Abstract
Purpose
Progression of lower urinary tract symptoms (LUTS) among community dwelling older men is not well described.
Materials and Methods
We evaluated 5,697 participants in MrOS, a prospective cohort study of community dwelling men aged 65 years and older. We characterized LUTS utilizing the American Urological Symptom Index (AUA-SI) at two time points: study entry and 2-year follow-up. Progression was examined in the overall cohort and within strata of baseline symptoms (AUA-SI ≤ 7 points and ≥8 points) using descriptive statistics.
Results
At baseline, the mean (SD) age was 73.5 (5.8) years and mean (SD) AUA-SI score was 8.3 (6.3). Mean (SD) and median total AUA-SI increased during follow-up by 1.1 (5.0) and 1.0 points, respectively. Of the 3092 men with AUA-SI ≤ 7 at baseline, 883 (29%) reported LUTS progression (AUA-SI ≥8 points) at follow-up. The frequency of LUTS progression increased with advancing baseline age. Of the 2605 men with AUA-SI ≥ 8 at baseline, 622 (24%) reported progression of at least 4 AUA-SI points at follow-up. Among the 2200 men with baseline AUA-SI ≤7 and no prior history of BPH or LUTS treatments, 94% remained untreated, 2% reported BPH surgery, and 4% reported medication use at follow-up.
Conclusions
Up to 29% of community dwelling older men with no or mild LUTS will develop clinically significant LUTS within 2 years. These data help elucidate the natural history of LUTS in the community and provide useful data for designing clinical trials of LUTS prevention.
Keywords: benign prostatic hyperplasia, lower urinary tract symptoms, risk factor, epidemiology, progression
Introduction
The male lower urinary tract symptoms complex (LUTS) occurs among 15% to 60% of men over the age of 40 years and may affect up to 80% of older, community dwelling men.1–6 LUTS is associated with an increased risk of falls, significantly diminished quality of life, depression, and impairment in instrumental activities of daily living.4, 7–10 The costs for diagnosis and treatment of LUTS-associated conditions total over $6 billion each year.1, 11, 12
Although LUTS prevalence has been described in detail, less is known of its incidence or progression. A previous study in the Olmsted County cohort observed that 14% of men without LUTS at baseline subsequently reported moderate or severe symptoms within 18 months of follow-up and 22% reported moderate or severe symptoms within 42 months of follow-up.13 Similarly, 21% of Japanese, 26% of black American and 20% of Austrian men with no or mild LUTS at baseline reported worsened symptoms after 3, 4 and 5 years of follow-up, respectively.14–16
These studies, however, focused on relatively younger (< 65 years) men. Neither LUTS onset nor symptom progression have been studied in detail among older men who may be disproportionately affected by urinary dysfunction.6 Further elucidation of LUTS natural history among elderly men would provide valuable information for improving treatment strategies and designing prevention trials. Therefore, we conducted a prospective study to determine the incidence and progression of LUTS during two years of follow-up in a cohort of community dwelling US men 65–100 years of age.
Materials and Methods
Study population
We conducted the present analysis as part of the Osteoporotic Fractures in Men (MrOS) Study, a prospective cohort study among older men that focuses on fractures, falls and prostate disease.17 From March 2000 through April 2002, 5995 participants were enrolled.17 Recruitment occurred at 6 US academic medical centers in Birmingham, AL, Minneapolis, MN, Palo Alto, CA, Pittsburgh, PA, Portland, OR, and San Diego, CA. Eligible participants were at least 65 years of age, able to walk without assistance from another person, and had at least one natural hip (for bone density measurement). All participants gave written informed consent.
Measurements
At baseline, participants completed a comprehensive questionnaire including demographic and lifestyle factors and medical history. LUTS were assessed with the validated American Urological Association Symptom Index (AUA-SI), including assessment of urinary bother.18 Men were queried about history of ‘enlarged prostate’ (BPH) diagnosed by a doctor or other health care provider, including history of surgery, use of prescription medications, or other treatment.
At the baseline in-person clinic visit, an inventory of prescription medications was made using methods for cohort studies.19 Study personnel recorded the product brand or generic name for medications taken during the past 30 days. Using an electronic database at the San Francisco Coordinating Center for the MrOS study, each medication was matched to its ingredient(s) based on the Iowa Drug Information Service (IDIS) Drub Vocabulary (College of Pharmacy, University of Iowa, Iowa City, IA).
