Abstract
Aims:
The nature of villitis of unknown etiology (VUE) is intriguing in terms of its etiology, origin of inflammatory cells and immunophenotype of T cells involved. The aim was to determine the origin of macrophages and the immunophenotype of T lymphocytes in VUE associated with various complications of pregnancy.
Methods and Results:
Placentas with VUE (n=45) were studied by chromogenic in-situ hybridization (CISH) for Y chromosome (DYZ1) and immunohistochemistry for CD14, CD68, Ki-67 (n=10; all from male neonates) and a panel of T-cell antigens (CD3, CD4 and CD8) (n=35). All of the placentas from male neonates showed CISH+ signals from Y chromosomes in the majority of macrophages, but not in lymphocytes, indicating that the macrophages were of fetal origin. Many macrophages of the affected chorionic villi were Ki67+, suggesting that they are hyperplastic Hofbauer cells. Among the lymphocytes, CD8+ T cells outnumbered CD4+ T cells in all placentas with different obstetrical conditions.
Conclusions:
We define primary components of VUE as maternal CD8+ T cells and hyperplastic Hofbauer cells. We propose that VUE is a unique inflammatory reaction where the leukocytes from two hosts are key partners, analogous to either allograft rejection or graft-versus-host disease.
Keywords: Villitis, Placenta, Graft-versus-host disease, Allograft rejection, In-situ hybridization
Introduction
Villitis of unknown etiology (VUE) is a destructive inflammatory lesion of the chorionic villi characterized by infiltration of chronic inflammatory cells, mainly lymphocytes and macrophages.1 It is intriguing in terms of its etiology, origin of inflammatory cells and immunophenotype of T cells involved. Chronic infections and abnormal immune responses comparable to allograft rejection have been implicated as causes and pathogeneic mechanisms.2 However, the epidemiological data (e.g. high prevalence, recurrence risk, lack of seasonal variation and absence of infection-related signs in the mother and fetus) suggests that it is more likely to be an immune-mediated process similar to allograft rejection rather than a specific infection.3 The lesion is not uncommon and can be found in term pregnancies. A recent prospective study of VUE as part of a population-based case-control study of infants at term has revealed that VUE is encountered in 17.3 % and 11.7 % of ‘small for gestational age’ and ‘appropriate for gestational age’ groups, respectively.4 The severe form of VUE is known as one of the placental lesions associated with neurological impairment 5,6 as well as with recurrent intrauterine fetal demise (IUFD).7
Previous studies have shown that the majority of cells in the inflammatory infiltrate in VUE are activated macrophages (HLA-DR+, −DQ+ or −DP+) and CD3+ T cells, 8 with CD4+ T lymphocytes constituting the main type of CD3+ T cell.9 Studies have also indicated that infiltrating inflammatory cells are primarily of maternal origin, 10,11 and that the maternal inflammatory cells are probably T cells.11 Therefore, invasion of the fetal tissue (chorionic villi) by maternal CD4+ T cells has been considered to be a central feature of VUE. Involvement of CD4+ T cells in the pathogenesis of VUE has also been supported by the demonstration of MHC class II HLA-DR, -DP, and -DQ reactivity (all of which are not expressed in normal placentas) in syncytiotrophoblast of the chorionic villi affected by VUE.12 However, whether there is a significant fetal inflammatory reaction, especially by macrophages, in VUE remains unclear. Furthermore, recent reports on the characteristics of inflammation in infectious chronic villitis, including syphilis and toxoplasmosis, and some cases of VUE indicate that CD8+ cells are present in greater numbers than CD4+ cells. The CD4+: CD8+ ratios were 1:2 and 1:7 in VUE and syphilitic villitis, respectively.13,14 This striking difference in the proportion of T-cell subsets in VUE among previous studies may be due to associated pregnancy complications and other confounding factors.
