Abstract
Methamphetamine (METH) is a psychostimulant that induces neural damage in experimental animals and humans. A binge (usually in the 5–10 mg/kg dose range 4× at 2 h intervals) and the acute bolus drug administration (20–40 mg/kg) of METH have been employed frequently to study neurotoxicity in the brain. In this study we have compared these drug delivery schedules to determine their efficacy to induce striatal apoptosis. Exposure of male mice to a binge of METH at 10 mg/kg 4× at 2 h intervals (cumulative dose of 40 mg/kg) was approximately four times less effective in inducing apoptotic cell death (TUNEL staining) 24 h after METH treatment in the striatum than a single bolus administration of 30 mg/kg of METH. The residual TUNEL staining observed three days after METH treatment is proportionately equivalent between a binge and the acute bolus drug administration. Interestingly, a binge of METH induces a hyperthermic response of longer duration. This study demonstrates that an acute bolus drug administration of METH is more effective inducing striatal apoptosis in mice, and therefore, is more suitable for studies assessing the impact of METH on sites post-synaptic to the striatonigral dopamine terminals.
Keywords: Methamphetamine, Neurotoxicity, Striatum, Cell death, Binge, Acute bolus drug administration
1. Introduction
The work of Escalante and Ellinwood (1970) was the first to demonstrate that METH induces neural damage to neurons of the cat brain. Exposure to a chronic high dose of METH produced depletion of dopamine (up to 80%) in the caudate nucleus of rhesus monkeys for up to six months post-treatment (Seiden et al., 1975). The same study reported loss of some noradrelanin as well. These seminal studies indicated that METH was neurotoxic leading to the systematic analysis of the impact of METH on monoaminergic systems of the brain. Conclusive evidence demonstrating METH-induced neuronal damage came from the application of the silver stain, the gold standard to assess neuronal degeneration. METH was shown to induce dopamine terminal degeneration in the forebrain with the silver stain (Ricaurte et al., 1982, 1984). METH-induced depletion of monoamines may be permanent since chronic exposure to METH in non-human primates led to reductions of dopamine and serotonin in the caudate four years after withdrawal from METH (Woolverton et al., 1989). Exposure to METH also causes cell body injury to neurons of the parietal cortex of rats (Eisch and Marshall, 1998). Studies with humans also demonstrated METH-induced deficits in monoaminergic systems of the forebrain. For example, a post-mortem study of METH users (unknown amount of consumption of the drug) reported significant reduction in the levels of dopamine transporters, tyrosine hydroxylase, and dopamine levels in the caudate and putamen (Wilson et al., 1996). These reductions may be due to toxicity (fewer dopamine terminals) rather than a neuroadaptation (diminished number of dopamine transporter sites against an unchanged number of dopamine terminals) since Positron Emission Tomography scanning involving METH abusers showed reduced levels of dopamine transporters in the caudate after three years of abstinence from METH (McCann et al., 1998). Moreover, detoxified METH abusers showed decreased levels of binding to dopamine transporters and decreased glucose utilization in the caudate and putamen (Volkow et al., 2001a, 2001b). The binge and the acute bolus drug administration of METH have been used in most studies assessing the neurotoxic effects of METH in the brain and both are equally effective inducing depletion of dopamine terminal markers in the striatum (for review see (Davidson et al., 2001). However, METH-induced apoptosis is a relatively recent discovery (Deng et al., 2001; Eisch and Marshall, 1998; Pu et al., 1996) that needs to be studied in more detail because it represents a model of striatal injury at the level of the postsynaptic neurons. In this study, we demonstrate that both the binge and an acute bolus drug administration of METH induce striatal apoptosis, but the latter is more effective. In contrast, the binge of METH induces a hyperthermic response of similar magnitude but of longer duration.
