Abstract
The rodent maternal separation (MS) model is frequently used to investigate the impact of early environmental factors on adult neurobiology and behavior. The majority of MS studies assess effects in the offspring and few address the consequences of repeated pup removal in the dam. Such studies are of interest since alterations detected in offspring subjected to MS may, at least in part, be mediated by variations in maternal behavior and the amount of maternal care provided by the dam. The aim of this study was to investigate how daily short (15 min; MS15) and prolonged (360 min; MS360) periods of MS affects the dam by examining postpartum behavioral profiles using the multivariate concentric square field™ (MCSF) test. The dams were tested on postpartum days 24–25, i.e., just after the end of the separation period and weaning. The results reveal a lower exploratory drive and lower risk-assessment behavior in MS15 dams relative to MS360 or animal facility reared dams. The present results contrast some of the previously reported findings and provide new information about early post-weaning behavioral characteristics in a multivariate setting. Plausible explanations for the results are provided including a discussion how the present results fit into the maternal mediation hypothesis.
Keywords: handling, maternal deprivation, animal facility rearing, non-handling, stress, multivariate concentric square field™ test, principal component analysis, trend analysis
Introduction
Together with the genetic makeup, the early life environment programs the development of neurobiobehavioral mechanisms and establishment of mental functions. In humans, adverse experiences early in life can alter brain development and result in enhanced vulnerability for adult psychopathology including depression and substance use disorders (e.g., Sinha, 2008; Loman and Gunnar, 2010). The neurobiological events mediating the effects are not fully understood and to further study these mechanisms the rodent maternal separation (MS) model is frequently used. Numerous studies have reported acute and long-term consequences of environmental influences during the postnatal period in the offspring (Ladd et al., 2000; Lehmann and Feldon, 2000; Pryce and Feldon, 2003; Roman and Nylander, 2005; Moffett et al., 2007). It has been suggested that these MS-induced alterations are mediated, at least in part, by the maternal behavior (Macri and Würbel, 2006). However, fewer investigations have addressed how the repeated removal of the pups during the MS procedure affects the dam and the results from these studies are not conclusive (Kalinichev et al., 2000, 2003; Boccia et al., 2007; Eklund et al., 2009; Maniam and Morris, 2010) Furthermore, there is an apparent lack of knowledge with regard to effects on the dam immediately after the MS period.
Many conventional behavior tests for rodents offer a limited choice of activities for the animal and thereby limited opportunities for more extended analyses of the various processes that presumably interact to establish complex behavioral traits and mental states. The multivariate concentric square field™ (MCSF) test is designed to include opportunity for exploration, risk assessment, risk taking, shelter seeking, and approach and avoidance behavior in rodents. Unlike many traditional tests the MCSF test is not designed to provide information relevant for a particular mental condition, e.g., anxiety. Instead, the test situation involves a free choice of different environmental settings and items that provide the opportunity to detect essential features of the animal's mentality. In this way a behavioral profile is generated in one and the same test situation (Meyerson et al., 2006; Roman et al., 2006, 2007; Roman and Colombo, 2009). In a battery combining the MCSF, open field and elevated plus maze tests, the MCSF test was found to be the most sensitive to previous experience and should be performed as the first test in order to eliminate the risk of carry over effects (Augustsson, 2004). Furthermore, the multivariate design of the MCSF generates more information than the open field and elevated plus maze tests, alone or in combination (Augustsson, 2004; Roman et al., 2007; Roman and Colombo, 2009), which suggests that the MCSF can be used as the sole test.
In previous studies we have established a MS protocol including short (15 min; MS15) and prolonged (360 min; MS360) periods of daily MS and demonstrated short- and long-term alterations in neurobiology and voluntary ethanol intake in rats subjected to the different rearing conditions (Roman and Nylander, 2005; Gustafsson, 2007; Oreland, 2009). In these MS studies, the MCSF test was utilized to characterize behavioral profiles in the offspring previously subjected to MS15 and MS360 (Roman et al., 2006). The aim of this study was to extend the use of the MCSF test to investigate how daily MS15 and MS360, respectively, affect the dams by examining post-weaning behavioral profiles (Meyerson et al., 2006; Roman and Colombo, 2009).
Materials and Methods
Animals
A total number of 18 Wistar dams were used. The litters of the dams were subjected to daily MS15, MS360 or standard animal facility rearing (AFR) during postpartum day 1–21 according to a protocol described in detail elsewhere (Roman and Nylander, 2005). Upon weaning on postpartum day 22 the dams were group housed (n = 3–4 dams/cage) in macrolon cages (59 cm × 38 cm × 20 cm) containing wood-chip bedding material and paper towels in temperature-controlled (21 ± 1°C) and humidity-controlled (50 ± 10%) cabinets in an animal room on a 12-h light/dark cycle with lights on at 06.00 hours. The behavioral profiling took place on postpartum days 24–25. All animal experiments were approved by the Uppsala Animal Ethical Committee and followed the guidelines of the Swedish Legislation on Animal Experimentation (Animal Welfare Act SFS1998:56) and the European Communities Council Directive (86/609/EEC).
