Abstract
Background
Extrapyramidal signs (EPS) are commonly accepted as a feature of Alzheimer’s disease (AD) and may influence both the profile of impairment and prognosis.
Objective
To examine rates of occurrence and risk factors for all types of EPS and to describe the impact of EPS over time on AD clinical course.
Methods
A total of 389 subjects with incident AD (mean age 79 years, 71% females), coming from an urban community (WHICAP), was followed up longitudinally for a mean of 3.6 years (range 0.7 to 13.1 years). EPS were rated using a standardized portion of the Unified Parkinson’s disease Rating scale. Prevalence and incidence rates and cumulative risk for non drug-induced EPS were calculated. Rates of change over time of EPS taking into account potential covariates were also estimated. Predictors of EPS were identified with a Cox model, controlling for age, sex, education and ethnicity.
Results
EPS were detected in 12.3% of patients at first evaluation and 22.6% for the last evaluation. In a multivariate-adjusted generalized estimating equation models of change, total EPS score increased at an annual rate of 1.3%. Women (RR 1.57, p=0.026), subjects with a higher age (RR 1.029, p=0.02) and with EPS (RR 2.07, p=0.001) at baseline had greater rates of cognitive decline.
Conclusion
EPS occur frequently and progress significantly in AD. Subjects with incident AD and concomitant EPS have a faster rate of cognitive decline than subjects with incident AD but without EPS.
Introduction
The frequency and severity of extrapyramidal signs (EPS), a common feature of Alzheimer’s disease (AD), appear to increase over time with disease severity.1 However, the clinical significance of EPS is poorly understood, as they may be the result of different underlying mechanisms, and location or type of lesions. At a clinical level they appear to influence both profile of impairment and prognosis, with several studies showing an association between increased EPS and more severe cognitive impairment, rapid cognitive decline, institutionalization, higher total annual cost and death.2-9
Most but not all previous studies were conducted in clinic-referred patients but not in population-based studies. Differences between studies with regard to diagnostic criteria and population sampling have led to considerable variability in prevalence estimates, which range between 6% to over 50%.1 Prospective studies of EPS have been rare, and have generally focused on changes in specific EPS in subjects with established AD. Little is currently known about the temporal relationship between EPS and AD onset, the relative frequency of different EPS, risk factors for different EPS profiles or the manner in which specific EPS may modulate clinical presentation.8, 10, 11
The present study is based on a community-based, prospective followed cohort of incident AD cases with baseline examinations before diagnosis. The rationale was based on the following considerations: (i) the pathological changes of AD are present in the brain many years before the clinical onset symptoms of dementia, (ii) AD patients may exhibit evidence of motor symptoms before clinical diagnosis of dementia (ref), (iii) rates of cognitive decline for incident AD patients in our study were similar before and after clinical diagnosis of dementia. The study aims to examine rates of occurrence and risk factors for all types of EPS, and to describe the impact of EPS on the rate of the cognitive decline in a population-based cohort where the date of onset of the dementia is known.
Methods
Participants
Subjects for the present study were recruited as part of the Washington Heights Hamilton Heights Inwood Columbia Aging Project (WHICAP), enrolled starting 1992. This cohort consists of elders identified from a probability sample of Medicare beneficiaries residing in an area of 3 contiguous census tracts in the northern Manhattan. Access to the names of individuals was provided by the Health Care Financing Administration. The proportion of individuals within each ethnic group and age stratum who participated in the study did not differ significantly from the source population. The study methodology is reported in detail elsewhere.12
The present study is based on subjects diagnosed with incident AD in the course of the follow-up, but who did not have AD at baseline. We did not include subjects whose last evaluation was the incidence one but only subjects on whom we had follow-up info after incidence. Three hundred and eighty nine subjects from the initial cohort study met these criteria.
Procedures
The study cohort was followed over a 14-year period beginning in 1992, during which time each participant received the same medical, neurological and neuropsychological evaluations. A physician elicited each subject’s medical and neurological history and conducted a standardized physical and neurological examination. All ancillary information (medical charts, clinical, CTs or MRIs) was considered in the evaluation, if available. Medical diagnoses were assigned when applicable. This examination was repeated at each follow-up. Past medical history was recorded with specific attention to stroke, trauma, medications, and recreational drug use. Participants’ medical co-morbidities were computed using a modified version of the Charlson Index of Comorbidity, which assessed conditions such as myocardial infarction, congestive heart failure, peripheral vascular disease, hypertension, chronic obstructive pulmonary disease, arthritis, gastrointestinal disease, mild liver disease, diabetes, chronic renal disease, and systemic malignancy.
