Abstract
The epithelial tissues of the C. elegans embryo provide a “minimalist” system for examining phylogenetically conserved proteins that function in epithelial polarity and cell-cell adhesion in a multicellular organism. In this review, we provide an overview of three major molecular complexes at the apical surface of epithelial cells in the C. elegans embryo: the cadherin-catenin complex, the more basal DLG-1/AJM-1 complex, and the apical membrane domain, which shares similarities with the subapical complex in Drosophila and the PAR/aPKC complex in vertebrates. We discuss how the assembly of these complexes contributes to epithelial polarity and adhesion, proteins that act as effectors and/or regulators of each subdomain, and how these complexes functionally interact during embryonic morphogenesis. Although much remains to be clarified, significant progress has been made in recent years to clarify the role of these protein complexes in epithelial morphogenesis, and suggests that C. elegans will continue to be a fruitful system in which to elucidate functional roles for these proteins in a living embryo.
Keywords: C. elegans, junction, cadherin, Discs large, cell adhesion
2. INTRODUCTION
The establishment of epithelial junctions is crucial for cell polarity and adhesion during development. The distinct apical and basolateral surfaces of epithelial cells allow different regions of the cell to develop specialized structures and functions (Figure 1). Adhesion between epithelial cells is essential for several processes, including the dramatic changes in cell shape, cell-cell contacts and cellular rearrangements that occur during morphogenesis (1). Because the molecules used to assemble and modulate adhesion and polarity complexes are similar in Caenorhabditis elegans, Drosophila, and humans (2), studies in invertebrate model organisms will shed light on general mechanisms used to regulate cell-cell adhesion in multicellular animals.
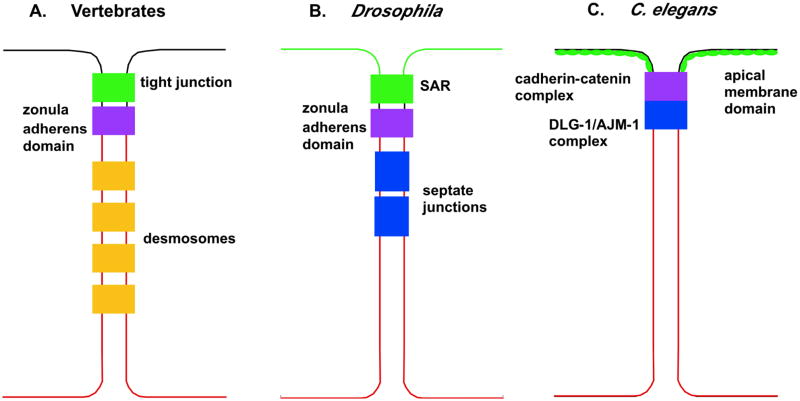
Figure 1.
Comparison of the organization of epithelial junctions in vertebrates, Drosophila, and C. elegans (A–C, respectively), with the apical membrane on top, and the basal membrane (in red) on the bottom. Whereas in vertebrates and Drosophila, different junctions are distinguishable by electron microscopy, the C. elegans junction consists of a single electron dense region called the apical junction, which consists of three molecular domains (2, 16). Despite differences in organization, there are some common features between the different junctional regions. At the apex is the apical membrane domain, which shares some of the same components as vertebrate tight junctions and the subapical region (SAR) of Drosophila (2, 95). Similar to the SAR in Drosophila, the C. elegans apical membrane domain localizes to both the marginal zone equivalent and the apical surface (6, 87). Next is the cadherin-catenin complex, which confers adhesion similar to the zonula adherens domain in vertebrates and Drosophila (2, 16). The most basal component of the junction is the DLG-1/AJM-1 complex, which contains homologues of several proteins found in Drosophila septate junctions (2, 16).
C. elegans is a powerful model system for investigating the formation and regulation of adhesive structures in vivo because of its optical transparency, invariant cell lineage, short generation time, and the large number of genetic tools that are available (3). In addition, C. elegans embryos, larvae, and adults possess several simple epithelia (Figure 2), including the epidermis, pharynx, intestine, and reproductive organs, which permit analysis of the establishment and maintenance of epithelial cell junctions (4–7). Forward genetic screening (4), as well as the use of specially designed transgenes and post-embryonic RNAi (7, 8) have allowed researchers to examine the role of adhesion and polarity proteins in these tissues. Such analysis is complemented by genomic analyses, which have identified numerous putative adhesion receptors, many of which have vertebrate homologues (9, 10). All of these advantages are supplemented by the ability to easily express fluorescently tagged proteins in living embryos, which enables the assessment of junction assembly, dynamics, and regulation at the level of single cells in vivo (8, 11–13). In this review, we present recent findings regarding the establishment and organization of apical junctions in C. elegans, which have in turn expanded our understanding of cell adhesion throughout the animal kingdom.
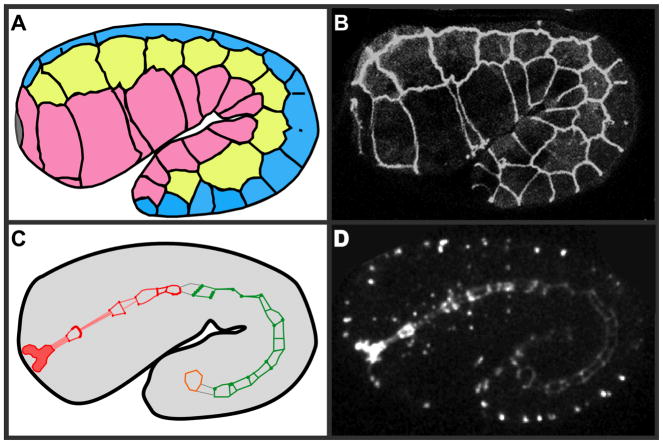
Figure 2.
Schematic representation of epithelial tissues in the C. elegans embryo. (A) At the 1.5-fold stage of C. elegans development, the embryo has already undergone enclosure, so that the epidermis completely surrounds the embryo. Dorsal cells are in blue, seam cells are in yellow, and ventral cells are in pink. Junctions are spread out along the borders between the different types of epidermal cells (outlined in black). At this stage of development, the dorsal epidermal cells are fusing to form a multicellular syncytium, so junctions that previously demarcate cell boundaries are being disassembled (11, 13, 26, 161). (B) An actual embryo expressing DLG-1::GFP, a convenient marker that highlights epidermal cell junctions and outlines neighboring cells in the C. elegans embryo. (C) Epithelial junctions are also present in the developing rectal valve (orange), intestine (green), and pharynx (red). The gray lines mark where the three organ systems will eventually connect to each other in later stage embryos (6, 95). (D) AJM-1::GFP in an actual embryo marks cell junctions in the digestive tract, so they can be followed throughout development.
3. THE C. ELEGANS APICAL JUNCTION
As in other organisms, cell-cell junctions in the C. elegans embryo have several diverse roles, including providing a permeability barrier, the mediation of strong adhesive linkages between neighboring epithelial cells, the establishment and maintenance of polarity, and the formation of connections to the cytoskeleton (14). In C. elegans, the apical and basolateral surfaces of cells are separated by continuous circumferential cellular junctions (15). Unlike Drosophila and vertebrates, however, epithelial junctions in C. elegans consist of a single discernable electron-dense structure, the apical junction, which has several distinct domains (Figure 1) (12, 16). In this section we introduce each of the major junctional complexes. Below, we discuss each in detail.
Like all higher eukaryotes, C. elegans epithelia contain a classical cadherin-catenin complex (Figure 3), which is part of the adherens junctional domain (2, 4, 15, 17). The cadherin-catenin complex is comprised of three essential proteins: a classic cadherin, encoded by hmr-1, which facilitates interactions with other cells, hmp-1/alpha-catenin, which interacts with the actin cytoskeleton, and hmp-2/beta-catenin, which mediates the interaction between HMR-1 and HMP-1 (4). While these proteins have low overall sequence similarity to vertebrate homologues, they have conserved functional domains and protein-protein interactions (2). Embryos that lack functional cadherin-catenin complexes fail to enclose and elongate during morphogenesis, resulting in embryonic lethality (4).
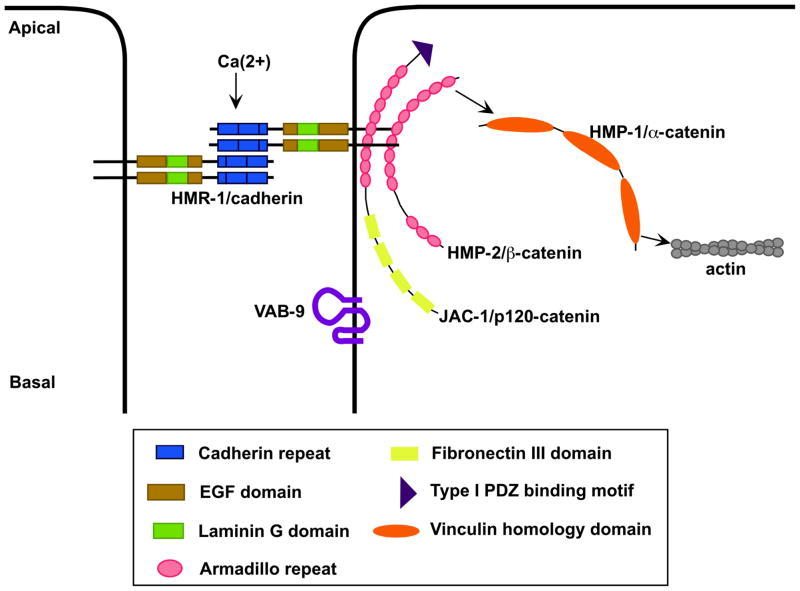
Figure 3.
A schematic overview of the cadherin-catenin complex in C. elegans. The classic cadherin, HMR-1, interacts intracellularly with JAC-1/p120 catenin and HMP-2/beta-catenin; HMP-2 recruits HMP-1/alpha-catenin to the junction (4). HMP-1 anchors circumferential actin filaments that transmit actomyosin generated forces required for elongation of the C. elegans embryo to the cell junction (2, 4, 26). The claudin-like protein VAB-9 also localizes to this region (14).
Basal to the cadherin-catenin complex is a complex that includes the MAGUK (Membrane Associated GUanylate Kinase) family protein DLG-1/Discs large, and its binding partner, AJM-1 (Figure 4) (12, 18). AJM-1 is a novel protein whose major predicted structural feature is a large coiled-coil domain (12). In addition, the Scribble homologue, LET-413, may also be present at the DLG-1/AJM-1 complex, but its localization also extends basally, well beyond this region (19).
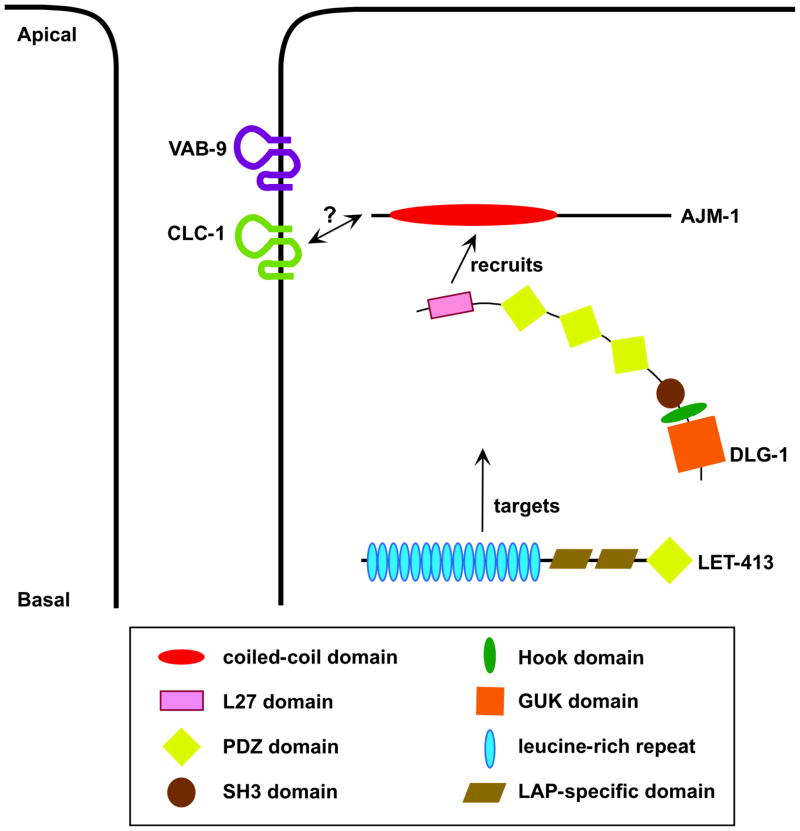
Figure 4.
