Abstract
Objective
The hyaluronan receptor CD44 provides chondrocytes with a mechanism for sensing and responding to changes in the extracellular matrix. The purpose of this study was to document the fragmentation and loss of CD44 and to determine the likely mechanisms involved.
Methods
A polyclonal anti-CD44 cytotail antibody was generated to detect CD44 fragmentation by Western blot analysis. Chondrocytes were isolated from human or bovine articular cartilage. Primary articular chondrocytes were treated with interleukin-1β (IL-1β), hyaluronan oligosaccharides, or phorbol myristate acetate or were passaged and subcultured in monolayer to induce dedifferentiation. Conditions that altered the capacity of CD44 to transit into lipid rafts, or pharmacologic inhibitors of metalloproteinase or γ-secretase activity were used to define the mechanism of fragmentation of CD44.
Results
Chondrocytes from osteoarthritic cartilage exhibited CD44 fragmentation as low molecular mass bands, corresponding to the CD44-EXT and CD44-ICD bands. Following dedifferentiation of chondrocytes or treatment of primary chondrocytes with hyaluronan oligosaccharides, IL-1β, or phorbol myristate acetate, CD44 fragmentation was enhanced. Subsequent culture of the dedifferentiated chondrocytes in 3-dimensional alginate beads rescued the chondrocyte phenotype and diminished the fragmentation of CD44. Fragmentation of CD44 in chondrocytes was blocked in the presence of the metalloproteinase inhibitor GM6001 and the γ-secretase inhibitor DAPT.
Conclusion
CD44 fragmentation, consistent with a signature pattern reported for sequential metalloproteinase/γ-secretase cleavage of CD44, is a common metabolic feature of chondrocytes that have undergone dedifferentiation in vitro and osteoarthritic chondrocytes. Transit of CD44 into lipid rafts may be required for its fragmentation.
In cartilage, cell–matrix interactions are the primary means by which chondrocytes sense changes in the extracellular environment and signal a reparative response. Hyaluronan (HA) binding to the CD44 receptor is a key player in such interactions. In articular cartilage, HA serves as the core filament of the proteoglycan aggregate, composed of HA, link protein, and the major cartilage proteoglycan aggrecan, establishing essential biomechanical properties of the cartilage extracellular matrix (1-3). Cell–matrix interactions promote tissue homeostasis and facilitate tissue remodeling (4). Disruption of this extracellular matrix, leading to the consistent loss of proteoglycans, results in degenerative changes in cartilage. CD44, through its interaction with actinbinding proteins, associates with the actin network (5,6). Also, a pool of CD44 is associated with lipid rafts; palmitoylation of 2 cysteine residues in CD44 is required for lipid raft association and, concomitantly, is a determinant of the rate of CD44 turnover from the cell surface (7). Therefore, in addition to chondrocyte integrins, CD44 represents another class of receptors that can participate in matrix–cell–cytoskeleton interactions (8). Because HA–CD44 interactions are required for the retention of proteoglycan in the matrix (9-11), the relationship between these 2 components is crucial to cartilage homeostasis.
Previous studies in several tumor cell lines determined that CD44 can be cleaved to generate 3 fragments. The first metalloproteinase-mediated cleavage ofCD44 generates extracellular fragments that are shed plus a C-terminal fragment (CD44-EXT); this subsequently becomes a substrate for γ-secretase cleavage by which the CD44 intracellular domain (CD44-ICD) is generated (5,12,13). Shedding of CD44 can be blocked by inhibitors of matrix metalloproteinases (MMPs) that also block membrane-type metalloproteases such as ADAM-10, ADAM-17, or membrane type 1 (MT1)–MMP (14-17), while inhibition of γ-secretase can block the formation of CD44-ICD (13).
Subculturing of adult human articular chondrocytes is often performed to expand cell numbers and facilitate autologous cell implantation (18,19). However, passaging of chondrocytes in monolayer results in changes in cell morphology, function, and gene expression; such changes are commonly termed “dedifferentiation” (20,21). Some of the phenotypic changes exhibited by dedifferentiated articular chondrocytes mimic osteoarthritic (OA) chondrocytes (22,23). These changes can be reversed (redifferentiation) by returning the chondrocytes to an environment that mimics cartilage, such as alginate bead culture, or by treatments that promote a round cell shape (20,24-26).
In this study, we observed that dedifferentiated chondrocytes exhibited a naturally occurring degradation of CD44. Furthermore, this cleavage activity was reversed upon redifferentiation within alginate bead culture, after which chondrocytes regained the capacity to retain a pericellular matrix. Additionally, we determined that chondrocytes from normal donor cartilage and from human OA cartilage generate multiple CD44 fragments, including CD44-EXT and CD44-ICD.
MATERIALS AND METHODS
Chondrocyte culture
Chondrocytes were isolated from the metacarpophalangeal joints of 18–24-month-old steers, from human knee cartilage following joint replacement, or from talocrural ankle joint cartilage obtained from tissue donors through the Gift of Hope Organ and Tissue Donor Network of Illinois. All human cartilage was obtained within 24 hours of death and with institutional approval. Chondrocytes were isolated from full-thickness slices of articular cartilage by sequential digestion with Pronase (EMD Biosciences) and collagenase P (Roche) (27). The primary chondrocytes were cultured in Dulbecco’s modified Eagle’s medium–F-12 medium (Mediatech) containing 10% fetal bovine serum (FBS; Hyclone). The chondrocytes were plated as high-density monolayers (2.0 × 106 cells/cm2) for analysis of primary cells or as low-density monolayers (5,000 cells/cm2) for particle exclusion assay analysis or to allow the cells to undergo dedifferentiation. When low-density chondrocytes reached confluence, the cells were passaged by treatment with 0.25% trypsin/2.21 mM EDTA (Sigma). In some experiments, passaged chondrocytes were subcultured in alginate beads as described previously (27). Cell viability was monitored by fluorescence of calcein AM/ethidium homodimer-1 (LIVE/DEAD assay; Invitrogen).
Treatment of cells
Chondrocyte cultures were treated with 10 ng/ml interleukin-1β (IL-1β) (R&D Systems), 250 μg/ml HA oligosaccharides (from rooster comb HA [Sigma], as described previously [27]), or 100 nM phorbol myristate acetate (PMA) in fresh culture medium with reduced serum (5% FBS). In some experiments, chondrocytes were pretreated for 30 minutes with 10 mM methyl-β-cyclodextrin (MCD) or 10 μM 2-bromopalmitate (2-BP), as described previously (7). In other experiments, cells were pretreated with varying concentrations of the γ-secretase inhibitor DAPT (N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester) or the general MMP inhibitor GM6001 (both from EMD Chemicals). For small interfering RNA (siRNA)–mediated inhibition of CD44, bovine articular chondrocytes were released from monolayer culture with 0.1% collagenase P/0.1% Pronase, mixed with Amaxa human chondrocyte solution (Lonza) containing 5 μg siRNA, and transfected using an Amaxa Nucleofector device, program U-028. The CD44 siRNA was constructed as the bovine ortholog of a human CD44 siRNA sequence originally described by Ghatak et al (28). The CD44 and control (D-001206-09-05) siRNA were obtained using Thermo Scientific Dharmacon RNA interference technologies. COS-7 cells (American Type Culture Collection) were transfected using an Amaxa Nucleofector device, program A-024. The experiments were initiated 24 hours posttransfection (7).
