Abstract
Objective
Adiponectin is an adipocyte-derived, secreted protein that is implicated in the protection against a cluster of related metabolic disorders. Mice lacking adiponectin display impaired hepatic insulin sensitivity and respond only partially to PPARγ agonists. Adiponectin has been associated with anti-inflammatory and anti-atherogenic properties, however, the direct involvement of adiponectin on the atherogenic process has not been studied.
Methods and Results
We crossed adiponectin knockout mice (Adn−/−) or mice with chronically elevated adiponectin levels (AdnTg) into the low-density lipoprotein receptor null (Ldlr−/−) and the apoliprotein E null (Apoe−/−) mouse models. Adiponectin levels did not correlate with a suppression of the atherogenic process. Plaque volume in the aortic root, cholesterol accumulation in the aorta and plaque morphology under various dietary conditions were not affected by circulating adiponectin levels. In light of the strong associations reported for adiponectin with cardiovascular disease in humans, the lack of a phenotype in gain- and loss-of-function studies in mice may suggests lack of causation for adiponectin in inhibiting the build up of atherosclerotic lesions.
Conclusion
These data indicate that the actions of adiponectin on the cardiovascular system are complex and multifaceted, with a minimal direct impact on atherosclerotic plaque formation in preclinical rodent models.
Keywords: Adiponectin, atherosclerosis, LDL receptor knockout mice
Introduction
Increasing prevalence of obesity and its association with the pathogenesis of cardiovascular disease has evoked great interest in understanding the impact of adipokines on vascular integrity, systemic inflammation and atherosclerosis.1 Adipokines are factors secreted from adipose tissue that play a key role in systemic energy homeostasis. Many of them, including leptin, resistin, retinol binding protein-4 are dysregulated in obesity 2. Similarly, pro-inflammatory cytokines tend to be upregulated in the obese state as well 3.
Adiponectin is a secreted adipokine abundantly expressed in differentiated adipocytes4. Adiponectin levels correlate negatively with BMI and enhanced inflammation within the adipose tissue 5. In humans, adiponectin levels correlate inversely with risk factors for cardiovascular disease including, visceral adiposity, hyperlipidemia and high density lipoprotein cholesterol (HDL-C) levels, underscoring a potential value of adiponectin as a biomarker6. Hotta et al. demonstrated that adiponectin levels of diabetics with coronary artery disease (CAD) are lower than those of diabetics without CAD or of non-diabetics7. In contrast, high adiponectin levels were associated with a reduced risk for myocardial infarction 8. A number of additional epidemiological studies suggest a link between adiponectin and the pathologies of cardiovascular disease, albeit no conclusions were drawn whether this relationship is correlative or causal.
Adiponectin may have anti-atherogenic and anti-inflammatory effects 9. While adiponectin is frequently measured as marker for cardiovascular disease in the clinical area, to date, only limited systematic studies have been published that address direct effects of adiponectin on atherosclerosis in pre-clinical models. We therefore embarked on a comprehensive analysis of commonly used atherosclerosis mouse models that were made adiponectin deficient or have mildly elevated levels of adiponectin by means of overexpression of an adiponectin transgene 10, 11. We exposed these mice to various diets in order to differentiate between correlative and causal effects of adiponectin. For example, we fed the mice a non-obesogenic diet intended to prevent excess adiposity or insulin resistance, yet causing significant hypercholesterolemia. Alternatively, we generated diet-induced obesity using a high fat ‘Western style’ diet. Interestingly, independently of the genetic or dietary modality we applied, we were unable to detect a prevention of atherosclerosis associated with adiponectin levels. Our observations may suggest lack of direct causation for adiponectin in protecting against the infiltration of macrophages, cholesterol deposition and necrosis within the atherosclerotic lesions.
Our studies also addressed effects of peroxisome proliferator-activated receptor γ (PPARγ). Pharmacologic activation of this nuclear receptor with a small molecule agonist is the single most potent means to induce adiponectin secretion 12. PPARγ activation improves insulin sensitivity and various cardiovascular risk factors. However, prolonged treatment with selective PPARγ agonists from the thiazolidinedione class is associated with increased adiposity and edema, and substantial concern has been raised regarding safety of these drugs 13, 14. Pro- and anti-atherogenic roles have been attributed to PPARγ activation. In fact, Thorp et al recently demonstrated that pioglitazone exposure can increase macrophage apoptosis and plaque necrosis in advanced atherosclerotic lesions 15.
In the present study, we analyzed adiponectin-dependent and independent effects of chronic PPARγ agonism on atherosclerosis in mice. We examined lesion size in adiponectin and LDL receptor deficient models upon long-term PPARγ agonist treatment. In our experimental paradigm, PPARγ activation did not reveal beneficial effects on atherosclerosis. In fact, we found a small but significant increase in average area of lesions specifically within the brachiocephalic branch of the aorta, related to the agonist treatment.
Methods
Detailed information about the materials and methods used can be found as supplementary data, available at http://atvb.ahajournals.org.
