Abstract
Background and Purpose
Deferoxamine (DFX) reduces brain edema, neurological deficits and brain atrophy after intracerebral hemorrhage (ICH) in aged as well as young rats. Our previous study found that 50 mg/kg is an effective dose in aged rats. In the present study, we explored potential therapeutic time windows and optimal therapeutic durations.
Methods
Aged male Fischer 344 rats (18-month old) sustained an intra-caudate injection of 100-µL autologous whole blood, followed by intramuscular DFX or vehicle beginning at different time points, or continuing for different durations. Subgroups of rats were sacrificed at day 3 for brain edema measurement and day 56 for brain atrophy determination. Behavioral tests were carried out on days 1, 28 and 56 post-ICH.
Results
Systemic administration of DFX, when begun within 12 hours after ICH, reduced brain edema. DFX treatment started 2 hours after ICH and administered for 7 days or more attenuated ICH-induced ventricle enlargement, caudate atrophy and neurological deficits. DFX attenuated ICH-induced brain atrophy and neurological deficits without detectable side effects when begun within 24 hours and administered for 7 days.
Conclusions
To the extent that these results can be translated to humans, the therapeutic time window and the optimal duration for DFX in this aged rat model of ICH may provide useful information for an ongoing DFX-ICH clinical trial.
Keywords: behavior, brain atrophy, brain edema, cerebral hemorrhage, deferoxamine, iron
Introduction
Intracerebral hemorrhage (ICH) is a common and often fatal subtype of stroke and produces severe neurologic deficits in survivors 1, 2. Experiments have shown that iron plays an important role in ICH-induced edema formation, neuronal death, brain atrophy and behavioral deficits3–5. Iron chelation with deferoxamine (DFX) could be a new therapy for ICH 6, 7. Studies in our laboratory have found that DFX reduces ICH- or hemoglobin-induced brain edema, neuronal death, neurological deficits and brain atrophy in young rats8–10 and reduces brain injury following ICH in young pigs 11. It is well known that age is an important factor affecting brain injury after ischemic and hemorrhagic stroke. Our recent study indicated that DFX higher than 10 mg/kg can reduce ICH-induced brain damage in aged rats 12.
To translate DFX therapy from animal models to ICH patients it is important to examine potentially therapeutic time windows and durations. We have found that DFX treatment begun within 6 hours but not 24 hours after ICH reduces ICH-induced brain edema in young rats8. In the present study, we examined DFX treatment windows for acute edema formation and delayed brain atrophy following ICH in aged rats. Effective therapeutic durations were also examined.
Materials and Methods
Animal Preparation and Intracerebral Infusion
Animal use protocols were approved by the University of Michigan Committee on the Use and Care of Animals. A total of 153 18-month old male Fischer 344 rats (weight, 380 to 450 g; NIH) were used in this study. Rats were anesthetized with pentobarbital (45 mg/kg, i.p.). The right femoral artery was catheterized for continuous blood pressure monitoring and blood sampling. Blood was obtained from the catheter for analysis of blood pH, PaO2, PaCO2, hematocrit, and blood glucose. Core temperature was maintained at 37°C with use of a feedback-controlled heating pad. Rats were positioned in a stereotactic frame (Kopf Instruments), and a cranial burr hole (1 mm) was drilled on the right coronal suture 3.5 mm lateral to the midline. A 26-gauge needle was inserted stereotactically into the right basal ganglia (coordinates: 0.2 mm anterior, 5.5 mm ventral, 3.5 mm lateral to the bregma). Autologous whole blood (100 µL) was injected at a rate of 10 µL/minute using a microinfusion pump. Sham controls had only an intracerebral needle insertion. After injection, the needle was removed, the burr hole was filled with bone wax, and the skin incision was closed with sutures.
Experimental Groups
A DFX concentration of 50 mg/kg was selected for this study. There were three sets of experiments. In the first set, DFX or vehicle (n=12) was injected intramuscularly 2, 4, 12, or 18 hours (n=9 for each) after ICH. A second injection was performed 4 hours after the first injection, and then DFX or vehicle was administrated every 12 hours for 3 days. Rats were sacrificed 3 days after ICH, and brain edema was measured.
In the second set, rats were treated with DFX 2 and 6 hours after ICH and then every 12 hours for either 2, 5, 7 or 14 days (n=9 for each) or treated with vehicle (n=12). Sham rats were treated with DFX (n=3) or vehicle (n=3) for 14 days. T2-weighted magnetic resonance images were taken at 2 months after ICH, after which the rats were sacrificed for brain atrophy assessment. Behavioral tests were undertaken pre-surgery and at 1, 28 and 56 days after ICH. Body weight and mean arterial blood pressure were measured at 1, 3, 7, 14, 21, 28, 42 and 56 days after surgery.
In the last set of experiments, rats were treated with DFX starting at 2, 4, 12, 24 or 48 hours (n=9 for each) after ICH or given vehicle (n=12), and then a second injection was given after a further 4 hours, followed by an injection every 12 hours for 7 days. Brain atrophy, behavioral and physiological assessments were undertaken as in the second set of experiments.
Body weight changes were calculated as: % change in body weight = (body weight at each time point-initial body weight)/initial body weight. Mean arterial blood pressure was measured using tail cuff plethysmography (IITC Life Science) 12.