Characterization of leukocytic infiltrates is crucial for an understanding of the pathogeneic mechanisms of inflammatory lesions. This study was performed to determine whether VUE is purely a maternal inflammatory reaction and to profile the major population of T cells (CD4+ T cells versus CD8+ T cells) in VUE associated with various pregnancy complications [e.g. IUFD, intrauterine growth restriction (IUGR), preterm premature rupture of membranes (PPROM), and preeclampsia] and normal term pregnancy. Chromogenic in-situ hybridization (CISH) using a Y chromosome-specific probe and immunohistochemistry for T-cell and macrophage markers were employed to address these questions.
Materials and methods
Tissue samples
A total of 45 cases with VUE were retrieved from the Bank of Biological Materials of the Perinatology Research Branch, National Institute of Child Health and Human Development. All of the specimens were obtained from patients at 22-42 weeks' gestation without any clinical evidence of infection in the mothers or babies. Ten cases of placentas from male neonates containing a prominent macrophage response were selected for CISH analysis of the Y chromosome in the macrophages. The cases included normal full-term deliveries (n=4), preeclampsia (n=3), preterm labor (n=2) and IUGR (n=1). Additionally, 35 cases for T-cell subset analysis included IUFD (n=5), IUGR (n=6), PPROM (n=7), preeclampsia (n=7) and normal term delivery (n=9).
Chromogenic in-situ hybridization
CISH was performed on paraffin sections using a Y chromosome-specific probe (DYZ1; Zymed, San Francisco, CA, USA) following the protocol provided by the manufacturer. Briefly, deparaffinized sections were incubated for 15 min at 121°C in Spot-Light Tissue Heat Pretreatment Buffer (Zymed) and were immediately washed in distilled water twice for 3 min each. Sections underwent enzymatic digestion at 37°C for 10 min using the Spot-Light Tissue Pretreatment Enzyme (Zymed), followed by washing and dehydration in a series of graded alcohols. After allowing the slides to air dry, 15 μl of the probe were added to the slides. Denaturation was performed at 95 °C for 5 min, followed by hybridization for 16 h at 37°C in a humidified chamber. Y chromosome immunodetection was observed using the CISH centromeric detection kit (Zymed). Slides were then briefly counterstained with hematoxylin and mounted.
Immunohistochemistry
Serial sections (5μm thick) were obtained from each case and subjected to immunohistochemistry using a panel of antibodies: murine monoclonal anti-CD3 (PS1; Novocastra, Newcastle, UK; 1:50), murine monoclonal anti-CD4 (1F6; DiNona, Seoul, Korea; 1:50) and murine monoclonal anti-CD8 (C8/144B; Dako, Carpinteria, CA, USA; 1:50). Deparaffinization, antigen retrieval and immunohistochemistry were performed with an automatic immunostainer (Ventana Discovery; Ventana Medical Systems, Inc., Tucson, AZ, USA). The signals were detected using a 3′3′-Diaminobenzidine (DAB) MAP™ kit (Ventana Medical Systems, Inc.). The number of specific antigen-positive cells was counted using image analysis software (Image-ProPlus; Media Cybernetics, Silver Spring, MD, USA) in five high-power fields of affected areas (one high-power field = 0.093 mm2). The proportion of CD4+ T cells and CD8+ T cells to CD3+ T cells was calculated. The inter- and intra-observer reproducibility for counting was evaluated by the intraclass correlation coefficient (ICC).15 The inter-observer ICC was >0.80 and the intra-observer ICCs for observers 1 and 2 were >0.90, indicating excellent reproducibility.
CD68 and CD14 double staining on consecutive sections was also performed using an automated immunostainer (Dako) in cases for CISH analysis. Endogenous peroxidase was quenched with 3% hydrogen peroxide. After blocking with normal mouse serum, the sections were incubated with murine monoclonal anti-human CD14 (7; Novocastra; 1:500). Sections were incubated with the secondary antibody, biotinylated antimouse immunoglobulin. Following incubation with streptavidin- horseradish peroxidase, DAB was used as a chromogen. Sections were then treated with proteinase K (Dako) prior to the incubation with a second primary antibody, murine monoclonal anti-human CD68 (PG-M1; Dako; 1:200). After incubation with anti-CD68, sections were incubated with biotinylated antimouse immunoglobulin, the secondary antibody. The sections were sequentially incubated with streptavidin-alkaline phosphatase as well as a second chromogen, Vector blue (Vector Laboratories, Burlingame, CA, USA). Double immunohistochemistry for mouse monoclonal Ki67 (MIB-1; Dako; 1:200) and CD68 was also performed using the Ventana Discovery autostainer.