Animals and Diets
Animals were maintained in a pathogen free facility and all experimental protocols were approved by the Institutes for Animal Studies of the Albert Einstein College of Medicine and the University of Texas Southwestern Medical Center. Mice were group housed and on alternating 12-hour light and dark cycles under controlled environmental conditions (22–25°C, 40–50% humidity) with free access to food and water. Adiponectin knockout mice were generated as described previously 10 and backcrossed for at least 6 generations onto a C57BL/6J background in all studies with exception of the first study for which the mice were backcrossed 5 times. Adiponectin transgenic mice (AdnTg) were generated as described by Combs et al 11.
Results
Adiponectin deficiency does not accelerate the formation of atherosclerotic lesions in lean, LDL receptor deficient mice
The majority of our studies were performed using the low density lipoprotein receptor knockout model (Ldlr−/−) 16, 17. Adiponectin knockout mice (Adn−/−) 10 were crossed with Ldlr−/− mice to generate double knockout mice (Ldlr/Adn−/−). Male mice were fed a low-fat diet (10% kcal from fat) containing 0.15% cholesterol, a regimen that causes substantial atherosclerotic lesions within three to four months, but does not evoke metabolic complications such as obesity and insulin resistance 18. Total cholesterol levels were elevated to approximately 800 mg/dL in all groups while adiponectin levels were not affected by the dietary intervention (Supplemental Table I). After 3 months on the diet, mice were lean and had normal glucose levels. The lack of adiponectin per se had no effect on total plasma lipids and lipoprotein distribution as seen in Adn−/− knockout mice in absence of the Ldlr−/− mutation (Supplemental Figure I).
To assess the influence of adiponectin on the vasculature, we determined atherosclerosis at the aortic root and in the brachiocephalic artery (BCA). Macroscopic lesions were observed underneath most of the valve leaflets and near the aortic branches but were generally absent from the thoracic or abdominal aorta. As shown in Figure 1, the lesion area within the aortic root (Figure 1A) and the BCA (Figure 1B) was not affected by adiponectin (no drug; Ldlr−/− vs. Ldlr/Adn−/−) suggesting that adiponectin does not affect the atherogenic process. To exclude the possibility of a phenomena unique to the Ldlr−/− model, we generated apolipoprotein E deficient, adiponectin deficient double knockout mice (Apoe−/−/Adn−/−). Total cholesterol was 116 ± 17 mg/dL in wildtype vs. 143 ± 17 in Adn−/− mice when fed a high fat, high cholesterol diet. Deletion of Apoe−/− dramatically increased plasma cholesterol levels (841 ± 27 mg/dL in wildtype vs. 808 ± 60 in Adn−/−) independently of adiponectin levels. More importantly, as in the Ldlr−/− model, adiponectin deficiency had no effect on atherosclerosis in Apoe−/− mice (Supplemental Figure II).
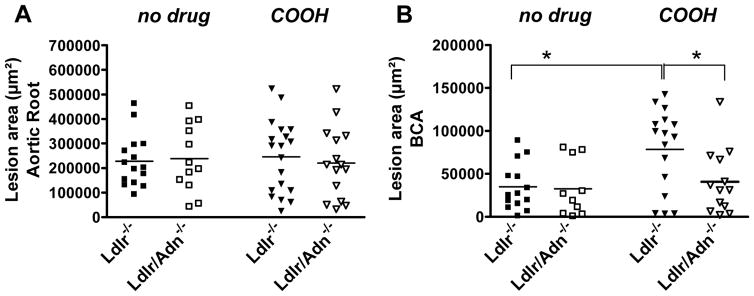
Figure 1.
Atherosclerosis in Ldlr−/− and in Ldlr/Adn−/− mice fed a non-diabetogenic diet supplemented with 0.15% cholesterol for 3 months. Mice were left untreated (no drug) or were dosed with a selective PPARγ agonist (COOH) in feed. A, Lesion area within aortic root or B, brachiocephalic artery (BCA). (* P<0.05), n = 12 to 18.
Pharmacological induction of adiponectin by PPARγ agonism does not prevent atherosclerosis in lean mice
PPARγ agonists have been evaluated in various long-term human studies for potential cardiovascular benefits with mixed results 19, 20. PPARγ activation causes an increase in plasma adiponectin levels, particularly the high molecular weight form 21. Selective PPARγ ligands of the thiazolidinedione class (troglitazone and rosiglitazone) have been shown to inhibit atherosclerosis in Ldlr−/− mice 22, 23. To assess whether these effects may be secondary to an up-regulation of adiponectin we included two parallel groups that received a PPARγ selective, non-thiazolidinedione agonist (COOH) at 30 mg/kg body weight per day in feed. As expected, COOH treatment increased plasma adiponectin levels in male mice approximately four fold over basal (Supplemental Table I). COOH has been shown previously to elicit a robust signature of hepatic PPARγ-responsive genes virtually identical to the profile obtained with rosiglitazone upon gene expression analysis on microarrays 24, 25.