Brain Water and Ion Contents
Animals were re-anesthetized, the brain removed, and a coronal tissue slice (3-mm thick) 4 mm from the frontal pole was cut. Five tissue samples from each brain were obtained: the ipsi- and contralateral cortex, the ipsi- and contralateral basal ganglia, and the cerebellum. Brain samples were immediately weighted to obtain the wet weight. Brain samples were dried at 100°C for 24 hours to obtain the dry weight and water content calculated as: (wet weight–dry weight)/wet weight. The dehydrated samples were digested in 1 ml of 1 mol/L nitric acid for 1 week. Sodium and potassium contents of this solution were measured by flame photometry. Sodium and potassium ion contents were expressed in microequivalents per gram of dehydrated brain tissue (mEq/kg dry wt).13
Behavioral Tests
ICH-induced neurological deficits were assessed using forelimb placing and corner turn tests.14 Both forelimb placing and corner turn tests are sensorimotor behavioral tests that are sensitive to injury in sensorimotor cortex and basal ganglia. In the vibrissae-elicited forelimb placing test, independent testing of each forelimb was conducted by brushing the respective vibrissae on the edge of a table top once per trial for 10 trials. A score of 1 was given each time the rat placed its forelimb onto the edge of the table in response to vibrissae stimulation. The percentage of successful placing responses was determined. A higher score equates to better function.
For the corner turn test, the rat was allowed to proceed into a corner for which the angle was 30°. To exit the corner, the animal could turn to either the left or the right, and the direction was recorded. This task was repeated 10 to 15 times, and the percentage of right turns calculated. In control animals, the score is ~50% and impairment leads to a greater % of right turns.
Magnetic Resonance Imaging (MRI)
MRIs were performed at 2 months after ICH. Axial T2-weighted images were acquired 12. Five MRI slices, from the slice showing the front tip of lateral ventricle to 5 mm posterior, were scanned on a computer. The bilateral ventricles were outlined for area measurement using Image J. Ventricle volume was obtained by combining the five ventricle areas and multiplying by the thickness (1 mm) of the sections. All measurements were repeated three times and the mean value was used. Ipsilateral ventricle volume was expressed as a percentage of contralateral.
Brain Atrophy Measurement
Rat brains were harvested for histology 8 weeks after ICH 12. Brain atrophy was assessed morphometrically. Coronal sections from 1 mm posterior to the blood injection site were stained with hematoxylin and eosin (H&E) and scanned. The caudate in each hemisphere was outlined on a computer and caudate size measured using Image J as described in the MRI section. To minimize the influence of tissue shrinkage on atrophy measurement, the ipsilateral caudate was expressed as a percentage of the contralateral caudate.
Statistical Analysis
All data in this study are presented as means ± SD. Data were analyzed with Student’s t-test or one-way analysis of variance (ANOVA). Differences were considered significant at p<0.05.
Results
Physiological Variables