Statistical analysis
Kruskal-Wallis and Mann-Whitney U-tests were employed to analyze differences in immunophenotype profiles of leukocytes among and between clinical groups. Using the statistical package SPSS version 12.0 (SPSS, Inc., Chicago, IL, USA), a P-value of <0.05 was considered to be significant.
Results
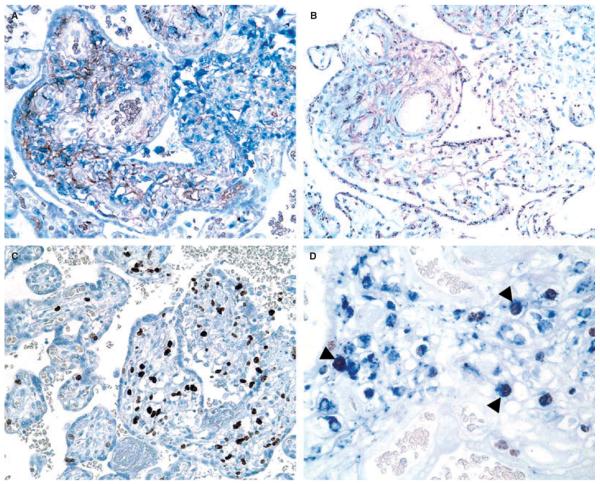
Macrophage origin was readily determined by the presence or absence of the CISH signal from Y chromosomes in the nuclei of macrophages defined as CD68+ or CD14+ cells in serial sections (Figure 1A,B). The signal appeared as a distinct granular spot in the nucleus. The majority of macrophages in the villous stroma were positive for CISH, indicating their fetal origin. Intervillous mononuclear inflammatory infiltrates (of maternal origin) were consistently negative (Figure 1B). A small number of CISH–macrophages were also encountered in the villous stroma. Immunohistochemistry for Ki67 revealed frequent nuclear labeling of macrophages and lymphocytes in the villous stroma and intervillous mononuclear cells. Prominent Ki67 immunoreactivity in the inflammatory cells of the villous stroma easily identified VUE-affected areas compared with unaffected areas (Figure 1C,D). CD14 immunoreactivity was much stronger in macrophages in the foci of VUE compared with that of Hofbauer cells in unaffected villi of the same placenta and in contrast to the constant CD68 immunoreactivity in macrophages of both affected and unaffected villi.
Figure 1.
A, Double staining for CD14 (brown) and CD68 (blue) detects an increased number of macrophages in the villitis of unknown etiology (VUE)-affected area. B, Chromogenic in-situ hybridization using a Y chromosome-specific probe (DYZ1) shows that a majority of the macrophages have a distinct intranuclear hybridization signal indicating that they are of fetal origin, whereas intervillous mononuclear inflammatory infiltrates of maternal origin are consistently negative (right upper part). C, VUE-affected areas are easily recognized by prominent Ki67 immunoreactivity in the inflammatory cells of the villous stroma (right side), when compared with unaffected areas (left side). D, Double immunohistochemistry for Ki67 (brown) and CD68 (blue) shows proliferating macrophages with Ki67 nuclear labeling (arrowheads).
The clinical characteristics of the cases for T-cell subset analysis are summarized in Table 1. CD8+ T cells were predominant among T lymphocytes in VUE associated with various obstetrical conditions, and the CD4+: CD8+ ratio ranged from 0.02 to 0.87 (median = 0.31) (Figure 2). The percentage of CD4+ T cells to CD3+ T cells, that of CD8+ T cells to CD3+ T cells, and CD4+ to CD8+ ratios were not significantly different among the study groups (Table 2; P > 0.05). There were no significant differences in those parameters between preterm (gestational age < 37 weeks; n=16) and term (gestational age ≥ 37 weeks; n=19) cases (P > 0.05).
