed either a control diet (E2-CD) or diet supplemented with 2.5% w/w black raspberry (E2-BRB), blueberry (E2-BB) or 400 ppm ellagic acid (E2-EA). The whole mammary mRNA was analyzed for CYP1B1 expression using qRTPCR as described in Materials and Methods. The relative fold change was calculated using the 2−ddcT method with a sample from SH-CD as the calibrator for each particular time point. a- significantly different from SH-CD, p<0.01; b- significantly different from E2-CD, p<0.01.
Dietary berries and ellagic acid counter the E2-induced increase in 17βHSD7 expression at early phase of carcinogenesis
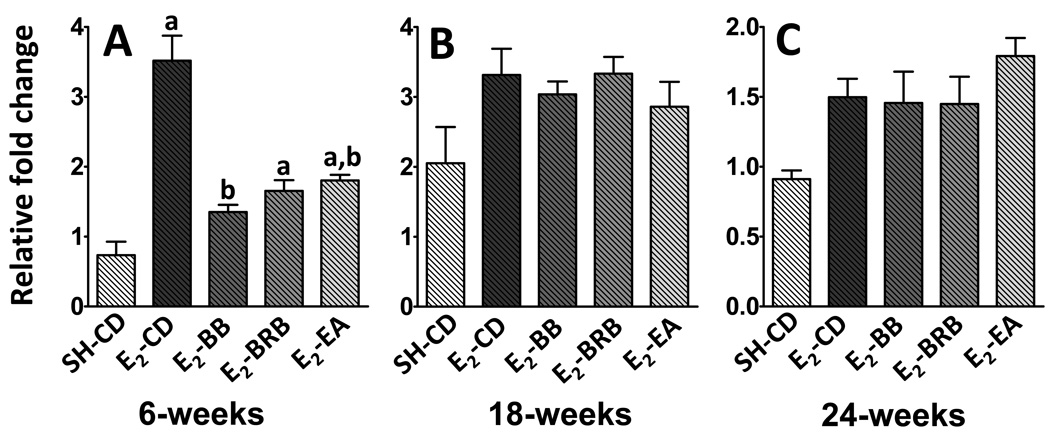
In the rat mammary, the enzyme 17βHSD7 plays an important role in in situ E2 synthesis, by converting E1 to E2. At 6 weeks, the expression of this enzyme increased by up to 5-fold after E2-treatment (0.73 ± 0.2 vs 3.5 ± 0.4; p<0.01; Figure 3A). This increase was returned close to baseline levels effectively by all dietary supplementation- BB (1.3 ± 0.1; p <0.01), BRB (1.6 ± 0.2; p <0.01) and EA (1.5 ± 0.4; p<0.01). This initial response to E2 treatment did not persist during the intermediate and late phases of carcinogenesis (Figure 3B and C). It appears that E2-induced increase in 17βHSD7 expression is an early phase phenomenon and is countered effectively by berries and ellagic acid also during the early phase. The expression of another enzyme involved in in situ E2 synthesis- aromatase- was undetectable in ACI rat mammary (data not shown).
Figure 3. (A–C). Effect of berry and ellagic acid diets on E2-induced elevation in 17βHSD7 expression.
ACI rats (n=6) were treated with E2 (27 mg) for 6 (A), 18 (B) or 24 (C) weeks and fed either a control diet (E2-CD) or diet supplemented with 2.5% w/w black raspberry (E2-BRB), blueberry (E2-BB) or 400 ppm ellagic acid (E2-EA). The whole mammary mRNA was analyzed for 17βHSD7 expression using qRTPCR as described in Materials and Methods. The relative fold change was calculated using the 2−ddcT method with a sample from SH-CD as the calibrator for each particular time point. a- significantly different from SH-CD, p<0.05; b- significantly different from E2-CD, p<0.05.