Lesion area was similar within the aortic root of Ldlr−/− and Ldlr/Adn−/− mice treated with COOH (Figure 1A). Interestingly, we found a small but significant increase in average area of lesions within the BCA related to COOH treatment (Ldlr−/−; no drug vs. COOH; Figure 1B). This effect was absent in Adn−/− mice (COOH, Ldlr−/− vs. Ldlr/Adn−/−; Figure 1B) suggesting that this increase is dependent on the elevation of plasma adiponectin induced by PPARγ. Lesions within the BCA tend to be more advanced than lesions in other areas, and analysis of this region has been used mainly for qualitative purposes 26. Further investigation of the role of COOH treatment on the quality and quantity of lesions within the BCA was out of scope for this investigation and should be presented elsewhere. Lack of adiponectin per se did not affect atherosclerotic lesion area or distribution. It is important to note that under these conditions (i.e. non-obesogenic, low fat diet) COOH treatment had no effect on overall plasma glucose levels or body weight. All groups gained similar amounts of body weight (average weight gain 9.9 ± 1.3 grams) over the course of the study. COOH treatment did not affect total and HDL cholesterol levels while triglycerides were slightly elevated at the end of the study (COOH; Supplemental Table I).
Adiponectin deficiency does not promote atherosclerosis secondary to metabolic disturbances in mice
Based on our initial studies, we concluded that the manifestation of several aspects of the metabolic syndrome, i.e. insulin resistance, increased adiposity and dyslipidemia may be necessary to unravel a putative role of adiponectin in the atherogenic process. This assumption is in line with the fact that genetic ablation of adiponectin causes a mild metabolic phenotype unless challenged with a high fat diet. We hypothesized that adiponectin may act indirectly through its overall effects on metabolic and inflammatory parameters. To address this question, we exposed Ldlr/Adn−/− mice to a high caloric Western type diet (WD, 42% kcal from fat, 42% kcal from carbohydrates and 0.2% cholesterol). We included females since plasma adiponectin levels are higher in females compared to males and one can assume that the extent of lesion formation may be gender related. After 4 months on WD, all groups were obese and hyperglycemic (Supplemental Table II). Both genders developed severe hypercholesterolemia and hyper-triglyceridemia independent of their respective adiponectin levels. Lipoprotein profiles were indistinguishable between Ldlr/Adn−/− and Ldlr−/− mice (data not shown). Adiponectin levels tended to be lower at the end of the study consistent with an adiposity-associated suppression of adiponectin secretion (Supplemental Table II) 27.
Atherosclerosis was assessed as cholesterol and cholesterol ester content in the aortic wall. This lipid extraction method has been validated and correlates well with the assessment of atherosclerotic lesions by en face lipid staining or intima/media thickness 28. The measurement considers surface area as well as volume and distribution of lesions including cholesterol depositions that may be undetectable by visual methods. At the end of the study all mice had increased body weight, hyperlipidemia and elevated basal glucose levels, and there was no difference in the deposition of cholesterol in aortas of female and male Ldlr/Adn−/− and Ldlr−/− mice (Figures 2A and 2B).
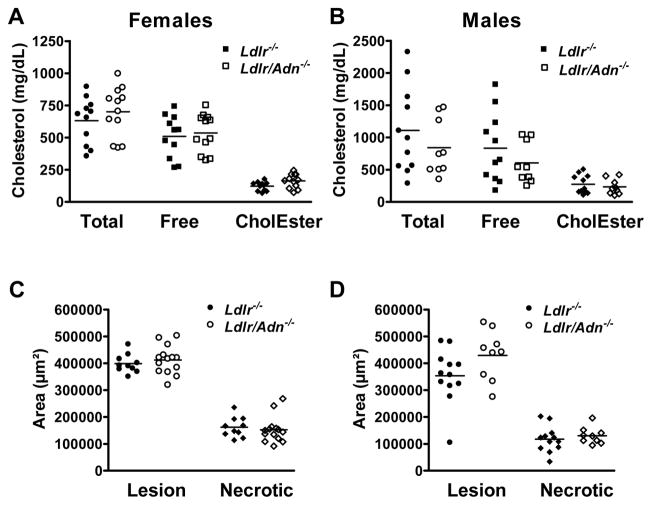
Figure 2.
Adiponectin deficiency has no impact on atherosclerosis in mice fed a WD. Total cholesterol, free cholesterol and cholesterol esters were measured from lipid extracts from aortas of Ldlr−/− and Ldlr/Adn−/− mice. A, female and B, male mice.. C, Lesion area and necrotic core area within aortic root of females. D, Lesion and necrotic core area in aortic root of males. Mean ± SEM, n = 11 to 14.
In a separate cohort, we assessed lesion area by morphological methods. We analyzed cross-sectional and necrotic lesion area in the aortic roots of both female and male mice (Figure 2C and 2D). Note that plaque necrosis is associated with vulnerable plaques and culprit lesions in humans 29. None of the measurements revealed a correlation between the adiponectin genotype and morphology of the plaques. We concluded that a) adiponectin does not directly suppress early stages of the atherogenic process, b) a role of adiponectin may be masked by compensatory mechanisms that could have evolved as a consequence of a germ line deletion of adiponectin 30, 31 or c) adiponectin may affect later stages of the atherosclerotic process, such as plaque rupture or lumenal thrombosis, which are not a component of these murine models 26.