A schematic overview of the DLG-1/AJM-1 complex. This complex, consisting of DLG-1/Discs large and its binding partner, AJM-1, is basal to the cadherin-catenin complex, but is required to stabilize the electron density associated with apical junctions (6, 12, 18). LET-413 targets the DLG-1/AJM-1 complex to apical junctions, and while it may also be present at apical junctions, its localization extends far more basally (18, 19, 93). DLG-1, specifically its L27 domain, is required for efficient spreading of AJM-1 along cell junctions (85). Experiments suggest that the DLG-1/AJM-1 complex acts synergistically with the cadherin-catenin complex to regulate cell adhesion (12, 18). This modulation of cell adhesion presumably involves an unidentified transmembrane protein with which the DLG-1/AJM-1 complex physically interacts. One such protein could be the claudin CLC-1, with which AJM-1 colocalizes in the pharynx (105).
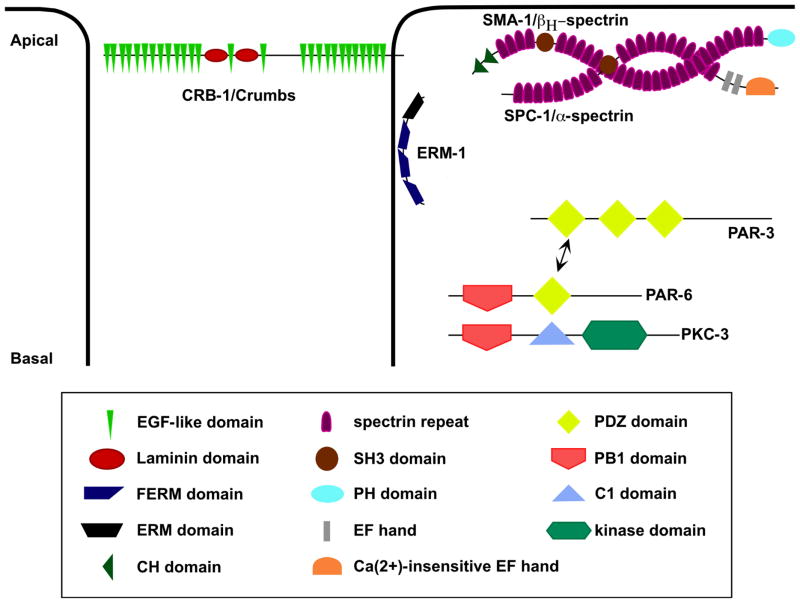
A third domain of the C. elegans apical junction is the apical membrane domain (Figure 5), which in some tissues contains transmembrane proteins of the Crumbs family (6, 20), the PAR/aPKC complex (7, 8), and proteins that stabilize cytoskeletal attachments to the apical membrane, including SMA-1/beta-heavy-spectrin (21). This region has a composition similar to that of the vertebrate tight junction (22) and the subapical region (SAR, or marginal zone) of Drosophila epithelia (23).
Figure 5.
A schematic overview of the apical membrane domain in C. elegans. The apical membrane domain comprises at least three groups of proteins: the PAR/aPKC complex, CRB-1/Crumbs, and the spectrins (115, 121, 138). The spectrins, SMA-1/beta-heavy-spectrin and SPC-1/alpha-spectrin, appear to be present in all epithelial tissues, where they mediate the attachment of actin to the apical membrane (116, 147). The PAR/aPKC proteins and CRB-1/Crumbs are only found in non-cuticular epithelia such as the intestine and parts of the pharynx (15). The PAR/aPKC complex consists of three proteins, PAR-3, PAR-6, and PKC-3, and constitutes an apical polarity complex in epithelia (124, 125). CRB-1/Crumbs appears to play a redundant role with LET-413 in the polarization of epithelia (95). ERM-1 genetically interacts with SMA-1 and appears to play a structural role in gut lumen formation (159, 160).
In the following sections, we describe in detail the structure and known functions of each of these apical junction subdomains. We begin with the cadherin-catenin complex, which is central to the function of the C. elegans apical junction.
4. THE C. ELEGANS CADHERIN-CATENIN COMPLEX
4.1 HMR-1/cadherin, HMP-2/beta-catenin, and HMP-1/alpha-catenin: core components of the classical cadherin-catenin complex
Before the C. elegans genome was sequenced, the C. elegans cadherin-catenin complex (Figure 3) was discovered through a genetic screen due to its role in regulating epidermal morphogenesis during ventral enclosure and elongation (4). During ventral enclosure, the free, ventral edges of the embryonic epidermis migrate around the embryo toward the ventral midline, where they meet and form nascent junctions (11, 13, 24). These events are mediated in part by HMR-1/cadherin, which, as in vertebrates (25) can form calcium-dependent homophilic bonds with cadherin molecules on neighboring cells (11). The embryo then elongates, a process that involves the development of circumferential actin filaments within the epidermis. These filaments, which presumably transmit the forces generated by actomyosin-mediated contraction to the epidermis, are anchored at epidermal cell-cell boundaries by the cadherin complex. Loss of either HMP-2/beta-catenin or HMP-1/alpha-catenin results in detachment of these filaments, failure of embryonic elongation, and death (3, 4, 26).
Traditionally, beta-catenins function in two important processes during development: cell-cell adhesion, and as downstream effectors of the Wnt signaling pathway (27). C. elegans represents an interesting example of evolutionary diversification of beta-catenins. Based on primary sequence data, two beta-catenin homologues were initially identified in addition to HMP-2: BAR-1 and WRM-1 (28–30). HMP-2 is clearly implicated in cell-cell adhesion (4), however, unlike beta-catenins in vertebrates and Drosophila (31, 32), HMP-2 does not interact with the single Tcf/Lef homologue in C. elegans, POP-1 (33, 34). BAR-1 appears to act canonically via a classical destruction complex in C. elegans larvae (29, 30, 35), but bar-1 null mutants exhibit no defects in embryonic morphogenesis (36; our unpublished observations).
In contrast to BAR-1, WRM-1 only weakly interacts with POP-1 (33, 34), but it interacts strongly with LIT-1, a Nemo-like kinase (NLK). LIT-1 is part of a MAPK-like pathway that leads to changes in the nuclear accumulation of POP-1 (28–30) in a pathway recently named the “Wnt/beta-catenin asymmetry” pathway (30). More recently, a fourth protein with Armadillo repeats, SYS-1, that can be considered a divergent beta-catenin based on functional data, has been shown to participate in Wnt/beta-catenin asymmetry (30, 37, 38). Like BAR-1 and WRM-1, there is no evidence linking SYS-1 to cell adhesion. This functional separation of known beta-catenin functions in C. elegans suggests that HMP-2, as a largely adhesion-specific beta-catenin, may provide unique opportunities in the future for studying features of beta-catenin that are important for its adhesive functions.
Subsequent to the sequencing of the genome, a p120-catenin homologue, JAC-1 (Juxtamembrane domain-Associated Catenin) was identified (17). JAC-1 exhibits the same 10 Armadillo repeats in its central region characteristic of other p120-catenins (17, 39). However, unlike the N-termini of mammalian and Drosophila p120-catenins, the N-terminus of JAC-1 contains four fibronectin type III domains (17). Whether this confers different functional properties on JAC-1 is unclear. Together, HMR-1, HMP-2, HMP-1, and JAC-1 appear to constitute a bona fide classical cadherin complex. HMR-1/cadherin is required for recruitment of JAC-1/p120-catenin, HMP-2/beta-catenin and HMP-1/alpha-catenin, and HMP-2/beta-catenin is required for proper localization of HMP-1/alpha-catenin (4, 17). All of this suggests that the core cadherin complex components in C. elegans have functions and interactions similar to their vertebrate counterparts.
4.2 Connecting to the cytoskeleton: HMP-1/alpha-catenin
The traditional view of the cadherin-catenin complex depicts its core components as assembled into a simultaneous complex (Figure 3): the transmembrane cadherin bound on its cytoplasmic tail to p120-catenin and beta-catenin, and beta-catenin in turn bound by alpha-catenin, which can interact with actin (2, 25, 40, 41). While C. elegans localization data supports the idea that all three cadherin-catenin proteins can be dynamically found together at apical junctions (4), this traditional view, at least in terms of the presence of a simultaneous, tertiary complex of cadherin/beta-catenin/alpha-catenin/F-actin, was recently challenged (42–44). In vertebrates, biochemical experiments show that alpha-catenin cannot simultaneously bind actin and a beta-catenin/E-cadherin complex (42, 43). Moreover, heterodimeric alpha-catenin/beta-catenin has a high affinity for E-cadherin, whereas dimeric alpha-catenin has a high affinity for actin, and that alpha-catenin bound to E-cadherin/beta-catenin can leave the complex and bind actin in solution (42, 43). These observations have led to a model in which E-cadherin and beta-catenin recruit alpha-catenin to junctions via transient interactions. The subsequent dissociation of alpha-catenin from the complex results in the formation of alpha-catenin homodimers in the vicinity of apical actin, where they can influence filament dynamics, possibly by antagonizing the branching effects of the Arp2/3 complex (42–44).
One of the features of this revised model is that actin at adherens junctions must either be recruited by another actin-binding protein at the junction (45), or via transient interactions of alpha-catenin itself with other actin-binding proteins (44). That the latter is a possibility is suggested by results from vertebrate tissue culture and Drosophila (46), which suggest that bypassing beta-catenin entirely can still lead to some adhesive function. Moreover, direct fusions between cadherins and the C terminus of alpha-catenin suggest that such constructs are sufficient to confer some adhesive activity (44, 45, 47).
C. elegans may be an outstanding system in which to address how alpha-catenin functions. Several domains of vertebrate alpha-catenin have previously been described, including an N-terminal beta-catenin-binding domain and a somewhat poorly defined F-actin binding site on the C-terminus (48, 49). Sequence comparison indicates C. elegans HMP-1/alpha-catenin contains all of these previously characterized functional domains (49, 50). In addition, the availability of multiple null alleles, the ability to easily express transgenes, and the variety of microscopy tools available make C. elegans a promising model organism for studying how alpha-catenin functions at epithelial junctions in vivo (2, 4, 14, 17).
4.3 Regulation of the cadherin complex: JAC-1/p120-catenin
Although the core components of the cadherin complex are all present in C. elegans, functional studies on JAC-1/p120-catenin suggest that there are differences in the importance of this member of the complex compared with vertebrates. In vertebrates, p120-catenin appears to have numerous functions, including kinesin-mediated transport of cadherins to the cell surface (51–53), modulation of Rho signaling (39, 54–56), and regulation of the transcription factor Kaiso (57–59). In contrast to these multiple roles for p120-catenin in vertebrates, RNAi depletion of jac-1/p120-catenin has no observable phenotypic effect, suggesting JAC-1 may not play an essential role in cadherin-catenin function in C. elegans (4, 17). Strikingly, as in C. elegans, knocking down the Drosophila p120-catenin homologue does not result in embryonic lethality (60). However, in both C. elegans and Drosophila, simultaneous knockdown of p120-catenin and other cadherin-catenin complex proteins does lead to dramatic phenotypes (17, 60). In C. elegans, jac-1(RNAi) increases the penetrance of defects associated with a hypomorphic allele of hmp-1/alpha-catenin, hmp-1(fe4), and enhances its disorganized actin phenotype (17). This indicates that JAC-1/p120-catenin does play a role as a modulator of cadherin-catenin function. How it does so is less clear. The lack of a jac-1 null mutant currently hampers further study of this modulatory role for JAC-1 at the apical junction.
4.4 Regulation of HMP-2/beta-catenin: a role for FRK-1/Fer kinase?
Another protein that may modulate the cadherin-catenin complex is FRK-1, an ortholog of the mammalian non-receptor tyrosine kinase, Fer. FRK-1 localizes to epithelial junctions and is reported to be necessary for embryonic enclosure and morphogenesis (61). Moreover, FRK-1 appears to mislocalize in hmp-2 mutants and jac-1(RNAi) embryos and coimmunoprecipitates with HMP-2 (61). Perhaps most interestingly however, FRK-1 may be required for nuclear exclusion of HMP-2, and hence maintenance at epithelial cell junctions (61). Some functions of FRK-1 appear to be independent of its function as a bona fide kinase, however, since a kinase-dead mutant form of FRK-1 is capable of rescuing morphogenetic defects in embryos homozygous for a deficiency that deletes frk-1 (61). This suggests that FRK-1 has kinase-independent functions during later embryogenesis. One possibility is that FRK-1 functions as a scaffolding protein, as has been shown for MAGUK (Membrane Associated GUanylate Kinase) proteins, which possess a non-functional guanylate kinase domain (62). Whether FRK-1 also plays roles in regulating the phosphorylation state of HMP-2 or other beta-catenins that are functionally separable from its kinase-independent functions is unknown. Fully understanding the role of FRK-1 at epithelial cell junctions is an important area of future research.