Rats supplemented with berries and ellagic acid show significantly smaller induction in COMT levels during the intermediate phase of tumorigenesis
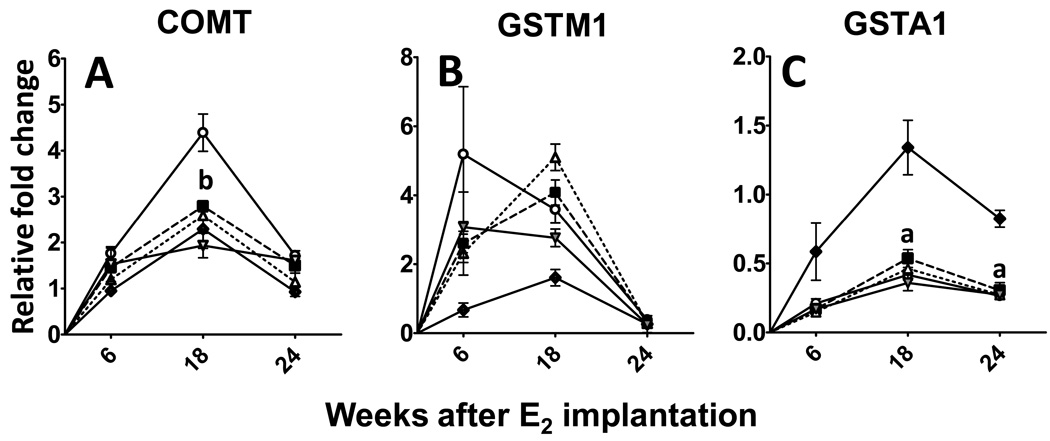
An important enzyme involved in the removal of harmful E2-metabolites is COMT. Unlike the phase I enzymes, COMT is not induced during the early phase. Instead, there is a 2-fold increase in COMT expression during the intermediate phase (SH-CD: 2.3 ± 0.4 vs E2-CD: 4.4 ± 0.4; p<0.05; Figure 4A). This increase is not seen in any of the supplemented groups (Figure 4A), with BB (2.8 ± 0.1), BRB (2.6 ± 0.1) and EA (1.9 ± 0.3) essentially showing expression levels close to baseline. Two other phase II enzymes were analyzed- GSTM1, was not altered by either E2 treatment or by supplementation (Figure 4B), whereas GSTA1 levels were found to be down-regulated after estrogen treatment by up to 3 fold (p<0.05) with no effect of intervention (Figure 4C).
Figure 4. (A–C). Effect of berry and ellagic acid diets on E2-induced changes in phase II enzyme expression.
ACI rats (n=6) were treated with either sham implants or E2 (27 mg) for 6, 18 or 24 weeks and fed a control diet (SH-CD, ◆; E2-CD, ○ ) or diet supplemented with 2.5% w/w black raspberry (E2-BRB, △), blueberry (E2-BB, ■ ) or 400 ppm ellagic acid (E2-EA,  ). The whole mammary mRNA was analyzed for A- Catechol-O-methyl transferase (COMT); B- Glutathione S transferase M1 (GSTM1); and C- GSTA1 expression using qRTPCR as described in Materials and Methods. The relative fold change was calculated using the 2−ddcT method with a sample from SH-CD as the calibrator for each particular time point. a- significantly different from SH-CD, p<0.05; b- significantly different from E2-CD, p<0.05.
). The whole mammary mRNA was analyzed for A- Catechol-O-methyl transferase (COMT); B- Glutathione S transferase M1 (GSTM1); and C- GSTA1 expression using qRTPCR as described in Materials and Methods. The relative fold change was calculated using the 2−ddcT method with a sample from SH-CD as the calibrator for each particular time point. a- significantly different from SH-CD, p<0.05; b- significantly different from E2-CD, p<0.05.
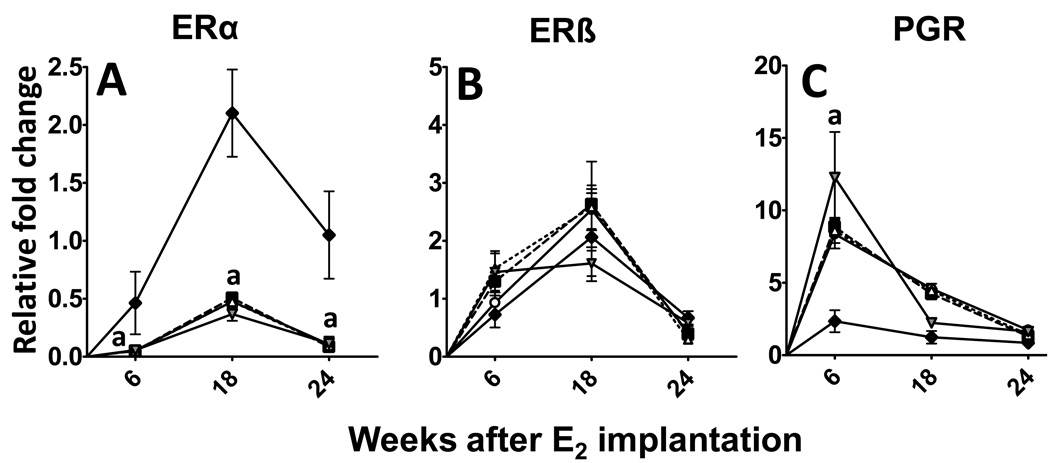
Berries or ellagic acid do not alter steroid signaling enzymes in E2-induced mammary tumors
The effect of E2-treatment on classic estrogen receptor (ER) pathway was analyzed by studying the expression of ERα, ERβ and progesterone receptor (PGR), a downstream gene of ERα activation. As expected E2-treatment had a remarkable down-regulatory effect on ERα expression at all time points (Figure 5A). On the other hand it had no effect of ERβ expression (Figure 5B). PGR levels were significantly elevated at 6 weeks, suggesting activation of classic ER signaling (Figure 5C). However, this increase was not sustained and fell to moderate levels during the intermediate phase and was very similar to sham treatment by the end of the study (Figure 5C).
