Abstract
Several studies have documented the effect of methamphetamine (METH) on the toxicity of the dopamine (DA) terminals of the striatum but only a few studies have assessed the damaging effects of METH on striatal neurons postsynaptic to the nigrostriatal DA terminals. In the present study, we employed histological methods to study the effect of METH on DA terminals and striatal neurons. We also assessed the role of the striatal neurokinin-1 (NK-1) receptor on pre- and post-synaptic METH-induced damage. Male mice were treated with METH (10 mg/kg) four times at 2-h intervals and were sacrificed 3 days after the treatment. A number of animals received the non-peptide NK-1 receptor antagonist WIN-51,708 (10 mg/kg) 30 min before the first and fourth injections of METH. Immunocytochemical staining for tyrosine hydroxylase (TH) showed significant deficits throughout all aspects of the caudate-putamen in animals exposed to METH. Pretreatment with WIN-51,708 prevented the METH-induced loss of TH immunostaining. Sections from a separate set of mice were stained with Fluoro-Jade B (FJB), a fluorochrome that binds specifically to degenerating fibers and cell bodies of neurons. Treatment with METH shows Fluoro-Jade B positive cell bodies in the striatum and pretreatment with WIN-51,708 abolished Fluoro-Jade B staining. Moreover, double labeling with Fluoro-Jade B and glial fibrillary acidic protein (GFAP) shows reactive astrocytosis in the area adjacent to the Fluoro-Jade B-positive cells but no Fluoro-Jade B staining of the astrocytes. This observation suggests that the degenerating cells must be striatal neurons and not astrocytes. The data demonstrate that METH induces pre- and post-synaptic damage in the striatum and the damage can be prevented with pharmacological blockade of the NK-1 receptor. These findings represent a new direction in the study of the mechanism of toxicity to METH and could be useful in the treatment of some neurological disorders.
Keywords: Methamphetamine; Neurokinin-1 receptor; Degeneration; Striatum; Substance P; WIN-51,708
1. Introduction
Methamphetamine (METH) causes pre-synaptic dopaminergic terminal degeneration in the striatum as demonstrated by deficits in various neurochemical and morphological markers [4,46]. Long-term toxic damage is evidenced by the degeneration of dopaminergic fibers in the neostriatum [41,47]. Despite the well-documented pre-synaptic dopamine (DA) terminal damage induced by METH, few studies have investigated the potentially damaging effects of METH exposure on neurons intrinsic to the striatum [7,9]. Most importantly, Cadet et al. [6] have demonstrated that METH also induces cell death by showing the presence of TUNEL-positive neurons in the striatum of mice treated with a neurotoxic dose of METH [9]. DA and glutamate have long been believed to mediate METH-induced neurotoxicity by facilitating the formation of reactive oxygen and nitrogen species as well as excitotoxic neurotransmission [1,5,22,33,37,49,55,56]. Recent evidence also suggests the involvement of neuropeptides, such as enkephalin and dynorphin, in the neural reactions induced by METH [13,58,59].
Tachykinin peptides and their receptors are intrinsic components of the DA system [2]. Substance P (SP) is the most abundant peptide of the tachykinin family and has the highest natural binding affinity for the neurokinin-1 (NK-1) receptor, which is a G-protein-coupled receptor. The basal ganglia is particularly rich in both SP neurons and NK-1 receptors [48]. The bulk of the striatal NK-1 receptors has been localized to interneurons [15,26,36]. SP/NK-1 is not only intimately involved in the modulation of neostriatal DA function [39,44,45], but also contributes to excitotoxic neurotransmission in various regions of the central nervous system [28,29,61]. One of the consequences of exposure to METH is elevation of the levels of SP peptide and the messenger RNA encoding the precursor to SP (preprotachykinin-A), within striatonigral projection neurons [3,54]. Depending on the site of its release and the dose of the releasing agent, SP may play different roles within the basal ganglia. For example, a study using in vivo microdialysis in the rat striatum demonstrates that local infusion of the non-peptide NK-1 receptor antagonist CP-96345 potentiates METH-induced DA overflow. This suggests that released SP plays a physiological role by dampening the activity of the nigrostriatal projection under conditions of excess DA [18]. However, we have recently reported that the pharmacological blockade of the NK-1 receptor protects against METH-induced pre-synaptic neurochemical deficits, suggesting a crucial role for the striatal NK-1 receptor in the pathogenesis of METH-induced neurotoxicity [66].
In order to confirm that NK-1 receptor antagonists could prevent METH-induced DA axonal degeneration, rather than facilitate the compensatory expression of phenotypic markers by surviving cells, we performed a histological assessment using tyrosine hydroxylase (TH) immunohistochemistry. In addition, to test the hypothesis that pretreatment with the NK-1 receptor antagonist WIN-51,708 also prevents METH-induced striatal tissue degeneration, we used a newly developed technique, Fluoro-Jade B staining double-labeled with immunofluorescent glial fibrillary acidic protein (GFAP), in order to visualize neuronal degeneration.
2. Materials and methods
2.1. Animals
Eight-week-old male ICR/CD-1 mice weighing 30–40 mg (Taconic, Germantown, NY) were singly housed on a 12-h light/dark cycle with food and water available ad libitum. They were habituated for 2 weeks before commencement of drug treatments. The animals were sacrificed by decapitation on day 3 post-treatment. All animal-use procedures were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the animal care committee at Hunter College of the City University of New York.
2.2. Drug administration
METH (Sigma, St. Louis, MO) was dissolved in saline and four injections (10 mg/kg) were intraperitoneally administered at 2-h intervals for 1 day. The highly selective NK-1 receptor antagonist WIN-51,708 (RBI/Sigma, Natick, MA) was dissolved in 45% 2-hydroxypropyl-β-cyclodextrin (RBI/Sigma, Natick, MA). Vehicle and WIN-51,708 (10 mg/kg) were given intraperitoneally 30 min prior to the 1st and 4th injections of METH or saline. WIN-51,708 is a highly selective non-peptide NK-1 receptor antagonist displaying an IC50 of 50 nM in rat forebrain membranes [42].