All physiological variables were measured immediately before an intracerebral infusion. Mean arterial blood pressure (MABP), blood pH, PaO2, PaCO2, hematocrit, and blood glucose level were within normal ranges (Table 1).
Table 1.
Physiological variables
| MABP (mmHg) |
pH | pO2 (mmHg) |
pCO2 (mmHg) |
Hct (%) |
Glucose (mg/dl) |
|
|---|---|---|---|---|---|---|
| Experiment Set 1 | ||||||
| ICH + Vehicle | 93.9±15.3 | 7.40±0.05 | 80.4±3.9 | 41.9±3.8 | 46.0±5.7 | 100.8±10.6 |
| ICH + DFX 50 mg/kg 2h-delay | 101.2±10.8 | 7.40±0.05 | 81.0±3.5 | 43.0±3.0 | 47.6±5.5 | 110.1±15.3 |
| ICH + DFX 50 mg/kg 4h-delay | 87.7±17.0 | 7.38±0.08 | 80.5±6.6 | 44.7±5.2 | 42.0±6.1 | 99.0±13.5 |
| ICH + DFX 50 mg/kg 12h-delay | 96.4±15.2 | 7.38±0.07 | 78.8±6.1 | 43.3±4.6 | 45.4±3.8 | 112.6±15.2 |
| ICH + DFX 50 mg/kg 18h-delay | 102.4±9.8 | 7.41±0.09 | 81.4±5.0 | 42.9±4.0 | 42.2±4.9 | 107.4±17.0 |
| Experiment Set 2 | ||||||
| ICH + Vehicle | 89.9±12.5 | 7.41±0.05 | 81.4±4.9 | 42.0±3.9 | 45.2±5.0 | 110.8±10.1 |
| ICH + DFX 50 mg/kg for 2 d | 98.3±13.3 | 7.41±0.08 | 79.5±6.5 | 42.8±6.0 | 43.3±6.1 | 113.4±12.2 |
| ICH + DFX 50 mg/kg for 5 d | 101.7±12.9 | 7.40±0.08 | 80.9±5.8 | 41.5±5.6 | 42.1±6.3 | 109.0±8.5 |
| ICH + DFX 50 mg/kg for 7 d | 106.4±8.2 | 7.38±0.10 | 80.8±4.3 | 41.4±4.8 | 43.8±5.2 | 112.4±12.2 |
| ICH + DFX 50 mg/kg for 14 d | 102.2±10.8 | 7.39±0.07 | 81.4±6.1 | 44.8±5.1 | 41.2±5.9 | 110.9±14.7 |
| Sham + Vehicle for 14 d | 99.7±12.4 | 7.41±0.05 | 80.9±4.7 | 41.4±4.4 | 43.9±4.2 | 109.2±7.5 |
| Sham + DFX 50 mg/kg for 14 d | 99.6±10.0 | 7.42±0.08 | 81.6±3.5 | 42.6±3.1 | 45.5±3.8 | 110.4±9.2 |
| Experiment Set 3 | ||||||
| ICH + Vehicle | 101.0±8.8 | 7.40±0.07 | 82.0±6.0 | 41.1±2.8 | 45.1±6.8 | 101.4±12.3 |
| ICH + DFX 50 mg/kg 2h-delay | 103.4±12.0 | 7.42±0.10 | 81.4±5.5 | 43.8±5.2 | 41.8±6.0 | 111.2±8.4 |
| ICH + DFX 50 mg/kg 4h-delay | 107.6±9.0 | 7.40±0.03 | 79.9±6.0 | 41.6±4.5 | 44.6±4.4 | 103.0±10.5 |
| ICH + DFX 50 mg/kg 12h-delay | 96.4±15.6 | 7.41±0.05 | 79.1±7.1 | 42.3±7.6 | 44.1±3.0 | 112.2±9.2 |
| ICH + DFX 50 mg/kg 24h-delay | 99.4±14.8 | 7.41±0.06 | 81.4±3.6 | 41.1±4.5 | 44.1±5.1 | 98.4±17.7 |
| ICH + DFX 50 mg/kg 48h-delay | 100.8±11.5 | 7.42±0.08 | 80.8±4.9 | 41.0±3.0 | 42.2±5.2 | 100.2±11.5 |
Values are expressed as the means ± SD.
Mortality
Mortality rate after ICH was low in the aged rats. A total of 6 rats (3 vehicle-treated and 3 DFX-treated) died during the experiments, including one in the first set of experiments, two in the second set and three in the third set.
DFX Therapeutic Time Window for Brain Edema Determination
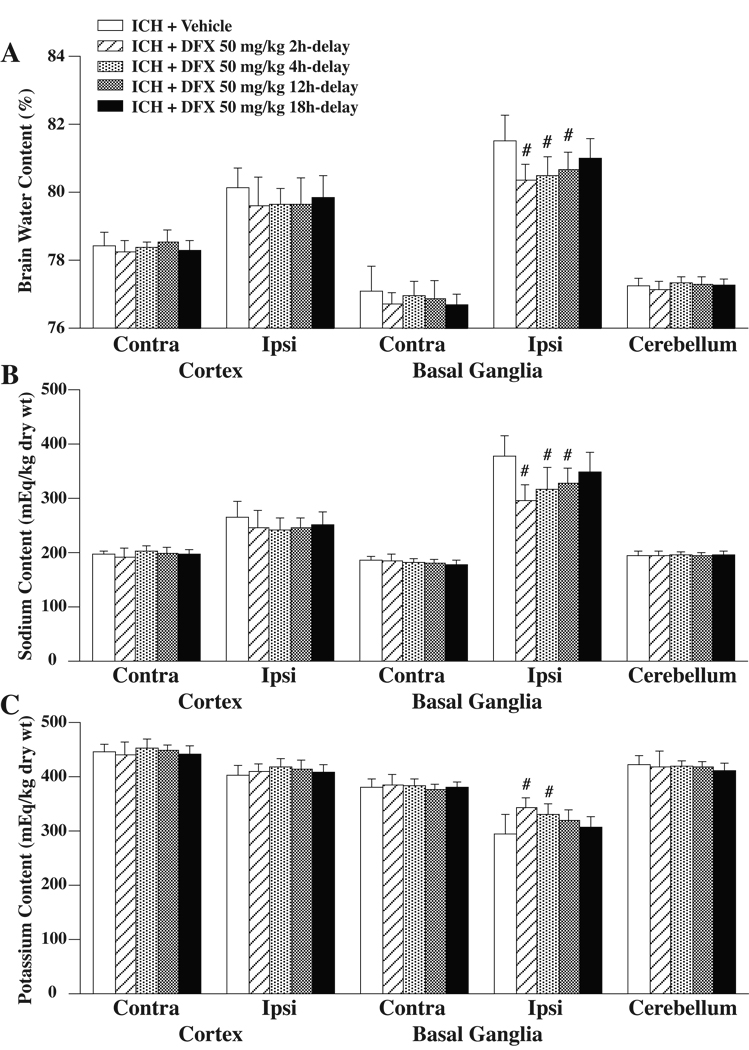
The effect of DFX treatment on brain edema formation was assessed 3 days after ICH in the aged rats. When DFX 50 mg/kg administration was begun 2, 4 or 12 hours after ICH, perihematomal brain edema was significantly reduced compared to vehicle-treated rats (80.4±0.5%, 80.5±0.5%, 80.7±0.5% vs. 81.5±0.8%, p<0.01, respectively, Figure 1A). DFX treatment with an 18 hours delay, however, did not result in a significant brain water content reduction (81.0±0.6% vs. 81.5±0.8% in the vehicle-treated group, Figure 1A). The amelioration in ICH-induced edema formation with DFX was associated with reduced accumulation of sodium and reduced loss of potassium in the ipsilateral basal ganglia (Figure 1B and C).