A standardized neuropsychological battery comprising evaluation of memory, orientation, abstract reasoning, language, and visuospatial abilities was administred to all subjects at each follow-up. The test battery included: The Selective Reminding Test (SRT);13 the Benton Visual Retention Test (BVRT);14 orientation items from the modified Mini-Mental State Examination (Columbia mMMS); the Boston Naming Test;15 verbal fluency using the Controlled Oral Word Association Test (CFL); the Boston Diagnostic Aphasia Examination (BDAE); the Complex Ideational Material and Repetition subtests; the Wechsler Adult Intelligence Scales-Revised (WAIS-R) Similarities subtest; the Mattis Dementia Rating Scale (DRS) - Identities and Oddities subtest; the Rosen Drawing Test and the Benton Visual Retention Test. Subjects were tested in English or Spanish according to their preference.
Subjects performing below specified cut-off scores for two memory measures, and in two other cognitive domains, were considered to have sufficient cognitive impairment to meet cognitive criteria for suspected AD. These cutoff scores have previously been shown to differentiate normal controls and patients with dementia.16 In addition to impaired cognitive performance, the diagnosis of AD required impairment in social or occupational functioning according to the Diagnostic and Statistical Manual of Mental Disorders (Association AP. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Press Inc; 1980). The longitudinal study design also permitted retrospective correction of initial AD diagnosis.
EPS were assessed by trained examiners using a modified Unified Parkinson’s Disease Rating Scale (UPDRS), whose inter-rater reliability has already been established.17, 9
Eleven EPS (speech, tremor at rest (in any limb), facial expression, neck rigidity, right arm rigidity, left arm rigidity, right leg rigidity, left leg rigidity, posture, gait, body bradykinesia/hypokinesia) were rated on a scale of 0 to 4, with 0 being normal and 4 indicating maximum impairment. These 11 items were then grouped into five domains 9: speech/facial expression (two items, range 0 to 8), tremor (one item, range 0 to 4), rigidity (five items, range 0 to 20), posture/gait (two items, range 0 to 8) and bradykinesia (one item, range 0 to 4). A total dichotomus EPS score was also calculated: total score≥2 vs total score<2, as an indicator of severity whose inter-rater reliability has been demonstrated in previous studies.17 A continuous total score was also derived from the sum of all EPS severity scores (range 0 to 44). A dichotomus form (less than 2 or 2 or more than 2) and continuous score was also calculated for each domain sub-score. Drug-induced EPS were excluded from the analyses.
Information derived from the neurological, psychiatric and neuropsychological assessments at each follow-up was reviewed by an expert consensus group comprising neurologists and neuropsychologists. Based on this review, all participants were assigned to one of three categories: dementia, mild cognitive impairment, or normal cognitive function. Only participants who were non demented at first evaluation and developed AD at follow-up (incident AD) were included in this study. All procedures were approved by the Institutional Review Board at Columbia University Medical Center.
Statistical Analyses
Baseline characteristics of patients who did and those who did not reach the outcomes of interest during the study period were compared using Student’s t test for continuous variables and χ2 test for categorical variables.
We calculated the prevalence of each EPS domain at base-line, time at incidence and last visit.
Given the clustered nature of the data and in order to use the full range of EPS scores and characterize rates of EPS change, we used generalized estimating equations (GEEs),18 which take into account multiple visits per subject and correlations due to repeated examinations. The repeated measures for each subject are treated as a cluster. For each domain, total continuous EPS scores were entered as the dependent variable. The main independent variable was time (years since the initial evaluation). A significant time effect indicates significant changes of EPS score over time. Models also controlled for age at baseline, sex, education in years and ethnicity (white / non Hispanic, black non Hispanic, Hispanic, other).
In order to determine predictors of incident EPS, we used Cox proportional hazards analysis with total EPS (dichotomus form) as the outcome and duration (in years) between the initial visit and either development of EPS or last evaluation without EPS as the timing variable. Patients with EPS at first evaluation were not included in the Cox analyses. The following predictors were included in the model: age at entry into the study, sex, education in years, initial composite cognitive score, ethnicity (white / non Hispanic, American American, Hispanic), ApoE genotype. We calculated similar Cox models for each EPS domain.
