Abstract
Using a one-trial procedure, preweanling rats exhibit robust sensitization regardless of whether drug pretreatment and testing occur in the same or different environments. The purpose of the present study was to determine whether one-trial context-specific and context-independent sensitization of preweanling rats could be dissociated by varying the pretreatment dose of cocaine, by varying the pretreatment drug, or by minimizing interoceptive cues. In Experiments 1a and 1b, rats were pretreated with a broad dose range of cocaine (0–40 mg/kg) before placement in a novel activity chamber or the home cage. In Experiment 2, rats were pretreated with a locomotor-enhancing drug (e.g., methylphenidate, U50,488, or MK-801) before placement in a novel activity or anesthesia chamber. In Experiment 3, rats were anesthetized with isoflurane prior to cocaine administration in order to minimize the effects of interoceptive and injection cues. In all experiments, rats were challenged with cocaine on the test day (24 hr later), with locomotion being measured in activity chambers. Results showed that: (a) the pretreatment dose of cocaine (10–40 mg/kg) did not differentially affect context-specific and context-independent sensitization; (b) cross-sensitization between methylphenidate and cocaine was observed in the context-specific condition, but not when using a context-independent procedure; and (c) sensitization was evident if injection and interoceptive cues were minimized. One possibility is that associative processes do not modulate the one-trial sensitization of preweanling rats. Alternatively, “unitization” may cause preweanling rats to treat the different environments as equivalent, thus permitting robust sensitization even when drug pretreatment and testing occur in different environments.
Keywords: behavioral sensitization, cocaine, isoflurane, environmental context, ontogeny
Behavioral sensitization occurs when rats repeatedly exposed to a psychostimulant drug (e.g., cocaine or amphetamine) show an augmented behavioral response after a challenge injection with the same drug (Kalivas & Stewart, 1991; Robinson & Becker, 1986). In this circumstance, adult rats will exhibit a sensitized response for several months after final psychostimulant exposure (Leith & Kuczenski, 1982; Paulson, Camp, & Robinson, 1991). Cross-sensitization between different compounds is also possible, because adult rats given repeated injections of formaldehyde, toluene, morphine, heroin, amphetamine, methamphetamine, GBR12909, or methylphenidate show an augmented locomotor response when challenged with cocaine (Baldo & Kelley, 1991; Beyer, Stafford, LeSage, Glowa, & Steketee, 2001; Bonate, Swann, & Silverman, 1997; Cador, Bjijou, & Stinus, 1995; Kazahaya, Akimoto, & Otsuki, 1989; Leri, Flores, Rajabi, & Stewart, 2003; Lett, 1989; Smith, Greene-Naples, Felder, Iordanou, Lyle, & Walker, 2009; Sorg, Willis, See, Hopkins, & Westberg, 1998; Torres-Reverón & Dow-Edwards, 2005).
When using a standard multi-trial behavioral sensitization paradigm (i.e., psychostimulant pretreatment occurs across multiple days), adult rats exhibit a more robust sensitized response when drug pretreatment and testing occur in the same previously novel environment (Badiani, Camp, & Robinson, 1997; Carey & Gui, 1998; Post, Lockfeld, Squillace, & Contel, 1981; Tirelli & Terry, 1998). Even so, sensitized responding has often been observed even when the psychostimulant is never associated with the testing environment (Battisti, Uretsky, & Wallace, 2000; Partridge & Schenk, 1999; Vezina & Stewart, 1990). Rather than representing two qualitatively different types of behavioral sensitization (context-specific vs context-independent), it seems more likely that sensitization is mediated by a common set of neural mechanisms that are capable of being modulated by associative learning processes (Anagnostaras & Robinson, 1996; Anagnostaras, Schallert, & Robinson, 2002). The relative impact of nonassociative and associative processes can be manipulated in a variety of ways. For example, nonassociative processes can be strengthened by repeatedly administering high doses of a psychostimulant across multiple days (Browman, Badiani, & Robinson, 1998a, b; Pert, Post, & Weiss, 1990).
Adult animals are also capable of exhibiting behavioral sensitization after only a single pretreatment administration of a psychostimulant drug, but in this experimental paradigm environmental conditioning factors gain in importance (see Pert et al., 1990; White, Joshi, Koeltzow, & Hu, 1998). For example, adult rats and mice pretreated with cocaine or amphetamine in a novel environmental context showed a sensitized response when subsequently challenged with a psychostimulant in the same environment (Battisti et al., 2000; Fontana, Post, Weiss, & Pert, 1993; Jackson & Nutt, 1993; McDougall, Reichel, Cyr, Karper, Nazarian, & Crawford, 2005). In contrast, behavioral sensitization was not evident if drug pretreatment and testing occurred in distinctly different environments or if drug pretreatment occurred in the home cage (Battisti, Chang, Uretsky, & Wallace, 1999; Battisti, Uretsky, & Wallace, 1999; McDougall, Baella, Stuebner, Halladay, & Crawford, 2007; McDougall, Cortez, et al., 2009; Weiss, Post, Pert, Woodward, & Murman, 1989). Therefore, it appears that adult rats will only exhibit context-specific behavioral sensitization, but not context-independent sensitization, when tested after a single pretreatment injection of cocaine or amphetamine.
Preweanling rats exhibit a very different pattern of responding when tested using the one-trial sensitization paradigm. Specifically, rats pretreated with cocaine on postnatal day (PD) 19 and tested on PD 20 showed robust context-independent behavioral sensitization in various experimental situations. For example, context-independent sensitization was evident on the test day (PD 20) if: (1) rats were exposed to the testing chamber on PD 19 and injected with cocaine 30 min after being returned to the home cage; (2) rats were pretreated with cocaine in a novel chamber on PD 19 and then given a challenge injection of cocaine in a different novel chamber on the test day; or (3) rats were injected with cocaine and restricted to the home cage on PD 19 (McDougall et al., 2007; McDougall, Charntikov, Cortez, Amodeo, Martinez, & Crawford, 2009; McDougall, Cortez, et al., 2009). In these situations, preweanling rats exhibited a sensitized response that was similar to rats pretreated and tested with cocaine in the same environment. Although this pattern of results was not initially anticipated, the most parsimonious conclusion is that environmental conditioning factors are unnecessary for one-trial behavioral sensitization during the preweanling period.
The overall purpose of the present study was to identify some of the critical determinants underlying the one-trial context-independent behavioral sensitization of preweanling rats. Because drug dose is an important factor determining whether adult rats exhibit context-independent sensitization (Browman et al., 1998a, b), we examined whether the context-specific and context-independent sensitization of preweanling rats could be dissociated by varying the pretreatment dose of cocaine. In Experiment 1a, context-independent sensitization was examined by administering cocaine (10–40 mg/kg) 30 min after rats were returned to the home cage from the novel activity chamber; whereas, in Experiment 1b, context-independent sensitization was assessed by injecting rats with cocaine (10–40 mg/kg) and restricting them to their home cage on the pretreatment day. In the second experiment, we tested whether simply elevating locomotor activity through pharmacological means (i.e., by administering methylphenidate, MK-801, or U50,488 on the pretreatment day) would result in context-specific or context-independent cross-sensitization to cocaine. The purpose of the third experiment was to determine whether interoceptive and injection cues are necessary for the development of context-independent behavioral sensitization in preweanling rats. To this end, some rats were briefly anesthetized with isoflurane prior to cocaine injection on the pretreatment day (i.e., to eliminate injection cues), while other rats were maintained in an anesthetized state until being returned to the home cage (i.e., to minimize interoceptive cues).
General Method
Subjects
Subjects were 240 male and female rats of Sprague-Dawley descent (Charles River, Hollister, CA) that were born and bred at California State University, San Bernardino (CSUSB). Litters were culled to 10 pups at three days of age. Preweanling rats were kept with the dam and littermates throughout behavioral testing and were housed in large polycarbonate maternity cages (56 × 34 × 22 cm) with wire lids and Tek-Fresh® bedding (Harlan, Indianapolis, IN). Food and water were freely available. The colony room was maintained at 22–24°C and kept under a 12-hr light–dark cycle, with behavioral testing occurring during the light phase of the cycle. All procedures were approved by the Institutional Animal Care and Use Committee of CSUSB.
Apparatus
Behavioral testing was done in commercially available (Coulbourn Instruments, Allentown, PA) activity monitoring chambers (25.5 × 25.5 × 41 cm, L × W × H), consisting of acrylic walls, a plastic floor, and an open top. Each chamber included an X–Y photobeam array, with 16 photocells and detectors, that was used to determine distance traveled (horizontal locomotor activity). Photobeam resolution was 0.76 cm, with the position of each rat being determined every 100 ms. Some experiments also used small animal anesthesia chambers (model: PY8 50-0108, Harvard Apparatus, Holliston, MA) made of clear Plexiglas with a sliding lid (23.5 × 10 × 10 cm, L × W × H). The activity monitoring chambers and anesthesia chambers were located in different rooms.
Drugs
(−)-Cocaine hydrochloride, methylphenidate hydrochloride, (+)-MK-801 hydrogen maleate, and (±)-trans-U50,488 methanesulfonate were purchased from Sigma (St. Louis, MO). All drugs were dissolved in saline and injected intraperitoneally (ip) at a volume of 5 ml/kg. Cocaine was administered to preweanling rats at doses ranging from 10 to 40 mg/kg. In comparison, one-trial behavioral sensitization experiments using adult rats most frequently employ a 40 mg/kg dose of cocaine on the pretreatment day (Fontana et al., 1993; Jackson & Nutt, 1993; Weiss et al., 1989).