The multivariate concentric square field™ test
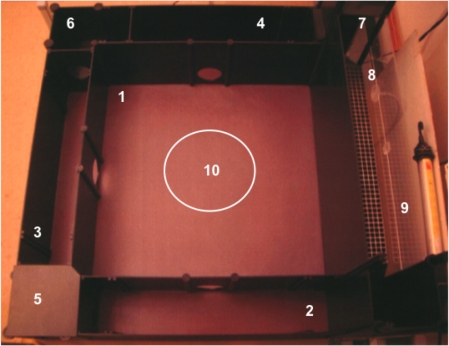
The MCSF test (Figure 1) has been described in detail elsewhere (Meyerson et al., 2006; Roman and Colombo, 2009). The entire arena is divided into zones (Figure 1), which forms the basis of the description and the variables of the animals’ performance in this test. The defined zones were: CENTER (#1), the center field of the arena; CORRIDORs (#2–4), the corridors surrounding the center field; DCR (#5), the covered room; HURDLE (#6), the high passage to a hole board with a photocell device; SLOPE (#7), the slope leading up to the BRIDGE; BRIDGE ENTRANCE (#8), the very first part of the BRIDGE where the illumination is lower and the animal can assess the risk of visiting the BRIDGE; BRIDGE (#9), the elevated and illuminated bridge construction; CENTRAL CIRCLE (#10), the circular zone (∅22 cm) in the middle of the CENTER. Visits to the defined zones were only scored as such if both hind legs had crossed over into that section. The animals were monitored with a TV-video set-up. The numbers of stretched attend postures (SAPs) from the CORRIDORs into CENTER, rearing actions, grooming actions, fecal boli, and urinations were recorded by direct observation. Manual scoring of the behavior in the MCSF test was performed using Score 3.3 (Pär Nyström, Copyright Soldis, Uppsala, Sweden). The latency (LAT, s) of first visiting a zone, frequency (FRQ) of visits, and duration (DUR, s) of time spent in a certain zone were all registered. The mean duration per visit to a zone (DUR/FRQ, s) and the percentage duration spent in each zone were calculated. The sum of frequencies to CORRIDORs A–C (FRQ TOTCORR) and to all zones (TOTACT) was used for assessment of general locomotor activity. The total time spent in CORRIDORs A–C was given the denomination DUR TOTCORR. An operational categorization of the various parameters generated from the MCSF with regard to function (i.e., general activity, exploration, risk assessment, risk taking, and shelter seeking) is used in the interpretation of results (Augustsson, 2004; Meyerson et al., 2006; Roman and Colombo, 2009).
Figure 1.
The MCSF test. The numbers indicate the defined zones CENTER (#1), CORRIDORs (#2–4), dark corner room (DCR; #5), HURDLE (#6), SLOPE (#7), BRIDGE ENTRANCE (#8), BRIDGE (#9), and CENTRAL CIRCLE (#10).
Experimental procedure
The dams in the MS15, MS360, and AFR groups (n = 6/group) were tested in a single trial in the MCSF test. The animal to be tested was transferred in a bucket from the home cage to the MCSF apparatus and released in the CENTER field (Figure 1, #1) facing the wall without openings. Animals from the different groups were alternated during testing. The MCSF test was performed in a room separate from the housing room, with a masking background noise. The test sessions lasted 20 min. Dimmed light was used during the testing, except for the BRIDGE area. The approximate light conditions (lx) in the MCSF arena were as follows: DCR: 0; CENTER, CORRIDORS and HURDLE: <10; BRIDGE: 600–650. After each test, the floor was wiped with a cloth containing 10% ethanol solution and sufficient time was allowed for the floor to dry before the next animal was placed in the arena.
Statistical analysis
The data was not normally distributed, and non-parametric statistics were used. Besides analyzing each MCSF parameter, a trend analysis was used. In the trend analysis, the individuals are ranked against each other and the rank values for each parameter are summed into a sum rank for each functional category. For all statistical analyses, the Kruskal–Wallis test was used to compare the performance of MS15, MS360, and AFR dams. When significant differences were detected, further pair-wise comparisons were conducted using the Mann–Whitney U-test. Statistica 8.0 (StatSoft Inc., Tulsa, OK, USA) was used for the statistical analyses. Differences were considered statistically significant at p ≤ 0.05. In addition to the conventional statistical analyses, a principal component analysis (PCA, Jackson, 2003; Eriksson et al., 2006) was performed in order to illustrate the relationship between MS15, MS360, and AFR dams. The PCA is a multivariate projection-based approach designed to extract and display the systemic variation in a data set. The most important use of PCA is to obtain an overview of the data, e.g., groups of observations, trends and outliers, and also to uncover the relationships between observations and variables, and among the variables themselves. The PCA creates a score plot showing a summary of the relationship between the individuals, and a loading plot identifying variables important for creating these relationships, i.e., parameters recorded in the MCSF. The direction of the score plot corresponds to the direction in the loading plot. The use of PCA on data generated in the MCSF test has been described in detail elsewhere (e.g., Meyerson et al., 2006; Roman et al., 2007; Roman and Colombo, 2009). The SIMCA-P+ software version 12.0 (Umetrics AB, Umeå, Sweden) was used.
