Abstract
The quality of life of people suffering from severe motor disabilities can benefit from the use of current assistive technology capable of ameliorating communication, house-environment management and mobility, according to the user's residual motor abilities. Brain Computer Interfaces (BCIs) are systems that can translate brain activity into signals that control external devices. Thus they can represent the only technology for severely paralyzed patients to increase or maintain their communication and control options.
Here we report on a pilot study in which a system was implemented and validated to allow disabled persons to improve or recover their mobility (directly or by emulation) and communication within the surrounding environment. The system is based on a software controller that offers to the user a communication interface that is matched with the individual's residual motor abilities. Patients (n=14) with severe motor disabilities due to progressive neurodegenerative disorders were trained to use the system prototype under a rehabilitation program carried out in a house-like furnished space. All users utilized regular assistive control options (e.g., microswitches or head trackers). In addition, four subjects learned to operate the system by means of a non-invasive EEG-based BCI. This system was controlled by the subjects' voluntary modulations of EEG sensorimotor rhythms recorded on the scalp; this skill was learnt even though the subjects have not had control over their limbs for a long time.
We conclude that such a prototype system, which integrates several different assistive technologies including a BCI system, can potentially facilitate the translation from pre-clinical demonstrations to a clinical useful BCI.
Keywords: EEG-based Brain-Computer Interfaces, Assistive Robotics, Severe Motor Impairment, Technologies for Independent Life
Introduction
The ultimate objective of a rehabilitation program is the reduction of the disability due to a given pathological condition, that is, the achievement for that clinical status of maximum independence by means of orthoses and by the management of the social disadvantages related to the disability by using different types of aids.
Recently, the development of electronic devices that are capable of assisting in communication and control needs (such as environmental control or Assistive Technology) has opened new avenues for patients affected by severe movement disorders. This development includes impressive advancements in the field of robotics. Indeed, the morphology of robots has remarkably mutated: from the fixed-base industrial manipulator, it has evolved into a variety of mechanical structures. These structures are are often capable of locomotion using either wheels or legs [1]. As a direct consequence, the domain of robots' application has increased substantially, including assistance to hospital patients and disabled people, automatic surveillance, space exploration and many others [2]. In the case of robotic assistive devices for severe motor impairments, they still suffer from limitations due to the necessity of residual motor ability (for instance, limb, head and/or eye movements, speech and/or vocalization). Patients in extreme pathological conditions (i.e., those that do not have any or only unreliable remaining muscle control) may in fact be prevented from use of such systems. Brain Computer Interface (BCI) technology “gives their users communication and control channels that do not depend on the brain's normal output channels of peripheral nerves and muscles.” [3], and can allow completely paralyzed individuals to communicate with the surrounding environment [4][5]. A BCI detects activation patterns in the brain that correspond to the subject's intent. Whenever the user induces a voluntary modification of these patterns, the BCI system is able to detect it and to translate it into an action that reflects the user's intent. Several animal and some human studies have shown the possibility to use electrical brain activity recorded within the brain to directly control the movement of robots or prosthetic devices in real time using microelectrodes implanted within the brain [6][7][8][9][10]. Other BCI systems depend on brain activity recorded non-invasively from the surface of the scalp using electroencephalography (EEG). EEG-based BCIs can be operated by modulations of EEG rhythmic activity located over scalp sensorimotor areas that are induced by motor imagery tasks [11]; these modulations can be used to control a cursor on a computer screen [12] or a prosthetic device for limited hand movements [13][14]. Thus, it has become conceivable to extend the communication between disabled individuals and the external environment from mere symbolic interaction (e.g. alphabetic spelling) to aid for mobility. A pioneering application of BCI consisted of controlling a small mobile robot through the rooms of a model house [15]. The recognition of mental activity could be put forward to guide devices (mobile robots) or to interact naturally with common devices within the external word (telephone, switch; etc). This possible application of BCI technology has not been studied yet. Its exploration was the principal aim of this study.
These considerations prompted us to undertake a study with the aim of integrating different technologies (including a BCI and a robotic platform) into a prototype assistive communication platform. The goal of this effort was to demonstrate that application of BCI technology in people's daily life is possible, including for people who suffer from diseases that affect their mobility. The current study, which is part of a project named ASPICE, addressed the implementation and validation of a technological aid that allows people with motor disabilities to improve or recover their mobility and communicate within the surrounding environment. The key elements of the system are:
Interfaces for easy access to a computer: mouse, joystick, eye tracker, voice recognition, and utilization of signals collected directly but non-invasively from the brain using an EEG-based BCI system. The rationale for the multiple access capacities was twofold: i) to widen the range of users, but tailoring the system to the different degrees of patient disability; ii) to track individual patient's increase or decrease (because of training or reduction of abilities, respectively) to interact with the system, according to the residual muscular activity present at the given moment of the disease course and eventually to learn to control the system with different accesses (up to the BCI) because of the nature of neurodegenerative diseases which provoke a time progressive loss of strength in different muscular segments.
Controllers for intelligent motion devices that can follow complex paths based on a small set of commands;
Information transmission and domotics that establish the information flow between subjects and the appliances they are controlling.
The goal pursued by designing this system was to fulfill needs (related to several aspects of daily activities) of a class of neuromuscular patients by blending several current technologies into an integrated framework. We strove to use readily available hardware components, so that the system could be practically replicated in other home settings.