Table 1.
Clinical characteristics of study population
| Diagnosis | IUFD (n=5) |
IUGR (n=6) |
PPROM (n=7) |
Preeclampsia (n=7) |
Normal Term (n=9) |
|---|---|---|---|---|---|
| Maternal age (years)* |
23 (19-29) | 26.5 (19-32) | 33 (20-37) | 29 (24-36) | 28 (21-37) |
| Gestational age at delivery (weeks)* |
30.2 (22-39) | 37.1 (36-40) | 34.6 (33-36) | 37 (35-41) | 38.4 (37-42) |
| Birth weight (g)* | 1740 (440-2980) | 2307.5 (1051-2590) | 2190 (1380-2700) | 2180 (1840-3750) | 3330 (2880-3910) |
| Small for gestational age† |
1 (20) | 6 (100) | 1 (14.3) | 5 (71.4) | 0 (0) |
Value expressed as median (range).
Value expressed as number (percentage).
IUFD, intrauterine fetal demise; IUGR, intrauterine growth restriction; PPROM: Preterm premature rupture of membranes.
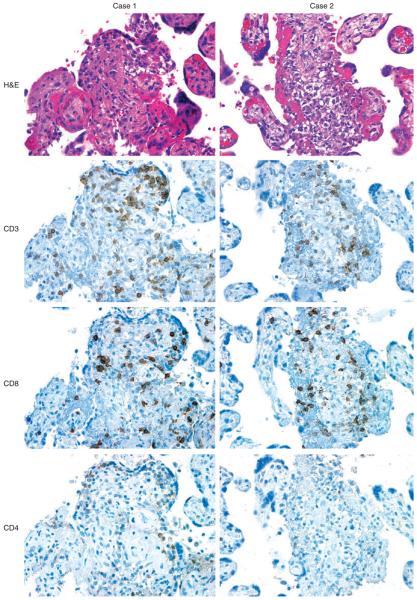
Figure 2.
Immunohistochemical features of villitis of unknown etiology (VUE) taken from two representative cases (case 1 and case 2). Necrotizing villous inflammation is present in both cases. Immunohistochemistry for a panel of T-cell antigens shows that the majority of infiltrating CD3+ T cells is positive for CD8. CD4+ T cells are sparsely distributed.
Table 2.
Immunophenotypic profile of lymphocytes in villitis of unknown etiology with various clinical conditions
| Diagnosis | IUFD (n=5) |
IUGR (n=6) |
PPROM (n=7) |
Preeclampsia (n=7) |
Normal Term (n=9) |
|---|---|---|---|---|---|
| CD4+ lymphocytes* | 26.6 (6.7-26.7) | 20.5 (3.1-32.5) | 17.4 (9.8-28.6) | 18.6 (4.3-32.2) | 17.6 (2.1-27.1) |
| CD8+ lymphocytes* | 51.8 (32.7-83.3) | 69.7 (27.2-98.1) | 60.7 (51.8-88.4) | 52.6 (27.1-79.6) | 60.8 (28.7-92.3) |
| CD4+:CD8+ ratio | 0.346 (0.15-0.51) | 0.319 (0.05-0.39) | 0.295 (0.18-0.47) | 0.277 (0.15-0.87) | 0.304 (0.02-0.55) |
The values are median (range).
CD4+ and CD8+ lymphocytes were quoted as a percentage to CD3+ lymphocytes.
IUFD, intrauterine fetal demise; IUGR, intrauterine growth restriction; PPROM, preterm premature rupture of membranes.