Updated information was obtained from each participant approximately 2 years after his baseline visit via a questionnaire mailed from July 2002 to March 2004. Prior to the mailing, 177 had died and 20 had voluntarily withdrawn. The questionnaire was completed by 5741 men (96% of the original cohort) and 57 refused. The interim questionnaire included the AUA-SI, queries regarding BPH diagnosis and treatment, and assessments of prescription medication use.
Classification of LUTS
At each assessment, symptom severity was categorized as no or mild (0 to 7 points), moderate (8–19 points), or severe (20 to 35 points).20 Change in the AUA-SI score was computed as the absolute difference between the interim and the baseline score.
There are no standardized definitions for LUTS incidence or progression. Therefore, we defined these outcomes based on clinical treatment guidelines and prior studies.21, 22 Among men with a baseline AUA-SI of ≤ 7 points, we defined LUTS incidence as a score of ≥8 on the next questionnaire assessment. We further distinguished those who had entered this symptom stratum by an increase of 1–3 points from those who had done so by an increase of ≥4 points.21 Among men with a baseline AUA-SI of ≥ 8 points, we defined LUTS progression as an increase in score of ≥ 4 points on the next questionnaire assessment. We defined clinically significant urinary bother as a score > 3.
Statistical analysis
Distributions of baseline characteristics between the 5741 men who did and the 254 men who did not return the interim mailed questionnaire were examined with Pearson chi-square tests for contingency tables and t-tests for continuous variables. From the 5,741 men who returned the interim questionnaire, the analytic cohort comprised 5,697 after excluding 44 who did not complete the AUA-SI items on either or both of the questionnaires. The distribution of change in AUA-SI score was described in the entire cohort. Subsequent analyses of LUTS incidence were restricted to the 3092 men with AUA-SI scores ≤ 7 at baseline and analyses of LUTS progression were restricted to the 2605 men with AUA-SI scores ≥8 at baseline. For each group, the proportions experiencing incidence and progression were computed. These outcomes were then examined after restricting to men with no surgical or prescription medication use for LUTS/BPH before baseline or during follow-up, according to history of previous prostate surgery obtained from report of radical prostatectomy or surgical treatment of BPH, and by category of baseline age (65–69, 70–79, ≥80 years). Descriptive statistics including means, standard deviations (SD), and proportions, were computed using SAS software (SAS Institute, Cary, NC).
Results
Study population
Mean (SD) age at baseline for the entire cohort was 73.1 (5.5) years. Compared with men who did not return the interim mailed questionnaire (because of death, withdrawal from the study or refusal), those who responded were younger, more likely to be of white race, and less likely to report poorer health, a history of heart disease, diabetes, or prostate cancer. The mean AUA-SI score was about 1 point lower among responders, who were more likely to be classified as having no or mild LUTS symptoms and to report higher urinary bother. The proportion reporting a previous diagnosis of BPH did not differ between the two groups (Table 1).
Table 1.
Distribution of baseline characteristics among men who did not and did complete the questionnaire mailed at the end of the second year of follow-up in the MrOS study.
| Number (%) Baseline Characteristic |
Completed questionnaire at 2 year follow-up |
P-value | |
|---|---|---|---|
| No* 254 (4.2%) |
Yes 5741 (95.8%) |
||
| Age, years (n,%) | |||
| 65–69 yr | 37 (14.6) | 1732 (30.2) | |
| 70–79 yr | 131 (51.6) | 3024 (52.7) | < 0.001 |
| ≥80 yr | 86 (33.9) | 985 (17.2) | |
| Caucasian race (n,%) | 213 (83.9) | 5149 (89.7) | 0.003 |
| BMI (kg/m2), mean (sd) | 27.4 (4.2) | 27.4 (3.8) | 0.91 |
| Alcohol consumption (drinks/week), mean (sd) | 3.9 (8.9) | 4.3 (6.7) | 0.37 |
| Smoking status (n,%) | |||
| Past | 160 (63.0) | 3379 (58.9) | 0.08 |
| Current | 13 (5.1) | 193 (3.4) | |
| Self-reported health (% fair-poor) | 87 (34.3) | 770 (13.4) | <0.001 |
| Medical history (n,%) | |||
| Any heart disease | 95 (37.4) | 1334 (23.2) | <0.001 |
| Self-reported diabetes | 51 (20.1) | 602 (10.5) | <0.001 |
| Self-reported hypertension | 129 (50.8) | 2452 (42.7) | 0.01 |
| Self-reported history of prostate cancer | 47 (18.5) | 552 (11.5) | 0.001 |
| Self-reported cancer, not prostate | 48 (18.9) | 989 (17.2) | 0.50 |
| AUA-SI symptom score, mean (sd) | 9.2 (6.9) | 8.3 (6.3) | 0.02 |
| AUA-SI symptom category (n,%) | 0.07 | ||
| None/Mild (0–7 pts) | 123 (48.4) | 3114 (54.2) | |
| Moderate (8–19 pts) | 107 (42.1) | 2249 (39.2) | |
| Severe (≥ 20 pts) | 24 (9.5) | 374 (6.5) | |
| Previous BPH diagnosis (n,%) | 123 (48.8) | 2806 (48.9) | 0.94 |
| Urinary satisfaction (n,%) | 0.003 | ||
| Delighted/pleased/mostly satisfied | 153 (60.2) | 3813 (66.4) | |
| Mixed | 48 (18.9) | 1159 (20.2) | |
| Unsatisfied/unhappy/terrible | 53 (20.9) | 768 (13.4) | |
Abbreviations: AUA-SI, American Urological Association Symptom Index; BMI, body mass index; BPH, benign prostatic hyperplasia.