The validation of the system prototype has been initially realized with the participation of healthy volunteers and subsequently with subjects with severe motor disabilities due to progressive neurodegenerative disorders. The disabled subjects described in this report were trained to use the system prototype with different types of access during a rehabilitation program carried out in a house-like furnished space.
Materials and Methods
Subjects and clinical experimental procedures
In this study, 14 able-bodied subjects and 14 subjects suffering from Spinal Muscular Atrophy type II (SMA II) or Duchenne Muscular Dystrophy (DMD) underwent system training. These neuromuscular diseases cause a progressive and severe global motor impairment that substantially reduces the subject's autonomy. Thus, these subjects required constant support by nursing staff. Subjects were informed regarding the general features and aims of the study, which was approved by the ethics committee of the Santa Lucia Foundation. All subjects (and their relatives when required) gave their written informed consent. In particular, an interactive discussion with the patients and their relatives allowed assessment of the needs of individual patients. This allowed for appropriate system customization. The characteristics of these patients are reported in Table 1. In general, all patients have been unable to walk since they were adolescent. They all relied on a wheelchair for mobility. All wheelchairs except two were electrically powered and were controlled by a modified joystick that could be manipulated by either the residual “fine” movements of the first and second finger or the residual movements of the wrist. All patients had poor residual muscular strength either of proximal or distal arm muscles. Also, all patients required a mechanical support to maintain neck posture. Finally, all patients retained effective eye movement control. Prior to the study, no patient used technologically advanced aids.
TABLE 1.
Characteristics of patients
| Users | Age | Diagnosis | BI | Electric Wheelchair Control (*) | Artificial Ventilation | Upper Limb Function | Speech | |
|---|---|---|---|---|---|---|---|---|
| 1 | (f) | 31 | SMA (II) | 33 | Yes (5) | No | Minimal | Yes |
| 2 | (f) | 25 | SMA (II) | 41 | Yes (5) | No | Minimal | Yes |
| 3 | (m) | 30 | DMD | 23 | No (0) | Yes | Minimal | Slow |
| 4 | (m) | 34 | DMD | 32 | Yes (5) | No | Minimal | Slow |
| 5 | (m) | 16 | DMD | 27 | Yes (5) | No | Minimal | Slow |
| 6 | (m) | 29 | SMA (II) | 27 | Yes (5) | Yes | Minimal | Yes |
| 7 | (m) | 35 | DMD | 23 | Yes (1) | Yes | Minimal | Slow |
| 8 | (f) | 35 | SMA (II) | 46 | Yes (4) | No | Weak | Yes |
| 9 | (f) | 44 | SMA (II-III) | 40 | Yes (4) | No | Yes | Yes |
| 10 | (m) | 16 | DMD | 26 | Yes (5) | No | Minimal | Yes |
| 11 | (m) | 12 | SMA (II) | 50 | Yes (5) | No | Yes | Yes |
| 12 | (m) | 32 | DMD | 23 | Yes (1) | No | Minimal | Yes |
| 13 | (m) | 16 | SMA (II) | 38 | Manual (3) | No | Weak | Yes |
| 14 | (f) | 55 | SMA (II) | 36 | Yes (5) | No | Weak | Yes |
0= completely dependent; 5= independent
The clinical experimentation took place at the Santa Lucia Foundation and Hospital where the system prototype (ASPICE) was installed in a three-room space that was furnished like a common house and devoted to Occupational Therapy. Patients were admitted to the hospital for a neurorehabilitation program. The first step in the clinical procedure consisted of an interview and physical examination performed by the clinicians. This interview determined several variables of interest as follows: the degree of motor impairment and reliance on the caregivers for everyday activities, as assessed by current standardized scale (Barthel Index -BI- for ability to perform daily activities [16]); the familiarity with transducers and aids (sip/puff, switches, speech recognition, joysticks) that could be used as input to the system; the ability to speak or communicate with an unfamiliar person; the level of informatics alphabetization measured by the number of hours / week spent in front of a computer. Corresponding questions were structured in a questionnaire that was administered to the patients at the beginning and end of the training. A level of system acceptance by the users was schematized by asking the users to indicate with a number ranging from 0 (not satisfied) to 5 (very satisfied) their degree of acceptance relative to each of the output devices controlled by the most individual adequate access. The training consisted of weekly sessions; for a period of time ranging from 3 to 4 weeks (except in the case of BCI training, see below), the patient and (when required) her/his caregivers were practicing with the system. During the whole period, patients had the assistance of an engineer and a therapist who facilitated interaction with the system.
System prototype input and output devices
The system architecture, with its input and output devices, is outlined in Figure 1. A three-room space in the hospital was furnished like a common house, and the actuators of the system were installed. Care was taken to make an installation that would be easily replicable in most houses. The place was provided with a portable computer to run the core program (see Results section). This core program was interfaced with several input devices that supported a wide range of motor capacities from a wide variety of users. For instance, keyboard, mouse, joystick, trackball touchpad and buttons allowed access to the system through upper limb residual motor abilities. Otherwise, microphone and head tracker could be used when motor disability was extremely impairing for the limbs but the neck muscles or comprehensible speech were preserved. Thus, we could customize these input devices to the users' residual motor abilities. In fact, users could utilize the aids they were already familiar with (if any), and that have been interfaced to provide a low level input to a more sophisticated assistive device. On the other hand, the variety of input devices provided robustness to the decrease of patient's ability, which is a typical consequence of degenerative diseases.
Figure 1.