Results
The results from the 20-min trial in the MCSF test revealed no or minor differences in general activity, exploration, risk-taking and shelter-seeking behavior between MS15, MS360, and AFR dams. With regard to risk-assessment behavior (Table 1) differences between the groups were revealed for the number of visits to the SLOPE (H = 8.08, p < 0.05) and the BRIDGE ENTRANCE (H = 7.35, p < 0.05), time spent on the SLOPE (H = 7.68, p < 0.05) and duration per visit on the BRIDGE ENTRANCE (H = 6.74, p < 0.05). The MS15 dams made fewer visits to the SLOPE (Z = 3.00; p < 0.01) and to the BRIDGE ENTRANCE (Z = 2.71; p < 0.01) and spent less time on the SLOPE (Z = 2.88; p < 0.01) than the AFR dams. Finally, the MS15 dams spent significantly shorter time per visit on the BRIDGE ENRANCE (Z = −2.40; p < 0.05) than the MS360 dams.
Table 1.
Behavioral parameters recorded during the 20-min trial of the MCSF test in post-weaning MS15, MS360, and AFR dams (n = 6/group).
| Functional categories | Parameters | MS15 | AFR | MS360 |
|---|---|---|---|---|
| General activity | TOTACT | 94.5 ± 9.7 | 103.0 ± 2.9 | 97.3 ± 7.5 |
| FRQ TOTCORR | 29.5 ± 2.8 | 33.2 ± 1.0 | 33.2 ± 2.5 | |
| FRQ CENTER | 21.2 ± 4.2 | 20.5 ± 1.5 | 19.8 ± 1.7 | |
| DUR CENTER | 224.4 ± 29.7 | 174.8 ± 10.0 | 182.0 ± 8.4 | |
| DUR/FRQ CENTER | 12.1 ± 1.6 | 8.7 ± 0.6 | 9.5 ± 0.9 | |
| Exploratory activity | LAT LEAVE CENTER | 17.9 ± 7.2 | 15.5 ± 3.9 | 30.2 ± 10.6 |
| DUR TOTCORR | 438.4 ± 22.3 | 383.3 ± 12.5 | 453.7 ± 53.2 | |
| DUR/FRQ TOTCORR | 15.8 ± 2.3 | 11.6 ± 0.5 | 14.1 ± 2.0 | |
| FRQ HURDLE | 8.2 ± 0.9 | 7.5 ± 0.4 | 8.0 ± 0.8 | |
| DUR HURDLE | 134.2 ± 19.8 | 138.6 ± 5.5 | 105.1 ± 11.0 | |
| DUR/FRQ HURDLE | 16.6 ± 1.7 | 18.7 ± 1.2 | 13.7 ± 1.9 | |
| HEAD DIPS HURDLE | 2.8 ± 1.2 | 5.3 ± 0.8 | 4.3 ± 1.9 | |
| REARING | 70.8 ± 6.4 | 75.5 ± 2.3 | 74.3 ± 6.6 | |
| Risk assessment | LAT SLOPE | 120.3 ± 29.3 | 73.5 ± 32.0 | 134.1 ± 35.5 |
| FRQ SLOPE | 8.5 ± 0.7** | 11.2 ± 0.2 | 9.3 ± 0.8 | |
| DUR SLOPE | 52.9 ± 5.5** | 91.8 ± 9.0 | 78.4 ± 12.0 | |
| DUR/FRQ SLOPE | 6.3 ± 0.6 | 8.2 ± 0.7 | 8.3 ± 0.9 | |
| LAT BRIDGE ENTRANCE | 134.9 ± 29.5 | 100.6 ± 28.4 | 156.9 ± 32.6 | |
| FRQ BRIDGE ENTRANCE | 8.7 ± 0.7** | 11.5 ± 0.22 | 8.7 ± 1.1 | |
| DUR BRIDGE ENTRANCE | 38.8 ± 6.0 | 65.2 ± 7.5 | 61.6 ± 8.7 | |
| DUR/FRQ BRIDGE ENTRANCE | 4.5 ± 0.6# | 5.7 ± 0.7 | 7.1 ± 0.5 | |
| SAP TO CENTER | 1.2 ± 1.0 | 0.2 ± 0.2 | 0.2 ± 0.2 | |
| Risk taking | LAT BRIDGE | 136.9 ± 29.5 | 102.4 ± 28.5 | 160.1 ± 33.0 |
| FRQ BRIDGE | 4.5 ± 0.4 | 5.7 ± 0.2 | 4.5 ± 0.7 | |
| DUR BRIDGE | 129.8 ± 9.8 | 148.1 ± 15.5 | 113.8 ± 19.9 | |
| DUR/FRQ BRIDGE | 29.4 ± 1.9 | 26.0 ± 2.3 | 25.1 ± 2.3 | |
| LAT CENTRAL CIRCLE | 397.6 ± 157.7 | 408.6 ± 144.8 | 218.7 ± 46.0 | |
| FRQ CENTRAL CIRCLE | 8.0 ± 2.0 | 6.8 ± 1.2 | 6.2 ± 1.1 | |
| DUR CENTRAL CIRCLE | 15.8 ± 4.6 | 9.3 ± 1.1 | 9.8 ± 2.8 | |
| DUR/FRQ CENTRAL CIRCLE | 1.8 ± 0.2 | 1.5 ± 0.2 | 1.5 ± 0.2 | |
| Shelter seeking | LAT DCR | 185.7 ± 51.4 | 189.4 ± 39.8 | 174.7 ± 55.9 |
| FRQ DCR | 6.0 ± 0.4 | 6.7 ± 0.4 | 7.7 ± 0.6 | |
| DUR DCR | 137.5 ± 21.8 | 157.3 ± 10.5 | 164.5 ± 16.1 | |
| DUR/FRQ DCR | 22.6 ± 2.6 | 23.9 ± 1.7 | 22.0 ± 2.4 | |
| Other | GROOMING | 0.8 ± 0.5 | 0.5 ± 0.2 | 0.5 ± 0.2 |
| URINE | 0.5 ± 0.2 | 0.3 ± 0.2 | 0.2 ± 0.2 | |
| BOLI | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.5 ± 0.3 |
Values represent mean ± SEM. **p < 0.01 compared to AFR rats; #p < 0.05 compared to MS360 rats (Kruskal–Wallis test, Mann–Whitney U-test).
