Abstract
Specific behavioral associative memory induced by stimulation of the cortically-projecting cholinergic nucleus basalis (NB) is dependent on intrinsic acetylcholine and shares with natural memory such features as associativity, specificity, rapid formation, consolidation and long-term retention. Herein, we examined extinction and the effects of stimulus pre-exposure. Two groups of adult male rats (n = 4 each) were first tested for behavioral responses (disruption of ongoing respiration) to tones (1–15 kHz), constituting a pre-training behavioral frequency generalization gradient (BFGG). They next received a first session of training, 200 trials of a tone (8.00 kHz, 70 dB, 2 s) either paired with electrical stimulation of the NB (100 Hz, 0.2 s, ~67 μA, NBstm) (group IP) or unpaired (group IU). Twenty-four hours later, they were tested for behavioral memory by obtaining post-training BFGGs. Then the contingencies were reversed yet another 24 h later; the IP group received tone and NBstm unpaired and the IU group received them paired. A final set of generalization gradients was obtained the next day. All stimuli were presented with subjects under state control indexed by regular respiration. Tested 24 h post-initial training, the IP group developed specific associative behavioral memory indicated by increased responses only to CS-band frequencies, while the IU group did not. After subsequent training with unpaired stimuli, the IP group exhibited experimental extinction. Furthermore, after initial exposure to the CS and NBstm unpaired, the IU group exhibited a tendency toward reduced conditioning to CS/NBstm pairing and a significant increase in latency of conditioned responses. The present findings provide additional support for the hypothesis that engagement of the NB is sufficient to induce natural associative memory and suggest that activation of the NB may be a normal component in the formation of natural associative memory.
Keywords: Acetylcholine, Association, Auditory system, Learning, Respiration, State control
1. Introduction
The cholinergic system has been implicated in learning and memory for more than 40 years. Understandably, pharmacological studies have been critical in delineating the role of acetylcholine in these processes. Cholinergic agonists and cholinesterase antagonists can facilitate memory (Introini-Collison & McGaugh, 1988; Stratton & Petrinovich, 1963), promote recovery of memory from brain damage (Russell, Escobar, Booth, & Bermúdez-Rattoni, 1994) and achieve rescue from memory deficits in transgenic mice (Fisher, Brandeis, Chapman, Pittel, & Michaelson, 1998). Lesion studies have also demonstrated the involvement of cholinergic mechanisms. Thus, lesions that reduce levels of acetylcholine (ACh) or choline–acetyl transferase (ChAT) impair learning and memory in a variety of tasks (e.g., Bartus, Flicker, Dean, Pontecorvo, & Figueiredo, 1985; Cabrera, Chavez, Corley, Kitto, & Butt, 2006; Everitt et al., 1987; Mandel, Gage, & Thal, 1989; McGaughy, Dalley, Morrison, Everitt, & Robbins, 2002). In addition, the cortical cholinergic input system was hypothesized to be necessary for the mediation of attentional functions and capacities (Sarter & Bruno, 1997; Sarter, Bruno, & Givens, 2003). To complement these approaches, enhance localization of critical cholinergic neurons and directly test the hypothesis that activation of the cholinergic system is sufficient to induce behavioral associative memory (Weinberger et al., 1990, chap. 3), we have been conducting a series of studies using electrical microstimulation of the nucleus basalis (NB) which is the major source of ACh to the cerebral cortex (Bigl, Woolf, & Butcher, 1982; Johnston, McKinney, & Coyle, 1979; Mesulam, Mufson, Wainer, & Levey, 1983).
We use the redundant term “behavioral memory” at the outset to distinguish genuine memory from learning-related neural plasticity, which many workers unfortunately equate with memory, which is a behavioral level construct. Thus, several laboratories have found that a tone paired with stimulation of the nucleus basalis (NBstm) induces CS-specific plasticity in the primary auditory cortex (e.g., Bakin & Weinberger, 1996; Kilgard & Merzenich, 1998; Moucha, Pandya, Engineer, Rathbun, & Kilgard, 2005; Zhang, Hakes, Bonfield, & Yan, 2005). However, given the hypothesis that ACh is involved in “behavioral memory”, demonstrations of cholinergically-related neural plasticity are insufficient. We explicitly distinguish between plasticity and memory and therefore have conducted studies in which behavioral memory (hereafter “memory”) is assessed.
To determine if NBstm can induce memory, we obtained behavioral frequency generalization gradients (BFGGs) to many tones following the pairing of a specific tone with NBstm compared to a control group that received the same stimuli in an unpaired or random manner. We used a highly sensitive behavioral measure (interruption of ongoing respiration) (Konorski, 1967) to reduce the probability of obtaining a false negative conclusion. Significantly larger behavioral responses (interruption of ongoing respiration) to tones per se (i.e., “flat” BFGGs) in the paired group indicated an associative effect, but not specific memory for CS frequencies. In contrast, “inverted V” gradients (i.e., peak at CS frequencies) indicated both associativity and specificity, i.e., the induction of a CS-specific associative memory.
Using this approach, we found that NBstm can induce specific, associative memory. Memory induced by NBstm has the following attributes: it is associative, highly specific, can be induced in a single session, becomes stronger over time (“consolidates”) and is retained for at least several days (longest retention period tested) (McLin, Miasnikov, & Weinberger, 2002a; Miasnikov, Chen, & Weinberger, 2006; Weinberger, Miasnikov, & Chen, in press). NBstm that induces memory is motivationally neutral (Miasnikov, Chen, Gross, Poytress, & Weinberger, 2008a), consistent with the nucleus basalis being functionally located “downstream” from motivational systems, and serving as a “final common path” to modulate the cerebral cortex by the release of ACh. Moreover, post-training scopolamine blocks the induction of memory, indicating the necessary involvement of muscarinic receptors (Miasnikov, Chen, & Weinberger, 2008b). In toto, these findings provide novel and strong support for the view that activation of the cholinergic system is a mechanism that is normally engaged in the formation of natural memory.
Additionally, a particular advantage of the “NB–memory induction” approach is that it provides a means by which the neurobiological substrates of the content or detail of memory can be studied. The use of BFGGs provides a straightforward, objective way of determining whether subjects learned about tones in general or about the specific frequency of the signal stimulus. Furthermore, this focus on the contents of memory has provided insight into the role of the cholinergic system in memory for details of an experience. Thus, the level of NBstm determines the level of detail in memory. Weak NBstm (~45 μA) produces a flat BFGG, i.e., memory for tones in general. In contrast, modest NBstm (~65 μA) induces memory of the CS frequencies themselves, as indicated by BFGGs that are peaked at these frequencies.
