Abstract
The continuing morbidity and mortality associated with Staphylococcus aureus (S. aureus) infections, especially methicillin-resistent Staphylococcus aureus (MRSA) infections, have motivated calls to make S. aureus vaccine development a research priority. We developed a decision analytic computer simulation model to determine the potential economic impact of a S. aureus vaccine for neonates. Our results suggest that a S. aureus vaccine for the neonatal population would be strongly cost-effective (and in many situations dominant) over a wide range of vaccine efficacies (down to 10%) for vaccine costs (≤$500), and S. aureus attack rates (≥1%).
Keywords: Staphylococcus aureus, Vaccine, Economics
1. Introduction
The continuing morbidity and mortality associated with Staphylococcus aureus infections, especially methicillin-resistent Staphylococcus aureus (MRSA) infections, have motivated calls to make S. aureus vaccine development a research priority. Indeed, over the past two decades, MRSA persists in many healthcare settings and has spread fairly rapidly throughout the community, despite control efforts and policies. In fact, an increasing number of S. aureus isolates are demonstrating antibiotic resistance.[1-5]
Quantifying the potential economic value of such a S. aureus vaccine can help us understand how much to emphasize and invest in its development. Such information can help policy makers determine their areas of emphasis, manufacturers plan their research and development portfolio, funding agencies allocate resources, and scientists establish goals. It can also help define and establish desired vaccine characteristics and establish price targets. Constructing economic models early in a vaccine's development can help all stakeholders anticipate potential obstacles and adjust research plans accordingly. Economic models also can aid in choosing initial target populations for the vaccine.
Neonates, who typically have naive immune systems that leave them more susceptible to infections, are a potential target population. For example, the cumulative incidence of Staphylococcus aureus bacteremia among premature infants is approximately 4% and overall nosocomial infections 15-20%.[6-9] Frequent handling by family members, friends, and healthcare providers can facilitate spread. Infection control interventions such as contact precautions, education, decolonization, cohort nursing, and hand hygiene may not be always be effective or easy and inexpensive to implement.[10-12] Neonatal intensive care unit (NICU)-associated nosocomial infections result in significant neonatal morbidity and mortality, prolonged hospitalization, and extensive hospital costs.[9, 13-15] Since the first reported case of MRSA in a hospitalized neonate in 1981, numerous outbreaks have occurred in the NICU population.[12, 16-20]. Even when control of MRSA outbreaks is achieved, enduring eradication is rarely achieved.[11-12] One other factor makes neonates a potential target for a S. aureus vaccine. Neonates do not remain in the higher at-risk state indefinitely, as their immune systems eventually mature. So a S. aureus vaccine does not need to confer immunity for an extensive period of time.
Immunization can be either active (i.e., stimulating the neonate's immune system) or passive (e.g., providing immunoglobulins) but a passive immunization strategy may be preferable in an immuno-naive population such as neonates. Neonates born before 32 weeks gestation have not acquired IgG across the placenta and will not have coverage afforded by endogenous synthesis until 4-6 months after birth. [8, 21] Passive immunization could immediately (but transiently) protect patients who cannot mount a timely or rapid enough response to active vaccination. Several candidate immunoglobulin preparations to prevent S. aureus infections or facilitate treatment of S. aureus associated bacteremia are currently under development.[8, 22-26]
We developed computer simulation models to evaluate the potential economic value of a S. aureus vaccine administered to neonates. The models simulated the decision of whether to immunize a neonate against S. aureus. Sensitivity analyses were conducted to assess how varying MRSA prevalence, vaccine cost and vaccine efficacy impacts the cost-effectiveness of a vaccination strategy. The results of our model may help guide policy making, research initiatives and design of future clinical studies.
2. Methods
2.1. Model Structure
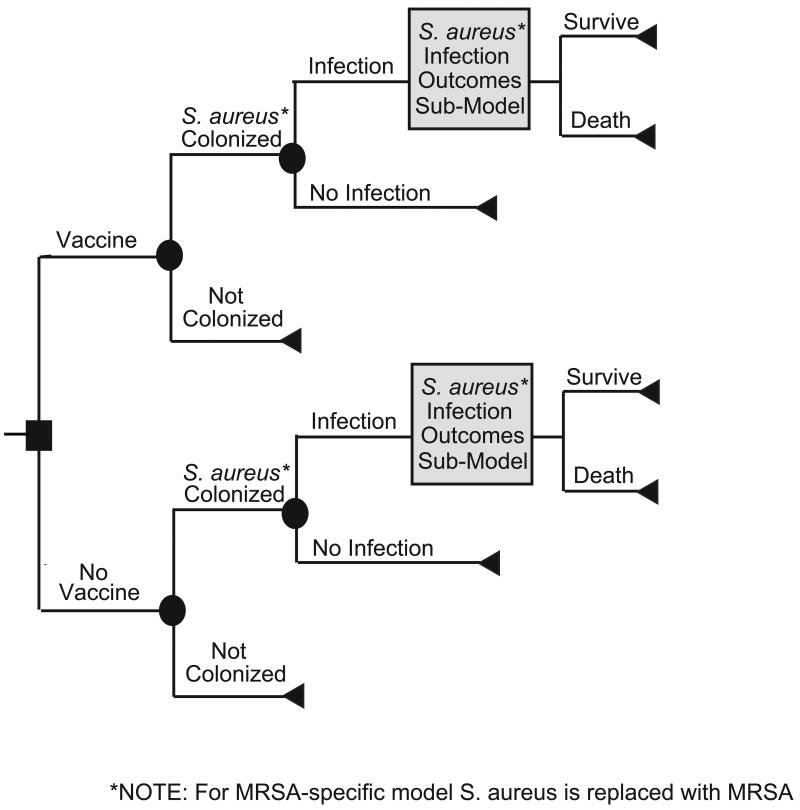
Using TreeAge Pro 2008 (TreeAge Software, Williamstown, MA), we developed two stochastic decision analytic computer simulation models depicting the decision of whether or not to administer a S. aureus vaccine to a neonate. The first model evaluated the effects of a S. aureus vaccine in preventing all types of S. aureus infections [including methicillin-sensitive S. aureus (MSSA) and MRSA] while the second focused specifically on MRSA, a subset of S. aureus infections that tends to have disproportionately worse outcomes than MSSA. Each model assumed both the societal and third party payor perspectives and simulated the potential cost-effectiveness outcomes of each scenario. The third party payor perspective included only direct medical costs, while the societal perspective accounted for both direct medical costs and patient productivity losses but did not include parent and caretaker productivity losses.