4.5 VAB-9: a divergent claudin family member regulated by the cadherin complex
Claudins are tetraspan, homotypic cell-cell adhesion receptors that mediate paracellular permeability in vertebrate tight junctions (63), and their function in C. elegans are just beginning to be understood (see below). Surprisingly, a divergent member of the claudin superfamily, vab-9 (Variably ABnormal) was identified as a regulator of epidermal morphogenesis in C. elegans using forward genetic approaches (14). Positional cloning showed that vab-9 encodes a divergent claudin-like molecule most similar to vertebrate BCMP1 (brain cell membrane protein 1) (14, 64). vab-9 mutants have a variety of body shape defects, and disrupted circumferential actin filaments, similar to hmp-1 mutants, suggesting vab-9 functions within the same pathway. Consistent with this possibility, VAB-9 localizes with the cadherin complex (Figure 3) and requires HMR-1 for localization to cell junctions and HMP-1 for its long-term stability at junctions (14). Taken together, these results indicate that VAB-9 is a novel, phylogenetically conserved component of the cadherin-based adhesion machinery. The small number of amino acids in the cytoplasmic domain of VAB-9 has thus far hampered progress toward identifying its binding partners; hopefully future work will identify in more detail how this conserved, poorly characterized protein functions in cell-cell adhesion.
4.6 The search for adhesion molecules that are functionally redundant with the cadherin complex
While important for morphogenesis, the cadherin-catenin complex in C. elegans is not essential for general cell adhesion in embryos (4). This is a pronounced departure from what is known about cadherin-based adhesion in other systems. Eliminating alpha-catenin, beta-catenin, or E-cadherin has dramatic effects on polarization, zonula adherens formation and epithelial structure in Drosophila (65–68). In mice and Xenopus, depleting cadherins or catenins similarly disrupts early embryonic cell adhesion (69, 70). Because this is not the case in C. elegans, other adhesion molecules presumably function redundantly with the cadherin-catenin complex in the early C. elegans embryo. Indeed, C. elegans may be a convenient model system for identifying such redundant adhesion systems.
One promising class of adhesion molecules that may function alongside the cadherin complex is the L1CAM family of proteins. L1CAMs are part of the immunoglobulin superfamily, conserved in vertebrates and invertebrates, and have already been implicated in cell adhesion in invertebrates (71). In Drosophila, hypomorphic mutations in the L1CAM neuroglian result in disrupted septate junctions, suggesting a role in cell adhesion (72). In C. elegans, the L1CAM SAX-7 localizes to cell-cell contacts in the early embryo and epithelia and can interact in vitro with UNC-44/ankyrin, part of the spectrin based membrane skeleton (73–75). Transgenic embryos expressing a dominant-negative SAX-7 display variably penetrant phenotypes, including cells with altered shapes and positions, which also implies a defect in cell-cell adhesion (73). The incomplete penetrance of sax-7 mutant phenotypes suggests that other adhesion molecules compensate for its loss (74). Given the existence of a hypomorphic, maternal effect allele of hmp-1 (17), it should be possible to test whether SAX-7 and the cadherin-catenin complex function synergistically in promoting general cell-cell adhesion in the early embryo. Moreover, similar tests could be performed using other putative cell adhesion receptors, such as other members of the cadherin superfamily that are encoded by the C. elegans genome (9).
4.7 Comparison with vertebrates suggests the C. elegans cadherin complex is a “minimalist” system
Although it is not particularly surprising that C. elegans has a core cadherin-catenin complex, what is surprising is how many proteins thought to be essential to the function of the vertebrate complex are missing from the cadherin-catenin complex in C. elegans. For example, vertebrate alpha-catenin can heterodimerize with vinculin (76), alpha-actinin (77), ZO-1 (78, 79) and l-afadin (80) to promote filament cross-linking, and can associate with the F-actin nucleating proteins formin-1 (81) and the mammalian Enabled homologue, Mena (82). Neither vinculin nor alpha-actinin is present with the cadherin-catenin complex in C. elegans (83, 84) and while the C. elegans genome contains homologues of ZO-1, l-afadin, and formin-1, their roles at junctions have yet to be described. In addition, other effectors such as C. elegans Enabled have surprisingly minor roles at cell-cell junctions (see below). Such surprises suggest that C. elegans may be extremely useful as a “minimalist” system for examining the core features of the cadherin complex, and indeed other conserved adhesion complexes, in animal embryos.
5. THE DLG-1/AJM-1 COMPLEX
5.1 DLG-1 and AJM-1: core components of the basolateral domain
In C. elegans, DLG-1 is essential for organizing the DLG-1/AJM-1 complex at epithelial junctions (85). DLG-1 is essential for the proper localization of AJM-1 in multiple epithelial tissues (Figure 4) and loss of either protein results in developmental arrest at the two-fold stage during elongation, often accompanied by swollen/ruptured cells that may be necrotic (12, 18, 85). Additionally, TEM shows that the integrity of the electron-dense region of the epithelial cell junction is perturbed in both dlg-1 and ajm-1 mutants. Loss of DLG-1 results in a complete loss of electron dense material (18), and loss of AJM-1 lead to the formation of bubble-like separations between junctions (12). These results suggest that the DLG-1/AJM-1 complex is a key stabilizer of this junctional domain at its cytoplasmic interface. Since it is a MAGUK family protein, DLG-1 may act as a scaffolding protein, allowing the integration of a wide variety of proteins into this subdomain (62).
DLG-1 is the homologue of Drosophila Discs large (Dlg), which localizes to and maintains septate junctions (Figure 1) (86). Dlg acts together with the proteins Scribble (Scrib) and Lethal-giant-larvae (Lgl) to control the localization of apical and adherens junction proteins and in regulating cell proliferation (62, 86). Dlg mutants exhibit disrupted septate junctions and an overall loss of cell polarity (87). This overall loss of cell polarity has made it difficult to demonstrate a specific role for Dlg in regulating proteins involved in maintaining the integrity of junctional domains in Drosophila (as described in 12). However, in C. elegans, the recruitment of proteins to the DLG-1/AJM-1 complex appears to be largely independent of HMR-1/E-cadherin, HMP-2/beta-catenin, and HMP-1/alpha-catenin, since none of these is required to localize AJM-1 (4, 12). Conversely, DLG-1 and AJM-1 are not required for largely normal recruitment of cadherin complex components (12). This has enabled functional studies of DLG-1 at cell-cell junctions.
5.2 The role of DLG-1 subdomains in its localization
A recent structure-function study of DLG-1 provides new insights into how domains of the protein function to maintain epithelial integrity during development (85). MAGUK proteins typically contain 1–3 PDZ domains at the N-terminus, followed by a SH3 domain, and a catalytically inactive GUK domain (62). In addition to these domains, C. elegans DLG-1 contains an L27 domain at the very N-terminus of the protein (Figure 4), which it shares with some isoforms of Drosophila Discs large (88). L27 domains have been shown to mediate heterodimerization between proteins containing the two L27 domain subtypes (89–91). To study domain function, GFP-tagged “dropout” dlg-1 constructs missing single or multiple domains (92) have been assessed for in vivo function in a dlg-1 mutant background (85). These experiments showed that the PDZ domains are needed for recruitment of DLG-1 to cell-cell junctions, although the L27 domain is required for the efficient spreading of AJM-1 along cell junctions (85).
Interestingly, DLG-1 constructs containing the SH3 domain are able to rescue some of the morphogenetic phenotypes seen in dlg-1 mutant embryos, although they cannot restore viability. Thus, the GUK domain is not required for localization, but it is necessary for full viability, although the mechanism behind the requirement is not yet known (85). Examining the role of the GUK domain, and identifying binding partners for both the PDZ and GUK domains will be important to further develop our understanding of the DLG-1/AJM-1 complex.
The LAP (leucine-rich repeat and PDZ) protein LET-413 is also necessary for the correct localization of DLG-1 and AJM-1 to cell junctions (Figure 4) (12, 18). LET-413 localizes to basolateral membranes of C. elegans epithelia through its leucine-rich repeats (93) and dynamic analysis of let-413 mutants shows a lack of apical-basal “focusing” of junctional proteins during junction formation in the epidermis and gut (12, 19, 94). What domain(s) of DLG-1 are required for this rapid focusing is not entirely clear. However, while a DLG-1::GFP construct missing only the GUK domain can localize normally, apical-basal focusing is blocked in dlg-1 mutant embryos carrying a DLG-1::GFP construct missing the SH3 and GUK domains (85). Additionally, the localization of a DLG-1 construct containing the SH3 domain, but not the GUK domain, is disrupted in let-413; dlg-1 mutant embryos in a manner that replicates the localization pattern of the DLG-1 construct without the SH3 and GUK domains in dlg-1 mutant embryos (85). Taken together, these results suggest that DLG-1 interacts with the LET-413-dependent polarization pathway through its SH3 domain for efficient distribution (85).
While a role for LET-413 in the correct localization of the DLG-1/AJM-1 complex is well established, there is evidence that LET-413 regulates apicobasal polarity more generally. let-413 mutant phenotypes suggest more widespread disruption of adhesion in the intestine and epidermis than effects on the DLG-1/AJM-1 complex alone can account for (19). Moreover, removal of LET-413 perturbs more apically localized proteins, although the mechanism by which this occurs is currently unknown (94). Continued studies into the other domains of LET-413 could provide additional information about its functions (93).
5.3 The DLG-1/AJM-1 complex: a self-reinforcing complex?
The original functional characterization of AJM-1 identified it as a physical binding partner of DLG-1 (12). More recent studies have refined this interaction to the N-terminus of DLG-1, to a region that includes the L27 domain (85). Examination of dlg-1 deletion constructs in dlg-1 mutant embryos shows that loss of the L27 domain of DLG-1 abrogates its ability to interact with AJM-1 in vivo, indicating that DLG-1 recruits AJM-1 to the junction. However, once there, studies suggest that AJM-1 may help to stabilize the DLG-1/AJM-1 complex against disruption. Loss of ajm-1 function may lead to loss of DLG-1 localization in the intestine (95) and although localization of DLG-1 in the epidermis of ajm-1 mutants has been reported to be normal (12, 18), DLG-1 deletion constructs that localize normally in wild-type embryos fail to do so in ajm-1 loss of function embryos (85). Taken together, these results suggest that AJM-1, once recruited to the complex, helps to maintain DLG-1 at junctions. The complex, in turn, stabilizes the electron density at the apical junction (12, 18, 92).
5.4 Do the DLG-1/AJM-1 and cadherin complexes interact?
Although there is abundant evidence that the classic cadherin-catenin complex in C. elegans regulates cell-cell adhesion during embryonic morphogenesis, there is tantalizing evidence that the DLG-1/AJM-1 complex may synergistically regulate cell adhesion alongside the HMR/HMP complex. In addition to the role of the DLG-1/AJM-1 complex in stabilizing the electron-dense region of epithelial cell junctions, analysis of mutants indicates that the complex is also involved in regulating adhesion. Loss of DLG-1 leads to leakage of cytoplasm from the tail and ventral midline during elongation, and loss of AJM-1 leads to the occasional separation of junctions (12, 18). Moreover, mutations affecting components of the cadherin-catenin complex synergize with removal of AJM-1 or DLG-1, resulting in a dramatic failure of cell adhesion (12, 18). Similarly, dlg-1 and ajm-1 mutants synergize with vab-9 loss of function: vab-9; dlg-1 and vab-9; ajm-1 double mutants frequently arrested with a ruptured epidermis, a more severe phenotype than any of the single mutants (14). Surprisingly, given that LET-413 acts upstream of DLG-1/AJM-1 localization (6, 19), the phenotype that results from knocking down both hmp-1 and let-413 resembles the hmp-1(RNAi) phenotype on its own (95). This suggests that cadherin-catenin mutants are phenotypically epistatic to mutations in let-413 (95). This raises serious questions regarding how these two complexes interact with each other, and is an important area for future research.
5.5 UNC-34/Enabled: a potential functional integrator of the cadherin and DLG-1/AJM-1 complexes
One way to understand how the cadherin-catenin and DLG-1/AJM-1 complexes interact with each other is to identify and study proteins that interact - physically or functionally - with both complexes. One such protein is UNC-34, the C. elegans homologue of Drosophila Enabled (Ena), a member of the Ena/VASP family of proteins (96). Ena/VASP proteins are actin binding proteins that promote actin filament elongation and bundling in vitro by binding free filament ends and inhibiting the activity of capping proteins that prevent filament extension (97) in contrast to WASP-family proteins that also promote F-actin formation, but via formation of new F-actin branches from existing filaments (98). In tissue culture, Ena/VASP proteins have been shown to play an important role in modulating the protrusion of the leading edge of migratory fibroblasts (99) and in epithelial sealing events in culture (82) and during neurulation (100, 101). Surprisingly, in C. elegans, UNC-34 plays only a minor role in modulating motility in epithelial cells (13). However, unc-34 is genetically redundant with the N-WASP homologue, WSP-1 (96). Both UNC-34 and WSP-1 are required for the successful completion of ventral enclosure in C. elegans, a process whereby epithelial cells migrate from the dorsal side to the ventral side of an embryo and seal the embryo in an epithelial sheet (11, 13, 96). During ventral enclosure, GFP-tagged UNC-34 localizes to the leading edges of migrating epidermal cells, but is also redistributed to junctions that are formed during epidermal sheet sealing, suggesting a role in modulating cell adhesion in C. elegans (13).