Statistics
For each experiment, omnibus repeated measures analyses of variance (ANOVAs) were used for the statistical analysis of distance traveled data. Significant higher-order interactions were further analyzed using one- or two-way ANOVAs. Post hoc analysis of behavioral data was made using Tukey tests (p < .05).
In all experiments, an equal number of male and female preweanling rats were assigned to each group. Unlike adults, prepubescent rats do not typically exhibit sex differences after treatment with dopamine agonists or other locomotor activating compounds (see also Bowman, Blatt, & Kuhn, 1997; Duke, Meier, Bolanos, Crawford, & McDougall, 1997; Frantz & Van Hartesveldt, 1999; McDougall et al., 2007; Scalzo & Holson, 1992; Snyder, Katovic, & Spear, 1998). Preliminary analyses indicated that distance traveled data did not differ according to sex, so this variable was not included in subsequent analyses. Litter effects were controlled through both experimental design and statistical procedures. In most circumstances no more than one subject per litter was assigned to a particular group. In cases where this procedure was not possible (e.g., analysis of the pretreatment day), a single litter mean was calculated from multiple littermates assigned to the same group (Holson & Pearce, 1992; Zorrilla, 1997). With only one exception (described below), litter was used as the unit of analysis for statistical purposes (Zorrilla, 1997). With this statistical model each litter, rather than each rat, is treated as an independent observation (i.e., a within analysis using one value/condition/litter). In order to compare the results of Experiments 1a and 1b, a between analysis was used (a 3 × 4 between-subjects ANOVA) because all groups were not represented within each litter.
Experiments 1a and 1b
Although adult rats do not exhibit context-independent behavioral sensitization when using the one-trial procedure (Fontana et al. 1993; Jackson & Nutt, 1993; McDougall et al., 2007; McDougall, Cortez, et al., 2009; Weiss et al., 1989), they are capable of showing context-independent sensitization when given repeated daily treatments with a psychostimulant (Battisti et al., 2000; Partridge & Schenk, 1999; Vezina & Stewart, 1990). In the latter circumstance, sensitization is more readily observed if adult rats are pretreated with a high dose of cocaine or amphetamine (Browman et al., 1998a, b). In the same manner, it is possible that the one-trial behavioral sensitization of preweanling rats is dependent on drug dose, with context-independent sensitization requiring a high dose of cocaine on the pretreatment day. In the first experiment, context-independent sensitization was assessed in two ways: by administering cocaine (10–40 mg/kg) 30 min after rats were returned to the home cage from the novel activity chamber (Experiment 1a) or by injecting rats with cocaine (10–40 mg/kg) and restricting them to their home cage on the pretreatment day (Experiment 1b). For comparison purposes, context-specific sensitization was assessed by pretreating rats with cocaine in a novel activity chamber (Experiment 1a).
Method
In Experiment 1a, eight litters of male and female rats (N = 72) were randomly assigned to one of nine pretreatment conditions on PD 19. Rats in the cocaine-activity groups were taken to the test room and injected with cocaine (10, 20, 30, or 40 mg/kg, ip) before being placed in activity chambers. Distance traveled was measured for 30 min. These rats were returned to the home cage and injected with saline 30 min later. Rats in the cocaine-home groups were injected with saline before being placed in the activity chambers and injected with cocaine (10, 20, 30, or 40 mg/kg, ip) 30 min after being returned to the home cage. The saline control group received saline in both the activity chamber and home cage. In all cases, “home” refers to the normal maternity cage that includes both the dam and littermates.
After 24 hr (i.e., on PD 20), all rats received a challenge injection of 20 mg/kg cocaine to determine the occurrence of behavioral sensitization. After drug administration, rats were placed in activity chambers where distance traveled was measured for 60 min. Distance traveled data from the pretreatment day were analyzed using a 5 × 6 (Drug Dose × Time Block) ANOVA. Test day data were initially analyzed using an omnibus 9 × 12 (Group × Time Block) ANOVA, while 3 × 12 (Condition × Time Block) ANOVAs were used to assess the effects of the condition variable at each dose of cocaine.
In Experiment 1b, an additional eight litters of male and female rats (N = 40) were used to determine the effects of cocaine on the sensitized responding of rats restricted to the home cage (i.e., the home-restricted groups). On PD 19, rats were injected with cocaine (0, 10, 20, 30, or 40 mg/kg, ip) in the home cage followed, 60 min later, by an injection of saline in the home cage (half of the rats received the saline injection first followed by the cocaine injection). None of these rats were removed from the colony room on the pretreatment day. After 24 hr, all rats were taken to the test room, injected with cocaine (20 mg/kg, ip), and immediately placed in activity chambers for 60 min. Distance traveled data from the test day were analyzed using a 5 × 12 (Drug Dose × Time Block) ANOVA.
Results and Discussion
Activity chamber groups (Experiment 1a)
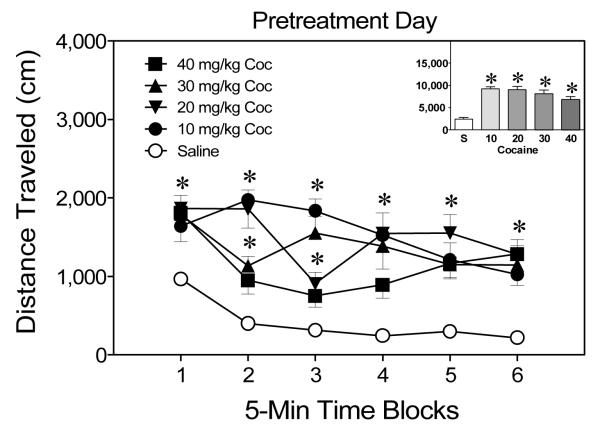
On the pretreatment day (i.e., PD 19), rats injected with cocaine (10–40 mg/kg) exhibited greater distance traveled scores than saline controls, Drug Dose main effect, F(4, 28) = 20.88, p < .05, and Tukey tests, p < .05. This effect varied across the 30-min session (see Figure 1) because rats given 40 mg/kg cocaine only differed significantly from the saline group on time blocks 1, 5, and 6, Drug Dose × Time Block interaction, F(20, 140) = 4.23, p < .05, and Tukey tests, p < .05. Rats injected with the lower doses of cocaine (10, 20, or 30 mg/kg) had greater distance traveled scores than saline controls on time blocks 1–6, Tukey tests, p < .05.
Figure 1.
Mean distance traveled scores (+SEM) of preweanling rats injected with cocaine (0, 10, 20, 30, or 40 mg/kg, ip) and placed in activity chambers on the pretreatment day (i.e., PD 19). The inset shows mean distance traveled collapsed across time blocks 1–6. *Significantly different from the saline group (p < .05).
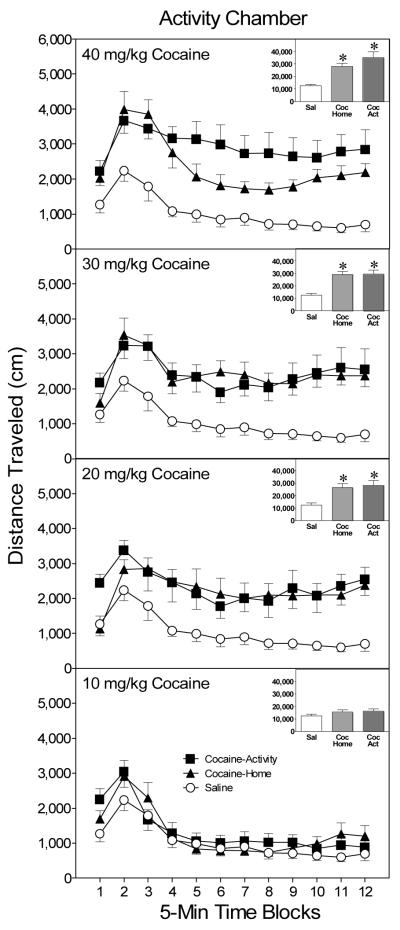
On the test day (i.e., PD 20), an omnibus ANOVA showed that distance traveled scores varied significantly according to treatment group and time block, Group main effect, F(8, 56) = 8.68, p < .05 and Time Block main effect, F(11, 77) = 11.85, p < .05, respectively. In the latter case, distance traveled scores increased across the first few time blocks and then gradually declined as the testing session progressed. Separate analysis of the 40 mg/kg groups showed that rats in the cocaine-activity and cocaine-home groups had greater distance traveled scores than rats in the saline control group, Condition main effect, F(2, 14) = 14.01, p < .05, and Tukey tests, p < .05 (see upper graph, Figure 2). Although a trend was apparent, the cocaine-activity and cocaine-home groups treated with 40 mg/kg cocaine did not differ significantly from each other. Context-independent sensitization was also apparent if preweanling rats were pretreated with lower doses of cocaine (see middle graphs, Figure 2). More specifically, rats exhibited elevated distance traveled scores on the test day if they had been pretreated with 20 or 30 mg/kg cocaine in the activity chambers (i.e., the cocaine-activity groups) or 30 min after being returned to the home cage (i.e., the cocaine-home groups), Condition main effects, F(2, 14) = 18.04, p < .05, F(2, 14) = 10.81, p < .05, respectively, and Tukey tests, p < .05]. Pretreating rats with 10 mg/kg cocaine in either the test chamber or home cage did not induce sensitized responding on the test day (see lower graph, Figure 2).