Figure 5. (A–C). Effect of berry and ellagic acid diets on E2-induced changes in steroid receptor expression.
ACI rats (n=6) were treated with either sham implants or E2 (27 mg) for 6, 18 or 24 weeks and fed a control diet (SH-CD, ◆; E2-CD, ○ ) or diet supplemented with 2.5% w/w black raspberry (E2-BRB, △ ), blueberry (E2-BB, ■) or 400 ppm ellagic acid (E2-EA,  ). The whole mammary mRNA was analyzed for A- Estrogen Receptor α (ERα); B- ERβ; and C- Progesterone receptor (PGR) expression using qRTPCR as described in Materials and Methods. The relative fold change was calculated using the 2−ddcT method with a sample from SH-CD as the calibrator for each particular time point. a- significantly different from SH-CD, p<0.01; b- significantly different from E2-CD, p<0.01.
). The whole mammary mRNA was analyzed for A- Estrogen Receptor α (ERα); B- ERβ; and C- Progesterone receptor (PGR) expression using qRTPCR as described in Materials and Methods. The relative fold change was calculated using the 2−ddcT method with a sample from SH-CD as the calibrator for each particular time point. a- significantly different from SH-CD, p<0.01; b- significantly different from E2-CD, p<0.01.
Effect of berry or ellagic acid supplementation on pituitary wet weight
E2-treatment caused an increase in liver, mammary and pituitary wet weights (Table 2). Compared to sham-treated animals, the most significant increase was seen in E2-treated animals on control diet for pituitary weight, with over 7-fold increase (p <0.005). Blueberry diet at neither dose affected this increase. However, in animals fed black raspberry, both doses inhibited this E2-induced increase in pituitary wet weight, with 2.5% dose (4-fold > SH-CD; p<0.05 compared to E2-CD) having a greater effect than 1% dose (6-fold > SH-CD; not significant). Ellagic acid had the same effect as 1% BRB (6-fold > SH-CD; not significant).
Diets supplemented with black raspberry, blueberry or ellagic acid significantly reduce tumor incidence
None of the sham-treated animals had any palpable or gross tumors. In animals fed the control diet, the first palpable tumor appeared at 18 weeks of E2- treatment (1 of 20 animals; 5% incidence). There was 50% incidence at just 20 weeks of treatment (10 of 20) and the linear trend continued until all animals in this group had palpable tumors by 26 weeks of treatment (Table 2). Although palpable mammary tumors were seen in animals fed 1% blueberry, 1% and 2.5% black raspberry diets ( 1 of 16; 6.25% for each group; Table 2), the incidence curves for all intervention groups except 1% blueberry were significantly different from the control diet (Table 2; log-rank test; p<0.05). Blueberry at 1% did not affect tumor incidence (Table 2). However, at 2.5% it had a highly significant effect and resulted in the lowest tumor incidence at 26 weeks (11 of 16; 69%; Table 2). Black raspberry at both 1% and 2.5% dose significantly shifted the tumor incidence curve to the right, resulting in 81% (13 of 16) and 87% (14 of 16) incidence at 26 weeks, respectively (Table 2). Ellagic acid (400 ppm) also had similar effects and significantly reduced incidence to 81% at 26 weeks (Table 2). The E2 treatment was continued until 32 weeks.
Effect of supplemented diets on tumor volume and multiplicity
Tumor indices were measured for each animal and are represented as mean ± SE (Table 2). In animals fed control diet the tumor volume and multiplicity were 2804 ± 547 mm3 and 11.7 ± 1.4, respectively. Tumor volume was reduced by all interventions, from significant effects of 2.5% berry (59% for BB and 67% for BRB; p< 0.05) and ellagic acid diet (65%; p<0.05) to marginal effects of 1% berry diets (41% for BB and 44% for BRB). The highest reduction in tumor multiplicity was achieved by 2.5% BRB (51%; p<0.007), followed by 1% BRB (43%; p<0.008), ellagic acid (41%; p<0.05) and 2.5% BB (38%; p< 0.05); 1% BB did not affect tumor multiplicity.
DISCUSSION
The results presented show that one of the main mechanisms by which berries and ellagic acid inhibit mammary tumors is by decreasing the levels of enzymes that can produce harmful E2 metabolites. Our time course analysis also suggests that this inhibition occurs mostly during the early phases of carcinogenesis. Further, data from our tumor study show that berries consistently inhibit E2-induced mammary tumors and black raspberry even at 1% dose shows significant chemopreventive efficacy.