2.3. TH immunohistochemistry
The animals were fully anesthetized with a Ketamine/Acepromazine mixture (100 mg/kg Ketamine/1 mg/kg Acepromazine) before perfusion and fixation through the heart. Mice perfused with 0.1M phosphate-buffered saline (PBS), pH 7.4, followed by fixation with 4% Paraformaldehyde (PFA) in 0.1 M PBS (pH 7.4). Brains were post-fixed overnight with 4% PFA/0.1M PBS at 4 °C and immersed in 30% sucrose for 24 h at 4 °C. Coronal sections (40 um in thickness) were cut in the vibratome at room temperature. The floating sections were washed 3 × 10 min in 0.1M PBS, followed by incubation with 10% Normal Sheep Serum (NSS) at room temperature for 30 min to block nonspecific binding. Then the sections were incubated with polyclonal rabbit anti-TH antibody (1:500, Chemicon, Temecula, CA) diluted in 2% blocking solution (NSS) at 4 °C overnight. The next day, the sections were washed with PBS 3 × 10 min and then incubated with sheep anti-rabbit 2nd antibody conjugated with FITC (1:1000, Chemicon) for 2 h at room temperature. Sections were washed three times with PBS before mounted on the slide. The slides were examined using the Nikon Eclipse E400 Fluorescent Microscope.
2.4. Double labeling of Fluoro-Jade B and GFAP
Slide-mounted sections were completely dried and postfixed in absolute ethanol at −20 °C for 10 min. After several washes in 0.01 M PBS, sections were incubated with the protein diluent from Vector Mouse on Mouse (M.O.M.) kit (Vector Laboratories, Burlingame, CA) for 5 min, followed by incubation with monoclonal anti-GFAP antibody (1:30, Sigma, St. Louis, MO) diluted in 0.3% Triton X-100 and M.O.M. protein diluent in PBS at 4 °C overnight. Sections were then incubated with biotinylated secondary antibody at room temperature for 30 min, followed by incubation with rhodamine avidin DCS (cell sorting grade; Vector Laboratories) for 5 min. After being washed for 5 min in PBS and 5 min in distilled water, sections were then placed in 0.0001% Fluoro-Jade B staining solution (Histo-Chem, Jefferson, AR) with 0.1% acetic acid at 4 °C for 1 h. After three washes in distilled water for 1 min each, sections were dried, immersed in xylene and overlayed with a glass cover with DPX. The tissue was examined using an epifluorescent microscope with blue (460–500 nm) and green (532–587 nm) excitation light. Fluoro-Jade B and GFAP positive cells were counted from four sections per animal (five to seven animals per group).
2.5. Statistical analysis
Experimental result values are presented as average values with ± S.E.M.. The significance of difference between means was analyzed by Student’s t-test or by ANOVA and post hoc analyses using the Fisher’s protected least significant difference test, with the significance level set at P < 0.05. The degree of neuronal degeneration was assigned a rank score depending on the average number of Fluoro-Jade B (FJB)-positive cells in the striatum per section by counting four coronal sections per animal: 0 (−), < 1 cell/section; 1 (+): 1–50 cells/section; 2 (++): 51–100 cells/section; 3 (+++): 101–150 cells/section; 4 (++++): >150 cells/section.
3. Results
3.1. Blockade of NK-1 receptor attenuated METH-induced loss of TH immunoreactive fibers
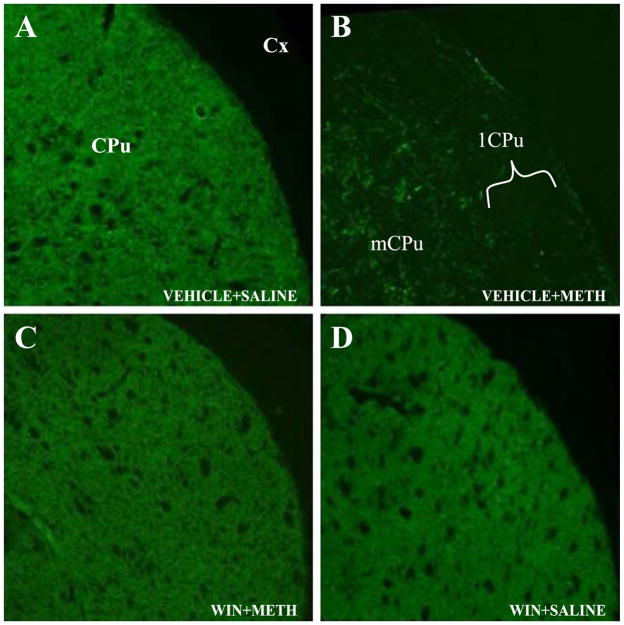
Deficits of TH immunohistochemical staining in the striatum, together with silver staining, have been reproducibly correlated in various published studies with DA terminal degeneration [43]. In the present study, we have used immunohistochemical staining of striatal TH as an index of METH-induced DA terminal toxicity in order to determine if the deficits are either uniformly distributed throughout the striatum or display regional selectivity. The vehicle-saline-treated control animals showed dense TH staining throughout the striatum. The animals treated with the NK-1 receptor antagonist WIN-51,708 alone demonstrated a pattern of TH immunostaining indistinguishable from those in the control group (Fig. 1A and D). However, intraperitoneal administration of 10 mg/kg METH four times at 2-h intervals for 1 day (n = 5) caused marked reduction in the intensity of striatal TH immunoreactivity (TH-IR) 3 days after drug treatment (Fig. 1B). Four animals of the five demonstrated extensive reduction of TH-IR over the entire striatum, but the lateral aspect of the striatum (lCPu) consistently displayed greater reduction of TH-IR than the medial aspect of the striatum (mCPu) (Fig. 1B). The lCPu displays higher levels of preprotachykinin-A mRNA [64] and delta opioid receptors [31]. In comparison with the animals treated with METH alone, pretreatment with WIN-51,708 (n = 5) at a dose of 10 mg/kg of body weight significantly prevented the decrease of TH-IR in four out of five treated animals (Fig. 1C).