Elevated adiponectin levels do not prevent atherosclerosis
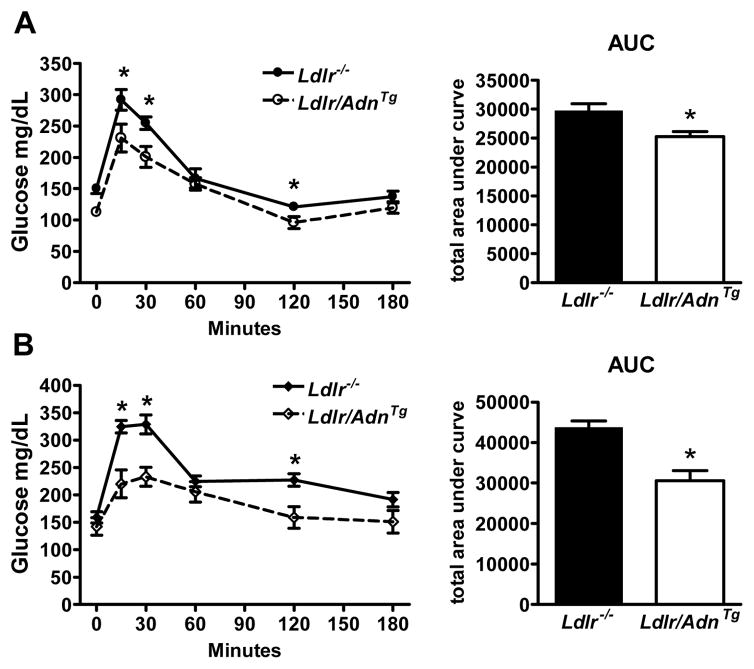
We have shown previously that adiponectin overexpression leads to a significant improvement of dyslipidemia in mice.5 The transgenic expression of an adiponectin deletion mutant (AdnTg) causes a moderate increase of steady state adiponectin concentrations in the circulation by approximately 2- to 3-fold over baseline, similar to levels achieved by activation of PPARγ5, 11. Adiponectin over-expression increases lipid clearance and lipoprotein lipase activity and improves the suppression of endogenous glucose production in the liver. We crossed AdnTg mice into the Ldlr−/− background (Ldlr/AdnTg) and determined atherosclerosis on a ‘Western style’ diet. Body weight, adiponectin, glucose levels, triglyceride and total and HDL cholesterol after 3 months on the diet are shown in Supplemental Table III. On average, males gained 16.5 ± 0.9 and females 11.4 ± 0.8 grams of weight within 4 months on WD independently of the genotype. Adiponectin levels were 3 to 3.5 fold higher in Ldlr/AdnTg compared to Ldlr−/− mice and slightly elevated in females, but not in males at the end of the study. Fasting glucose was significantly lower in Ldlr/AdnTg mice as compared to Ldlr−/− mice throughout the study (Supplemental Table III) and correlated with an improved response to an oral glucose challenge in both genders at the end of the study (females; Figure 3A, males; Figure 3B). Ldlr−/− mice were mildly glucose intolerant and this defect was significantly improved in Ldlr/AdnTg mice. Before feeding of WD total cholesterol and triglyceride levels were significantly lower in Ldlr/AdnTg compared to Ldlr−/− mice, while HDL cholesterol was independent of the genotype (Supplemental Table III; Beginning of study, males n = 11 to 12, females n = 15 to 17). After 4 months on the WD, total cholesterol and triglyceride levels were dramatically increased in all groups independent of the genotype (Supplemental Table III; End of study). In males, HDL cholesterol was significantly increased Ldlr/AdnTg compared to Ldlr−/− mice, and a similar trend was observed in female mice.
Figure 3.
Overexpression of adiponectin improves glucose tolerance. A, female and B, male Ldlr/AdnTg and Ldlr−/− mice after feeding of WD for 4 months. Oral glucose tolerance test was performed after 4 hours of fasting. Circulating glucose was measured at times 0, 20, 40, 60, 120 and 180 minutes after an oral glucose challenge. Data are mean ± SEM, * P<0.05, n = 9 to 13.