In order to characterize rates of cognitive changes, we also applied GGEs to the composite cognitive score. The composite cognitive measure was derived as follows: each of the above 12 raw scores were transformed into z-scores using means and standard deviations of scores from 272 controls without dementia in WHICAP with a similar distribution of age, education and ethnicity to the patients with AD. Z-scores from individual tests were then average to create a z-score for each cognitive domain. If more than half of the tests were missing, the domain score was considered missing and excluded from the analysis. The composite z-score was derived by averaging five domain scores (memory, abstract reasoning, visuo-spatial skills, language, executive speed), with missing data treated in the manner described above. The composite cognitive score was entered as the dependant variable. Independant variables were EPS (in its dichotomus form), time (years from first evaluation or at time at incidence), and EPS X time interaction. A significant EPS effect would suggest a difference in cognitive performances at initial diagnosis for different EPS levels. A significant time effect would suggest a change in cognitive scores over time. A significant interaction term would suggest differential rates of cognitive change for different EPS levels. Models also adjusted for age at baseline, sex, education, ethnicity.
Lastly, in order to determine predictors of cognitive decline, we used Cox proportional hazards analysis with composite cognitive score as the outcome and duration (in years) between the initial visit and either development of Cs<-1.5 or last evaluation without Cs<-1.5 as the timing variable. Patients with Cs<-1.5 at first evaluation were not included in the Cox analyses. The following predictors were included in the model: age at entry into the study, sex, education in years, initial EPS score, ethnicity.
Results
Characteristics of the sample
Three hundred and eighty nine subjects with incident AD were included in the study. Basic demographic and clinical characteristics are presented in table 1. Subjects were followed for a mean of 3.6 (SD=2.6) years on average, with total follow-up times (from diagnosis to final visit or death) ranging from 0.7 to 13.1 years and an average of 4.1 visits/assessments per patient.
Table 1. Demographic and clinical characteristics.
| Age at baseline, y | 79.18 (6.91) |
| Age at the time of diagnosis of dementia, y | 82.78 (6.93) |
| Men / Women (%) | 111 (28.6) / 277 (71.4) |
| Education, mean (SD), y | 6.9 (4.6) |
| At least one ApoE4 allele (n=322 patiens) | 31.9 |
| Duration of follow-up, mean (SD), y | 3.6 (2.6) |
| Number of visit, mean (SD) | 4.1 (1.8) |
| Number of visit after time of diagnosis of dementia, mean (SD) | 2,7 (1,3) |
| Ethnicity: white / non hispanic | 11.9% |
| Ethnicity: black / non hispanic | 31.4% |
| Ethnicity: hispanic | 55.4% |
| Ethnicity: others | 1.3% |
EPS: descriptive statistics
Prevalence of any EPS at base-line, time at incidence and final evaluation are presented in table 2. An increase was observed across time in the frequency of all EPS domains with the exception of resting tremor, which was also less frequent. Similar frequencies were observed across EPS domains (2 to 6% at base-line, 5 to 10% at time to incidence and 8 to 13% at final evaluation).
Table 2. Prevalence for baseline, last and time of diagnosis of dementia of each EPS.
| Baseline evaluation n (%) | Time of incident dementia n (%) | Last evaluation n (%) | |
|---|---|---|---|
| EPS dichotomus Total score | 48 (12.3) | 72 (18.5) | 88 (22.6) |
| Speech / Facial expression | 9 (2.3) | 20 (5.1) | 32 (8.2) |
| Tremor at rest | 5 (1.3) | 7 (1.8) | 6 (1.5) |
| Rigidity | 12 (3.1) | 20 (5.1) | 41 (10.5) |
| Posture / Gait | 27 (6.9) | 42 (10.8) | 53 (13.6) |
| Bradykinesia | 9 (2.3) | 23 (5.9) | 29 (7.5) |
EPS over time
With regard to changes across time, the GEEs models indicate an increase in all EPS (continuous score) from both baseline and time at incidence. The rate of change is an increase of 0.4 and 0.6 respectively in total EPS score per year of follow-up. The results for each domain are presented in table 3. Only tremor did not significantly increase over time from baseline or from time at incidence.