Fig. 1.
Effects of METH and WIN-51,708 (WIN) on TH immunoreactivity in the striatum. Administration of 10 mg/kg METH resulted in a decrease in the intensity of TH immunoreactivity in four out of five animals 3 days after treatment. Pretreatment with 10 mg/kg WIN-51,708 prevented the METH-induced depletion of TH staining. Note that METH induces greater deficits of TH in the lCPu relative to the mCPu. Scale bar, 100 μm. CPu, caudate-putamen; lCPu and mCPu refer to lateral and medial aspects of the caudate-putamen, respectively; Cx, cortex.
3.2. Toxic doses of METH caused post-synaptic neuronal degeneration in the striatum
There is a paucity of information on the damaging effects of METH on neurons post-synaptic to the DA terminals of the striatum. In order to assess the deleterious effects of METH on striatal neurons, we employed a histological method using fluorescent staining of degenerating processes and cell bodies with Fluoro-Jade B. Fluoro-Jade B is a newly developed anionic fluorochrome that selectively stains degenerating neurons after a variety of neurotoxic insults. It is more sensitive, reproducible and reliable than almost all the other conventional histochemical techniques in localizing neuronal degeneration [50,51]. In our previous work on neurochemical assessment, we demonstrated that pretreatment with WIN-51,708 prevented the induction of GFAP protein level in astrocytes [67]. Therefore, reactive astrocytosis, assessed by immunofluorescence of GFAP, double-labeled with Fluoro-Jade B, was used as the main index of METH-induced neural damage.
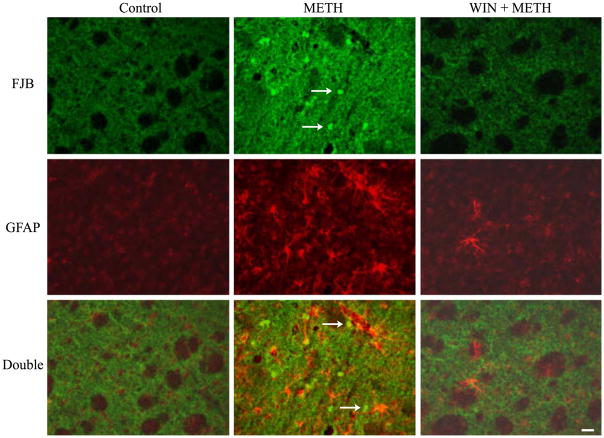
No positive Fluoro-Jade B staining was observed in either control animals (Fig. 2) or in the group receiving WIN-51,708 alone (data not shown). However, four injections of 10 mg/kg METH resulted in prominent staining of Fluoro-Jade B positive cells in the striatum of five out of seven treated animals (n = 7; Fig. 2). Fluoro-Jade B positive cells were seen throughout the striatum of two animals, relatively concentrated at the ventral lateral striatum of the other two animals, and at the lateral striatum in one animal. METH exposure caused a substantial increase in the number of astrocytes with larger cell bodies and longer processes. The reactive astrocytes were present in proximity to the degenerating neurons as well as in regions surrounding the degenerating area. No doubly labeled cells were observed, suggesting that the Fluoro-Jade B positive cells represent some striatal neurons. The degenerating bodies (neurons) appear small (indicated by arrows in Fig. 2 METH group) because they may represent the remnant of the cell body or perhaps just the nucleus.
Fig. 2.
A NK-1 receptor antagonist prevents reactive astrocytosis and degeneration in the striatum. Multiple exposure to METH (10 mg/kg) led to the staining of degenerating Fluoro-Jade B (FJB) positive neurons (green) in the striatum, and the reactive astrocytes (red GFAP; hypertrophic cell body and increased number) adjacent to FJB positive neurons; pretreatment with WIN-51,708 (10 mg/kg; WIN) afforded protection from METH as evidenced by the lack of astrocytosis and FJB staining. Arrows refer to degenerating cell bodies or a fragment of the latter such as the nucleus of the cell. DOUBLE refers to the overlay of FJB and GFAP images. Scale bar 20 μm.
3.3. NK-1 receptor antagonist attenuated METH-induced striatal neuronal degeneration
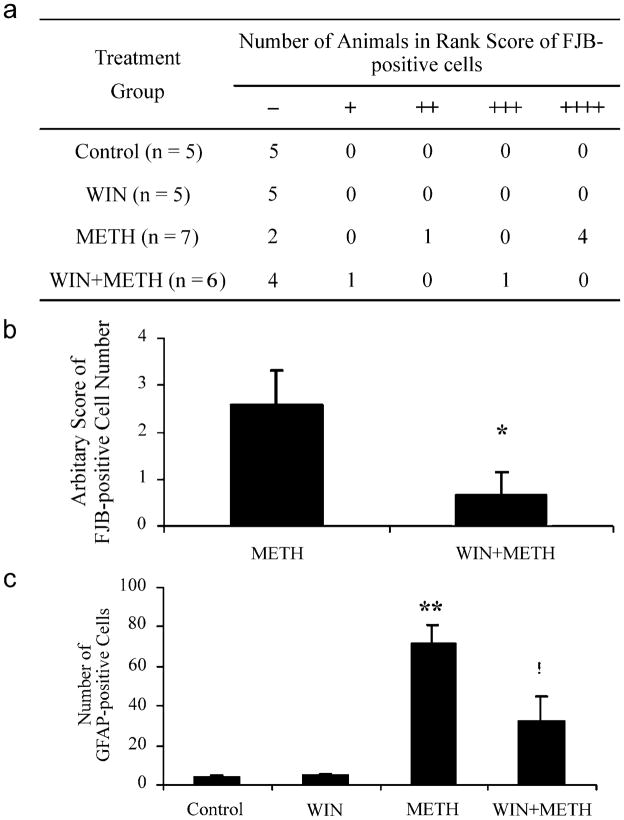
In order to assess the role of the striatal NK-1 receptor on METH-induced neuronal degeneration, a group of animals was pretreated with the NK-1 receptor antagonist and then injected with METH (the animals were sacrificed 3 days after the treatment). In contrast to animals treated with METH alone, no Fluoro-Jade B positive cells were seen in four out of six animals pretreated with the NK-1 receptor antagonist WIN-51,708 (n = 6; Fig. 2), while the other two showed Fluoro-Jade B staining either in the ventral lateral region or throughout the striatum. The degree of neuronal degeneration was assigned an arbitrary rank score depending on the average number of Fluoro-Jade B positive cells in the striatum per section by counting four sections per animal (Fig. 3a). Pretreatment of WIN-51,708 caused a quantitative decrease in the average rank score (Fig. 3b) as well as in the number of astrocytes (Fig. 3c) compared to the group treated with METH alone.