CTRCI, central circle; DCR, dark corner room; DUR, duration (s); DUR/FRQ, duration per visit (s); FRQ, frequency; LAT, latency (s); SAP, stretched attend posture; TOTACT, total activity, i.e., the sum of all frequencies; TOTCORR; total corridor, i.e., the sum of all CORRIDORs. The functional interpretation has been adapted from previous studies (Augustsson, 2004; Meyerson et al., 2006; Roman and Colombo, 2009).
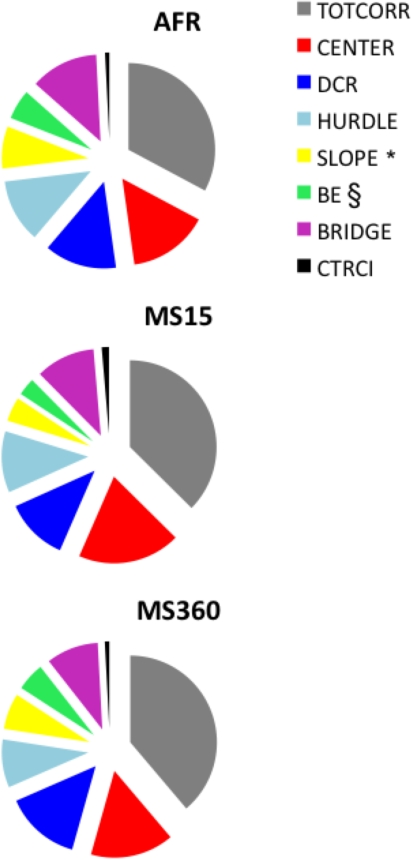
Figure 2 illustrates the percentage duration of time spent in the different zones of the MCSF and revealed that all zones were visited. A significant difference was demonstrated for the SLOPE (H = 7.68; p < 0.05) and a trend toward significance for the BRIDGE ENTRANCE (H = 5.63; p = 0.06; both areas associated with risk assessment). MS15 dams spent less amount of time on the SLOPE (Z = 2.88; p < 0.01) and BRIDGE ENTRANCE (Z = 2.08; p < 0.05) than AFR dams.
Figure 2.
The percentage of time spent in the defined zones of the MCSF test in AFR, MS15, and MS360 dams (n = 6/group). §p < 0.10, *p < 0.05 (Kruskal–Wallis test); MS15 dams spent significantly less amount of time on the SLOPE (Z = 2.88; p < 0.01) and BRIDGE ENTRANCE (Z = 2.08; p < 0.05) than AFR dams (Mann–Whitney U-test).
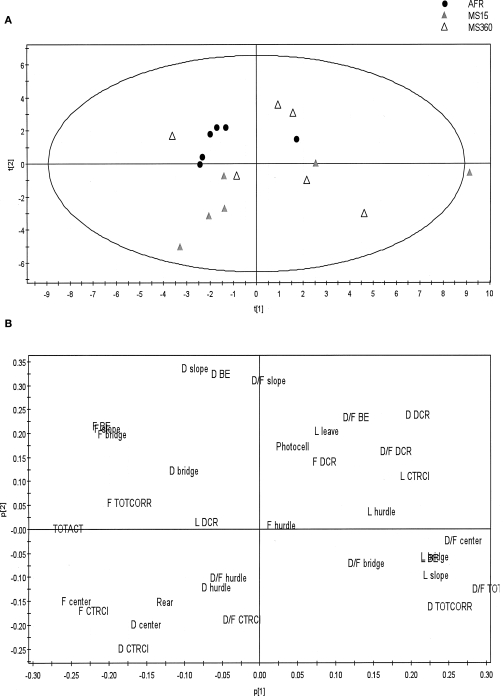
The PCA (Figure 3) illustrates and confirms the findings from the traditional statistical analysis. The PCA score plot reveals that the AFR dams were grouped close together indicating low within-group variance. The largest within-group variance was found in the MS360 group of dams. The loading plot identifies parameters included in the functional categories general activity (TOTACT and TOTCORR), risk assessment (SLOPE and BRIDGE ENTRANCE) and risk taking (BRIDGE) of importance for the grouping of AFR dams.