Generation of rabbit polyclonal anti-human CD44 cytotail antibody
A rabbit polyclonal anti-human CD44 cytotail antiserum (anti-cytotail) was generated as described previously (29). Briefly, a specific synthetic peptide (DQFMTADETRNLQNVDMKIGV) representing the 21 C-terminal amino acids of the human CD44 sequence was synthesized by Affinity BioReagents, coupled to keyhole limpet hemocyanin, and used for immunization. Immunoaffinity-purified antisera (9.3 mg/ml IgG by enzyme-linked immunosorbent assay) were typically used at 1:10,000 dilution.
Western blotting for detection of CD44 fragmentation
Total protein was extracted using Cell Lysis Buffer (Cell Signaling Technology) containing protease inhibitor cocktail. For each sample, 20 μg total protein was loaded and separated on Novex 4–12% gradient sodium dodecyl sulfate–polyacrylamide gel electrophoresis gels (Invitrogen). Following electroblot transfer onto nitrocellulose membranes and blocking in 5% nonfat dry milk, CD44, β-actin, or GAPDH was detected with primary antibodies followed by horseradish peroxidase–conjugated secondary antibodies. Antibodies specific for the CD44 ectodomain (BU52; Calbiochem) and the CD44 cytoplasmic tail (anti-cytotail) were used. Detection was performed using chemiluminescence (Novex ECL; Invitrogen). In some cases, the blots were treated with stripping buffer and reprobed using another primary antibody.
Sucrose gradient ultracentrifugation for isolation of lipid rafts from bovine articular chondrocytes
Bovine articular chondrocytes were preincubated overnight with or without 10 μM 2-BP or 10 mM MCD, washed, and then lysed with 25 mM Tris HCl, pH 7.6, 150 mM NaCl, 1 mM dithiothreitol, 10% sucrose, 1% Triton X-100, and 1× protease and phosphatase inhibitor cocktails (Sigma). The cell lysate was mixed with an 80% stock solution of sucrose in 25 mM Tris HCl, pH 7.6, 150 mM NaCl, 1 mM dithiothreitol, to bring the final concentration to 40%. This was layered at the bottom of the ultracentrifuge tube and overlaid with a 2.4-ml aliquot of 30% sucrose solution followed by a 1.6-ml aliquot of 0% sucrose solution in lysis buffer without Triton X-100. The samples were centrifuged at 114,000g for 20 hours at 4°C in a SW50.1 rotor and then recovered as 6 equal-volume fractions. CD44 within each fraction was characterized by Western blotting, using the anti-cytotail antibody.
Real-time reverse transcription–polymerase chain reaction (RT-PCR)
Total RNA was isolated from chondrocyte cultures with TRIzol reagent (Invitrogen), according to the manufacturer’s instructions. The RNA was reverse transcribed with qScript cDNA Supermix reagents (Quanta BioSciences) and amplified at 42°C for 30 minutes. For real-time RT-PCR, the PCR products were detected using RT2 Real-Time SYBR Green reagents (SABiosciences). Primer-specific amplification was performed at 60°C for 30 seconds. However, fluorescence quantification was performed at a higher temperature (72°C). The primers pair sequences are as follows: for GAPDH, forward 5′-ATTCTGGCAAAGTGGACATCGTCG-3′, reverse 5′-ATGGCCTTTCCATTGATGACGAGC-3′; for CD44, forward 5′-TCTGCAAGGCCTTTAATAGCACGC-3′, reverse 5′-GTTCGCAGCACAGATGGAATTGG-3′; for aggrecan, forward 5′-AAATATCACTGAGGGTGAAGCCCG-3′, reverse 5′-ACTTCAGGGACAAACGTGAAAGGC-3′; for hyaluronan synthase 2 (HAS-2), forward 5′-GAGGACGACTTTATGACCAAGAGC-3′, reverse 5′-TAAGCAGCTGTGATTCCAAGGAGG-3′; for SOX9, forward 5′-AAGAAGGAGAGCGAGGAGGACAAGTT-3′, reverse 5′-TTGTTCTTGCTCGAGCCGTTGA-3′. The primers for COL1A2 (forward 5′-ACATGCCGAGACTTGAGACTCA-3′, reverse 5′-GCATCCATAGTACATCCTTGGTTAGG-3′) and COL2A1 (forward 5′-AGCAGGTTCACATATACCGTTCTG-3′, reverse 5′-CGATCATAGTCTTGCCCCACTT-3′) were described by Shintani et al (30). All primers were obtained from Integrated DNA Technologies. Thermal cycling and fluorescence detection were performed using the SmartCycler System (Cepheid). Real-time PCR efficiencies and the fold increase in copy numbers of messenger RNA (mRNA) were calculated as described previously (27).
Particle exclusion assay
Chondrocytes were cultured overnight in 35-mm wells. The medium was replaced with a suspension of formalin-fixed erythrocytes in phosphate buffered saline (PBS)/0.1% bovine serum albumin (31). Cells were photographed using a Nikon TE2000 inverted phase-contrast microscope, and images were captured digitally in real time using a SPOT RT camera. The presence of cell-bound extracellular matrix is seen as the particle-excluded zone surrounding the chondrocytes.
Generation of a CD44-ICD construct
PCR primers were designed to amplify the human sequence corresponding to CD44-ICD, CD44 Ala288 to the stop codon that follows Val361, using the primer pairs 5′-GTCGACGCAGTCAACAGTCGAAGAAGGTGTGG-3′ (including a Sal I restriction site) and 5′-TTACACCCCAATCTTCATGTCCACATTC-3′. The primers were used to amplify human CD44H complementary DNA within a previously described pCDM8 plasmid (31). The PCR product was first inserted into the pcDNA3.1/V5-His-TOPO vector and then subcloned into a pCMV/myc/cyto plasmid (pShooter; Invitrogen) that provides the ATG sequence as part of a Kozak consensus sequence (ANNATGG) for expression of the C-terminal fragment. The DNA sequence for the CD44-ICD insert was verified at the East Carolina University Sequencing Facility. The insert was subcloned again into a pcDNA5/FRT shuttle vector (Flp-In System; Invitrogen) for preparation of a stable Flp-In–293 cell line, as described previously (7).
Fluorescence microscopy
Primary chondrocytes, passaged chondrocytes, or chondrocytes released from alginate beads were cultured overnight in 4-well chamber slides (Titertek). The cells were rinsed with PBS, fixed, and permeabilized as described previously (7) and then incubated with rhodamine phalloidin (Invitrogen)/PBS (1:150) for 30 minutes at 4°C, rinsed with PBS, and mounted using a medium containing 4′,6-diamidino-2-phenylindole nuclear stain (Invitrogen). In other studies, chondrocytes cultured on chamber slides were incubated for 1 hour on ice with 16 μg/ml fluorescein isothiocyanate–labeled cholera toxin B subunit (FITC–CTxB; Sigma) to indirectly detect lipid rafts in living cells. For colocalization with CD44, cells were first labeled using FITC–CTxB as described above, fixed in 4% paraformaldehyde, and then incubated with biotinylated anti-CD44 monoclonal antibody IM7.8.1 (Invitrogen) followed by streptavidin-conjugated rhodamine red. Cells were visualized using a Nikon Eclipse E600 microscope equipped with a Y-FL EPI-Fluorescence attachment. Images were captured digitally in real time, using a SPOT RT camera, and were processed using Nikon NIS-Elements BR imaging software.