Fig. 3.
Analysis of the effect of WIN-51,708 on degeneration and reactive astrocysotsis in the striatum. (a) Number of animals showing FJB positive cells in each arbitrary rank score (0 [−], < 1 cell/section; 1 [+]: 1 – 50 cells/section; 2 [++]: 51– 100 cells/section; 3 [+++]: 101– 150 cells/section; 4 [++++]: >150 cells/section); (b) average rank score in METH-treated mice with or without WIN-51,708 pretreatment; and (c) pretreatment of WIN-51,708 also caused quantitative decrease in the number of astrocytes. *p < 0.05 and !p < 0.01 as compared to METH-treated animals, **p < 0.001 compared to control.
4. Discussion
The histological results presented here support previous neurochemical studies from our laboratory [66]. The histological studies on TH immunohistochemistry and Fluoro-Jade B staining double-labeled with GFAP immunofluorescence demonstrate that the pharmacological blockade of the NK-1 receptor attenuates METH-induced neurodegeneration in the striatum of the mouse brain. In addition to presynaptic DA terminal damage, our data also provide direct histological evidence that METH exposure can cause degenerative damage to the post-synaptic striatal neurons. As demonstrated in the METH-treated group, acute treatment with multiple injections of METH has greater impact on the pre-synaptic terminals than it does on the post-synaptic neurons, implying that pre-synaptic terminals are subjected to more damaging influences or that they themselves are more vulnerable to toxic injury.
The protective effects afforded by the NK-1 receptor antagonist WIN-51,708 on both pre-synaptic DA terminal damage and post-synaptic neuronal degeneration suggest that the NK-1 receptor participates in an initial step (upstream) in the cascade leading to long-term neural degeneration in the striatum. These results also suggest the possibility that there may be links between the mechanisms causing DA terminal degeneration and those giving rise to intrinsic neuronal damage in the striatum. Some published work suggests a causal link between post- and pre-synaptic mechanisms of METH-induced neural damage. For example, it has been shown that excitotoxic destruction of post-synaptic striatal neurons protect against METH-induced DA depletion [40], and the administration of toxic doses of METH, are associated with increased p53-like immunoreactivity [20], as well as prolonged activation of AP-1 in striatal cell bodies [52]. More experiments are needed in order to elucidate the mechanism by which WIN-51,708 protects the striatum from METH. For example, the WIN-51,708 compound may hinder the accumulation of METH in brain tissue. This possibility appears to be remote since WIN-51,708 does not prevent METH-induced hyperthermia [66]. Moreover, the non-peptide NK-1 receptor antagonist L-733,060 also protects DA terminal markers from METH [66].
An alternative hypothesis is that METH-induced post-synaptic degeneration has no causal relationship to pre-synaptic terminal damage. For example, we did not observe Fluoro-Jade B staining at 5 mg/kg of METH (personal observation), a dose that has been shown to induce loss of DA transporter sites and TH in the striatum of rats [11]. A similar dose of METH produced Fluoro-Jade B staining of neurons in the parietal cortex but not in the striatum [12], suggesting that cortical neurons are more sensitive to METH than striatal neurons. In order to resolve these discrepancies, more systematic studies are needed at various doses of METH and temporal assessment, especially a comparison of early with late events in the neurotoxic cascade induced by METH.
The intracellular mechanism by which METH induces toxicity and neural damage may be activated from more than one direction. For example, METH administration resulted in the induction of immediate early gene (IEG) expression [9,52]. It has also been demonstrated that DA/glutamate interactions occur not only at the circuit level but also at the level of intracellular signaling within individual striatal neurons, which requires functional NMDA receptor and Ca2 + to regulate the expression of IEGs [23]. Therefore, in addition to the synergistic actions of DA and glutamate on the formation of reactive oxygen and nitrogen species causing acute damage, the molecular events involving the activation or inhibition of cell-death-related genes may also be responsible for METH-induced long-term degeneration.
Ladenheim et al. [24] have recently demonstrated that METH-induced terminal degeneration and apoptotic cell death were attenuated in interleukin-6 knockout mice, suggesting the involvement of cytokines in the pathogenic process of METH neurotoxicity. Converging evidence reveals that neuroglial cells, including astrocytes and microglia, contain nitric oxide synthase, and that cytokines can mediate nitric oxide production as well as activation of apoptotic pathways in various neurodegenerative diseases including Parkinson’s Disease [8,21,63]. One possibility is that the reactive gliosis, as a phenomenon secondary to the primary insults, may cause necrosis or apoptosis in the neighboring areas via the increased production of nitric oxide or cytokines [14,25,62]. It is worth noting that reactive astrocytes and microglia express SP receptors [27,30,32,57] and SP has been shown to stimulate the secretion of various cytokines, such as interleukin-1, interleukin-6 and tumor necrosis factor-α from neuroglial cells in a number of central nervous system disorders [16,30,34,39]. Furthermore, non-peptide NK-1 receptor antagonists selectively blocked SP-induced production of interleukin-6 in astrocytoma cell lines [10].