Table 3. Changes in EPS score across time (GEEs models).
| From baseline | From time to incidence | |
|---|---|---|
| Speech / facial expression | β=0.068, p=0.000 | β=0.073, p=0.000 |
| rigidity | β=0.192, p=0.000 | β=0.270, p=0.000 |
| bradykinesia | β=0.069, p=0.000 | β=0.079, p=0.000 |
| Posture / gait | β=0.103, p=0.000 | β=0.122, p=0.000 |
| Tremor | β=0.006, p=0.092 * | β=0.006, p=0.079 * |
no significance
All covariates: age, sex, education, ethnicity, ApoE
An other way to present these results is to calculate the annual increase in EPS total and domain scores. From time of incident dementia, the annual increase in scores is of 1.30% for EPS total, 0.91% for speech and facial expression, 1.35% for rigidity, 1.97% for bradykinesia, 1.52% for posture and gait and only 0.15% for tremor.
The incidence and risk factors for EPS were explored with Cox model (table 4). Subjects with higher cognitive score at baseline were less likely to develop EPS (any EPS and 3 domains, including speech - facial expression, rigidity and posture gait). Subjects with higher age were most likely to develop EPS (any EPS and 3 domains, including rigidity, posture gait and bradykinesia). Subjects with higher education level were most likely to develop EPS (any EPS and 2 domains, including rigidity, posture gait). Of note, there was no influence of sex and ethnicity.
Table 4. Cox models predicting occurrence of individual EPS and any EPS overall.
| Parameter | Speech/facial expression | Tremor | Rigidity | Posture/Gait | Bradykinesia | Any EPS |
|---|---|---|---|---|---|---|
| Females | 0.80 (0.43-1.51) | 1.42 (0.52-3.83) | 0.88 (0.48-1.59) | 0.65 (0.39-1.08) | 0.57 (0.30-1.09) | 0.82 (0.56-1.19) |
| Age | 1.01 (0.97-1.05) | 1.05 (0.97-1.12) | 1.04* (1.01-1.08) | 1.06* (1.03-1.09) | 1.05* (1.01-1.09) | 1.05* (1.03-1.08) |
| Education | 1.04 (0.92-1.12) | 0.96 (0.84-1.09) | 1.10* (1.03-1.18) | 1.07* (1.01-1.13) | 1.07 (0.40-1.05) | 1.05* (1.0161.10) |
| Cs | 0.57* (0.34-0.96) | 0.947 (0.36-2.47) | 0.37* (0.23-0.59) | 0.64* (0.44-0.94) | 0.65 (0.40-1.05) | 0.58* (0.43-0.78) |
Risk ratios (95%CI) are tabulated.
Significant (95% CI not including 1) association.
EPS: extrapyramidal signs; Cs: Composite cognitive score
Cognition over time and the effect of EPS on cognition
We assessed the effect of EPS on cognition (composite cognitive score) with GEEs model (table 5 and 6). The presence of EPS was associated with a lower composite cognitive score (baseline model β=-0.224, p=0.013 and incidence model β=-0.165, p=0.055). Moreover, in the baseline model, subjects with tremor (β=-0.300, p=0.000) and rigidity (β=-0.407, p=0.027) had lower composite cognitive score whereas in the time of incidence model, impaired speech/facial expression was associated with lower composite cognitive (β=-0.342, p=0.019). There was no significant interaction with time, indicating that presence of EPS does not affect rates of cognitive decline over time.
Table 5. cognition over time and the effect of EPS (continuous score) on cognition (GEE’s model).
| Baseline model | Time of incidence model | |
|---|---|---|
| EPS continuous total score | β=- 0.055, p=0.000* | β=0.003, p=0.725 |
| Speech / facial expression (continuous score) | β=-0.171, p=0.003* | β=-0.065, p=0.085 |
| Rigidity (continuous score) | β=-0.115, p=0.000* | β=-0.008, p=0.203 |
| Bradykinesia (continuous score) | β=-0.003, p=0.890 | β=0.018, p=0.572 |
| Posture / gait (continuous score) | β=-0.103, p=0.014* | β=0.109, p=0.258 |
| Tremor (continuous score) | β=-0.138, p=0.012* | β=0.113, p=0.858 |
Significant (p<0.05) coefficients for interaction terms.