Discussion
Pregnancy inevitably presents an interaction of cells between mother and fetus. VUE is an inflammatory reaction in which the presence of maternal leukocytes in fetal tissues (chorionic villi) has been clearly documented. This study has unveiled two additional aspects of VUE: first, fetal placental macrophages (Hofbauer cells) are another primary histological component of VUE; second, maternal CD8+ T cells are the predominant T cells involved in VUE associated with a variety of obstetrical conditions, including normal pregnancy.
Hofbauer cells are resident tissue macrophages of the placenta. Their population remains constant throughout gestation, occupying around 40% of villous stromal cells.16 Along with their close anatomical relationship to villous trophoblast and fetal capillaries, the expression of Class I and Class II major histocompatibility complex antigens and Fc receptors support an important role for them in immunological reactions, including antigen presentation.17,18 Frequent Ki67 labeling of macrophages involved in VUE suggests that they are hyperplastic Hofbauer cells, as blood-derived ‘exudate’ macrophages are less proliferative and resident macrophages have more proliferative potential.19 Previous studies have demonstrated mitosis in Hofbauer cells and suggested that mitosis of Hofbauer cells could allow for a rapid increase in numbers when required.20 Increased immunoreactivity of CD14 in the activated Hofbauer cells of the affected villi is similar to up-regulation of CD14 in Kupffer cells after lipopolysaccharide stimulation21 and in microglia of ischemic or traumatic brain lesions.22,23 Although we cannot exclude the possibility that some maternal macrophages are involved (a few CISH– cells could be of either fetal or maternal origin due to limitations in the interpretation of CISH results in a given plane of tissue section), it is evident that the majority of the macrophages are Y chromosome-positive fetal cells. CISH provided an excellent and definitive platform for sex typing of the cells with preservation of morphological details, as has been demonstrated in other studies.24,25
The relevance of CD8+ T-cell predominance found in this study is supported by previous observations. CD8+ cells were dominant over CD4+ cells in limited examples of VUE and infectious chronic villitis.13,14 In chronic chorioamnionitis, which is commonly associated with VUE, CD3+/CD8+ T cells have been found more frequently than CD3+/CD4+ T cells.26 One previous study has reported a rarity of CD8+ T cells with an excess CD4+ T cells in VUE.9 The discrepancy with our observation might be due to differences in applied immunohistochemical methods, sensitivity of antibodies used, or other confounding factors. A weakness of our study is that a limited number of cases was available in each group, making matching for gestational age among the groups impossible. However, CD8+ predominance was found in all the groups compared, showing no difference between preterm and term cases.
The key feature of VUE, based on the observations in this study, is the involvement of hyperplastic Hofbauer cells and maternal T cells. The involvement of Hofbauer cells raises nosological issues about how the inflammatory reaction in VUE should be defined. VUE has been likened to an allograft rejection of the mother (recipient) to fetal antigens (donor). Donor macrophages are involved in allograft rejection, especially in the early stage, along with recipient T cells and macrophages.27 However, the placenta is unique from conventional organ transplantation models, in that true vascular anastomosis between the mother and the placenta is never established, and that it is also part of another viable host—the fetus. Therefore, infiltration of chorionic villi by maternal T cells is comparable to the transplantation of immunocompetent allogeneic immunocytes into a fetal organ, which would potentially establish an analogous situation to graft-versus-host disease (GVHD) by maternal T cells (graft) in the fetal (host) compartment. Either active immune suppression by fetal regulatory T cells or a passive form of immune suppression by a functional deficit in the fetus might contribute to the development of GVHD by immunocompetent maternal T cells.28
Graft-versus-host disease is associated with the activation, proliferation and differentiation of allogeneic T cells into effector cells by antigen-presenting cells (APCs), and tissue damage. Animal studies clearly indicate that host APCs are required to initiate T-cell-dependent acute GVHD and local tissue-resident APCs control the recruitment of alloreactive donor CD4+ or CD8+ T cells during the effector phase.29-31 Chemokines and their receptors play a major role in the taxis of alloreactive T cells by macrophages and dendritic cells in GVHD and are important for the migration of donor-derived T cells into GVHD target organs.32,33 A rat GVHD model has demonstrated that there is activation and expansion of the responding microglia, the resident macrophage of the central nervous system, following CD4+ T-cell infiltration in the brain.34 It is noteworthy that the central nervous system is similar to the placental environment in that the resident lymphocytic population is minimal. The model of interaction between T cells and microglia might be relevant for VUE where maternal T cells interact with Hofbauer cells of fetal origin. Whereas the role of resident macrophages is fundamental for the development of GVHD, microglia can induce both final effector functions and apoptosis of T cells, indicating that resident macrophages induce apoptosis of effector T cells in vivo.35 It is possible that Hofbauer cells play a similar role in inducing the effector function of maternal T cells and eventual apoptosis, so that the fetus can be rescued from detrimental propagation of GVHD.