If natural associative memory involves activation of the nucleus basalis, then memory for stimuli paired with stimulation of the nucleus basalis (hereafter “NB-induced memory”) should exhibit cardinal attributes of natural memory. The present experiment constitutes one of our final characterizations of NB-induced memory. As noted above, NB-induced memory is associative, specific, can form rapidly, consolidates over time and is retained over days. This study investigated its flexibility by determining the effects of a reversal of CS–NBstm contingencies on extinction and latent inhibition. Specifically, extinction was examined in a group receiving paired CS and NBstm by later presenting the CS and NBstm unpaired. Maintaining the presence of the US avoided possible state confounds due to the removal of cortical arousal induced by NBstm.
The effects of pre-exposure to unpaired CS and NBstm was studied in a second group that later received these stimuli paired. This procedure is thought to produce “learned irrelevance” (LIRR) (Mackintosh, 1973). However, this interpretation is controversial and other workers believe that the retardation of learning following pre-exposure of unpaired CS and US can be explained by context-specific latent inhibition (LI) (e.g., Bonardi & Hall, 1996). As the theoretical interpretation of this pre-exposure effect is not of critical importance to the current study, we maintain a neutral, descriptive stance and use the label “LI/LIRR” throughout.
2. Materials and methods
The materials and methods were the same as those previously reported (Miasnikov et al., 2006) and will be described only briefly. All procedures were performed in accordance with the University of California Irvine Animal Research Committee and the NIH Animal Welfare guidelines. During training and testing, subjects were continuously monitored by video cameras.
2.1. Subjects and surgery
The subjects were eight adult male Sprague–Dawley rats (112 ± 24 days of age, 439 ± 44 g, mean ± s.d.), housed individually with ad libitum food and water, on a 12/12 h light–dark cycle (lights on at 7:15 AM). They had been studied previously during acquisition training (paired or unpaired), and the findings reported for nine animals (Miasnikov et al., 2006). The current study concerns the effects of reversing paired and unpaired training protocols for these two groups. The original report was for nine animals but data on reversed contingencies are unavailable for one subject; the complete experimental protocol for eight subjects is shown in Fig. 1 (see Section 2.3 for detailed description). Under general anesthesia (sodium pentobarbital, 40 mg/kg i.p.), an 0.8-mm diameter stainless steel epidural screw recording electrode was inserted over the right primary auditory cortex and two screws over the frontal sinus served as reference electrodes. A concentric bipolar stainless steel stimulating electrode was implanted through the contralateral (left) hemisphere (45° angle in the frontal plane at A–P –2.2, M–L 3.2; Paxinos & Watson, 1997) into the right nucleus basalis. The final locus was determined by obtaining 1–5 s of auditory cortical EEG activation to stimulation (pairs of 0.2 ms opposite polarity pulses, 100 Hz (Rasmusson, Clow, & Szerb, 1992), 200–300 ms trains; S88 stimulator, linked pair of the PSIU6 isolation units, Grass Instrument Co., Quincy, MA). A dental acrylic pedestal was built with two aluminum hex threaded standoffs embedded therein, and all leads connected to a miniature socket that could be led to a commutator via a multi-conductor cable. Subjects were adapted to allow attachment of a thermistor assembly to their pedestal and allowed 1–2 weeks to recover from surgery.
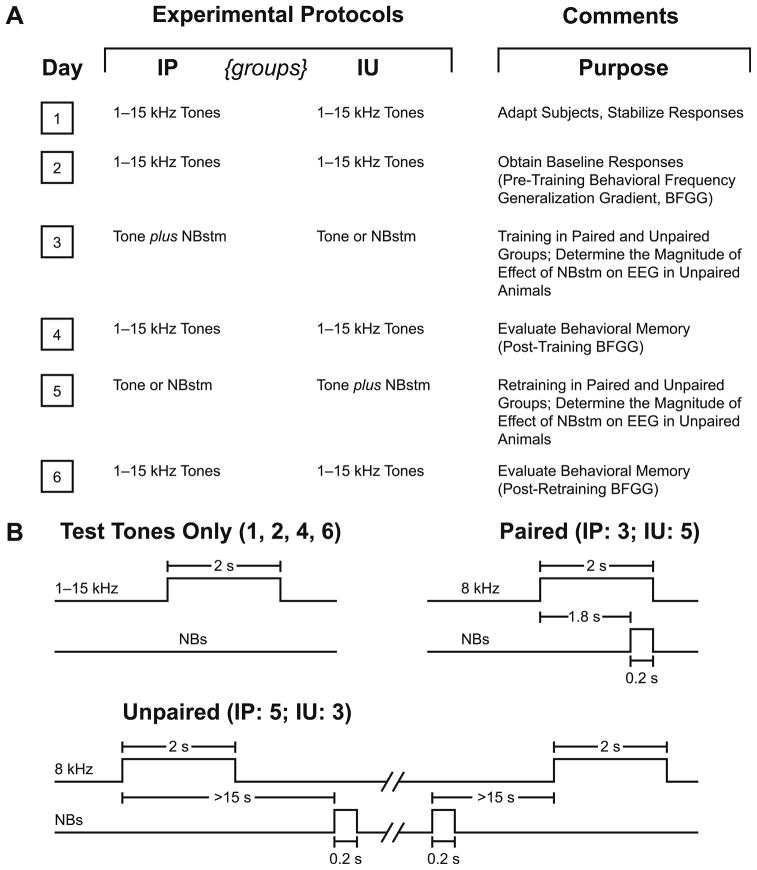
Fig. 1.
Experimental design. (A) The six main stages of the experiment used to obtain pre-training and post-training behavioral frequency generalization gradients (BFGG) for the IP and IU groups. (B) Detailed temporal relationships between the stimuli presentation in the various phases of the experiment: delivery of test tones (Days 1, 2, 4 and 6), tone–NBstm pairing (Day 3 in IP and Day 5 in IU groups) and unpaired tone/NBstm (Day 3 in IU and Day 5 in IP groups).