177 died during follow-up, 20 withdrew from the study, and 57 refused.
Change in AUA-SI Scores
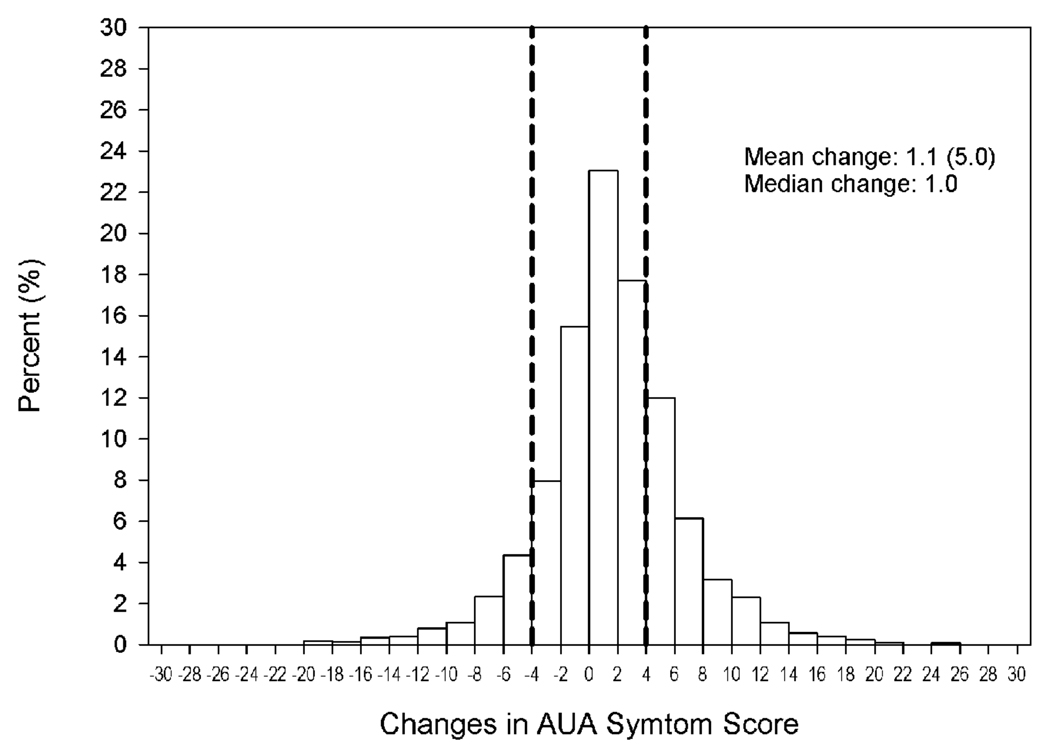
At baseline, the mean (SD) AUA-SI score was 8.3 (6.3) (Table 1). Mean and median changes in AUA-SI score during the 2-year follow-up period were 1.1 (5.0) and 1.0, respectively (Figure 1). Scores ≤ 4 points lower than at baseline were observed among 13%, whereas scores that increased by ≥ 4 points was observed among 26%.
Figure 1.