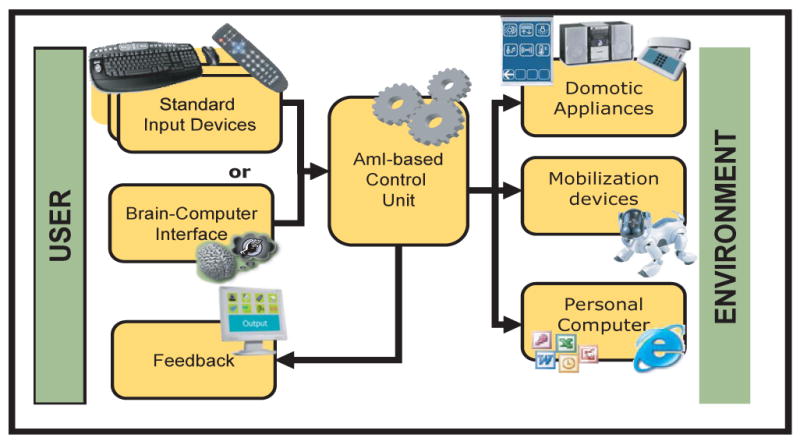
Outline of the architecture of the ASPICE project. The figure shows that the system interfaces the user to the surrounding environment. The modularity is assured by the use of a core unit that takes inputs by one of the possible input devices and sends commands to one or more of the possible actuators. Feedback is provided to keep the user informed about the status of the system.
When the user was not able to master any of the above mentioned devices, or when the nature of a degenerative disease suggested that the patient may not be able to use any of the devices in the future, the support team proposed to the patient to start training on the use of a BCI.
As for the system output devices, we considered (also based upon patient's needs/wishes), a basic group of domotics appliances such as neon lights and bulbs, TV and stereo sets, motorized bed, acoustic alarm, front door opener, telephone and wireless cameras (to monitor the different rooms of the house ambient). The system also included a robotic platform (a Sony AIBO) to act as an extension of the ability of the patient to move around the house (“virtual” mobility). The AIBO was meant to be controlled from the system control unit in order to accomplish few simple tasks with a small set of commands. As previously mentioned, the system should cope with a variety of disabilities depending on the patient conditions. Therefore, three possible navigation systems were designed for robot control: single step, semi-autonomous, and autonomous mode. Each navigation mode was associated with a Graphical User Interface in the system control unit (see Results section).
Brain Computer Interface (BCI) framework and subject training
As described, the system contained a BCI module meant to translate commands from users that cannot use any of the conventional aids. This BCI system was based on detection of simple motor imagery (mediated by modulation of sensorimotor EEG rhythms) and was realized using the BCI2000 software system [17]. Users needed to learn to modulate their sensorimotor rhythms to achieve more robust control than the simple imagination of limb movements can produce. Using a simple binary task as performance measure, training is meant to improve performances from 50-70% to 80-100%. An initial screening session suggested, for each subject, the signal features (i.e., amplitudes at particular brain locations and frequencies) that could best discriminate between imagery and rest. The BCI system was then configured to use these brain signal feature, and to thus translate the user's brain signals into output control signals that were communicated to the Aspice central unit.
During the initial screening session, subjects were comfortably seated on a reclining chair (or when necessary a wheelchair), in an electrically shielded, dimly lit room. Scalp activity was collected with a 96 channel EEG system (BrainAmp, Brainproducts GmbH, Germany). EEG data sampling frequency was 200 Hz; signals were bandpass-filtered between 0.1 and 50 Hz before digitization. In this screening session, the subject was not provided with any feedback (any representation of her/his brain signals). The screening session consisted of alternate and random presentation of cues on opposite sides of the screen (either up/down, i.e., vertical, or left/right, i.e., horizontal). In coupled runs, the subject was asked to execute (first run) or to image (second run) movements of her/his hands or feet upon the appearance of top or bottom target, respectively. In horizontal runs, the targets appeared on the left or right side of the screen and the subject was asked to move (odd trials) or to imagine (even trials) his/her left or right hand. In vertical runs, the targets appeared on top or bottom of the screen, and the subject had to concentrate on his/her upper or lower limbs. This sequence was repeated three times for a total of 12 trials.
We then analyzed the brain signals recorded during these tasks offline. In these analyses, we compared brain signals associated with the top target to those associated with the bottom target, and did the same for left and right targets. These analyses aimed at detecting a set of EEG features that maximized prediction of the current cue. The analysis was carried out by replicating the same signal conditioning and feature extraction that was subsequently used in on-line processing (training session). Data sets were divided into epochs (usually 1 second long) and spectral analysis is performed by means of a Maximum Entropy algorithm with a resolution of 2Hz. Differently from the on-line processing, when the system only computes the few features relevant for BCI control, all possible features in a reasonable range (i.e., 0-60 Hz in 2 Hz bins) were extracted and analyzed simultaneously. A feature vector was extracted from each epoch. This vector was composed of the spectral amplitude at each frequency bin for each channel. When all features in the two datasets under contrast were extracted, a statistical analysis (r2, i.e., the proportion of the total variance of the signal amplitude accounted for by target position [18]) was performed to assess significant differences in the values of each feature in the two conditions. At the end of this process, r2 values were compiled in a channel-frequency matrix and head topography (examples are shown in Figures 3 and 4 in the Results section) and evaluated to identify the set of candidate features to be enhanced with training.
Figure 3.