2.2. Stimuli, recording and data analyses
Training and testing took place while each subject was in a box (23 × 23 × 31 cm) supplied with fresh bedding and lined inside with acoustic-damping tile, contained in a double-walled acoustic chamber. Acoustic stimuli were pure tones (1.0–15.0 kHz, 2 s duration, cosine 10 ms rise/fall time [10–90%], 70 dB SPL) produced by Tucker–Davis Technologies System 3 components (TDT, Alachua, FL) and delivered to a calibrated loudspeaker positioned 35 cm above the floor of the box. After recovery from surgery, NBstm thresholds were determined while subjects were in a quiet waking state. NBstm was delivered every few minutes at increasing levels starting at 35 μA (100 Hz bipolar, 200 ms train) until stimulation reliably elicited 1–5 s epoch of cortical activation (decrease in low frequency activity often accompanied by increase in gamma activity). The current levels used in subsequent training with NB stimulation did not elicit body movements.
The induction of memory was determined by comparing behavioral frequency generalization gradients to various tones before and after training (below). The behavioral measure was the magnitude of disruption of ongoing respiration. The rationale for using respiration to detect learning is that the pairing of a tone with another stimulus constitutes classical (Pavlovian) conditioning. Changes in respiration (and several autonomic measures) are sensitive indices of successful classical conditioning, i.e., they reveal the association between two sequentially-paired stimuli, and are widely employed in the field of learning and memory (reviewed in Lennartz & Weinberger, 1992; Winters, McCabe, & Schneiderman, 2002). Respiration was detected as breathing-related thermal fluctuations by a glass-encapsulated thermistor attached to a lightweight pedestal-mounted assembly positioned in front of a naris (Miasnikov et al., 2006).
The amplified output of the thermistor probe was fed to analog-to-digital converting modules and respiration patterns were visualized on a computer screen. Stimuli were given only when respiration was regular, during a state of calm waking. State control was employed to avoid presenting stimuli during periods of very high levels of cortical ACh (e.g., exploration, grooming or REM sleep), or very low levels of ACh (e.g., slow wave [SW] sleep) (Giovannini et al., 2001; Jasper & Tessier, 1971; Kametani & Kawamura, 1990; Marrosu et al., 1995).
The rationale for using such state control is that we induce specific memory by pairing a tone with stimulation of the nucleus basalis (see Section 2.3). If NBstm were delivered when the tonic level of ACh in the cortex were very high, then it would probably be ineffective, due to a “ceiling effect”. In such cases, negative results, i.e., failure to induce specific memory, would be uninterpretable. Similarly, if the NB was stimulated when the tonic level of cortical ACh was too low, then the relatively low level of stimulation which we employ (to preclude spread of current and possible elicitation of motor responses or other confounding effects) could be insufficient to adequately engage cholinergic targets in the cortex. Thus any negative findings also would be uninterpretable. State control thus insured that NBstm during training occurred on a baseline of moderate levels of ACh in the cortex, enabling interpretation of any failure to induce memory as genuine evidence against our hypothesis that properly-timed activation of the NB following tone presentation is sufficient to induce behavioral specific associative memory.
Major evoked changes in respiration occurred within the first 13 s after tone onset. The collected data were used to calculate a “Respiration Change Index” (RCI), on a second-by-second basis. The index was sensitive to increases and decreases of both frequency and amplitude. RCIs were calculated as: RCIi = (|Posti –Pre|)/(Posti + Pre) where the “Post” and “Pre” were the values of power of respiration signal (Weinberger, Miasnikov, & Chen, 2006). An RCI value of zero would indicate no change and a value of 1.0 would indicate complete cessation of respiration. Statistical analyses used SPSS v.16.0 software (SPSS, Chicago, IL).
2.3. Experimental design
The subjects were assigned to two groups, “Initially Paired” (IP, n = 4) and “Initially Unpaired” (IU, n = 4). After initial training and testing, the contingencies were reversed in the second training phase, i.e., the IP group received tone and NBstm unpaired and the IU received tone and NBstm paired. The former “reversal” constitutes experimental extinction while the latter provides a test for the effects of latent inhibition, i.e., conditioning following exposure to unpaired tone and NBstm.
The protocol required six consecutive days (Fig. 1A). Days 1–2 were for obtaining the pre-training baseline response to test tones; the Day 1 session was used to acclimatize subjects to the testing environment and thus data from this session were not analyzed. Day 3 was the first training session, in which a CS tone was paired with NBstm in the IP group (200 trials per day). The IU group received 200 tones and 200 NBstm in random order with the restriction that no more than three of the same type could occur consecutively and that NBstm could not occur during a 15-s period either immediately following or preceding presentation of the tone (Fig. 1B). (This arrangement has sometimes been termed “pseudo-random”, but we use “unpaired” to distinguish this procedure from “strictly unpaired”, which can produce inhibitory conditioning.) Potential transfer between training and frequency testing sessions was reduced by using different contexts for the two types of session. Thus, animals were delivered to the lab via different circuitous routes and they were trained in the dark (red light) but tested (pre- and post-training) in the light. On Day 4, post-initial-training responses to tones were obtained. The animals were then trained with reversed contingencies (Day 5) and re-tested on Day 6.
During pairing, the animals in each group received the CS tone (8.00 kHz, 2 s, 70 dB SPL) followed by NBstm (same level as determined post-operatively) that overlapped CS presentation and coterminated with CS offset (i.e., the CS–NBstm interval was 1.8 s). During unpaired training, also presented to the animals of each group, the stimuli, tone and NBstm, never overlapped. There was no significance difference in stimulation current between groups (t(6) = 1.06, p >.30; mean ± s.d.; for all subjects = 67 ± 9 μA). Inter-trial intervals averaged 80 s (range ~25–150 s). On frequency test days, subjects received random presentation of tones of nine different frequencies (1.00, 2.75, 4.50, 6.25, 8.00, 9.75, 11.50, 13.25 and 15.00 kHz; 70 dB SPL; constrained only by presenting not more than two stimuli of the same frequency in a row) for 200 trials total. Intervals between tone presentations averaged 94 s. The initial statistical analyses of respiration responses were based on averaging the data for triplets of frequencies: 1.00–4.50 kHz (lower band), 6.25–9.75 kHz (middle band) and 11.50–15.00 kHz (upper band). The middle frequency band (6.25, 8.00 and 9.75 kHz) is referred to as the “CS band”.