RESULTS
Association of CD44 fragmentation with chondrocyte dedifferentiation
Subculture of adult human articular chondrocytes results in changes in cell morphology and gene expression; this change is commonly termed dedifferentiation (20,21). In this study, for example, primary cultures of chondrocytes exhibited a more rounded morphology, driven in part by cortical organization of the actin cytoskeleton (Figure 1A, top). With passage in culture, the chondrocytes became elongated and exhibited a fibroblast-like morphology, including the appearance of stress fibers (Figure 1A, middle). In addition, we observed that with dedifferentiation, chondrocytes lost the capacity to assemble and retain a pericellular matrix (Figure 1B, middle), a property that is characteristic of primary cells. No change in cell viability was observed in cells from passages 1–4 (results not shown).
Figure 1.
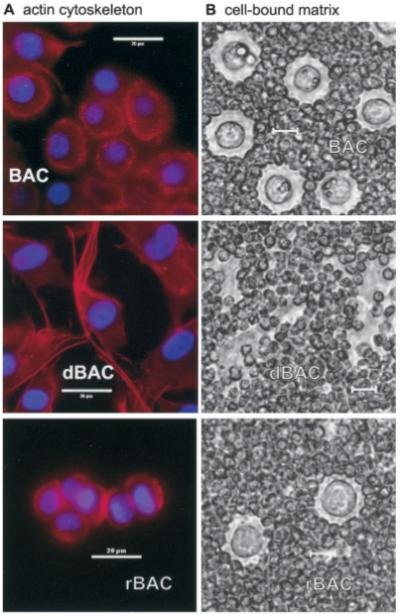
A, Rhodamine–phalloidin staining of permeabilized chondrocytes. Top, Primary bovine articular chondrocytes (BACs) exhibited a more rounded morphology, driven in part by cortical organization of the actin cytoskeleton. Middle, After passage in culture, dedifferentiated BACs (dBAC) became elongated and exhibited a fibroblast-like morphology, including the appearance of stress fibers. Bottom, Following culture in alginate beads for 2 weeks, redifferentiated BACs (rBAC) regained a spherical morphology, lacked stress fibers, and established a fine cortical actin network. B, Pericellular matrix on living chondrocytes, as revealed by particle exclusion assay. Top, Primary BACs synthesized and retained a pericellular matrix. Middle, With dedifferentiation, chondrocytes lost the capacity to assemble and retain a pericellular matrix. Bottom, When dedifferentiated chondrocytes were subcultured in alginate beads for 2 weeks and released from the beads, the redifferentiated chondrocytes regained a pericellular matrix. All bars = 20 μm.
Subculturing chondrocytes also effects changes in gene expression. Compared with primary chondrocytes (passage 0), subcultured chondrocytes from passage 1 to passage 4 exhibited a classic progressive reduction in aggrecan and SOX9 mRNA expression and a reduction in type II collagen mRNA, coupled with an increase in type I collagen mRNA (Figure 2A). Our previous studies have suggested that the assembly and retention of a chondrocyte-associated matrix also depend on the presence of sufficient HA and the HA receptor, CD44 (3,10,32). As shown in Figure 2A, the expression of HAS-2 mRNA, the enzyme primarily responsible for HA production in chondrocytes (3), also decreased as the passage number increased. The expression of CD44 mRNA, however, appeared to increase as the passage number increased. This passage-dependent increase in CD44 mRNA was next verified at the protein level.
Figure 2.
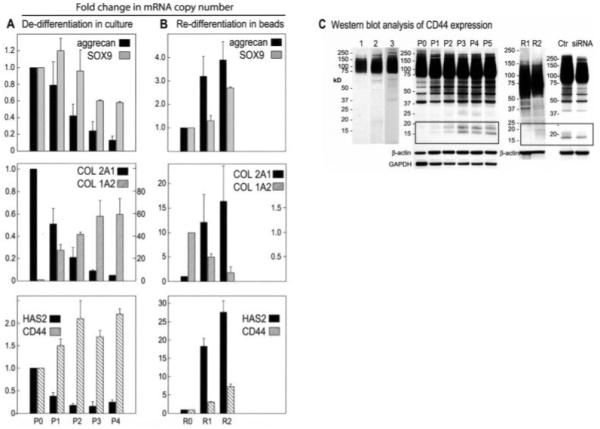
Changes in gene expression and CD44 fragmentation by subculturing chondrocytes in monolayer or alginate beads. A, Dedifferentiation in culture. Total RNA from cultures of primary bovine articular chondrocytes (P0; set at 1.0) or subcultured chondrocytes from P1 to P4 were subjected to real-time reverse transcription–polymerase chain reaction (RT-PCR) analysis. B, Redifferentiation in alginate beads. Chondrocytes from passage 4 were subcultured in monolayer (R0; set at 1.0) or in alginate beads for 1 week (R1) or 2 weeks (R2). Total RNA was isolated and subjected to RT-PCR analysis. C, Western blot analysis of CD44 expression. CD44 in lysates from human knee articular chondrocytes, grown in alginate beads, was detected with BU52 (lane 1) or anti-CD44 cytotail (lane 2). CD44 in lysates from primary bovine articular chondrocytes was detected with anti-CD44 cytotail (lane 3). CD44 in lysates from primary bovine chondrocytes (P0), subcultured chondrocytes (P1–P5), alginate bead–redifferentiated chondrocytes (R1 and R2), or lysates from dedifferentiated bovine articular chondrocytes treated with control small interfering RNA (siRNA; Ctr) or CD44-specific siRNA was detected with anti-CD44 cytotail antibody. Bars represent the mean and SD fold change in mRNA copy number. HAS2 = hyaluronan synthase 2.
In Western blots, CD44 is typically observed as a broad, heterogeneous glycoprotein of ~85 kd (29,33). To improve detection of bovine CD44, we generated an antibody directed to the C-terminal ICD of CD44, because this domain is nonglycosylated and highly homologous between vertebrate species. As shown in Figure 2C, the expression of human (lane 2) and bovine (lane 3) chondrocyte CD44, detected using the C-terminal antibody, appears similar to that of human chondrocyte CD44 detected with a conventional extracellular domain antibody (lane 1). When the C-terminal antibody was applied to lysates of bovine chondrocytes from varying passages, additional CD44 bands were observed (Figure 2C, lanes P0–P5). All of the lysates displayed bands between 37 kd and 85 kd, which likely represents posttranslation processing of CD44 from a 37-kd precursor (33-35) to the mature ~85-kd glycosylated protein. However, lysates from subcultured chondrocytes revealed not only a passage-dependent increase in CD44 expression but also the appearance of lower molecular mass bands between 17 kd and 20 kd (Figure 2C, lanes P0–P5). Thus, using the C-terminal anti-CD44 cytotail antibody, enhanced CD44 fragmentation was observed in association with chondrocyte dedifferentiation. All of the bands observed on these Western blots were partially reduced after CD44 siRNA transfection as compared with control (irrelevant) siRNA transfection (Figure 2C). Given that this siRNA knocks down only 1 gene product (CD44) with a high degree of specificity, all of the bands displayed on the Western blots are related to CD44.