In conclusion, the findings reported here strongly suggest that VUE is analogous to either an allograft rejection or a placental GVHD, where maternal T cells and fetal macrophages are key partners. A recent report on VUE in the placenta from a male fetus demonstrating the predominance of maternal T cells and fetal macrophages using a new conjoint immunohistochemistry-in situ hybridization procedure is quite consistent with our data.36 Further investigations on the interaction between maternal T cells and Hofbauer cells, especially on the role of Hofbauer cells in the initiation, progression and resolution of VUE, are necessary for an understanding of the clinical and biological significance of this intriguing inflammatory lesion of the human placenta.
Acknowledgements
This research was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), NIH, DHHS, USA.
Abbreviations
- APC
antigen-presenting cell
- CISH
chromogenic in-situ hybridization
- GVHD
graft-versus-host disease
- ICC
intraclass correlation coefficient
- IUFD
intrauterine fetal demise
- IUGR
intrauterine growth restriction
- PPROM
preterm premature rupture of membranes
- VUE
villitis of unknown etiology
References
- 1.Russell P. Inflammatory lesions of the human placenta. III. The histopathology of villitis of unknown aetiology. Placenta. 1980;1:227–244. doi: 10.1016/s0143-4004(80)80005-1. [DOI] [PubMed] [Google Scholar]
- 2.Benirschke K, Coen R, Patterson B, Key T. Villitis of known origin: varicella and toxoplasma. Placenta. 1999;20:395–399. doi: 10.1053/plac.1999.0405. [DOI] [PubMed] [Google Scholar]
- 3.Redline RW. Placental inflammation. Semin. Neonatol. 2004;9:265–274. doi: 10.1016/j.siny.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 4.Becroft DM, Thompson JM, Mitchell EA. Placental villitis of unknown origin: epidemiologic associations. Am. J. Obstet. Gynecol. 2005;192:264–271. doi: 10.1016/j.ajog.2004.06.062. [DOI] [PubMed] [Google Scholar]
- 5.Redline RW, O'Riordan MA. Placental lesions associated with cerebral palsy and neurologic impairment following term birth. Arch. Pathol. Lab Med. 2000;124:1785–1791. doi: 10.5858/2000-124-1785-PLAWCP. [DOI] [PubMed] [Google Scholar]
- 6.Redline RW. Severe fetal placental vascular lesions in term infants with neurologic impairment. Am. J. Obstet. Gynecol. 2005;192:452–457. doi: 10.1016/j.ajog.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 7.Kraus FT, Redline RW, Gersell DJ, Nelson DM, Diche JM. Inflammation and infection. In: Kraus FT, Redline RW, Gersell DJ, Nelson DM, Diche JM, editors. Placental Pathology Atlas of Non Tumor Pathology 3. AFIP; Washington: 2004. pp. 75–116. [Google Scholar]
- 8.Altemani AM. Immunohistochemical study of the inflammatory infiltrate in villitis of unknown etiology. A qualitative and quantitative analysis. Pathol. Res. Pract. 1992;188:303–309. doi: 10.1016/S0344-0338(11)81208-2. [DOI] [PubMed] [Google Scholar]
- 9.Labarrere CA, McIntyre JA, Faulk WP. Immunohistologic evidence that villitis in human normal term placentas is an immunologic lesion. Am. J. Obstet. Gynecol. 1990;162:515–522. doi: 10.1016/0002-9378(90)90421-3. [DOI] [PubMed] [Google Scholar]