2.4. Histology
Following the completion of the experiment, an electrolytic lesion (4 ms pulses at 100 Hz, 500 μA for 60 s) was made with bipolar current through the stimulating electrode while the animal was under deep sodium pentobarbital anesthesia. The animal was then given an overdose of sodium pentobarbital and perfused through the heart with saline followed with 3.7% paraformaldehyde in 0.1 M phosphate buffer (pH 7.3). The location of the cortical recording electrode relative to the primary auditory cortex was accomplished by determining its Anterior-to-Posterior (A–P) and Medial-to-Lateral (M–L) coordinates relative to Bregma and midline, respectively, and plotting them onto a stereotaxic map of the auditory and surrounding areas of cortex derived from the Paxinos and Watson (1997) atlas. Histological processing of the brain to locate the sites of the NB stimulating electrodes used conventional methods and has been reported (Miasnikov et al., 2006).
3. Results
3.1. Location of electrodes
The electrode sites were re-evaluated from the preceding report (Miasnikov et al., 2006) because of the difference in subject population. All of the cortical recording electrodes were located above the primary auditory cortex. The recording sites of the IP and IU groups were intermingled and differed neither in the A–P (t(6) = 0.57, p >.55) nor the M–L (t(6) = 0.97, p >.35) dimensions (two-tailed t-tests). The stimulation sites of the IP and IU groups were intermingled and did not differ in the A–P (t(6) = 0.33, p >.75), M–L (t(6) = 0.44, p >.65), or the D–V (t(6) = 0.00, p >.99) dimensions (two-tailed t-tests) (see also Fig. 1 in Miasnikov et al., 2006). In general, the coordinates of the area of stimulation, as referenced to the coronal plane, were as follows: A–P, –1.74 ± 0.50 mm (mean ± s.d.); M–L, 3.08 ± 0.61 mm; D–V, 7.28 ± 0.32 mm. All stimulation sites were in the basal forebrain within structures containing corticopetal cholinergic cells, including those that project to the auditory cortex (Bigl et al., 1982; Johnston et al., 1979; Luiten, Gaykema, Traber, & Spencer, 1987; Mesulam, Mufson, Levey, & Wainer, 1983; Rye, Wainer, Mesulam, Mufson, & Saper, 1984).
3.2. Effect of initial training and reversal of tone–NBstm contingencies
Behavioral frequency generalization gradients (BFGG) were obtained before and after initial training and after reversal of the contingencies in the second training session. The IP and IU groups exhibited the same respiratory responses to tones of 1–15 kHz before training. In contrast, 24 h after initial training, they exhibited differential responses. Fig. 2 provides examples of behavior for single presentations of three tones for an IP animal (Fig. 2A) and an IU animal (Fig. 2B). Both subjects exhibited little response to either the CS tone (8.00 kHz) or a lower (2.75 kHz) or higher (15.00 kHz) tone prior to training. However, after training, the IP animal displayed a large response to the CS frequency, although still no responses to the higher and lower frequencies, indicating CS-specific response augmentation. In contrast, the IU subject displayed no post-training response to the CS frequency, or to the sideband frequencies. Following reversal of the contingencies, their response profiles were inverted. There were no responses of the IP animal to either the CS or the sideband tones. In contrast, pairing the CS and NBstm for the IU animal led to development of CS-specific conditioned behavior: the animal showed clear behavioral response to the CS frequency but no response to the lower and higher test frequencies.
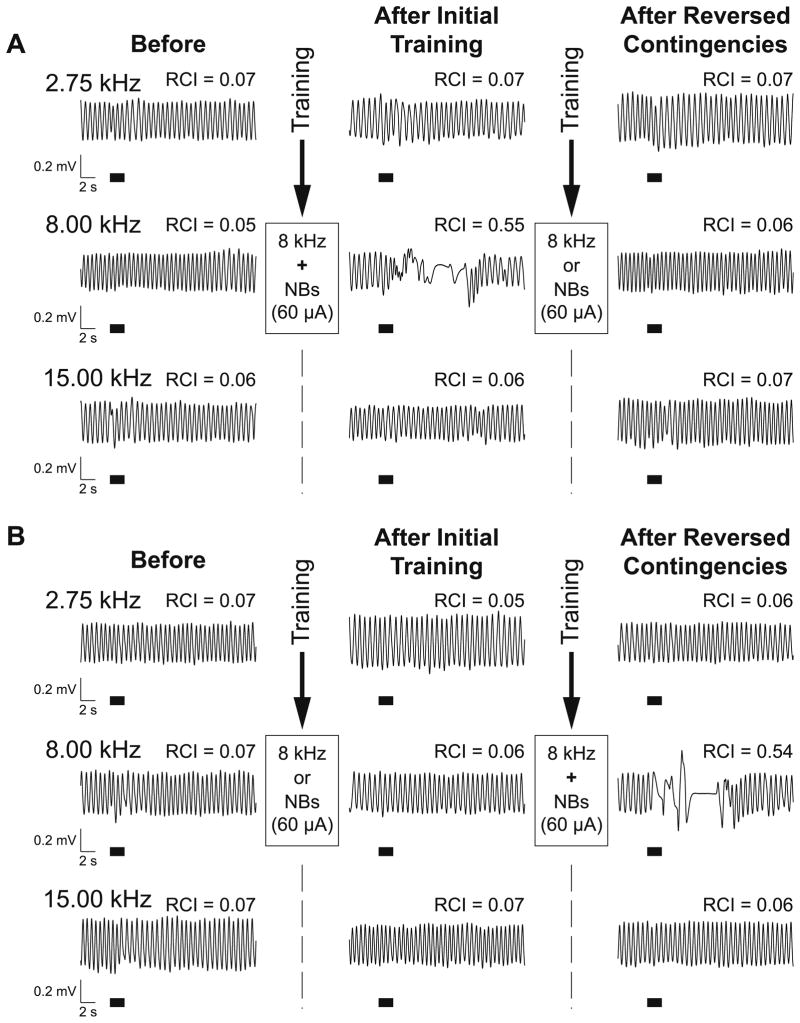
Fig. 2.