Table 6. cognition over time and the effect of EPS (dichotomus score) on cognition (GEE’s model).
| Baseline model | Time of incidence model | |
|---|---|---|
| EPS dichotomus total score | β=-0.224, p=0.013* | β=-0.165, p=0.055* |
| Speech / facial expression (dichotomus score) | β=-0.376, p=0.108 | β=-0.342, p=0.019* |
| Rigidity (dichotomus score) | β=-0.407, p=0.000* | β=0.531, p=0.026* |
| Bradykinesia (dichotomus score) | β=-0.124, p=0.546 | β=-0.342, p=0.080 |
| Posture / gait (dichotomus score) | β=-0.114, p=0.332 | β=0.109, p=0.258 |
| Tremor (dichotomus score) | β=-0.300, p=0.000* | β=0.016, p=0.927 |
Significant (p<0.05) coefficients for interaction terms.
The cognitive decline was explored with Cox models. There was a significant gender effect (RR= 1.570, 95% CI, p=0.026), age at baseline effect (RR= 1.029, 95% CI, p=0.022), education effect (RR= 0.895, 95% CI, p=0.000) and baseline EPS score effect (RR= 2.070, 95% CI, p=0.001).
Subjects were thus observed to be more likely to develop cognitive decline if they were being female, were older at baseline or presented some EPS at baseline. Subjects with a higher level of education were less likely to develop a cognitive decline.
Discussion
This large community-based prospective study has permitted the observation of the emergence of EPS in incident AD cases.
We found EPS to be present even in very early stages of AD, even before confirmation of the diagnosis, with their prevalence increasing over time. Compared to other forms of EPS, resting tremor was less frequent and did not increase over time. This observation has also been made by previous studies.1, 2, 8, 11, 19 EPS symptoms other than resting tremor were seen to progress rapidly over time as previously noted, 1, 2, 10, 11, 19 with an annual increase in EPS scores of between 0.85 to 2%, with only 0.15 for tremor. These calculated rates are smaller or quite close to previously reported ones.10, 11 However, differences with regard to population sampling and baseline levels of EPS limit direct comparisons.
EPS and AD may share similar pathogenesis. The presence of one increases the probability of having the other.3, 20 Whether EPS represent the presence of AD pathology, Lewy body disease or vascular pathology is still uncertain. Explanations other than Lewy bodies for the existence of EPS in AD include the presence of senile plaques in the putamen, caudate and substantia nigra, the presence of neurofibrillary tangles in the substantia nigra and a neuronal loss.21, 22 A main limitation of our study is that the findings are based on a clinical diagnosis of probable AD, without neuropathological examination.
It is important to note that EPS may pre-date the clinical diagnosis of dementia as observed in previous studies. Mild parkinsonian symptoms have been described in subjects with Mild cognitive Impairment.20, 23 In a French general population study 24, where 30% of an elderly cohort free of dementia were seen to have at least one EPS. Their clinical significance remains unclear. Decline in nigro-striatal dopaminergic regulation with advancing age is probably implicated 25, however the observed relationship between EPS and disability appears to be independent of age, which suggests that EPS are not a benign feature of a normal aging process. 24 They could be associated to early cognitive symptoms in the course of probable AD and have also been linked to depression possibly through common effects of underlying dopaminergic changes.
In our study, EPS occured in AD in the absence of psychotropic medications, particularly neuroleptics. Wilson and McLehnnan 26 found no difference between idiopathic and iatrogenic EPS except for higher number of gait EPS. Regarding the difficulties in separating relative contribution of neuroleptic use and AD-related sensitivity to neuroleptics, we analyzed non drug induced EPS in order to increase our confidence that the occurrence of EPS is strictly related to underlying disease process.
Baseline EPS symptoms were associated with lower baseline cognitive performance suggesting either early impact of the underlying neurological changes related to EPS on cognition or that persons with higher performance have increased resistance to EPS due to a greater number of synaptic connections permitting longer resistance to clinical manifestations of dopaminergic or serotoninergic loss.
Moreover, studies concerning the cognitive reserve hypothesis suggest that there are individual differences in the ability to compensate AD lesions.27, 28, 29, 30 Subjects with more cognitive reserve may have AD pathology longer before or without clinical expression.30,31 When AD pathology is clinically expressed, AD pathology is already quite advanced and the disease is more severe. Our study is consistent with this hypothesis, regarding the results of EPS and the level of education. Subjects with a higher educational attainment have a higher cognitive reserve.27, 29, 30 In this study, subjects with higher level of education are most likely to develop EPS, which may be a hallmark of a more severe disease.
Furthermore, our study is in accordance with the previous studies, it enables us to validate a link between the risk of cognitive decline and a more advanced age at time at inclusion, a lower socio-cultural level and the presence of EPS at time at inclusion.