It has previously been shown that Ena/VASP proteins associate with cell-cell junctions in cultured mammalian cells and Drosophila epithelial cells (82, 102, 103). In primary keratinocytes, this association is dependent on cadherin-catenin function (82). This does not appear to be the case in C. elegans however, because disrupting cadherin-catenin function has no effect on the localization of UNC-34 (13). Somewhat surprisingly, normal UNC-34 junctional localization requires the activity of AJM-1, which contains a putative consensus Ena/VASP binding motif (13). In ajm-1 mutants, the intensity of junctional signal is greatly decreased and UNC-34::GFP distribution is non-uniform along junctions (13). That UNC-34 is not completely missing from cell-cell junctions suggests that there are other factors influencing the localization of UNC-34.
To understand how UNC-34 influences epithelial junctions, Sheffield et al. (2007) took advantage of the hypomorphic allele of C. elegans alpha-catenin, hmp-1(fe4), that shows variable defects in epidermal morphogenesis (17). Depleting UNC-34 in hmp-1(fe4) embryos results in frequent failure of ventral enclosure, suggesting that UNC-34 might be able to partially compensate for reduced HMP-1 activity (13). If alpha-catenin acts to regulate actin dynamics partly by antagonizing actin filament branching activity, it is possible that UNC-34 could compensate for compromised alpha-catenin function (17, 42, 43, 104). Interestingly, the loss of abl-1/Abelson tyrosine kinase, an actin capping protein that regulates the localization of Enabled in Drosophila, partially suppresses the morphogenetic defects seen in unc-34; hmp-1(fe4) double mutants (13, 103). A loss of abl-1 function could result in a reduction of actin capping, thus partially relieving the requirement for UNC-34 in hmp-1(fe4) homozygous embryos (13). Further experiments aimed at characterizing molecules that modulate UNC-34 function will be necessary to test these models.
5.6 Remaining questions: What links DLG-1 to the membrane?
Experiments suggesting that the DLG-1/AJM-1 complex acts synergistically with the cadherin-catenin complex do not address how this synergy is achieved. In order to modulate cell adhesion, there must presumably be a transmembrane protein associated with the DLG-1/AJM-1 complex; however no such protein has yet been identified in C. elegans. One class of candidate proteins that could provide such a transmembrane linkage is the claudins. In addition to vab-9, the C. elegans genome encodes several claudin-like proteins: CLC-1, CLC-2, CLC-3, and CLC-4 (105). Interestingly, CLC-1 colocalizes with AJM-1 to epithelial cell junctions in the pharynx where it regulates barrier function (105). Due to the colocalization of AJM-1 and CLC-1 in the pharynx (Figure 4), it is tempting to speculate that claudins are responsible for mediating the synergy between the DLG-1/AJM-1 and cadherin-catenin complexes; however, further experiments would be needed to substantiate this claim. If such a connection between claudins and DLG-1 can be found, it would further underscore some of the similarities between the DLG-1/AJM-1 region of the apical junction and the Drosophila septate junction, since the latter contains the claudin family member Sinuous (106) as well as Discs large (86). There are other possible transmembrane linkages to DLG-1, if the DLG-1/AJM-1 complex truly is in some ways analogous to the Drosophila septate junction. In addition to the L1CAM family member Neuroglian (72), Drosophila septate junctions also contain the neurexin, Neurexin IV (Nrx-IV) (87, 107). While the C. elegans genome encodes two Neurexin homologues, NLR-1 and ITX-1 (9), their functions are poorly characterized; itx-1(RNAi) yields no obvious phenotypes (M. Köppen, C. Lockwood, and J. Hardin, unpublished).
While functional examination of such candidate proteins may identify a transmembrane protein that interacts with DLG-1, the lack of convincing functional data implicating any thus far may reflect differences between the DLG-1/AJM-1 complex in C. elegans and the septate junction of Drosophila. As one example, septate junctions contain proteins that have no obvious C. elegans homologue, such as the recently identified MAGUK protein Varicose, which is a binding partner for Nrx-IV (107). In addition to the obvious ultrastructural difference between septate junctions, which have discernable septa, and the C. elegans apical junction, which do not, these differences suggest that the junctional roles of these complexes could be different as well. If this is true, C. elegans could provide a unique system for discovering new roles for conserved proteins in the basolateral domain.
6. THE APICAL MEMBRANE DOMAIN
6.1 Organization and function
A conserved feature of epithelia across the animal kingdom is the presence of proteins near the apical surface that are important for maintenance or establishment of apicobasal polarity, and hence formation of stratified junctional complexes precisely localized along the apicobasal axis (108). Components of this domain include the PAR/aPKC complex and members of the Crumbs family of proteins and their interactors (109, 110). In vertebrates, the PAR/aPKC and Crumbs complexes are associated with tight junctions, whereas in Drosophila and other invertebrates, these components are at the extreme apex of epithelial cells, where the apical membrane ends and the lateral membrane begins (Figure 1) (23, 111). Work in mammalian and invertebrate systems has demonstrated that the Crumbs and PAR complexes have conserved functions in conferring polarizing cues to the epithelium (108). A third class of proteins present is spectrin proteins, members of the apical membrane-associated actin cytoskeleton in epithelial cells (112–114). This apical cytoskeleton is crucial for providing mechanical support and mediating epithelial cell shape changes (115, 116).
All three classes of apical membrane domain proteins are present in C. elegans, but the presence of these proteins seems to vary among different epithelial tissues. The intestine and the excretory cell express transmembrane proteins of the Crumbs family, a PAR complex, and spectrin proteins (6, 116–118). As is true for at least some epithelia in Drosophila (110), not all tissues in C. elegans require, or even express, Crumbs family proteins. Epidermal cells and regions of the pharynx, while still expressing spectrins, do not appear to contain Crumbs family proteins (116). And while a recent report indicates that PAR-3 and PAR-6 are in young embryonic epidermis, they do not appear to be retained in older epidermis (119). One main difference between C. elegans epithelial tissues is that the epidermis and some pharyngeal cells are surrounded by cuticle, whereas the intestine and excretory cell are not (15). In Drosophila, cuticular epithelia have been shown to require several additional proteins for proper organization (120). These combined observations raise two distinct possibilities for how epithelia are organized in C. elegans: the cuticle itself provides support and organizational cues, or other proteins are involved in establishing polarity in the epidermis and pharynx.
In the following sections we discuss what is known about the three complexes associated with the apex, and what is understood about how they interact with other apical membrane proteins.
6.2 The PAR/aPKC complex: regulator of junctional organization
The six par (PARtitioning defective) genes and pkc-3 (atypical Protein Kinase C3) were originally uncovered in a screen for defects in early asymmetric cell divisions during C. elegans development (109, 118, 121). PAR/aPKC proteins have subsequently been identified in other systems and work in mammalian tissue culture and Drosophila has shown that three of these proteins constitute an apical polarity complex in epithelia: the PDZ-containing scaffold proteins PAR-3 and PAR-6, and the serine/threonine kinase PKC-3 (122–125). In mammals and Drosophila, these three proteins physically interact to form the PAR/aPKC complex, which is thought to be important for promoting epithelia junction structure (126–129). Antibody staining reveals that PAR-3, PAR-6, and PKC-3 are expressed in developing epithelial tissues in C. elegans, indicating the PAR/aPKC complex might be a component of the epithelial apical membrane in C. elegans as well (Figure 5) (8, 18, 130).
While the localization of the PAR/aPKC complex depends in part on the proteins within the complex, other cues are also involved. In Drosophila, the Lgl/Dlg/Scrib group of proteins is required to maintain apical localization of the PAR/aPKC complex, which in turn is essential for restricting Lgl localization to the basolateral membrane (131–133). In MDCK cells, mammalian Lgl is thought to antagonize cell polarization by sequestering the PAR-6/aPKC complex (134). Similarly, removing LET-413/Scrib by RNAi allows lateral expansion of PAR-3 and PAR-6 in intestinal cells in C. elegans (18, 19). Genetic studies in C. elegans have putatively identified another protein that acts upstream of localization of the PAR/aPKC complex. While RNAi against hmp-1, ajm-1, or dlg-1 has little effect on the disposition of PAR-3, PAR-6, or PKC-3 in the intestine, mutants lacking ZEN-4, an MKLP1 kinesin, express the polarity markers PAR-3 and PKC-3, but fail to target these proteins to the cell cortex in arcade region of the pharynx (5, 6, 12). Interestingly, in C. elegans, as is the case in Drosophila, while the PAR/aPKC proteins are dependent on each other for correct localization during development, they do not always colocalize, with PAR-3 having a tendency to segregate from PAR-6 and PKC-3 (8, 119, 135–137). These observations suggest that PAR-3 and PAR-6/PKC-3 can function independently in many situations (108).
Recent technological advances, which bypass the requirements for the PAR/aPKC complex in the early embryo, have enabled studies of how the PAR/aPKC proteins contribute to epithelial junction assembly in C. elegans. Post-embryonic RNAi was used to demonstrate a functional role for par-3 in the proper polarization of spermathecal cells, including positioning of the DLG-1/AJM-1 complex (7). Nance et al. (2003) designed constructs possessing a C-terminal sequence that allows PAR proteins to persist in early embryos, but leads to their destruction well before the birth of epithelial cells to study the role of PAR proteins in epithelia. They used these constructs to show that PAR-3 has a role in cell adhesion and gastrulation, even when early embryonic polarity requirement is normal.
More recently, this method was used to provide insights into the role of PAR-6 during the establishment and maintenance of epithelial cell polarity in C. elegans embryos. Totong et al. (2007) showed that depletion of PAR-6 prior to gastrulation results in elongation failure during embryogenesis. The embryos can still enclose, which suggests that adhesion molecules are still at junctions, but their epithelia are unorganized, hence the embryo cannot elongate (119). In wild-type embryos, DLG-1 and other junctional proteins accumulate in puncta at the apicobasal surface of cells and then condense into belt-like junctions (6, 12, 18). In embryos depleted of PAR-6 prior to gastrulation, DLG-1::GFP was expressed at the correct time and formed apical puncta; however, these puncta failed to condense into continuous junctions (119). This demonstrates a role for PAR-6 in C. elegans epithelial junction organization, but not apicobasal polarization (119). The fact that junction proteins can still localize apically suggests there is a mechanism independent of PAR-6 that can be used to polarize epithelial cells in C. elegans (119).
6.3 The Crumbs complex: surprisingly minor roles
In the tight junctions of vertebrates and the subapical region of Drosophila (Figure 1), the Crumbs complex is a key regulator of junction formation and apical-basal polarity (138). Disrupting the normal expression of the Crumbs complex in vertebrates leads to severe defects in tight junction formation and cell polarity and affects localization of the PAR/aPKC complex (139, 140). PALS1, a protein that interacts with mammalian CRB3/Crumbs, can also regulate E-cadherin exocytosis (140). In Drosophila, cells that lack crumbs do not organize a continuous zonula adherens and fail to maintain polarity (110, 141, 142). In apparent contrast to vertebrates, the Drosophila Crumbs and PAR/aPKC complexes interact differently at distinct times during development. During mid- to late-embryogenesis, Crumbs is expressed normally in zygotic Bazooka/PAR-3 mutants; conversely, Bazooka/PAR-3 is found in the apical membrane of epithelial vesicles in Crumbs mutants (132). However, the Crumbs and PAR/aPKC complexes are mutually dependent on one another for proper localization in the photoreceptor (143).
In order to achieve apical-basal polarity, the Crumbs complex interacts with several apical membrane domain proteins. In tissue culture, biochemical studies have demonstrated that the Crumbs and PAR/aPKC complex physically interact to define the apical domain of epithelial cells (144, 145). Intriguingly, Drosophila Crumbs genetically interacts and is physically associated with the spectrin cytoskeleton, but the nature of this interaction is unclear (115, 141, 142). What is more clear is that the Crumbs complex functions competitively with the Lgl/Dlg/Scrib complex to define the apical and basolateral surfaces of epithelial cells, respectively (132). The crumbs mutant phenotype can even be partially rescued by lgl mutations, indicating that Crumbs is only part of the story in defining apical polarity (132, 146). In addition, Crumbs mutant embryos can regain normal polarity on their own in epithelial cells during mid-embryogenesis, albeit much later than is required for viability (132). These observations indicate the existence of functionally redundant mechanisms in epithelial polarization in Drosophila.