- 10.Labarrere CA, Faulk WP. Maternal cells in chorionic villi from placentae of normal and abnormal human pregnancies. Am. J. Reprod. Immunol. 1995;33:54–59. doi: 10.1111/j.1600-0897.1995.tb01138.x. [DOI] [PubMed] [Google Scholar]
- 11.Redline RW, Patterson P. Villitis of unknown etiology is associated with major infiltration of fetal tissue by maternal inflammatory cells. Am. J. Pathol. 1993;143:473–479. [PMC free article] [PubMed] [Google Scholar]
- 12.Labarrere CA, Faulk WP. MHC class II reactivity of human villous trophoblast in chronic inflammation of unestablished etiology. Transplantation. 1990;50:812–816. doi: 10.1097/00007890-199011000-00014. [DOI] [PubMed] [Google Scholar]
- 13.Kapur P, Rakheja D, Gomez AM, Sheffield J, Sanchez P, Rogers BB. Characterization of inflammation in syphilitic villitis and in villitis of unknown etiology. Pediatr. Dev. Pathol. 2004;7:453–458. doi: 10.1007/s10024-004-2124-3. [DOI] [PubMed] [Google Scholar]
- 14.Brito H, Juliano P, Altemani C, Altemani A. Is the immunohistochemical study of the inflammatory infiltrate helpful in distinguishing villitis of unknown etiology from non-specific infection villitis? Placenta. 2005;26:839–841. doi: 10.1016/j.placenta.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 15.Rosner B. Fundamentals of Biostatics. Duxbury Press; Belmont, CA: 1995. [Google Scholar]
- 16.Goldstein J, Braverman M, Salafia C, Buckley P. The phenotype of human placental macrophages and its variation with gestational age. Am. J. Pathol. 1988;133:648–659. [PMC free article] [PubMed] [Google Scholar]
- 17.Sutton L, Gadd M, Mason DY, Redman CW. Cells bearing class II MHC antigens in the human placenta and amniochorion. Immunology. 1986;58:23–29. [PMC free article] [PubMed] [Google Scholar]
- 18.Saji F, Koyama M, Matsuzaki N. Current topic: human placental Fc receptors. Placenta. 1994;15:453–466. doi: 10.1016/s0143-4004(05)80415-1. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi K, Naito M, Takeya M. Development and heterogeneity of macrophages and their related cells through their differentiation pathways. Pathol. Int. 1996;46:473–485. doi: 10.1111/j.1440-1827.1996.tb03641.x. [DOI] [PubMed] [Google Scholar]
- 20.Castellucci M, Celona A, Bartels H, Steininger B, Benedetto V, Kaufmann P. Mitosis of the Hofbauer cell: possible implications for a fetal macrophage. Placenta. 1987;8:65–76. doi: 10.1016/0143-4004(87)90040-3. [DOI] [PubMed] [Google Scholar]
- 21.Matsuura K, Ishida T, Setoguchi M, Higuchi Y, Akizuki S, Yamamoto S. Upregulation of mouse CD14 expression in Kupffer cells by lipopolysaccharide. J. Exp. Med. 1994;179:1671–1676. doi: 10.1084/jem.179.5.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beschorner R, Schluesener HJ, Gozalan F, Meyermann R, Schwab JM. Infiltrating CD14+ monocytes and expression of CD14 by activated parenchymal microglia/macrophages contribute to the pool of CD14+ cells in ischemic brain lesions. J. Neuroimmunol. 2002;126:107–115. doi: 10.1016/s0165-5728(02)00046-2. [DOI] [PubMed] [Google Scholar]
- 23.Beschorner R, Nguyen TD, Gozalan F, et al. CD14 expression by activated parenchymal microglia/macrophages and infiltrating monocytes following human traumatic brain injury. Acta Neuropathol. (Berl) 2002;103:541–549. doi: 10.1007/s00401-001-0503-7. [DOI] [PubMed] [Google Scholar]