Distribution of changes in LUTS form baseline during 2 years of follow-up in the MrOS study
LUTS onset
Among men with AUA-SI ≤7 at baseline, 29% had onset of LUTS at 2-year follow-up (Table 2). Although onset could potentially be attributed to chance variation associated with smaller (1–3 point) increases in AUA-SI, over 20% reported an increase of ≥4 points during follow-up, a robust indicator of clinically significant symptom progression.21 When this study group was restricted to those men who reported no BPH treatment before baseline or during follow-up, 26% reported LUTS onset. The proportions with LUTS onset were similar among those with no prostate surgery compared to prostate surgery prior to baseline. However, the proportion with LUTS onset was about somewhat higher among men who had prostate surgery during follow-up, but there were few men in this category. Finally, LUTS onset significantly increased with baseline age: 27% of men 65–69 years, 28% of men 70–79 years, and 34% of men ≥ 80 years who had no or mild LUTS at baseline reported moderate or severe LUTS at 2-year follow-up (p = 0.009) (Table 2).
Table 2.
Change in AUA-SI score after 2 years of follow-up among elderly men reporting no or mild LUTS at baseline in the MrOS Study
| Change in score at follow-up | ||||
|---|---|---|---|---|
| AUA score ≤ 7 pts | AUA score ≥ 8pts with: | |||
| 1–3 points increase from baseline |
≥ 4 point increase from baseline |
|||
| Baseline AUA-SI score ≤7 points | (No.) | No. (%) | No. (%) | No. (%) |
| All in category at baseline | 3092 | 2209 (71.4%) | 219 (7.1%) | 664 (21.5%) |
| No treatment (medication or surgery) before baseline or during follow-up |
2059 | 1524 (74.2%) | 139 (6.8%) | 396 (19.2%) |
| Prostate surgery* | ||||
| None ever reported | 2452 | 1754 (71.5%) | 175 (7.1%) | 523 (21.3%) |
| Surgery before baseline | 564 | 407 (72.2%) | 38 (6.7%) | 119 (21.1%) |
| Surgery during follow-up | 73 | 45 (61.6%) | 6 (8.2%) | 22 (30.1%) |
| Age group (years) | ||||
| 65 – 69 | 1036 | 761 (73.5%) | 68 (6.6%) | 207 (20.0%) |
| 70 – 79 | 1594 | 1144 (71.8%) | 115 (7.2%) | 335 (21.0%) |
| ≥ 80 | 462 | 304 (65.8%) | 36 (7.8%) | 122 (26.4%) |
Abbreviations: AUA-SI, American Urological Association Symptom Index.
Prostate surgery was defined as self-report of removal of the prostate gland to treat prostate cancer or surgery to BPH. Surgery status for 2 men could not be determined.
LUTS progression
Of the men who reported AUA-SI of ≥8 points at baseline, 25% reported scores at least 4 points lower at follow-up, 51% had minor change, and 24% worsened by at least 4 points (Table 3). Comparable proportions were observed in analyses restricted to men who reported no treatment for BPH before or during follow-up. Among men with moderate symptoms at baseline, the progression was as frequent as in the entire group, whereas about 45% of men with severe baseline symptoms reported a change consistent with improvement and 17% reported change consistent with symptom progression.
Table 3.
Change in AUA-SI score after 2 years of follow-up among elderly men reporting moderate or severe LUTS at baseline in the MrOS Study
| Change in AUA-SI score during follow-up | ||||
|---|---|---|---|---|
| ≤ -4 points | -3 to 3 points | ≥ 4 points | ||
| Baseline AUA-SI score ≥8 points | (No.) | No. (%) | No. (%) | No. (%) |
| All in category | 2605 | 659 (25.3%) | 1324 (50.8%) | 622 (23.9%) |
| No treatment before baseline or during follow-up |
1275 | 317 (24.9%) | 683 (53.6%) | 275 (21.6%) |
| Baseline LUTS severity | ||||
| Moderate (AUA-SI 8–19 pts) | 2233 | 492 (22.0%) | 1181 (52.9%) | 560 (25.1%) |
| Severe (AUA-SI ≥ 20 pts) | 372 | 167 (44.9%) | 143 (38.4%) | 62 (16.7%) |
| Prostate surgery** | ||||
| None ever reported | 2081 | 513 (24.7%) | 1085 (52.1%) | 483 (23.2%) |
| Surgery before baseline | 407 | 86 (21.1%) | 202 (49.6%) | 119 (29.2%) |
| Surgery during follow-up | 114 | 59 (51.8%) | 36 (31.6%) | 19 (16.7%) |
| Age group (years) | ||||
| 65 – 69 | 688 | 175 (25.4%) | 361 (52.5%) | 152 (22.1%) |
| 70 – 79 | 1410 | 361 (25.6%) | 712 (50.5%) | 337 (23.9%) |
| ≥ 80 | 507 | 123 (24.3%) | 251 (49.5%) | 133 (26.2%) |
Abbreviations: AUA-SI, American Urological Association Symptom Index.