C. elegans has one Crumbs homologue, CRB-1, and one Crumbs-like gene, CRL-1 or EAT-20 (2). Loss-of-function studies indicate that EAT-20 is required for normal muscle pumping in the pharynx (20). C. elegans CRB-1 localizes more closely with HMP-1 than DLG-1 at cell junctions, suggesting it also acts as an apical membrane protein (Figure 5) (67, 95). Given the dramatic phenotypes that result from the removal of Drosophila Crumbs, it is surprising that C. elegans CRB-1 does not appear to be an essential junctional protein, although it does affect junction polarity (67, 95). In the C. elegans intestine, CRB-1 appears to play a redundant role in the polarization of epithelia, where it acts as a positional cue for the localization of DLG-1 after depletion of let-413 (95). The fact that crb-1 mutant embryos are viable suggests that C. elegans may be an ideal system for studying how CRB-1 functions redundantly with other apical membrane proteins to establish polarity.
6.4 Spectrin-based membrane skeletons: structural stabilizers of the apex
Spectrins, first identified in erythrocytes, mediate attachments between cell membranes and actin (21, 147). Typically, alpha- and beta-spectrins form heterotetramers that cross-link actin to the cell membrane using adapter proteins such as ankyrin and protein 4.1 family members (FERM domain proteins) that interact with integral membrane proteins and phospholipids (115, 141). Early studies in vertebrates suggested that spectrin proteins play a role in either the initial formation or maintenance of epithelial polarity following cadherin-based adhesion. Vertebrate spectrin is recruited to sites of cell-cell contact following expression of E-cadherin in fibroblasts, and spectrin exists in a complex with cadherin in MDCK cells (148–150). Furthermore, overexpression of the ankyrin- or actin-binding domain of β-spectrin resulted in cells lacking a polarized distribution of membrane proteins (151, 152).
In Drosophila and C. elegans, spectrin proteins do not appear to be necessary for the initial establishment of epithelial polarity, but they are needed for normal development (153, 154). Compared to vertebrates, Drosophila and C. elegans have fewer spectrin genes: a single alpha-spectrin, a generally expressed beta-spectrin and a beta-heavy-spectrin (115). Beta-heavy-spectrin, a beta-spectrin isoform originally identified in Drosophila, can still form heterotetramers with alpha-spectrin (112–114, 155), but does not require ankyrin to associate with the plasma membrane (75). In Drosophila, mutations in the beta-heavy-spectrin gene karst result in extensive larval lethality and cause cell shape change and cell adhesion defects; in humans a homologue of beta-heavy-spectrin, beta-V-spectrin, may play a similar role (156–158). In C. elegans, the spectrin proteins SMA-1/beta-heavy-spectrin and SPC-1/alpha-spectrin appear to be present in all epithelial tissues (Figure 5) and moreover, both SMA-1 and SPC-1 are necessary to mediate epidermal cell shape changes during morphogenesis (21, 147).
In C. elegans, SMA-1’s primary function appears to be to link actin to the apical membrane, and not at sites of adhesion (116). During elongation, SMA-1 redistributes to the apical membrane in a speckled pattern that shows increased intensity near cell boundaries (116). At this time during C. elegans development, cortical arrays of actin redistribute to form circumferential actin filament bundles (4). In both wild-type and sma-1 mutant animals, the actin fibers remain intact at cell boundaries during morphogenesis, unlike the phenotypes observed in strains carrying defects in other proteins of the apical cell junction (4, 6, 14, 17). However, sma-1 mutants fail to elongate, as actin filaments dissociate from the apical membrane during morphogenesis (116). The N-terminus of SMA-1 is sufficient to organize actin at the apical membrane during cell elongation, but cannot rescue a null mutation (116). This indicates that other domains of SMA-1 mediate additional functions necessary for embryonic elongation.
The proteins that are required for the interaction between SMA-1 and the apical membrane have yet to be identified (116). In contrast to the Drosophila homologues, SPC-1/alpha-spectrin is not required for the normal localization of SMA-1, although it is needed for proper organization of SMA-1 in C. elegans (116). This means there are additional C. elegans proteins necessary for the normal assembly of the apical membrane-associated spectrin-based cytoskeleton. Candidates include CRB-1/Crumbs, which coimmunoprecipitates with the Drosophila beta-heavy-spectrin (141) and ERM-1, which shows a genetic interaction with SMA-1 (159). ERM-1, the Ezrin, Radixin, Moesin homologue appears to function as a structural molecule in lumen formation in the gut (159). However, when knocked down together with hmr-1, the apical junction is missing in many places (160). This is similar to the phenotype seen after a simultaneous loss of DLG-1/AJM-1 and cadherin-catenin complex components (95). These observations suggest that the spectrin cytoskeleton, along with other strata of the apical junction, plays a role in stabilizing the entire apical junction in C. elegans.
7. SUMMARY AND PERSPECTIVES
There are striking similarities between the assembly and regulation of junction complexes in C. elegans and higher organisms, making C. elegans a useful system for studying the organization of adhesion complexes in vivo. Much of the work to date has centered on defining the roles of individual adhesion or polarity proteins during development. For example, C. elegans has been useful in uncovering additional roles for known junctional proteins, such as HMP-1/alpha-catenin (11) and DLG-1/Discs large (85). However, there are still many worm homologues of vertebrate adhesion proteins, such as the neurexin family proteins mentioned above, whose functions have yet to be characterized in epithelia (9).
In addition to such analyses, however, several areas of research have just begun to be addressed. In particular, understanding how domains of the apical epithelial junction engage in functional and physical cross-talk requires much more attention. Here, too, C. elegans may present some advantages. For example, elongation of the C. elegans embryo requires functional catenins, DLG-1 and AJM-1, as well as an intact apical membrane domain, leading to several questions. Why are all three domains of a C. elegans epithelial apical junction required for elongation? How do different components of the apical junction function mechanistically relative to one another during elongation? What signaling pathways are involved? How does each of these domains contribute to the overall biomechanical properties of epithelia during morphogenesis? Using the genetic and molecular tools available in C. elegans, it should be possible to explore answers to these questions. The fruits of such investigation should, in turn, provide general insights into how each of the conserved apical junction domains contributes to the completion of complex tasks by epithelial tissues during embryonic development.
Acknowledgments
We thank Ryan King for sharing materials for use in the figures, and Theresa Grana and Ronen Zaidel-Bar for critical reading of the manuscript. This work was supported by NIH grant GM058038 to J.H.
Abbreviations
- MAGUK
membrane associated guanylate kinase protein family
- NLK
Nemo-like kinase
- Dlg
Discs large
- Scrib
Scribble
- Lgl
Lethal-giant-larvae
- PDZ
PSD95/Discs large/ZO-1 domain
- SH3
Src homology 3 domain
- GUK
guanylate kinase domain
- L27
LIN-2 and LIN-7 domain
- LAP
leucine-rich repeat and PDZ domain
- Ena
Enabled
- WASP
Wiskott-Aldrich syndrome protein
- MDCK
Madin-Darby canine kidney cells
- FERM
Protein 4.1/Ezrin/Radixin/Moesin domain
References
- 1.Goodwin M, Yap A. Classical cadherin adhesion molecules: coordinating cell adhesion, signaling and the cytoskeleton. J Mol Histol. 2004;35(8–9):839–44. doi: 10.1007/s10735-004-1833-2. [DOI] [PubMed] [Google Scholar]
- 2.Cox E, Hardin J. Sticky worms: adhesion complexes in C. elegans. J Cell Sci. 2004;117(Pt 10):1885–97. doi: 10.1242/jcs.01176. [DOI] [PubMed] [Google Scholar]
- 3.Labouesse M. Epithelial junctions and attachments. WormBook. 2006:1–21. doi: 10.1895/wormbook.1.56.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Costa M, Raich W, Agbunag C, Leung B, Hardin J, Priess J. A putative catenin-cadherin system mediates morphogenesis of the Caenorhabditis elegans embryo. J Cell Biol. 1998;141(1):297–308. doi: 10.1083/jcb.141.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Portereiko M, Saam J, Mango S. ZEN-4/MKLP1 is required to polarize the foregut epithelium. Curr Biol. 2004;14(11):932–41. doi: 10.1016/j.cub.2004.05.052. [DOI] [PubMed] [Google Scholar]
- 6.Bossinger O, Klebes A, Segbert C, Theres C, Knust E. Zonula adherens formation in Caenorhabditis elegans requires dlg-1, the homologue of the Drosophila gene discs large. Dev Biol. 2001;230(1):29–42. doi: 10.1006/dbio.2000.0113. [DOI] [PubMed] [Google Scholar]
- 7.Aono S, Legouis R, Hoose W, Kemphues K. PAR-3 is required for epithelial cell polarity in the distal spermatheca of C. elegans. Development. 2004;131(12):2865–74. doi: 10.1242/dev.01146. [DOI] [PubMed] [Google Scholar]
- 8.Nance J, Munro E, Priess J. C. elegans PAR-3 and PAR-6 are required for apicobasal asymmetries associated with cell adhesion and gastrulation. Development. 2003;130(22):5339–50. doi: 10.1242/dev.00735. [DOI] [PubMed] [Google Scholar]
- 9.Cox E, Tuskey C, Hardin J. Cell adhesion receptors in C. elegans. J Cell Sci. 2004;117(Pt 10):1867–70. doi: 10.1242/jcs.01177. [DOI] [PubMed] [Google Scholar]
- 10.Hutter H, Vogel B, Plenefisch J, Norris C, Proenca R, Spieth J, Guo C, Mastwal S, Zhu X, Scheel J, Hedgecock E. Conservation and novelty in the evolution of cell adhesion and extracellular matrix genes. Science. 2000;287(5455):989–94. doi: 10.1126/science.287.5455.989. [DOI] [PubMed] [Google Scholar]
- 11.Raich W, Agbunag C, Hardin J. Rapid epithelial-sheet sealing in the Caenorhabditis elegans embryo requires cadherin-dependent filopodial priming. Curr Biol. 1999;9(20):1139–46. doi: 10.1016/S0960-9822(00)80015-9. [DOI] [PubMed] [Google Scholar]
- 12.Köppen M, Simske J, Sims P, Firestein B, Hall D, Radice A, Rongo C, Hardin J. Cooperative regulation of AJM-1 controls junctional integrity in Caenorhabditis elegans epithelia. Nat Cell Biol. 2001;3(11):983–91. doi: 10.1038/ncb1101-983. [DOI] [PubMed] [Google Scholar]
- 13.Sheffield M, Loveless T, Hardin J, Pettitt J. C. elegans Enabled exhibits novel interactions with N-WASP, Abl, and cell-cell junctions. Curr Biol. 2007;17(20):1791–6. doi: 10.1016/j.cub.2007.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simske J, Köppen M, Sims P, Hodgkin J, Yonkof A, Hardin J. The cell junction protein VAB-9 regulates adhesion and epidermal morphology in C. elegans. Nat Cell Biol. 2003;5(7):619–25. doi: 10.1038/ncb1002. [DOI] [PubMed] [Google Scholar]
- 15.Hardin J, Lockwood C. Skin tight: cell adhesion in the epidermis of Caenorhabditis elegans. Curr Opin Cell Biol. 2004;16(5):486–92. doi: 10.1016/j.ceb.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 16.Knust E, Bossinger O. Composition and formation of intercellular junctions in epithelial cells. Science. 2002;298(5600):1955–9. doi: 10.1126/science.1072161. [DOI] [PubMed] [Google Scholar]