2. Materials and methods
2.1. Animals, drugs, and drug administration
Single (30 mg/kg) or multiple (10 mg/kg × 4 at 2 h intervals) intraperitoneal (i.p.) injections of METH (Sigma, St. Louis, MI) were given to 11-week-old male ICR mice (Taconic, Germantown, NY). Mice receiving the multiple injections of METH were sacrificed 24 h after the fourth and last injection. All animals were housed singly with food and water available ad libitum on a 12 h light/dark cycle. Animals were habituated for approximately two weeks prior to any drug treatment. Animals were sacrificed by decapitation at 24 h or three days post-treatment. Brains were dissected, placed on dry ice, and stored at −70°C until use. All animal use procedures were according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of Hunter College of the City University of New York.
2.2. Terminal deoxyncleotidyl transferase-mediated dUTP nick end labeling (TUNEL) histochemistry and quantification
The method for the TUNEL assay was adapted from (Deng et al., 2001) with minor modifications. In brief, fresh frozen 20 μm serial coronal sections were taken from bregma 0.38 ± 0.1 mm and fixed in 4% paraformaldehyde for 30 min. After washing with phosphate-buffered saline, pH 7.6 (PBS), the sections were immersed in 0.4% Triton-X-100 in PBS for 5–10 min at 70°C. The sections were washed again and TUNEL reagents (Roche Applied Science, Indianapolis, IN) were applied directly onto the tissue and incubated for 1 h in a humidified chamber. Sections were washed and counter-stained with DAPI. Stained sections were washed in PBS and coverslipped with Vectashield (Vector Laboratories, Burlingame, CA). Images were taken with a Nikon Eclipse TE200 epifluorescent scope attached with a CE 3.2.0 digital camera using FITC filters.
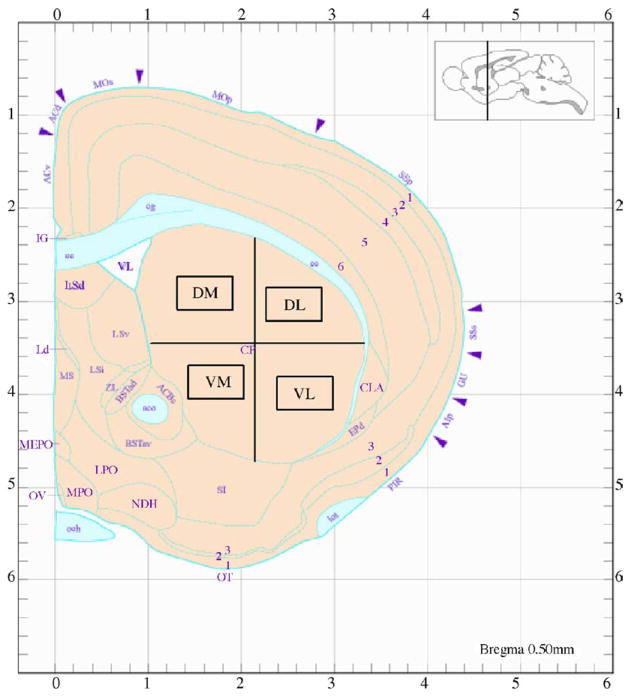
TUNEL-positive cells were quantified from 20 μm sections in an area of 260 μm2 for each quadrant of the caudate-putamen (Fig. 1)(dorsal-medial (DM), dorsal-lateral (DL), ventral-medial (VM), ventral-lateral (VL)). Average counts were taken from five serial sections per animal. Percentage of TUNEL-positive cells relative to the total number of neurons stained with NeuN in each quadrant was calculated from total neuronal cell counts as previously described (Xu et al., 2005).
Fig. 1.
Striatal subregions assessed in this study. Cross sectional representation in schematic form of the striatum in one hemisphere of the mouse brain. DM, dorsal-medial; DL, dorsal-lateral; VM, ventral-medial; VL, ventral-lateral. Cell counts were taken from the areas within the rectangular enclosures. Reproduced from Hof et al. (2000).