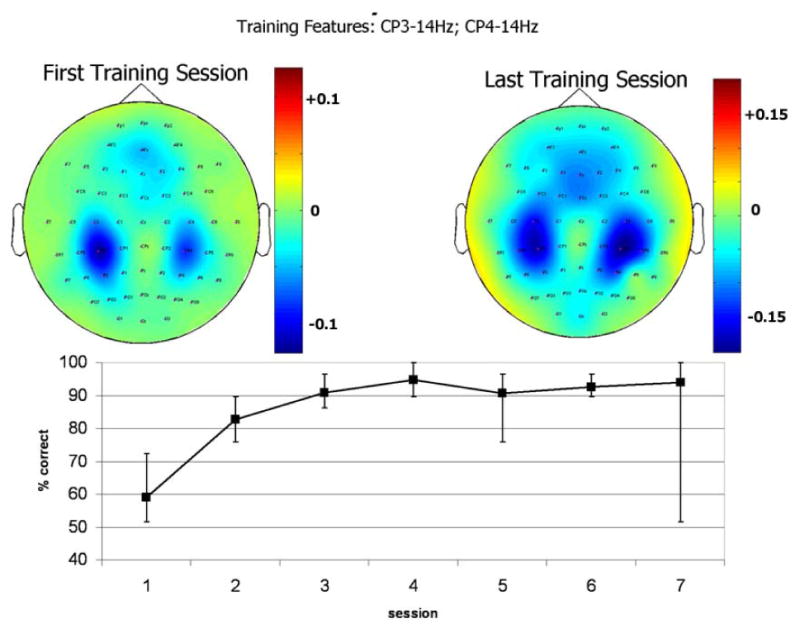
Relevant EEG features and learning curve of a representative able-bodied user. Top panel: topographical maps of r2 values during the first (to the left) and the last (to the right) training sessions, for EEG spectral features extracted at 14 Hz. The patterns changed both in spatial distribution and in absolute value (note the different color scales). Bottom panel: time course of BCI performance over training sessions, as measured by the percentage of correctly selected targets. Error bars indicate the best and the worst experimental run in each session.
Figure 4.

EEG patterns related to the intentional brain control in a SMA patient. Left panel: spectral power density of the EEG of the most responsive channel. Red and blue lines correspond to the subset of trials in which the user tried to hit the top and the bottom target, respectively. Right Panel: Topographical distributions of r2 values at the most responsive frequency (33Hz). The red colored region corresponds to those regions of the brain that exhibited brain control.
During the following training sessions, the subjects were provided feedback of these features, so that they could learn how to improve their modulation. A subset of electrodes (out of the 59 placed on the scalp according to an extension of the 10-20 International System) were used to control the movement of a computer cursor, whose position was controlled in real time by the amplitude or the subject's sensorimotor rhythms. Each session lasted about 40 minutes and consists of eight 3-minutes runs of 30 trials each. We collected a total of 5-12 training sessions for each patient; training ended when performance was stabilized. Each subject's performance was assessed by accuracy (i.e., the percentage of trials in which the target was hit) and by r2 value. The training outcome was monitored over sessions. Upon successful training, the BCI was connected to the prototype system, and the subject was asked to utilize its button interface using BCI control.
During experimentation with the Aspice system, BCI2000 was configured to stream its output (current cursor position) in real time over a TCP/IP connection. Goals of the cursor were dynamically associated with an action of the system, similarly to commands issued through the other input devices (e.g. button presses).
Results
System prototype and robotic platform implementation
Implementation of the prototype system core started at the beginning of this study, and its successive releases took advantage of advice and daily interaction with the users. It was eventually realized as follows.
The core unit received the logical signals from the input devices and converted them into commands that could be used to drive the output devices. Its operation was organized as a hierarchical structure of possible actions, whose relationship could be static or dynamic. In the static configuration, it behaved as a “cascaded menu” choice system and was used to feed the Feedback module only with the options available at the moment (i.e. current menu). In the dynamic configuration, an intelligent agent tried to learn from use which would have been the most probable choice the user will make. The user could select the commands and monitor the system behavior through a graphical interface. Figure 2.A shows a possible appearance of the feedback screen, including a feedback stimulus from the BCI. The prototype system allowed the user to operate remotely electric devices (e.g. TV, telephone, lights, motorized bed, alarm, and a front door opener) as well as monitoring the environment with remotely controlled video cameras. While input and feedback signals were carried over a wireless communication, so that mobility of the patient was minimally affected, most of the actuation commands were carried via a powerline-based control system.
Figure 2.
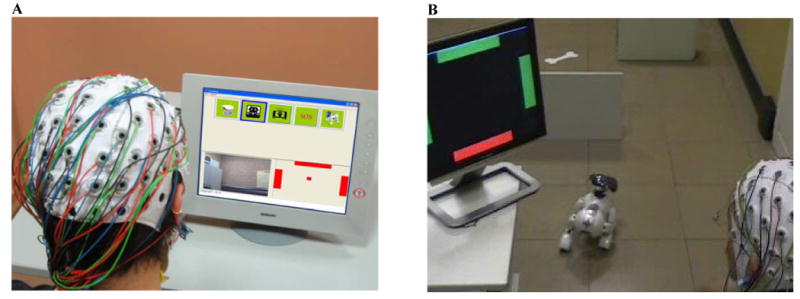
Panel A: Appearance of the feedback screen. In the feedback application, the screen is divided into three panels. In the top panel, the available selections (commands) appear as icons. In the bottom right panel, a feedback stimulus by the BCI (matching the one the subject has been training with) is provided. The user uses modulation of brain activity to move the cursor at the center to hit either the left or the right bars – in order to focus the previous or following icon in the top panel – or to hit the top bar – to select the current icon. In the bottom left panel, the feedback module displays the video stream from the video camera that was chosen beforehand in the operation. Panel B: An experiment of BCI-controlled navigation of the AIBO mobile robot. Here, the user is controlling the BCI to emulate a continuous directional joystick mode which drives the robot to its target (the bone). The robot automatically avoids obstacles.