- 17.Pettitt J, Cox E, Broadbent I, Flett A, Hardin J. The Caenorhabditis elegans p120 catenin homologue, JAC-1, modulates cadherin-catenin function during epidermal morphogenesis. J Cell Biol. 2003;162(1):15–22. doi: 10.1083/jcb.200212136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McMahon L, Legouis R, Vonesch J, Labouesse M. Assembly of C. elegans apical junctions involves positioning and compaction by LET-413 and protein aggregation by the MAGUK protein DLG-1. J Cell Sci. 2001;114(Pt 12):2265–77. doi: 10.1242/jcs.114.12.2265. [DOI] [PubMed] [Google Scholar]
- 19.Legouis R, Gansmuller A, Sookhareea S, Bosher J, Baillie D, Labouesse M. LET-413 is a basolateral protein required for the assembly of adherens junctions in Caenorhabditis elegans. Nat Cell Biol. 2000;2(7):415–22. doi: 10.1038/35017046. [DOI] [PubMed] [Google Scholar]
- 20.Shibata Y, Fujii T, Dent J, Fujisawa H, Takagi S. EAT-20, a novel transmembrane protein with EGF motifs, is required for efficient feeding in Caenorhabditis elegans. Genetics. 2000;154(2):635–46. doi: 10.1093/genetics/154.2.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKeown C, Praitis V, Austin J. sma-1 encodes a betaH-spectrin homolog required for Caenorhabditis elegans morphogenesis. Development. 1998;125(11):2087–98. doi: 10.1242/dev.125.11.2087. [DOI] [PubMed] [Google Scholar]
- 22.Shin K, Fogg V, Margolis B. Tight junctions and cell polarity. Annu Rev Cell Dev Biol. 2006;22:207–35. doi: 10.1146/annurev.cellbio.22.010305.104219. [DOI] [PubMed] [Google Scholar]
- 23.Tepass U. Adherens junctions: new insight into assembly, modulation and function. Bioessays. 2002;24(8):690–5. doi: 10.1002/bies.10129. [DOI] [PubMed] [Google Scholar]
- 24.Williams-Masson E, Malik A, Hardin J. An actin-mediated two-step mechanism is required for ventral enclosure of the C. elegans hypodermis. Development. 1997;124(15):2889–901. doi: 10.1242/dev.124.15.2889. [DOI] [PubMed] [Google Scholar]
- 25.Nagafuchi A. Molecular architecture of adherens junctions. Curr Opin Cell Biol. 2001;13(5):600–3. doi: 10.1016/s0955-0674(00)00257-x. [DOI] [PubMed] [Google Scholar]
- 26.Simske J, Hardin J. Getting into shape: epidermal morphogenesis in Caenorhabditis elegans embryos. Bioessays. 2001;23(1):12–23. doi: 10.1002/1521-1878(200101)23:1<12::AID-BIES1003>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 27.Brembeck F, Rosário M, Birchmeier W. Balancing cell adhesion and Wnt signaling, the key role of beta-catenin. Curr Opin Genet Dev. 2006;16(1):51–9. doi: 10.1016/j.gde.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 28.Korswagen H. Canonical and non-canonical Wnt signaling pathways in Caenorhabditis elegans: variations on a common signaling theme. Bioessays. 2002;24(9):801–10. doi: 10.1002/bies.10145. [DOI] [PubMed] [Google Scholar]
- 29.Herman M, Wu M. Noncanonical Wnt signaling pathways in C. elegans converge on POP-1/TCF and control cell polarity. Front Biosci. 2004;9:1530–9. doi: 10.2741/1306. [DOI] [PubMed] [Google Scholar]
- 30.Mizumoto K, Sawa H. Two betas or not two betas: regulation of asymmetric division by beta-catenin. Trends Cell Biol. 2007;17(10):465–73. doi: 10.1016/j.tcb.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 31.Moon R, Bowerman B, Boutros M, Perrimon N. The promise and perils of Wnt signaling through beta-catenin. Science. 2002;296(5573):1644–6. doi: 10.1126/science.1071549. [DOI] [PubMed] [Google Scholar]
- 32.Hoppler S, Kavanagh C. Wnt signalling: variety at the core. J Cell Sci. 2007;120(Pt 3):385–93. doi: 10.1242/jcs.03363. [DOI] [PubMed] [Google Scholar]
- 33.Korswagen H, Herman M, Clevers H. Distinct beta-catenins mediate adhesion and signalling functions in C. elegans. Nature. 2000;406(6795):527–32. doi: 10.1038/35020099. [DOI] [PubMed] [Google Scholar]
- 34.Natarajan L, Witwer N, Eisenmann D. The divergent Caenorhabditis elegans beta-catenin proteins BAR-1, WRM-1 and HMP-2 make distinct protein interactions but retain functional redundancy in vivo. Genetics. 2001;159(1):159–72. doi: 10.1093/genetics/159.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Korswagen H, Coudreuse D, Betist M, van de Water S, Zivkovic D, Clevers H. The Axin-like protein PRY-1 is a negative regulator of a canonical Wnt pathway in C. elegans. Genes Dev. 2002;16(10):1291–302. doi: 10.1101/gad.981802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eisenmann D, Maloof J, Simske J, Kenyon C, Kim S. The beta-catenin homolog BAR-1 and LET-60 Ras coordinately regulate the Hox gene lin-39 during Caenorhabditis elegans vulval development. Development. 1998;125(18):3667–80. doi: 10.1242/dev.125.18.3667. [DOI] [PubMed] [Google Scholar]
- 37.Kidd Ar, Miskowski J, Siegfried K, Sawa H, Kimble J. A beta-catenin identified by functional rather than sequence criteria and its role in Wnt/MAPK signaling. Cell. 2005;121(5):761–72. doi: 10.1016/j.cell.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 38.Phillips B, Kidd Ar, King R, Hardin J, Kimble J. Reciprocal asymmetry of SYS-1/beta-catenin and POP-1/TCF controls asymmetric divisions in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2007;104(9):3231–6. doi: 10.1073/pnas.0611507104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anastasiadis P, Reynolds A. Regulation of Rho GTPases by p120-catenin. Curr Opin Cell Biol. 2001;13(5):604–10. doi: 10.1016/s0955-0674(00)00258-1. [DOI] [PubMed] [Google Scholar]; Perez-Moreno M, Fuchs E. Catenins: keeping cells from getting their signals crossed. Dev Cell. 2006;11(5):601–12. doi: 10.1016/j.devcel.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scott J, Yap A. Cinderella no longer: alpha-catenin steps out of cadherin’s shadow. J Cell Sci. 2006;119(Pt 22):4599–605. doi: 10.1242/jcs.03267. [DOI] [PubMed] [Google Scholar]
- 41.Drees F, Pokutta S, Yamada S, Nelson W, Weis W. Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell. 2005;123(5):903–15. doi: 10.1016/j.cell.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamada S, Pokutta S, Drees F, Weis W, Nelson W. Deconstructing the cadherin-catenin-actin complex. Cell. 2005;123(5):889–901. doi: 10.1016/j.cell.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gates J, Peifer M. Can 1000 reviews be wrong? Actin, alpha-Catenin, and adherens junctions. Cell. 2005;123(5):769–72. doi: 10.1016/j.cell.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 44.Weis W, Nelson W. Re-solving the cadherin-catenin-actin conundrum. J Biol Chem. 2006;281(47):35593–7. doi: 10.1074/jbc.R600027200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pacquelet A, Lin L, Rorth P. Binding site for p120/delta-catenin is not required for Drosophila E-cadherin function in vivo. J Cell Biol. 2003;160(3):313–9. doi: 10.1083/jcb.200207160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Niessen C, Gottardi C. Molecular components of the adherens junction. Biochim Biophys Acta. 2008 doi: 10.1016/j.bbamem.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rimm D, Koslov E, Kebriaei P, Cianci C, Morrow J. Alpha 1(E)-catenin is an actin-binding and -bundling protein mediating the attachment of F-actin to the membrane adhesion complex. Proc Natl Acad Sci U S A. 1995;92(19):8813–7. doi: 10.1073/pnas.92.19.8813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pappas D, Rimm D. Direct interaction of the C-terminal domain of alpha-catenin and F-actin is necessary for stabilized cell-cell adhesion. Cell Commun Adhes. 2006;13(3):151–70. doi: 10.1080/15419060600726142. [DOI] [PubMed] [Google Scholar]
- 49.Pokutta S, Weis W. Structure of the dimerization and beta-catenin-binding region of alpha-catenin. Mol Cell. 2000;5(3):533–43. doi: 10.1016/s1097-2765(00)80447-5. [DOI] [PubMed] [Google Scholar]
- 50.Peifer M, Yap A. Traffic control: p120-catenin acts as a gatekeeper to control the fate of classical cadherins in mammalian cells. J Cell Biol. 2003;163(3):437–40. doi: 10.1083/jcb.200310090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xiao K, Oas R, Chiasson C, Kowalczyk A. Role of p120-catenin in cadherin trafficking. Biochim Biophys Acta. 2007;1773(1):8–16. doi: 10.1016/j.bbamcr.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 52.Chen X, Kojima S, Borisy G, Green K. p120 catenin associates with kinesin and facilitates the transport of cadherin-catenin complexes to intercellular junctions. J Cell Biol. 2003;163(3):547–57. doi: 10.1083/jcb.200305137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Noren N, Liu B, Burridge K, Kreft B. p120 catenin regulates the actin cytoskeleton via Rho family GTPases. J Cell Biol. 2000;150(3):567–80. doi: 10.1083/jcb.150.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Magie C, Pinto-Santini D, Parkhurst S. Rho1 interacts with p120ctn and alpha-catenin, and regulates cadherin-based adherens junction components in Drosophila. Development. 2002;129(16):3771–82. doi: 10.1242/dev.129.16.3771. [DOI] [PubMed] [Google Scholar]
- 55.Franz C, Ridley A. p120 catenin associates with microtubules: inverse relationship between microtubule binding and Rho GTPase regulation. J Biol Chem. 2004;279(8):6588–94. doi: 10.1074/jbc.M312812200. [DOI] [PubMed] [Google Scholar]
- 56.Daniel J, Reynolds A. The catenin p120(ctn) interacts with Kaiso, a novel BTB/POZ domain zinc finger transcription factor. Mol Cell Biol. 1999;19(5):3614–23. doi: 10.1128/mcb.19.5.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Daniel J, Spring C, Crawford H, Reynolds A, Baig A. The p120(ctn)-binding partner Kaiso is a bi-modal DNA-binding protein that recognizes both a sequence-specific consensus and methylated CpG dinucleotides. Nucleic Acids Res. 2002;30(13):2911–9. doi: 10.1093/nar/gkf398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Park J, Kim S, Lyons J, Ji H, Nguyen T, Cho K, Barton M, Deroo T, Vleminckx K, Moon R, McCrea P. Kaiso/p120-catenin and TCF/beta-catenin complexes coordinately regulate canonical Wnt gene targets. Dev Cell. 2005;8(6):843–54. doi: 10.1016/j.devcel.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 59.Myster S, Cavallo R, Anderson C, Fox D, Peifer M. Drosophila p120catenin plays a supporting role in cell adhesion but is not an essential adherens junction component. J Cell Biol. 2003;160(3):433–49. doi: 10.1083/jcb.200211083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Putzke A, Hikita S, Clegg D, Rothman J. Essential kinase-independent role of a Fer-like non-receptor tyrosine kinase in Caenorhabditis elegans morphogenesis. Development. 2005;132(14):3185–95. doi: 10.1242/dev.01900. [DOI] [PubMed] [Google Scholar]
- 61.Funke L, Dakoji S, Bredt D. Membrane-associated guanylate kinases regulate adhesion and plasticity at cell junctions. Annu Rev Biochem. 2005;74:219–45. doi: 10.1146/annurev.biochem.74.082803.133339. [DOI] [PubMed] [Google Scholar]
- 62.Tsukita S, Furuse M. Claudin-based barrier in simple and stratified cellular sheets. Curr Opin Cell Biol. 2002;14(5):531–6. doi: 10.1016/s0955-0674(02)00362-9. [DOI] [PubMed] [Google Scholar]
- 63.Tepass U. Claudin complexities at the apical junctional complex. Nat Cell Biol. 2003;5(7):595–7. doi: 10.1038/ncb0703-595. [DOI] [PubMed] [Google Scholar]
- 64.Cox R, Kirkpatrick C, Peifer M. Armadillo is required for adherens junction assembly, cell polarity, and morphogenesis during Drosophila embryogenesis. J Cell Biol. 1996;134(1):133–48. doi: 10.1083/jcb.134.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Müller H, Wieschaus E. armadillo, bazooka, and stardust are critical for early stages in formation of the zonula adherens and maintenance of the polarized blastoderm epithelium in Drosophila. J Cell Biol. 1996;134(1):149–63. doi: 10.1083/jcb.134.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tepass U. Crumbs, a component of the apical membrane, is required for zonula adherens formation in primary epithelia of Drosophila. Dev Biol. 1996;177(1):217–25. doi: 10.1006/dbio.1996.0157. [DOI] [PubMed] [Google Scholar]