As shown previously by other investigators, the chondrocyte phenotype can be rescued, in part, by subculture of dedifferentiated cells in 3-dimensional alginate bead culture (19,24,26). As the length of time in alginate bead culture increased, chondrocyte type I collagen mRNA expression diminished, and the expression of type II collagen, aggrecan, SOX9, HAS-2, and CD44 increased (Figure 2B). Chondrocytes removed from alginate bead culture lacked actin stress fibers, reestablished a fine cortical actin network (Figure 1A, bottom), and displayed the capacity to retain a particle-excluding pericellular matrix (Figure 1B, bottom). Interestingly, the low molecular mass CD44 fragments that were present in the dedifferentiated chondrocytes (Figure 2C, lanes P1–P5) were diminished in the lysates of redifferentiated cells (Figure 2C, lanes R1 and R2). This suggests that the generation of the low molecular mass CD44 bands is reversible.
Enhanced CD44 fragmentation in human OA chondrocytes
Unlike what was observed with bovine articular chondrocytes, primary cultures of adult human articular chondrocytes from 3 separate experiments all exhibited low molecular mass bands for CD44 (Figure 3). Chondrocytes derived from the normal ankle or knee cartilage of donors (modified Collins grade 1 or 2 [36]) exhibited a CD44 profile (Figure 3A, lanes 1–4) similar to that of lysates from dedifferentiated bovine chondrocytes. Interestingly, lysates from primary cultures of chondrocytes derived from OA cartilage (Figures 3A and B, lanes 5–10) displayed a more pronounced banding pattern, including protein bands of <20 kd. Lysates from OA chondrocytes exhibited a single 15-kd band (Figure 3B, lanes 7–10) or multiple bands (Figure 3A, lane 6). The presence of multiple CD44 protein bands, especially those in the range of 15 kd and 17–20 kd, are indicative of a signature pattern reported for the sequential metalloproteinase/γ-secretase cleavage of CD44 in other cell types (5,12); such cleavage can be detected only with use of the C-terminal–directed antibody. The ~15-kd band observed in lysates of OA chondrocytes is equivalent in size to a recombinant human CD44-ICD expressed in Flp-In–293 cells (Figure 3B, lane ICD), detected using the same anti-CD44 cytotail antibody. The 17–20-kd bands are similar to those shown to represent the CD44 extracellular truncation fragments generated following an initial MMP-mediated cleavage of CD44 (CD44-EXT) (12).
Figure 3.
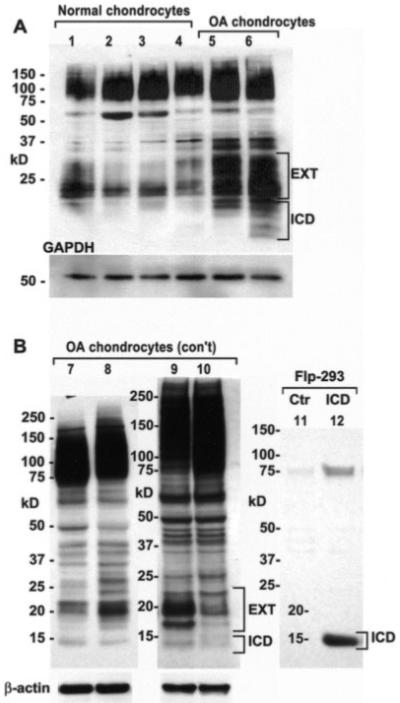
Western blots showing that adult human articular chondrocytes exhibit low molecular mass bands for CD44. Cell lysates were analyzed by Western blotting using anti-CD44 cytotail antisera in 3 separate experiments. A, Lysates from chondrocytes derived from ankle cartilage (grade 1 [lanes 1 and 2] and grade 2 [lane 3]) or knee cartilage (grade 1 [lane 4]) exhibited a CD44 profile with low molecular mass bands. Lysates from primary cultures of chondrocytes derived from osteoarthritic (OA) cartilage displayed a more pronounced banding pattern, with bands for both CD44-EXT and CD44-ICD (lanes 5 and 6). B, Lysates from primary cultures of chondrocytes derived from OA cartilage displayed a more pronounced banding pattern, with a CD44-ICD band at ~15 kd (lanes 7–10). The ~15-kd band observed in lysates of OA chondrocytes is equivalent in size to a recombinant human CD44-ICD (lane ICD) expressed in Flp-In–293 cells, detected using the same anti-CD44 cytotail antisera. Ctr = control.
Induction of CD44 fragmentation
Treatment of normal bovine or human articular chondrocytes with inflammatory cytokines such as IL-1β induces a state of enhanced catabolism that mimics some of the properties of OA chondrocytes (22,37). Upon treatment of primary bovine chondrocytes with 10 ng/ml IL-1β, CD44 fragmentation was enhanced, with maximal CD44-EXT formation occurring on day 2 of treatment (Figure 4A). However, IL-1β also stimulates overall CD44 mRNA and protein expression (38), which raises the possibility that the CD44-EXT bands are observed only because of the increased proportion of full-length CD44. A dilution of lysates from chondrocytes treated with IL-1β for 48 hours was analyzed on Western blots and scanned by densitometry (Figure 4C). The full-length CD44 band in IL-1β–treated chondrocytes increased by 2.9-fold as compared with untreated control chondrocytes, similar to our previous observations (38). However, the CD44-EXT band increased by an average of 5.6-fold after IL-1β treatment as compared with control. Thus, taking into consideration the overall increase in CD44, CD44-EXT expression was enhanced by IL-1β treatment (~2-fold greater than control cultures). We have also previously demonstrated that treatment of bovine or human articular chondrocytes with small HA oligosaccharides induces increased expression of MMP-3 and MMP-13 mRNA, increased protein and increased enzymatic activity (27,39,40). Treatment of bovine articular chondrocytes with HA oligosaccharides also effected enhanced expression of CD44-EXT bands without an overall fold increase of intact CD44 (Figure 4B).
Figure 4.
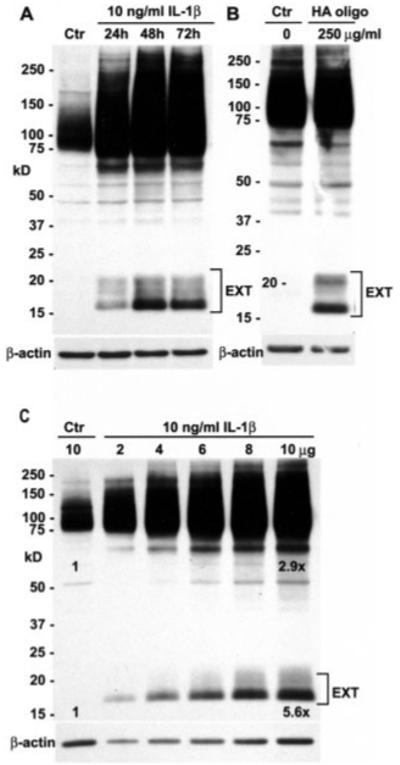
Induction of CD44 fragmentation in bovine articular chondrocytes. Cell lysates were analyzed by Western blotting using anti-CD44 cytotail antisera. A, Upon treatment of primary bovine articular chondrocytes with 10 ng/ml interleukin-1β (IL-1β), CD44 fragmentation was enhanced, with maximal CD44-EXT formation occurring on day 2 of treatment. B, Treatment of bovine articular chondrocytes with hyaluronan oligosaccharides (HA oligo) also effected enhanced expression of CD44-EXT bands. C, Lysates (2–10 μg protein) from chondrocytes treated for 48 hours with IL-1β were analyzed on Western blots and scanned by densitometry. With values in untreated control chondrocytes (Ctr; 10 μg) set to 1.0, the full-length CD44 band in IL-1β–treated chondrocytes (after correcting for dilutions of the lysates) increased by 2.9-fold (y = 0.16x + 1.36; R2 = 0.96). After IL-1β treatment, the CD44-EXT band increased by an average of 5.6-fold (y = 0.43x + 1.30; R2 = 0.99) compared with control.