The robotic platform (AIBO, Figure 2.B) was capable of three navigation modes that allowed us to serve the different needs of the users. The first mode was single-step navigation. In this mode, the user had complete control of robot movement. This was useful for fine motion in cluttered areas. The second mode was semi-autonomous navigation. In this mode, the user specified the main direction of motion and the robot automatically avoided obstacles. The third and final mode was autonomous navigation. In this mode, the user specified the target destination in the apartment (e.g., the living room, the bedroom, the bathroom, and the battery charging station). The robot autonomously traveled to the target. This mode was useful for quickly reaching some important locations, and for enabling AIBO to charge its battery autonomously when needed. We expected that this mode would be particularly useful for severely impaired patients who may be unable to send frequent commands. All three navigation modes contained some level of obstacle avoidance based on a two-dimensional occupancy grid (OG) built by the on-board laser range sensor, with the robot either stationary or in motion.
In single-step mode, the robot was driven, with a fixed step size, in one of six directions (forward, backward, lateral left/right, clockwise or counter clockwise rotations). Before performing the motion command, the robot generated an appropriate OG (oriented along the intended direction of motion) to verify whether the step could be performed without colliding with obstacles. Depending on the result of the collision check, the robot decided whether or not to step in the desired direction.
In semi-autonomous mode, the user specified a general direction of motion. Instead of executing a single step, the robot walked continuously in the specified direction until it received a new command (either a new direction or a stop). Autonomous obstacle avoidance was obtained by the use of artificial potential fields. The OG was generated as the robot moved, and then used to compute the robot velocities. Our algorithm used vortex and repulsive fields to build the velocity field. The velocity field was mapped to the configuration space velocities either with omnidirectional translational motion or by enforcing nonholonomic-like motion. The first conversion was consistent with the objective of maintaining as much as possible the robot orientation specified by the user whereas with the second kind of conversion, the OG provided more effective collision avoidance.
In autonomous navigation mode, the user controlled robot movement towards a fixed set of destinations. To allow the robot to autonomously reach these destinations, we designed a physical roadmap that connected all relevant destinations in the experimental arena. The robot used a computer vision algorithm to navigate. The roadmap consisted of streets and crossings, which were marked on the floor using white adhesive tape. Edge detection algorithms were used to visually identify and track streets (i.e., straight white lines) and crossings (i.e., coded squares), while path approaching and following algorithms were used to drive the robot. The robot behavior was represented by a Petri Nets based plan. The robot traveled towards the selected destination using a series of cascaded actions. Initially, the robot sought a street. When it detected a street, the AIBO approached it and subsequently followed it until at least one crossing was detected. Then, the robot identified its position and orientation on the roadmap. The robot then used a Dijkstra-based graph search to find the shortest path to its destination. Depending on the result of the graph search, the robot approached and followed another street (repeat the corresponding actions in the plan), or stop if the crossing corresponded to the desired destination.
The three navigation modes were compared in a set of experiments in which some of the able-bodied users controlled the robot to move from a source to a destination. The task was repeated 5 times for each of the three navigation modes and results were averaged. A mouse was used as input device for all modes. In semi-autonomous navigation, omnidirectional translational motion was used for mapping desired user velocities to the configuration space. Comparison between the three modes was based on execution time and user intervention (i.e., number of times the user had to intervene by clicking on the GUI for updating the commands; Table 2). According to the average execution time and user intervention, the qualitative properties expected for each mode were confirmed.
TABLE 2.
Comparison between the three navigation modes for robot platform control.
| Navigation mode | Execution time (sec) | User intervention (clicks) |
|---|---|---|
| single step | 107 | 11 |
| semi-autonomous | 83 | 5 |
| autonomous | 90 | 1 |
User feedback drew our attention to the noise produced by AIBO's walking. We minimized the noise by reducing the velocity of the legs' tips during ground contact.
Finally, the robot could assist the users in visually monitoring the environment and communicating with the caregiver. Visual monitoring was achieved by transmitting a video stream acquired by the robot camera to the control unit over a wireless connection; image compression was performed onboard before transmission. The robot could also be utilized for communication with the caregiver by requesting it to play pre-recorded vocal sentences (e.g., “I am thirsty” or “Please come”) on its speakers.
More information about the control strategy implemented for the AIBO, is available at [19].
Clinical validation
All 14 able-bodied subjects tested the successive releases of the system for 8-12 sessions. The purpose of system use by able-bodied subjects was to validate system security and safety. The system input devices were all functionally effective in controlling the domotic appliances and the small robotic device (AIBO). At the time of the study, these subjects were also enrolled in the BCI training with and without interfacing it with the system prototype. Early results on BCI training will be reported in the pertinent section of this paper.