Effects of the tone and NBstm presentations on behavioral memory in the IP and IU groups following training. (A) Examples of respiratory waveforms obtained from the subject in the IP group. Shown are baseline responses to the CS (8.00 kHz) and a lower (2.75 kHz) and higher (15.00 kHz) frequency during Day 2 (left column, “Before”), Day 4, 24 h post-training (middle column, “After Initial Training”), and Day 6, 24 h post-second-training (right column, “After Reversed Contingencies”). RCI values indicate the averaged (over the 13-s post-stimulus onset) quantified effect of tone on respiration. Before training, responses to all three frequencies were minimal (RCI = 0.05–0.07). After initial training (paired), the CS frequency produced a large disruption of respiration (RCI = 0.55) while not changing responses to lower (RCI = 0.07) and higher (RCI = 0.06) frequencies thus indicating specific associative learning. Following re-training (unpaired), responses to all three frequencies returned to the pre-training baseline levels (RCI = 0.06–0.07) thus supporting the conclusion that the effects of the previous learning were erased. (B) Examples of respiratory waveforms from a subject in the IU group. Similar to the IP group, responses before training were minimal (RCIs = 0.07). In contrast to the IP group, there was also a minimal response to the sideband and the CS frequencies 24 h after initial (unpaired) training (CS RCI = 0.06; lower sideband RCI = 0.05; higher sideband RCI = 0.07). However, the responses obtained 24 h following reversed contingencies training (paired) were quite different. While they still were minimal at both sidebands (RCI = 0.06), the response at the CS was strong (RCI = 0.54), comparable to the one obtained following initial paired training in the IP subject (see [A]). That suggests that any potential memory about the CS formed after unpaired training was overwritten and had no visible residual decremental effect on the formation of the new one. Note that both subjects had same level of NBstm (60 μA in both cases) during training. The thick horizontal bars indicate tone presentation.
Fig. 3 summarizes the group data. Before initial training, the responses of both IP and IU groups were similar to the lower (1.0–4.5 kHz), the CS (6.25–9.75 kHz) and the higher (11.5–15.0 kHz) frequency bands. The animals were differentially sensitive to different frequencies, as expected from the rat’s audiogram (Heffner, Heffner, Contos, & Ott, 1994) (2-way ANOVA, frequency factor: F(2,1550) = 15.77, p <.0001). However, there was no significant difference between the IP and IU groups (F(1, 1550) = 0.40, p >.50) (Fig. 3A). Conversely, the groups did differ after initial training (F(1, 1600) = 15.06, p <.0002). The frequency factor remained significant (F(2, 1600) = 1638, p <.0001) and the Group × Frequency interaction was significant (F(2, 1600) = 8.54, p <.0003). Post-hoc tests revealed that the between-group difference was limited to the CS frequency band (Tukey’s test: p <.0001); groups did not differ in response to the lower (p >.99) or higher (p >.95) frequency bands. These findings indicate that the IP group had acquired an associative CS-specific memory following tone–NBstm pairing that could be detected 24 h after training, whereas the IU group did not (Fig. 3B and D).
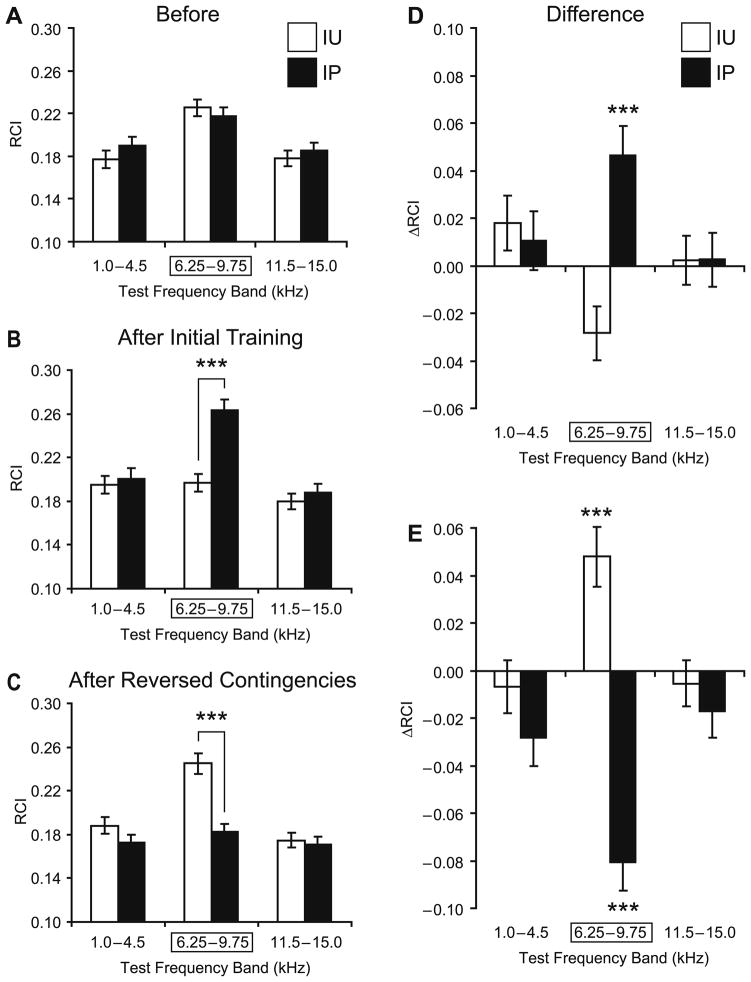
Fig. 3.
Effects of sequential training on NB-induced memory. (A) Pre-training (“Before”) frequency generalization gradients to tones in three frequency bands: “Low” (1.0–4.5 kHz), “CS” (6.25–9.75 kHz [framed with rectangle]; the CS was 8.00 kHz), and “High” (11.5–15.0 kHz). There were no significant differences between the IP (black bars) and IU (opened bars) groups before training. Graph bars show mean ± s.e. of Respiration Change Index, RCI (Y-axis). (B) Post-initial-training (“After Initial Training”) generalization gradients for the IP and IU groups. Note the significant difference in response between the groups in the CS frequency band, and no difference in response within both lower and higher sidebands, indicating CS-specific associative behavioral learning. (C) Post-second-training (“After Reversed Contingencies”) generalization gradients for the IP and IU groups. Note the significant difference in response in the CS band between the two experimental groups. There were no significant differences in responses at the Low and High frequency bands. (D) Differential plots reflecting the changes from the baseline (“Before”) to the post-initial-training (“After Initial Training”) responses to tones for the IP (black bars) and IU (opened bars) groups. Post-pairing responses (IP group) were significantly larger within the CS frequency band while showing no significant differences at lower and higher bands with respect to the baseline response levels. In contrast, post-unpaired responses (IU group) were smaller within the CS frequency band while responses within the lower and higher bands did not change much relative to the baseline. (E) Following reversal of contingencies, the group responses showed the reversal as well. Now the responses of the IU animals were stronger within the CS frequency band while remaining unchanged within the lower and higher bands. Conversely, responses of the IP animals declined from the level achieved as a result of initial training. The decline was statistically significant and most pronounced at the CS. There were no significant changes in responses at lower and higher frequency bands. ***p <.005.