Figure 2.
Mean distance traveled scores (+SEM) of preweanling rats (n = 8 per group) given a challenge injection of cocaine (20 mg/kg, ip) prior to placement in activity chambers on PD 20. Rats in the cocaine-activity groups (filled squares) had been pretreated with cocaine (10–40 mg/kg, ip) before being placed in the activity chamber on PD 19, while rats in the cocaine-home groups (filled triangles) had been injected with cocaine 30 min after being returned to the home cage. The saline group (open circles) was injected with saline at both time points. The inset shows mean distance traveled collapsed across time blocks 1–12. *Significantly different from the saline group (p < .05).
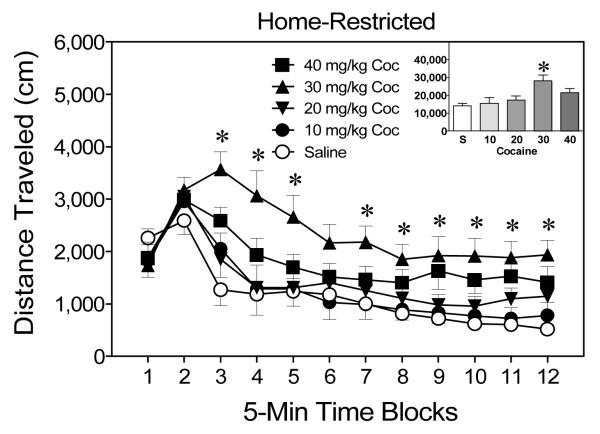
Home-restricted groups (Experiment 1b)
When assessed on the test day, rats pretreated with 30 mg/kg cocaine in the home cage exhibited significantly greater distance traveled scores than rats pretreated with 0 mg/kg cocaine, Drug Dose main effect, F(4, 28) = 5.60, p < .05, and Tukey tests, p < .05 (see Figure 3). Rats pretreated with lower (10 or 20 mg/kg) or higher (40 mg/kg) doses of cocaine did not differ from controls. The effects of the pretreatment variable differed across the testing session, because rats pretreated with 30 mg/kg cocaine had greater distance traveled scores than the 0 mg/kg group on time blocks 3–5 and 7–12, Condition × Time Block interaction, F(44, 308) = 2.35, p < .05, and Tukey tests, p < .05.
Figure 3.
Mean distance traveled scores (+SEM) of preweanling rats (n = 8 per group) given a challenge injection of cocaine (20 mg/kg, ip) prior to placement in activity chambers on PD 20. Rats were pretreated with cocaine (0–40 mg/kg, ip) while being maintained in the home cage on PD 19. The inset shows mean distance traveled collapsed across time blocks 1–12. *Significantly different from the 0 mg/kg group (p < .05).
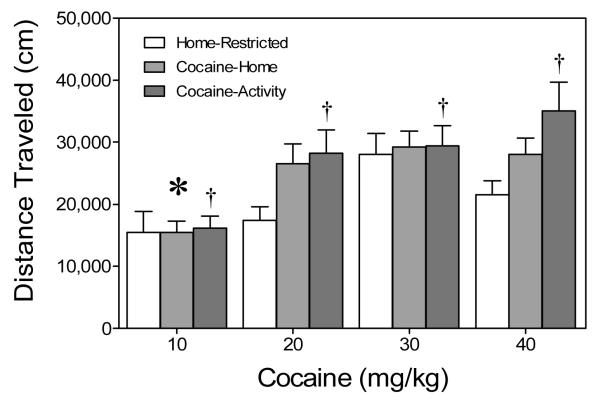
Comparison of activity chamber and home-restricted groups
Mean distance traveled scores of activity chamber groups (Experiment 1a) and home-restricted groups (Experiment 1b) are shown in Figure 4. A 3 × 4 between-subjects ANOVA indicated that distance traveled scores varied according to dose, with the 10 mg/kg cocaine group exhibiting less locomotor activity than groups pretreated with higher doses of cocaine (20, 30, or 40 mg/kg), Drug Dose main effect, F(3, 84) = 12.14, p < .001, and Tukey tests, p < .05. Overall, rats in the cocaine-activity groups (dark gray bars) had greater distance traveled scores than rats in the home-restricted groups (open bars), with the cocaine-home groups (light gray bars) being intermediate between the other groups, Condition main effect, F(2, 84) = 4.90, p < .01, and Tukey tests, p < .05. The Condition × Drug Dose interaction was nonsignificant.
Figure 4.
Mean total distance traveled scores (+SEM) of preweanling rats (n = 8 per group) given a challenge injection of cocaine (20 mg/kg, ip) prior to placement in activity chambers on PD 20. These are the same rats as described in Figures 2 and 3. The cocaine-activity groups (dark gray bars) were pretreated with cocaine (10–40 mg/kg, ip) before being placed in the activity chamber on PD 19, while the cocaine-home groups (light gray bars) were injected with cocaine 30 min after being returned to the home cage. The home-restricted groups (open bars) were pretreated with cocaine (10–40 mg/kg, ip) while being maintained in the home cage on PD 19. *Significantly different from the other cocaine groups (Drug Dose main effect, p < .05). †Significantly different from the home-restricted condition (Condition main effect, p < .05).
Discussion
In Experiment 1a, rats pretreated with 20–40 mg/kg cocaine in the novel activity chambers showed a sensitized locomotor response when challenged with cocaine on PD 20. The same pattern of results was apparent if rats were pretreated with cocaine (20–40 mg/kg) 30 min after they were returned to the home cage. When considered together, these two sets of findings indicate that the context-independent sensitization of preweanling rats is not dependent on a high dose of cocaine. In Experiment 1b, context-independent sensitization was also evident when cocaine-pretreated rat were maintained in the home cage on the pretreatment day, but the sensitized responding appeared weaker than if rats were exposed to the activity chambers on PD 19.
Experiment 2
The purpose of Experiment 2 was to determine whether pretreating rats with a compound other than cocaine would differentially affect the expression of context-specific and context-independent behavioral sensitization. MK-801 (an NMDA antagonist), U50,488 (a kappa opioid agonist), and methylphenidate (a psychostimulant) were used because all three drugs typically induce substantial locomotor activity in preweanling rats (Duke, Meier, et al., 1997; Frantz & Van Hartesveldt, 1999; Jackson & Kitchen, 1989; McDougall, Collins, Karper, Watson, & Crawford, 1999) and are capable of inducing a sensitized locomotor response (Collins, Zavala, Ingersoll, Duke, Crawford, & McDougall, 1998; Duke, O’Neal, & McDougall, 1997; McDougall et al., 1999). On PD 19, rats were pretreated with MK-801, U50,488, methylphenidate, or cocaine prior to placement in activity chambers or small, enclosed anesthesia chambers. On PD 20, all rats were challenged with 20 mg/kg cocaine and distance traveled scores were measured in the activity chambers. Thus, context-independent sensitization (or cross-sensitization) was tested in a different manner than in Experiment 1 (i.e., context-independent sensitization was previously assessed by pretreating rats with cocaine in the home cage rather than in a separate environment). The reasons for using different procedures to assess context-independent sensitization were twofold: (a) to better determine the generality of context-independent sensitization in preweanling rats and (b) design constraints involving Experiment 3 required that context-independent sensitization (or the lack thereof) be established using a separate, novel environment (i.e., anesthesia chambers).
Method
On PD 19, eight litters of male and female rats (N = 80) were randomly assigned to one of ten pretreatment conditions. Specifically, preweanling rats were injected with a test compound or saline and then immediately placed in activity or anesthesia chambers for 30 min (no anesthesia was administered). The test compounds were MK-801 (0.3 mg/kg), U50,488 (5 mg/kg), methylphenidate (10 mg/kg), or cocaine (30 mg/kg). After 24 hr, all rats were challenged with 20 mg/kg cocaine and placed in activity chambers where distance traveled was measured for 60 min.
Distance traveled data from the pretreatment day were analyzed using a 5 × 6 (Drug × Time Block) ANOVA. Test day data were analyzed using separate 5 × 12 (Drug × Time Block) ANOVAs for each pretreatment environment.
Results and Discussion
Activity chamber groups
On the pretreatment day (i.e., PD 19), rats injected with MK-801 (M = 7,429 cm, SEM = ±1,096), methylphenidate (M = 10,547 cm, SEM = ±1,561), or cocaine (M = 6,487 cm, SEM = ±823) had significantly greater distance traveled scores than saline controls (M = 2,502 cm, SEM = ±324), Drug main effect, F(4, 28) = 11.61, p < .05, and Tukey tests, p < .05. Unexpectedly, U50,488 (M = 3,660 cm, SEM = ±545) did not enhance the locomotor activity of preweanling rats when compared to the saline group.
On the test day (i.e., PD 20), rats pretreated with methylphenidate (10 mg/kg) or cocaine (30 mg/kg) showed a sensitized locomotor response when challenged with 20 mg/kg cocaine (see upper graph, Figure 5). Specifically, when collapsed across the test session the distance traveled scores of the methylphenidate- and cocaine-pretreated rats were significantly greater than the saline controls, Drug main effect, F(4, 28) = 10.48, p < .05, and Tukey tests, p < .05. Rats pretreated with MK-801 or U50,488 did not show a sensitized locomotor response after cocaine challenge, nor did the methylphenidate and cocaine groups differ from each other. Although distance traveled scores showed a general decline across the testing session, Time Block main effect, F(11, 77) = 19.08, p < .05, the drug variable did not interact with time to affect performance.