The current study is the first to show that E2 significantly affects the expression levels of enzymes that are involved in E2 metabolism in the ACI rat. Previously published reports show that agents that alter E2 metabolism and reduce oxidative stress can cause a reduction in mammary tumors in ACI rats (37–38). Further, this is also the first report to show that berries and ellagic acid significantly reverse the effect of E2 on these enzymes, thus potentially affecting the levels of harmful E2 metabolites in the ACI rat mammary. Another significant finding is the analysis of gene expression changes throughout the course of carcinogenesis. The E2-induced animal model varies vastly from the classic 7,12-dimethyl benzanthrazene (DMBA)-induced mammary tumor model in that the estrogen treatment is continuous, albeit in much lower doses. Thus, the conventional model of initiation, promotion and progression does not apply. However, from the results presented, it appears that most of the detrimental effects of E2 occur during the early phase and seem to level off during the later phases.
In this study, we show that E2 significantly and consistently elevates CYP1A1 expression to various levels throughout the carcinogenesis. CYP1A1 is primarily known to catalyze the conversion of E2 to the less harmful metabolite 2-hydroxy estradiol (2E2). Nevertheless, 15–20% of the E2 metabolite produced by CYP1A1 is 4E2 (10, 39). Bhat and coworkers have shown that E2 treatment causes a higher ratio of 4E2/2E2 in the ACI rat mammary (37). However, these studies were done using microsomes isolated from whole mammary and these investigators did not show exactly which enzymes are responsible. It is not clear whether the significant elevation of CYP1A1 in our study actually leads to increased production of 4E2. Efforts are currently underway in our laboratory to identify the different metabolites using mass spectrometry. Future studies are planned to study effect of E2 with and without supplementation on the levels of various E2 metabolites.
A surprising finding of this study was the effect of E2 on CYP1B1 levels, especially during the intermediate and late phases. It is well documented that the primary CYP1B1 metabolite 4E2 is more harmful with respect to mammary tumorigenesis (40). To this date only one study has shown a clear increase in 4E2/2E2 ratio in the ACI rat mammary (37). However, no study has as yet shown a clear increase in CYP1B1 levels after E2-treatment in ACI rats. E2-treated mammary largely consists of proliferating cells of epithelial origin, whereas sham-treated tissue consists of a much higher percentage of stromal cells (31, 38). It is reported that CYP1B1 expression is constitutively higher in the rat mammary stroma, whereas CYP1A1 can be induced by estrogenic agents only in the epithelial cells (42). Thus differences in the cell composition between sham and treated rats may potentially confound the results as these analyses were done from total tissue RNA. Thus, the higher CYB1B1 in untreated animals reflects the constitutive expression in the stromal compartment, whereas CYP1A1 is up-regulated by estrogen predominantly in epithelial cells and is thus increased by over 40-fold.
Regardless of the effect of E2, berries and ellagic acid significantly reduce the levels of both CYP1A1 and CYP1B1 expression at 6 weeks. The single most significant finding of this report is that berries and ellagic acid cause a net reduction in the expression of phase I enzymes responsible for converting E2 to harmful metabolites, which in turn may lead to a net reduction in metabolites themselves, especially in the early stages. This is substantiated by the effect of both berries and ellagic acid on COMT expression at 18 weeks. The significant reduction in the COMT expression may be due to the constant suppression in the production of catechol-estrogen metabolites by sustained down-regulation of CYP1A1 and to a lesser extent of CYP1B1. It remains to be seen if the actual levels of E2 metabolites are lower in animals fed berry and ellagic acid diets. Ellagic acid does not alter CYP1A1 expression, suggesting that it differs from other berry phytochemicals (anthocyanins) in its mechanism of action. Previous reports suggest that ellagic acid does not alter the expression of hepatic CYP1A1, but inhibits its activity both in vitro and in vivo (43). It has also been shown α-napthoflavone, a CYP-inhibitor, prevents mammary tumors in ACI rats (37).
Another interesting finding is the up-regulation of 17βHSD7 by estradiol. This enzyme has high specificity for the conversion of estrone to estradiol in the mammary, suggesting that estradiol may influence in-situ estrogen synthesis. However, 17HSD7 expression is affected by both E2 and prolactin in the rat corpus luteum (9, 44), and E2 induces pituitary prolactinomas in this model (33). Thus, E2 either directly influences the expression of 17βHSD7 or this may be a downstream effect of increased prolactin secretion. This enzyme is involved in the conversion of estrone to estradiol, however, the expression of aromatase which forms estrone from androgen precursors, is almost undetectable in the mammary tissue of the ACI rat (data not shown). Thus the exact role of 17βHSD7 in in-situ E2 synthesis in ACI rat mammary remains to be demonstrated.
Berries and ellagic acid also down-regulate 17βHSD7, which may further reduce in-situ E2 formation. Also, 17βHSD7 is modulated by prolactin (9). In the current tumor study, we show that berries and ellagic acid significantly lower pituitary prolactinoma growth as evidenced by lower pituitary wet weight (Table 2). This finding suggests that berries regulate 17βHSD7 expression by possibly altering prolactin levels during the early phase or that they inhibit E2-induced pituitary proliferation. There is support for the latter since both berries and ellagic acid significantly reduced pituitary associated mortality in the previous tumor study (24).
