Abstract
Atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP) are important biomarkers in the diagnosis and risk stratification for heart failure (HF). These peptides are synthesized as inactive precursors, pro-ANP and pro-BNP, which are converted to biologically active 28-amino-acid ANP and 32-amino-acid BNP, respectively. Most immunoassays currently used in the clinical setting, however, do not determine precise molecular forms of these natriuretic peptides, which may vary depending on the pathophysiological state of HF. Analysis from chromatography-based studies reveals that in HF, inactive pro-ANP and pro-BNP forms often predominate. This indicates that the bioactive forms of natriuretic peptides may not be processed proportionally in patients with advanced HF. Distinguishing the bioactive natriuretic peptides from their inactive forms in plasma may help to define the role of these peptides in the pathogenesis of HF and provide new insights into the treatment of the disease.
Keywords: biomarker, atria natriuretic peptide, B-type or brain natriuretic peptide, heart failure
Introduction
Atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP) are key regulators in the homeostasis of salt and water balance and in maintaining peripheral vessel tone. In the nearly thirty years since ANP was discovered by de Bold,1 substantial knowledge has been advanced in this field. ANP and BNP expression and secretion increase significantly in response to pathophysiological stressors, such as hypertension, hypoxia, infection, cardiac ischemia, ventricular hypertrophy, heart failure (HF), and cardiac allograft rejection. Radioimmunoassay and enzyme-linked immunosorbent assay for natriuretic peptides have been developed and extensively used as biomarkers in a variety of clinical settings,2,3 particularly in acute myocardial infarction (AMI) and HF diagnosis, risk stratification, and treatment response monitoring. Most assays currently used in the clinical setting, however, cannot determine precise molecular forms of the natriuretic peptides. In contrast, chromatography-based methods, such as high-performance liquid chromatography (HPLC) and gel filtration chromatography (GFC) followed by Western blotting or mass spectrometry, provide more accurate information about molecular forms of these peptides. In this article, we review the molecular forms of pro-ANP and pro-BNP and their derivatives, analyze available data in the literature, and discuss their potential clinical implications.
ANP and BNP Synthesis, Function, and Modulation
Synthesis
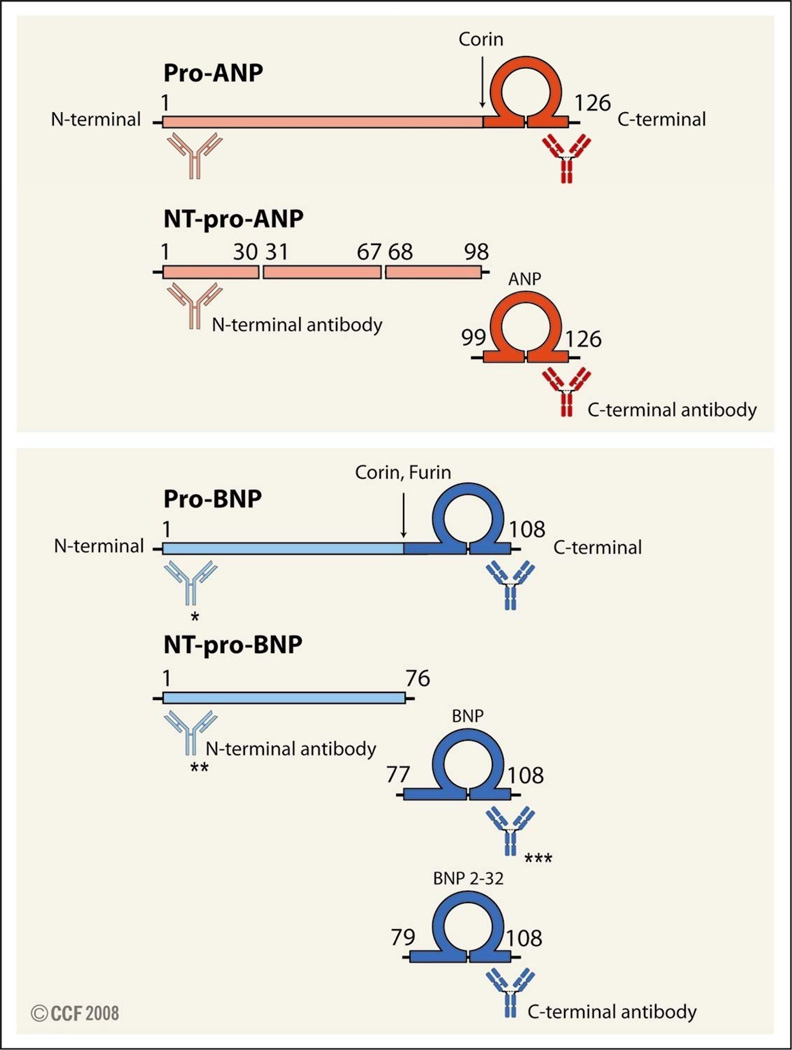
During embryonic development, ANP and BNP genes are expressed in both the atrium and ventricle. After birth, these peptides are expressed mainly in the atrium, but downregulated in the ventricle. In cardiomyocytes, ANP is synthesized as an inactive prepropeptide. After removal of the signal peptide, a 126-amino-acid precursor, pro-ANP, is derived. Upon secretion from myocytes, pro-ANP is processed by the transmembrane enzyme corin at arginine 98, generating the amino-terminal (NT)-pro-ANP fragment of 98 amino acids and the carboxyl terminal (C-terminal) bioactive ANP of 28 amino acids.4,5 Pro-ANP, NT-pro-ANP, and ANP are all released into circulation (Figure 1). Pro-BNP is synthesized in both the atrium and ventricle and cleaved at arginine 76 to yield NT pro-BNP and C-terminal bioactive BNP of 32 amino acids. In in vitro experiments, both corin and furin cleaved pro-BNP into BNP.5, 6 However, the mechanism for pro-BNP post-translational maturation in vivo has not been established. In circulation, a small portion of BNP is further processed to BNP79–108 by dipeptidyl peptidase IV. Like pro-ANP derivatives, pro-BNP, NT-pro-BNP, and BNP all enter the bloodstream.