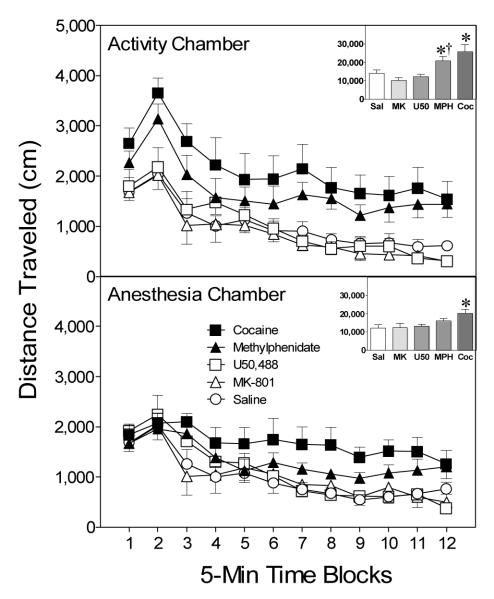
Figure 5.
Mean distance traveled scores (+SEM) of preweanling rats (n = 8 per group) given a challenge injection of cocaine (20 mg/kg, ip) prior to placement in activity chambers on PD 20. Rats had been pretreated with saline, MK-801 (0.3 mg/kg, ip), U50,488 (5 mg/kg, ip), methylphenidate (10 mg/kg, ip), or cocaine (30 mg/kg, ip) before being placed in activity chambers or anesthesia chambers on PD 19. The inset shows mean distance traveled collapsed across time blocks 1–12. *Significantly different from the saline group from the same chamber (p < .05). †Significantly different from the methylphenidate group from the anesthesia chamber (p < .05).
Anesthesia chamber groups
Rats pretreated with cocaine (30 mg/kg) in the anesthesia chambers exhibited significantly more locomotor activity than control rats on the test day, Drug main effect, F(4, 28) = 4.95, p < .05, and Tukey tests, p < .05 (see lower graph, Figure 5). In contrast, rats pretreated with MK-801, U50,488, or methylphenidate did not differ significantly from saline controls. Overall, distance traveled scores declined across the session, Time Block main effect, F(11, 77) = 14.71, p < .05.
Separate statistical analyses showed that rats pretreated with methylphenidate in the activity chambers had significantly greater distance traveled scores on the test day than rats given an injection of methylphenidate in the anesthesia chambers, Condition main effect, F(1, 7) = 5.67, p < .05 (compare the upper and lower graphs, Figure 5). None of the other compounds (cocaine, U50,488, or MK-801) induced differential amounts of locomotor activity in the two chambers.
Discussion
When drug pretreatment occurred in the activity chambers, a single exposure to cocaine or methylphenidate was sufficient to induce behavioral sensitization on the test day. In contrast, a sensitized locomotor response was only evident when cocaine, but not methylphenidate, was administered in the anesthesia chambers (see also McDougall, Cortez, et al., 2009). Simply enhancing locomotor activity on the pretreatment day was not sufficient to induce behavioral sensitization, because rats pretreated with MK-801 (in either the activity or anesthesia chambers) did not show a sensitized locomotor response when challenged with cocaine on the test day.
Experiment 3
Results from Experiments 1 and 2 suggest that environmental conditioning is unnecessary for the one-trial behavioral sensitization of preweanling rats. Instead, interoceptive and/or injection cues may be critical factors modulating the context-independent behavioral sensitization of preweanling rats (see Crombag, Badiani, & Robinson, 1996; Pert et al., 1990). According to this hypothesis, changes in the internal state of the rat or the injection procedure itself could serve as potent conditioned stimuli (CS) or occasion-setters necessary for behavioral sensitization. To test this idea, rats were pretreated with saline or cocaine in the same anesthesia chambers as described previously. Some of these rats were briefly anesthetized with isoflurane prior to the injection procedure; other rats were maintained in an anesthetized state until being returned to the home cage; while still other rats were never exposed to isoflurane. Behavioral sensitization was assessed one day later in the novel activity chambers.
Method
On PD 19, eight litters of male and female rats (N = 48) were randomly assigned to one of six groups. Rats in the extended isoflurane condition were placed in the anesthesia chambers and given isoflurane (a 5% concentration mixed with oxygen) prior to cocaine (30 mg/kg) or saline injections. These rats were then maintained under 1% isoflurane anesthesia for the entire pretreatment period and remained unresponsive until they were returned to the home cage. Rats in the brief isoflurane condition were placed in the anesthesia chambers and given isoflurane before being injected with cocaine or saline. Isoflurane administration was quickly discontinued and rats became responsive soon after the injection procedure was completed (approximately 5 min). Rats in the no-isoflurane condition were placed in the anesthesia chambers and injected with cocaine or saline, with no isoflurane being administered. In all conditions, rats were kept in the anesthesia chambers for 30 min and then returned to the home cage.
After 24 hr (i.e., on PD 20), all rats were challenged with 20 mg/kg cocaine and placed in activity chambers where distance traveled was measured for 60 min. Distance traveled data from the test day were analyzed using a 3 × 2 × 12 (Drug × Condition × Time Block) ANOVA.
Results and Discussion
Anesthesia chamber groups
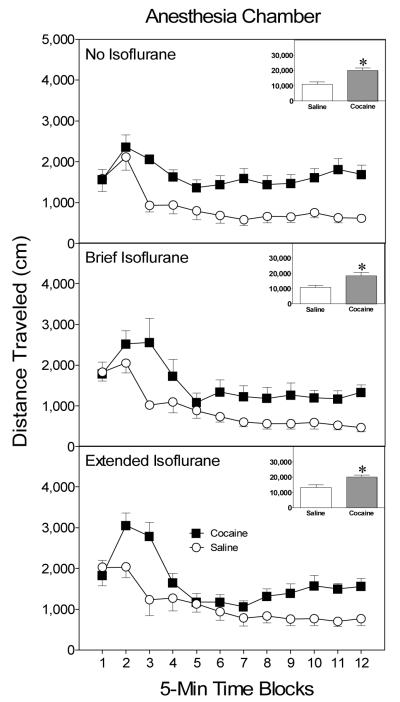
On the test day (i.e., PD 20), an omnibus 3 × 2 × 12 ANOVA indicated that there were significant effects for only time block, F(11, 77) = 16.02, p < .05, and drug. Regardless of anesthesia condition, cocaine-pretreated rats showed greater distance traveled scores on the test day than saline-pretreated rats, Drug main effect, F(1, 7) = 17.84, p < .05 (see Figure 6). To ensure that this overall main effect was truly representative, we performed separate 2 × 12 ANOVAs for each anesthesia condition. When no isoflurane was administered on the pretreatment day, rats previously exposed to cocaine had greater test day distance traveled scores than saline-pretreated rats, Drug main effect, F(1, 7) = 10.59, p < .05 (see upper graph, Figure 6). This finding replicated results from Experiment 2. Likewise, cocaine-pretreated rats from the brief isoflurane condition (i.e., rats were under isoflurane anesthesia during the injection procedure) had elevated distance traveled scores when compared to saline controls, Drug main effect, F(1, 7) = 8.51, p < .05 (see middle graph, Figure 6). Rats maintained under isoflurane anesthesia for the entire pretreatment session (i.e., from before the injection procedure until 30 min after cocaine or saline treatment) also exhibited behavioral sensitization (see lower graph, Figure 6), because cocaine-pretreated rats had significantly greater distance traveled scores on the test day than saline-pretreated controls, Drug main effect, F(1, 7) = 9.57, p < .05. In no case did the drug variable interact with time block to affect performance.
Figure 6.
Mean distance traveled scores (+SEM) of preweanling rats (n = 8 per group) given a challenge injection of cocaine (20 mg/kg, ip) prior to placement in activity chambers on PD 20. Rats in the various isoflurane conditions had been pretreated with saline or cocaine (30 mg/kg, ip) before being placed in the anesthesia chambers on PD 19. The inset shows mean distance traveled collapsed across time blocks 1–12. *Significantly different from the saline group (p < .05).
Discussion
Preweanling rats showed a sensitized locomotor response on the test day regardless of whether they had been anesthetized with isoflurane during the entire pretreatment session or only during the injection procedure. Thus, these results suggest that interoceptive and injection cues are not necessary for the one-trial context-independent behavioral sensitization of preweanling rats.
General Discussion
The ability of associative processes to modulate psychostimulant-induced behavioral sensitization appears to vary across ontogeny. Unlike adult rats, preweanling rats express robust context-independent behavioral sensitization if they previously received a single 30 mg/kg cocaine injection after return to the home cage (McDougall et al., 2007; McDougall, Charntikov, et al., 2009; McDougall, Cortez, et al., 2009). Some interpretive difficulties arise from using a high dose of psychostimulant, because repeatedly administering a high dose of amphetamine or cocaine in the home cage is sufficient to induce context-independent behavioral sensitization in adult rats, even when drug administration is unsignalled (Browman et al., 1998a, b). That being said, results from Experiment 1a show that the one-trial context-independent sensitization of preweanling rats is not uniquely dependent on a high dose of cocaine, because sensitized responding was apparent regardless of whether cocaine (20, 30, or 40 mg/kg) was administered in the activity chamber or 30 min after being returned to the home cage. A lower dose of cocaine (10 mg/kg) did not induce either context-specific or context-independent behavioral sensitization. When results from these developmental and nondevelopmental studies are considered together, it appears that the ability of preweanling rats to exhibit one-trial context-independent behavioral sensitization represents a true qualitative ontogenetic difference that is not dependent on drug dose.