A neurodegenerative function may be associated with the NK-1 receptor in the hippocampus of the rodent brain. It has been demonstrated by several laboratories that excessive levels of excitatory amino acids, namely glutamate, induce excitotoxicity and cell death in several brain regions [53]. In the hippocampus, the neuropeptide SP is known to induce the release of glutamate [29]. Moreover, kainate induces cell death in the hippocampus of wild type mice but not in the hippocampus of a mouse lacking the preprotachykinin-A gene encoding the neuropeptide SP [28]. These observations lend support to our hypothesis that METH-induced neural damage in the striatum is initiated by signaling through the NK-1 receptor. These observations assume that METH induces the release of SP. A recent study utilizing in vivo microdialysis in freely behaving rats reported that a high dose of METH failed to evoke detectable levels of SP [19]. It is conceivable that very low levels of SP are released, below the level of detection by microdialysis. An alternative interpretation is that SP is degraded very quickly having sufficient time to signal through the NK-1 receptor but never accumulating at levels high enough to be picked up by the microdialysis probe [35]. More work is needed using an alternative method to demonstrate the release of SP and the activation of the NK-1 receptor by METH.
It is possible that the neuropeptide SP mediates some of the neurotoxic effects of METH in the striatum via glutamate by a mechanism similar to the one described above for the hippocampus [28,29]. A role for glutamate in METH-induced neurotoxicity in the striatum has been suggested by some studies. For example, the non-competitive NMDA receptor antagonist MK-801 protects dopaminergic and serotonergic terminals from METH in the striatum [41]. Moreover, pretreatment with MK-801 significantly reduced METH-induced dopamine overflow in the striatum of the rat brain [60]. In the light of the results presented here and in other published work from this laboratory [66,67], it is reasonable to speculate that the NK-1 receptor may be located upstream of the glutamate receptors in the neurotoxic cascade induced by METH in the striatum. This mechanism necessitates the release of SP by METH. A recent study using in vivo microdialysis in rats failed to detect augmented SP overflow in the presence of METH [19]. However, the rate of decomposition of SP in striatal tissue is extremely high [35]. We have observed that METH induces the internalization of the NK-1 receptor in striatal tissue suggesting that in the presence of METH, SP is released and signals through the NK-1 receptor (unpublished results).
Some studies suggest that SP released by METH in striatal tissue serves to dampen the activity of the nigrostriatal pathway. For example, administration of the NK-1 receptor antagonist CP-96345 enhances METH-induced dopamine overflow in the striatum [18]. However, this was observed using a very low dose of METH (0.5 mg/kg). Similarly, effects of NK-1 receptor blockade on METH-induced behavior and neuropeptide mRNA expression [17] may not correlate with models of toxicity where high doses of METH are used. This study employs acute METH (2.5 mg/kg) and assesses behavior immediately after METH and neuropeptide mRNA expression 3 h later [17]. Early events at low doses of METH may not correlate with changes that appear days after the exposure to a high dose of METH.
In summary, blockade of the striatal NK-1 receptor with a non-peptide antagonist prevents the loss of DA transporter sites, tissue DA content, TH and blocks the induction of GFAP by METH in the striatum [66]. Here we demonstrate that METH induces the degeneration of neuronal elements and cells post-synaptic to DA in the striatum. Moreover, METH-induced degeneration in the striatum can be prevented by pretreatment with an NK-1 receptor antagonist. Work in progress will determine the cell type undergoing degeneration and possibly cell death in the striatum of mice exposed to a neurotoxic dose of METH.
Acknowledgments
We would like to thank Gertrude Rivera for her help in the preparation of the manuscript and Adele Kudish for the editorial assistance. This work was supported by ‘Specialized Neuroscience Research Program’ grant NS41073 from the National Institute for Neurological Disorders and Stroke and DA12136 from the National Institute on Drug Abuse to JAA. Support has also come from the ‘Research Centers in Minority Institutions’ award to Hunter College.
References
- 1.Abekawa T, Ohmori T, Koyama T. Effects of repeated administration of a high dose of methamphetamine on dopamine and glutamate release in rat striatum and nucleus accumbens. Brain Res. 1994;643:276–281. doi: 10.1016/0006-8993(94)90033-7. [DOI] [PubMed] [Google Scholar]
- 2.Angulo JA, McEwen BS. Molecular aspects of neuropeptide regulation and function in the corpus striatum and nucleus accumbens. Brain Res Brain Res Rev. 1994;19:1–28. doi: 10.1016/0165-0173(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 3.Bannon MJ, Elliott PJ, Bunney EB. Striatal tachykinin biosynthesis: regulation of mRNA and peptide levels by dopamine agonists and antagonists. Brain Res. 1987;427:31–37. doi: 10.1016/0169-328x(87)90041-6. [DOI] [PubMed] [Google Scholar]