- 67.Uemura T, Oda H, Kraut R, Hayashi S, Kotaoka Y, Takeichi M. Zygotic Drosophila E-cadherin expression is required for processes of dynamic epithelial cell rearrangement in the Drosophila embryo. Genes Dev. 1996;10(6):659–71. doi: 10.1101/gad.10.6.659. [DOI] [PubMed] [Google Scholar]
- 68.Larue L, Antos C, Butz S, Huber O, Delmas V, Dominis M, Kemler R. A role for cadherins in tissue formation. Development. 1996;122(10):3185–94. doi: 10.1242/dev.122.10.3185. [DOI] [PubMed] [Google Scholar]
- 69.Heasman J, Crawford A, Goldstone K, Garner-Hamrick P, Gumbiner B, McCrea P, Kintner C, Noro C, Wylie C. Overexpression of cadherins and underexpression of beta-catenin inhibit dorsal mesoderm induction in early Xenopus embryos. Cell. 1994;79(5):791–803. doi: 10.1016/0092-8674(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 70.Kenwrick S, Watkins A, De Angelis E. Neural cell recognition molecule L1: relating biological complexity to human disease mutations. Hum Mol Genet. 2000;9(6):879–86. doi: 10.1093/hmg/9.6.879. [DOI] [PubMed] [Google Scholar]
- 71.Genova J, Fehon R. Neuroglian, Gliotactin, and the Na+/K+ ATPase are essential for septate junction function in Drosophila. J Cell Biol. 2003;161(5):979–89. doi: 10.1083/jcb.200212054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen L, Ong B, Bennett V. LAD-1, the Caenorhabditis elegans L1CAM homologue, participates in embryonic and gonadal morphogenesis and is a substrate for fibroblast growth factor receptor pathway-dependent phosphotyrosine-based signaling. J Cell Biol. 2001;154(4):841–55. doi: 10.1083/jcb.200009004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang X, Kweon J, Larson S, Chen L. A role for the C. elegans L1CAM homologue lad-1/sax-7 in maintaining tissue attachment. Dev Biol. 2005;284(2):273–91. doi: 10.1016/j.ydbio.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 74.Dubreuil R. Functional links between membrane transport and the spectrin cytoskeleton. J Membr Biol. 2006;211(3):151–61. doi: 10.1007/s00232-006-0863-y. [DOI] [PubMed] [Google Scholar]
- 75.Weiss E, Kroemker M, Rüdiger A, Jockusch B, Rüdiger M. Vinculin is part of the cadherin-catenin junctional complex: complex formation between alpha-catenin and vinculin. J Cell Biol. 1998;141(3):755–64. doi: 10.1083/jcb.141.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Knudsen K, Soler A, Johnson K, Wheelock M. Interaction of alpha-actinin with the cadherin/catenin cell-cell adhesion complex via alpha-catenin. J Cell Biol. 1995;130(1):67–77. doi: 10.1083/jcb.130.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Watabe-Uchida M, Uchida N, Imamura Y, Nagafuchi A, Fujimoto K, Uemura T, Vermeulen S, van Roy F, Adamson E, Takeichi M. alpha-Catenin-vinculin interaction functions to organize the apical junctional complex in epithelial cells. J Cell Biol. 1998;142(3):847–57. doi: 10.1083/jcb.142.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Itoh M, Nagafuchi A, Moroi S, Tsukita S. Involvement of ZO-1 in cadherin-based cell adhesion through its direct binding to alpha catenin and actin filaments. J Cell Biol. 1997;138(1):181–92. doi: 10.1083/jcb.138.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pokutta S, Drees F, Takai Y, Nelson W, Weis W. Biochemical and structural definition of the l-afadin- and actin-binding sites of alpha-catenin. J Biol Chem. 2002;277(21):18868–74. doi: 10.1074/jbc.M201463200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kobielak A, Pasolli H, Fuchs E. Mammalian formin-1 participates in adherens junctions and polymerization of linear actin cables. Nat Cell Biol. 2004;6(1):21–30. doi: 10.1038/ncb1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vasioukhin V, Bauer C, Yin M, Fuchs E. Directed actin polymerization is the driving force for epithelial cell-cell adhesion. Cell. 2000;100(2):209–19. doi: 10.1016/s0092-8674(00)81559-7. [DOI] [PubMed] [Google Scholar]
- 82.Barstead R, Waterston R. Vinculin is essential for muscle function in the nematode. J Cell Biol. 1991;114(4):715–24. doi: 10.1083/jcb.114.4.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Barstead R, Kleiman L, Waterston R. Cloning, sequencing, and mapping of an alpha-actinin gene from the nematode Caenorhabditis elegans. Cell Motil Cytoskeleton. 1991;20(1):69–78. doi: 10.1002/cm.970200108. [DOI] [PubMed] [Google Scholar]
- 84.Lockwood C, Lynch A, Hardin J. Dynamic analysis identifies novel roles for DLG-1 subdomains in AJM-1 recruitment and LET-413-dependent apical focusing. J Cell Sci. 2008;121(Pt 9):1477–87. doi: 10.1242/jcs.017137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Woods D, Hough C, Peel D, Callaini G, Bryant P. Dlg protein is required for junction structure, cell polarity, and proliferation control in Drosophila epithelia. J Cell Biol. 1996;134(6):1469–82. doi: 10.1083/jcb.134.6.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tepass U, Tanentzapf G, Ward R, Fehon R. Epithelial cell polarity and cell junctions in Drosophila. Annu Rev Genet. 2001;35:747–84. doi: 10.1146/annurev.genet.35.102401.091415. [DOI] [PubMed] [Google Scholar]
- 87.Mendoza C, Olguín P, Lafferte G, Thomas U, Ebitsch S, Gundelfinger E, Kukuljan M, Sierralta J. Novel isoforms of Dlg are fundamental for neuronal development in Drosophila. J Neurosci. 2003;23(6):2093–101. doi: 10.1523/JNEUROSCI.23-06-02093.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Feng W, Long J, Fan J, Suetake T, Zhang M. The tetrameric L27 domain complex as an organization platform for supramolecular assemblies. Nat Struct Mol Biol. 2004;11(5):475–80. doi: 10.1038/nsmb751. [DOI] [PubMed] [Google Scholar]
- 89.Harris B, Venkatasubrahmanyam S, Lim W. Coordinated folding and association of the LIN-2, -7 (L27) domain. An obligate heterodimerization involved in assembly of signaling and cell polarity complexes. J Biol Chem. 2002;277(38):34902–8. doi: 10.1074/jbc.M205856200. [DOI] [PubMed] [Google Scholar]
- 90.Petrosky K, Ou H, Löhr F, Dötsch V, Lim W. A general model for preferential hetero-oligomerization of LIN-2/7 domains: mechanism underlying directed assembly of supramolecular signaling complexes. J Biol Chem. 2005;280(46):38528–36. doi: 10.1074/jbc.M506536200. [DOI] [PubMed] [Google Scholar]
- 91.Firestein B, Rongo C. DLG-1 is a MAGUK similar to SAP97 and is required for adherens junction formation. Mol Biol Cell. 2001;12(11):3465–75. doi: 10.1091/mbc.12.11.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Legouis R, Jaulin-Bastard F, Schott S, Navarro C, Borg J, Labouesse M. Basolateral targeting by leucine-rich repeat domains in epithelial cells. EMBO Rep. 2003;4(11):1096–102. doi: 10.1038/sj.embor.7400006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bossinger O, Fukushige T, Claeys M, Borgonie G, McGhee J. The apical disposition of the Caenorhabditis elegans intestinal terminal web is maintained by LET-413. Dev Biol. 2004;268(2):448–56. doi: 10.1016/j.ydbio.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 94.Segbert C, Johnson K, Theres C, van Fürden D, Bossinger O. Molecular and functional analysis of apical junction formation in the gut epithelium of Caenorhabditis elegans. Dev Biol. 2004;266(1):17–26. doi: 10.1016/j.ydbio.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 95.Withee J, Galligan B, Hawkins N, Garriga G. Caenorhabditis elegans WASP and Ena/VASP proteins play compensatory roles in morphogenesis and neuronal cell migration. Genetics. 2004;167(3):1165–76. doi: 10.1534/genetics.103.025676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Scott J, Shewan A, den Elzen N, Loureiro J, Gertler F, Yap A. Ena/VASP proteins can regulate distinct modes of actin organization at cadherin-adhesive contacts. Mol Biol Cell. 2006;17(3):1085–95. doi: 10.1091/mbc.E05-07-0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Takenawa T, Suetsugu S. The WASP-WAVE protein network: connecting the membrane to the cytoskeleton. Nat Rev Mol Cell Biol. 2007;8(1):37–48. doi: 10.1038/nrm2069. [DOI] [PubMed] [Google Scholar]
- 98.Bear J, Svitkina T, Krause M, Schafer D, Loureiro J, Strasser G, Maly I, Chaga O, Cooper J, Borisy G, Gertler F. Antagonism between Ena/VASP proteins and actin filament capping regulates fibroblast motility. Cell. 2002;109(4):509–21. doi: 10.1016/s0092-8674(02)00731-6. [DOI] [PubMed] [Google Scholar]
- 99.Menzies A, Aszodi A, Williams S, Pfeifer A, Wehman A, Goh K, Mason C, Fassler R, Gertler F. Mena and vasodilator-stimulated phosphoprotein are required for multiple actin-dependent processes that shape the vertebrate nervous system. J Neurosci. 2004;24(37):8029–38. doi: 10.1523/JNEUROSCI.1057-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vanderzalm P, Garriga G. Losing their minds: Mena/VASP/EVL triple knockout mice. Dev Cell. 2007;13(6):757–8. doi: 10.1016/j.devcel.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 101.Baum B, Perrimon N. Spatial control of the actin cytoskeleton in Drosophila epithelial cells. Nat Cell Biol. 2001;3(10):883–90. doi: 10.1038/ncb1001-883. [DOI] [PubMed] [Google Scholar]
- 102.Grevengoed E, Fox D, Gates J, Peifer M. Balancing different types of actin polymerization at distinct sites: roles for Abelson kinase and Enabled. J Cell Biol. 2003;163(6):1267–79. doi: 10.1083/jcb.200307026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sawa M, Suetsugu S, Sugimoto A, Miki H, Yamamoto M, Takenawa T. Essential role of the C. elegans Arp2/3 complex in cell migration during ventral enclosure. J Cell Sci. 2003;116(Pt 8):1505–18. doi: 10.1242/jcs.00362. [DOI] [PubMed] [Google Scholar]
- 104.Asano A, Asano K, Sasaki H, Furuse M, Tsukita S. Claudins in Caenorhabditis elegans: their distribution and barrier function in the epithelium. Curr Biol. 2003;13(12):1042–6. doi: 10.1016/s0960-9822(03)00395-6. [DOI] [PubMed] [Google Scholar]
- 105.Wu V, Schulte J, Hirschi A, Tepass U, Beitel G. Sinuous is a Drosophila claudin required for septate junction organization and epithelial tube size control. J Cell Biol. 2004;164(2):313–23. doi: 10.1083/jcb.200309134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wu V, Yu M, Paik R, Banerjee S, Liang Z, Paul S, Bhat M, Beitel G. Drosophila Varicose, a member of a new subgroup of basolateral MAGUKs, is required for septate junctions and tracheal morphogenesis. Development. 2007;134(5):999–1009. doi: 10.1242/dev.02785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang Q, Margolis B. Apical junctional complexes and cell polarity. Kidney Int. 2007;72(12):1448–58. doi: 10.1038/sj.ki.5002579. [DOI] [PubMed] [Google Scholar]
- 108.Kemphues K, Priess J, Morton D, Cheng N. Identification of genes required for cytoplasmic localization in early C. elegans embryos. Cell. 1988;52(3):311–20. doi: 10.1016/s0092-8674(88)80024-2. [DOI] [PubMed] [Google Scholar]
- 109.Tepass U, Theres C, Knust E. crumbs encodes an EGF-like protein expressed on apical membranes of Drosophila epithelial cells and required for organization of epithelia. Cell. 1990;61(5):787–99. doi: 10.1016/0092-8674(90)90189-l. [DOI] [PubMed] [Google Scholar]
- 110.Margolis B, Borg J. Apicobasal polarity complexes. J Cell Sci. 2005;118(Pt 22):5157–9. doi: 10.1242/jcs.02597. [DOI] [PubMed] [Google Scholar]
- 111.de Cuevas M, Lee J, Spradling A. alpha-spectrin is required for germline cell division and differentiation in the Drosophila ovary. Development. 1996;122(12):3959–68. doi: 10.1242/dev.122.12.3959. [DOI] [PubMed] [Google Scholar]
- 112.Dubreuil R, Maddux P, Grushko T, MacVicar G. Segregation of two spectrin isoforms: polarized membrane-binding sites direct polarized membrane skeleton assembly. Mol Biol Cell. 1997;8(10):1933–42. doi: 10.1091/mbc.8.10.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lee J, Brandin E, Branton D, Goldstein L. alpha-Spectrin is required for ovarian follicle monolayer integrity in Drosophila melanogaster. Development. 1997;124(2):353–62. doi: 10.1242/dev.124.2.353. [DOI] [PubMed] [Google Scholar]