Similar to a previous report (McDougall, Cortez, et al., 2009), results from Experiment 1b showed that cocaine-pretreated rats restricted to the home cage on PD 19 exhibited a sensitized locomotor response when challenged with cocaine on the test day (PD 20). Two features of these data are of special note: first, the strength of the sensitized response did not increase linearly according to dose. Specifically, only home-restricted rats injected with 30 mg/kg cocaine on the pretreatment day exhibited a sensitized response on the test day (see Figure 3). It is unclear why a greater dose of cocaine (40 mg/kg) did not induce a stronger or at least a statistically significant sensitized response, especially since there was no evidence that stereotypy was elevated in rats pretreated with 40 mg/kg cocaine (data not shown). Second, the sensitized responding of home-restricted rats was weaker than when rats were injected with saline in the novel chamber and cocaine in the home cage (the cocaine-home groups). This conclusion is based on the finding that the cocaine-home groups pretreated with 20, 30, or 40 mg/kg cocaine exhibited behavioral sensitization; whereas, rats in the home-restricted groups only showed a sensitized response when pretreated with 30 mg/kg cocaine. Pert et al. (1990) state that “conditioning would not be expected to occur in a paradigm in which animals were injected in their home environment (p. 215),” because the “entire stimulus complex” (i.e., the environmental context, injection procedure, transportation to the testing room, interoceptive cues, etc.) is less discriminable than when rats are conditioned in a novel environment. In the present study, transporting preweanling rats from the colony room and placing them in a novel chamber (regardless of whether it was the environment they were ultimately tested in) seemed to promote stronger behavioral sensitization. One possibility is that a more robust sensitized response was evident on the test day because additional salient cues were incorporated into the “entire stimulus complex” on the pretreatment day.
Alternatively, stress may have been responsible for the different patterns of responding exhibited by the cocaine-home and home-restricted groups. In adult rats, stress is known to both enhance responsivity to psychostimulants and induce behavioral sensitization (Anisman, Hahn, Hoffman, & Zacharko, 1985; de Jong, Wasilewski, van der Vegt, Buwalda, & Koolhaas, 2005; Prasad, Sorg, Ulibarri, & Kalivas, 1995). Similarly, rats postnatally exposed to repeated isolation stress show a sensitized response when challenged with a psychostimulant drug during early ontogeny (PD 10) or in adulthood (Kehoe, Shoemaker, Arons, Triano, & Suresh, 1998; Kehoe, Shoemaker, Triano, Callahan, & Rappolt, 1998; Kikusui, Faccidomo, & Miczek, 2005). In the present study, therefore, the stress due to extended removal (30 min) from the home cage, transport, and placement in a novel environment may have contributed to the strength of the sensitized response. Of course, this explanation assumes that stress interacted with cocaine to affect performance, otherwise stress-induced sensitization should have been observed in the saline-pretreated groups as well.
Although environmental context does not appear to determine whether one-trial behavioral sensitization will be expressed by preweanling rats, the importance of other cues (e.g., injection and interoceptive cues) had not been previously assessed in young rats. In adult rats, the “entire stimulus complex” impacts the induction and ultimate expression of behavioral sensitization. In an illustrative set of studies, a modest sensitized response was evident if adult rats were injected intraperitoneally with amphetamine in the home environment (Badiani, Anagnostaras, & Robinson, 1995; Badiani, Browman, & Robinson, 1995); however, behavioral sensitization did not occur if injection and handling cues were eliminated by administering amphetamine via an indwelling catheter (Crombag et al., 1996; Fraioli, Crombag, Badiani, & Robinson, 1999). Anesthetizing adult rats with pentobarbital or a ketamine/xylazine mixture prior to daily cocaine treatments also eliminated the expression of context-independent behavioral sensitization (Torres, Rivier, & Weiss, 1994), thus suggesting that interoceptive cues may be an important component of the stimulus complex modulating behavioral sensitization. In contrast, Wang and Hsiao (2003) reported that adult rats had a normal sensitized response on the test day even if they had been anesthetized with chloral hydrate prior to daily amphetamine administration. Various procedural differences could be responsible for these inconsistent results (e.g., the psychostimulant used, rat strain, number of pretreatment days, etc.), but of greatest relevance may be the unique actions of each anesthetic agent on neurotransmitter system functioning (e.g., ketamine is a noncompetitive NMDA receptor antagonist; for a discussion, see Torres et al., 1994).
In the present study, we attempted to minimize the impact of interoceptive and injection cues by anesthetizing preweanling rats with isoflurane during either the injection procedure (approximately 5 min) or for the duration of the pretreatment session (approximately 35 min). Consistent with the results reported by Wang and Hsiao (2003), preweanling rats exhibited a sensitized locomotor response on the test day (PD 20) even if anesthesia had been administered on the pretreatment day (PD 19). Thus, it appears that eliminating injection cues and reducing interoceptive cues (i.e., anesthesia was only administered for 35 min) does not abolish the context-independent behavioral sensitization of preweanling rats. Isoflurane was used in the present study because an inhalable anesthetic avoids the injection process entirely (i.e., ketamine, pentobarbital, and chloral hydrate are injectables) and it does not antagonize NMDA receptors. Isoflurane has its own limitations, however, because dopamine synthesis and release are altered after prolonged exposure (Adachi, Yamada, Satomoto, Higuchi, Watanabe, & Kazama, 2005). Even so, isoflurane has only minimal impact on cocaine-induced Fos expression in the caudate-putamen and nucleus accumbens of adult rats (Kufahl, Pentkowski, Heintzelman, & Neisewander, 2009).
In a further attempt to dissociate the one-trial context-specific and context-independent sensitization of preweanling rats, we pretreated rats with various locomotor-enhancing compounds (MK-801, U50,488, or methylphenidate) in activity or anesthesia chambers on PD 19. Interpretation of this experiment is somewhat limited because only one dose of each compound was tested (due to constraints caused by litter size). Nonetheless, cross-sensitization between cocaine and either MK-801 or U50,488 (both nonpsychostimulants) was not evident on PD 20, thus showing that merely elevating locomotor activity on the pretreatment day was not sufficient to induce an augmented locomotor response on the test day. Interestingly, methylphenidate (a psychostimulant) and cocaine cross-sensitized, but only in the context-specific situation. The ability of drugs to cross-sensitize is often interpreted to mean that a common neural substrate underlies the sensitization process (Aizenstein, Segal, & Kuczenski, 1990; Cadoni, Valentini, & Di Chiara, 2008). In the present circumstance, however, the lack of cross-sensitization in the context-independent situation should not be taken as evidence that the neural mechanisms mediating context-specific and context-independent sensitization are separate and discrete (see Anagnostaras & Robinson, 1996; Anagnostaras et al., 2002). Instead, it is possible that associative/perceptual processes might be responsible for the lack of cross-sensitization in the context-independent situation (this explanation is discussed below).
Interestingly, young and adult animals appear to perceive stimuli differently, with rats and humans showing an age-dependent decline in stimulus generalization across ontogeny (Chotro & Alonso, 1999; Gibson, 1969; Spear & McKenzie, 1994). For example, adult rats treat multiple CSs as discrete and often competitive events (Spear & McKenzie, 1994), whereas preweanling rats treat two distinguishable stimuli as if they were equivalent (i.e., components of a single event or object) as long as both stimuli were paired with the same US (Kraemer, Kraemer, Smoller, & Spear, 1989; Lariviere, Chen, & Spear, 1990; Molina, Hoffmann, Serwatka, & Spear, 1991; Spear, Kraemer, Molina, & Smoller, 1988). This process is referred to as “unitization” and may explain why preweanling rats showed context-independent sensitization to cocaine, yet did not exhibit context-independent cross-sensitization after methylphenidate pretreatment. In the former case, preweanling rats may have shown context-independent behavioral sensitization because the different environmental contexts (e.g., the activity chamber, anesthesia chamber, and home cage), although discriminable, were treated as equivalent units. In other words, the two environments where cocaine was experienced (e.g., the anesthesia chamber and the activity chamber) may have been organized as a single integrated event (i.e., components of a single CS or occasion-setter). If unitization occurred, rats would be expected to show a sensitized response regardless of the location where cocaine was initially administered.
Spear and colleagues have shown that the unitization process will not occur if separate CSs are paired with either qualitatively different unconditioned stimuli (US) or with a single US that differs in intensity (Molina et al., 1991; Spear et al., 1988). Assuming that preweanling rats did not treat cocaine and methylphenidate as a common US, unitization may explain the results from the cross-sensitization experiment. More specifically, if cocaine and methylphenidate were sufficiently discriminable (e.g., as a result of pharmacokinetic differences or differential activity at the serotonin transporter) then rats should have perceived the anesthesia and activity chambers as separate and isolated events. In this situation, cross-sensitization would not be evident because the pretreatment and testing chambers would be recognized as different environments. Although the potential relationship between unitization and one-trial sensitization is still speculative, it is possible that this phenomenon may explain why preweanling rats, but not adults, show context-independent behavioral sensitization when using the one-trial procedure.