Figure 1.
Molecular forms of ANP and BNP. Pro-ANP (top panel) and Pro-BNP (lower panel) are processed by proteases corin and furin. Fragments derived from pro-ANP and pro-BNP are indicated. Potential cross-reactivity of C- and N-terminal antibodies with pro-NP, NT-pro-NP, and NP is illustrated. Antibodies used in commercial assays: * in Biosite® pro-BNP assay, antibodies recognizing the end of N-terminal, amino acids 82–90 and 79–108 were used. ** in Elesys® NT-pro-BNP assay, antibodies recognizing amino acids 1–21 and 39–50 were used; *** in Biosite® BNP assay, antibodies recognizing amino acids 82–90 and 79–108 were used.36
Biological Function
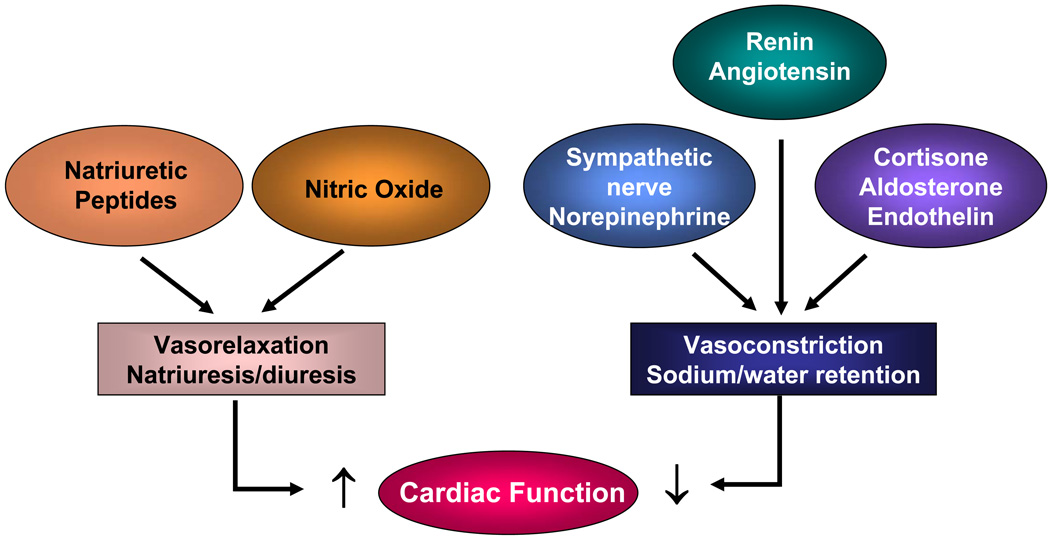
ANP and BNP bind to their receptor natriuretic peptide receptor A (NPR-A) in target organs, activate the intrinsic guanylyl cyclase activity of NPR-A, and generate intracellular cGMP.7 ANP and BNP also antagonize the renin-angiotensin system, inhibit endothelin secretion, and reduce systemic and renal sympathetic activity, thereby increasing natriuretic, diuretic, and vasorelaxant activity (Figure 2). Thus, the natriuretic peptides act as a compensatory mechanism to promote cardiac function. These peptides also suppress myocardial fibrosis and hypertrophy in cultured cells and animal models. In contrast, pro-ANP and pro-BNP have minimal biological activities.8 ANP and BNP are metabolized by at least two different mechanisms. Both peptides bind to their clearance receptor NPR-C and are internalized and hydrolyzed by lysosomal enzymes. ANP and BNP also are degraded by neutral endopeptidase 24.11.
Figure 2.
Neurohormonal regulation of cardiac function in heart failure.
Modulation
The natriuretic peptide system is upregulated under pathophysiological stress. In acute biomechanical stress, such as stretch, pro-ANP is rapidly released from atrial granules. Under conditions of chronic neurohumoral activation or increased hemodynamic load, such as hypertension, many cardiac genes, which normally are expressed during embryonic development, could be activated. This phenomenon is known as reinduction of the “fetal gene program.” ANP and BNP gene expression is enhanced in both the atrium and ventricle in myocardial ischemia, AMI, and HF.9 The increase is associated with disease type and proportional to disease severity. Most cardiac pathophysiological stresses stimulate both ANP and BNP production, but in certain conditions, the response may differ. In the early phase of AMI, for example, BNP gene expression increases considerably, whereas ANP gene expression increases only slightly. In human cardiac allograft acute rejection, BNP, but not ANP, gene expression has been shown to increase.10 In cardiac hypertrophy, pro-ANP is made mainly in the atrium, whereas pro-BNP is made mainly in the ventricle. Plasma ANP level starts to increase as atrial pressure elevates, whereas plasma BNP level rises when ventricular hypertrophy develops.