In this study, with lower dose E2, we also show that dietary berries reduce tumor incidence, tumor multiplicity and tumor volume in a dose-dependant manner. Black raspberry (2.5% w/w) with the highest concentration of both anthocyanins and ellagic acid had the greatest effect on all three end-points, followed by ellagic acid (400 ppm). The higher dose of blueberry (2.5%) had effects similar to that of low-dose black raspberry (1%). The lower dose of blueberry (1%) showed a marginal reduction in tumor volume but no effect on multiplicity or incidence (Table 2). Any confounding effect of caloric restriction on mammary tumor development can be safely ruled out as no differences were seen in either the weight gain or the feed intake among control and supplemented diet fed groups (data not shown). Further, the supplemented diets were shown to be isocaloric to the AIN-93M diet (24). These results are highly consistent with our previous report in which 2.5% dietary berries or 400ppm ellagic acid significantly diminished mammary tumors induced by high dose E2 regimen (24) . However, in the current study the 2.5% dose elicits a higher reduction of tumor volume and multiplicity than those observed in the previous model, suggesting that the toxicity due to the higher E2 dose obscured the beneficial effects of supplementation.
Another important organ in this framework is the liver. The liver is responsible for the metabolism of circulating E2 and it has been shown that metabolites of E2 may play a significant role in mammary tumor development (13, 45). Mesia-Vela et al demonstrated that altering the liver metabolism of E2 significantly affected the mammary tumor development (46). We have previously published that both dietary berries and ellagic acid significantly inhibit E2-induced hepatic DNA adducts, showing that berries have a distinctive effect on the liver (25). It remains to be shown whether berries and berry phytochemicals cause a change of E2 metabolism in the liver to bring about a change in mammary tumor development.
The differential effects of the two types of berries could be due to their distinctive anthocyanin profiles and contents. At a comparable dose, blueberry has only 2/3rd the anthocyanin content and less than 1/20th of the ellagic acid content as that in black raspberry. Further, malvidin and delphinidin are the major anthocyanidins in blueberry followed by petunidin and peonidin, whereas black raspberry contains almost exclusively cyanidin (21, 47). Ellagic acid, the pure compound consistently exhibits very similar effects regardless of the E2 dosage. This suggests that, regardless of the E2-dose used, similar mechanisms are involved in the prevention of E2-induced mammary tumors by ellagic acid. Even though the calculated levels of ellagic acid are 8-times lower in 2.5% BRB diet (24), both ellagic acid and 2.5% BRB elicited very similar effects in reducing tumor volume, suggesting that whole-food source is more efficient than a purified component. This is theory is supported by results from Stoner and colleagues, who showed that the insoluble fraction of BRB containing just ellagitannins is as effective as either whole BRB or the anthocyanin-rich fraction in reducing esophageal tumors (48).
In summary, this is the first report to show both the changes in expression of E2-metabolizing enzymes during the course of E2-carcinogenesis in ACI rats and the effect of dietary berries/ellagic acid on the same. The changes that occur during the early phase in E2-induced carcinogenesis are indicative of the efficacy of chemopreventive agents in reducing mammary tumor indices. CYP1A1 may play an important role in the E2-induced tumorigenesis in the ACI rats. Dietary berries and ellagic acid cause a net reduction in the expression of phase I E2 metabolizing enzymes. Black raspberry is so far the most effective in reducing tumor incidence at 1% and 2.5%. It also has the greatest effect on phase I enzyme reduction at early phases. Blueberry, which has significantly lower levels of total phenolics than black raspberry, has much less effect on the enzyme expression, although effect on tumor indices are more comparable suggesting different anthocyandins (e.g., delphinidin) may be acting via alternative mechanism. Ellagic acid, may act via mechanisms other than modulating CYP1A1 to significantly deter mammary tumor growth.
Acknowledgements
The authors gratefully acknowledge Dr. Barb Mickelson at Harlan Teklad for her continued technical support and Dr. Cidambi Srinivasan and Dr.Howard P. Glauert at the University of Kentucky, for performing part of the statistical analyses using the SAS software. We are also thankful to Dr. Wendy Spencer for the review of this manuscript. This work was supported by USPHS grant CA-90892, CA-118114 and Agnes Brown Duggan Foundation. Dr. Ramesh Gupta holds Agnes Brown Duggan Endowed Chair in Oncological Research.
Footnotes
Disclosure: Authors have no potential conflicts of interest.