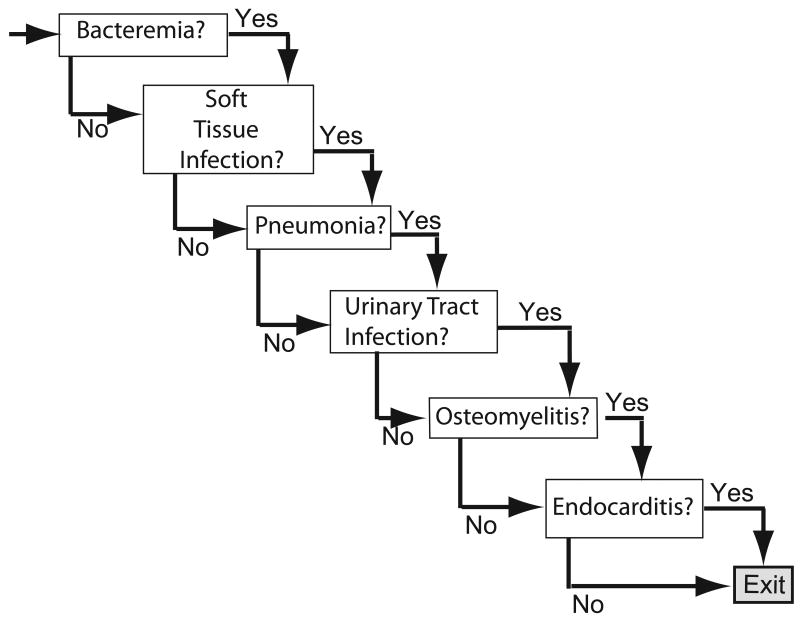
Figures 1 and 2 depict the general structure of both the general S. aureus and the MRSA specific vaccine decision models. Each neonate entering the S. aureus model receives or does not receive a S. aureus vaccine. The neonate then has a probability of developing a S. aureus infection (the attack rate in unvaccinated neonates not vaccinated; the attack rate multiplied by 1-vaccine efficacy in vaccinated neonates). A neonate with a S. aureus infection then has a probability of developing one or more of the following clinical syndromes: skin and soft tissue infection (SSTI), urinary tract infection (UTI), bacteremia, pneumonia, or endocarditis. For example, one newborn traveling through the model could develop a soft tissue infection whereas another could exhibit multiple clinical manifestations including SSTI, pneumonia, and endocarditis. Each clinical syndrome is accompanied by a probability of requiring essential diagnostic and therapeutic procedures. The MRSA-specific model is similar in structure, except that the attack rate and the probabilities of developing each clinical syndrome are specific to MRSA.
FIGURE 1.
Main Model Structure
FIGURE 2.
S. aureus or MRSA Infection Outcomes Sub-Model
For each simulation run, the following equation determined the incremental cost-effectiveness ratio (ICER) of neonate vaccination:
2.2. Data Inputs
Table 1 lists the input parameters for the S. aureus and MRSA vaccine models, respectively, including probabilities, costs, and utilities, as well as the distribution parameters and data sources used for each variable. Probabilities assume beta distributions, except the probability of developing an MRSA-attributable abscess and the probability of developing MRSA osteomyelitis, which assume triangular distributions. We use triangular distributions for all costs, except for the cost of death which is a fixed $5,000.[27] All costs are in 2008 U.S. dollars. A discount rate of 3% is used to convert past and future costs into 2008 dollars.
TABLE 1.
Data Inputs for Model Variables
| Description (units) | Mean | Range | Source | |
|---|---|---|---|---|
| Lower Limit | Upper Limit | |||
| COSTS ($US) | ||||
| Procedures: | ||||
| Blood Culture | 14.41 | 9.51 | 19.31 | Hospitals |
| Complete Blood Count | 12.24 | 8.08 | 16.40 | Hospitals |
| Chest Radiograph | 42.36 | 27.96 | 56.76 | [41] |
| C-Reactive Protein | 74.00 | 48.84 | 99.16 | Hospitals |
| Computed Tomography | 362.98 | 239.57 | 486.39 | [41] |
| Echocardiogram | 295.28 | 194.88 | 395.68 | [41] |
| Erythrocyte Sedimentation Rate | 4.93 | 3.25 | 6.61 | Hospitals |
| Incision and Drainage | 451.29 | 297.85 | 604.73 | [41] |
| Lumbar Puncture | 142.59 | 94.11 | 191.07 | [41] |
| Lumbar Puncture Tests | 50.06 | 33.04 | 67.08 | Hospitals |
| Magnetic Resonance Imaging | 359.06 | 236.98 | 481.14 | [41] |
| Nuclear Scan | 298.14 | 196.77 | 399.51 | [41] |
| Triple Scan | 269.86 | 178.11 | 361.61 | [41] |
| Urinalysis | 14.41 | 9.51 | 19.31 | Hospitals |
| Urine Culture | 14.47 | 9.55 | 19.39 | Hospitals |
| Vancomycin Level | 47.71 | 31.49 | 63.93 | Hospitals |
| Voiding Cystourogram | 275.87 | 182.07 | 369.67 | [41] |
| Plain Radiograph for Osteomyelitis | 28.04 | 18.51 | 37.57 | [41] |
| Side Effects from Vaccination | ||||
| Major Side Effects, Societal Perspective | 667363 | 578778 | 755947 | [42] |
| Minor Side Effects | 0.76 | 0.68 | 3.62 | [43] |
| Hospitalization for: | ||||
| Bacteremia | 7258.06 | 5302.62 | 9213.49 | [44] |
| Endocarditis | 46521.69 | 33988.00 | 59055.38 | [44] |
| Osteomyelitis | 15572.98 | 11377.37 | 19768.59 | [44] |
| Pneumonia | 3617.27 | 2642.72 | 4591.83 | [44] |
| Soft Tissue Infection | 4070.58 | 2973.90 | 5167.26 | [44] |
| Urinary Tract Infection | 6292.67 | 4597.32 | 7988.01 | [44] |
| PROBABILITIES (%) | ||||
| Given S. aureus Infection | ||||
| Bacteremia | 24.00 | 19.72* | [45-50] | |
| Endocarditis | 4.48 | 2.71* | [7, 47] | |
| Pneumonia | 33.33 | 14.06* | [7, 45, 48-50] | |
| Urinary Tract Infection | 2.40 | 2.27* | [46, 50] | |
| Soft Tissue Infection | 42.39 | 22.65* | [7, 45-47] | |
| Mortality | 23.26 | 9.87* | [47, 51] | |
| Given MRSA Infection: | ||||
| Bacteremia | 27.64 | 25.88* | [20, 52-56] | |
| Endocarditis | 1.54 | 0.52* | [57] | |
| Osteomyelitis | 3.33 | 1.13* | [20] | |
| Pneumonia | 16.67 | 12.82* | [20, 52, 57] | |
| Urinary Tract Infection | 18.50 | 9.19* | [55, 58] | |
| Soft Tissue Infection | 24.37 | 26.14* | [52, 54, 57, 59-61] | |
| Mortality | 0.1935 | 0.12* | [20, 51, 54, 57] | |
Standard Deviation
Our models measure effectiveness of vaccination in quality-adjusted life-years (QALYs). The probability distributions of projected life expectancy come from the Human Mortality Database.[22] Each clinical outcome results in an attendant QALY decrement that is assumed to persist for the duration of the condition. A urinary tract infection resulted in QALY decrement down to 0.73, an abscess down to 0.642, pneumonia down to 0.58, and bacteremia, endocarditis, or osteomyelitis down to 0.53.[23-24, 28] Death results in a loss of QALYs equal to the projected QALY-adjusted life expectancy of a newborn.[29]
Our models assume that infective endocarditis and osteomyelitis are treated with a 42-day course of vancomycin and soft tissue infections with a 10-day course of vancomycin. All other MRSA infections are assumed to necessitate a 14-day course of antibiotic treatment. Vancomycin is dosed by weight with its cost being $0.014472 per milligram. The distribution of newborn birth weights is drawn from the Centers for Disease Control and Prevention VitalStats Birth information web database.[30]