Figure 1.
Brain water (A), sodium (B) and potassium (C) content at 3 days after ICH. Values are expressed as the means ± SD. Contra=contralateral, Ipsi=ipsilateral. #p<0.01 vs. ICH+Vehicle group.
Optimal Therapeutic Duration of DFX
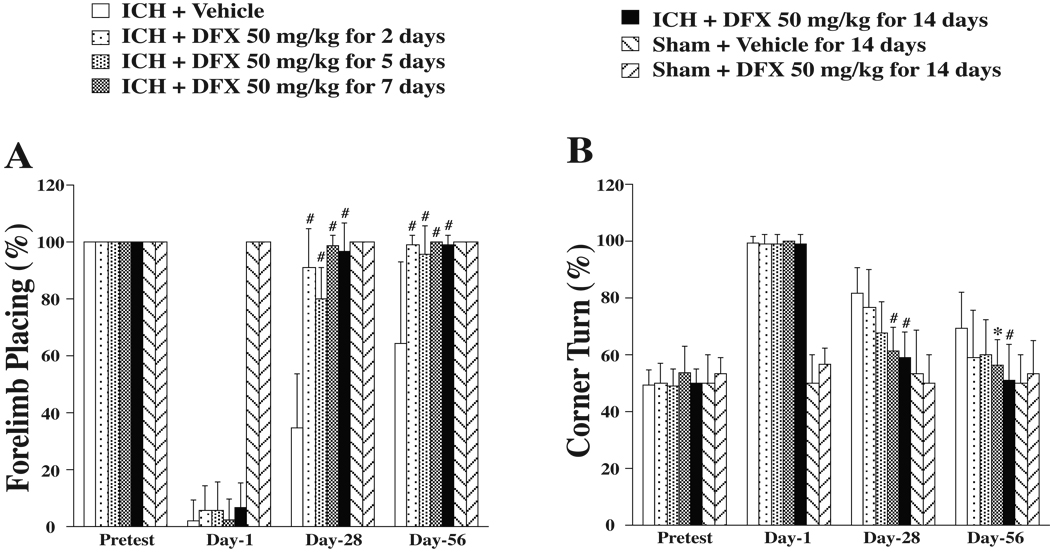
To examine potential optimal durations of DFX therapy, behavior and brain atrophy were assessed after ICH. Systemic administration of DFX lasted for 2, 5, 7 or 14 days. All DFX-treated ICH rats, regardless of therapeutic durations, had reduced forelimb placing deficits at days 28 and 56 compared to vehicle-treated rats (p<0.01, Figure 2A). DFX treatment for 7 days or 14 days reduced corner turn deficits. However, DFX treatment for only 2 days or 5 days failed to reduce corner turn deficits significantly (Figure 2B).
Figure 2.
Forelimb placing (A) and corner turn (B) scores prior to ICH, and 1, 28, 56 days after ICH. Values are expressed as the means ± SD. *p<0.05, #p<0.01 vs. ICH+Vehicle group. For forelimb placing, 100%=no deficit, 0%=maximal deficit. For corner turn, 50%=no deficit, 100%=maximal deficit.
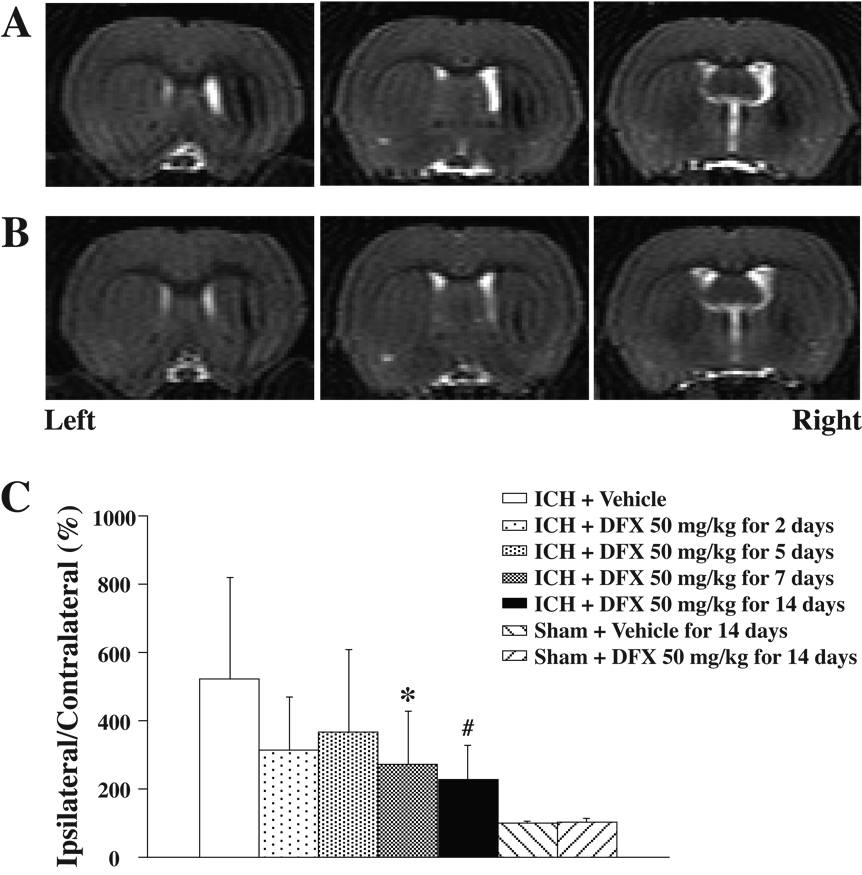
To estimate brain atrophy, we measured ventricle enlargement and caudate tissue loss. T2-weighted MR imaging was carried out at 2 months after ICH. MRI showed marked enlargement of the ipsilateral ventricle after ICH in vehicle-treated rats (Figure 3A). When DFX treatment lasted for 7 days or more, ipsilateral ventricle enlargement was significantly reduced compared to the vehicle-treated group (p<0.05, Figure 3B and 3C). Atrophy of the ipsilateral compared to contralateral caudate was found in coronal brain sections stained with H&E at 2 months after ICH in vehicle-treated rats (Figure 4A and 4C). DFX treatment for 7 or 14 days significantly reduced the ipsilateral caudate atrophy compared to vehicle treatment (p<0.01, Figure 4B and 4C). DFX treatment for 2 days or 5 days failed to reduce brain atrophy significantly, although there was a tendency for less brain tissue loss in the DFX-treated animals (Figure 3C and 4C).
Figure 3.
A and B: T2-weighted magnetic resonance images at eight weeks after ICH treated with vehicle (A) or DFX 50 mg/kg starting 4 hours after ICH for 7 days (B); C: Bar graph showing ventricle volume expressed as a percentage of the contralateral side. Values are expressed as the means±SD. *p<0.05, #p<0.01 vs. ICH+Vehicle group.
Figure 4.