References
- 1.Yabroff KR, Bradley CJ, Mariotto AB, Brown ML, Feuer EJ. Estimates and projections of value of life lost from cancer deaths in the United States. J Natl Cancer Inst. 2008;100(24):1755–1762. doi: 10.1093/jnci/djn383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernstein L, Ross RK. Endogenous hormones and breast cancer risk. Epidemiol Rev. 1993;15(1):48–65. doi: 10.1093/oxfordjournals.epirev.a036116. [DOI] [PubMed] [Google Scholar]
- 3.Lippman ME, Krueger KA, Eckert S, Sashegyi A, Walls EL, Jamal S, et al. Indicators of lifetime estrogen exposure: effect on breast cancer incidence and interaction with raloxifene therapy in the multiple outcomes of raloxifene evaluation study participants. J Clin Oncol. 2001;19(12):3111–3116. doi: 10.1200/JCO.2001.19.12.3111. [DOI] [PubMed] [Google Scholar]
- 4.Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006;354(3):270–282. doi: 10.1056/NEJMra050776. [DOI] [PubMed] [Google Scholar]
- 5.Sasano H, Suzuki T, Nakata T, Moriya T. New development in intracrinology of breast carcinoma. Breast cancer (Tokyo, Japan) 2006;13(2):129–136. doi: 10.2325/jbcs.13.129. [DOI] [PubMed] [Google Scholar]
- 6.Simpson ER. Sources of estrogen and their importance. The Journal of steroid biochemistry and molecular biology. 2003;86(3–5):225–230. doi: 10.1016/s0960-0760(03)00360-1. [DOI] [PubMed] [Google Scholar]
- 7.Luu-The V. Analysis and characteristics of multiple types of human 17beta-hydroxysteroid dehydrogenase. The Journal of steroid biochemistry and molecular biology. 2001;76(1–5):143–151. doi: 10.1016/s0960-0760(00)00155-2. [DOI] [PubMed] [Google Scholar]
- 8.Miettinen MM, Poutanen MH, Vihko RK. Characterization of estrogen-dependent growth of cultured MCF-7 human breast-cancer cells expressing 17beta-hydroxysteroid dehydrogenase type 1. Int J Cancer. 1996;68(5):600–604. doi: 10.1002/(SICI)1097-0215(19961127)68:5<600::AID-IJC8>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 9.Duan WR, Parmer TG, Albarracin CT, Zhong L, Gibori G. PRAP, a prolactin receptor associated protein: its gene expression and regulation in the corpus luteum. Endocrinology. 1997;138(8):3216–3221. doi: 10.1210/endo.138.8.5336. [DOI] [PubMed] [Google Scholar]
- 10.Liehr JG. Is estradiol a genotoxic mutagenic carcinogen? Endocrine reviews. 2000;21(1):40–54. doi: 10.1210/edrv.21.1.0386. [DOI] [PubMed] [Google Scholar]
- 11.McFadyen MC, Breeman S, Payne S, Stirk C, Miller ID, Melvin WT, et al. Immunohistochemical localization of cytochrome P450 CYP1B1 in breast cancer with monoclonal antibodies specific for CYP1B1. J Histochem Cytochem. 1999;47(11):1457–1464. doi: 10.1177/002215549904701111. [DOI] [PubMed] [Google Scholar]
- 12.Rogan EG, Badawi AF, Devanesan PD, Meza JL, Edney JA, West WW, et al. Relative imbalances in estrogen metabolism and conjugation in breast tissue of women with carcinoma: potential biomarkers of susceptibility to cancer. Carcinogenesis. 2003;24(4):697–702. doi: 10.1093/carcin/bgg004. [DOI] [PubMed] [Google Scholar]
- 13.Liehr JG. Dual role of oestrogens as hormones and pro-carcinogens: tumour initiation by metabolic activation of oestrogens. Eur J Cancer Prev. 1997;6(1):3–10. doi: 10.1097/00008469-199702000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Abel EL, Lyon RP, Bammler TK, Verlinde CL, Lau SS, Monks TJ, et al. Estradiol metabolites as isoform-specific inhibitors of human glutathione S-transferases. Chemico-biological interactions. 2004;151(1):21–32. doi: 10.1016/j.cbi.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 15.Lakhani NJ, Venitz J, Figg WD, Sparreboom A. Pharmacogenetics of estrogen metabolism and transport in relation to cancer. Current drug metabolism. 2003;4(6):505–513. doi: 10.2174/1389200033489244. [DOI] [PubMed] [Google Scholar]
- 16.Gallicchio L, Berndt SI, McSorley MA, Newschaffer CJ, Thuita LW, Argani P, et al. Polymorphisms in estrogen-metabolizing and estrogen receptor genes and the risk of developing breast cancer among a cohort of women with benign breast disease. BMC cancer. 2006;6:173. doi: 10.1186/1471-2407-6-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu BT, Conney AH. Functional role of estrogen metabolism in target cells: review and perspectives. Carcinogenesis. 1998;19(1):1–27. doi: 10.1093/carcin/19.1.1. [DOI] [PubMed] [Google Scholar]