History of prostate surgery was defined as self-report of removal of the prostate gland to treat prostate cancer or surgery to treat BPH. Surgery status for 3 men could not be determined.
Urinary bother
The majority of men reported clinically insignificant bother scores both at baseline and follow-up (Table 4). However, among men without clinically significant bother at baseline, the proportions with onset of clinically significant bother increased with increased baseline symptom severity or increase in ≥4 points in AUA-SI score during follow-up. Significant bother persisted in over 40% of those reporting significant bother at baseline. The report of more bothersome symptoms was substantially increased among men with severe symptoms at baseline and those who reported an increase of ≥ 4 points on the AUA-SI. Onset of bother did not vary substantially by age (Table 4).
Table 4.
Change in urinary bother score after 2 years of follow-up among elderly men in the MrOS Study
| Bother score at end of follow-up* | ||
|---|---|---|
| Insignificant (≤ 3 points) | Significant (> 3 points) | |
| Baseline bother status | No. (%) | No. (%) |
| Entire cohort | ||
| Insignificant bother (N = 4941) | 4615 (93.5) | 323 (6.5) |
| Significant bother (N = 756) | 432 (57.2) | 323 (42.8) |
| Baseline LUTS Severity | ||
| None / Mild | ||
| Insignificant bother (N = 3013) | 2907 (96.5) | 105 (3.5) |
| Significant bother (N = 79) | 54 (69.2) | 24 (30.8) |
| Moderate | ||
| Insignificant bother (N = 1776) | 1587 (89.5) | 187 (10.5) |
| Significant bother (N = 457) | 281 (61.5) | 176 (38.5) |
| Severe | ||
| Insignificant bother (N = 152) | 121 (79.6) | 31 (20.4) |
| Significant bother (N = 220) | 97 (44.1) | 123 (55.9) |
| Baseline age group (years) | ||
| 65 – 69 years | ||
| Insignificant bother (N = 1530) | 1428 (93.4) | 101 (6.6) |
| Significant bother (N = 194) | 111 (57.5) | 82 (42.5) |
| 70 –79 years | ||
| Insignificant bother (N = 2616) | 2453 (93.8) | 162 (6.2) |
| Significant bother (N = 388) | 224 (57.7) | 164 (42.3) |
| ≥ 80 years | ||
| Insignificant bother (N = 795) | 734 (92.4) | 60 (7.6) |
| Significant bother (N = 174) | 97 (55.8) | 77 (44.3) |
| AUA-SI change during follow-up | ||
| ≤ -4 points | ||
| Insignificant bother (N = 519) | 504 (97.1) | 15 (2.9) |
| Significant bother (N = 217) | 163 (75.5) | 53 (24.5) |
| -3 to 3 points | ||
| Insignificant bother (N = 3119) | 3014 (96.7) | 104 (3.3) |
| Significant bother (N = 350) | 204 (58.3) | 146 (41.7) |
| ≥ 4 points | ||
| Insignificant bother (N = 1303) | 1097 (84.3) | 204 (15.7) |
| Significant bother (N = 189) | 65 (34.4) | 124 (65.6) |
Four men had missing values for the LUTS bother score on the follow-up questionnaire.
Treatment during follow-up
During follow-up among the 2200 men with baseline AUA-SI ≤7 who reported no history of treatment for BPH or prostate symptoms at baseline, 94% remained untreated, 2% reported new BPH surgery, and 4% reported new prescription medication use. Among these men, proportions undergoing new treatment were similar regardless of AUA-SI score category at follow-up. No treatment was reported among 95% of those whose score remained ≤7 points and among 91% of those whose AUA-SI score increased to ≥ 8 points.
During follow-up among the 1528 men with baseline AUA-SI ≥8 who reported no history of treatment at baseline, 83% remained untreated, 4% reported new surgery, and 13% reported new prescription medication use.
Discussion
Our study describes the considerable extent to which LUTS affects community-dwelling older men: in this cohort, nearly 1 in 3 older men without substantial urinary symptoms developed clinically significant LUTS within 2 years and about 1 in 4 men with moderate or severe symptoms experienced progression. While the overall onset of clinically significant urinary bother was generally low (<10%), it is notable that bother persisted in nearly half of the men who reported it at baseline.