Molecular Forms of Natriuretic Peptides in Circulation
To date, a great deal of information on ANP and BNP levels in various clinical settings has been generated. However, most studies use antibody-based immunoassays, which do not distinguish the molecular forms of the peptides. For example, an antibody that detects bioactive ANP may also recognize pro-ANP (Figure 1). In contrast, HPLC or GFC in combination with immunoassays can differentiate natriuretic peptide fragments based on their molecular masses. The results provide insights into the molecular form of these peptides under various pathophysiological conditions. Therefore, we decided to analyze data on the molecular form of natriuretic peptides from such studies. We searched Ovid Medline using the keywords chromatography and natriuretic peptide for publications since 1981, then selected the studies in which molecular forms of ANP and BNP were determined for further analysis. In this review, iANP and iBNP are used to refer to immunoreactive ANP and BNP.
Pro-ANP and ANP
Studies of pro-ANP and ANP forms in human plasma are summarized in Table 1. Three major molecular forms—2.6, 6, and 13–15 kDa—were identified by chromatography-based methods. The 2.6 kDa low-molecular-weight form corresponded to ANP, and the 6 and 13–15 kDa forms corresponded to NT-pro-ANP and the 126-amino-acid pro-ANP, respectively 11, 12 (Figure 1). In normal human plasma, both pro-ANP and ANP levels are low, mostly in the fmol/ml range. ANP appeared to be the major form,13 although pro-ANP also was detected in some studies.12 In patients with normal heart function who were under volume challenges, such as pregnancy14 or hemodialysis,15–18 ANP was found to be the dominant form in the blood.
Table 1.
Plasma ANP molecular forms in normal controls and patients with heart disease
| Study | Plasma Sample (n) |
Diagnosis | Pro-ANP | ANP | Prop-ANP /ANP |
|---|---|---|---|---|---|
| Thesis et al.13 | 1 | normal control | none | major | |
| Akiba et al. 18 | 25 | normal control | minor | major | |
| Hunter et al. 12 | 10, pooled plasma | normal control | equal | equal | |
| Ohashi et al.39 | 1 | normal control | none | present | |
| Sato et al.40 | 7, pooled plasma | normal control | minor | major | |
| Meleagros et al.19 | 1 | normal control | minor | major | 0.24 |
| Ando et al.21 | 1 | normal control | minor | major | |
| Tan et al.41 | 2 | normal control | minor | major | |
| Espiner et al.42 | 1 | normal control | minor | major | |
| Azizi et al.22 | pooled plasma | normal control | minor | major | |
| Yamaji et al. 14 | 1 | normal heart, pregnant woman |
minor | major | |
| Predel et al.43 | 4 | CRF, normotension | minor | major | 0.25 |
| Ishizaka et al.15 | 1 | HD, normal heart | minor | major | 0.47 |
| Cannella et al.16 | 10, pooled | HD, normal heart | minor | major | |
| Akiba et al.18 | 10 | HD, normal heart | minor | major | |
| Hasegawa et al.17 | 1 | HD, normal heart | minor | major | |
| Lindberg et al.44 | 2 | HD, MV stenosis; HD, MI | minor | major | |
| Hunter et al.12 | 7, pooled plasma | HF | equal | equal | Pro-ANP=ANP |
| Yamaji et al.14 | 3, pooled plasma | HF | minor | major | |
| Meleagros et al.19 | 1 | HF | minor | major | 0.16 |
| Mukoyama et al.11 | 1 | HF | minor | major | Pro-ANP<<ANP |
| Ando et al.21 | 4 | NYHA class I | minor | major | 0.087 |
| 11 | NYHA class II | minor | major | 0.45 | |
| 5 | NYHA class III | major | minor | 1.63 | |
| 8 | NYHA class IV | major | minor | 1.27 | |
| Azizi et al.22 | pooled plasma | HF | major | minor | Pro-ANP>>ANP |
Key: CRF, chronic renal failure; HD, hemodialysis; HF, heart failure; Ir, immunoreactive; MI, myocardial infarction; MV, mitral valve; NYHA, New York Heart Association.
In patients with HF, plasma pro-ANP and ANP levels appear to vary dramatically. Hunter et al.12 revealed that in patients with HF (functional class unspecified), pro-ANP was the predominant form. In three other studies, on the other hand, ANP appeared to be the major form in plasma samples from patients with HF.11, 19, 20 The difference may reflect the disease state of the patients, from which samples were collected. Ando et al.21 reported that patients of New York Heart Association (NYHA) class I to II had ANP as the main form in their plasma, whereas pro-ANP predominated in samples from class III to IV patients. Studying pooled plasma samples, Azizi et al.22 found that in patients with an average ejection fraction of 28% and HF (NYHA class I–IV), circulating iANP was mainly pro-ANP, and only a small fraction was ANP. These findings suggest that at the late stage of HF, plasma pro-ANP and ANP may not increase proportionally and that elevated iANP levels detected by immunoassays may mostly represent inactive pro-ANP. This notion is supported by other functional studies. For example, de Bold et al. showed that intravenous infusion of atrial extracts from normal rats incited a rapid and potent diuretic and vasodilating response.1 However, such a response was not observed when atrial extracts from BIO 14.6 hamsters with congestive HF were used,23 although plasma iANP levels were remarkably high in these cardiomyopathic hamsters. These results indicate that atria in failing hearts may contain much less bioactive ANP compared to that in normal atria.