A and B: Coronal gross H&E sections eight weeks after ICH treated with vehicle (A) or DFX 50 mg/kg starting 4 hours after ICH for 7 days (B); C: Bar graph showing caudate size expressed as a percentage of the contralateral side. Values are expressed as the means±SD. *p<0.05, #p<0.01 vs. ICH+Vehicle group.
Blood pressure and body weight were monitored during the experiment to observe whether DFX causes adverse reactions. Blood pressure did not change significantly after ICH induction with or without DFX treatment (group mean values from 108 to 114 mmHg). In all treatment groups, ICH induction and sham surgery caused a significant reduction in body weight (peaking at 7–14 days with a ~10–12% loss in vehicle-treated rats). When DFX treatment was continued for 14 days (but not 7 days) after ICH, body weight loss was slightly greater than in vehicle-treated rats at day 14 and 21 (−12.8±1.0 vs. −9.2±2.4%, p<0.01 and −9.2±1.7 vs. −7.1±2.5%, p<0.05). There was no effect of 14 days DFX treatment in sham-operated rats. Taken together with the functional and tissue pathological data described above, these data suggest that 7 days may represent a beneficial duration for DFX in this model without body weight side effects.
DFX Therapeutic Time Window for Brain Atrophy and Functional Outcome
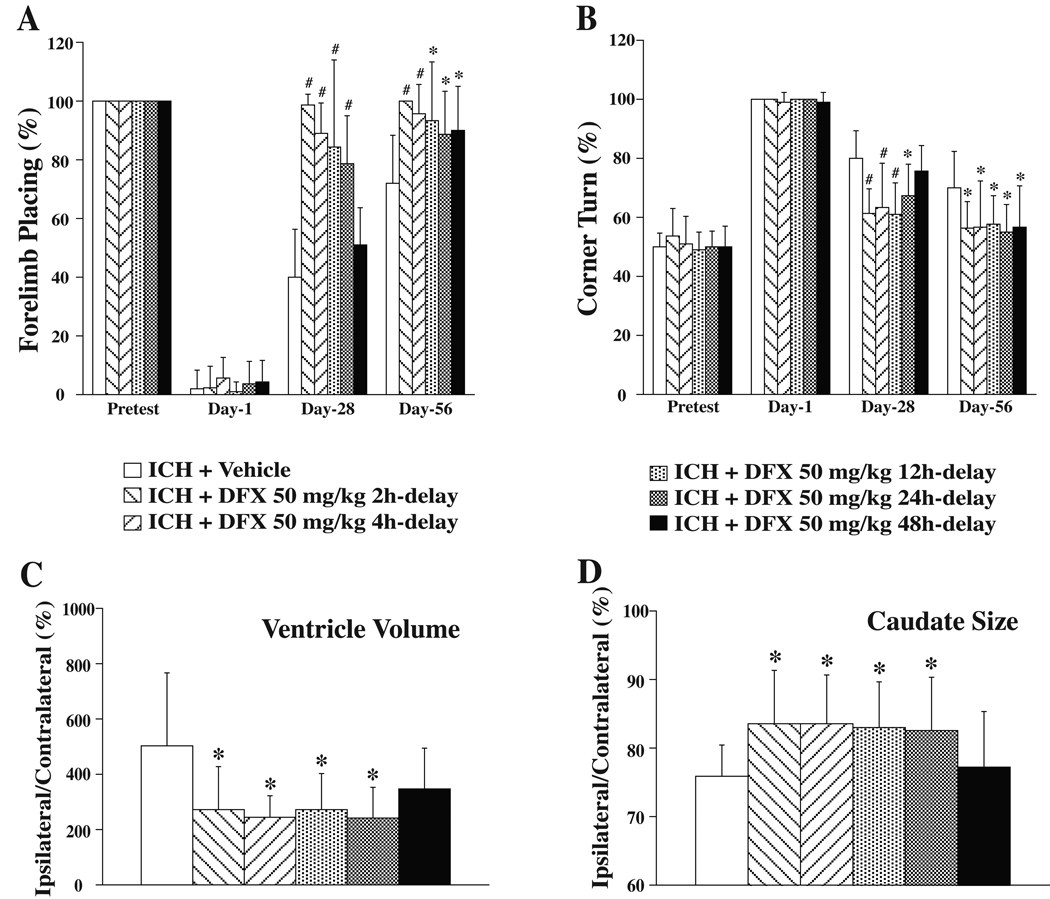
Rats were treated with DFX for 7 days starting at 2, 4, 12, 24 or 48 hours after ICH to determine potentially beneficial DFX therapeutic time windows for long-term outcome, including brain atrophy and behavior. When DFX administration was begun within 24 hours, DFX improved both of forelimb placing and corner turn scores significantly, compared to those in the vehicle-treated rats when tested at day 28 and day 56. A 48 hours delayed treatment, however, reduced neurological deficits at day 56 but failed to improve the behavioral score at day 28 (Figure 5A and 5B). DFX treatment, when delayed within 24 hours after ICH, also reduced the ipsilateral ventricle enlargement (e.g. 244±78% in ICH + DFX 50 mg/kg 4h-delay group vs. 503±264% in ICH + vehicle group, p<0.05, Figure 5C), and reduced caudate tissue loss (e.g. 83.5±7.2% in ICH + DFX 50 mg/kg 4h-delay group vs. 75.9±4.6% in ICH + vehicle group, p<0.05, Figure 5D).
Figure 5.
DFX therapeutic time window for brain atrophy and functional outcome. A: Forelimb placing test; B: Corner turn test; C: Ventricle volume expressed as a percentage of the contralateral side at eight weeks after ICH; D: Caudate size expressed as a percentage of the contralateral side at eight weeks after ICH. Values are expressed as the means±SD. *p<0.05, #p<0.01 vs. ICH+Vehicle group.