Several patients (see Table 1) were also able to master the final release of the system within 5 sessions, performed once or twice a week. According to the score of the BI, all patients depended almost completely on caregivers, especially those with the diagnosis of DMD (n=6 subjects; BI score < 35) who required artificial ventilation, had minimal residual mobility of the upper limbs and very slow speech. Because of the high level of muscular impairment, five of the DMD patients had the best access to the system via joystick, which required minimal efficiency of the residual muscular contraction at the distal muscular segments of the upper limbs (minimal flexion-extension of the hand fingers). One additional DMD patient found a trackball to be most comfortable for her level of distal muscle strength (third patient in Table 1). The level of dependency for the 8 type I/II SMA patients was slightly higher compared to the DMA patients. Nevertheless, the SMA patients also required continuous assistance for daily life activity (BI ≤50). These patients had optimum access to the system via a joystick (3 patients), touchpad (2 patients), keyboard (1 patient), and button (2 patients). The variety in the access devices in this class of patients was related to a still functionally effective residual motor abilities of the upper limbs (mainly proximal muscles), both in terms of muscular strength and range of movements preserved. None of the patients was comfortable in accessing the system via head-tracker because of the weakness of the neck muscles. At the end of the training, all patients were able to control the domotic appliances and the robotic platform using one of the mentioned input methodologies. According to the early results of the questionnaire, all patients were independent in the use of the system at the end of the training and they experienced (as they reported) “the possibility to interact with the environment by myself.” A schematic evaluation of the degree of the system acceptance by the users revealed that amongst the several system outputs, the front door opener was the most accepted controlled device (mean score 4.93 in a range 1-5) whereas the robotic platform (AIBO) received the lowest score (mean 3.64). Four of the motor impaired users had interacted with the system via BCI (see below).
We documented this overall clinical experience in a system manual for future use by users and installers, and also described suggested training guidelines. This manual will eventually be available to the community.
Brain Computer Interface (BCI) application
Over the 8-12 sessions of training, subjects acquired brain control with an average accuracy higher than 75% (accuracy expected by chance alone was 50%) in a binary selection task. Table 3 shows the average accuracy for the last 3 of the 8-12 training sessions for each subject. As shown in Figure 3 for one representative normal subject (Subject 1 in Table 3), the topographical and spectral analysis of r2 values revealed that since the beginning of the training, motor cortical reactivity was localized over sensorimotor scalp areas. This pattern persisted over training and corresponded to good performance in cursor control. Four patients out of 14 underwent a standard BCI training (Table 3, P1-4). Similar to healthy subjects, these patients acquired brain control that supported as accuracies above 60% in the standard binary decision task. The patients employed imagery of foot or hand movements. Brain signal changes associated with these imagery tasks were mainly located at midline centro-parietal electrode positions. Figure 4 shows for one representative patient (second row in Table1; P1 in Table 2) in a session near the end of training, the scalp topography of r2 at the frequency used to control the cursor with an average accuracy of 80%. In this case, control was focused at Cz (i.e., the vertex of the head).
TABLE 3.
Brain control in normal subjects: motor imagery used, scalp location, frequency band and average accuracy over the last three sessions
| User | Task | Location | Frequency | Accuracy(%) |
|---|---|---|---|---|
| S01 | R Hand-Up L Hand-Down | CP4-CP3 | 12-14 | 86.1 |
| S02 | Hands-Up Feet-Down | C4-Cz | 14-26 | 82.2 |
| S03 | Hands-Up Feet-Down | C3-C4 | 12 | 93.1 |
| S04 | Hands-Up Feet-Down | C4 | 12 | 85.0 |
| S05 | Hands-Up Feet-Down | CP3-C4 | 16 | 84.1 |
| S06 | Hands-Up Feet-Down | Cz | 20 | 90.1 |
| S07 | Hands-Up Feet-Down | C3 | 26 | 79.7 |
| S08 | Hands-Up Feet-Down | C3 | 24 | 80.1 |
| S09 | Hands-Up Feet-Down | C3-CP3 | 14 | 95.1 |
| S10 | Hands-Up Feet-Down | C4 | 14 | 89.5 |
| S11 | Hands-Up Feet-Down | Cz | 20 | 80.2 |
| S12 | Hands-Up Feet-Down | CP3-CP4 | 12 | 100 |
| S13 | Hands-Up Feet-Down | C4-C3 | 16 | 79.2 |
| S14 | Hands-Up Feet-Down | C1-CP3 | 18 | 90.1 |
| P01 | Hands-Up Feet-Down | Cz-CPz | 26-29 | 74.0 |
| P02 | Hands-Up Feet-Down | Cz | 20-22 | 60.8 |
| P03 | Hands-Up Feet-Down | CPz-Cz | 18-29 | 66.5 |
| P04 | Hands-Up Feet-Down | Cz-CP4 | 20-14 | 65.0 |
When BCI training was performed in the system environment, the visual feedback from the BCI input device was included into the usual application screen (bottom right panel of the screen in Figure 2.A.) Through this alternative input, healthy subjects could control the interface by using two targets to scroll through the icons and to select the current icon, respectively. One more icon was added to disable selection of commands (turn off BCI input) and a combination of BCI targets was programmed to re-establish BCI control of the system. All 4 patients were able to successfully control the system. However, system performance achieved in these patients using the BCI input was lower than hat for muscle-based input.