This study is one of the largest prospective studies on a multiethnic cohort allowing a detailed and longitudinal analysis of the correlation between EPS and cognitive decline in AD. The number of incident cases led to analyses with good statistical power and allowed us to examine many covariates. The AD diagnosis is based on a complete analysis of the clinical and neuropsychological data and the final validation of AD cases is conducted by a multidisciplinary expert team. The EPS evaluation could be limited since it is a subjective assessment of the various symptoms but it was based on a short assessment scale validated in other cohort studies. Finally, the present study confirms the association between EPS and early AD.
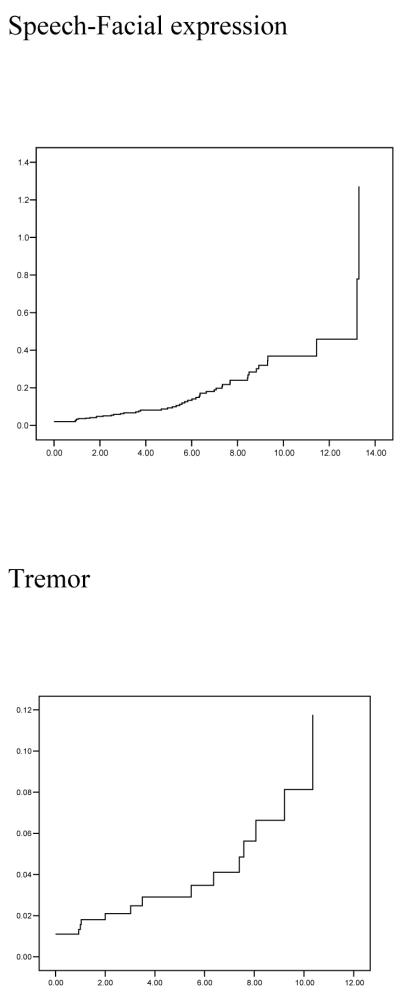
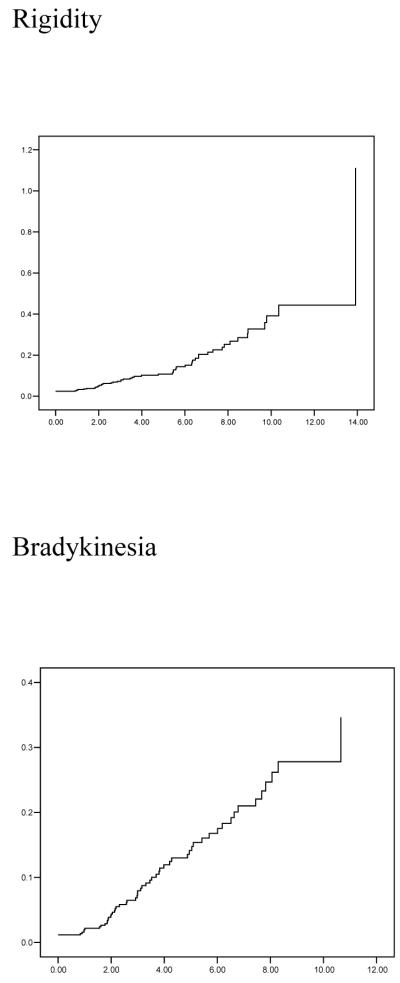
Figure 1.

Cumulative risk (1 - cumulative survival) curves of developing any EPS and individual domain EPS y-axes. The time axes (x) show from first evaluation until development of motor signs (or last evaluation).