Pro-BNP and BNP
HPLC- and GFC-based studies also have been conducted to determine pro-BNP-derived peptides in human plasma (Table 2). Tateyama et al.24 used reverse-phase C-18 column and an anti-BNP antibody and identified two peptides—3.5 and 12 kDa, respectively—in human plasma. Further analysis by reverse-phase HPLC showed that the 12-kDa fragment was 108-amino-acid pro-BNP, and the 3.5-kDa fragment was 32-amino-acid BNP. Several other studies showed that in plasma samples from healthy subjects, BNP is the predominant form,15, 25, 26 although pro-BNP also was detected in plasma extracts from normal individuals.27Ishizaka et al.15 showed that in 5 patients who underwent hemodialysis but had normal heart function, plasma iBNP increased immediately before dialysis, most likely because of volume expansion. Under this condition, BNP was found to be the predominant form. In another study with one hypertensive patient, BNP also was found to be the predominant form.25 These results suggest that in patients with normal heart function with or without hypertension, increased pro-BNP is properly processed into BNP. Interestingly, in a study of 3 patients with HF, all plasma samples had significantly increased iBNP, but the composition of pro-BNP and BNP varied in each case.27 A 50-year-old woman with aortic stenosis and regurgitation, mitral stenosis, and in NYHA class II had similar amounts of plasma pro-BNP and BNP. In a 75-year-old man with AMI, ventricular septal perforation, congestive HF, and in NYHA class III, BNP appeared to be the dominant form. In a 77-year-old man with aortic stenosis, infective endocarditis, congestive HF, and in NYHA class III–IV, pro-BNP predominated in his samples. In another study, Seferian et al.28 found that 3 patients with AMI, ejection fraction <30%, and pulmonary edema had pro-BNP as the predominant form in their plasma, with an average ratio of pro-BNP to BNP of 3.3. The same study also reported that 4 other patients with ischemic cardiomyopathy and acute HF had an even higher pro-BNP to BNP ratio of (8.5).28 Similar results were reported by several other groups.29–31 For example, Yandle et al.30 demonstrated a strong inverse relationship between pro-BNP and BNP components (y = −8.9+2.4x; r=0.99).
Table 2.
Plasma BNP molecular forms in normal controls and patients with heart disease
| Study | n | Diagnosis | Pro-BANP | BNP | Pro-BNP/BNP |
|---|---|---|---|---|---|
| Yandle et al.30 | 3 | normal control | none | none | |
| Tateyama et al.27 | 10 | normal control | major | 1.2, 1.3, 1.7, 1.7, 2.8, 3.0, 3.1, 3.3, 5.2 |
|
| Kohno et al.25 | 1 | normal control | major (only) | ||
| Togashi et al.26 | 1 | normal control | minor | major | |
| Ishizaka et al.15 | 5 | HD, normal heart | minor | major | |
| Kohno et al.25 | 1 | hypertension | minor | major | |
| Mukoyama et al.11 | 1 | HF, NYHA not doc. |
minor | major | |
| Tateyama et al. 27 | 1 | AV stenosis, MV stenosis, NYHA class II |
minor (slightly) | major (slightly) | |
| 1 | AMI, VSP, NYHA class III |
minor | major | ||
| 1 | AS, IE, CHF, NYHA class III-IV |
major | minor | ||
| Hejmdal et al.45 | 1 | MI, cardiogenic shock | major | minor | |
| Seferian et al.28 | 3 | AMI, EF <30%, pulmonary edema |
major | minor | 3.9, 1.8, 4.2 |
| 4 | AMI, EF <30%, NYHA class IV |
major | minor | 7.8, 4.7, 10.6, 10.8 | |
| Shimizu et al.46 | 2 | HF, NYHA class not doc. |
major | minor | |
| 1 | HF, NYHA class not doc. |
minor (slightly) | major (slightly ) | 0.85 | |
| Shimizu et al.29 | 3 | HF, NYHA class not doc. |
major | minor | 1.1, 2.4, 4.8 |
| 1 | HF, NYHA class not doc. |
minor (slightly) | major (slightly) | 0.98 | |
| Yandle et al.30 | 7, pooled plasma |
HF, NYHA class II–IV |
major | minor | average 1.92 |
| Hunt et al.12 | 1 | HF | major | none |
Key: AMI, acute myocardial infarction; AV, aortic valve; CHF, congestive heart failure; HD, hemodialysis; HF, heart failure; MV, mitral valve; not doc., not documented; NYHA, New York Heart Association; VSP, ventricular septal perforation.
The above observations are supported by recent studies using most advanced techniques. Hawkridge et al.32 used nano-liquid chromatography electrospray ionization Fourier transform ion cyclotron resonance and quantitative mass spectrometry to study 4 patients with advanced HF (NYHA class IV).32 Despite plasma iBNP levels of 420–1156 fmol/mL, endogenous BNP was not detected in their plasma samples, indicating that antigens detected by the immunoassays were mostly unprocessed pro-BNP molecules. Consistently, Liang et al.8 used immunoprecipitation and Western blotting to examine plasma pro-BNP and BNP in samples from 5 patients with decompensated HF (NYHA class IV). Results showed significantly elevated levels of iBNP (1270–4010 pg/mL) but low amounts of BNP. Together, these data indicate that patients with severe HF have significantly elevated levels of plasma iBNP. In these patients, however, pro-BNP appears to be the main form, whereas BNP represented as a minor form.