None of the 7 day DFX treatment paradigms affected body weight or blood pressure.
Correlations Between Brain Atrophy and Behavioral Scores
We examined whether there was a correlation between brain atrophy and behavioral scores. There was a weak correlation, with increased deficits with greater caudate atrophy (forelimb placing: R=0.305, n=96, p<0.01; corner turn: R=0.254, n=96; p<0.05).
Discussion
There are several important findings in this study. First, the therapeutic window of DFX for acute perihematomal brain edema formation is about 12 hours. Second, the DFX treatment window for long-term brain atrophy is about 24 hours. Third, the optimal therapeutic duration is about 7 days.
Our previous study showed that DFX can reduce ICH-induced brain edema, neurological deficits and brain atrophy in an aged rat model, and that the optimal dose of DFX is higher than 10 mg/kg12. The present study used 50 mg/kg to determine the therapeutic time window and optimal therapeutic duration for DFX. We chose 50 mg/kg dose for this study because DFX doses of 50 mg/kg and 100 mg/kg produce same protection 12. The intervals between doses (12 hours after loading doses) was based on the half-life of DFX, which is ~3 hours after intravenous infusion 15.
We determined the therapeutic time window for DFX in aged male rats. For brain edema measurement, deferoxamine treatment started at 2, 4, 12 or 18 hours after ICH. Acute brain edema was measured at day 3 after ICH. Brain edema develops within minutes after an ICH, peaking several days later 16, 17. Edema development after ICH can elevate intracranial pressure and cause herniation and death 18. In experimental models, perihematomal edema peaks around the third or fourth day after the hemorrhage, then declines slowly 13, 19, 20. Evidence suggests that iron has an important role in perihematomal edema formation. For example, iron accumulation occurs in the perihematomal zone within 24 hours following ICH and intracerebral injection of iron results in brain edema 4, 21. DFX can attenuate brain edema in the perihematomal zone in both young and aged rats 8, 12. In the present study, the therapeutic time window of DFX for acute edema was 12 hours. This result is supported by one of our previous studies. In that study, DFX treatment, when begun within 6 hours, attenuates perihematomal brain edema 3 days after ICH, but 24 hours delayed treatment failed to reduce brain edema in young rats 8.