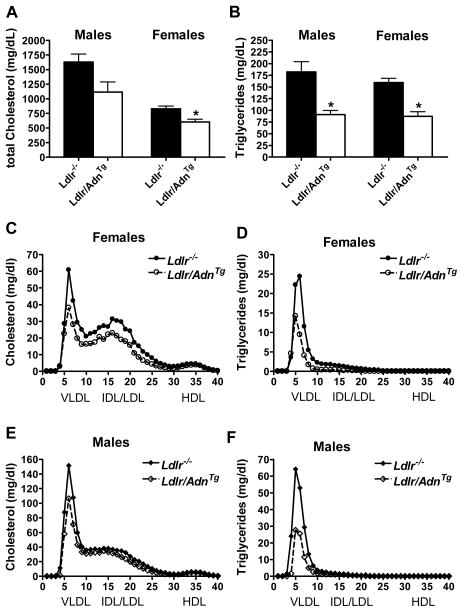
Lipoprotein distribution and atherosclerosis was measured in a separate cohort of mice after 4 months on WD. In this study we measured a trend in male and a significant reduction of total cholesterol in female Ldlr/AdnTg compared to Ldlr−/− mice (Figure 4A). Triglycerides were reduced significantly in both female and male Ldlr/AdnTg mice (Figure 4B). This reduction in total lipid levels was not seen in plasma samples collected from tail blood in the previous study (Supplemental Table III). The discrepancy may be explained by variations in sample preparation or among cohorts. The lipoprotein profile measured by size exclusion chromatography revealed reduced cholesterol levels in VLDL and IDL/LDL fractions from both female and male Ldlr/AdnTg mice when compared to Ldlr−/− mice while HDL fractions were unaffected (Figures 4C and 4E, females and males, respectively). Lower triglycerides were observed in female and male Ldlr/AdnTg mice and were mostly accounted for by VLDL fractions (Figures 4D and 4F, females and males, respectively).
Figure 4.
Adiponectin improves lipoprotein profile. A, Total cholesterol and B, triglycerides in female and male Ldlr/AdnTg and Ldlr−/− mice after feeding of WD for 4 months (* P<0.05, n = 6). C – F, Lipoprotein profiles were analyzed by gel filtration chromatography at end of study. Cholesterol and triglyceride content in each fraction from females (C, D) and males (E, F).
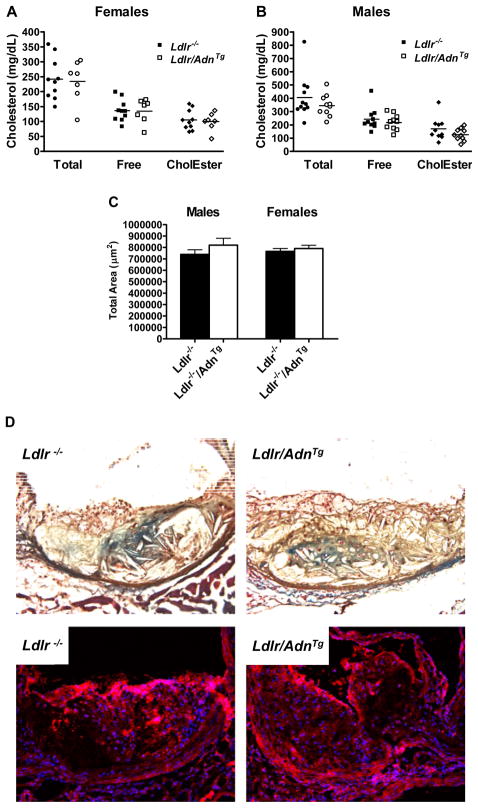
As before atherosclerosis was quantified by the lipid extraction method. Despite an improved lipoprotein profile we could not detect differences in cholesterol deposited in the aortic wall between Ldlr/AdnTg and Ldlr−/− (females; Figure 5A, males; Figure 5B). These results were confirmed by histological assessment of lesions within the aortic root (n= 9) after staining with oil red O (Figure 5C). Lesion area was independent of plasma adiponectin levels in both female and male mice. Overexpression of adiponectin did not alter the number of macrophages within in the lesion or the amount of collagen deposition (Figure 5D and 5E). Positive staining for collagen was localized to regions surrounding the necrotic core in plaques from both Ldlr−/− and Ldlr−/−/AdnTg mice. Visual analysis of the staining using a score from 0 to 10 with ten being the highest intensity revealed no significant differences within the genotypes (scores of 5.5 ± 4.9 for Ldlr−/− and 3.4 ± 2.8 for Ldlr/AdnTg mice). CD68 positive macrophages were localized throughout the plaques with greatest abundance near the sub-endothelial space (Figure 5E) but the content and distribution was unaffected by the genotype (scores of 5.3 ± 3.2 for Ldlr−/− and 4.7 ± 3.8 for Ldlr/AdnTg mice). Our data demonstrate that genetic elevation of adiponectin has no effect on the formation of lesions despite some beneficial effects on lipid clearance and plasma lipids in the Ldlr−/− atherosclerosis model.
Figure 5.
Adiponectin overexpression does not inhibit macrophage infiltration, collagen deposition and atherosclerosis. Aortic cholesterol content in Ldlr/AdnTg and Ldlr−/− mice A, females, B, males. Total, free cholesterol and cholesterol ester content in aorta (n = 7–11). C, Lesion area in aortic root (n = 3). D, collagen content by Mason’s trichrome stain (blue). E, CD68-macrophages (red, blue DAPI-positive nuclei).