Clinical Implications of Molecular Forms of Natriuretic Peptides
Pathologically, congestive HF is a complex mechanical and neurohumoral syndrome, with excessive sodium and water accumulation and increased plasma levels of norepinephrine, renin, aldosterone, cortisol, and endothelin (Figure 2). As discussed under the section “Molecular Forms,” it appears that in HF, the myocardium preserves its ability to synthesize natriuretic peptides in response to chronic fluid expansion and increasing afterload. Significantly increased expression of pro-ANP and pro-BNP is commonly observed in patients with HF. However, production of the bioactive ANP and BNP appears to vary considerably with HF severity. Plasma levels of active ANP and BNP were low in most cases,23, 32 although increased levels of ANP and BNP also were reported in some patients with HF.9 Because ANP and BNP, but not their inactive pro-forms, have biological activity in promoting vasodilatation, diuresis, and natriuresis and preventing cardiac fibrosis, the level of respective molecular forms of these peptides may be associated with clinical presentation and progression in patients with HF. Clinically, a subset of patients with HF has severe left ventricular (LV) dysfunction with increased end-diastolic wall stress, but exhibits no obvious fluid retention. The lack of a direct link between HF and body fluid retention in these patients is intriguing, but the underlying mechanism is unknown. Is it possible that sufficient amounts of active natriuretic peptides were produced to antagonize detrimental neurohumoral effects? If so, then conversely, lack of active ANP and BNP may contribute to the sodium and fluid retention commonly seen in patients with advanced HF.
The increased immature pro-ANP and pro-BNP, but not mature ANP and BNP, in plasma samples from patients with HF suggest that these natriuretic peptides may not be appropriately processed in failing hearts. Plasma cGMP, which is produced by activation of NPR-A and its cGMP cyclase, is a useful biomarker, reflecting the biologic activity of ANP and BNP in vivo. In a study of patients with acutely decompensated HF, iANP and iBNP were shown to increase significantly as symptoms worsened and to decrease as symptoms resolved.33 However, cGMP levels at the worsening and improving phases remained unchanged. In another study of 175 patients with end-stage HF, corin mRNA and protein and BNP mRNA were found to be increased, but the altered corin expression inversely correlated with the increase in BNP mRNA expression.34 These results might indicate that natriuretic peptide processing becomes rate-limiting in advanced HF, and therefore, production of active ANP and BNP may not increase further even though their activities are needed under this stress condition.
ANP and BNP are important biomarkers in the diagnosis and risk stratification for HF.2, 3 Plasma levels of pro-BNP may increase to a much higher level than that of pro-ANP in the pathologic condition.11 Currently, BNP and NT-pro-BNP are the preferred biomarkers for HF.2 As shown in Figure 1, there are at least three molecular forms, each derived from pro-ANP and pro-BNP. Further proteolytic processing and/or degradation by plasma enzymes may generate additional fragments of various lengths.35 As a result, accurately identifying and measuring each form are extremely challenging using antibody-based radioimmunoassays or enzyme-linked immunosorbent assays. These convenient and rapid assays have been widely used in clinical settings for quick diagnostic screening and assessing therapeutic effectiveness for HF.2 However, the principle, on which these assays are based, needs to be carefully considered. As illustrated in Figure 1, antibodies against ANP and BNP may also recognize pro-ANP and pro-BNP. Conversely, antibodies recognizing NT-fragments may bind to pro-NP. Commercial Triage BNP assay and Advia Centaur BNP assay are designed to detect bioactive forms of BNP by using antibodies against the C-terminal amino acids 82–90 and 79–108. Elecsys NT-pro-BNP assay is designed to measure the inactive form of NT-pro-BNP by using antibodies targeting N-terminal amino acids 1–21 and 39–50.36 Liang et al.8 used recombinant human pro-BNP and BNP to examine the accuracy and cross-reactivity of commercially available assays. The study found that both forms were recognized by the Triage BNP assay and the Advia Centaur BNP assay, and recombinant pro-BNP was also recognized by the Elecsys NT-pro-BNP assay, although its concentration was significantly underestimated.8 The study indicates that these common clinical assays may not adequately distinguish pro-BNP from BNP, and thus the data based on these immunoassays may not be conclusive. In contrast, HPLC- and GFC-based methods provide more accurate information about the molecular forms of these peptides. To date, however, these methods are not routinely used in clinical settings because such assays require a large blood volume (5 to 15 ml), and the processes are much more complicated and time consuming. Thus, new assays that can precisely identify the molecular forms of natriuretic peptides in clinical settings are acutely needed.
The ability to identify premature and active molecular forms of natriuretic peptides in individuals with HF may be clinically important in tailoring treatment. For example, plasma levels of the pro-peptides could be used to guide HF treatment. Therapeutic approaches to enhance pro-peptide to active peptide conversion could be developed to promote vasorelaxation, natriuresis, and diuresis. Synthetic ANP and BNP have been used to treat patients with cardiovascular diseases. These exogenous natriuretic peptides may be therapeutically beneficial for patients with low levels of ANP or BNP. In patients with AMI, ANP infusion decreased LV remodeling and preserved LV systolic function. In HF, infusion ANP lowered pulmonary wedge pressure and systemic blood pressure.37 However, renal response to ANP infusion in patients with severe congestive HF was markedly attenuated, a phenomenon known as renal hyporesponsiveness.37 In experimental models of HF as well as in patients with HF, the effect of ANP on urine output, urinary sodium excretion, and free-water clearance was reduced compared with that in normal controls.37 There are several possible explanations for this phenomenon. In part, hormone-dependent activities of NPR-A can be reduced by chronic exposure to natriuretic peptides, a process known as homologous desensitization. Prior receptor occupation by endogenous active natriuretic peptides is expected to decrease availability of the receptor for subsequent exogenous natriuretic peptides. Nakajima et al.38 infused ANP into two groups of patients with low and high iBNP levels. The low-iBNP group had much greater improvement in LV contractility and reduction of arterial pressure when compared with the response of the high-iBNP group. This suggests that the low-iBNP group may have more unoccupied NPR-A sites to be activated by exogenous ANP, whereas in the high-iBNP group, most receptor sites are expected to be occupied. Additionally, renal ANP resistance might be affected by down-regulation of NPR-A. Therefore, we may predict that patients with lower levels of active forms of natriuretic peptides might be more sensitive to ANP or BNP treatment.