Figure 3.
The PCA generated from behavioral parameters recorded during the 20-min trial of the MCSF test in post-weaning MS15, MS360, and AFR dams (n = 6/group). The PCA creates a score plot (A) showing a summary of the relationship between the individuals, and a loading plot (B) identifying variables important for creating these relationships, i.e., parameters recorded in the MCSF. The direction of the score plot corresponds to the direction of the loading plot. Variables located further away from the origin in the loading plot are of larger importance to the model. The two principal components explained 47% of the variance and values of explained variation and predicted variation were within an appropriate range [R2X(cum) = 0.468 and Q2(cum) = 0.113, respectively]. BE, bridge entrance; CTRCI, central circle; DCR, dark corner room; D, duration (s); D/F, duration per visit (s); F, frequency; L, latency (s); TOTACT, total activity, i.e., the sum of all frequencies; TOTCORR; total corridor, i.e., the sum of all corridors.
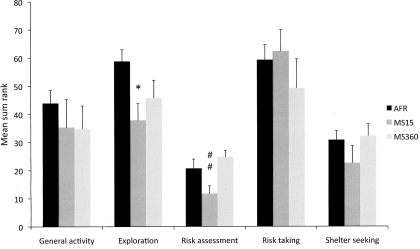
The results from the trend analysis are shown in Figure 4. MS15 dams were found to be significantly less explorative than AFR dams and showed significantly less risk-assessment behavior compared to MS360 dams. General activity, risk taking and shelter seeking did not differ between the groups.
Figure 4.
The trend analysis in AFR, MS15 and MS360 dams (n = 6/group). In the trend analysis, the individual rank values for the parameters included in the functional categories general activity (TOTAL ACTIVITY, FRQ TOTCORR and CENTER, and DUR/FRQ TOTCORR), exploratory activity (DUR TOTCORR, CENTER and HUDLE, REARING, and number of PHOTOCELL COUNTS in the hole board), risk assessment (SAP to CENTER, and DUR/FRQ SLOPE and BRIDGE ENTRANCE), risk-taking behavior (FRQ BRIDGE and CENTRAL CIRCLE, DUR BRIDGE and CENTRAL CIRCLE, and DUR/FRQ BRIDGE and CENTRAL CIRCLE) and shelter-seeking behavior (FRQ, DUR, and DUR/FRQ DCR) are summed. *p < 0.05 compared to AFR dams; ##p < 0.01 compared to MS360 dams (Kruskal–Wallis, Mann–Whitney U-test).
Discussion
The impact of different MS procedures on behavior in dams after weaning is scarcely investigated. So far no consistent evidence exist, which may be due to factors including variations in maternal care, different MS protocols, behavioral tests used and postpartum time points for testing the dams, as well as contrasting reference groups (i.e., non-handled or AFR rats). Research on the postpartum behavior at the time point evaluated in the present study, i.e., just after the end of the separation period and weaning, in dams reared under MS conditions is sparse. Previous studies, using various prolonged MS procedures, have indicated acute (Maniam and Morris, 2010) and long-term (Kalinichev et al., 2000, 2003; Boccia et al., 2007; Eklund et al., 2009; Maniam and Morris, 2010) effects that were interpreted as elevations in anxiety- and depression-like behavior in dams. The advantage of a behavioral profiling of the animals using an ethologically founded, multivariate approach unprejudiced with regard to mental conditions, i.e., the MCSF, is here presented. The MCSF has been validated with regard to areas associated with risk and safety, respectively. Lactating dams retrieve their pups from the hypothesized risk area, i.e., the bridge, to a sheltered area, the DCR, but do not move the pups out of the sheltered area. Similarly, food pellets are hoarded from the risk area and consumed in the sheltered area (Meyerson et al., 2006). Furthermore, preliminary results indicate that benzodiazepines have effects at different doses in this multivariate setting compared to those reported in the literature using more conventional tests (Roman and Meyerson, 2007). Anxiety-like behavior in the MCSF test is usually interpreted based on the relationship between risk-taking and shelter-seeking behavior, i.e., low risk taking and high shelter seeking would be interpreted as higher anxiety-like behavior (Roman et al., 2007; Roman and Colombo, 2009), keeping in mind the difference between behavior and mental states. Thus, applying this interpretation there was no evidence for anxiety-like behavior in any of the experimental groups in the present experiment. Besides the use of different behavioral tests, also the different time points for behavioral assessment may explain these contrasting results. In the present study the behavior of dams was investigated just after weaning while the majority of the previous studies report long-term impact of MS on behavior in dams assessed several weeks after weaning. The consequences of factors such as varying laboratory conditions and animal housing are well described (Crabbe et al., 1999) and may result in larger variation in dam behavior when investigated in a long-term perspective. The immediate consequence of exposure to different environmental conditions could thereby be lost. The results presented herein demonstrate that MS15 dams are less explorative and less risk assessing than MS360 and AFR dams in a multivariate setting when tested on postpartum days 24–25, i.e., 2–3 days post weaning. The differences between the groups in risk assessment are interesting from a risk and gain point of view. Taking into account that risk assessment is influenced by exploratory drive, this implies that the risk/benefit assessment (Blanchard and Blanchard, 1988; Lima and Dill, 1990) is altered in MS15 dams relative to the other groups. Risk assessment and risk-taking behaviors are central traits in the behavioral repertoire of the rat. Impaired risk assessment has some relevance for aspects of impulsivity-like behavior and can be a serious disadvantage to survival, but an over expressed risk assessment can also be detrimental (Lima and Dill, 1990).