2.3. Sensitivity Analyses
Sensitivity analyses look at varying the values of all parameters simultaneously across their distributions in Table 1 as well as focusing on certain key parameters. We systematically test a wide range of vaccine efficacies (10% to 99%) and a wide range of S. aureus and MRSA attack rates (from 0.1% to 10%). Varying the cost of vaccination from $100-$1,000 per neonate helps us understand how different price points would affect the vaccine's economic value. We also evaluate the potential impact of minor and major vaccine side effects. Minor side effects include both local and self-limited systemic side effects that only require home treatment with over the counter medications. Since, currently, the potential major side effects of a S. aureus vaccine are unknown, we use cost data from a major potentially debilitating vaccine side effect, Guillain-Barré Syndrome (GBS). (Note that this does not imply that GBS will be a side effect of a S. aureus vaccine). Probabilistic (Monte Carlo) sensitivity analyses examine the effects of varying all parameters simultaneously using all the distributions on Table 1.
3. Results
3.1 Overall Results
Each simulation run consisted of 1,000 trials of 1,000 neonates (or 1,000,000 total newborns traveling through the model). The top half of Table 2 compiles select key simulation scenarios results from the all S. aureus model, and how they trend with the S. aureus infection attack rate, vaccine efficacy, and vaccine cost. The lower half of Table 2 lists analogous results from the MRSA-specific model. (Not displayed are numerous other simulation runs utilizing intermediate vaccine cost and efficacy values.)
Table 2.
Incremental Cost-Effectiveness Ratios (ICERs) for Neonatal Staphylococcus aureus (S. aureus) Vaccination
| All Staphylococcus. aureus Model | ||||||
|---|---|---|---|---|---|---|
| Cost of Vaccine | S. aureus Attack Rate (AR) | Vaccine Efficacy | ||||
| 0.1 | 0.25 | 0.5 | 0.75 | 0.9 | ||
| $100 | ||||||
| 0.1% | 71,609 | 33,293 | 15,406 | 8,405 | 78,99 | |
| 1% | 6,252 | 2,450 | 996 | 566 | 364 | |
| 2% | 3,182 | 1,031 | 299 | 47 | *** | |
| 5% | 956 | 138 | *** | *** | *** | |
| 10% | 292 | *** | *** | *** | *** | |
| $200 | ||||||
| 0.1% | 128,392 | 48,743 | 25,668 | 21,873 | 16,753 | |
| 1% | 13,321 | 5,522 | 2,625 | 1,514 | 1,187 | |
| 2% | 6,715 | 2,542 | 1,064 | 542 | 376 | |
| 5% | 2,520 | 716 | 150 | *** | *** | |
| 10% | 1,041 | 137 | *** | *** | *** | |
| $300 | ||||||
| 0.1% | 175,146 | 84,725 | 38,733 | 29,151 | 23,423 | |
| 1% | 23,178 | 7,756 | 4,025 | 2,545 | 1,959 | |
| 2% | 10,214 | 3,959 | 1,819 | 987 | 785 | |
| 5% | 4,056 | 1,382 | 434 | 149 | 47 | |
| 10% | 1,782 | 420 | *** | *** | *** | |
| $500 | ||||||
| 0.1% | 320,971 | 176,788 | 63,407 | 50,172 | 47,581 | |
| 1% | 39,368 | 14,358 | 6,892 | 4,384 | 3,636 | |
| 2% | 17,842 | 6,879 | 3,053 | 1,882 | 1,672 | |
| 5% | 6,878 | 2,388 | 1,030 | 522 | 377 | |
| 10% | 3,284 | 1,034 | 285 | 58 | *** | |
| $1,000 | ||||||
| 0.1% | 813,431 | 28,3074 | 143,605 | 96,925 | 81,078 | |
| 1 | 65,770 | 27,265 | 13,885 | 9,117 | 7,611 | |
| 2% | 37,497 | 14,347 | 6,673 | 4,678 | 3,601 | |
| 5% | 13,064 | 5,539 | 2,448 | 1,514 | 1,197 | |
| 10% | 6,668 | 2,492 | 1,015 | 544 | 359 | |
| Methicillin Resistant Staphylococcus aureus (MRSA) –specific Model | ||||||
| Cost of Vaccine | MRSA Attack Rate (AR) | Vaccine Efficacy | ||||
| 0.1 | 0.25 | 0.5 | 0.75 | 0.9 | ||
| $100 | ||||||
| 0.1% | 113,220 | 33,489 | 13,317 | 9,110 | 6,745 | |
| 1% | 5,564 | 2,213 | 861 | 470 | 334 | |
| 2% | 3,122 | 889 | 308 | 72 | 5 | |
| 5% | 912 | 173 | *** | *** | *** | |
| 10% | 252 | *** | *** | *** | *** | |
| $200 | ||||||
| 0.1% | 622,854 | 33,580 | 25,443 | 16,698 | 14,459 | |
| 1% | 10,251 | 5,166 | 2,014 | 1,287 | 1,062 | |
| 2% | 6,397 | 1,926 | 973 | 497 | 324 | |
| 5% | 4,230 | 711 | 158 | *** | *** | |
| 10% | 851 | 138 | *** | *** | *** | |
| $300 | ||||||
| 0.1% | 152,563 | 207,014 | 54,506 | 23,525 | 20,621 | |
| 1% | 26,561 | 7,974 | 3,478 | 2,187 | 1,783 | |
| 2% | 8,232 | 4,414 | 1,454 | 931 | 703 | |
| 5% | 3,210 | 1,296 | 379 | 153 | 69 | |
| 10% | 1,617 | 431 | 22 | *** | *** | |
| $500 | ||||||
| 0.1% | 492,442 | 97,543 | 56,458 | 48,635 | 32,205 | |
| 1% | 25,355 | 12,792 | 6,244 | 3,813 | 3,273 | |
| 2% | 16,960 | 5,924 | 2,764 | 1,689 | 1,475 | |
| 5% | 5,404 | 2,151 | 944 | 478 | 349 | |
| 10% | 2,578 | 910 | 268 | 62 | 2 | |
| $1,000 | ||||||
| 0.1% | 503,609 | 273,739 | 195,166 | 87,632 | 73,933 | |
| 1% | 88,168 | 24,679 | 12,854 | 8,391 | 6,628 | |
| 2% | 31,983 | 11,657 | 6,264 | 3,741 | 3,115 | |
| 5% | 12,949 | 4,826 | 2,059 | 1,383 | 1,029 | |
| 10% | 5,521 | 2359 | 975 | 482 | 346 | |
Values shaded in light grey are cost-effective (≤$50,000/QALY)
Values shaded in dark grey with *** indicate that vaccination is the dominant strategy
While some debate exists over the exact ICER threshold at which an intervention becomes cost-effective, traditionally ICERs under $50,000/QALY suggest that an intervention may be relatively cost-effective.[31] In general, interventions costing less than $20,000 per QALY have very good evidence for adoption while those costing greater than $100,000 per QALY have fairly poor evidence for adoption.[32] When vaccination is both less costly and more effective than no vaccination, vaccination is the dominant strategy (i.e., choosing to vaccinate is clearly beneficial).