Blocking of CD44 fragmentation by inhibitors of MMP and γ-secretase activity
In primary bovine articular chondrocytes, CD44 fragmentation could also be induced by pretreatment of the cells with PMA, as has been described in some tumor cells by other investigators (41); the enhanced fragmentation included the 15-kd putative CD44-ICD band. Upon pretreatment of PMA-stimulated bovine chondrocytes with varying concentrations of the γ-secretase inhibitor DAPT, the 15-kd band was no longer detectable at a DAPT concentration >1 μM (Figure 5A), concomitant with an increase in the 17–20-kd doublet bands (CD44-EXT). In another series of experiments, the γ-secretase inhibitor DAPT was again partially effective at 0.1 μM but completely inhibited generation of the 15-kd band at 5.0 μM (Figure 5B, left). In high-density cultures of OA chondrocytes, expression of a 15-kd band of CD44 was also blocked when these cells were treated with 5 μM DAPT (Figure 5B, right). In all cases, blocking generation of the down-stream product (~15 kd CD44-ICD) resulted in an accumulation of the presumed intermediate, the 17–20-kd CD44-EXT. Even when little CD44-EXT was apparent, such as in primary bovine articular chondrocytes (Figure 5C), pretreatment with 5 μM DAPT resulted in an accumulation of CD44-EXT (Figure 5C). When the DAPT-treated chondrocytes were also treated with varying concentrations of GM6001, a general MMP inhibitor, generation of the presumed CD44-EXT bands was blocked at GM6001 concentrations ≥10.0 μM (Figure 5C). These results are consistent with sequential 2-step proteolysis of CD44 in activated chondrocytes.
Figure 5.
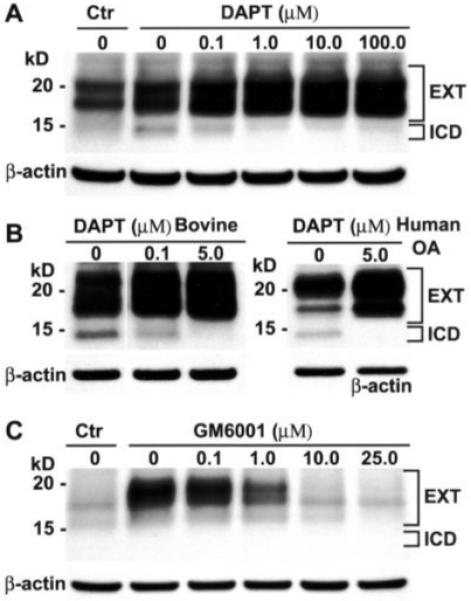
Blocking of CD44 fragmentation by inhibitors of matrix metalloproteinase and γ-secretase activities. A, CD44 fragmentation was induced by pretreatment of primary bovine articular chondrocytes either without (Ctr) or with phorbol myristate acetate (PMA). The PMA-stimulated bovine articular chondrocytes were pretreated with varying concentrations (0–100 μM) of the γ-secretase inhibitor DAPT. At a DAPT concentration of >1 μM, the 15-kd band (CD44-ICD) was no longer detectable, concomitant with an increase in the 17-20-kd doublet bands (CD44-EXT). B, In another series of experiments with PMA-stimulated primary bovine articular chondrocytes, DAPT was again partially effective at 0.1 μM but completely inhibited generation of the 15-kd band at 5.0 μM (left). In high-density cultures of human osteoarthritis (OA) chondrocytes, expression of the ~15-kd band of CD44 was blocked when the cells were treated with 5.0 μM DAPT (right). C, Primary bovine articular chondrocytes were preincubated either without (Ctr) or with 5.0 μM DAPT followed by treatment with 0–25 μM GM6001, a general matrix metalloproteinase inhibitor. Generation of the CD44-EXT bands was blocked by GM6001.
Requirement of CD44 transit into lipid rafts for CD44 fragmentation
Our group and other investigators have shown that a fraction of CD44 is present in plasma membrane lipid raft microdomains (7,42-44). In chondrocytes, lipid rafts can be detected indirectly using a FITC–CTxB probe that binds to GM1 gangliosides present in lipid rafts (Figure 6A, control). Preincubation with the cholesterol chelator MCD eliminated surface labeling by FITC–CTxB. In control chondrocytes, CD44 codistributed with FITC–CTxB in some regions but not in others. This was discerned by analyzing CD44 distribution within sucrose density gradients of chondrocyte lysates (Figure 6B). In control chondrocytes, a substantial proportion of CD44 was detected in the 2 upper fractions of the gradients (the raft-containing fractions), as determined previously (7). This pool of CD44 was released from the raft microdomain by pretreatment of cells with either MCD or the palmitate analog, 2-BP. If bovine articular chondrocytes were pretreated with MCD or 2-BP for 30 minutes prior to stimulation with 10 ng/ml IL-1β, the enhanced expression of CD44-EXT bands was reduced (Figure 6C). This suggests that at least the first step in CD44 fragmentation occurs within a membrane microdomain dependent on cholesterol and protein palmitoylation. However, both of these inhibitors are not specific for CD44 and may also block the transit of one of the proteases into the appropriate lipid raft domain.
Figure 6.
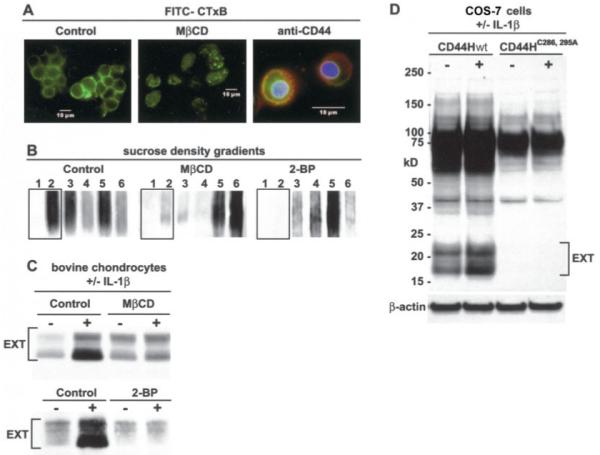
CD44 fragmentation requires transit of CD44 into lipid rafts. A, Bovine articular chondrocytes were incubated with a fluorescein isothiocyanate–labeled cholera toxin B subunit (FITC–CTxB) probe to detect lipid rafts. Preincubation with methyl-β-cyclodextrin (MβCD) eliminated surface expression of FITC–CTxB. In control chondrocytes (right panel), CD44 (red immunofluorescence) codistributed with FITC–CTxB in some regions. B, The distribution of CD44 within sucrose density gradients of lysates derived from chondrocytes pretreated with or without MβCD or 2-bromopalmitate (2-BP) was assessed by Western blotting, using anti-CD44 cytotail antisera. C, Bovine articular chondrocytes pretreated in the absence or presence of MβCD or 2-BP were stimulated with 10 ng/ml interleukin-1β (IL-1β). Both MβCD and 2-BP reduced the enhanced expression of CD44-EXT bands detected by Western blotting using anti-CD44 cytotail antisera. D, COS-7 cells were transfected with a full-length human CD44 construct (CD44Hwt) or a full-length human CD44 containing 2 cysteine–alanine mutations (CD44H-C286, 295A) followed by incubation with or without 10 ng/ml IL-1β and Western blotting using anti-CD44 cytotail antisera. Control CD44Hwt underwent cleavage, but enhanced generation of CD44-EXT followed IL-1β treatment. However, the CD44H-C286, 295A construct displayed no capacity for fragmentation in control or IL-1β–treated COS-7 transfectants.