Discussion
The role of adiponectin in cardiovascular disease has been met with considerable interest and examined in a large number of epidemiological studies. A picture has emerged suggesting that high adiponectin levels are predictive of a lower incidence of cardiovascular disease in the general population. However, when examined at later stages of cardiovascular disease, high adiponectin levels are indicative of an increased rate of mortality from cardiovascular disease in humans 32. This may reflect a compensatory upregulation of a beneficial factor in light of a generally disadvantageous cardiovascular environment a mechanism that is far from understood.
Studies in preclinical models also suggest that adiponectin is more than an innocent bystander and convenient marker for cardiovascular integrity. An association between low adiponectin and increased vascular thickening has been suggested based on a mouse cuff-injury model 33. In vitro experiments attributed a potent anti-inflammatory role to the globular form of adiponectin and Libby et al. demonstrated that globular adiponectin suppressed lipopolysaccharide-induced cytokine release in macrophages and suppressed T-lymphocyte accumulation in atherogenesis in Apoe−/− mice 34. Our study is the first to address the function of the full length form of adiponectin secreted in its native conformation and in physiological concentrations. We are also the first to address the role of adiponectin in the Ldlr−/− mouse model which develops atherosclerosis only after supplementation of high levels of dietary cholesterol16. We used diets that cause substantial hypercholesterolemia but differentially affect the degree of adiposity, insulin resistance and inflammation. Surprisingly, while observing the expected differential susceptibility to the diets with respect to insulin sensitivity, we failed to detect an effect on atherosclerosis in both genetic adiponectin gain- and loss-of-function mice. Our data differ from previous reports derived from the Apoe−/− model which may be best explained by differences in the experimental paradigm, the form of adiponectin and diets used.
As described preciously 5, introduction of the AdnTg caused reduction of plasma lipids in our studies however, mice on a Ldlr−/− background remained extremely hypercholesterolemic. We conclude that adiponectin plays an important role in the regulation of plasma lipids, however the severity of hyperlipidemia in Ldlr−/− mice may prevent conclusions about the role of adiponectin under physiological conditions. Similarly, elevation adiponectin levels by pharmacological means did not affect atherosclerosis in the Ldlr−/− model. In fact, COOH treatment caused a small but significant increase in lesion area in the BCA in males. PPARγ is highly expressed in macrophage-derived foam cells and has been implicated in the development of atherosclerosis. Most, but not all pre-clinical studies described a reduction of atherosclerosis coinciding with an improvement of overall metabolic parameters 22, 23, 35. Another study suggested that pioglitazone increased macrophage apoptosis and plaque necrosis in non-diabetic Ldlr−/− mice 15. We chose to study lean, insulin sensitive mice using a non-obesogenic diet in the hope of dissociating direct actions of adiponectin on the vascular wall from secondary effects related to a metabolic improvement. Notably, we did not observe any signs of reduction of atherosclerosis upon treatment with COOH, despite a large number of samples.
Our findings underline potential limitations of the LDL receptor mouse model in reproducing associations that have been established in humans. Mice are generally resistant to plaque rupture and myocardial infarction. In our studies, genetic or pharmacologic manipulation of adiponectin levels in traditional rodent models did not correlate with atherosclerosis suggesting that adiponectin is not involved in advanced plaque progression.
Supplementary Material
Acknowledgments
Sources of Funding
The authors were supported by NIH grants R01-DK55758, R01-CA112023 (PES), 8UL1DE019584-03 (Jay Horton) and the UT Southwestern Mouse Phenotyping Core (1PL1DK081182-03) as well as P01-HL087123 and P01-HL054591 (IT). ARN was supported by a postdoctoral fellowship from the Swiss National Science Foundation and the American Heart Association (Heritage Affiliate; 0425800T). SMH was supported by the American Heart Association (0635079N).