When animals were re-tested after reversing the contingencies in the second training period, the response profiles were inverted. The difference between the IP and IU groups was again significant (F(1, 1599) = 18.87, p <.0001). However, the direction of change was opposite to that found after initial training (compare Figs. 3B, D and C, E). Both the frequency factor (F(2, 1599) = 16.19, p <.0001) and the Group × Frequency interaction (F(2, 1599) = 8.11, p <.0004) were significant. Post-hoc tests revealed that the between-group difference was once again limited to the CS frequency band (Tukey’s test: p <.0001); responses did not differ at lower (p >.70) and higher (p >.99) frequency bands (Fig. 3C and E). These findings indicate that the IP group had undergone experimental extinction of the CS-specific memory that had been induced during initial training.
It is noticeable that the decline in CS-band responses following extinction was deep enough to overshoot the baseline (“Before”) responses on the downside by 16% (that is the overshoot represented 43% of the entire decline manifested in the extinction). However, the overshoot (the CS-band post-extinction responses being compared to the “Before” baseline) did not reach the level of significance (p >.10). None of the sideband responses showed statistically significant overshoot either.
We also determined the effects of pre-exposure to the CS and NBstm on learning in the IU group. We compared the IU responses to the CS Frequency band after subsequent pairing, relative to its baseline responses (“Before”), with the post-pairing responses of the IP group relative to its own “Before” baseline. This analysis showed that pre-exposure tended to retard learning. The IU group exhibited a 9% increase in response after pairing, compared to a 21% increase in the IP group. However, this difference was not statistically significant (p >.10; two-tailed t-test). Neither of the sideband responses exhibited significant differences either (p >.50, higher band; p >.80, lower band).
3.3. Temporal pattern of behavioral responses
The measurement of the interruption of respiration, as a sensitive index of the formation of specific memory for up to 20 s following tone onset, provides a distinct opportunity to determine the temporal dynamics of behavior that is not afforded by most other behavioral analyses. As testing to obtain behavioral generalization gradients requires the presentation of many test frequencies, it was possible to obtain a spectro-temporal (S–T) profile of behavior that transcended the 2-s period of tone presentation. Although seldom studied, behavior that occurs after stimulus offset may also be indicative of mnemonic processes. To visualize the over-all S–T pattern of differences between groups, we subtracted the S–T pattern of the groups (details below) to yield either any pre-training differences, or the associative differences produced by both initial training and training after reversal of the contingencies. The findings are shown in Fig. 4 in two displays: 3-D differences in magnitude of S–T patterns (Figs. 4A–C) and 2-D differences divided into quintiles of response differences (Figs. 4D–F).
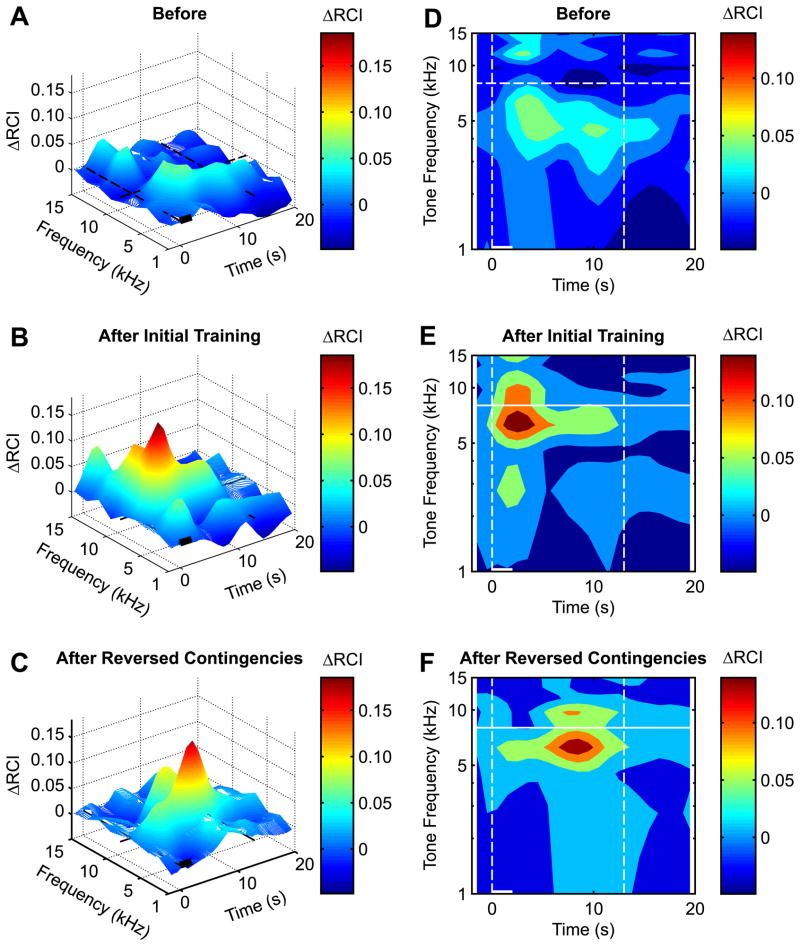
Fig. 4.
Differential spectro-temporal response profiles for the pre-training baseline (A and D), 24 h post-initial-training (B and E) and 24 h post-reversed-contingencies-training (C and F). The plots represent original 3-D response surfaces (A–C) and 2-D response maps (D–F) as areas delineating quintiles; each color represents the area containing 20% of responses measured according to their amplitudes ranked top to bottom. (A and D) Pre-training response differences (IP minus IU) were small: they were slightly smaller around the CS frequency (horizontal dashed line) and modestly larger at sidebands in the IP animals compared to the IU group. The color of the peaks corresponds to high positive differential values (responses in IP animals greater than responses in IU animals); the color of the valleys corresponds to negative differential values (responses in IU animals greater than responses in IP animals). (B and E) Initial training, as measured 24 h later (IP minus IU), resulted in a CS-specific associative effect in the IP compared to the IU groups. The peak on the response surface (B) occupies the space only at and around the CS frequency while the valleys occupy the sideband frequencies’ territory. (E) The quantified response profile (IP minus IU) provides further detail of the spectro-temporal domain of the conditioning effect. It shows that the maximum behavioral associative effect took place at and around the CS, with maximum occurring ~1–3 s after tone onset. These changes indicate the formation of specific associative behavioral memory following tone–NBstm pairing. (C and F) After reversal of the contingencies (IU minus IP on this plot), the IU group acquired specific associative memory compared with the IP group. This time, however, the maximum response occurred with a longer latency of ~5–10 s. Such an evolution indicates that associative training after unpaired CS and NBstm presentation is still effective, although delayed. This process is accompanied by a concomitant decrement in responses to tones surrounding the CS-band frequencies. Such a center/periphery response combination, being more precise in frequency and time dimensions, enhances the signal-to-noise resolution of the system in detecting and responding to the CS frequency.