3.2 S. aureus Vaccine Cost of $100 per Patient
When vaccine cost is $100 per patient and vaccine efficacy is as low as 10%, vaccination is cost-effective as long as S. aureus attack rate is at least 1%. Increasing vaccine efficacy to 25% means that the vaccine is cost-effectiveness down to a lower S. aureus attack rate: 0.1%. At this vaccine cost, vaccination becomes the dominant strategy at the following combinations: efficacy is 25% and the S. aureus attack rate is at least 10%; efficacy is 50%-75% and the S. aureus attack rate is equal to or greater than 5%; efficacy reaches 90% and the S. aureus attack is at least 2%.
3.3 S. aureus Vaccine Cost of $200 per Patient
Although increasing per patient vaccine cost to $200 does change some of cost-effectiveness thresholds, vaccination remains cost-effective at a wide range of efficacy and prevalence levels. Even at a vaccine efficacy as low as 10%, vaccination is cost-effective when the S. aureus attack rate is 1%. Raising vaccine efficacy levels to the range of 25%-50% lowers the S. aureus attack rate threshold for cost-effectiveness to 0.1%. Vaccination remains dominant for a wide range of vaccine efficacy and S. aureus attack rates. Even when vaccine efficacy is 50%, vaccination is dominant when the S. aureus attack rate is greater than or equal to 10%. When vaccine efficacy crosses 75%, vaccination dominates when the S. aureus attack rate is at least 5%.
3.4 S. aureus Vaccine Cost of $1000 per Patient
Even when vaccine cost is raised to $1000 per patient, vaccination remains relatively cost-effective for a vast majority of the scenarios. At an efficacy of 10%, vaccination is cost-effective when the S. aureus attack rate is at least 2%. For vaccine efficacy anywhere between 25% and 25%, vaccination is cost-effective as long as the S. aureus attack rate is at least 1%. Vaccination is never economically dominant when vaccine cost $1,000 per patient.
3.5 MRSA-specific Results
As the bottom half of Table 2 shows, a S. aureus vaccine is cost-effective under a wide variety of circumstances even when considering prevention of only MRSA (and not MSSA). A fairly low efficacy vaccine is still fairly cost-effective at a cost as high as $1000 vaccine as long as the MRSA attack rate is at least 1%.
3.6 Minor and Major Vaccine Side Effects
Adding vaccine minor side effects to the model has little effect, even when minor side effects are very common. Adding major side effects has little impact as long as the probability of major side effects does not exceed 0.5%. For example, when vaccine costs $1000 per patient, ICER values do not change significantly even with a minor side effect probability of 90%. At the same vaccine cost, vaccination remains cost-effective when the probability of major vaccine side effects <0.5%, even when the S. aureus infection attack rate is as low as 1% and vaccine efficacy is as low as 50%.
4. Discussion
4.1 Study Implications
Our results suggest that a S. aureus vaccine would be strongly cost-effective (and in many situations economically dominant) over a wide range of vaccine efficacies, vaccine costs, and S. aureus attack rates. It is fairly compelling that vaccination is still cost-effective at fairly low vaccine efficacies (as low as 25%) and S. aureus attack rates (as low as 0.1%), well below those seen in many neonatal units. It is also noteworthy that even when the vaccine is fairly costly ($1000 per patient), it is still cost-effective. Our analyses may be more consistent with a passive immunization approach (or an unusually rapidly acting active immunization) since they did not account for a potential delay before the neonate can mount an adequate response to active immunization. In fact, passive immunization may be a more favorable or practical approach since neonates' immune systems may not be adequately developed to respond to a vaccine.
The target population for a S. aureus vaccine could be either the overall neonatal population or more specifically low birth weight (≤1,000 g), who appear to have the highest rates of MRSA infection, potentially because of their immature immune systems, prolonged hospital stays, and exposure to invasive devices and procedures. In our model, attack rates of 2-10% are more consistent with low birth weight (≤1,000 g), while lower attack rates of 0.1-1% are consistent with higher birth weight neonates (>1,000 g).[6-9] If a S. aureus vaccine targeted low birth weight neonates (≤1,000 g) with a median 5% S. aureus attack rate, with a vaccine that costs $200 and has a 50% efficacy, then our model suggests that the ICER value would be approximately $158 per QALY, well below suggested thresholds for cost-effectiveness. Lowering the cost of the vaccine to $100 would make the immunizing all low birth weight neonates economically dominant, i.e., both saving costs and providing health benefits, strong evidence for its adoption.