Footnotes
Disclosures
None
References
- 1.Abel ED, Litwin SE, Sweeney G. Cardiac remodeling in obesity. Physiol Rev. 2008;88:389–419. doi: 10.1152/physrev.00017.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scherer PE. Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes. 2006;55:1537–1545. doi: 10.2337/db06-0263. [DOI] [PubMed] [Google Scholar]
- 3.Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96:939–949. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- 4.Shetty S, Kusminski CM, Scherer PE. Adiponectin in health and disease: evaluation of adiponectin-targeted drug development strategies. Trends Pharmacol Sci. 2009;30:234–239. doi: 10.1016/j.tips.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Kim JY, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, Schraw T, Durand JL, Li H, Li G, Jelicks LA, Mehler MF, Hui DY, Deshaies Y, Shulman GI, Schwartz GJ, Scherer PE. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. 2007;117:2621–2637. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Menzaghi C, Trischitta V, Doria A. Genetic influences of adiponectin on insulin resistance, type 2 diabetes, and cardiovascular disease. Diabetes. 2007;56:1198–1209. doi: 10.2337/db06-0506. [DOI] [PubMed] [Google Scholar]
- 7.Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, Iwahashi H, Kuriyama H, Ouchi N, Maeda K, Nishida M, Kihara S, Sakai N, Nakajima T, Hasegawa K, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Hanafusa T, Matsuzawa Y. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20:1595–1599. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- 8.Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB. Plasma adiponectin levels and risk of myocardial infarction in men. Jama. 2004;291:1730–1737. doi: 10.1001/jama.291.14.1730. [DOI] [PubMed] [Google Scholar]
- 9.Takemura Y, Walsh K, Ouchi N. Adiponectin and cardiovascular inflammatory responses. Curr Atheroscler Rep. 2007;9:238–243. doi: 10.1007/s11883-007-0025-4. [DOI] [PubMed] [Google Scholar]
- 10.Nawrocki AR, Rajala MW, Tomas E, Pajvani UB, Saha AK, Trumbauer ME, Pang Z, Chen AS, Ruderman NB, Chen H, Rossetti L, Scherer PE. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor gamma agonists. J Biol Chem. 2006;281:2654–2660. doi: 10.1074/jbc.M505311200. [DOI] [PubMed] [Google Scholar]
- 11.Combs TP, Pajvani UB, Berg AH, Lin Y, Jelicks LA, Laplante M, Nawrocki AR, Rajala MW, Parlow AF, Cheeseboro L, Ding YY, Russell RG, Lindemann D, Hartley A, Baker GR, Obici S, Deshaies Y, Ludgate M, Rossetti L, Scherer PE. A transgenic mouse with a deletion in the collagenous domain of adiponectin displays elevated circulating adiponectin and improved insulin sensitivity. Endocrinology. 2004;145:367–383. doi: 10.1210/en.2003-1068. [DOI] [PubMed] [Google Scholar]
- 12.Pajvani UB, Hawkins M, Combs TP, Rajala MW, Doebber T, Berger JP, Wagner JA, Wu M, Knopps A, Xiang AH, Utzschneider KM, Kahn SE, Olefsky JM, Buchanan TA, Scherer PE. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione -mediated improvement in insulin sensitivity. J Biol Chem. 2004;279:12152–12162. doi: 10.1074/jbc.M311113200. [DOI] [PubMed] [Google Scholar]
- 13.Stafylas PC, Sarafidis PA, Lasaridis AN. The controversial effects of thiazolidinediones on cardiovascular morbidity and mortality. Int J Cardiol. 2009;131:298–304. doi: 10.1016/j.ijcard.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Patel C, Wyne KL, McGuire DK. Thiazolidinediones, peripheral oedema and congestive heart failure: what is the evidence? Diab Vasc Dis Res. 2005;2:61–66. doi: 10.3132/dvdr.2005.010. [DOI] [PubMed] [Google Scholar]
- 15.Thorp E, Kuriakose G, Shah YM, Gonzalez FJ, Tabas I. Pioglitazone increases macrophage apoptosis and plaque necrosis in advanced atherosclerotic lesions of nondiabetic low-density lipoprotein receptor-null mice. Circulation. 2007;116:2182–2190. doi: 10.1161/CIRCULATIONAHA.107.698852. [DOI] [PubMed] [Google Scholar]
- 16.Ishibashi S, Brown MS, Goldstein JL, Gerard RD, Hammer RE, Herz J. Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J Clin Invest. 1993;92:883–893. doi: 10.1172/JCI116663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zadelaar S, Kleemann R, Verschuren L, de Vries-Van der Weij J, van der Hoorn J, Princen HM, Kooistra T. Mouse models for atherosclerosis and pharmaceutical modifiers. Arterioscler Thromb Vasc Biol. 2007;27:1706–1721. doi: 10.1161/ATVBAHA.107.142570. [DOI] [PubMed] [Google Scholar]
- 18.Teupser D, Persky AD, Breslow JL. Induction of Atherosclerosis by Low-Fat, Semisynthetic Diets in LDL Receptor-Deficient C57BL/6J and FVB/NJ Mice: Comparison of Lesions of the Aortic Root, Brachiocephalic Artery, and Whole Aorta (En Face Measurement) Arterioscler Thromb Vasc Biol. 2003;23:1907–1913. doi: 10.1161/01.ATV.0000090126.34881.B1. [DOI] [PubMed] [Google Scholar]