References
- 1.Ellis RJ, Caligiuri M, Galasko D, Thal LJ. Extrapyramidal motor signs in clinically diagnosed Alzheimer disease. Alzheimer Dis Assoc Disord. 1996;10(2):103–114. doi: 10.1097/00002093-199601020-00008. [DOI] [PubMed] [Google Scholar]
- 2.Soininen H, Helkala EL, Laulumaa V, Soikkeli R, Hartikainen P, Riekkinen PJ. Cognitive profile of Alzheimer patients with extrapyramidal signs: a longitudinal study. J Neural Transm Park Dis Dement Sect. 1992;4(3):241–254. doi: 10.1007/BF02260907. [DOI] [PubMed] [Google Scholar]
- 3.Richards M, Stern Y, Mayeux R. Subtle extrapyramidal signs can predict the development of dementia in elderly individuals. Neurology. 1993;43(11):2184–2188. doi: 10.1212/wnl.43.11.2184. [DOI] [PubMed] [Google Scholar]
- 4.Stern Y, Albert M, Brandt J, Jacobs DM, Tang MX, Marder K, Bell K, Sano M, Devanand DP, Bylsma F, et al. Utility of extrapyramidal signs and psychosis as predictors of cognitive and functional decline, nursing home admission, and death in Alzheimer’s disease: prospective analyses from the Predictors Study. Neurology. 1994;44(12):2300–2307. doi: 10.1212/wnl.44.12.2300. [DOI] [PubMed] [Google Scholar]
- 5.Chui HC, Lyness SA, Sobel E, Schneider LS. Extrapyramidal signs and psychiatric symptoms predict faster cognitive decline in Alzheimer’s disease. Arch Neurol. 1994;51(7):676–681. doi: 10.1001/archneur.1994.00540190056015. [DOI] [PubMed] [Google Scholar]
- 6.Richards M, Stern Y, Mayeux R. Subtle extrapyramidal signs and incident dementia: a follow-up analysis. Neurology. 1995;45(10):1942. doi: 10.1212/wnl.45.10.1942. [DOI] [PubMed] [Google Scholar]
- 7.Stern Y, Liu X, Albert M, Brandt J, Jacobs DM, Del Castillo-Castaneda C, Marder K, Bell K, Sano M, Bylsma F. Modeling the influence of extrapyramidal signs on the progression of Alzheimer disease. Arch Neurol. 1996;53(11):1121–6. doi: 10.1001/archneur.1996.00550110061013. [DOI] [PubMed] [Google Scholar]
- 8.Lopez OL, Wisnieski SR, Becker JT, Boller F, DeKosky ST. Extrapyramidal signs in patients with probable Alzheimer disease. Arch Neurol. 1997;54(8):969–975. doi: 10.1001/archneur.1997.00550200033007. [DOI] [PubMed] [Google Scholar]
- 9.Scarmeas N, Albert M, Brandt J, Blacker D, Hadjigeorgiou G, Papadimitriou A, Dubois B, Sarazin M, Wegesin D, Marder K, Bell K, Honig L, Stern Y. Motor signs predict poor outcomes in Alzheimer disease. Neurology. 2005;64(10):1696–1703. doi: 10.1212/01.WNL.0000162054.15428.E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson RS, Bennett DA, Gilley DW, Beckett LA, Schneider JA, Evans DA. Progression of parkinsonian signs in Alzheimer’s disease. Neurology. 2000;54(6):1284–1289. doi: 10.1212/wnl.54.6.1284. [DOI] [PubMed] [Google Scholar]
- 11.Scarmeas N, Hadjigeorgiou GM, Papadimitriou A, Dubois B, Sarazin M, Brandt J, Albert M, Marder K, Bell K, Honig LS, Wegesin D, Stern Y. Motor signs during the course of Alzheimer disease. Neurology. 2004;63(6):975–982. doi: 10.1212/01.wnl.0000138440.39918.0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang MX, Cross P, Andrews H, Jacobs DM, Small S, Bell K, Merchant C, Lantigua R, Costa R, Stern Y, Mayeux R. Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology. 2001;56(1):49–56. doi: 10.1212/wnl.56.1.49. [DOI] [PubMed] [Google Scholar]
- 13.Buschke H, Fuld PA. Evaluating storage, retention, and retrieval in disordered memory and learning. Neurology. 1974;24(11):1019–1025. doi: 10.1212/wnl.24.11.1019. [DOI] [PubMed] [Google Scholar]
- 14.Benton AL. A multiple choice type of the visual retention test. AMA Arch Neurol Psychiatry. 1950;64(5):699–707. doi: 10.1001/archneurpsyc.1950.02310290095010. [DOI] [PubMed] [Google Scholar]
- 15.Williams BW, Mack W, Henderson VW. Boston Naming Test in Alzheimer’s disease. Neuropsychologia. 1989;27(8):1073–1079. doi: 10.1016/0028-3932(89)90186-3. [DOI] [PubMed] [Google Scholar]