2.3. Body temperature
Rectal body temperature was determined with a BAT-12 thermometer coupled to a RET-3 mouse rectal probe (Physitemp Instruments, Clifton, NJ). Ambient room temperature was maintained at 20–22°C.
2.4. Statistical analysis
Analysis is performed from mean ± S.E.M. Differences between groups were analyzed by ANOVA followed by post hoc comparison using Fisher’s protected least significance test. Significance criteria set at p < 0.05.
3. Results
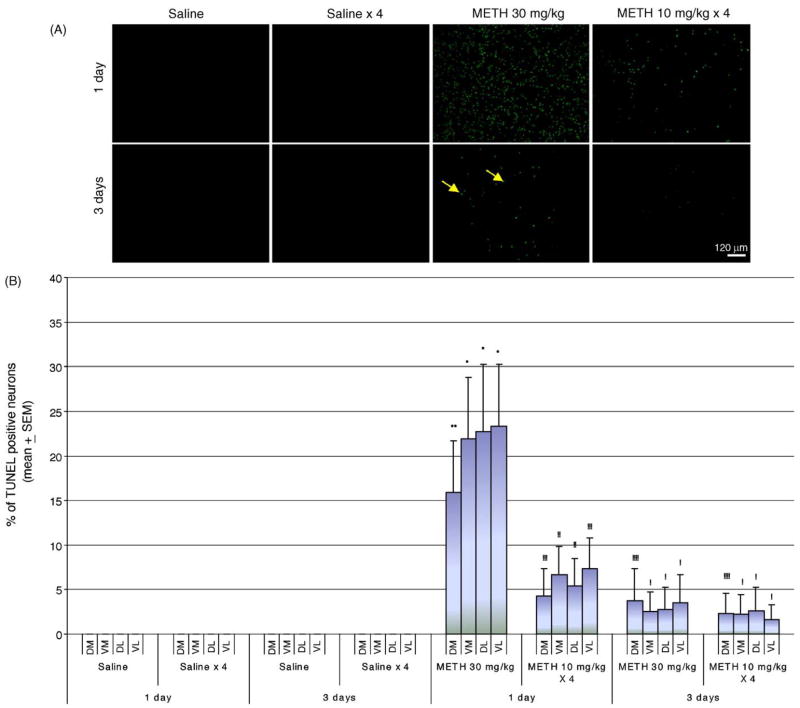
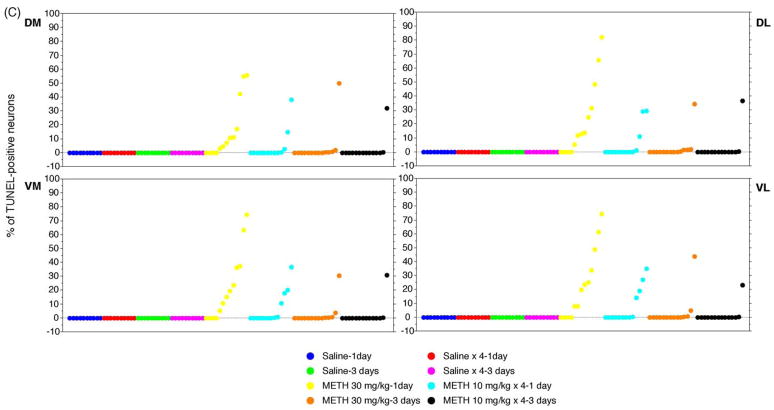
METH-induced apoptotic cell death was assessed in coronal sections using the TUNEL assay. Cell counts were taken from the striatal areas within the rectangular enclosures shown in Fig. 1. Cells that are TUNEL-positive display green fluorescent nuclei against a dark background (Fig. 2A). An acute bolus drug administration (30 mg/kg of body weight, injected i.p.) of METH induces considerably more TUNEL staining 24 h after the treatment than a binge of METH at 10 mg/kg 4× at 2 h intervals (Fig. 2A). A few scattered TUNEL-positive cells are visible three days after the treatment for both drug delivery schedules (Fig. 2A). The bulk of the cell death occurs 24 h after METH treatment (Fig. 2A). Nuclei staining positive for TUNEL are observed only in the striata of mice injected with METH. In order to quantify the amount of apoptotic cell death in sections of striatal tissue, the amount of TUNEL staining was expressed as the ratio of TUNEL-positive nuclei relative to the total number of neurons stained with the neuronal specific marker NeuN. All TUNEL-positive nuclei also stained positive for NeuN, demonstrating that METH-induced apoptosis occurs in neurons (Xu et al., 2005; Zhu et al., submitted for publication). The striatum was subdivided into four quadrants: dorsal-medial, dorsal-lateral, ventral-medial, ventral-lateral (see Fig. 1). An acute bolus drug administration of METH induced approximately 20% TUNEL-staining while a binge induced just an average of 5–7% cell death 24 h after treatment with METH (Fig. 2B). METH-induced apoptotic cell death at day 3 post-treatment fell between averages of 2–4% of total neurons (Fig. 2B). All striatal quadrants displayed proportional levels of TUNEL staining although not all animals displayed proportional levels of apoptosis within treatment groups (Fig. 2C). Interestingly, an acute bolus drug administration of METH induces apoptosis in the striata of more mice than a binge of METH (Fig. 2C).