- 18.Mauvais-Jarvis P, Kuttenn F, Gompel A. Estradiol/progesterone interaction in normal and pathologic breast cells. Annals of the New York Academy of Sciences. 1986;464:152–167. doi: 10.1111/j.1749-6632.1986.tb16002.x. [DOI] [PubMed] [Google Scholar]
- 19.Stoner GD. Foodstuffs for preventing cancer: the preclinical and clinical development of berries. Cancer Prev Res (Phila Pa) 2009;2(3):187–194. doi: 10.1158/1940-6207.CAPR-08-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daniel EM, Krupnick AS, Heur Y, Blinzler JA, Nims RW, Stoner GD. Extraction, stability and quantitation of ellagic acid in various fruits and nuts. J Food composition and analysis. 1989;2:338–349. [Google Scholar]
- 21.Wu X, Beecher GR, Holden JM, Haytowitz DB, Gebhardt SE, Prior RL. Concentrations of anthocyanins in common foods in the United States and estimation of normal consumption. J Agric Food Chem. 2006;54(11):4069–4075. doi: 10.1021/jf060300l. [DOI] [PubMed] [Google Scholar]
- 22.Seeram NP. Berry fruits: compositional elements, biochemical activities, and the impact of their intake on human health, performance, and disease. J Agric Food Chem. 2008;56(3):627–629. doi: 10.1021/jf071988k. [DOI] [PubMed] [Google Scholar]
- 23.Seeram NP. Berry fruits for cancer prevention: current status and future prospects. J Agric Food Chem. 2008;56(3):630–635. doi: 10.1021/jf072504n. [DOI] [PubMed] [Google Scholar]
- 24.Aiyer HS, Srinivasan C, Gupta RC. Dietary berries and ellagic acid diminish estrogen-mediated mammary tumorigenesis in ACI rats. Nutr Cancer. 2008;60(2):227–234. doi: 10.1080/01635580701624712. [DOI] [PubMed] [Google Scholar]
- 25.Aiyer HS, Kichambare S, Gupta RC. Prevention of oxidative DNA damage by bioactive berry components. (Suppl 1).Nutr Cancer. 2008;60:36–42. doi: 10.1080/01635580802398448. [DOI] [PubMed] [Google Scholar]
- 26.Schmitt E, Stopper H. Estrogenic activity of naturally occurring anthocyanidins. Nutr Cancer. 2001;41(1–2):145–149. doi: 10.1080/01635581.2001.9680625. [DOI] [PubMed] [Google Scholar]
- 27.Larrosa M, Gonzalez-Sarrias A, Garcia-Conesa MT, Tomas-Barberan FA, Espin JC. Urolithins, ellagic acid-derived metabolites produced by human colonic microflora, exhibit estrogenic and antiestrogenic activities. J Agric Food Chem. 2006;54(5):1611–1620. doi: 10.1021/jf0527403. [DOI] [PubMed] [Google Scholar]
- 28.Borges G, Roowi S, Rouanet JM, Duthie GG, Lean ME, Crozier A. The bioavailability of raspberry anthocyanins and ellagitannins in rats. Mol Nutr Food Res. 2007;51(6):714–725. doi: 10.1002/mnfr.200700024. [DOI] [PubMed] [Google Scholar]
- 29.Kay CD. Aspects of anthocyanin absorption, metabolism and pharmacokinetics in humans. Nutr Res Rev. 2006;19(1):137–146. doi: 10.1079/NRR2005116. [DOI] [PubMed] [Google Scholar]
- 30.Talavera S, Felgines C, Texier O, Besson C, Manach C, Lamaison JL, et al. Anthocyanins are efficiently absorbed from the small intestine in rats. The Journal of nutrition. 2004;134(9):2275–2279. doi: 10.1093/jn/134.9.2275. [DOI] [PubMed] [Google Scholar]
- 31.Ravoori S, Vadhanam MV, Sahoo S, Srinivasan C, Gupta RC. Mammary tumor induction in ACI rats exposed to low levels of 17beta-estradiol. Int J Oncol. 2007;31(1):113–120. [PubMed] [Google Scholar]
- 32.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 33.Shull JD, Spady TJ, Snyder MC, Johansson SL, Pennington KL. Ovary-intact, but not ovariectomized female ACI rats treated with 17beta-estradiol rapidly develop mammary carcinoma. Carcinogenesis. 1997;18(8):1595–1601. doi: 10.1093/carcin/18.8.1595. [DOI] [PubMed] [Google Scholar]