- 4.Brunswick DJ, Benmansour S, Tejani-Butt SM, Hauptmann M. Effects of high-dose methamphetamine on monoamine uptake sites in rat brain measured by quantitative autoradiography. Synapse. 1992;11:287–293. doi: 10.1002/syn.890110404. [DOI] [PubMed] [Google Scholar]
- 5.Cadet JL, Brannock C. Free radicals and the pathobiology of brain dopamine systems. Neurochem Int. 1998;32:117–131. doi: 10.1016/s0197-0186(97)00031-4. [DOI] [PubMed] [Google Scholar]
- 6.Cadet JL, Ordonez SV, Ordonez JV. Methamphetamine induces apoptosis in immortalized neural cells: protection by the proto-oncogene, bcl-2. Synapse. 1997;25:176–184. doi: 10.1002/(SICI)1098-2396(199702)25:2<176::AID-SYN8>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 7.Cadet JL, Ladenheim B, Hirata H. Effects of toxic doses of methamphetamine (METH) on dopamine D1 receptors in the mouse brain. Brain Res. 1998;786:240–242. doi: 10.1016/s0006-8993(97)01432-7. [DOI] [PubMed] [Google Scholar]
- 8.Choi C, Park JY, Lee J, Lim JH, Shin EC, Ahn YS, Kim CH, Kim SJ, Kim JD, Choi IS, Choi IH. Fas ligand and Fas are expressed constitutively in human astrocytes and the expression increases with IL-1, IL-6, TNF-alpha, or IFN-gamma. J Immunol. 1999;162:1889–1895. [PubMed] [Google Scholar]
- 9.Deng X, Ladenheim B, Tsao LI, Cadet JL. Null mutation of c-fos causes exacerbation of methamphetamine-induced neurotoxicity. J Neurosci. 1999;19:10107–10115. doi: 10.1523/JNEUROSCI.19-22-10107.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Derocq JM, Segui M, Blazy C, Emonds-Alt X, Le Fur G, Brelire JC, Casellas P. Effect of substance P on cytokine production by human astrocytic cells and blood mononuclear cells: characterization of novel tachykinin receptor antagonists. FEBS Lett. 1996;399:321–325. doi: 10.1016/s0014-5793(96)01346-4. [DOI] [PubMed] [Google Scholar]
- 11.Eisch AJ, Marshall JF. Methamphetamine neurotoxicity: dissociation of striatal dopamine terminal damage from parietal cortical cell body injury. Synapse. 1998;30:433–445. doi: 10.1002/(SICI)1098-2396(199812)30:4<433::AID-SYN10>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 12.Eisch AJ, Schmued LC, Marshall JF. Characterizing cortical neuron injury with Fluoro-Jade labeling after a neurotoxic regimen of methamphetamine. Synapse. 1998;30:329–333. doi: 10.1002/(SICI)1098-2396(199811)30:3<329::AID-SYN10>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 13.El Daly E, Chefer V, Sandill S, Shippenberg TS. Modulation of the neurotoxic effects of methamphetamine by the selective kappa-opioid receptor agonist U69593. J Neurochem. 2000;74:1553–1562. doi: 10.1046/j.1471-4159.2000.0741553.x. [DOI] [PubMed] [Google Scholar]
- 14.Eliasson MJ, Huang Z, Ferrante RJ, Sasamata M, Molliver ME, Snyder SH, Moskowitz MA. Neuronal nitric oxide synthase activation and peroxynitrite formation in ischemic stroke linked to neural damage. J Neurosci. 1999;19:5910–5918. doi: 10.1523/JNEUROSCI.19-14-05910.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerfen CR. Substance P (neurokinin-1) receptor mRNA is selectively expressed in cholinergic neurons in the striatum and basal forebrain. Brain Res. 1991;556:165–170. doi: 10.1016/0006-8993(91)90563-b. [DOI] [PubMed] [Google Scholar]
- 16.Gitter BD, Regoli D, Howbert JJ, Glasebrook AL, Waters DC. Interleukin-6 secretion from human astrocytoma cells induced by substance P. J Neuroimmunol. 1994;51:101–108. doi: 10.1016/0165-5728(94)90134-1. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez-Nicolini V, McGinty JF. NK-1 receptor blockade decreases amphetamine-induced behavior and neuropeptide mRNA expression in the striatum. Brain Res. 2002;931:41–49. doi: 10.1016/s0006-8993(02)02250-3. [DOI] [PubMed] [Google Scholar]
- 18.Gygi SP, Gibb JW, Johnson M, Hanson GR. Blockade of tachykinin NK1 receptors by CP-96345 enhances dopamine release and the striatal dopamine effects of methamphetamine in rats. Eur J Pharmacol. 1993;250:177–180. doi: 10.1016/0014-2999(93)90639-y. [DOI] [PubMed] [Google Scholar]
- 19.Hanson GR, Bush L, Keefe KA, Alburges ME. Distinct responses of basal ganglia substance P systems to low and high doses of methamphetamine. J Neurochem. 2002;82:1171–1178. doi: 10.1046/j.1471-4159.2002.01053.x. [DOI] [PubMed] [Google Scholar]
- 20.Hirata H, Cadet JL. p53-knockout mice are protected against the long-term effects of methamphetamine on dopaminergic terminals and cell bodies. J Neurochem. 1997;69:780–790. doi: 10.1046/j.1471-4159.1997.69020780.x. [DOI] [PubMed] [Google Scholar]
- 21.Hirsch EC, Hunot S, Damier P, Faucheux B. Glial cells and inflammation in Parkinson’s disease: a role in neurodegeneration? Ann Neurol. 1998;44:S115–S120. doi: 10.1002/ana.410440717. [DOI] [PubMed] [Google Scholar]
- 22.Itzhak Y, Gandia C, Huang PL, Ali SF. Resistance of neuronal nitric oxide synthase-deficient mice to methamphetamine-induced dopaminergic neurotoxicity. J Pharmacol Exp Ther. 1998;284:1040–1047. [PubMed] [Google Scholar]
- 23.Konradi C, Leveque JC, Hyman SE. Amphetamine and dopamine-induced immediate early gene expression in striatal neurons depends on postsynaptic NMDA receptors and calcium. J Neurosci. 1996;16:4231–4239. doi: 10.1523/JNEUROSCI.16-13-04231.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ladenheim B, Krasnova IN, Deng X, Oyler JM, Polettini A, Moran TH, Huestis MA, Cadet JL. Methamphetamine-induced neurotoxicity is attenuated in transgenic mice with a null mutation for interleukin-6. Mol Pharmacol. 2000;58:1247–1256. doi: 10.1124/mol.58.6.1247. [DOI] [PubMed] [Google Scholar]