Discussion
The quality of life of an individual suffering from severe motor impairments is importantly affected by its complete dependence upon the caregivers. An assistive device, even the most advanced, cannot substitute – at the state of the art – the assistance provided by a human. Nevertheless, it can contribute to relieve the caregiver from continuous presence in the patient's room since the patient can perform some simple activities on his/her own. Most importantly, because the patient can call the attention of the caregiver using some form of alarm. This suggests that the cost of care for patients in stable conditions could be reduced since the same number of paramedics or assistants can care after a higher number of patients. In a home environment, the life of familiars can be less hardly affected by the presence of the impaired relative. In this respect, the preliminary findings we reported would innovate the concept of assistive technology device and they may bring it to a system level, that is - the user is no more given many devices to perform separate activities but the system provides unified (though flexible) access to all controllable appliances. Moreover, we succeeded in the effort of including many commercially available components in the system, so that affordability and availability of components is maximized.
From a clinical perspective, the perception of the patient, as revealed by the analysis of questionnaires, is that he/she does not have to rely on the caregiver for all tasks. This may increase the patient's sense of independence. In addition, this independence grants a sense of privacy that is absent when patients have to rely on caregivers. For these two reasons, the patients reported to expect that their quality of life would substantially improve if they could use such a system in their homes. As an additional indication that supports this notion, the patients selected the front door opener as their favorite output device. The ability to decide autonomously or at least to participate to the decision on who can be part of their life environment at any given moment was systematically reported as highest in system acceptance. The possibility to control the robot received a lower acceptance score, although the patients were well aware of the potential usefulness of the device as virtual mobility in the house. At least one main aspect has to be considered in interpreting these findings: the higher level of demand in controlling the robot, that in turn increases the probability of failure and the level of the related sense of frustration. Although further studies are needed in which a larger cohort of patients is confronted with the system and a systematic categorization of the system impact on the quality of life should take into account a range of outcomes (e.g. mood, motivation, caregiver burden; employability; satisfaction) [20][21][22], the results obtained from this pilot study are encouraging for the establishment of a solid link between the field of human machine interaction and neurorehabilitation strategy [23].
Exploration of potential impact of BCI on the users' interaction with the environment is peculiar to this work when compared to the previous studies on the usefulness of the BCI-based interfaces, i.e. [5][12][14]. Although the improvement of quality-of-life brought by such an interface is expected to be relevant only for those patients who are not able to perform any voluntarily controlled movement, the advances in the BCI field are expected to increase the performance of this communication channel, thus making it effective for a broader population of individuals. Upon training, the able-bodied subjects enrolled in this study were able to control a standard application of the BCI (i.e. a cursor moving on a screen as implemented in the BCI2000 framework) by modulating their brain activity recorded over the scalp centro-parietal regions, with an overall accuracy over 70%. Similar levels of performance were achieved by the patients who underwent BCI training with standard cursor control application. All patients displayed brain signal modulations over the expected centro-parietal scalp positions. This confirms findings in [5][12][14] and extends them to other neurological disorders (DMD and SMA). Our study is thus additional evidence that people with severely disabling neuromuscular or neurological disorders can acquire and maintain control over detectable aspects of brain signals, and use this control to drive output devices. When patients and control subjects were challenged with a different application of the BCI, i.e., the system prototype rather than the cursor used in the training period, performance in mastering the system were substantially maintained. This shows that an EEG-based BCI can be integrated into an environmental control system. Several important aspects yet remain to be addressed. This includes the influence on BCI performance of the visual channel as the natural vehicle of information (in our case the set of icons to be selected) and as BCI feedback channel (which is mandatory for the training and performing processes in the actual “BCI task”). As mentioned above, motivation, mood, and other psychological variables are of relevance for a successful user-machine interaction based on her/his residual muscle activity. This becomes crucial in the case of severely paralyzed patients who are the eligible candidate for the BCI approach.
In conclusion, in this pilot study, we integrated an EEG-based BCI and a robotic platform in an environmental control system. This provides a first application of this integrated technology platform towards its eventual clinical significance. In particular, the BCI application is promising in enabling people to operate an environmental control system, including those who are severely disabled and have difficulty using conventional devices that rely on muscle control.
Acknowledgments
This work has been partially supported by the Italian Telethon Foundation (Grant: GUP03562) and by the National Institutes of Health in the USA (Grants: HD30146, EB00856, and EB006356).