Fig. 4A and D shows the S–T differences for the baseline (pre-training) period (IP minus IU groups). There was little difference between groups, mainly a relatively small difference in the first 10 s for ~3.0–8.0 kHz. The S–T pattern was markedly changed after initial training. Fig. 4B and E (IP minus IU groups) show that the IP group had a substantially greater response than the IU group at and near the CS frequency. While the maximum difference was short-latency (1–3 s), this difference continued for the 20-s recording period and became more specific to the CS frequency band. Thus, initial training produced specific associative behavior of extended duration.
Reversing the contingencies in the second training session produced major changes in the S–T pattern. This is shown in Fig. 4C and F in which the IP group (now unpaired) data were subtracted from the IU group (now paired) data. This analysis yields the associative effects when the contingencies were reversed. Once again, pairing produced specific associative memory that was evident in behavior in a particular S–T pattern. However, the pattern differed from that of initial training. The associative effect was shifted in the temporal domain; the maximum associative effects were of longer latency, ~5–10 s vs. ~1–3 s following initial training. Thus, although the prior standard analysis, which collapsed behavior across time within a trial, failed to reveal interference by stimulus pre-exposure, the S–T analysis did reveal an effect, in the form of delayed conditioned responses.
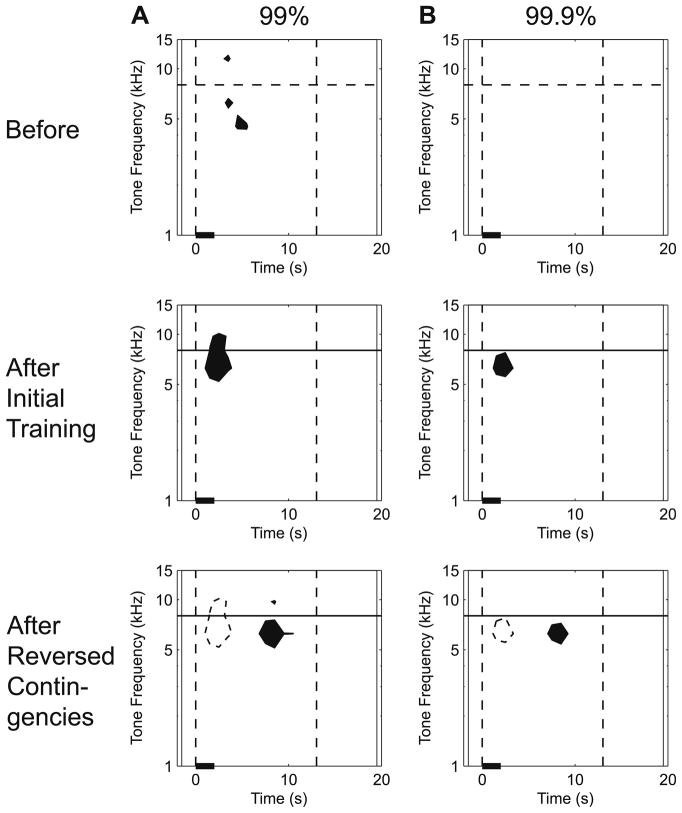
Fig. 5 shows the results of a statistical evaluation of the S–T patterns of behavioral responses, at both the 99% and 99.9% confidence levels (p <.01 and p <.001, respectively). Note the statistical significance of the 3-D response peaks (Fig. 4B and C) and corresponding 2-D response maps (Fig. 4E and F) at both levels of confidence. This analysis shows that the increase in latency of associative responses following contingency reversal was statistically significant. In other words, although stimulus pre-exposure of the IU group did not significantly reduce the specificity or magnitude of associative learning, it did significantly increase the latency of its associative responses.
Fig. 5.
Statistical evaluation of the effects of reversed contingences on the spectro-temporal profile of NB-induced memory. This analysis revealed statistically significant CS-specific associative responses at different latencies for initial pairing and reversed contingencies. Statistical analysis was accomplished by calculating confidence levels for the corresponding data shown in Fig. 4D–F. The data were thresholded at two levels, (A) 99% (p <.01) and (B) 99.9% (p <.001). The confidence intervals were calculated individually for each plot based on the data representing RCI values from within the 22 × 9 points X–Y matrix (X-axis = time: 2 s before to 20 s following stimulus onset; Y-axis = tone frequency, 1.00, 2.75, 4.50, 6.25, 8.00, 9.75, 11.50, 13.25 and 15.00 kHz). Black-filled areas on the plots represent spectro-temporal regions that equal or exceed the values of the mean plus either the 99% (A) or the 99.9% (B) confidence intervals; none of the points in the matrices fell below the mean minus the confidence intervals. Before training, groups IP and IU differed little (A) or not at all (B). After initial training (IP paired, IU unpaired), the IP group had significantly larger responses than the IU group at and immediately adjacent to the CS frequency (horizontal line) with a latency of ~1–3 s (A); at the 99.9% confidence interval, the difference was confined to the CS frequency band, at ~6.25 kHz, with the same short-latency (B). Following contingency reversal (IU minus IP group data), associative responses were also confined to the CS band ~6.25 kHz, but at a much longer latency of ~7–9 s. (To directly compare the shift in latency, the significant S–T regions in the middle row are projected onto the bottom row as dashed outlines.) Vertical dashed lines at tone onset and 13 s delineate the area of respiration responses where they were most pronounced (see Fig. 4D–F), and are provided as references.