In conclusion, the sensitized responding of rodents is characterized by a number of changes across ontogeny (for a review, see Tirelli, Laviola, & Adriani, 2003), not the least important of which is the ability of preweanling rats to show one-trial context-independent behavioral sensitization. An obvious possibility is that these various ontogenetic differences are due to the immaturity of neural systems underlying the nonassociative components of behavioral sensitization (e.g., the mesocorticolimbic dopamine system and glutamatergic systems). Available evidence suggests otherwise, however, because dopamine (D1 and D2) and glutamate (NMDA and nonNMDA) receptor levels, and other indices of dopaminergic and glutamatergic functioning (e.g., coupling with G proteins and adenylyl cyclase, etc.), have reached nearly adult values by PD 15 (Broaddus & Bennett, 1990; Gelbard, Teicher, Faedda, & Baldessarini, 1989; Insel, Miller, & Gelhard, 1990; Jung & Bennett, 1996; Miller, Johnson, Gelhard, & Insel, 1990; Nansen, Jokel, Lobo, Micevych, Ariano, & Levine, 2000; Rao, Molinoff, & Joyce, 1991; Sales, Martes, Bouthenet, & Schwartz, 1991). Moreover, NMDA receptor antagonists modulate the development of psychostimulant-induced sensitization in a similar manner in preweanling and adult rats (Duke, O’Neal, et al., 1997). Instead, ontogenetic differences in behavioral sensitization may be due to age-dependent changes in associative learning. An obvious possibility is that associative processes are incapable of modulating the neural (nonassociative) mechanisms responsible for mediating the one-trial behavioral sensitization of preweanling rats. Another possibility is that the associative/perceptual process of unitization, which is largely restricted to early development, allows preweanling rats to perceive the different cocaine-paired environments as an integrated event or object. If preweanling rats do, in fact, treat the different chambers as equivalent, then sensitized responding should occur independent of environmental context.
Acknowledgments
This research was supported by NIDA research grant DA027985 (SAM).
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/pha.
References
- Adachi YU, Yamada S, Satomoto M, Higuchi H, Watanabe K, Kazama T. Isoflurane anesthesia induces biphasic effect on dopamine release in the rat striatum. Brain Research Bulletin. 2005;67:176–181. doi: 10.1016/j.brainresbull.2005.06.020. doi: 10.1016/j.brainresbull.2005.06.020. [DOI] [PubMed] [Google Scholar]
- Aizenstein ML, Segal DS, Kuczenski R. Repeated amphetamine and fencamfamine: sensitization and reciprocal cross-sensitization. Neuropsychopharmacology. 1990;3:283–290. [PubMed] [Google Scholar]
- Anagnostaras SG, Robinson TE. Sensitization to the psychomotor stimulant effects of amphetamine: modulation by associative learning. Behavioral Neuroscience. 1996;110:1397–1414. doi: 10.1037//0735-7044.110.6.1397. doi: 10.1037/0735-7044.110.6.1397. [DOI] [PubMed] [Google Scholar]
- Anagnostaras SG, Schallert T, Robinson TE. Memory processes governing amphetamine-induced psychomotor sensitization. Neuropsychopharmacology. 2002;26:703–715. doi: 10.1016/S0893-133X(01)00402-X. doi: 10.1038/S0893-133X(01)00402-X. [DOI] [PubMed] [Google Scholar]
- Anisman H, Hahn B, Hoffman D, Zacharko RM. Stressor invoked exacerbation of amphetamine-elicited perseveration. Pharmacology, Biochemistry and Behavior. 1985;23:173–183. doi: 10.1016/0091-3057(85)90552-0. doi: 10.1016/0091-3057(85)90552-0. [DOI] [PubMed] [Google Scholar]
- Badiani A, Anagnostaras SG, Robinson TE. The development of sensitization to the psychomotor stimulant effects of amphetamine is enhanced in a novel environment. Psychopharmacology. 1995;117:443–452. doi: 10.1007/BF02246217. doi: 10.1007/BF02246217. [DOI] [PubMed] [Google Scholar]
- Badiani A, Browman KE, Robinson TE. Influence of novel versus home environments on sensitization to the psychomotor stimulant effects of cocaine and amphetamine. Brain Research. 1995;674:291–298. doi: 10.1016/0006-8993(95)00028-o. doi: 10.1016/0006-8993(95)00028-O. [DOI] [PubMed] [Google Scholar]
- Badiani A, Camp DM, Robinson TE. Enduring enhancement of amphetamine sensitization by drug-associated environmental stimuli. Journal of Pharmacology and Experimental Therapeutics. 1997;282:787–794. [PubMed] [Google Scholar]
- Baldo BA, Kelley AE. Cross-sensitization between cocaine and GBR 12909, a dopamine uptake inhibitor. Brain Research Bulletin. 1991;27:105–108. doi: 10.1016/0361-9230(91)90289-v. doi: 10.1016/0361-9230(91)90289-V. [DOI] [PubMed] [Google Scholar]
- Battisti JJ, Chang CH, Uretsky NJ, Wallace LJ. Sensitization of stereotyped behavior to amphetamine is context and response dependent. Pharmacology, Biochemistry and Behavior. 1999;63:263–269. doi: 10.1016/s0091-3057(98)00259-7. doi: 10.1016/S0091-3057(98)00259-7. [DOI] [PubMed] [Google Scholar]
- Battisti JJ, Uretsky NJ, Wallace LJ. Sensitization of apomorphine-induced stereotyped behavior in mice is context dependent. Psychopharmacology. 1999;146:42–48. doi: 10.1007/s002130051086. doi: 10.1007/s002130051086. [DOI] [PubMed] [Google Scholar]
- Battisti JJ, Uretsky NJ, Wallace LJ. Importance of environmental context in the development of amphetamine- or apomorphine-induced stereotyped behavior after single and multiple doses. Pharmacology, Biochemistry and Behavior. 2000;66:671–677. doi: 10.1016/s0091-3057(00)00214-8. doi: 10.1016/S0091-3057(00)00214-8. [DOI] [PubMed] [Google Scholar]
- Beyer CE, Stafford D, LeSage MG, Glowa JR, Steketee JD. Repeated exposure to inhaled toluene induces behavioral and neurochemical cross-sensitization to cocaine in rats. Psychopharmacology. 2001;154:198–204. doi: 10.1007/s002130000614. doi: 10.1007/s002130000614. [DOI] [PubMed] [Google Scholar]
- Bonate PL, Swann A, Silverman PB. Context-dependent cross-sensitization between cocaine and amphetamine. Life Sciences. 1997;60:PL1–7. doi: 10.1016/s0024-3205(96)00591-7. doi: 10.1016/S0024-3205(96)00591-7. [DOI] [PubMed] [Google Scholar]
- Bowman BP, Blatt B, Kuhn CM. Ontogeny of the behavioral response to dopamine agonists after chronic cocaine. Psychopharmacology. 1997;129:121–127. doi: 10.1007/s002130050171. doi: 10.1007/s002130050171. [DOI] [PubMed] [Google Scholar]
- Broaddus WC, Bennett JP., Jr. Postnatal development of striatal dopamine function. I. An examination of D1 and D2 receptors, adenylate cyclase regulation and presynaptic dopamine markers. Developmental Brain Research. 1990;52:265–271. doi: 10.1016/0165-3806(90)90244-s. doi: 10.1016/0165-3806(90)90244-S. [DOI] [PubMed] [Google Scholar]
- Browman KE, Badiani A, Robinson TE. The influence of environment on the induction of sensitization to the psychomotor activating effects of intravenous cocaine in rats is dose-dependent. Psychopharmacology. 1998a;137:90–98. doi: 10.1007/s002130050597. doi: 10.1007/s002130050597. [DOI] [PubMed] [Google Scholar]
- Browman KE, Badiani A, Robinson TE. Modulatory effect of environmental stimuli on the susceptibility to amphetamine sensitization: a dose-effect study in rats. Journal of Pharmacology and Experimental Therapeutics. 1998b;287:1007–1014. [PubMed] [Google Scholar]
- Cadoni C, Valentini V, Di Chiara G. Behavioral sensitization to delta Δ9-tetrahydrocannabinol and cross-sensitization with morphine: differential changes in accumbal shell and core dopamine transmission. Journal of Neurochemistry. 2008;106:1586–1593. doi: 10.1111/j.1471-4159.2008.05503.x. doi: 10.1111/j.1471-4159.2008.05503.x. [DOI] [PubMed] [Google Scholar]
- Cador M, Bjijou Y, Stinus L. Evidence of a complete independence of the neurobiological substrates for the induction and expression of behavioral sensitization to amphetamine. Neuroscience. 1995;65:385–395. doi: 10.1016/0306-4522(94)00524-9. doi: 10.1016/0306-4522(94)00524-9. [DOI] [PubMed] [Google Scholar]
- Carey RJ, Gui J. Cocaine conditioning and cocaine sensitization: what is the relationship? Behavioural Brain Research. 1998;92:67–76. doi: 10.1016/s0166-4328(97)00126-5. doi: 10.1016/S0166-4328(97)00126-5. [DOI] [PubMed] [Google Scholar]