An effective S. aureus vaccine could have a substantial market. The models' results highlight the substantial burden of MRSA infections in the neonatal population. Neonates are at increased-risk for Staphylococcal infections. Currently, MRSA exposure is very possible in the healthcare setting. For instance, data from the National Healthcare Safety Network at the Centers for Disease Control and Prevention (CDC) show that 78% of healthcare-associated infections in patients under the age of 3 are central line-associated bloodstream infections (CLABSI) and 20.6% of all CLABSIs occur in NICU patients. S. aureus was the pathogenic isolate in 9.9% of all CLABSIs, and 56.8% of those isolates exhibited methicillin resistance.[5] According to a study by Lessa et al. that analyzed data from the National Nosocomial Infections Surveillance System (NNIS), 1995–2004, there were 4831 S. aureus hospital-acquired infections among 578,521 neonates. Of the 4302 of the S. aureus isolated had susceptibility tests performed 975 (23%) were MRSA. Additionally, there has been a significant increase in both S. aureus and MRSA since 1995.[33] Additionally MRSA exposure in the community is becoming a growing problem. A single death of a neonate from MRSA can be devastating. Add the variety of other possible infection outcomes and it is clear that MRSA may be a significant threat for neonates. Preventing even only a fraction of these infections can pay significant dividends.
The considerable potential benefits of a S. aureus vaccine supports further investment into its development. Realizing that the market may support relatively high vaccine price points could encourage more vaccine developers to pursue this area. Higher price points with reasonable adoption could translate into ample revenues, justifying upfront investment into research and development. Additionally, the target efficacy window is fairly wide. Scientists and developers do not necessarily have to design the “perfect” vaccine that provides close to 100% protection. Even vaccines that offer low protection may be valuable. Moreover, our study suggests that third party payors would benefit from covering the S. aureus vaccine, even when the cost of the vaccine is fairly high. Anticipating insurance coverage for a vaccine can be additional motivation for a manufacturer to develop the vaccine.
While several S. aureus vaccine candidates have emerged, none have reached the market. StaphVAX®, a promising capsular bivalent polysaccharide-protein conjugate vaccine, passed an initial phase III trial evaluating safety and efficacy for the prevention of S. aureus associated bacteremia in end-stage renal disease patients undergoing chronic hemodialysis. However, when the vaccine failed to meet its primary efficacy endpoint in a second, larger phase III trial, development halted.[34] The manufacturer is currently developing a vaccine intended to confer protection against an additional capsular polysaccharide type and two toxins. Several immunoglobulin preparations are in various stages of pre-clinical and early clinical development for the prevention of staphylococcal infection and as adjunctive treatment of S. aureus bacteremia.[13, 25, 35-36]
Intercell (Vienna, Austria) in cooperation with Merck and Company (Whitehouse Station, NJ, USA) are developing V710, which is in Phase II testing in end stage renal disease patients. Nabi also is currently in the process of developing PentaStaph™, a multi-target S. aureus polysaccharide conjugate and toxoid vaccine. In addition to coverage for capsular polysaccharide types 5 and 8 that were included in the original StaphVAX® vaccine, TriStaph™ also targets type 336. These three polysaccharide conjugates have been implicated in a majority of S. aureus infections. Nabi also plans to add coverage for two toxins, one of which is associated with the severe SSTIs common to CA-MRSA infections, to the product in order to produce the PentaStaph™ vaccine.[27] The hemodialysis population is a challenging population for vaccine efficacy testing since they exhibit a suboptimal response to immunoprophylaxis and are unlikely to exhibit or maintain a substantial increase in antibody levels. Selection of a different population for a proof-of-principle study may be an important consideration for the production and efficacy testing of future vaccine candidates.[36] The neonate population shares the characteristic of compromised immunity with the dialysis study population and may pose the same challenge to vaccine development.
Of course, bringing a S. aureus vaccine to market would involve surmounting a variety of scientific hurdles. More than a decade of vaccine research and development has resulted in notable scientific advances, including the increasing availability of genomic sequences of S. aureus strains, but there is still a great deal unknown about the complex interaction between Staphylococcus aureus and the human host.[25] MRSA possesses a variety of virulence factors that complicate vaccine development, including factors influencing bacterial attachment, penetration of bacteria into tissue, and evasion of host defenses.[37-38] S. aureus has exhibited capsular variations, multiple toxins, and the ability to persist in biofilms and as small-colony variants.[39] Moreover, MRSA colonization and disease manifestation may not be the result of a single protein product.[36] The complex nature of MRSA virulence and the pathogen-host interaction makes it unlikely that a single immunologic target will be sufficient to confer protection against antibiotic resistant strains of S. aureus. In addition, many candidate vaccines have failed to eliminate MRSA and instead only been able to reduce infection severity.
While researchers have successfully conferred protection against Staphylococcus aureus in murine subjects, they have struggled to do so in humans. One possible explanation for this discrepancy is the inherent difference between human and murine immune response to S. aureus. Another observation is that some murine infection models rely on such high levels of bacteria that overwhelm the innate immune response of the host. Human infection may be due to much smaller amounts of bacteria, frequently introduced through broken skin or a medical device, that are not effectively recognized and eliminated by the immune system.[15]
In developing our model, we endeavored to remain very conservative about the benefits of a vaccine. It did not consider how the vaccine may reduce the transmission of S. aureus (e.g., herd immunity effects). Furthermore, by decreasing the incidence of S. aureus infections, a vaccine could reduce antibiotic use, which in turn could curb the development of antibiotic resistance among various pathogens. This includes curtailing the use of and resistance to MRSA decolonization regimens, such as mupirocin.[1, 13, 40]
4.2. Limitations
All mathematical and computational models are simplifications of real life and cannot account for every possible scenario that may arise from S. aureus vaccination or infection. Our model assumed that a vaccine will be safe and that administration will result in few side effects or adverse events. Safety is paramount for neonates, and regulatory bodies such as the Food and Drug Administration are unlikely to license a risky vaccine for neonates. As a result, neonates may not be the initial target population for a S. aureus vaccine. A requirement for successful completion of safety trials en route to FDA licensure is preserving neonatal safety, but it bears repeating. The data inputs (Table 1) used for this model were compiled from reports and studies of varying quality, but represent the best available approximations of these values. QALY values may not capture all the potential benefits of vaccination and illness prevention, such as obviating parental emotional pain and suffering from having an infected child, caretaker productivity costs, or S. aureus transmission to family members. Our goal was to remain conservative about the benefits of a vaccine. So, our model did not include the impact that an ill neonate would have on family members and other caretakers, which underestimates the potential value of a S. aureus vaccine.