- 34.Kurz SG, Hansen KK, McLaughlin MT, Shivaswamy V, Schaffer BS, Gould KA, et al. Tissue-specific actions of the Ept1, Ept2, Ept6, and Ept9 genetic determinants of responsiveness to estrogens in the female rat. Endocrinology. 2008;149(8):3850–3859. doi: 10.1210/en.2008-0173. PMCID: 2488241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruhlen RL, Willbrand DM, Besch-Williford CL, Ma L, Shull JD, Sauter ER. Tamoxifen induces regression of estradiol-induced mammary cancer in the ACI.COP-Ept2 rat model. Breast Cancer Res Treat. 2008 doi: 10.1007/s10549-008-0169-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shull JD, Lachel CM, Murrin CR, Pennington KL, Schaffer BS, Strecker TE, et al. Genetic control of estrogen action in the rat: mapping of QTLs that impact pituitary lactotroph hyperplasia in a BNXACI intercross. Mamm Genome. 2007;18:657–669. doi: 10.1007/s00335-007-9052-2. [DOI] [PubMed] [Google Scholar]
- 37.Mense SM, Singh B, Remotti F, Liu X, Bhat HK. Vitamin C and alpha-naphthoflavone prevent estrogen-induced mammary tumors and decrease oxidative stress in female ACI rats. Carcinogenesis. 2009;30(7):1202–1208. doi: 10.1093/carcin/bgp093. PMCID: 2704283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh B, Mense SM, Remotti F, Liu X, Bhat HK. Antioxidant butylated hydroxyanisole inhibits estrogen-induced breast carcinogenesis in female ACI rats. J Biochem Mol Toxicol. 2009;23(3):202–211. doi: 10.1002/jbt.20281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cribb AE, Knight MJ, Dryer D, Guernsey J, Hender K, Tesch M, et al. Role of polymorphic human cytochrome P450 enzymes in estrone oxidation. Cancer Epidemiol Biomarkers Prev. 2006;15(3):551–558. doi: 10.1158/1055-9965.EPI-05-0801. [DOI] [PubMed] [Google Scholar]
- 40.Liehr JG. Genotoxicity of the steroidal oestrogens oestrone and oestradiol: possible mechanism of uterine and mammary cancer development. Human reproduction update. 2001;7(3):273–281. doi: 10.1093/humupd/7.3.273. [DOI] [PubMed] [Google Scholar]
- 41.Wilson AM, Reed GA. Predominant 4-hydroxylation of estradiol by constitutive cytochrome P450s in the female ACI rat liver. Carcinogenesis. 2001;22(2):257–263. doi: 10.1093/carcin/22.2.257. [DOI] [PubMed] [Google Scholar]
- 42.Christou M, Savas U, Schroeder S, Shen X, Thompson T, Gould MN, et al. Cytochromes CYP1A1 and CYP1B1 in the rat mammary gland: cell-specific expression and regulation by polycyclic aromatic hydrocarbons and hormones. Molecular and cellular endocrinology. 1995;115(1):41–50. doi: 10.1016/0303-7207(95)03668-w. [DOI] [PubMed] [Google Scholar]
- 43.Barch DH, Rundhaugen LM, Thomas PE, Kardos P, Pillay NS. Dietary ellagic acid inhibits the enzymatic activity of CYP1A1 without altering hepatic concentrations of CYP1A1 or CYP1A1 mRNA. Biochem Biophys Res Commun. 1994;201(3):1477–1482. doi: 10.1006/bbrc.1994.1870. [DOI] [PubMed] [Google Scholar]
- 44.Risk M, Shehu A, Mao J, Stocco CO, Goldsmith LT, Bowen-Shauver JM, et al. Cloning and characterization of a 5' regulatory region of the prolactin receptor-associated protein/17{beta} hydroxysteroid dehydrogenase 7 gene. Endocrinology. 2005;146(6):2807–2816. doi: 10.1210/en.2004-1673. [DOI] [PubMed] [Google Scholar]
- 45.Li KM, Todorovic R, Devanesan P, Higginbotham S, Kofeler H, Ramanathan R, et al. Metabolism and DNA binding studies of 4-hydroxyestradiol and estradiol-3,4-quinone in vitro and in female ACI rat mammary gland in vivo. Carcinogenesis. 2004;25(2):289–297. doi: 10.1093/carcin/bgg191. [DOI] [PubMed] [Google Scholar]
- 46.Mesia-Vela S, Sanchez RI, Roberts KG, Reuhl KR, Conney AH, Kauffman FC. Dietary clofibrate stimulates the formation and size of estradiol-induced breast tumors in female August-Copenhagen Irish (ACI) rats. Toxicology. 2008;246(1):63–72. doi: 10.1016/j.tox.2007.12.025. PMCID: 2441444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moyer RA, Hummer KE, Finn CE, Frei B, Wrolstad RE. Anthocyanins, phenolics, and antioxidant capacity in diverse small fruits: vaccinium, rubus, and ribes. J Agric Food Chem. 2002;50(3):519–525. doi: 10.1021/jf011062r. [DOI] [PubMed] [Google Scholar]
- 48.Wang LS, Hecht SS, Carmella SG, Yu N, Larue B, Henry C, et al. Anthocyanins in black raspberries prevent esophageal tumors in rats. Cancer Prev Res (Phila Pa) 2009;2(1):84–93. doi: 10.1158/1940-6207.CAPR-08-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]