Because different natriuretic peptide forms have different levels of biological activity, development of novel assays that can precisely identify them is imperative. Quantitative and longitudinal information could further aid in our understanding of the role of natriuretic peptides in the pathogenesis, progression, and treatment of HF, one of the most burdensome diseases and challenging conditions to treat.
ACKNOWLEDGMENTS
We thank Drs. Franklin Michota, Richard Lang, and Brian Harte for their support, Mrs. Tess Parry for editing the manuscript, and Mr. Jeff Loerch for preparing Figure 1. This work is supported in part by grants from the Research Program Committee of the Cleveland Clinic, the NIH (R01 HL089298), the Ralph Wilson Medical Research Foundation, and the Bakken Heart-Brain Institute.
Footnotes
Neither author has a conflict of interest with the material reported herein.
“The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence (or non exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd and its Licensees to permit this article (if accepted) to be published in HEART editions and any other BMJPGL products to exploit all subsidiary rights, as set out in our licence http://heart.bmjjournals.com/ifora/licence.pdf”
REFERENCES
- 1.de Bold AJ, Borenstein HB, Veress AT, Sonnenberg H. A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Life Sci. 1981;28(1):89–94. doi: 10.1016/0024-3205(81)90370-2. [DOI] [PubMed] [Google Scholar]
- 2.Mueller C, Scholer A, Laule-Kilian K, Martina B, Schindler C, Buser P, et al. Use of B-type natriuretic peptide in the evaluation and management of acute dyspnea. N Engl J Med. 2004;350(7):647–654. doi: 10.1056/NEJMoa031681. [DOI] [PubMed] [Google Scholar]
- 3.Januzzi JL, Jr, Camargo CA, Anwaruddin S, Baggish AL, Chen AA, Krauser DG, et al. The N-terminal Pro-BNP investigation of dyspnea in the emergency department (PRIDE) study. Am J Cardiol. 2005;95(8):948–954. doi: 10.1016/j.amjcard.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 4.Wu Q, Xu-Cai YO, Chen S, Wang W. Corin: new insights into the natriuretic peptide system. Kidney Int. 2009;75:142–146. doi: 10.1038/ki.2008.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yan W, Wu F, Morser J, Wu Q. Corin, a transmembrane cardiac serine protease, acts as a pro-atrial natriuretic peptide-converting enzyme. Proc Natl Acad Sci U S A. 2000;97(15):8525–8529. doi: 10.1073/pnas.150149097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sawada Y, Inoue M, Kanda T, Sakamaki T, Tanaka S, Minamino N, et al. Co-elevation of brain natriuretic peptide and proprotein-processing endoprotease furin after myocardial infarction in rats. FEBS Lett. 1997;400(2):177–182. doi: 10.1016/s0014-5793(96)01385-3. [DOI] [PubMed] [Google Scholar]
- 7.Koller KJ, Lowe DG, Bennett GL, Minamino N, Kangawa K, Matsuo H, et al. Selective activation of the B natriuretic peptide receptor by C-type natriuretic peptide (CNP) Science. 1991;252(5002):120–123. doi: 10.1126/science.1672777. [DOI] [PubMed] [Google Scholar]
- 8.Liang F, O'Rear J, Schellenberger U, Tai L, Lasecki M, Schreiner GF, et al. Evidence for functional heterogeneity of circulating B-type natriuretic peptide. J Am Coll Cardiol. 2007;49(10):1071–1078. doi: 10.1016/j.jacc.2006.10.063. [DOI] [PubMed] [Google Scholar]
- 9.Giuliani I, Rieunier F, Larue C, Delagneau JF, Granier C, Pau B, et al. Assay for measurement of intact B-type natriuretic peptide prohormone in blood. Clin Chem. 2006;52(6):1054–1061. doi: 10.1373/clinchem.2005.061770. [DOI] [PubMed] [Google Scholar]
- 10.Ogawa T, Veinot JP, Davies RA, Haddad H, Smith SJ, Masters RG, et al. Neuroendocrine profiling of humans receiving cardiac allografts. J Heart Lung Transplant. 2005;24(8):1046–1054. doi: 10.1016/j.healun.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 11.Mukoyama M, Nakao K, Hosoda K, Suga S, Saito Y, Ogawa Y, et al. Brain natriuretic peptide as a novel cardiac hormone in humans. Evidence for an exquisite dual natriuretic peptide system, atrial natriuretic peptide and brain natriuretic peptide. J Clin Invest. 1991;87(4):1402–1412. doi: 10.1172/JCI115146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hunter EFM, Kelly PA, Prowse C, Woods RJ, Lowry PJ. Analysis of peptides derived from Pro Atrial Natriuretic Peptide that circulate in man and increase in heart disease. Scand J Clin Lab Invest. 1998;58:205–216. doi: 10.1080/00365519850186599. [DOI] [PubMed] [Google Scholar]