Fig. 2.
Comparison of an acute bolus drug administration (30 mg/kg, i.p.) with a binge (10 mg/kg × 4 at 2 h intervals, i.p.) of METH on induction of apoptosis in the striatum of mice. Cell death was detected using the TUNEL assay. (A) Epifluorescent micrographs of TUNEL-stained mouse striata. Scale bar = 120 μm. Note the appearance of green fluorescent nuclei against a dark background in the striatum of mice treated with METH. (B) Percent of TUNEL-positive staining (mean ± S.E.M.) relative to total neuronal cell counts (data not shown) are shown for the dorsal-medial (DM), dorsal-lateral (DL), ventral-medial (VM), and ventral-lateral (VL) aspects of the striatum. Note that an acute bolus drug administration of METH is significantly more effective in inducing striatal apoptosis. *p < 0.001 compared to all saline treatments. #p < 0.005 compared to METH 30 mg/kg. (C) Scattergraphs for DM, DL, VM, and VL regions of the striatum demonstrate the percent of TUNEL-positive neurons is variable within treatment groups. Each dot represents one animal within each treatment group. ( ) Saline-1 day, (
) Saline-1 day, ( ) Saline × 4–1 day, (
) Saline × 4–1 day, ( ) Saline-3 days, (
) Saline-3 days, ( ) Saline × 4–3 days, (
) Saline × 4–3 days, ( ) METH 30 mg/kg-1 day, (
) METH 30 mg/kg-1 day, ( ) METH 10 mg/kg × 4–1 day, (
) METH 10 mg/kg × 4–1 day, ( ) METH 30 mg/kg-3 days, (●) METH 30 mg/kg-3 days. n = 10 for all saline groups. n = 13 for METH treatment groups at day 1. n = 14 for METH treatment groups at day 3.
) METH 30 mg/kg-3 days, (●) METH 30 mg/kg-3 days. n = 10 for all saline groups. n = 13 for METH treatment groups at day 1. n = 14 for METH treatment groups at day 3.
We measured body core temperature utilizing a mouse rectal probe. A single bolus administration of METH elevated body core temperature from 36.6 to 39.1°C 2 h after METH. Body core temperature returned to pre-injection levels 4 h after METH (Fig. 3A). In contrast, a binge of 10 mg/kg of METH at 2 h intervals elevated body core temperature from 36.2 to 38.2°C 1 h after METH. This elevation was sustained for seven consecutive hours (Fig. 3B). Thus, one notable feature between these two dose schedules of METH is that the binge induces a hyperthermic response that is of longer duration than that observed after a single bolus administration of METH.