- 19.Home PD, Pocock SJ, Beck-Nielsen H, Gomis R, Hanefeld M, Jones NP, Komajda M, McMurray JJ. Rosiglitazone evaluated for cardiovascular outcomes--an interim analysis. N Engl J Med. 2007;357:28–38. doi: 10.1056/NEJMoa073394. [DOI] [PubMed] [Google Scholar]
- 20.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 21.Pajvani UB, Hawkins M, Combs TP, Rajala MW, Doebber T, Berger JP, Wagner JA, Wu M, Knopps A, Xiang AH, Utzschneider KM, Kahn SE, Olefsky JM, Buchanan TA, Scherer PE. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione -mediated improvement in insulin sensitivity. J Biol Chem. 2003 doi: 10.1074/jbc.M311113200. [DOI] [PubMed] [Google Scholar]
- 22.Collins AR, Meehan WP, Kintscher U, Jackson S, Wakino S, Noh G, Palinski W, Hsueh WA, Law RE. Troglitazone inhibits formation of early atherosclerotic lesions in diabetic and nondiabetic low density lipoprotein receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2001;21:365–371. doi: 10.1161/01.atv.21.3.365. [DOI] [PubMed] [Google Scholar]
- 23.Li AC, Brown KK, Silvestre MJ, Willson TM, Palinski W, Glass CK. Peroxisome proliferator-activated receptor gamma ligands inhibit development of atherosclerosis in LDL receptor-deficient mice. J Clin Invest. 2000;106:523–531. doi: 10.1172/JCI10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muise ES, Azzolina B, Kuo DW, El-Sherbeini M, Tan Y, Yuan X, Mu J, Thompson JR, Berger JP, Wong KK. Adipose fibroblast growth factor 21 is up-regulated by peroxisome proliferator-activated receptor gamma and altered metabolic states. Mol Pharmacol. 2008;74:403–412. doi: 10.1124/mol.108.044826. [DOI] [PubMed] [Google Scholar]
- 25.Chang CH, McNamara LA, Wu MS, Muise ES, Tan Y, Wood HB, Meinke PT, Thompson JR, Doebber TW, Berger JP, McCann ME. A novel selective peroxisome proliferator-activator receptor-gamma modulator-SPPARgammaM5 improves insulin sensitivity with diminished adverse cardiovascular effects. Eur J Pharmacol. 2008;584:192–201. doi: 10.1016/j.ejphar.2007.12.036. [DOI] [PubMed] [Google Scholar]
- 26.Rosenfeld ME, Carson KG, Johnson JL, Williams H, Jackson CL, Schwartz SM. Animal models of spontaneous plaque rupture: the holy grail of experimental atherosclerosis research. Curr Atheroscler Rep. 2002;4:238–242. doi: 10.1007/s11883-002-0025-3. [DOI] [PubMed] [Google Scholar]
- 27.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 28.Sparrow CP, Burton CA, Hernandez M, Mundt S, Hassing H, Patel S, Rosa R, Hermanowski-Vosatka A, Wang PR, Zhang D, Peterson L, Detmers PA, Chao YS, Wright SD. Simvastatin has anti-inflammatory and antiatherosclerotic activities independent of plasma cholesterol lowering. Arterioscler Thromb Vasc Biol. 2001;21:115–121. doi: 10.1161/01.atv.21.1.115. [DOI] [PubMed] [Google Scholar]
- 29.Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the vulnerable plaque. J Am Coll Cardiol. 2006;47:C13–18. doi: 10.1016/j.jacc.2005.10.065. [DOI] [PubMed] [Google Scholar]
- 30.Davis KE, Scherer PE. Adiponectin: no longer the lone soul in the fight against insulin resistance? Biochem J. 2008;416:e7–9. doi: 10.1042/BJ20082033. [DOI] [PubMed] [Google Scholar]
- 31.Wong GW, Krawczyk SA, Kitidis-Mitrokostas C, Revett T, Gimeno R, Lodish HF. Molecular, biochemical and functional characterizations of C1q/TNF family members: adipose-tissue-selective expression patterns, regulation by PPAR-gamma agonist, cysteine-mediated oligomerizations, combinatorial associations and metabolic functions. Biochem J. 2008;416:161–177. doi: 10.1042/BJ20081240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cavusoglu E, Ruwende C, Chopra V, Yanamadala S, Eng C, Clark LT, Pinsky DJ, Marmur JD. Adiponectin is an independent predictor of all-cause mortality, cardiac mortality, and myocardial infarction in patients presenting with chest pain. Eur Heart J. 2006;27:2300–2309. doi: 10.1093/eurheartj/ehl153. [DOI] [PubMed] [Google Scholar]
- 33.Kubota N, Terauchi Y, Yamauchi T, Kubota T, Moroi M, Matsui J, Eto K, Yamashita T, Kamon J, Satoh H, Yano W, Nagai R, Kimura S, Kadowaki T, Noda T. Disruption of adiponectin causes insulin resistance and neointimal formation. J Biol Chem. 2002;277:25863–25866. doi: 10.1074/jbc.C200251200. [DOI] [PubMed] [Google Scholar]
- 34.Okamoto Y, Folco EJ, Minami M, Wara AK, Feinberg MW, Sukhova GK, Colvin RA, Kihara S, Funahashi T, Luster AD, Libby P. Adiponectin inhibits the production of CXC receptor 3 chemokine ligands in macrophages and reduces T-lymphocyte recruitment in atherogenesis. Circ Res. 2008;102:218–225. doi: 10.1161/CIRCRESAHA.107.164988. [DOI] [PubMed] [Google Scholar]
- 35.Hennuyer N, Tailleux A, Torpier G, Mezdour H, Fruchart JC, Staels B, Fievet C. PPARalpha, but not PPARgamma, activators decrease macrophage-laden atherosclerotic lesions in a nondiabetic mouse model of mixed dyslipidemia. Arterioscler Thromb Vasc Biol. 2005;25:1897–1902. doi: 10.1161/01.ATV.0000175756.56818.ee. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.