The observed behavioral differences in the dam may either be caused by absence of the pups during a time period when pup-dam interactions are vital or be a result of an altered behavior in the pups due to repeated MS. Both these scenarios could by themselves or in combination induce endocrinological and biological responses in the dam that may linger and affect her behavior also beyond the MS period (e.g., Macri and Würbel, 2006; Eklund et al., 2009; Maniam and Morris, 2010). Another possibility is that the previous experience of either short or prolonged absence of the pups during the separations results in altered sensitivity to later life events or challenges and thereby different responses in the dams when tested at weaning. The maternal behavior may thus depend on the different repeated disturbances in maternal contact with the litter, i.e., the MS procedures. The complex and flexible nature of pup-dam interactions in response to environmental disturbances, which partially is under genetic control, is well established but not fully understood. There is for instance still no consensus as to the effects of different MS procedures on maternal care. Generally, short periods of MS result in increased maternal care upon reunion. No conclusive data are available on the effects of prolonged periods of MS on maternal care. Recent evidence indicates that prolonged periods of MS induce increased levels of active maternal care, of the same magnitude as that induced by short periods of MS (Macri and Würbel, 2006). The effects observed in offspring subjected to MS may be mediated, at least in part, by transmission of information from the dam to her offspring (Macri and Würbel, 2006). However, the hypothesis that the maternal behavior is mediating the effects observed after different environmental rearing conditions has been questioned. Accumulating evidence indicates that many of the effects observed after different postnatal manipulations cannot be accounted for via altered maternal care (Pryce and Feldon, 2003; Millstein and Holmes, 2007).
Several MS protocols are currently in use and the results are sometimes inconsistent (Lehmann and Feldon, 2000; Pryce and Feldon, 2003; Roman and Nylander, 2005; Moffett et al., 2007). We have focused on one protocol, i.e., litter-wise MS15, MS360, and AFR as used herein, and demonstrated short- and long-term neurochemical alterations and effects on voluntary ethanol intake and behavior in male offspring while less or no effects were found in female offspring (Roman and Nylander, 2005; Roman et al., 2006; Gustafsson, 2007; Oreland, 2009). If the maternal behavior was mediating the effects observed after MS one would expect similar outcomes in males and females. However, the level of maternal care provided by the dam has been suggested to impact on the sex-dependent outcomes (Moore and Morelli, 1979), although conflicting results have been reported (Champagne et al., 2003). In our paradigm, MS360 is hypothesized to exert a more adverse environment than MS15, which to a larger extent resembles the condition in the naturalistic environment (Calhoun, 1962; Roman and Nylander, 2005). The PCA reveals that the highest variance was found among MS360 dams. This finding may explain the high variance among male MS360 offspring, which for instance has suggested the presence of subgroups such as responder and non-responder rats (Roman and Nylander, 2005; Gustafsson, 2007; Oreland, 2009).
The question about the proper reference group in MS studies is a matter of debate. The non-handled and AFR conditions, i.e., two commonly used groups for comparisons, have been suggested to be artificial since they both provide little external stimuli to the dam and her offspring, contradictory to the naturalistic setting (Calhoun, 1962; Lehmann and Feldon, 2000; Pryce and Feldon, 2003; Macri and Würbel, 2006). Hence, it is worth noting that the “common laboratory rat” is reared according to the present AFR rats. In the present study, MS15 dams displayed a different behavioral profile than MS360 and AFR dams when investigated at postpartum days 24–25. Furthermore, as revealed by the trend analysis, similar functional outcomes were seen in MS360 and AFR dams. This novel finding in MS360 and AFR dams is in agreement with our results in male offspring (Roman and Nylander, 2005; Gustafsson, 2007; Oreland, 2009) and the results of others using prolonged periods of MS (Lehmann and Feldon, 2000; Pryce and Feldon, 2003; Moffett et al., 2007). The similar outcome between prolonged MS and AFR is well known and may be a remaining consequence of these hypothesized un-naturalistic rearing conditions.
So far animal models of resilience are less researched compared to manipulations inducing negative outcomes (Lyons et al., 2010). With regard to resilience and vulnerability for excessive intake of drugs of abuse, compelling evidence support the fact that short periods of MS during the postnatal period have long-term consequences serving as a protective factor against high voluntary drug intake (Roman and Nylander, 2005; Moffett et al., 2007), i.e., these animals have the lowest voluntary intake of drugs of abuse. As revealed herein, MS15 dams differ from MS360 and AFR dams, which may be related to provision of resilience.