- 114.Bennett V, Baines A. Spectrin and ankyrin-based pathways: metazoan inventions for integrating cells into tissues. Physiol Rev. 2001;81(3):1353–92. doi: 10.1152/physrev.2001.81.3.1353. [DOI] [PubMed] [Google Scholar]
- 115.Praitis V, Ciccone E, Austin J. SMA-1 spectrin has essential roles in epithelial cell sheet morphogenesis in C. elegans. Dev Biol. 2005;283(1):157–70. doi: 10.1016/j.ydbio.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 116.Hung T, Kemphues K. PAR-6 is a conserved PDZ domain-containing protein that colocalizes with PAR-3 in Caenorhabditis elegans embryos. Development. 1999;126(1):127–35. doi: 10.1242/dev.126.1.127. [DOI] [PubMed] [Google Scholar]
- 117.Tabuse Y, Izumi Y, Piano F, Kemphues K, Miwa J, Ohno S. Atypical protein kinase C cooperates with PAR-3 to establish embryonic polarity in Caenorhabditis elegans. Development. 1998;125(18):3607–14. doi: 10.1242/dev.125.18.3607. [DOI] [PubMed] [Google Scholar]
- 118.Totong R, Achilleos A, Nance J. PAR-6 is required for junction formation but not apicobasal polarization in C. elegans embryonic epithelial cells. Development. 2007;134(7):1259–68. doi: 10.1242/dev.02833. [DOI] [PubMed] [Google Scholar]
- 119.Denholm B, Skaer H. Tubulogenesis: a role for the apical extracellular matrix? Curr Biol. 2003;13(23):R909–11. doi: 10.1016/j.cub.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 120.Nance J. PAR proteins and the establishment of cell polarity during C. elegans development. Bioessays. 2005;27(2):126–35. doi: 10.1002/bies.20175. [DOI] [PubMed] [Google Scholar]
- 121.Cox D, Seyfried S, Jan L, Jan Y. Bazooka and atypical protein kinase C are required to regulate oocyte differentiation in the Drosophila ovary. Proc Natl Acad Sci U S A. 2001;98(25):14475–80. doi: 10.1073/pnas.261565198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Suzuki A, Ishiyama C, Hashiba K, Shimizu M, Ebnet K, Ohno S. aPKC kinase activity is required for the asymmetric differentiation of the premature junctional complex during epithelial cell polarization. J Cell Sci. 2002;115(Pt 18):3565–73. doi: 10.1242/jcs.00032. [DOI] [PubMed] [Google Scholar]
- 123.Goldstein B, Macara I. The PAR proteins: fundamental players in animal cell polarization. Dev Cell. 2007;13(5):609–22. doi: 10.1016/j.devcel.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Suzuki A, Ohno S. The PAR-aPKC system: lessons in polarity. J Cell Sci. 2006;119(Pt 6):979–87. doi: 10.1242/jcs.02898. [DOI] [PubMed] [Google Scholar]
- 125.Noda Y, Takeya R, Ohno S, Naito S, Ito T, Sumimoto H. Human homologues of the Caenorhabditis elegans cell polarity protein PAR6 as an adaptor that links the small GTPases Rac and Cdc42 to atypical protein kinase C. Genes Cells. 2001;6(2):107–19. doi: 10.1046/j.1365-2443.2001.00404.x. [DOI] [PubMed] [Google Scholar]
- 126.Nagai-Tamai Y, Mizuno K, Hirose T, Suzuki A, Ohno S. Regulated protein-protein interaction between aPKC and PAR-3 plays an essential role in the polarization of epithelial cells. Genes Cells. 2002;7(11):1161–71. doi: 10.1046/j.1365-2443.2002.00590.x. [DOI] [PubMed] [Google Scholar]
- 127.Mizuno K, Suzuki A, Hirose T, Kitamura K, Kutsuzawa K, Futaki M, Amano Y, Ohno S. Self-association of PAR-3-mediated by the conserved N-terminal domain contributes to the development of epithelial tight junctions. J Biol Chem. 2003;278(33):31240–50. doi: 10.1074/jbc.M303593200. [DOI] [PubMed] [Google Scholar]
- 128.Ebnet K, Suzuki A, Ohno S, Vestweber D. Junctional adhesion molecules (JAMs): more molecules with dual functions? J Cell Sci. 2004;117(Pt 1):19–29. doi: 10.1242/jcs.00930. [DOI] [PubMed] [Google Scholar]
- 129.Leung B, Hermann G, Priess J. Organogenesis of the Caenorhabditis elegans intestine. Dev Biol. 1999;216(1):114–34. doi: 10.1006/dbio.1999.9471. [DOI] [PubMed] [Google Scholar]
- 130.Bilder D, Perrimon N. Localization of apical epithelial determinants by the basolateral PDZ protein Scribble. Nature. 2000;403(6770):676–80. doi: 10.1038/35001108. [DOI] [PubMed] [Google Scholar]
- 131.Tanentzapf G, Tepass U. Interactions between the crumbs, lethal giant larvae and bazooka pathways in epithelial polarization. Nat Cell Biol. 2003;5(1):46–52. doi: 10.1038/ncb896. [DOI] [PubMed] [Google Scholar]
- 132.Hutterer A, Betschinger J, Petronczki M, Knoblich J. Sequential roles of Cdc42, Par-6, aPKC, and Lgl in the establishment of epithelial polarity during Drosophila embryogenesis. Dev Cell. 2004;6(6):845–54. doi: 10.1016/j.devcel.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 133.Yamanaka T, Horikoshi Y, Sugiyama Y, Ishiyama C, Suzuki A, Hirose T, Iwamatsu A, Shinohara A, Ohno S. Mammalian Lgl forms a protein complex with PAR-6 and aPKC independently of PAR-3 to regulate epithelial cell polarity. Curr Biol. 2003;13(9):734–43. doi: 10.1016/s0960-9822(03)00244-6. [DOI] [PubMed] [Google Scholar]
- 134.Petronczki M, Knoblich J. DmPAR-6 directs epithelial polarity and asymmetric cell division of neuroblasts in Drosophila. Nat Cell Biol. 2001;3(1):43–9. doi: 10.1038/35050550. [DOI] [PubMed] [Google Scholar]
- 135.Wodarz A, Ramrath A, Grimm A, Knust E. Drosophila atypical protein kinase C associates with Bazooka and controls polarity of epithelia and neuroblasts. J Cell Biol. 2000;150(6):1361–74. doi: 10.1083/jcb.150.6.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rolls M, Albertson R, Shih H, Lee C, Doe C. Drosophila aPKC regulates cell polarity and cell proliferation in neuroblasts and epithelia. J Cell Biol. 2003;163(5):1089–98. doi: 10.1083/jcb.200306079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Médina E, Lemmers C, Lane-Guermonprez L, Le Bivic A. Role of the Crumbs complex in the regulation of junction formation in Drosophila and mammalian epithelial cells. Biol Cell. 2002;94(6):305–13. doi: 10.1016/s0248-4900(02)00004-7. [DOI] [PubMed] [Google Scholar]
- 138.Roh M, Fan S, Liu C, Margolis B. The Crumbs3-Pals1 complex participates in the establishment of polarity in mammalian epithelial cells. J Cell Sci. 2003;116(Pt 14):2895–906. doi: 10.1242/jcs.00500. [DOI] [PubMed] [Google Scholar]
- 139.Straight S, Shin K, Fogg V, Fan S, Liu C, Roh M, Margolis B. Loss of PALS1 expression leads to tight junction and polarity defects. Mol Biol Cell. 2004;15(4):1981–90. doi: 10.1091/mbc.E03-08-0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Médina E, Williams J, Klipfell E, Zarnescu D, Thomas G, Le Bivic A. Crumbs interacts with moesin and beta(Heavy)-spectrin in the apical membrane skeleton of Drosophila. J Cell Biol. 2002;158(5):941–51. doi: 10.1083/jcb.200203080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pellikka M, Tanentzapf G, Pinto M, Smith C, McGlade C, Ready D, Tepass U. Crumbs, the Drosophila homologue of human CRB1/RP12, is essential for photoreceptor morphogenesis. Nature. 2002;416(6877):143–9. doi: 10.1038/nature721. [DOI] [PubMed] [Google Scholar]
- 142.Hong Y, Ackerman L, Jan L, Jan Y. Distinct roles of Bazooka and Stardust in the specification of Drosophila photoreceptor membrane architecture. Proc Natl Acad Sci U S A. 2003;100(22):12712–7. doi: 10.1073/pnas.2135347100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lemmers C, Michel D, Lane-Guermonprez L, Delgrossi M, Médina E, Arsanto J, Le Bivic A. CRB3 binds directly to Par6 and regulates the morphogenesis of the tight junctions in mammalian epithelial cells. Mol Biol Cell. 2004;15(3):1324–33. doi: 10.1091/mbc.E03-04-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hurd T, Gao L, Roh M, Macara I, Margolis B. Direct interaction of two polarity complexes implicated in epithelial tight junction assembly. Nat Cell Biol. 2003;5(2):137–42. doi: 10.1038/ncb923. [DOI] [PubMed] [Google Scholar]
- 145.Bilder D, Schober M, Perrimon N. Integrated activity of PDZ protein complexes regulates epithelial polarity. Nat Cell Biol. 2003;5(1):53–8. doi: 10.1038/ncb897. [DOI] [PubMed] [Google Scholar]
- 146.Norman K, Moerman D. Alpha spectrin is essential for morphogenesis and body wall muscle formation in Caenorhabditis elegans. J Cell Biol. 2002;157(4):665–77. doi: 10.1083/jcb.200111051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.McNeill H, Ozawa M, Kemler R, Nelson W. Novel function of the cell adhesion molecule uvomorulin as an inducer of cell surface polarity. Cell. 1990;62(2):309–16. doi: 10.1016/0092-8674(90)90368-o. [DOI] [PubMed] [Google Scholar]
- 148.Nelson W, Shore E, Wang A, Hammerton R. Identification of a membrane-cytoskeletal complex containing the cell adhesion molecule uvomorulin (E-cadherin), ankyrin, and fodrin in Madin-Darby canine kidney epithelial cells. J Cell Biol. 1990;110(2):349–57. doi: 10.1083/jcb.110.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yeaman C, Grindstaff K, Nelson W. New perspectives on mechanisms involved in generating epithelial cell polarity. Physiol Rev. 1999;79(1):73–98. doi: 10.1152/physrev.1999.79.1.73. [DOI] [PubMed] [Google Scholar]
- 150.Das A, Base C, Dhulipala S, Dubreuil R. Spectrin functions upstream of ankyrin in a spectrin cytoskeleton assembly pathway. J Cell Biol. 2006;175(2):325–35. doi: 10.1083/jcb.200602095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hu R, Moorthy S, Bennett V. Expression of functional domains of beta G-spectrin disrupts epithelial morphology in cultured cells. J Cell Biol. 1995;128(6):1069–80. doi: 10.1083/jcb.128.6.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Moorthy S, Chen L, Bennett V. Caenorhabditis elegans beta-G spectrin is dispensable for establishment of epithelial polarity, but essential for muscular and neuronal function. J Cell Biol. 2000;149(4):915–30. doi: 10.1083/jcb.149.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lee J, Coyne R, Dubreuil R, Goldstein L, Branton D. Cell shape and interaction defects in alpha-spectrin mutants of Drosophila melanogaster. J Cell Biol. 1993;123(6 Pt 2):1797–809. doi: 10.1083/jcb.123.6.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Dubreuil R, Byers T, Stewart C, Kiehart D. A beta-spectrin isoform from Drosophila (beta H) is similar in size to vertebrate dystrophin. J Cell Biol. 1990;111(5 Pt 1):1849–58. doi: 10.1083/jcb.111.5.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Thomas G, Williams J. Dynamic rearrangement of the spectrin membrane skeleton during the generation of epithelial polarity in Drosophila. J Cell Sci. 1999;112(Pt 17):2843–52. doi: 10.1242/jcs.112.17.2843. [DOI] [PubMed] [Google Scholar]
- 156.Zarnescu D, Thomas G. Apical spectrin is essential for epithelial morphogenesis but not apicobasal polarity in Drosophila. J Cell Biol. 1999;146(5):1075–86. doi: 10.1083/jcb.146.5.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Stabach P, Morrow J. Identification and characterization of beta V spectrin, a mammalian ortholog of Drosophila beta H spectrin. J Biol Chem. 2000;275(28):21385–95. doi: 10.1074/jbc.C000159200. [DOI] [PubMed] [Google Scholar]
- 158.Göbel V, Barrett P, Hall D, Fleming J. Lumen morphogenesis in C. elegans requires the membrane-cytoskeleton linker erm-1. Dev Cell. 2004;6(6):865–73. doi: 10.1016/j.devcel.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 159.Van Fürden D, Johnson K, Segbert C, Bossinger O. The C. elegans ezrin-radixin-moesin protein ERM-1 is necessary for apical junction remodelling and tubulogenesis in the intestine. Dev Biol. 2004;272(1):262–76. doi: 10.1016/j.ydbio.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 160.Williams-Masson E, Heid P, Lavin C, Hardin J. The cellular mechanism of epithelial rearrangement during morphogenesis of the Caenorhabditis elegans dorsal hypodermis. Dev Biol. 1998;204(1):263–76. doi: 10.1006/dbio.1998.9048. [DOI] [PubMed] [Google Scholar]