- 13.Theiss G, John A, Morich F, Neuser D, Schroder W, Stasch JP, et al. Alpha-h-ANP is the only form of circulating ANP in humans. FEBS Lett. 1987;218(1):159–162. doi: 10.1016/0014-5793(87)81038-4. [DOI] [PubMed] [Google Scholar]
- 14.Yamaji T, Hirai N, Ishibashi M, Takaku F, Yanaihara T, Nakayama T. Atrial natriuretic peptide in umbilical cord blood: evidence for a circulating hormone in human fetus. J Clin Endocrinol Metab. 1986;63(6):1414–1417. doi: 10.1210/jcem-63-6-1414. [DOI] [PubMed] [Google Scholar]
- 15.Ishizaka Y, Yamamoto Y, Tanaka M, Kato F, Ishizaka Y, Yokota N, et al. Molecular forms of human brain natriuretic peptide (BNP) in plasma of patients on hemodialysis (HD) Clin Nephrol. 1995;43(4):237–242. [PubMed] [Google Scholar]
- 16.Cannella G, Albertini A, Assanelli D, Ghielmi S, Poiesi C, Gaggiotti M, et al. Effects of changes in intravascular volume on atrial size and plasma levels of immunoreactive atrial natriuretic peptide in uremic man. Clin Nephrol. 1988;30(4):187–192. [PubMed] [Google Scholar]
- 17.Hasegawa K, Matsushita Y, Inoue T, Morii H, Ishibashi M, Yamaji T. Plasma levels of atrial natriuretic peptide in patients with chronic renal failure. J Clin Endocrinol Metab. 1986;63(4):819–822. doi: 10.1210/jcem-63-4-819. [DOI] [PubMed] [Google Scholar]
- 18.Akiba T, Ando K, Marumo F. Changes in molecular pattern of atrial natriuretic peptide in hemodialysis patients. Int J Artif Organs. 1994;17(11):585–590. [PubMed] [Google Scholar]
- 19.Meleagros L, Ghatei MA, Anderson JV, Wharton J, Taylor KM, Krikler DM, et al. The presence and molecular forms of cardiodilatin immunoreactivity in the human and rat right atrium. Clin Chim Acta. 1988;172(2–3):199–209. doi: 10.1016/0009-8981(88)90324-5. [DOI] [PubMed] [Google Scholar]
- 20.Yamaji T, Ishibashi M, Takaku F, Nakaoka H, Imataka K, Kitahara Y, et al. Nature of atrial natriuretic peptide in plasma from patients with congestive heart failure. Lancet. 1986;2(8503):402–403. doi: 10.1016/s0140-6736(86)90093-0. [DOI] [PubMed] [Google Scholar]
- 21.Ando K, Hirata Y, Emori T, Shichiri M, Kurosawa T, Sato K, et al. Circulating forms of human atrial natriuretic peptide in patients with congestive heart failure. J Clin Endocrinol Metab. 1990;70(6):1603–1607. doi: 10.1210/jcem-70-6-1603. [DOI] [PubMed] [Google Scholar]
- 22.Azizi C, Maistre G, Kalotka H, Isnard R, Barthelemy C, Masson F, et al. Plasma levels and molecular forms of proatrial natriuretic peptides in healthy subjects and in patients with congestive heart failure. J Endocrinol. 1996;148(1):51–57. doi: 10.1677/joe.0.1480051. [DOI] [PubMed] [Google Scholar]
- 23.Chimoskey JE, Spielman WS, Brandt MA, Heidemann SR. Cardiac atria of BIO 14.6 hamsters are deficient in natriuretic factor. Science. 1984;223(4638):820–822. doi: 10.1126/science.6538050. [DOI] [PubMed] [Google Scholar]
- 24.Tateyama H, Hino J, Minamino N, Kangawa K, Ogihara T, Matsuo H. Characterization of immunoreactive brain natriuretic peptide in human cardiac atrium. Biochem Biophys Res Commun. 1990;166(3):1080–1087. doi: 10.1016/0006-291x(90)90977-u. [DOI] [PubMed] [Google Scholar]
- 25.Kohno M, Horio T, Yokokawa K, Murakawa K, Yasunari K, Akioka K, et al. Brain natriuretic peptide as a cardiac hormone in essential hypertension. Am J Med. 1992;92(1):29–34. doi: 10.1016/0002-9343(92)90011-y. [DOI] [PubMed] [Google Scholar]
- 26.Togashi K, Hirata Y, Ando K, Takei Y, Kawakami M, Marumo F. Brain natriuretic peptide-like immunoreactivity is present in human plasma. FEBS Lett. 1989;250(2):235–237. doi: 10.1016/0014-5793(89)80728-8. [DOI] [PubMed] [Google Scholar]
- 27.Tateyama H, Hino J, Minamino N, Kangawa K, Minamino T, Sakai K, et al. Concentrations and molecular forms of human brain natriuretic peptide in plasma. Biochem Biophys Res Commun. 1992;85(2):760–767. doi: 10.1016/0006-291x(92)91691-i. [DOI] [PubMed] [Google Scholar]
- 28.Seferian KR, Tamm NN, Semenov AG, Mukharyamova KS, Tolstaya AA, Koshkina EV, et al. The brain natriuretic peptide (BNP) precursor is the major immunoreactive form of BNP in patients with heart failure. Clin Chem. 2007;53(5):866–873. doi: 10.1373/clinchem.2006.076141. [DOI] [PubMed] [Google Scholar]
- 29.Shimizu H, Masuta K, Asada H, Sugita K, Sairenji T. Characterization of molecular forms of probrain natriuretic peptide in human plasma. Clin Chim Acta. 2003;334(1–2):233–239. doi: 10.1016/s0009-8981(03)00240-7. [DOI] [PubMed] [Google Scholar]