It is important to note that the natural history of LUTS within populations is a dynamic process.23 LUTS may progress as well regress or resolve, either spontaneously or with treatment. Our data corroborate this pattern. Nevertheless, the overall increase in mean AUA-SI score, clinically significant urinary bother, and LUTS progression that we observed strongly suggest that—with or without regression of symptoms in a proportion of the men—the overall urinary health of this population deteriorated during the study period. In addition, because only a single AUA-SI score was utilized to characterize baseline LUTS, regression to the mean may have potentially contributed to measurement error and misclassification bias. However, there is no evidence that differential misclassification bias occurred.
The incidence of LUTS onset in the MrOS cohort is substantially greater than in the Olmsted County cohort.13 There are several possible explanations for this observation. First, LUTS onset may occur more frequently among older men. The average age of MrOS participants in this study was 73 years, and none of the participants was younger than 65 years. The Olmsted County cohort included men as young as 40 years. Second, the likelihood of LUTS onset may have increased during the 10 years that elapsed between the two studies. LUTS prevalence increases with age,2, 3, 5, 6 and the male population is aging: current estimates are that the number of individuals 80 years and older in the U.S. will rise from 9.3 million in 2000 to 19.5 million in 2030, an increase of over 100%.24 Finally, the Olmsted County cohort utilized a different instrument to collect urinary symptom data—a process that may have led to minor variations in scores.
Despite a shorter follow-up interval in MrOS, which potentially allowed for less time to characterize temporal trends, LUTS onset was also more common in MrOS than in the aforementioned Japanese, black American and Austrian cohorts, which measured symptoms with the International Prostate Symptom Score (I-PSS). These cohorts, however, were substantially younger and smaller; moreover, racial variations potentially confound comparisons of our cohort with the black American cohort.14–16 Notably, since MrOS men are generally healthy, it is possible that LUTS prevalence, onset and progression may be even higher in a wider population of older men. A persistent conundrum in designing population-based studies of urinary symptoms in older men is case definition. LUTS describes a distinct phenotype of a group of disorders affecting the prostate and bladder that share a common clinical manifestation. Our study represents a broad, epidemiological description of LUTS; our purpose was to study urinary symptoms at a population level without identifying organ-or disease- specific causes. This approach allows consideration of LUTS within a macroscopic context using a uniform definition unbiased by variable definitions of BPH and other diseases. In recent observational studies, LUTS has become the preferred term for studying urinary symptoms in populations.3, 4, 6, 25
Safe, low cost, and effective prevention of LUTS and BPH among asymptomatic or mildly symptomatic individuals may substantially improve the public health. Emerging data indicate that modifiable metabolic and lifestyle factors—including obesity, diabetes, diet, and physical activity—are strongly associated with BPH and LUTS.26–30 These factors represent novel and feasible targets for prevention. A necessary first step of LUTS prevention is to describe the incidence of LUTS in at-risk populations such as MrOS. Therefore, these data may contribute to the development of clinical strategies to prevent LUTS. Our data may inform the design of prospective clinical trials in at least 2 ways: first, by identifying vulnerable yet asymptomatic men most likely to benefit from targeted preventive therapies; second, by providing the frequency of LUTS onset in untreated men to allow for precise power calculations.
For many health care providers, it is tempting to dismiss LUTS as a relatively harmless disorder representing more an inexorable consequence of aging and less a salient public health issue. This interpretation, however, belies the substantial medical, psychological, and economic burdens of this condition and does a great disservice to this population. Our data demonstrate that greater attention to the possibility of LUTS progression should be an essential component of clinical care for elderly men. From a clinical perspective, these data may prove useful for advising older men about the prospect of worsening (or waning) symptoms or the possibility that treatment may not prevent symptom progression. Given the considerable number of men who could be expected to experience symptom worsening, efforts between urologists and geriatricians to ameliorate possible consequences on quality of life may prove useful.
Conclusions
In this cohort of community dwelling older men, 29% of those without significant urinary symptoms at baseline developed clinically significant LUTS within 2 years of follow-up. The majority of men with moderate or severe LUTS at baseline reported persistent moderate or severe LUTS at 2-year follow-up and a large proportion reported symptom progression. These results underscore the formidable challenges LUTS poses to the public health and provide useful data for designing clinical trials of LUTS prevention in the community.