- Chotro MG, Alonso G. Effects of stimulus preexposure on the generalization of conditioned taste aversions in infant rats. Developmental Psychobiology. 1999;35:304–317. doi: 10.1002/(sici)1098-2302(199912)35:4<304::aid-dev5>3.0.co;2-o. doi: 10.1002/(SICI)1098-2302(199912)35:4<304::AID-DEV5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Collins RL, Zavala AR, Ingersoll VY, Crawford CA, Duke MA, McDougall SA. Kappa opioid mediated behavioral sensitization in the rat: relationship to Fos immunoreactivity. Psychopharmacology. 1998;137:282–291. doi: 10.1007/s002130050621. doi: 10.1007/s002130050621. [DOI] [PubMed] [Google Scholar]
- Crombag HS, Badiani A, Robinson TE. Signalled versus unsignalled intravenous amphetamine: large differences in the acute psychomotor response and sensitization. Brain Research. 1996;722:227–231. doi: 10.1016/0006-8993(96)00066-2. doi: 10.1016/0006-8993(96)00066-2. [DOI] [PubMed] [Google Scholar]
- de Jong JG, Wasilewski M, van der Vegt BJ, Buwalda B, Koolhaas JM. A single social defeat induces short-lasting behavioral sensitization to amphetamine. Physiology & Behavior. 2005;83:805–811. doi: 10.1016/j.physbeh.2004.10.002. doi: 10.1016/j.physbeh.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Duke MA, Meier TL, Bolanos CA, Crawford CA, McDougall SA. Paradoxical effects of kappa opioid stimulation on the locomotor activity and Fos immunoreactivity of the preweanling rat: role of dopamine receptors. Behavioral Neuroscience. 1997;111:1114–1122. doi: 10.1037//0735-7044.111.5.1114. doi: 10.1037/0735-7044.111.5.1114. [DOI] [PubMed] [Google Scholar]
- Duke MA, O’Neal J, McDougall SA. Ontogeny of dopamine agonist-induced sensitization: role of NMDA receptors. Psychopharmacology. 1997;129:153–160. doi: 10.1007/s002130050175. doi: 10.1007/s002130050175. [DOI] [PubMed] [Google Scholar]
- Fontana D, Post RM, Weiss SRB, Pert A. The role of D1 and D2 dopamine receptors in the acquisition and expression of cocaine-induced conditioned increases in locomotor activity. Behavioural Pharmacology. 1993;4:375–387. [PubMed] [Google Scholar]
- Fraioli S, Crombag HS, Badiani A, Robinson TE. Susceptibility to amphetamine-induced locomotor sensitization is modulated by environmental stimuli. Neuropsychopharmacology. 1999;20:533–541. doi: 10.1016/S0893-133X(98)00079-7. doi: 10.1038/sj.npp.1395262. [DOI] [PubMed] [Google Scholar]
- Frantz K, Van Hartesveldt C. Locomotion elicited by MK801 in developing and adult rats: temporal, environmental, and gender effects. European Journal of Pharmacology. 1999;369:145–157. doi: 10.1016/s0014-2999(99)00070-9. doi: 10.1016/S0014-2999(99)00070-9. [DOI] [PubMed] [Google Scholar]
- Gelbard HA, Teicher MH, Faedda G, Baldessarini RJ. Postnatal development of dopamine D1 and D2 receptor sites in rat striatum. Developmental Brain Research. 1989;49:123–130. doi: 10.1016/0165-3806(89)90065-5. doi: 10.1016/0165-3806(89)90065-5. [DOI] [PubMed] [Google Scholar]
- Gibson EJ. Principles of perceptual learning and development. Appleton-Century-Crofts; New York: 1969. [Google Scholar]
- Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicology and Teratology. 1992;14:221–228. doi: 10.1016/0892-0362(92)90020-b. doi: 10.1016/0892-0362(92)90020-B. [DOI] [PubMed] [Google Scholar]
- Insel TR, Miller LP, Gelhard RE. The ontogeny of excitatory amino acid receptors in rat forebrain—I. N-methyl-D-aspartate and quisqualate receptors. Neuroscience. 1990;35:31–43. doi: 10.1016/0306-4522(90)90117-m. doi: 10.1016/0306-4522(90)90117-M. [DOI] [PubMed] [Google Scholar]
- Jackson HC, Kitchen I. Behavioural effects of selective μ-, κ-, and δ-opioid agonists in neonatal rats. Psychopharmacology. 1989;97:404–409. doi: 10.1007/BF00439459. doi: 10.1007/BF00439459. [DOI] [PubMed] [Google Scholar]
- Jackson HC, Nutt DJ. A single preexposure produces sensitization to the locomotor effects of cocaine in mice. Pharmacology, Biochemistry and Behavior. 1993;45:733–735. doi: 10.1016/0091-3057(93)90533-y. doi: 10.1016/0091-3057(93)90533-Y. [DOI] [PubMed] [Google Scholar]
- Jung AB, Bennett JP., Jr. Development of striatal dopaminergic function. I. Pre- and postnatal development of mRNAs and binding sites for striatal D1 (D1a) and D2 (D2a) receptors. Developmental Brain Research. 1996;94:109–120. doi: 10.1016/0165-3806(96)00033-8. doi: 10.1016/S0165-3806(96)80002-2. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Research Reviews. 1991;16:223–244. doi: 10.1016/0165-0173(91)90007-u. doi: 10.1016/0165-0173(91)90007-U. [DOI] [PubMed] [Google Scholar]
- Kazahaya Y, Akimoto K, Otsuki S. Subchronic methamphetamine treatment enhances methamphetamine- or cocaine-induced dopamine efflux in vivo. Biological Psychiatry. 1989;25:903–912. doi: 10.1016/0006-3223(89)90270-9. doi: 10.1016/0006-3223(89)90270-9. [DOI] [PubMed] [Google Scholar]
- Kehoe P, Shoemaker WJ, Arons C, Triano L, Suresh G. Repeated isolation stress in the neonatal rat: relation to brain dopamine systems in the 10-day-old rat. Behavioral Neuroscience. 1998;112:1466–1474. doi: 10.1037//0735-7044.112.6.1466. doi: 10.1037/0735-7044.112.6.1466. [DOI] [PubMed] [Google Scholar]
- Kehoe P, Shoemaker WJ, Triano L, Callahan M, Rappolt G. Adult rats stressed as neonates show exaggerated behavioral responses to both pharmacological and environmental challenges. Behavioral Neuroscience. 1998;112:116–125. doi: 10.1037//0735-7044.112.1.116. doi: 10.1037/0735-7044.112.1.116. [DOI] [PubMed] [Google Scholar]
- Kikusui T, Faccidomo S, Miczek KA. Repeated maternal separation: differences in cocaine-induced behavioral sensitization in adult male and female mice. Psychopharmacology. 2005;178:202–210. doi: 10.1007/s00213-004-1989-1. doi: 10.1007/s00213-004-1989-1. [DOI] [PubMed] [Google Scholar]
- Kraemer PJ, Kraemer EL, Smoller DE, Spear NE. Enhancement of flavor aversion conditioning in weanling but not adult rats by prior conditioning to an odor. Psychobiology. 1989;17:34–42. [Google Scholar]
- Kufahl PR, Pentkowski NS, Heintzelman K, Neisewander JL. Cocaine-induced Fos expression is detectable in the frontal cortex and striatum of rats under isoflurane but not α-chloralose anesthesia: implication for FMRI. Journal of Neuroscience Methods. 2009;181:241–248. doi: 10.1016/j.jneumeth.2009.05.012. doi: 10.1016/j.jneumeth.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lariviere NA, Chen W-J, Spear NE. The influence of olfactory context on Pavlovian conditioning and its expression in preweanling (16-day-old) and adult rats. Animal Learning & Behavior. 1990;18:179–190. [Google Scholar]
- Leith NJ, Kuczenski R. Two dissociable components of behavioral sensitization following repeated amphetamine administration. Psychopharmacology. 1982;76:310–315. doi: 10.1007/BF00449116. doi: 10.1007/BF00449116. [DOI] [PubMed] [Google Scholar]
- Leri F, Flores J, Rajabi H, Stewart J. Effects of cocaine in rats exposed to heroin. Neuropsychopharmacology. 2003;28:2102–2116. doi: 10.1038/sj.npp.1300284. doi: 10.1038/sj.npp.1300284. [DOI] [PubMed] [Google Scholar]
- Lett BT. Repeated exposures intensify rather than diminish the rewarding effects of amphetamine, morphine, and cocaine. Psychopharmacology. 1989;98:357–362. doi: 10.1007/BF00451687. doi: 10.1007/BF00451687. [DOI] [PubMed] [Google Scholar]
- McDougall SA, Baella SA, Stuebner NM, Halladay LM, Crawford CA. Cocaine-induced behavioral sensitization in preweanling and adult rats: effects of a single drug-environment pairing. Psychopharmacology. 2007;193:323–332. doi: 10.1007/s00213-007-0788-x. doi: 10.1007/s00213-007- 0788-x. [DOI] [PubMed] [Google Scholar]
- McDougall SA, Charntikov S, Cortez AM, Amodeo DA, Martinez CE, Crawford CA. Persistence of one-trial cocaine-induced behavioral sensitization in young rats: regional differences in Fos immunoreactivity. Psychopharmacology. 2009;203:617–628. doi: 10.1007/s00213-008-1407-1. doi: 10.1007/s00213-008-1407-1. [DOI] [PubMed] [Google Scholar]
- McDougall SA, Collins RL, Karper PE, Watson JB, Crawford CA. Effects of repeated methylphenidate treatment in the young rat: sensitization of both locomotor activity and stereotyped sniffing. Experimental and Clinical Psychopharmacology. 1999;7:208–218. doi: 10.1037//1064-1297.7.3.208. doi: 10.1037/1064-1297.7.3.208. [DOI] [PubMed] [Google Scholar]