- 24.Hanna WM, Kwok K. Chromogenic in-situ hybridization: a viable alternative to fluorescence in-situ hybridization in the HER2 testing algorithm. Mod. Pathol. 2006;19:481–487. doi: 10.1038/modpathol.3800555. [DOI] [PubMed] [Google Scholar]
- 25.Thorner PS, Ho M, Chilton-MacNeill S, Zielenska M. Use of chromogenic in situ hybridization to identify MYCN gene copy number in neuroblastoma using routine tissue sections. Am. J. Surg. Pathol. 2006;30:635–642. doi: 10.1097/01.pas.0000202163.82525.5c. [DOI] [PubMed] [Google Scholar]
- 26.Jacques SM, Qureshi F. Chronic chorioamnionitis: a clinicopathologic and immunohistochemical study. Hum. Pathol. 1998;29:1457–1461. doi: 10.1016/s0046-8177(98)90016-8. [DOI] [PubMed] [Google Scholar]
- 27.Grau V, Herbst B, Steiniger B. Dynamics of monocytes/macrophages and T lymphocytes in acutely rejecting rat renal allografts. Cell Tissue Res. 1998;291:117–126. doi: 10.1007/s004410050985. [DOI] [PubMed] [Google Scholar]
- 28.Michaelsson J, Mold JE, McCune JM, Nixon DF. Regulation of T cell responses in the developing human fetus. J. Immunol. 2006;176:5741–5748. doi: 10.4049/jimmunol.176.10.5741. [DOI] [PubMed] [Google Scholar]
- 29.Xia G, Truitt RL, Johnson BD. Graft-versus-leukemia and graft-versus-host reactions after donor lymphocyte infusion are initiated by host-type antigen-presenting cells and regulated by regulatory T cells in early and long-term chimeras. Biol. Blood Marrow Transplant. 2006;12:397–407. doi: 10.1016/j.bbmt.2005.11.519. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Shlomchik WD, Joe G, et al. APCs in the liver and spleen recruit activated allogeneic CD8+ T cells to elicit hepatic graft-versus-host disease. J. Immunol. 2002;169:7111–7118. doi: 10.4049/jimmunol.169.12.7111. [DOI] [PubMed] [Google Scholar]
- 31.Duffner UA, Maeda Y, Cooke KR, et al. Host dendritic cells alone are sufficient to initiate acute graft-versus-host disease. J. Immunol. 2004;172:7393–7398. doi: 10.4049/jimmunol.172.12.7393. [DOI] [PubMed] [Google Scholar]
- 32.Terwey TH, Kim TD, Kochman AA, et al. CCR2 is required for CD8-induced graft-versus-host disease. Blood. 2005;106:3322–3330. doi: 10.1182/blood-2005-05-1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wysocki CA, Panoskaltsis-Mortari A, Blazar BR, Serody JS. Leukocyte migration and graft-versus-host disease. Blood. 2005;105:4191–4199. doi: 10.1182/blood-2004-12-4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sedgwick JD, Ford AL, Foulcher E, Airriess R. Central nervous system microglial cell activation and proliferation follows direct interaction with tissue-infiltrating T cell blasts. J. Immunol. 1998;160:5320–5330. [PubMed] [Google Scholar]
- 35.Ford AL, Foulcher E, Lemckert FA, Sedgwick JD. Microglia induce CD4 T lymphocyte final effector function and death. J. Exp. Med. 1996;184:1737–1745. doi: 10.1084/jem.184.5.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Myerson D, Parkin RK, Benirschke K, Tschetter CN, Hyde SR. The pathogenesis of villitis of unknown etiology: analysis with a new conjoint immunohistochemistry-in situ hybridization procedure to identify specific maternal and fetal cells. Pediatr. Dev. Pathol. 2006;9:257–265. doi: 10.2350/08-05-0103.1. [DOI] [PubMed] [Google Scholar]