To examine the role of CD44 specifically, COS-7 cells (CD44 negative) were transfected with a full-length human CD44 wild-type construct (CD44Hwt) or a full-length human CD44 construct (CD44H-C286, 295A) in which the critical CD44 cysteine amino acids involved in thioester formation were mutated to alanines (7). As shown in Figure 6D, control CD44Hwt underwent cleavage even without treatment with IL-1β, but fragmentation was nonetheless enhanced following IL-1β treatment of COS-7 cells. However, the CD44H-C286, 295A construct displayed no capacity for fragmentation in control or IL-1β-treated cells (Figure 6D). These results suggest that only CD44 that has transitted into a lipid raft microdomain is a substrate for cleavage by an MMP.
DISCUSSION
This report is the first to describe fragmentation of CD44 in adult human articular chondrocytes. Although it is premature to conclude that there is a correlation with disease, it does appear that CD44 fragmentation in OA chondrocytes can be extensive. Future comparative studies are needed to determine whether this degradative capacity is correlated with the disease state and the resultant effects on chondrocyte metabolism. Our laboratory and those of other investigators (21,24) often use dedifferentiated chondrocytes as a model of OA, because the flattened cells display morphologic and metabolic features of OA cells (22). CD44 fragmentation similar to that observed in OA cells could be induced in chondrocytes by dedifferentiation. An interesting aspect of this study was that fragmentation in bovine chondrocytes could be reversed by culturing the dedifferentiated cells under conditions that effected redifferentiation. Whether CD44 fragmentation in OA chondrocytes is also reversible by culture in alginate beads will be determined in future studies.
CD44 cleavage associated with adult human articular chondrocytes can be inhibited by preincubation with an inhibitor of MMPs as well as by an inhibitor of γ-secretase. Similar proteolytic cleavage of CD44 has been documented previously as being associated with certain human tumors (12,29,45). The CD44 fragments generated in chondrocytes include multiple bands that coincide with what other investigators have documented to be CD44-EXT bands as well as CD44-ICD. For example, Nakamura et al (15) identified 3 extracellular cleavage sites in CD44, Gly192→Tyr193, Gly233→Ser234, and Gly249→Gln250, attributable to the activity of MT1-MMP (MMP-14) or ADAM-17. The 2–3 CD44-EXT bands expressed by chondrocytes are consistent with cleavage at these sites. The MMP inhibitor GM6001 is thought to block the activity of multiple MMP enzymes via its bidentate interaction with active-site zinc atoms (46). In this study, GM6001 blocked the generation of CD44-EXT fragments. The ~15-kd CD44 bands detected from chondrocytes were tentatively identified as CD44-ICD, because they migrated at a size equivalent to a recombinant human CD44-ICD that we prepared, which matches the putative γ-secretase cleavage site between CD44 Ile287 and Ala288 (47). Second, CD44-ICD can be blocked by pretreatment of chondrocytes with the γ-secretase inhibitor DAPT. DAPT is an optimized N-arylalanine ester compound with low micromolar inhibitor potency to block cellular γ-secretase–mediated generation of amyloid β peptide (48).
MT1-MMP modulates composition of the pericellular matrix as well as cellular behavior. A complex can form between the PEX domain of MT1-MMP and the stem region of CD44 (49). Both CD44 and MT1-MMP are palmitoylated, and both partition in lipid rafts as well as non-raft fractions, with these transitions modulating function and establishing microdomains consisting of signaling platforms (7,50). Our results suggest that only CD44 that has transitted into a lipid raft microdomain is a substrate for MMP cleavage. Whether γ-secretase cleavage also occurs within this membrane microdomain, or whether CD44 palmitoylation is required, remains to be determined.
In many of our experiments, the CD44-ICD band was faint, even in PMA-treated cells. However, in preliminary studies, incubation of chondrocytes with the proteosome inhibitor MG132 substantially increased the level of detectable CD44-ICD (data not shown). At present, it remains unknown whether the CD44-ICD bands that are readily observable in lysates of OA chondrocytes are attributable to increased γ-secretase activity or to reduced ubiquitination or proteosomal function.
When γ-secretase was inhibited with DAPT, the expression of CD44-EXT increased substantially, even when little or no CD44-ICD was detected. This may be attributable to the rapid turnover of CD44-ICD once it is generated. We predict that, unlike turnover of CD44-ICD, turnover of CD44-EXT is substantially slower. When chondrocytes are treated with trypsin, a single cell–associated CD44 fragment is generated, migrating at ~25 kd (data not shown), which is well above the 17–20-kd bands of naturally occurring CD44-EXT. Moreover, the 25-kd bands can persist for >48 hours. Thus, our approach to in vitro dedifferentiation of chondrocytes was to replate the trypsinized chondrocytes at lower density and allow these cultures to reach confluence over a 1–2-week period, thereby eliminating the presence of the 25-kd CD44 fragment. We predict that CD44-EXT bands undergo turnover via endocytosis in a manner similar to that in full-length CD44 (7).
At this time, the full biologic ramifications of CD44 cleavage remain unknown. In addition to the physical loss of the cell-associated pericellular matrix seen in OA and dedifferentiated chondrocytes, the biologic consequence of this disruption of the interaction of HA with functional CD44 receptors (9,10,27,41,42) would include the induction of both matrix turnover as well as matrix biosynthesis, replicating a chondrocyte response that partners attempted repair with enhanced catabolism, both of which are hallmarks of early OA (22). Fragmentation and loss of the CD44 extracellular domain itself may activate these pathways, but the generation of a CD44 ICD inside the chondrocytes may initiate additional responses. The CD44-ICD has been reported to undergo nuclear translocation and, with CREB binding protein/p300, regulates transcription activation (47). In our study, overexpression of CD44-ICD in Flp-In–293 cells also resulted in an increase in the expression of full-length CD44 (Figure 3B). Thus, the stimulation of CD44 mRNA that occurs in association with the passage number or in OA may, in part, represent a response to translocated CD44-ICD.
ACKNOWLEDGMENTS
We thank the donor families and the Gift of Hope Organ and Tissue Donor Network of Illinois. The generosity and beneficence of the donor families for allowing access to the human tissues is greatly appreciated. We thank Dr. Larry J. Dobbs, Jr., of the Department of Pathology and Laboratory Medicine at East Carolina University for his assistance in the appropriation of human OA tissue. We also thank Dr. Xin Li, Mr. Jarret DeVine, Ms Christy Holland, Ms Nadege Etienne, and Ms Srabani Mondal for their technical assistance.
Dr. C. Knudson’s work was supported by NIH grant R01-AR-39507. Dr. Im’s work was supported by NIH grant R01-AR-053220. Dr. W. Knudson’s work was supported by NIH grant R01-AR-43384.