- 30.Yandle TG, Richards AM, Gilbert A, Fisher S, Holmes S, Espiner EA. Assay of brain natriuretic peptide (BNP) in human plasma: evidence for high molecular weight BNP as a major plasma component in heart failure. J Clin Endocrinol Metab. 1993;76(4):832–838. doi: 10.1210/jcem.76.4.8473392. [DOI] [PubMed] [Google Scholar]
- 31.Hunt PJ, Espiner EA, Nicholls MG, Richards AM, Yandle TG. The role of the circulation in processing pro-brain natriuretic peptide (proBNP) to amino-terminal BNP and BNP-32. Peptides. 1997;18(10):1475–1481. doi: 10.1016/s0196-9781(97)00245-3. [DOI] [PubMed] [Google Scholar]
- 32.Hawkridge AM, Heublein DM, Bergen HR, 3rd, Cataliotti A, Burnett JC, Jr, Muddiman DC. Quantitative mass spectral evidence for the absence of circulating brain natriuretic peptide (BNP-32) in severe human heart failure. Proc Natl Acad Sci U S A. 2005;102(48):17442–17447. doi: 10.1073/pnas.0508782102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takahashi M, Takeda S, Kurokawa S, Kubo T, Fukuda N, Izumi T. Cyclic GMP production by ANP, BNP, and NO during worsening and improvement of chronic heart failure. Jpn Heart J. 2003;44(5):713–724. doi: 10.1536/jhj.44.713. [DOI] [PubMed] [Google Scholar]
- 34.Dries DL. Corin, the "pro-ANP/BNP convertase" is up-regulated in human heart failure, but inversely conrrelated with BNP expression. J Cardiac Failure. 2005;11:S128. [Google Scholar]
- 35.Gower WR, Jr, Chiou S, Skolnick KA, Vesely DL. Molecular forms of circulating atrial natriuretic peptides in human plasma and their metabolites. Peptides. 1994;15(5):861–867. doi: 10.1016/0196-9781(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 36.Lam CS, Burnett JC, Jr, Costello-Boerrigter L, Rodeheffer RJ, Redfield MM. Alternate circulating pro-B-type natriuretic peptide and B-type natriuretic peptide forms in the general population. J Am Coll Cardiol. 2007;49(11):1193–1202. doi: 10.1016/j.jacc.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 37.Crozier IG, Nicholls MG, Ikram H, Espiner EA, Gomez HJ, Warner NJ. Haemodynamic effects of atrial peptide infusion in heart failure. Lancet. 1986;2(8518):1242–1245. doi: 10.1016/s0140-6736(86)92675-9. [DOI] [PubMed] [Google Scholar]
- 38.Nakajima K, Onishi K, Dohi K, Tanabe M, Kurita T, Yamanaka T, et al. Effects of human atrial natriuretic peptide on cardiac function and hemodynamics in patients with high plasma BNP levels. Int J Cardiol. 2005;104(3):332–337. doi: 10.1016/j.ijcard.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 39.Ohashi M, Fujio N, Nawata H, Kato K, Ibayashi H, Kangawa K, et al. High plasma concentrations of human atrial natriuretic polypeptide in aged men. J Clin Endocrinol Metab. 1987;64(1):81–85. doi: 10.1210/jcem-64-1-81. [DOI] [PubMed] [Google Scholar]
- 40.Sato F, Kamoi K, Wakiya Y, Ozawa T, Arai O, Ishibashi M, et al. Relationship between plasma atrial natriuretic peptide levels and atrial pressure in man. J Clin Endocrinol Metab. 1986;63(4):823–827. doi: 10.1210/jcem-63-4-823. [DOI] [PubMed] [Google Scholar]
- 41.Tan AC, Hoefnagels WH, Swinkels LM, Kloppenborg PW, Benraad TJ. The effect of volume expansion on atrial natriuretic peptide and cyclic guanosine monophosphate levels in young and aged subjects. J Am Geriatr Soc. 1990;38(11):1215–1219. doi: 10.1111/j.1532-5415.1990.tb01502.x. [DOI] [PubMed] [Google Scholar]
- 42.Espiner EA, Nicholls MG, Yandle TG, Crozier IG, Cuneo RC, McCormick D, et al. Studies on the secretion, metabolism and action of atrial natriuretic peptide in man. J Hypertens Suppl. 1986;4(2):S85–S91. [PubMed] [Google Scholar]
- 43.Predel HG, Kipnowski J, Meyer-Lehnert H, Arendt RM, Kramer HJ. Human atrial natriuretic peptide in non-dialyzed patients with chronic renal failure. Clin Nephrol. 1989;31(3):150–155. [PubMed] [Google Scholar]
- 44.Lindberg BF, Nilsson LG, Bergquist S, Andersson KE. Radio-immunoassay of atrial natriuretic peptide (ANP) and characterization of ANP immunoreactivity in human plasma and atrial tissue. Scand J Clin Lab Invest. 1992;52(6):447–456. doi: 10.3109/00365519209090121. [DOI] [PubMed] [Google Scholar]
- 45.Hejmdal A, Boesgaard S, Lindholm MG, Goetze JP. B-type natriuretic peptide and its molecular precursor in myocardial infarction complicated by cardiogenic shock. J Card Fail. 2007;13(3):184–188. doi: 10.1016/j.cardfail.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 46.Shimizu H, Masuta K, Aono K, Asada H, Sasakura K, Tamaki M, et al. Molecular forms of human brain natriuretic peptide in plasma. Clin Chim Acta. 2002;316(1–2):129–135. doi: 10.1016/s0009-8981(01)00745-8. [DOI] [PubMed] [Google Scholar]