- 16.Stern Y, Andrews H, Pittman J, Sano M, Tatemichi T, Lantigua R, Mayeux R. Diagnosis of dementia in a heterogeneous population. Development of a neuropsychological paradigm-based diagnosis of dementia and quantified correction for the effects of education. Arch Neurol. 1992;49(5):453–460. doi: 10.1001/archneur.1992.00530290035009. [DOI] [PubMed] [Google Scholar]
- 17.Richards M, Marder K, Bell K, Dooneief G, Mayeux R, Stern Y. Interrater reliability of extrapyramidal signs in a group assessed for dementia. Arch Neurol. 1991;48(11):1147–1149. doi: 10.1001/archneur.1991.00530230055021. [DOI] [PubMed] [Google Scholar]
- 18.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrica. 1986;73:13–22. [Google Scholar]
- 19.Chen JY, Stern Y, Sano M, Mayeux R. Cumulative risks of developing extrapyramidal signs, psychosis, or myoclonus in the course of Alzheimer’s disease. Arch Neurol. 1991;48(11):1141–1143. doi: 10.1001/archneur.1991.00530230049020. [DOI] [PubMed] [Google Scholar]
- 20.Louis ED, Schupf N, Manly J, Marder K, Tang MX, Mayeux R. Association between mild parkinsonian signs and mild cognitive impairment in a community. Neurology. 2005;12(64(7)):1157–1161. doi: 10.1212/01.WNL.0000156157.97411.5E. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y, Stern Y, Chun MR, Jacobs DM, Yau P, Goldman JE. Pathological correlates of extrapyramidal signs in Alzheimer’s disease. Ann Neurol. 1997;41(3):368–74. doi: 10.1002/ana.410410312. [DOI] [PubMed] [Google Scholar]
- 22.Burns JM, Galvin JE, Roe CM, Morris JC, McKeel DW. The pathology of the substantia nigra in Alzheimer disease with extrapyramidal signs. Neurology. 2005;26(64(8)):1397–1403. doi: 10.1212/01.WNL.0000158423.05224.7F. [DOI] [PubMed] [Google Scholar]
- 23.Boyle PA, Wilson RS, Aggarwal NT, Arvanitakis Z, Kelly J, Bienias JL, Bennett DA. Parkinsonian signs in subjects with mild cognitive impairment. Neurology. 2005;27(65(12)):1901–1906. doi: 10.1212/01.wnl.0000188878.81385.73. [DOI] [PubMed] [Google Scholar]
- 24.Richards M, Touchon J, Ledesert B, Ritchie K. Mild extrapyramidal signs and functional impairment in ageing. Int J Geriatr Psychiatry. 2002;17(2):150–153. doi: 10.1002/gps.548. [DOI] [PubMed] [Google Scholar]
- 25.Katzman R, Brown T, Fuld P, Thal L, Davies P, Terry R. Significance of neurotransmitter abnormalities in Alzheimer’s disease. Res Publ Assoc Res Nerv Ment Dis. 1986;64:279–286. [PubMed] [Google Scholar]
- 26.Wilson JA, MacLennan WJ. Review: drug-induced parkinsonism in elderly patients. Age Ageing. 1989;18(3):208–210. doi: 10.1093/ageing/18.3.208. [DOI] [PubMed] [Google Scholar]
- 27.Stern Y, Albert S, Tang MX, Tsai WY. Rate of memory decline in AD is related to education and occupation: cognitive reserve? Neurology. 1999;10(53(9)):1942–7. doi: 10.1212/wnl.53.9.1942. [DOI] [PubMed] [Google Scholar]
- 28.Stern Y, Gurland B, Tatemichi TK, Tang MX, Wilder D, Mayeux R. Influence of education and occupation on the incidence of Alzheimer’s disease. JAMA. 1994;271(13):1004–1010. [PubMed] [Google Scholar]
- 29.Scarmeas N, Zarahn E, Anderson KE, Habeck CG, Hilton J, Flynn J, Marder KS, Bell KL, Sackeim HA, Van Heertum RL, Moeller JR, Stern Y. Association of life activities with cerebral blood flow in Alzheimer disease: implications for the cognitive reserve hypothesis. Arch Neurol. 2003;60(3):359–365. doi: 10.1001/archneur.60.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stern Y. Cognitive reserve and Alzheimer disease. Alzheimer Dis Assoc Disord. 2006;20(3 Suppl 2):S69–74. doi: 10.1097/00002093-200607001-00010. [DOI] [PubMed] [Google Scholar]
- 31.Bennett DA. Postmortem indices linking risk factors to cognition: results from the Religious Order Study and the Memory and Aging Project. Alzheimer Dis Assoc Disord. 2006;20(3 Suppl 2):S63–68. doi: 10.1097/00002093-200607001-00009. [DOI] [PubMed] [Google Scholar]