- 25.Le W, Rowe D, Xie W, Ortiz I, He Y, Appel SH. Microglial activation and dopaminergic cell injury: an in vitro model relevant to Parkinson’s disease. J Neurosci. 2001;21:8447–8455. doi: 10.1523/JNEUROSCI.21-21-08447.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li JL, Kaneko T, Mizuno N. Colocalization of neuronal nitric oxide synthase and neurokinin-1 receptor in striatal interneurons in the rat. Neurosci Lett. 2001;310:109–112. doi: 10.1016/s0304-3940(01)02097-3. [DOI] [PubMed] [Google Scholar]
- 27.Lin RC. Reactive astrocytes express substance-P immunoreactivity in the adult forebrain after injury. NeuroReport. 1995;7:310–312. [PubMed] [Google Scholar]
- 28.Liu H, Cao Y, Basbaum AI, Mazarati AM, Sankar R, Wasterlain CG. Resistance to excitotoxin-induced seizures and neuronal death in mice lacking the preprotachykinin A gene. Proc Natl Acad Sci U S A. 1999;96:12096–12101. doi: 10.1073/pnas.96.21.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu H, Mazarati AM, Katsumori H, Sankar R, Wasterlain CG. Substance P is expressed in hippocampal principal neurons during status epilepticus and plays a critical role in the maintenance of status epilepticus. Proc Natl Acad Sci U S A. 1999;96:5286–5291. doi: 10.1073/pnas.96.9.5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luber-Narod J, Kage R, Leeman SE. Substance P enhances the secretion of tumor necrosis factor-alpha from neuroglial cells stimulated with lipopolysaccharide. J Immunol. 1994;152:819–824. [PubMed] [Google Scholar]
- 31.Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ. Autoradiographic differentiation of mu, delta, and kappa opioid receptors in the rat forebrain and midbrain. J Neurosci. 1987;7:2445–2464. [PMC free article] [PubMed] [Google Scholar]
- 32.Mantyh PW, Johnson DJ, Boehmer CG, Catton MD, Vinters HV, Maggio JE, Too HP, Vigna SR. Substance P receptor binding sites are expressed by glia in vivo after neuronal injury. Proc Natl Acad Sci U S A. 1989;86:5193–5197. doi: 10.1073/pnas.86.13.5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marshall JF, O’Dell SJ, Weihmuller FB. Dopamine – glutamate interactions in methamphetamine-induced neurotoxicity. J Neural Transm Gen Sect. 1993;91:241–254. doi: 10.1007/BF01245234. [DOI] [PubMed] [Google Scholar]
- 34.Martin FC, Charles AC, Sanderson MJ, Merrill JE. Substance P stimulates IL-1 production by astrocytes via intracellular calcium. Brain Res. 1992;599:13–18. doi: 10.1016/0006-8993(92)90846-2. [DOI] [PubMed] [Google Scholar]
- 35.Michael-Titus AT, Fernandes K, Setty H, Whelpton R. In vivo metabolism and clearance of substance P and co-expressed tachykinins in rat striatum. Neuroscience. 2002;110:277–286. doi: 10.1016/s0306-4522(01)00530-9. [DOI] [PubMed] [Google Scholar]
- 36.Morello M, Reiner A, Sancesario G, Karle EJ, Bernardi G. Ultra-structural study of nitric oxide synthase-containing striatal neurons and their relationship with parvalbumin-containing neurons in rats. Brain Res. 1997;776:30–39. doi: 10.1016/s0006-8993(97)00997-9. [DOI] [PubMed] [Google Scholar]
- 37.O’Dell SJ, Weihmuller FB, Marshall JF. Multiple methamphetamine injections induce marked increases in extracellular striatal dopamine which correlate with subsequent neurotoxicity. Brain Res. 1991;564:256–260. doi: 10.1016/0006-8993(91)91461-9. [DOI] [PubMed] [Google Scholar]
- 39.Palma C, Manzini S. Substance P induces secretion of immunomodulatory cytokines by human astrocytoma cells. J Neuroimmunol. 1998;81:127–137. doi: 10.1016/s0165-5728(97)00167-7. [DOI] [PubMed] [Google Scholar]
- 40.Petit F, Glowinski J. Stimulatory effect of substance P on the spontaneous release of newly synthesized [3H]dopamine from rat striatal slices: a tetrodotoxin-sensitive process. Neuropharmacology. 1986;25:1015–1021. doi: 10.1016/0028-3908(86)90196-6. [DOI] [PubMed] [Google Scholar]
- 41.Pu C, Vorhees CV. Protective effects of MK-801 on methamphetamine-induced depletion of dopaminergic and serotonergic terminals and striatal astrocytic response: an immunohistochemical study. Synapse. 1995;19:97–104. doi: 10.1002/syn.890190205. [DOI] [PubMed] [Google Scholar]
- 42.Quartara L, Maggi CA. The tachykinin NK1 receptor: Part I. Ligands and mechanisms of cellular activation. Neuropeptides. 1997;31:537–563. doi: 10.1016/s0143-4179(97)90001-9. [DOI] [PubMed] [Google Scholar]
- 43.Rabinovic AD, Lewis DA, Hastings TG. Role of oxidative changes in the degeneration of dopamine terminals after injection of neurotoxic levels of dopamine. Neuroscience. 2000;101:67–76. doi: 10.1016/s0306-4522(00)00293-1. [DOI] [PubMed] [Google Scholar]
- 44.Reid MS, Herrera-Marschitz M, Hokfelt T, Ohlin M, Valentino KL, Ungerstedt U. Effects of intranigral substance P and neurokinin A on striatal dopamine release: I. Interactions with substance P antagonists. Neuroscience. 1990;36:643–658. doi: 10.1016/0306-4522(90)90007-q. [DOI] [PubMed] [Google Scholar]
- 45.Reid MS, Herrera-Marschitz M, Kehr J, Ungerstedt U. Striatal dopamine and glutamate release: effects of intranigral injections of substance P. Acta Physiol Scand. 1990;140:527–537. doi: 10.1111/j.1748-1716.1990.tb09030.x. [DOI] [PubMed] [Google Scholar]