Animal experiments are crucial for the understanding of the interaction between the early life environment and the genome on the acute and long-term impact on the phenotype. It has been shown that there is dissociation in the effects of different postnatal rearing conditions on levels of maternal care during the separation period, dam behavior after weaning as well as offspring phenotype (Macri and Würbel, 2006). The present study focused on the behavior in dams previously exposed to either repeated short or prolonged loss of pup contact, i.e., the MS dams, or constant presence of pups with no option to leave the litter during the first 3 weeks post partum, the AFR dams. A protocol that previously has been used to describe consequences in the offspring was used. The results show that the experiences during the lactation period also affect the behavior in the dam after weaning The maternal behavior toward the pups during the separation period was not investigated so with the present results it was not possible to provide direct evidence for or against the maternal mediation hypothesis. The amount of maternal care provided by the dam is a function of the dam's capabilities, while levels of maternal care received by the pups are a function of factors including within-litter competition. For a better understanding of how these processes relate to the MS paradigm, to the dam's behavior after weaning, and the associated offspring phenotype further studies are now warranted.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors are grateful for the excellent technical assistance provided by Ms Marita Berg, and to Professor Bengt J Meyerson for introducing the trend analysis used herein. Funding from theSwedish Research Council K2008-62X-12588-11-3 (Ingrid Nylander), the Swedish Society for Medical Research (SSMF) and the Facias Foundation (Erika Roman) supported this study.
References
- Augustsson H. (2004). Ethoexperimental studies of behaviour in wild and laboratory mice. Risk assessment, emotional reactivity and animal welfare. Acta Univ. Agric. Sueciae Vet. 174, 7–62 10.1016/S0031-9384(04)00100-3 [DOI] [Google Scholar]
- Blanchard D. C., Blanchard R. J. (1988). Ethoexperimental approaches to the biology of emotion. Annu. Rev. Psychol. 39, 43–68 10.1146/annurev.psych.39.1.43 [DOI] [PubMed] [Google Scholar]
- Boccia M. L., Razzoli M., Vadlamudi S. P., Trumbull W., Caleffie C., Pedersen C. A. (2007). Repeated long separations from pups produce depression-like behavior in rat mothers. Psychoneuroendocrinology 32, 65–71 10.1016/j.psyneuen.2006.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun J. B. (1962). The Ecology and Sociology of the Norway Rat Bethesda: Public Health Service; (Publication No. 1008). 10.1021/i560129a032 [DOI] [Google Scholar]
- Champagne F. A., Francis D. D., Mar A., Meaney M. J. (2003). Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiol. Behav. 79, 359–371 10.1016/S0031-9384(03)00149-5 [DOI] [PubMed] [Google Scholar]
- Crabbe J. C., Wahlsten D., Dudek B. C. (1999). Genetics of mouse behavior: interactions with laboratory environment. Science 284, 1670–1672 10.1126/science.284.5420.1670 [DOI] [PubMed] [Google Scholar]
- Eklund M. B., Johansson L. M., Uvnäs-Moberg K., Arborelius L. (2009). Differential effects of repeated long and brief maternal separation on behaviour and neuroendocrine parameters in Wistar dams. Behav. Brain Res. 203, 69–75 10.1016/j.bbr.2009.04.017 [DOI] [PubMed] [Google Scholar]
- Eriksson L., Johansson E., Kettaneh-Wold N., Trygg J., Wikström C., Wold S. (2006). Multi- and Megavariate Data analysis. Part I: Basic Principles and Applications, Second revised and enlarged edition. Umeå: Umetrics AB; 10.1016/0169-7439(92)80092-I [DOI] [Google Scholar]
- Gustafsson L. (2007). “Endogenous opioids and voluntary ethanol drinking. Consequences of postnatal environmental influences in rats,” in Acta Universitatis Upsaliensis. Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Pharmacy, Vol. 51 (Uppsala: Uppsala University; ), 80 p. 10.3997/1873-0604.2006032 [DOI] [Google Scholar]
- Jackson J. E. (2003). A User's Guide to Principal Components Hoboken, NJ: Wiley-Interscience; 10.1144/gsjgs.134.3.0343 [DOI] [Google Scholar]
- Kalinichev M., Easterling K. W., Holtzman S. G. (2000). Periodic postpartum separation from the offspring results in long-lasting changes in anxiety-related behaviors and sensitivity to morphine in Long-Evans mother rats. Psychopharmacology (Berl.) 152, 431–439 10.1007/s002130000556 [DOI] [PubMed] [Google Scholar]
- Kalinichev M., Easterling K. W., Holtzman S. G. (2003). Long-lasting changes in morphine-induced locomotor sensitization and tolerance in Long-Evans mother rats as a result of periodic postpartum separation from the litter: a novel model of increased vulnerability to drug abuse? Neuropsychopharmacology 28, 317–328 10.1038/sj.npp.1300068 [DOI] [PubMed] [Google Scholar]