To avoid potential side effects and to reduce the cost of treatment, we examined the optimal duration for DFX; i.e., when DFX treatment can be stopped without affecting efficacy. Although our initial experiments indicated the time course of iron release from the hematoma 4, this may not directly correlate with the timing of iron toxicity. Thus, with time, there is an upregulation in endogenous iron chelators after ICH which may limit iron toxicity and limit the need for DFX for extended time points. On the other hand, however, the delayed DFX-inhibitable tissue atrophy suggests that DFX should be maintained for a long duration. In these experiments, therefore, we compared DFX efficacy when administered for 2, 5, 7 and 14 days. We evaluated brain atrophy at 2 month since brain atrophy after ICH develops gradually and peaks between 1 and 2 months in young rats 9. We found that systemic treatment of 50 mg/kg DFX (begun 2 hours after onset) lasting for 7 days or 14 days significantly reduced ICH-induced brain atrophy, and improved behavioral outcomes after ICH. We propose, at least in this animal model, 7 days as the optimal treatment duration because DFX treatment for 14 days causes significant body weight drop in aged rats.
We hypothesized that the therapeutic time window for brain atrophy reduction might be longer than that for reducing brain edema. Brain atrophy with prolonged neurological deficits was associated with iron accumulation in brain tissue. For brain atrophy measurement, DFX therapy was initiated at 2, 4, 12, 24 or 48 hours after ICH. Our current data showed that DFX (50 mg/kg dosage for 7 days), when begun within 24 hours after ICH induction, attenuated neurological deficits and brain atrophy. However, DFX treatment with a 48 hour delay failed to reduce brain damage. These results suggest the therapeutic time window for brain atrophy and functional outcome is 24 hours in aged rats. It should be noted that it is dangerous to impute ICH therapeutic time windows from cerebral ischemia studies. The mechanisms involved are very different (e.g., there is no ischemia in our ICH animal models; 3, 22). Thus, it is possible, and our results would indicate, that treatment for ICH might be given at time points much later than would be efficacious in ischemic stroke. The difficulty in getting stroke patients to the hospital soon after ictus means that it is important to know whether DFX has a long therapeutic time window.
Our previous study demonstrated that forelimb placing correlates with brain edema formation during the acute phase of ICH. In this study, we found a weak correlation between behavioral deficits and delayed brain atrophy. DFX reduces brain edema, brain atrophy and behavioral deficits and we recently we found that DFX can reduce neuronal death in a rat model of ICH10. The causal interplay between these different elements of ICH-induced brain injury is still uncertain (e.g. does neuronal death lead to brain edema or does brain edema exacerbate neuronal death). The time window for DFX treatment of neurological deficits and brain atrophy was longer than that for treating brain edema, but this might reflect the sensitivity of our assays.
Although it might be expected that increasing dose would increase protection, it should be noted that this is not the case for all drugs 23 and that DFX at high concentrations is not without side effects. DFX may cause hypersensitivity reactions, systemic allergic reactions and some serious adverse events including significant hypotension and marked body weight loss. Thus, as well as examining whether DFX reduces ICH-induced brain injury, we examined the incidence of side effects, including body weight loss and hypotension. Our results demonstrated that no dose of DFX used in this study caused hypotension, but prolonged DFX treatment can cause body weight loss. Although the causes of DFX-induced body weight loss are still unknown, DFX has side effects on digestive system including abdominal discomfort and nausea.
In summary, systemic administration of DFX reduced ICH-induced brain edema, neurological deficits and brain atrophy in aged rats. Optimal durations and therapeutic windows might reasonably be estimated from these data. Potential therapeutic windows appear to be substantially longer than has been typical of preclinical studies of neuroprotection interventions after cerebral ischemia24. A phase I clinical trial called “Safety and Tolerability of Deferoxamine in Acute Cerebral Hemorrhage” is ongoing. The information generated in this paper may be useful for that, and future, DFX trials.
Acknowledgment
This study was supported by grants NS-052510, NS-017760, NS-039866, NS-047245, and NS-057539 from the National Institutes of Health (NIH) and 0755717Z and 0840016N from American Heart Association (AHA). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH and AHA.
References
- 1.Qureshi AI, Mendelow AD, Hanley DF. Intracerebral haemorrhage. Lancet. 2009;373:1632–1644. doi: 10.1016/S0140-6736(09)60371-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Broderick JP, Brott T, Tomsick T, Miller R, Huster G. Intracerebral hemorrhage more than twice as common as subarachnoid hemorrhage. J Neurosurg. 1993;78:188–191. doi: 10.3171/jns.1993.78.2.0188. [DOI] [PubMed] [Google Scholar]