Fig. 3.
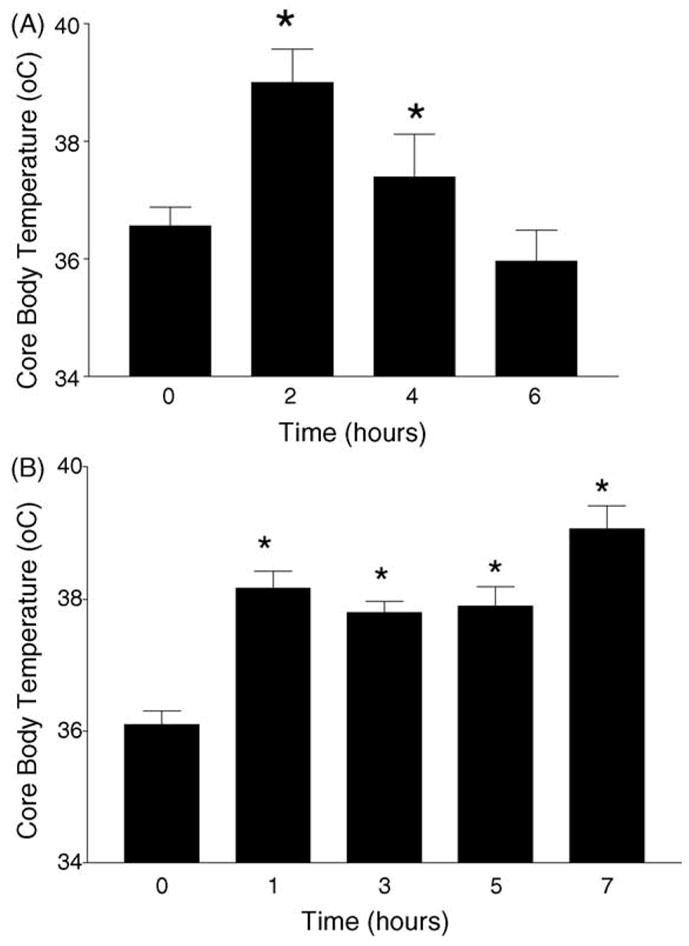
METH-induced hyperthermia. Body core temperature was measured with a mouse rectal probe. (A) The mice were injected i.p. with METH (30 mg/kg) at time 0 and body core temperature was measured at 2 h intervals up to 6 h post-METH. (B) Mice received four i.p. injections of METH (10 mg/kg each) at 2 h intervals. The mice were given METH at 0, 2, 4, and 6 h. Body core temperature was measured at 1, 3, 5, and 7 h. Note that the hyperthermic response elicited by the binge of METH is sustained longer than that observed with a single bolus administration (compare Fig. 2A and B). *p < 0.05 compared to control group (Student’s t-test).
4. Discussion
Our results demonstrate that a binge or a single bolus administration of METH induces striatal apoptosis in the mouse. While the binge schedule of METH administration models METH use by humans, the single high bolus administration may be a model of severe METH use (Bowyer et al., 2004). However, the single bolus administration is used in animal models studying the mechanism of METH-induced neural damage in the brain (Deng et al., 2001; Fumagalli et al., 1999; Imam et al., 2001; Ricaurte et al., 1982; Xie et al., 2002). Similarities exist between these two schedules of METH administration. For example, the distribution of METH-induced apoptosis is consistent with previous observations that the ventral aspects of the striatum are more susceptible to the damaging effects of METH (Pu et al., 1994). This study also found residual astrocytosis in the ventral striatum in the absence of tyrosine hydroxylase depletion (Pu et al., 1994), suggesting that the residual astrocytosis may have been induced by degenerating cortical fibers. There is histological evidence demonstrating degeneration of corticostriatal fibers caused by D-amphetamine (Ryan et al., 1990). More recent evidence demonstrates degeneration of cortical neurons by METH (Eisch and Marshall, 1998; Pu et al., 1996). Thus, it is plausible to suspect that the striatal apoptosis induced by METH may be mediated by excessive release of glutamate. METH-induced overflow of glutamate in the striatum has been demonstrated (Nash and Yamamoto, 1992), and METH-induced dopamine terminal injury is prevented by NMDA receptor antagonists (Marshall et al., 1993; Muraki et al., 1992; Sonsalla et al., 1991).