REFERENCES
- 1.Hardingham TE, Fosang AJ. Proteoglycans: many forms and many functions. FASEB J. 1992;6:861–70. [PubMed] [Google Scholar]
- 2.Knudson W, Knudson C. An update on hyaluronan and CD44 in cartilage. Current Opin Orthop. 2004;15:369–75. [Google Scholar]
- 3.Nishida Y, Knudson CB, Nietfeld JJ, Margulis A, Knudson W. Antisense inhibition of hyaluronan synthase-2 in human articular chondrocytes inhibits proteoglycan retention and matrix assembly. J Biol Chem. 1999;274:21893–9. doi: 10.1074/jbc.274.31.21893. [DOI] [PubMed] [Google Scholar]
- 4.Knudson CB, Knudson W. Hyaluronan-binding proteins in development, tissue homeostasis and disease. FASEB J. 1993;7:1233–41. [PubMed] [Google Scholar]
- 5.Thorne RF, Legg JW, Isacke CM. The role of the CD44 transmembrane and cytoplasmic domains in co-ordinating adhesive and signalling events. J Cell Sci. 2004;117:373–80. doi: 10.1242/jcs.00954. [DOI] [PubMed] [Google Scholar]
- 6.Mori T, Kitano K, Terawaki S, Maesaki R, Fukami Y, Hakoshima T. Structural basis for CD44 recognition by ERM proteins. J Biol Chem. 2008;283:29602–12. doi: 10.1074/jbc.M803606200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thankamony SP, Knudson W. Acylation of CD44 and its association with lipid rafts are required for receptor and hyaluronan endocytosis. J Biol Chem. 2006;281:34601–9. doi: 10.1074/jbc.M601530200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knudson W, Loeser RF. CD44 and integrin matrix receptors participate in cartilage homeostasis. Cell Mol Life Sci. 2002;59:36–44. doi: 10.1007/s00018-002-8403-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knudson W, Casey B, Nishida Y, Eger W, Kuettner KE, Knudson CB. Hyaluronan oligosaccharides perturb cartilage matrix homeostasis and induce chondrogenic chondrolysis. Arthritis Rheum. 2000;43:1165–74. doi: 10.1002/1529-0131(200005)43:5<1165::AID-ANR27>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 10.Chow G, Nietfeld J, Knudson CB, Knudson W. Antisense inhibition of chondrocyte CD44 expression results in cartilage chondrolysis. Arthritis Rheum. 1998;41:1411–9. doi: 10.1002/1529-0131(199808)41:8<1411::AID-ART10>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 11.Nishida Y, Knudson CB, Knudson W. Osteogenic protein-1 inhibits matrix depletion in a hyaluronan hexasaccharide-induced model of osteoarthritis. Osteoarthritis Cartilage. 2004;12:374–82. doi: 10.1016/j.joca.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 12.Nagano O, Saya H. Mechanism and biological significance of CD44 cleavage. Cancer Science. 2004;95:930–5. doi: 10.1111/j.1349-7006.2004.tb03179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lammich S, Okochi M, Takeda M, Kaether C, Capell A, Zimmer AK, et al. Presenilin-dependent intramembrane proteolysis of CD44 leads to the liberation of its intracellular domain and the secretion of an Aβ-like peptide. J Biol Chem. 2002;277:44754–9. doi: 10.1074/jbc.M206872200. [DOI] [PubMed] [Google Scholar]
- 14.Nagano O, Murakami D, Hartmann D, de Strooper B, Saftig P, Iwatsubo T, et al. Cell-matrix interaction via CD44 is independently regulated by different metalloproteinases activated in response to extracellular Ca2+ influx and PKC activation. J Cell Biol. 2004;165:893–902. doi: 10.1083/jcb.200310024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakamura H, Suenaga N, Taniwaki K, Matsuki H, Yonezawa K, Fujii M, et al. Constitutive and induced CD44 shedding by ADAM-like proteases and membrane-type 1 matrix metalloproteinase. Cancer Res. 2004;64:876–82. doi: 10.1158/0008-5472.can-03-3502. [DOI] [PubMed] [Google Scholar]
- 16.Stoeck A, Keller S, Riedle S, Sanderson MP, Runz S, Le Naour F, et al. A role for exosomes in the constitutive and stimulus-induced ectodomain cleavage of L1 and CD44. Biochem J. 2006;393:609–18. doi: 10.1042/BJ20051013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kajita M, Itoh Y, Chiba T, Mori H, Okada A, Kinoh H, et al. Membrane-type 1 matrix metalloproteinase cleaves CD44 and promotes cell migration. J Cell Biol. 2001;153:893–904. doi: 10.1083/jcb.153.5.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peterson L, Brittberg M, Kiviranta I, Akerlund EL, Lindahl A. Autologous chondrocyte transplantation: biomechanics and longterm durability. Am J Sports Med. 2002;30:2–12. doi: 10.1177/03635465020300011601. [DOI] [PubMed] [Google Scholar]
- 19.Hardingham TE, Oldershaw RA, Tew SR. Cartilage, SOX9 and Notch signals in chondrogenesis. J Anatomy. 2006;209:469–80. doi: 10.1111/j.1469-7580.2006.00630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benya PD, Shaffer JD. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982;30:215–24. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- 21.Tew SR, Hardingham TE. Regulation of SOX9 mRNA in human articular chondrocytes involving p38 MAPK activation and mRNA stabilization. J Biol Chem. 2006;281:39471–9. doi: 10.1074/jbc.M604322200. [DOI] [PubMed] [Google Scholar]
- 22.Goldring MB, Goldring SR. Osteoarthritis. J Cell Physiol. 2007;213:626–34. doi: 10.1002/jcp.21258. [DOI] [PubMed] [Google Scholar]
- 23.Stokes DG, Liu G, Coimbra IB, Piera-Velazquez S, Crowl RM, Jimenez SA. Assessment of the gene expression profile of differentiated and dedifferentiated human fetal chondrocytes by microarray analysis. Arthritis Rheum. 2002;46:404–19. doi: 10.1002/art.10106. [DOI] [PubMed] [Google Scholar]
- 24.Schulze-Tanzil G, Mobasheri A, de Souza P, John T, Shakibaei M. Loss of chondrogenic potential in dedifferentiated chondrocytes correlates with deficient Shc-Erk interaction and apoptosis. Osteoarthritis Cartilage. 2004;12:448–58. doi: 10.1016/j.joca.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 25.Gan L, Kandel RA. In vitro cartilage tissue formation by co-culture of primary and passaged chondrocytes. Tissue Eng. 2007;14:831–42. doi: 10.1089/ten.2006.0231. [DOI] [PubMed] [Google Scholar]
- 26.Bonaventure J, Kadhom N, Cohen-Solal L, Ng KH, Bourguignon J, Lasselin C, et al. Reexpression of cartilage-specific genes by dedifferentiated human articular chondrocytes cultured in alginate beads. Exp Cell Res. 1994;212:97–104. doi: 10.1006/excr.1994.1123. [DOI] [PubMed] [Google Scholar]