- Ladd C. O., Huot R. L., Thrivikraman K. V., Nemeroff C. B., Meaney M. J., Plotsky P. M. (2000). Long-term behavioral and neuroendocrine adaptations to adverse early experience. Prog. Brain Res. 122, 81–103 10.1016/S0079-6123(08)62132-9 [DOI] [PubMed] [Google Scholar]
- Lehmann J., Feldon J. (2000). Long-term biobehavioral effects of maternal separation in the rat: consistent or confusing? Rev. Neurosci. 11, 383–408 10.1016/S0166-4328(99)00122-9 [DOI] [PubMed] [Google Scholar]
- Lima S. L., Dill L. M. (1990). Behavioral decisions made under the risk of predation: a review and prospectus. Can. J. Zool. 68, 619–640 10.1139/z90-092 [DOI] [Google Scholar]
- Loman M. M., Gunnar M. R. (2010). Early experience and the development of stress reactivity and regulation in children. Neurosci. Biobehav. Rev. 34, 867–876 10.1016/j.neubiorev.2009.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons D. M., Parker K. J., Schatzberg A. F. (2010). Animal models of early life stress: implications for understanding resilience. Dev. Psychobiol. [Epub ahead of print]. 10.1002/dev.20429 [DOI] [PMC free article] [PubMed]
- Macri S., Würbel H. (2006). Developmental plasticity of HPA and fear responses in rats: a critical review of the maternal mediation hypothesis. Horm. Behav. 50, 667–680 10.1016/j.yhbeh.2006.06.015 [DOI] [PubMed] [Google Scholar]
- Maniam J., Morris M. J. (2010). Long-term postpartum anxiety and depression-like behavior in mother rats subjected to maternal separation are ameliorated by palatable high fat diet. Behav. Brain Res. 208, 72–79 10.1016/j.bbr.2009.11.005 [DOI] [PubMed] [Google Scholar]
- Meyerson B. J., Augustsson H., Berg M., Roman E. (2006). The concentric square field: a multivariate test arena for analysis of explorative strategies. Behav. Brain Res. 168, 100–113 10.1016/j.bbr.2005.10.020 [DOI] [PubMed] [Google Scholar]
- Millstein R. A., Holmes A. (2007). Effects of repeated maternal separation on anxiety- and depression-related phenotypes in different mouse strains. Neurosci. Biobehav. Rev. 31, 3–17 10.1016/j.neubiorev.2006.05.003 [DOI] [PubMed] [Google Scholar]
- Moffett M. C., Vicentic A., Kozel M., Plotsky P., Francis D. D., Kuhar M. J. (2007). Maternal separation alters drug intake patterns in adulthood in rats. Biochem. Pharmacol. 73, 321–330 10.1016/j.bcp.2006.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore C. L., Morelli G. A. (1979). Mother rats interact differently with male and female offspring. J. Comp. Physiol. Psychol. 93, 677–684 10.1037/h0077599 [DOI] [PubMed] [Google Scholar]
- Oreland S. (2009). “Maternal separation in the rat. The short- and long-term effects of early-life experience on neuropeptides, monoamines and voluntary ethanol consumption,” in Acta Universitatis Upsaliensis. Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Pharmacy, Vol. 106 (Uppsala: Uppsala University; ), 87 p. 10.3109/03009739609178921 [DOI] [Google Scholar]
- Pryce C. R., Feldon J. (2003). Long-term neurobehavioural impact of the postnatal environment in rats: manipulations, effects and mediating mechanisms. Neurosci. Biobehav. Rev. 27, 57–71 10.1016/S0149-7634(03)00009-5 [DOI] [PubMed] [Google Scholar]
- Roman E., Colombo G. (2009). Lower risk taking and exploratory behavior in alcohol-preferring sP rats than in alcohol nonpreferring sNP rats in the multivariate concentric square field™ (MCSF) test. Behav. Brain Res. 205, 249–258 10.1016/j.bbr.2009.08.020 [DOI] [PubMed] [Google Scholar]
- Roman E., Gustafsson L., Berg M., Nylander I. (2006). Behavioral profiles and stress-induced corticosteroid secretion in male Wistar rats subjected to short and prolonged periods of maternal separation. Horm. Behav. 50, 736–747 10.1016/j.yhbeh.2006.06.016 [DOI] [PubMed] [Google Scholar]
- Roman E., Meyerson B. J. (2007). The multivariate concentric square field test – behavioural profiles after pre-treatment with diazepam. Behav Pharmacol 18(Suppl. 1), S45. 10.1016/j.bbr.2007.06.009 [DOI] [Google Scholar]
- Roman E., Meyerson B. J., Hyytiä P., Nylander I. (2007). The multivariate concentric square field test reveals different behavioural profiles in male AA and ANA rats with regard to risk taking and environmental reactivity. Behav. Brain Res. 183, 195–205 10.1016/j.bbr.2007.06.009 [DOI] [PubMed] [Google Scholar]
- Roman E., Nylander I. (2005). The impact of emotional stress early in life on adult voluntary ethanol intake – results of maternal separation in rats. Stress 8, 157–174 10.1080/10253890500188666 [DOI] [PubMed] [Google Scholar]
- Sinha R. (2008). Chronic stress, drug use, and vulnerability to addiction. Ann. N. Y. Acad. Sci. 1141, 105–130 10.1196/annals.1441.030 [DOI] [PMC free article] [PubMed] [Google Scholar]