The extent of METH-induced neural damage displays interanimal variability. Approximately 3 of 10 mice die within the first 24 h after exposure by either binge or single bolus administration of METH. Of the animals that survive, the amount and regional distribution of apoptosis varies within compartments of the striatum with the ventral aspects being the most affected. This type of interanimal variability is not peculiar to METH-induced striatal apoptosis. For example, although to a lesser degree, variability between animals has been reported for METH-induced depletion of dopamine terminal markers using a binge of METH 10 mg/kg 4× at 2 h intervals (Pu et al., 1994; Yu et al., 2002, 2004). A factor contributing to the large interanimal variability observed in our results might be the concentration of METH in plasma. It is conceivable that the greater magnitude of apoptosis induced by the single high dose of METH (30 mg/kg) is due to higher plasma levels of METH compared to the 4× 10 mg/kg at 2 h intervals schedule of drug administration. This is an important issue that needs to be investigated further.
The present study shows that both the binge and the single bolus administration of METH induce hyperthermia; however, a binge of four injections of METH induces a hyperthermic response that persists for at least 7 h. This is in sharp contrast to the transient (lasting 3–4 h) elevation of body core temperature induced by a single bolus administration of METH. Interestingly, the latter induces more apoptotic cell death in the mouse striatum than the former, demonstrating dissociation between the severity of METH-induced striatal apoptosis and the hyperthermic response. We have demonstrated that neuroprotection of the dopamine terminals from METH by antagonists of the neurokinin-1 receptor occurs without preventing METH-induced hyperthermia (Yu et al., 2002, 2004).
Our data demonstrate that both schedules of METH administration induce striatal apoptosis; however, the single bolus administration is more effective and may be more suitable for animal model studies that assess METH-induced striatal injury at sites postsynaptic to the nigrostriatal dopamine terminals. A recent study using magnetic resonance imaging suggests the presence of cell death in the brain of METH users (Ernst et al., 2000). Work from our laboratory demonstrates that METH induces apoptosis in some striatal neurons but not glial cells. Moreover, the apoptosis in the aftermath of METH occurs in some medium spiny projection neurons, cholinergic, and GABA-parvalbumin interneurons. The somatostatin/nitric oxide synthase interneurons are refractory to METH-induced apoptosis (Zhu et al., submitted for publication). In conclusion, a single bolus administration of METH is more effective inducing striatal apoptosis in the mouse brain than a binge administration. Although the former represents a high dose of METH, it may be more useful in studies investigating the mechanism of METH-induced striatal neuronal injury.
Acknowledgments
We would like to thank Gertrude Rivera for her help in the preparation of the manuscript. This work was supported by ‘Specialized Neuroscience Research Program’ grant NS41073 from the National Institute for Neurological Disorders and Stroke and DA12136 from the National Institute on Drug Abuse to JAA. Support has also come from the ‘Research Centers in Minority Institutions’ award for infrastructure to Hunter College.