- McDougall SA, Cortez AM, Palmer AG, Herbert MS, Martinez CE, Charntikov S, Amodeo DA. Importance of environmental context for one- and three-trial cocaine-induced behavioral sensitization. Psychopharmacology. 2009;206:377–388. doi: 10.1007/s00213-009-1616-2. doi: 10.1007/s00213-009-1616-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall SA, Reichel CM, Cyr MC, Karper PE, Nazarian A, Crawford CA. Importance of D1 receptors for associative components of amphetamine-induced behavioral sensitization and conditioned activity: A study using D1 receptor knockout mice. Psychopharmacology. 2005;183:20–30. doi: 10.1007/s00213-005-0146-9. doi: 10.1007/s00213-005-0146-9. [DOI] [PubMed] [Google Scholar]
- Miller LP, Johnson AE, Gelhard RE, Insel TR. The ontogeny of amino acid receptors in the rat forebrain—II. Kainic acid receptors. Neuroscience. 1990;35:45–51. doi: 10.1016/0306-4522(90)90118-n. doi: 10.1016/0306-4522(90)90118-N. [DOI] [PubMed] [Google Scholar]
- Molina JC, Hoffmann H, Serwatka J, Spear NE. Establishing intermodal equivalence in preweanling and adult rats. Journal of Experimental Psychology: Animal Behavior Processes. 1991;17:433–447. doi: 10.1037//0097-7403.17.4.433. doi: 10.1037/0097-7403.17.4.433. [DOI] [PubMed] [Google Scholar]
- Nansen EA, Jokel ES, Lobo MK, Micevych PE, Ariano MA, Levine MS. Striatal ionotropic glutamate receptor ontogeny in the rat. Developmental Neuroscience. 2000;22:329–340. doi: 10.1159/000017457. doi: 10.1159/000017457. [DOI] [PubMed] [Google Scholar]
- Partridge B, Schenk S. Context-independent sensitization to the locomotor-activating effects of cocaine. Pharmacology, Biochemistry and Behavior. 1999;63:543–548. doi: 10.1016/s0091-3057(99)00013-1. doi: 10.1016/S0091-3057(99)00013-1. [DOI] [PubMed] [Google Scholar]
- Paulson PE, Camp DM, Robinson TE. Time course of transient behavioral depression and persistent behavioral sensitization in relation to regional brain monoamine concentrations during amphetamine withdrawal in rats. Psychopharmacology. 1991;103:480–492. doi: 10.1007/BF02244248. doi: 10.1007/BF02244248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pert A, Post R, Weiss SR. Conditioning as a critical determinant of sensitization induced by psychomotor stimulants. NIDA Research Monographs. 1990;97:208–241. [PubMed] [Google Scholar]
- Post RM, Lockfeld A, Squillace KM, Contel NR. Drug-environment interaction: context dependency of cocaine-induced behavioral sensitization. Life Sciences. 1981;28:755–760. doi: 10.1016/0024-3205(81)90157-0. doi: 10.1016/0024-3205(81)90157-0. [DOI] [PubMed] [Google Scholar]
- Prasad BM, Sorg BA, Ulibarri C, Kalivas PW. Sensitization to stress and psychostimulants. Involvement of dopamine transmission versus the HPA axis. Annals of the New York Academy of Sciences. 1995;771:617–625. doi: 10.1111/j.1749-6632.1995.tb44714.x. doi: 10.1111/j.1749- 6632.1995.tb44714.x. [DOI] [PubMed] [Google Scholar]
- Rao PA, Molinoff PB, Joyce JN. Ontogeny of dopamine D1 and D2 receptor subtypes in rat basal ganglia: A quantitative autoradiographic study. Developmental Brain Research. 1991;60:161–177. doi: 10.1016/0165-3806(91)90045-k. doi: 10.1016/0165-3806(91)90045-K. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Research Reviews. 1986;11:157–198. doi: 10.1016/s0006-8993(86)80193-7. doi: 10.1016/0165-0173(86)90002-0. [DOI] [PubMed] [Google Scholar]
- Sales N, Martes MP, Bouthenet ML, Schwartz JC. Ontogeny of dopaminergic D2 receptors in the rat nervous system: Characterization and detailed autoradiographic mapping with [125]iodosulpiride. Neuroscience. 1991;28:673–700. doi: 10.1016/0306-4522(89)90014-6. doi: 10.1016/0306-4522(89)90014-6. [DOI] [PubMed] [Google Scholar]
- Scalzo FM, Holson RR. The ontogeny of behavioral sensitization to phencyclidine. Neurotoxicology and Teratology. 1992;14:7–14. doi: 10.1016/0892-0362(92)90023-4. doi: 10.1016/0892-0362(92)90023-4. [DOI] [PubMed] [Google Scholar]
- Smith MA, Greene-Naples JL, Felder JN, Iordanou JC, Lyle MA, Walker KL. The effects of repeated opioid administration on locomotor activity: II. Unidirectional cross-sensitization to cocaine. Journal of Pharmacology and Experimental Therapeutics. 2009;330:476–486. doi: 10.1124/jpet.108.150037. doi: 10.1124/jpet.108.150037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder KJ, Katovic NM, Spear LP. Longevity of the expression of behavioral sensitization to cocaine in preweanling rats. Pharmacology, Biochemistry, and Behavior. 1998;60:909–914. doi: 10.1016/s0091-3057(98)00078-1. doi: 10.1016/S0091-3057(98)00078-1. [DOI] [PubMed] [Google Scholar]
- Sorg BA, Willis JR, See RE, Hopkins B, Westberg HH. Repeated low-level formaldehyde exposure produces cross-sensitization to cocaine: possible relevance to chemical sensitivity in humans. Neuropsychopharmacology. 1998;18:385–394. doi: 10.1016/S0893-133X(97)00179-6. doi: 10.1038/sj.npp.1395160. [DOI] [PubMed] [Google Scholar]
- Spear NE, Kraemer PJ, Molina JC, Smoller DE. Developmental change in learning and memory: infantile disposition for unitization. In: Delacour J, Levy JCS, editors. Systems with learning and memory abilities. Elsevier; New York: 1988. pp. 27–52. [Google Scholar]
- Spear NE, McKenzie DL. Intersensory integration in the infant rat. In: Lewkowicz DJ, Terrace H, editors. The development of intersensory perception: comparative perspectives. Erlbaum; Hillsdale, NJ: 1994. pp. 133–161. [Google Scholar]
- Tirelli E, Laviola G, Adriani W. Ontogenesis of behavioral sensitization and conditioned place preference induced by psychostimulants in laboratory rodents. Neuroscience and Biobehavioral Reviews. 2003;27:163–178. doi: 10.1016/s0149-7634(03)00018-6. doi: 10.1016/S0149-7634(03)00018-6. [DOI] [PubMed] [Google Scholar]
- Tirelli E, Terry P. Amphetamine-induced conditioned activity and sensitization: the role of habituation to the test context and the involvement of Pavlovian processes. Behavioural Pharmacology. 1998;9:409–419. doi: 10.1097/00008877-199809000-00004. [DOI] [PubMed] [Google Scholar]
- Torres G, Rivier C, Weiss F. A ketamine mixture anesthetic inhibits neuroendocrine and behavioral consequences of cocaine administration. Brain Research. 1994;656:33–42. doi: 10.1016/0006-8993(94)91363-3. doi: 10.1016/0006-8993(94)91363-3. [DOI] [PubMed] [Google Scholar]
- Torres-Reverón A, Dow-Edwards DL. Repeated administration of methylphenidate in young, adolescent, and mature rats affects the response to cocaine later in adulthood. Psychopharmacology. 2005;181:38–47. doi: 10.1007/s00213-005-2221-7. doi: 10.1007/s00213-005-2221-7. [DOI] [PubMed] [Google Scholar]
- Vezina P, Stewart J. Amphetamine administered to the ventral tegmental area but not to the nucleus accumbens sensitizes rats to systemic morphine: lack of conditioned effects. Brain Research. 1990;516:99–106. doi: 10.1016/0006-8993(90)90902-n. doi: 10.1016/0006-8993(90)90902-N. [DOI] [PubMed] [Google Scholar]
- Wang YC, Hsiao S. Amphetamine sensitization: nonassociative and associative components. Behavioral Neuroscience. 2003;117:961–969. doi: 10.1037/0735-7044.117.5.961. doi: 10.1037/0735-7044.117.5.961. [DOI] [PubMed] [Google Scholar]
- Weiss SRB, Post RM, Pert A, Woodward R, Murman D. Context-dependent cocaine sensitization: differential effect of haloperidol on development versus expression. Pharmacology, Biochemistry and Behavior. 1989;34:655–661. [PubMed] [Google Scholar]
- White FJ, Joshi A, Koeltzow TE, Hu X-T. Dopamine receptor antagonists fail to prevent induction of cocaine sensitization. Neuropsychopharmacology. 1998;18:26–40. doi: 10.1016/S0893-133X(97)00093-6. doi: 10.1038/sj.npp.1395074. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP. Multiparous species present problems (and possibilities) to developmentalists. Developmental Psychobiology. 1997;30:141–150. doi: 10.1002/(sici)1098-2302(199703)30:2<141::aid-dev5>3.0.co;2-q. doi: 10.1002/(SICI)1098-2302(199703)30:2<141::AID-DEV5>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]