- 3.Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral hemorrhage. Lancet Neurol. 2006;5:53–63. doi: 10.1016/S1474-4422(05)70283-0. [DOI] [PubMed] [Google Scholar]
- 4.Wu J, Hua Y, Keep RF, Nakamura T, Hoff JT, Xi G. Iron and iron-handling proteins in the brain after intracerebral hemorrhage. Stroke. 2003;34:2964–2969. doi: 10.1161/01.STR.0000103140.52838.45. [DOI] [PubMed] [Google Scholar]
- 5.Lou M, Lieb K, Selim M. The relationship between hematoma iron content and perihematoma edema: An mri study. Cerebrovasc Dis. 2009;27:266–271. doi: 10.1159/000199464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Selim M. Deferoxamine mesylate: A new hope for intracerebral hemorrhage: From bench to clinical trials. Stroke. 2009;40:S90–S91. doi: 10.1161/STROKEAHA.108.533125. [DOI] [PubMed] [Google Scholar]
- 7.Hua Y, Keep RF, Hoff JT, Xi G. Deferoxamine therapy for intracerebral hemorrhage. Acta Neurochirurgica - Supplement. 2008;105:3–6. doi: 10.1007/978-3-211-09469-3_1. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura T, Keep R, Hua Y, Schallert T, Hoff J, Xi G. Deferoxamine-induced attenuation of brain edema and neurological deficits in a rat model of intracerebral hemorrhage. J Neurosurg. 2004;100:672–678. doi: 10.3171/jns.2004.100.4.0672. [DOI] [PubMed] [Google Scholar]
- 9.Hua Y, Nakamura T, Keep RF, Wu J, Schallert T, Hoff JT, Xi G. Long-term effects of experimental intracerebral hemorrhage: The role of iron. J Neurosurg. 2006;104:305–312. doi: 10.3171/jns.2006.104.2.305. [DOI] [PubMed] [Google Scholar]
- 10.Song S, Hua Y, Keep RF, Hoff JT, Xi G. A new hippocampal model for examining intracerebral hemorrhage-related neuronal death: Effects of deferoxamine on hemoglobin-induced neuronal death. Stroke. 2007;38:2861–2863. doi: 10.1161/STROKEAHA.107.488015. [DOI] [PubMed] [Google Scholar]
- 11.Gu Y, Hua Y, Keep RF, Morgenstern LB, Xi G. Deferoxamine reduces intracerebral hematoma-induced iron accumulation and neuronal death in piglets. Stroke. 2009;40:2241–2243. doi: 10.1161/STROKEAHA.108.539536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okauchi M, Hua Y, Keep RF, Morgenstern LB, Xi G. Effects of deferoxamine on intracerebral hemorrhage-induced brain injury in aged rats. Stroke. 2009;40:1858–1863. doi: 10.1161/STROKEAHA.108.535765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xi G, Keep RF, Hoff JT. Erythrocytes and delayed brain edema formation following intracerebral hemorrhage in rats. J Neurosurg. 1998;89:991–996. doi: 10.3171/jns.1998.89.6.0991. [DOI] [PubMed] [Google Scholar]
- 14.Hua Y, Schallert T, Keep RF, Wu J, Hoff JT, Xi G. Behavioral tests after intracerebral hemorrhage in the rat. Stroke. 2002;33:2478–2484. doi: 10.1161/01.str.0000032302.91894.0f. [DOI] [PubMed] [Google Scholar]
- 15.Porter JB. Deferoxamine pharmacokinetics. Semin Hematol. 2001;38 suppl 1:63–68. doi: 10.1016/s0037-1963(01)90061-7. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki J, Ebina T. Sequential changes in tissue surrounding ICH. In: Pia HW, Longmaid C, Zierski J, editors. Spontaneous intracerebral hematomas. Berlin: Springer; 1980. pp. 121–128. [Google Scholar]
- 17.Wagner KR, Xi G, Hua Y, Kleinholz M, de Courten-Myers GM, Myers RE, Broderick JP, Brott TG. Lobar intracerebral hemorrhage model in pigs: Rapid edema development in perihematomal white matter. Stroke. 1996;27:490–497. doi: 10.1161/01.str.27.3.490. [DOI] [PubMed] [Google Scholar]
- 18.Ropper AH. Lateral displacement of the brain and level of consciousness in patients with an acute hemispheral mass. New England Journal of Medicine. 1986;314:953–958. doi: 10.1056/NEJM198604103141504. [DOI] [PubMed] [Google Scholar]
- 19.Enzmann DR, Britt RH, Lyons BE, Buxton JL, Wilson DA. Natural history of experimental intracerebral hemorrhage: Sonography, computed tomography and neuropathology. Ajnr: Am J Neuroradiol. 1981;2:517–526. [PMC free article] [PubMed] [Google Scholar]
- 20.Tomita H, Ito U, Ohno K, Hirakawa K. Chronological changes in brain edema induced by experimental intracerebral hematoma in cats. Acta Neurochir - Suppl. 1994;60:558–560. doi: 10.1007/978-3-7091-9334-1_154. [DOI] [PubMed] [Google Scholar]
- 21.Huang F, Xi G, Keep RF, Hua Y, Nemoianu A, Hoff JT. Brain edema after experimental intracerebral hemorrhage: Role of hemoglobin degradation products. J Neurosurg. 2002;96:287–293. doi: 10.3171/jns.2002.96.2.0287. [DOI] [PubMed] [Google Scholar]
- 22.Wagner KR, Xi G, Hua Y, Kleinholz M, de Courten-Myers GM, Myers RE. Early metabolic alterations in edematous perihematomal brain regions following experimental intracerebral hemorrhage. J Neurosurg. 1998;88:1058–1065. doi: 10.3171/jns.1998.88.6.1058. [DOI] [PubMed] [Google Scholar]
- 23.Du C, Hu R, Hsu CY, Choi D. Dextrorphan reduces infarct volume, vascular injury, and brain edema after ischemic brain injury. J Neurotrauma. 1996;13:215–222. doi: 10.1089/neu.1996.13.215. [DOI] [PubMed] [Google Scholar]
- 24.Ginsberg MD. Neuroprotection for ischemic stroke: Past, present and future. Neuropharmacology. 2008;55:363–389. doi: 10.1016/j.neuropharm.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]