Acknowledgments
Funding: The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Institute on Aging (NIA), the National Center for Research Resources (NCRR), and NIH Roadmap for Medical Research under the following grant numbers: U01 AR45580, U01 AR45614, U01 AR45632, U01 AR45647, U01 AR45654, U01 AR45583, U01 AG18197, U01-AG027810, and UL1 RR024140.
Dr. Parsons is supported by Department of Defense Physician Research Training Award PC073412 and National Cancer Institute Awards CA32102 and P30 CA23100-23.
Abbreviations
- AUA-SI
American Urological Symptom Index
- BPH
benign prostatic hyperplasia
- LUTS
lower urinary tract symptoms
- MrOS
Osteoporotic Fractures in Men
- SD
standard deviation
References
- 1.Wei JT, Calhoun E, Jacobsen SJ. Urologic diseases in America project: benign prostatic hyperplasia. J Urol. 2005;173:1256. doi: 10.1097/01.ju.0000155709.37840.fe. [DOI] [PubMed] [Google Scholar]
- 2.Boyle P, Robertson C, Mazzetta C, et al. The prevalence of lower urinary tract symptoms in men and women in four centres. The UrEpik study. BJU Int. 2003;92:409. doi: 10.1046/j.1464-410x.2003.04369.x. [DOI] [PubMed] [Google Scholar]
- 3.Kupelian V, Wei JT, O'Leary MP, et al. Prevalence of lower urinary tract symptoms and effect on quality of life in a racially and ethnically diverse random sample: the Boston Area Community Health (BACH) Survey. Arch Intern Med. 2006;166:2381. doi: 10.1001/archinte.166.21.2381. [DOI] [PubMed] [Google Scholar]
- 4.Taylor BC, Wilt TJ, Fink HA, et al. Prevalence, severity, and health correlates of lower urinary tract symptoms among older men: the MrOS study. Urology. 2006;68:804. doi: 10.1016/j.urology.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 5.Trueman P, Hood SC, Nayak US, et al. Prevalence of lower urinary tract symptoms and self-reported diagnosed 'benign prostatic hyperplasia', and their effect on quality of life in a community-based survey of men in the UK. BJU Int. 1999;83:410. doi: 10.1046/j.1464-410x.1999.00966.x. [DOI] [PubMed] [Google Scholar]
- 6.Parsons JK, Bergstrom J, Silberstein J, et al. Prevalence and characteristics of lower urinary tract symptoms in men aged > or = 80 years. Urology. 2008;72:318. doi: 10.1016/j.urology.2008.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engstrom G, Henningsohn L, Steineck G, et al. Self-assessed health, sadness and happiness in relation to the total burden of symptoms from the lower urinary tract. BJU Int. 2005;95:810. doi: 10.1111/j.1464-410X.2005.05406.x. [DOI] [PubMed] [Google Scholar]
- 8.Engstrom G, Henningsohn L, Walker-Engstrom ML, et al. Impact on quality of life of different lower urinary tract symptoms in men measured by means of the SF 36 questionnaire. Scand J Urol Nephrol. 2006;40:485. doi: 10.1080/00365590600830862. [DOI] [PubMed] [Google Scholar]
- 9.Engstrom G, Walker-Engstrom ML, Henningsohn L, et al. Prevalence of distress and symptom severity from the lower urinary tract in men: a population-based study with the DAN-PSS questionnaire. Fam Pract. 2004;21:617. doi: 10.1093/fampra/cmh607. [DOI] [PubMed] [Google Scholar]
- 10.Parsons JK, Mougey J, Lambert L, et al. Lower urinary tract symptoms increase the risk of falls in older men. BJU Int. 2009 doi: 10.1111/j.1464-410X.2008.08317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu TW, Wagner TH, Bentkover JD, et al. Estimated economic costs of overactive bladder in the United States. Urology. 2003;61:1123. doi: 10.1016/s0090-4295(03)00009-8. [DOI] [PubMed] [Google Scholar]
- 12.Saigal CS, Joyce G. Economic costs of benign prostatic hyperplasia in the private sector. J Urol. 2005;173:1309. doi: 10.1097/01.ju.0000152318.79184.6f. [DOI] [PubMed] [Google Scholar]
- 13.Jacobsen SJ, Girman CJ, Guess HA, et al. Natural history of prostatism: longitudinal changes in voiding symptoms in community dwelling men. J Urol. 1996;155:595. doi: 10.1016/s0022-5347(01)66461-9. [DOI] [PubMed] [Google Scholar]