References
- Bowyer JF, Harris AJ, Delongchamp RR, Jakab RL, Miller DB, Little AR, et al. Selective changes in gene expression in cortical regions sensitive to amphetamine during the neurodegenerative process. Neurotoxicology. 2004;25:555–72. doi: 10.1016/j.neuro.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Davidson C, Gow AJ, Lee TH, Ellinwood EH. Methamphetamine neurotoxicity: necrotic and apoptotic mechanisms and relevance to human abuse and treatment. Brain Res Brain Res Rev. 2001;36:1–22. doi: 10.1016/s0165-0173(01)00054-6. [DOI] [PubMed] [Google Scholar]
- Deng X, Wang Y, Chou J, Cadet JL. Methamphetamine causes widespread apoptosis in the mouse brain: evidence from using an improved TUNEL histochemical method. Brain Res Mol Brain Res. 2001;93:64–9. doi: 10.1016/s0169-328x(01)00184-x. [DOI] [PubMed] [Google Scholar]
- Eisch AJ, Marshall JF. Methamphetamine neurotoxicity: dissociation of striatal dopamine terminal damage from parietal cortical cell body injury. Synapse. 1998;30:433–45. doi: 10.1002/(SICI)1098-2396(199812)30:4<433::AID-SYN10>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Ernst T, Chang L, Leonido-Yee M, Speck O. Evidence for long-term neurotoxicity associated with methamphetamine abuse: a 1H MRS study. Neurology. 2000;54:1344–9. doi: 10.1212/wnl.54.6.1344. [DOI] [PubMed] [Google Scholar]
- Escalante OD, Ellinwood EH., Jr Central nervous system cytopathological changes in cats with chronic methedrine intoxication. Brain Res. 1970;21:151–5. doi: 10.1016/0006-8993(70)90033-8. [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Gainetdinov RR, Wang YM, Valenzano KJ, Miller GW, Caron MG. Increased methamphetamine neurotoxicity in heterozygous vesicular monoamine transporter 2 knock-out mice. J Neurosci. 1999;19:2424–31. doi: 10.1523/JNEUROSCI.19-07-02424.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hof PR, Young WG, Bloom FE, Belichenko PV, Celio MR. Comparative Cytoarchitectonic Atlas of the C57BL/6 and 129/Sv Mouse Brains. Elsevier; 2000. p. 45. [Google Scholar]
- Imam SZ, Newport GD, Itzhak Y, Cadet JL, Islam F, Slikker W, Jr, et al. Peroxynitrite plays a role in methamphetamine-induced dopaminergic neurotoxicity: evidence from mice lacking neuronal nitric oxide synthase gene or overexpressing copper–zinc superoxide dismutase. J Neurochem. 2001;76:745–9. doi: 10.1046/j.1471-4159.2001.00029.x. [DOI] [PubMed] [Google Scholar]
- Marshall JF, O’Dell SJ, Weihmuller FB. Dopamine-glutamate interactions in methamphetamine-induced neurotoxicity. J Neural Transm Gen Sect. 1993;91:241–54. doi: 10.1007/BF01245234. [DOI] [PubMed] [Google Scholar]
- McCann UD, Wong DF, Yokoi F, Villemagne V, Dannals RF, Ricaurte GA. Reduced striatal dopamine transporter density in abstinent methamphetamine and methcathinone users: evidence from positron emission tomography studies with [11C]WIN-35, 428. J Neurosci. 1998;18:8417–22. doi: 10.1523/JNEUROSCI.18-20-08417.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraki A, Koyama T, Nakayama M, Ohmori T, Yamashita I. MK-801, a noncompetitive antagonist of NMDA receptor, prevents methamphetamine-induced decrease of striatal dopamine uptake sites in the rat striatum. Neurosci Lett. 1992;136:39–42. doi: 10.1016/0304-3940(92)90642-k. [DOI] [PubMed] [Google Scholar]
- Nash JF, Yamamoto BK. Methamphetamine neurotoxicity and striatal glutamate release: comparison to 3,4-methylenedioxymethamphetamine. Brain Res. 1992;581:237–43. doi: 10.1016/0006-8993(92)90713-j. [DOI] [PubMed] [Google Scholar]