4.3 Conclusions and Future Directions
Our results suggest that a S. aureus vaccine for the neonatal population would be strongly cost-effective (and in many situations dominant) over a wide range of vaccine efficacies, vaccine costs, and MRSA prevalence levels. The considerable potential benefits of a S. aureus vaccine supports further investment into its development. Realizing that the market may support relatively high vaccine price points could encourage more vaccine developers to pursue this area. Additionally, scientists and developers do not necessarily have to design the “perfect” vaccine that provides close to 100% protection, since even vaccines that offer low protection may be valuable. Moreover, third party payors may benefit from covering the S. aureus vaccine, even when the cost of the vaccine is fairly high. As vaccine research and development continue to evolve, emerging data from clinical trials could be used to further refine our model predictions.
Acknowledgments
Supported by the National Institute of General Medical Sciences Models of Infectious Disease Agent Study (MIDAS) through grant 5U01GM070708-05. The funder had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bal AM, Gould IM. Antibiotic resistance in Staphylococcus aureus and its relevance in therapy. Expert Opin Pharmacother. 2005 Oct;6(13):2257–69. doi: 10.1517/14656566.6.13.2257. [DOI] [PubMed] [Google Scholar]
- 2.Cunha BA, Pherez FM. Daptomycin resistance and treatment failure following vancomycin for methicillin-resistant Staphylococcus aureus (MRSA) mitral valve acute bacterial endocarditis (ABE) Eur J Clin Microbiol Infect Dis. 2009 Jul;28(7):831–3. doi: 10.1007/s10096-008-0692-2. [DOI] [PubMed] [Google Scholar]
- 3.Kirby A, Mohandas K, Broughton C, Neal TJ, Smith GW, Pai P, et al. In vivo development of heterogeneous glycopeptide-intermediate Staphylococcus aureus (hGISA), GISA and daptomycin resistance in a patient with meticillin-resistant S. aureus endocarditis. J Med Microbiol. 2009 Mar;58(Pt 3):376–80. doi: 10.1099/jmm.0.006486-0. [DOI] [PubMed] [Google Scholar]
- 4.Skiest DJ. Treatment failure resulting from resistance of Staphylococcus aureus to daptomycin. J Clin Microbiol. 2006 Feb;44(2):655–6. doi: 10.1128/JCM.44.2.655-656.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hidron AI, Edwards JR, Patel J, Horan TC, Sievert DM, Pollock DA, et al. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006-2007. Infect Control Hosp Epidemiol. 2008 Nov;29(11):996–1011. doi: 10.1086/591861. [DOI] [PubMed] [Google Scholar]
- 6.Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002 Aug;110(2 Pt 1):285–91. doi: 10.1542/peds.110.2.285. [DOI] [PubMed] [Google Scholar]
- 7.Healy CM, Palazzi DL, Edwards MS, Campbell JR, Baker CJ. Features of invasive staphylococcal disease in neonates. Pediatrics. 2004 Oct;114(4):953–61. doi: 10.1542/peds.2004-0043. [DOI] [PubMed] [Google Scholar]
- 8.Benjamin DK, Schelonka R, White R, Holley HP, Bifano E, Cummings J, et al. A blinded, randomized, multicenter study of an intravenous Staphylococcus aureus immune globulin. J Perinatol. 2006 May;26(5):290–5. doi: 10.1038/sj.jp.7211496. [DOI] [PubMed] [Google Scholar]
- 9.Carey AJ, Saiman L, Polin RA. Hospital-acquired infections in the NICU: epidemiology for the new millennium. Clin Perinatol. 2008 Mar;35(1):223–49. x. doi: 10.1016/j.clp.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 10.Harbarth S, Sax H, Fankhauser-Rodriguez C, Schrenzel J, Agostinho A, Pittet D. Evaluating the probability of previously unknown carriage of MRSA at hospital admission. Am J Med. 2006 Mar;119(3):275 e15–23. doi: 10.1016/j.amjmed.2005.04.042. [DOI] [PubMed] [Google Scholar]
- 11.Lepelletier D, Corvec S, Caillon J, Reynaud A, Roze JC, Gras-Leguen C. Eradication of methicillin-resistant Staphylococcus aureus in a neonatal intensive care unit: which measures for which success? Am J Infect Control. 2009 Apr;37(3):195–200. doi: 10.1016/j.ajic.2008.09.024. [DOI] [PubMed] [Google Scholar]
- 12.Gregory ML, Eichenwald EC, Puopolo KM. Seven-year experience with a surveillance program to reduce methicillin-resistant Staphylococcus aureus colonization in a neonatal intensive care unit. Pediatrics. 2009 May;123(5):e790–6. doi: 10.1542/peds.2008-1526. [DOI] [PubMed] [Google Scholar]
- 13.John CC, Schreiber JR. Therapies and vaccines for emerging bacterial infections: learning from methicillin-resistant Staphylococcus aureus. Pediatr Clin North Am. 2006 Aug;53(4):699–713. doi: 10.1016/j.pcl.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 14.Beretta AL, Trabasso P, Stucchi RB, Moretti ML. Use of molecular epidemiology to monitor the nosocomial dissemination of methicillin-resistant Staphylococcus aureus in a university hospital from 1991 to 2001. Braz J Med Biol Res. 2004 Sep;37(9):1345–51. doi: 10.1590/s0100-879x2004000900009. [DOI] [PubMed] [Google Scholar]
- 15.Lindsay JA. Prospects for a MRSA vaccine. Future Microbiol. 2007 Feb;2:1–3. doi: 10.2217/17460913.2.1.1. [DOI] [PubMed] [Google Scholar]
- 16.Andersen BM, Lindemann R, Bergh K, Nesheim BI, Syversen G, Solheim N, et al. Spread of methicillin-resistant Staphylococcus aureus in a neonatal intensive unit associated with understaffing, overcrowding and mixing of patients. J Hosp Infect. 2002 Jan;50(1):18–24. doi: 10.1053/jhin.2001.1128. [DOI] [PubMed] [Google Scholar]
- 17.Weeks JL, Garcia-Prats JA, Baker CJ. Methicillin-resistant Staphylococcus aureus osteomyelitis in a neonate. JAMA. 1981 Apr 24;245(16):1662–4. [PubMed] [Google Scholar]
- 18.Saiman L, Cronquist A, Wu F, Zhou J, Rubenstein D, Eisner W, et al. An outbreak of methicillin-resistant Staphylococcus aureus in a neonatal intensive care unit. Infect Control Hosp Epidemiol. 2003 May;24(5):317–21. doi: 10.1086/502217. [DOI] [PubMed] [Google Scholar]