Footnotes
Conflict of Interest Declaration: I had full access to all the data in the study and I take responsibility for the integrity of the data and the accuracy of the data analysis. I also state that all authors have read and approved submission of the manuscript; the manuscript contains original material that has not been published and has not being considered for publication elsewhere.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegwart R, Nourbakhsh IR. Introduction to Autonomous Mobile Robots. The MIT Press; 2004. [Google Scholar]
- 2.Zelinsky A. Field and Service Robotics. Springer Verlag; 1997. [Google Scholar]
- 3.Wolpaw JR, Birbaumer N, McFarland DJ, Pfurtscheller G, Vaughan TM. Brain-computer interfaces for communication and control. Clin Neurophysiol. 2002;113(6):767–791. doi: 10.1016/s1388-2457(02)00057-3. [DOI] [PubMed] [Google Scholar]
- 4.Birbaumer N, Ghanayim N, Hinterberger T, Iversen I, Kotchoubey B, Kubler A, Perelmouter J, Taub E, Flor H. A spelling device for the paralyzed. Nature. 1999;398:297–298. doi: 10.1038/18581. [DOI] [PubMed] [Google Scholar]
- 5.Kübler A, Nijboer F, Mellinger J, Vaughan TM, Pawelzik H, Schalk G, McFarland DJ, Birbaumer N, Wolpaw JR. Patients with ALS can use sensorimotor rhythms to operate a brain-computer interface. Neurology. 2005;64:1775–1777. doi: 10.1212/01.WNL.0000158616.43002.6D. [DOI] [PubMed] [Google Scholar]
- 6.Chapin JK, Moxon KA, Markowitz RS, Nicolelis MA. Real-time control of a robot arm using simultaneously recorded neurons in the motor cortex. Nat Neurosi. 1999;2:664–670. doi: 10.1038/10223. [DOI] [PubMed] [Google Scholar]
- 7.Wessberg J, Stambaugh CR, Kralik JD, Beck PD, Laubach M, Chapin JK, Kim J, Biggs J, Srinivasan MA, Nicolelis MA. Real-time prediction of hand trajectory by ensemble of cortical neurons in primates. Nature. 2000;408:361–365. doi: 10.1038/35042582. [DOI] [PubMed] [Google Scholar]
- 8.Serruya MD, Hatsopoulos NG, Paninski L, Fellows MR, Donoghue JP. Brain-machine interface: Instant neural control of a movement signal. Nature. 2002;416:141–142. doi: 10.1038/416141a. [DOI] [PubMed] [Google Scholar]
- 9.Hochberg LR, Serruya MD, Friehs GM, Mukand JA, Saleh M, Caplan AH, Branner A, Chen D, Penn RD, Donoghue JP. Neuronal ensemble control of prosthetic device by a human with tetraplegia. Nature. 2006;442:164–171. doi: 10.1038/nature04970. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz AB, Cui XT, Weber DJ, Moran DW. Brain-Controlled Interfaces: Movement Restoration with Neural Prosthetics. Neuron. 2006;52:205–220. doi: 10.1016/j.neuron.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 11.Wolpaw JR, Birbaumer N, Heetderks WJ, McFarland DJ, Peckham PH, Schalk G, Donchin E, Quatrano LA, Robinson CJ, Vaughan TM. Brain–computer interface technology: a review of the first international meeting. IEEE Trans Rehabil Eng. 2000;8:161–163. doi: 10.1109/tre.2000.847807. [DOI] [PubMed] [Google Scholar]
- 12.Wolpaw JR, McFarland DJ. Control of a two-dimensional movement signal by a noninvasive brain-computer interface in humans. Proc Natl Acad Sci U S A. 2004;51:17849–54. doi: 10.1073/pnas.0403504101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pfurtscheller G, Muller GR, Pfurtscheller J, Gerner HJ, Rupp R. ‘Thought’-control of functional electrical stimulation to restore hand grasp in a patient with tetraplegia. Neurosci Lett. 2003;351(1):33–36. doi: 10.1016/s0304-3940(03)00947-9. [DOI] [PubMed] [Google Scholar]
- 14.Muller-Putz GR, Scherer R, Pfurtscheller G, Rupp R. EEG-based neuroprosthesis control: a step towards clinical practice. Neurosci Lett. 2005;1-8;382(1-2):169–74. doi: 10.1016/j.neulet.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 15.del J, Millán R, Renkens F, Mouriño J, Gerstner W. Non-invasive brain-actuated control of a mobile robot by human EEG. IEEE Trans on Biomedical Engineering. 2004;51:1026–1033. doi: 10.1109/TBME.2004.827086. [DOI] [PubMed] [Google Scholar]
- 16.Mahoney F, Barthel D. Functional evaluation: the Barthel Index. Md State Med J. 1965;14:61– 65. [PubMed] [Google Scholar]
- 17.Schalk G, McFarland D, Hinterberger T, Birbaumer N, Wolpaw J. BCI2000: A general-purpose brain-computer interface (BCI) system. IEEE Trans Biomed Eng. 2004;51:1034–1043. doi: 10.1109/TBME.2004.827072. [DOI] [PubMed] [Google Scholar]
- 18.McFarland DJ, et al. Spatial filter selection for EEG-based communication. Electroencephalogr Clin Neurophysiol. 1997;103(3):386–394. doi: 10.1016/s0013-4694(97)00022-2. [DOI] [PubMed] [Google Scholar]
- 19.The Aspice Project web page at the LabRob web site. 2006 http://www.dis.uniroma1.it/∼labrob/research/ASPICE.html.
- 20.Kohler M, Clarenbach CF, Boni L, Brack T, Russi EW, Bloch KE. Quality of life, physical disability, and respiratory impairment in Duchenne muscular dystrophy. Am J Respir Crit Care Med. 2005;172(8):1032–1036. doi: 10.1164/rccm.200503-322OC. [DOI] [PubMed] [Google Scholar]
- 21.Bach JR, Vega J, Majors J, Friedman A. Spinal muscular atrophy type 1 quality of life. Am J Phys Med Rehabil. 2003;82(2):137–142. doi: 10.1097/00002060-200302000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Natterlund B, Gunnarsson LG, Ahlstrom G. Disability, coping and quality of life in individuals with muscular dystrophy: a prospective study over five years. Disabil Rehabil. 2000;22(17):776–85. doi: 10.1080/09638280050200278. [DOI] [PubMed] [Google Scholar]
- 23.Hammel J. Technology and the environment: supportive resource or barrier for people with developmental disabilities? Nurs Clin North Am. 2003;38(2):331–49. doi: 10.1016/s0029-6465(02)00053-1. [DOI] [PubMed] [Google Scholar]