- Pu C, Broening HW, Vorhees CV. Effect of methamphetamine on glutamate-positive neurons in the adult and developing rat somatosensory cortex. Synapse. 1996;23:328–34. doi: 10.1002/(SICI)1098-2396(199608)23:4<328::AID-SYN11>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Pu C, Fisher JE, Cappon GD, Vorhees CV. The effects of amfonelic acid, a dopamine uptake inhibitor, on methamphetamine-induced dopaminergic terminal degeneration and astrocytic response in rat striatum. Brain Res. 1994;649:217–24. doi: 10.1016/0006-8993(94)91067-7. [DOI] [PubMed] [Google Scholar]
- Ricaurte GA, Guillery RW, Seiden LS, Schuster CR, Moore RY. Dopamine nerve terminal degeneration produced by high doses of methylamphetamine in the rat brain. Brain Res. 1982;235:93–103. doi: 10.1016/0006-8993(82)90198-6. [DOI] [PubMed] [Google Scholar]
- Ricaurte GA, Seiden LS, Schuster CR. Further evidence that amphetamines produce long-lasting dopamine neurochemical deficits by destroying dopamine nerve fibers. Brain Res. 1984;303:359–64. doi: 10.1016/0006-8993(84)91221-6. [DOI] [PubMed] [Google Scholar]
- Ryan LJ, Linder JC, Martone ME, Groves PM. Histological and ultrastructural evidence that D-amphetamine causes degeneration in neostriatum and frontal cortex of rats. Brain Res. 1990;518:67–77. doi: 10.1016/0006-8993(90)90955-b. [DOI] [PubMed] [Google Scholar]
- Seiden LS, MacPhail RC, Oglesby MW. Catecholamines and drug-behavior interactions. Fed Proc. 1975;34:1823–31. [PubMed] [Google Scholar]
- Sonsalla PK, Riordan DE, Heikkila RE. Competitive and noncompetitive antagonists at N-methyl-D-aspartate receptors protect against methamphetamine-induced dopaminergic damage in mice. J Pharmacol Exp Ther. 1991;256:506–12. [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler MJ, et al. Higher cortical and lower subcortical metabolism in detoxified methamphetamine abusers. Am J Psychiatry. 2001a;158:383–9. doi: 10.1176/appi.ajp.158.3.383. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D, et al. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am J Psychiatry. 2001b;158:377–82. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Kalasinsky KS, Levey AI, Bergeron C, Reiber G, Anthony RM, et al. Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat Med. 1996;2:699–703. doi: 10.1038/nm0696-699. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Ricaurte GA, Forno LS, Seiden LS. Long-term effects of chronic methamphetamine administration in rhesus monkeys. Brain Res. 1989;486:73–8. doi: 10.1016/0006-8993(89)91279-1. [DOI] [PubMed] [Google Scholar]
- Xie T, Tong L, Barrett T, Yuan J, Hatzidimitriou G, McCann UD, et al. Changes in gene expression linked to methamphetamine-induced dopaminergic neurotoxicity. J Neurosci. 2002;22:274–83. doi: 10.1523/JNEUROSCI.22-01-00274.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Zhu JPQ, Angulo JA. Induction of striatal pre- and postsynaptic damage by methamphetamine requires the dopamine receptors. Synapse. 2005;58:110–21. doi: 10.1002/syn.20185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Cadet JL, Angulo JA. Neurokinin-1 (NK-1) receptor antagonists abrogate methamphetamine-induced striatal dopaminergic neurotoxicity in the murine brain. J Neurochem. 2002;83:613–22. doi: 10.1046/j.1471-4159.2002.01155.x. [DOI] [PubMed] [Google Scholar]
- Yu J, Wang J, Cadet JL, Angulo JA. Histological evidence supporting a role for the striatal neurokinin-1 receptor in methamphetamine-induced neurotixicity in the mouse brain. Brain Res. 2004;1007:124–31. doi: 10.1016/j.brainres.2004.01.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JPQ, Xu W, Cadet JL, Angulo JA. Methamphetamine-induced cell death: Selective vulnerability in neuronal subpopulations of the striatum in mice. Neuroscience. doi: 10.1016/j.neuroscience.2006.02.055. submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]