- 27.Ohno S, Im HJ, Knudson CB, Knudson W. Hyaluronan oligosaccharides induce matrix metalloproteinase 13 via transcriptional activation of NFκB and p38 MAP kinase in articular chondrocytes. J Biol Chem. 2006;281:17952–60. doi: 10.1074/jbc.M602750200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghatak S, Misra S, Toole BP. Hyaluronan constitutively regulates ErbB2 phosphorylation and signaling complex formation in carcinoma cells. J Biol Chem. 2005;280:8875–83. doi: 10.1074/jbc.M410882200. [DOI] [PubMed] [Google Scholar]
- 29.Okamoto I, Kawano Y, Tsuiki H, Sasaki J, Nakao M, Matsumoto M, et al. CD44 cleavage induced by a membrane-associated metalloprotease plays a critical role in tumor cell migration. Oncogene. 1999;18:1435–46. doi: 10.1038/sj.onc.1202447. [DOI] [PubMed] [Google Scholar]
- 30.Shintani N, Kurth T, Hunziker EB. Expression of cartilage-related genes in bovine synovial tissue. J Orthop Res. 2007;25:813–9. doi: 10.1002/jor.20345. [DOI] [PubMed] [Google Scholar]
- 31.Jiang H, Peterson RS, Wang W, Bartnik E, Knudson CB, Knudson W. A requirement for the CD44 cytoplasmic domain for hyaluronan binding, pericellular matrix assembly and receptor mediated endocytosis in COS-7 cells. J Biol Chem. 2002;277:10531–8. doi: 10.1074/jbc.M108654200. [DOI] [PubMed] [Google Scholar]
- 32.Knudson W, Aguiar DJ, Hua Q, Knudson CB. CD44-anchored hyaluronan-rich pericellular matrices: an ultrastructural and biochemical analysis. Exp Cell Res. 1996;228:216–28. doi: 10.1006/excr.1996.0320. [DOI] [PubMed] [Google Scholar]
- 33.Gunthert U. CD44: a multitude of isoforms with diverse functions. Curr Top Microbiol Immunol. 1993;184:47–63. doi: 10.1007/978-3-642-78253-4_4. [DOI] [PubMed] [Google Scholar]
- 34.Goldstein LA, Zhou DF, Picker LJ, Minty CN, Bargatze RF, Ding JF, et al. A human lymphocyte homing receptor, the Hermes antigen, is related to cartilage proteoglycan core and link proteins. Cell. 1989;56:1063–72. doi: 10.1016/0092-8674(89)90639-9. [DOI] [PubMed] [Google Scholar]
- 35.Stamenkovic I, Amiot M, Pesando JM, Seed B. A lymphocyte molecule implicated in lymph node homing is a member of the cartilage link protein family. Cell. 1989;56:1057–62. doi: 10.1016/0092-8674(89)90638-7. [DOI] [PubMed] [Google Scholar]
- 36.Muehleman C, Bareither D, Huch K, Cole AA, Kuettner KE. Prevalence of degenerative morphological changes in the joints of the lower extremity. Osteoarthritis Cartilage. 1997;5:23–37. doi: 10.1016/s1063-4584(97)80029-5. [DOI] [PubMed] [Google Scholar]
- 37.Flannery CR, Little CB, Caterson B, Hughes CE. Effects of culture conditions and exposure to catabolic stimulators (IL-1 and retinoic acid) on the expression of matrix metalloproteinases (MMPs) and disintegrin metalloproteinases (ADAMs) by articular cartilage chondrocytes. Matrix Biol. 1999;18:225–37. doi: 10.1016/s0945-053x(99)00024-4. [DOI] [PubMed] [Google Scholar]
- 38.Chow G, Knudson CB, Homandberg G, Knudson W. Increased expression of CD44 in bovine articular chondrocytes by catabolic cellular mediators. J Biol Chem. 1995;270:27734–41. doi: 10.1074/jbc.270.46.27734. [DOI] [PubMed] [Google Scholar]
- 39.Ohno S, Ohno-Nakahara M, Knudson CB, Knudson W. Induction of MMP-3 by hyaluronan oligosaccharides in temporomandibular joint chondrocytes. J Dental Res. 2005;84:1005–9. doi: 10.1177/154405910508401107. [DOI] [PubMed] [Google Scholar]
- 40.Ohno S, Im HJ, Knudson CB, Knudson W. Hyaluronan oligosaccharide–induced activation of transcription factors in bovine articular chondrocytes. Arthritis Rheum. 2005;52:800–9. doi: 10.1002/art.20937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sugahara KN, Murai T, Nishinakamura H, Kawashima H, Saya H, Miyasaka M. Hyaluronan oligosaccharides induce CD44 cleavage and promote cell migration in CD44-expressing tumor cells. J Biol Chem. 2003;278:32259–65. doi: 10.1074/jbc.M300347200. [DOI] [PubMed] [Google Scholar]
- 42.Oliferenko S, Paiha K, Harder T, Gerke V, Schwarzler C, Schwarz H, et al. Analysis of CD44-containing lipid rafts: recruitment of annexin II and stabilization by the actin cytoskeleton. J Cell Biol. 1999;146:843–54. doi: 10.1083/jcb.146.4.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Foger N, Marhaba R, Zoller M. Involvement of CD44 in cytoskeleton rearrangement and raft reorganization in T cells. J Cell Sci. 2001;114:1169–78. doi: 10.1242/jcs.114.6.1169. [DOI] [PubMed] [Google Scholar]
- 44.Bourguignon LY, Kalomiris EL, Lokeshwar VB. Acylation of the lymphoma transmembrane glycoprotein, GP85, may be required for GP85-ankyrin interaction. J Biol Chem. 1991;266:11761–5. [PubMed] [Google Scholar]
- 45.Okamoto I, Tsuiki H, Kenyon LC, Godwin AK, Emlet DR, Holgado-Madruga M, et al. Proteolytic cleavage of the CD44 adhesion molecule in multiple human tumors. Am J Pathol. 2002;160:441–7. doi: 10.1016/S0002-9440(10)64863-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Galardy RE, Cassabonne ME, Giese C, Gilbert JH, Lapierre F, Lopez H, et al. Low molecular weight inhibitors in corneal ulceration. Ann N Y Acad Sci. 1994;732:315–23. doi: 10.1111/j.1749-6632.1994.tb24746.x. [DOI] [PubMed] [Google Scholar]
- 47.Okamoto I, Kawano Y, Murakami D, Sasayama T, Araki N, Miki T, et al. Proteolytic release of CD44 intracellular domain and its role in the CD44 signaling pathway. J Cell Biol. 2001;155:755–62. doi: 10.1083/jcb.200108159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dovey HF, John V, Anderson JP, Chen LZ, de Saint Andrieu P, Fang LY, et al. Functional γ-secretase inhibitors reduce β-amyloid peptide levels in brain. J Neurochem. 2001;76:173–81. doi: 10.1046/j.1471-4159.2001.00012.x. [DOI] [PubMed] [Google Scholar]
- 49.Mori H, Tomari T, Koshikawa N, Kajita M, Itoh Y, Sato H, et al. CD44 directs membrane-type 1 matrix metalloproteinase to lamellipodia by associating with its hemopexin-like domain. EMBO J. 2002;21:3949–59. doi: 10.1093/emboj/cdf411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anilkumar N, Uekita T, Couchman JR, Nagase H, Seiki M, Itoh Y. Palmitoylation at Cys574 is essential for MT1-MMP to promote cell migration. FASEB J. 2005;19:1326–8. doi: 10.1096/fj.04-3651fje. [DOI] [PubMed] [Google Scholar]