- 19.Khoury J, Jones M, Grim A, Dunne WM, Jr, Fraser V. Eradication of methicillin-resistant Staphylococcus aureus from a neonatal intensive care unit by active surveillance and aggressive infection control measures. Infect Control Hosp Epidemiol. 2005 Jul;26(7):616–21. doi: 10.1086/502590. [DOI] [PubMed] [Google Scholar]
- 20.Chuang YY, Huang YC, Lee CY, Lin TY, Lien R, Chou YH. Methicillin-resistant Staphylococcus aureus bacteraemia in neonatal intensive care units: an analysis of 90 episodes. Acta Paediatr. 2004 Jun;93(6):786–90. doi: 10.1080/08035250410028084. [DOI] [PubMed] [Google Scholar]
- 21.DeJonge M, Burchfield D, Bloom B, Duenas M, Walker W, Polak M, et al. Clinical trial of safety and efficacy of INH-A21 for the prevention of nosocomial staphylococcal bloodstream infection in premature infants. J Pediatr. 2007 Sep;151(3):260–5. 5 e1. doi: 10.1016/j.jpeds.2007.04.060. [DOI] [PubMed] [Google Scholar]
- 22.Wilmoth JR, Shkolnikov V. Human Mortality Database. 2008 doi: 10.1093/ije/dyv105. [updated January 21, 2008]; Available from: www.humanmortality.de, www.mortality.org. [DOI] [PMC free article] [PubMed]
- 23.Tengs TO, Wallace A. One thousand health-related quality-of-life estimates. Med Care. 2000 Jun;38(6):583–637. doi: 10.1097/00005650-200006000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Selai C, Rosser R. Eliciting EuroQol descriptive data and utility scale values from inpatients. A feasibility study. Pharmacoeconomics. 1995 Aug;8(2):147–58. doi: 10.2165/00019053-199508020-00006. [DOI] [PubMed] [Google Scholar]
- 25.Deresinski S. Antistaphylococcal vaccines and immunoglobulins: current status and future prospects. Drugs. 2006;66(14):1797–806. doi: 10.2165/00003495-200666140-00002. [DOI] [PubMed] [Google Scholar]
- 26.Weisman LE, Thackray HM, Garcia-Prats JA, Nesin M, Schneider JH, Fretz J, et al. Phase 1/2 double-blind, placebo-controlled, dose escalation, safety, and pharmacokinetic study of pagibaximab (BSYX-A110), an antistaphylococcal monoclonal antibody for the prevention of staphylococcal bloodstream infections, in very-low-birth-weight neonates. Antimicrob Agents Chemother. 2009 Jul;53(7):2879–86. doi: 10.1128/AAC.01565-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith KJ, Roberts MS. Cost-effectiveness of newer treatment strategies for influenza. Am J Med. 2002 Sep;113(4):300–7. doi: 10.1016/s0002-9343(02)01222-6. [DOI] [PubMed] [Google Scholar]
- 28.Sackett DL, Torrance GW. The utility of different health states as perceived by the general public. J Chronic Dis. 1978;31(11):697–704. doi: 10.1016/0021-9681(78)90072-3. [DOI] [PubMed] [Google Scholar]
- 29.Gold MR, Franks P, McCoy KI, Fryback DG. Toward consistency in cost-utility analyses: using national measures to create condition-specific values. Med Care. 1998 Jun;36(6):778–92. doi: 10.1097/00005650-199806000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Control CfD. Vitalstat Birth Weight 2005. Atlanta: CDC; 2005. [updated 9/10/2008; cited 2008 9/10/2008]; 2005:[CDC National Center for Health Statistics Birth weight 2005]. Available from: http://www.cdc.gov/nchs/datawh/vitalstats/VitalStatsbirths.htm. [Google Scholar]
- 31.Braithwaite RS, Meltzer DO, King JT, Jr, Leslie D, Roberts MS. What does the value of modern medicine say about the $50,000 per quality-adjusted life-year decision rule? Med Care. 2008 Apr;46(4):349–56. doi: 10.1097/MLR.0b013e31815c31a7. [DOI] [PubMed] [Google Scholar]
- 32.Laupacis A, Feeny D, Detsky AS, Tugwell PX. How attractive does a new technology have to be to warrant adoption and utilization? Tentative guidelines for using clinical and economic evaluations. CMAJ. 1992 Feb 15;146(4):473–81. [PMC free article] [PubMed] [Google Scholar]
- 33.Lessa FC, Edwards JR, Fridkin SK, Tenover FC, Horan TC, Gorwitz RJ. Trends in incidence of late-onset methicillin-resistant Staphylococcus aureus infection in neonatal intensive care units: data from the National Nosocomial Infections Surveillance System, 1995-2004. Pediatr Infect Dis J. 2009 Jul;28(7):577–81. doi: 10.1097/INF.0b013e31819988bf. [DOI] [PubMed] [Google Scholar]
- 34.Shinefield H, Black S, Fattom A, Horwith G, Rasgon S, Ordonez J, et al. Use of a Staphylococcus aureus conjugate vaccine in patients receiving hemodialysis. N Engl J Med. 2002 Feb 14;346(7):491–6. doi: 10.1056/NEJMoa011297. [DOI] [PubMed] [Google Scholar]
- 35.Patti JM. Vaccines and immunotherapy for staphylococcal infections. Int J Artif Organs. 2005 Nov;28(11):1157–62. doi: 10.1177/039139880502801113. [DOI] [PubMed] [Google Scholar]
- 36.Projan SJ, Nesin M, Dunman PM. Staphylococcal vaccines and immunotherapy: to dream the impossible dream? Curr Opin Pharmacol. 2006 Oct;6(5):473–9. doi: 10.1016/j.coph.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 37.Shinefield HR, Black S. Prospects for active and passive immunization against Staphylococcus aureus. Pediatr Infect Dis J. 2006 Feb;25(2):167–8. doi: 10.1097/01.inf.0000199887.18267.9a. [DOI] [PubMed] [Google Scholar]
- 38.Shinefield HR. Use of a conjugate polysaccharide vaccine in the prevention of invasive staphylococcal disease: is an additional vaccine needed or possible? Vaccine. 2006 Apr 12;24 2:S2-65–9. doi: 10.1016/j.vaccine.2005.01.126. [DOI] [PubMed] [Google Scholar]
- 39.Schaffer AC, Lee JC. Vaccination and passive immunisation against Staphylococcus aureus. Int J Antimicrob Agents. 2008 Nov;32 1:S71–8. doi: 10.1016/j.ijantimicag.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 40.Gaudreau MC, Lacasse P, Talbot BG. Protective immune responses to a multi-gene DNA vaccine against Staphylococcus aureus. Vaccine. 2007 Jan 15;25(5):814–24. doi: 10.1016/j.vaccine.2006.09.043. [DOI] [PubMed] [Google Scholar]
- 41.Common Procedural Terminology (CPT) Code and Relative Value Search. 2009 [cited 2009 July 1]; Available from: https://catalog.ama-assn.org/Catalog/cpt/cpt_search.jsp.