- 46.Ricaurte GA, Guillery RW, Seiden LS, Schuster CR, Moore RY. Dopamine nerve terminal degeneration produced by high doses of methylamphetamine in the rat brain. Brain Res. 1982;235:93–103. doi: 10.1016/0006-8993(82)90198-6. [DOI] [PubMed] [Google Scholar]
- 47.Ricaurte GA, Seiden LS, Schuster CR. Further evidence that amphetamines produce long-lasting dopamine neurochemical deficits by destroying dopamine nerve fibers. Brain Res. 1984;303:359–364. doi: 10.1016/0006-8993(84)91221-6. [DOI] [PubMed] [Google Scholar]
- 48.Saria A. The tachykinin NK1 receptor in the brain: pharmacology and putative functions. Eur J Pharmacol. 1999;375:51–60. doi: 10.1016/s0014-2999(99)00259-9. [DOI] [PubMed] [Google Scholar]
- 49.Schmidt CJ, Ritter JK, Sonsalla PK, Hanson GR, Gibb JW. Role of dopamine in the neurotoxic effects of methamphetamine. J Pharmacol Exp Ther. 1985;233:539–544. [PubMed] [Google Scholar]
- 50.Schmued LC, Hopkins KJ. Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000;874:123–130. doi: 10.1016/s0006-8993(00)02513-0. [DOI] [PubMed] [Google Scholar]
- 51.Schmued LC, Albertson C, Slikker W., Jr Fluoro-Jade: a novel fluorochrome for the sensitive and reliable histochemical localization of neuronal degeneration. Brain Res. 1997;751:37–46. doi: 10.1016/s0006-8993(96)01387-x. [DOI] [PubMed] [Google Scholar]
- 52.Sheng P, Ladenheim B, Moran TH, Wang XB, Cadet JL. Methamphetamine-induced neurotoxicity is associated with increased striatal AP-1 DNA-binding activity in mice. Brain Res Mol Brain Res. 1996;42:171–174. doi: 10.1016/s0169-328x(96)00192-1. [DOI] [PubMed] [Google Scholar]
- 53.Snider BJ, Choi DW. Glutamate and neurotoxicity. In: Herman BH, editor. Glutamate and Addiction. Humana Press; New Jersey: 2002. pp. 51–61. [Google Scholar]
- 54.Sonsalla PK, Gibb JW, Hanson GR. Nigrostriatal dopamine actions on the D2 receptors mediate methamphetamine effects on the striatonigral substance P system. Neuropharmacology. 1986;25:1221–1230. doi: 10.1016/0028-3908(86)90139-5. [DOI] [PubMed] [Google Scholar]
- 55.Sonsalla PK, Nicklas WJ, Heikkila RE. Role for excitatory amino acids in methamphetamine-induced nigrostriatal dopaminergic toxicity. Science. 1989;243:398–400. doi: 10.1126/science.2563176. [DOI] [PubMed] [Google Scholar]
- 56.Sulzer D, Chen TK, Lau YY, Kristensen H, Rayport S, Ewing A. Amphetamine redistributes dopamine from synaptic vesicles to the cytosol and promotes reverse transport. J Neurosci. 1995;15:4102–4108. doi: 10.1523/JNEUROSCI.15-05-04102.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Torrens Y, Beaujouan JC, Saffroy M, Daguet de Montety MC, Bergstrom L, Glowinski J. Substance P receptors in primary cultures of cortical astrocytes from the mouse. Proc Natl Acad Sci U S A. 1986;83:9216–9220. doi: 10.1073/pnas.83.23.9216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tsao LI, Ladenheim B, Andrews AM, Chiueh CC, Cadet JL, Su TP. Delta opioid peptide [D-Ala2,D-leu5]enkephalin blocks the long-term loss of dopamine transporters induced by multiple administrations of methamphetamine: involvement of opioid receptors and reactive oxygen species. J Pharmacol Exp Ther. 1998;287:322–331. [PubMed] [Google Scholar]
- 59.Tsao LI, Hayashi T, Su TP. Blockade of dopamine transporter and tyrosine hydroxylase activity loss by [D-Ala(2), D-Leu(5)]enkephalin in methamphetamine-treated CD-1 mice. Eur J Pharmacol. 2000;404:89–93. doi: 10.1016/s0014-2999(00)00616-6. [DOI] [PubMed] [Google Scholar]
- 60.Weihmuller FB, O’Dell SJ, Marshall JF. MK-801 protection against methamphetamine-induced striatal dopamine terminal injury is associated with attenuated dopamine overflow. Synapse. 1992;11:155–163. doi: 10.1002/syn.890110209. [DOI] [PubMed] [Google Scholar]
- 61.Woolf CJ, Doubell TP. The pathophysiology of chronic pain-increased sensitivity to low threshold A beta-fibre inputs. Curr Opin Neurobiol. 1994;4:525–534. doi: 10.1016/0959-4388(94)90053-1. [DOI] [PubMed] [Google Scholar]
- 62.Wu DC, Jackson-Lewis V, Vila M, Tieu K, Teismann P, Choi C, Choi DK, Ischiropoulos H, Przedborski S. Blockade of microglial activation is neuroprotective in the 1-Methyl-4-Phenyl-1,2,3,6-Tetra-hydropyridine mouse model of Parkinson Disease. J Neurosci. 2002;22:1763–1771. doi: 10.1523/JNEUROSCI.22-05-01763.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xiao BG, Link H. Immune regulation within the central nervous system. J Neurol Sci. 1998;157:1–12. doi: 10.1016/s0022-510x(98)00049-5. [DOI] [PubMed] [Google Scholar]
- 64.Young WS, III, Bonner TI, Brann MR. Mesencephalic dopamine neurons regulate the expression of neuropeptide mRNAs in the rat forebrain. Proc Natl Acad Sci U S A. 1986;83:9827–9831. doi: 10.1073/pnas.83.24.9827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yu J, Cadet JL, Angulo JA. Neurokinin-1 (NK-1) receptor antagonists abrogate methamphetamine-induced striatal dopaminergic neurotoxicity in the murine brain. J Neurochem. 2002;83:613–622. doi: 10.1046/j.1471-4159.2002.01155.x. [DOI] [PubMed] [Google Scholar]
- 67.Zhang Y, Landas K, Mueller H, Angulo JA. Progressive augmentation of striatal and accumbal preprotachykinin mRNA levels by chronic treatment with methamphetamine and effect of concurrent administration of the N-methyl-D-aspartate receptor antagonist MK-801. Neuropharmacology. 1997;36:325–334. doi: 10.1016/s0028-3908(97)00005-1. [DOI] [PubMed] [Google Scholar]