- 14.Masumori N, Tsukamoto T, Rhodes T, et al. Natural history of lower urinary tract symptoms in men--result of a longitudinal community-based study in Japan. Urology. 2003;61:956. doi: 10.1016/s0090-4295(02)02594-3. [DOI] [PubMed] [Google Scholar]
- 15.Temml C, Brossner C, Schatzl G, et al. The natural history of lower urinary tract symptoms over five years. Eur Urol. 2003;43:374. doi: 10.1016/s0302-2838(03)00084-8. [DOI] [PubMed] [Google Scholar]
- 16.Sarma AV, McLaughlin JC, Jacobsen SJ, et al. Longitudinal changes in lower urinary tract symptoms among a cohort of black American men: the Flint Men's Health Study. Urology. 2004;64:959. doi: 10.1016/j.urology.2004.06.043. [DOI] [PubMed] [Google Scholar]
- 17.Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study--a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26:569. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Barry MJ, Fowler FJ, Jr, O'Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148:1549. doi: 10.1016/s0022-5347(17)36966-5. [DOI] [PubMed] [Google Scholar]
- 19.Pahor M, Chrischilles EA, Guralnik JM, et al. Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol. 1994;10:405. doi: 10.1007/BF01719664. [DOI] [PubMed] [Google Scholar]
- 20.Barry MJ, Fowler FJ, Jr, O'Leary MP, et al. Correlation of the American Urological Association symptom index with self-administered versions of the Madsen-Iversen, Boyarsky and Maine Medical Assessment Program symptom indexes. Measurement Committee of the American Urological Association. J Urol. 1992;148:1558. doi: 10.1016/s0022-5347(17)36967-7. [DOI] [PubMed] [Google Scholar]
- 21.McConnell JD, Roehrborn CG, Bautista OM, et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med. 2003;349:2387. doi: 10.1056/NEJMoa030656. [DOI] [PubMed] [Google Scholar]
- 22.AUA guideline on management of benign prostatic hyperplasia (2003). Chapter 1: Diagnosis and treatment recommendations. J Urol. 2003;170:530. doi: 10.1097/01.ju.0000078083.38675.79. [DOI] [PubMed] [Google Scholar]
- 23.Sarma AV, Jacobsen SJ, Girman CJ, et al. Concomitant longitudinal changes in frequency of and bother from lower urinary tract symptoms in community dwelling men. J Urol. 2002;168:1446. doi: 10.1016/S0022-5347(05)64471-0. [DOI] [PubMed] [Google Scholar]
- 24.Control CfD. Public Health and Aging: Trends in Aging: United States and Worldwide. 2003 [Google Scholar]
- 25.Rosen R, Altwein J, Boyle P, et al. Lower urinary tract symptoms and male sexual dysfunction: the multinational survey of the aging male (MSAM-7) Eur Urol. 2003;44:637. doi: 10.1016/j.eururo.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 26.Kristal AR, Arnold KB, Schenk JM, et al. Dietary Patterns, Supplement Use, and the Risk of Symptomatic Benign Prostatic Hyperplasia: Results from the Prostate Cancer Prevention Trial. Am J Epidemiol. 2008;167:925. doi: 10.1093/aje/kwm389. [DOI] [PubMed] [Google Scholar]
- 27.Kristal AR, Arnold KB, Schenk JM, et al. Race/ethnicity, obesity, health related behaviors and the risk of symptomatic benign prostatic hyperplasia: results from the prostate cancer prevention trial. J Urol. 2007;177:1395. doi: 10.1016/j.juro.2006.11.065. [DOI] [PubMed] [Google Scholar]
- 28.Parsons JK. Modifiable risk factors for benign prostatic hyperplasia and lower urinary tract symptoms: new approaches to old problems. J Urol. 2007;178:395. doi: 10.1016/j.juro.2007.03.103. [DOI] [PubMed] [Google Scholar]
- 29.Parsons JK, Carter HB, Partin AW, et al. Metabolic factors associated with benign prostatic hyperplasia. J Clin Endocrinol Metab. 2006;91:2562. doi: 10.1210/jc.2005-2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parsons JK, Kashefi C. Physical activity, benign prostatic hyperplasia, and lower urinary tract symptoms. Eur Urol. 2008;53:1228. doi: 10.1016/j.eururo.2008.02.019. [DOI] [PubMed] [Google Scholar]