- 42.Frenzen PD. Economic cost of Guillain-Barre syndrome in the United States. Neurology. 2008 Jul 1;71(1):21–7. doi: 10.1212/01.wnl.0000316393.54258.d1. [DOI] [PubMed] [Google Scholar]
- 43.PDR, editor. Red book 2009. 2009. Montvale, N.J.: Thompson Healthcare Inc.; 2009. [Google Scholar]
- 44.Levit K, R K. HCUP Facts and Figures, 2006: Statistics HCUP Kids' Inpatient Database. Rockville, MD: Agency for Healthcare Research and Quality; 2006. [PubMed] [Google Scholar]
- 45.Drews MB, Ludwig AC, Leititis JU, Daschner FD. Low birth weight and nosocomial infection of neonates in a neonatal intensive care unit. J Hosp Infect. 1995 May;30(1):65–72. doi: 10.1016/0195-6701(95)90250-3. [DOI] [PubMed] [Google Scholar]
- 46.Fortunov RM, Hulten KG, Hammerman WA, Mason EO, Jr, Kaplan SL. Evaluation and treatment of community-acquired Staphylococcus aureus infections in term and late-preterm previously healthy neonates. Pediatrics. 2007 Nov;120(5):937–45. doi: 10.1542/peds.2007-0956. [DOI] [PubMed] [Google Scholar]
- 47.Carey AJ, Duchon J, Della-Latta P, Saiman L. The epidemiology of methicillin-susceptible and methicillin-resistant Staphylococcus aureus in a neonatal intensive care unit, 2000-2007. J Perinatol. 2010 Feb;30(2):135–9. doi: 10.1038/jp.2009.119. [DOI] [PubMed] [Google Scholar]
- 48.van der Zwet WC, Kaiser AM, van Elburg RM, Berkhof J, Fetter WP, Parlevliet GA, et al. Nosocomial infections in a Dutch neonatal intensive care unit: surveillance study with definitions for infection specifically adapted for neonates. J Hosp Infect. 2005 Dec;61(4):300–11. doi: 10.1016/j.jhin.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 49.Sohn AH, Garrett DO, Sinkowitz-Cochran RL, Grohskopf LA, Levine GL, Stover BH, et al. Prevalence of nosocomial infections in neonatal intensive care unit patients: Results from the first national point-prevalence survey. J Pediatr. 2001 Dec;139(6):821–7. doi: 10.1067/mpd.2001.119442. [DOI] [PubMed] [Google Scholar]
- 50.Jeong IS, Jeong JS, Choi EO. Nosocomial infection in a newborn intensive care unit (NICU), South Korea. BMC Infect Dis. 2006;6:103. doi: 10.1186/1471-2334-6-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kuint J, Barzilai A, Regev-Yochay G, Rubinstein E, Keller N, Maayan-Metzger A. Comparison of community-acquired methicillin-resistant Staphylococcus aureus bacteremia to other staphylococcal species in a neonatal intensive care unit. Eur J Pediatr. 2007 Apr;166(4):319–25. doi: 10.1007/s00431-006-0238-5. [DOI] [PubMed] [Google Scholar]
- 52.Reboli AC, John JF, Jr, Levkoff AH. Epidemic methicillin-gentamicin-resistant Staphylococcus aureus in a neonatal intensive care unit. Am J Dis Child. 1989 Jan;143(1):34–9. doi: 10.1001/archpedi.1989.02150130044013. [DOI] [PubMed] [Google Scholar]
- 53.Adedeji A, Gray JW. MRSA at an English children's hospital from 1998 to 2003. Arch Dis Child. 2005 Jul;90(7):720–3. doi: 10.1136/adc.2004.065235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gerber SI, Jones RC, Scott MV, Price JS, Dworkin MS, Filippell MB, et al. Management of outbreaks of methicillin-resistant Staphylococcus aureus infection in the neonatal intensive care unit: a consensus statement. Infect Control Hosp Epidemiol. 2006 Feb;27(2):139–45. doi: 10.1086/501216. [DOI] [PubMed] [Google Scholar]
- 55.McDonald JR, Carriker CM, Pien BC, Trinh JV, Engemann JJ, Harrell LJ, et al. Methicillin-resistant Staphylococcus aureus outbreak in an intensive care nursery: potential for interinstitutional spread. Pediatr Infect Dis J. 2007 Aug;26(8):678–83. doi: 10.1097/INF.0b013e3180616ce4. [DOI] [PubMed] [Google Scholar]
- 56.Huang YC, Su LH, Wu TL, Lin TY. Molecular surveillance of clinical methicillin-resistant Staphylococcus aureus isolates in neonatal intensive care units. Infect Control Hosp Epidemiol. 2005 Feb;26(2):157–60. doi: 10.1086/502520. [DOI] [PubMed] [Google Scholar]
- 57.Isaacs D, Fraser S, Hogg G, Li HY. Staphylococcus aureus infections in Australasian neonatal nurseries. Arch Dis Child Fetal Neonatal Ed. 2004 Jul;89(4):F331–5. doi: 10.1136/adc.2002.009480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nambiar S, Herwaldt LA, Singh N. Outbreak of invasive disease caused by methicillin-resistant Staphylococcus aureus in neonates and prevalence in the neonatal intensive care unit. Pediatr Crit Care Med. 2003 Apr;4(2):220–6. doi: 10.1097/01.PCC.0000059736.20597.75. [DOI] [PubMed] [Google Scholar]
- 59.Ish-Horowicz MR, McIntyre P, Nade S. Bone and joint infections caused by multiply resistant Staphylococcus aureus in a neonatal intensive care unit. Pediatr Infect Dis J. 1992 Feb;11(2):82–7. doi: 10.1097/00006454-199202000-00005. [DOI] [PubMed] [Google Scholar]
- 60.Mitsuda T, Arai K, Fujita S, Yokota S. Epidemiological analysis of strains of methicillin-resistant Staphylococcus aureus (MRSA) infection in the nursery; prognosis of MRSA carrier infants. J Hosp Infect. 1995 Oct;31(2):123–34. doi: 10.1016/0195-6701(95)90167-1. [DOI] [PubMed] [Google Scholar]
- 61.Fortunov RM, Hulten KG, Hammerman WA, Mason EO, Jr, Kaplan SL. Community-acquired Staphylococcus aureus infections in term and near-term previously healthy neonates. Pediatrics. 2006 Sep;118(3):874–81. doi: 10.1542/peds.2006-0884. [DOI] [PubMed] [Google Scholar]