4. Discussion
4.1. Summary and validity of the findings
The goal of this study is to further characterize behavioral memory induced by pairing a tone with stimulation of the cholinergic nucleus basalis. This line of research was initiated by the discovery that tone paired with stimulation of the nucleus basalis induced CS-specific shifts of frequency receptive fields in the primary auditory cortex (Bakin & Weinberger, 1996). Subsequently, several laboratories replicated and extended this finding, all within a framework of cholinergic mechanisms underlying learning and memory (e.g., Chen & Yan, 2007; Kilgard & Merzenich, 1998; Moucha et al., 2005; Zhang, Hamilton, Nathanson, & Yan, 2006). Although extensive pharmacological studies had, and continue to, implicate the cholinergic system in learning and memory, they are limited in the ability to pinpoint specific cholinergic neural structures or circuits and to actually induce memory. Thus, such nucleus basalis stimulation studies are valuable in their ability to locate critical cholinergic loci. However, demonstrations of NB-induced specific plasticity provide a presumptive, rather than a direct, link between the cholinergic system and actual memory. Even granting that specific associative plasticity is at least part of a substrate of memory, a general tendency to more-or-less equate learning-related neural plasticity with memory can be considered to constitute a “category error”, i.e., attribution to a part the properties of the whole (Ryle, 1949). Thus, the rationale for determining if NB stimulation can induce behavioral memory, as opposed merely to inducing neural plasticity, is that allegations of cholinergic sufficiency for memory formation can be directly tested.
If activation of the NB during natural learning is sufficient to induce memory, as well as neural plasticity, then appropriately-timed direct stimulation of the NB should be sufficient to also induce memory. Moreover, NB-induced associative memory should have major characteristics of natural associative memory. Previous experiments had revealed that this type of memory is associative, highly specific, rapidly acquired, can be induced in a single session, becomes more precise over time (“consolidates”) and is retained for at least several days (Introduction; see also Weinberger, 2007; Weinberger et al., in press). They also revealed that the NB-induced memory does require the engagement of muscarinic cholinergic receptors (Miasnikov et al., 2008b). The current study examined experimental extinction and the effects of CS and NBstm unpaired pre-exposure (i.e., potential effects of LI/LIRR, latent inhibition or learned irrelevance).
4.2. Extinction
It is now almost universally agreed that behavioral extinction indexes the learning of a new “inhibitory” contingency rather than the loss of the original association. Such inhibitory learning is itself both expressed by the reduction of response to the CS and by its ability to interfere with new learning to the CS (Bouton, 2007; Mackintosh, 1974). Thus, extinction is indicative of the flexibility of mnemonic processes in adjusting behavior to changing circumstances. The current findings show that after pairing a tone with stimulation of the NB in the IP group, which induces specific associative memory, disrupting the initial contingency produces a loss of frequency-specific response. We interpret this change in behavior as indexing experimental extinction as it encompasses the key elements of extinction, i.e., elimination of the contingency between the CS and NBstm. However, the procedures that we employed are different from standard extinction protocols, in which the US is simply absent while the CS continues to be presented. Instead of removing the NBstm, which is a proxy for the US, we retained the “US” but removed its ability to be predicted by the CS tone.
Our rationale was twofold. First, this permitted a balanced design with the IU group so that both groups experienced the same stimuli in both phases of the experiment, undergoing only a change in the CS–NBstm relationship. Second, and generally overlooked, is the fact that removal of the US also produces a change in the state of subjects. For example, removing a shock US in fear conditioning also reduces the arousal level of the animals. We wished to avoid this confound, not only because it complicates the interpretation of the results, but because it would lead to grossly different levels of ACh released into the cerebral cortex, including the auditory cortex. There is close relationship between arousal level and the level of ACh in the cortex, the greater the arousal, the greater the level of ACh (e.g., Cape & Jones, 1998, 2000; Celesia & Jasper, 1966; Metherate, Cox, & Ashe, 1992; Phillis, 1968; Phillis & Chong, 1965; Rasmusson & Szerb, 1976). Therefore, removing NBstm from the IP group during the second phase of the experiment would have had a detrimental consequence: subjects’ lowered arousal level would have meant that comparisons between the IP and IU groups during the second phase would engender a confound in arousal level, so that any differences in behavior could not be attributable exclusively to the training contingencies.
That the IP group exhibited behavioral extinction when its contingency was changed indicates that NB-induced memory possesses another important attribute of natural associative memory. This finding adds to the evidence that engagement of the NB during normal learning is sufficient to induce natural associative memory. That NB-induced memory possess attributes of natural memory is important because it reduces the possibility that induced memory is simply a demonstration of what the brain can be “forced to do” by an intervention such as electrical microstimulation. We do realize that in principle, one could examine all associative phenomena to determine if NB-induced memory has all of the attributes of normal associative memory. However, we believe that this would not be a good strategy, and address this issue in the final section of this paper.
4.3. Effect of pre-exposure to unpaired tone and NBstm
The IU group first received tone and NBstm in unpaired and later received them in a standard, paired relationship. This constitutes pre-exposure to both the CS and the “US”, i.e., NBstm which was presented at the time that a standard US ordinarily would be given. With reference to such “proxy” status, it is important to note that NBstm that induces specific associative memory is motivationally neutral, i.e., it has neither positive nor negative valence (Miasnikov et al., 2008a) although it can elicit changes in respiration and heart rate (McLin, Miasnikov, & Weinberger, 2002b). Thus, the current experiment is not merely a demonstration that brain stimulation which is appetitive or aversive can serve as a US, as it is often used in tracing a conditioning circuit (e.g., Chapman, Steinmetz, & Thompson, 1988; Cruikshank, Edeline, & Weinberger, 1992; Steinmetz, Lavond, & Thompson, 1989). Rather, it is a test of a particular model of natural sensory associative learning, which posits a “final common path” for the long-term, specific storage of information via activation of the nucleus basalis, its release of ACh into the auditory cortex and the subsequent engagement of cholinergic receptors in the auditory cortex (Weinberger, 1998, 2007).
Pre-exposure to a CS generally retards subsequent acquisition to that stimulus, a process known as “latent inhibition” (e.g., Lubow & Moore, 1959). Less well known, but also established, are the effects of exposure to the US prior to attempting conditioning. This “US pre-exposure effect” also is indexed by retardation of acquisition, when the US is later used in conditioning (Kremer, 1971).
Additionally, retardation of learning when there is pre-exposure to both the CS and US has been interpreted as “learned irrelevance” (LIRR). (e.g., Baker, 1976; Baker & Mackintosh, 1977; Bennett, Maldonado, & Mackintosh, 1995). However, LIRR has been disputed; other workers have been arguing that the CS/US pre-exposure effect can be explained by recourse to the sum of latent inhibition and the “US alone pre-exposure effect” (e.g., Bonardi & Hall, 1996; Bonardi, Hall, & Ong, 2005; Bonardi & Ong, 2003). Regardless of the ultimate theoretical explanation of the effects of CS and US unpaired pre-exposure on later associative learning, the role of the NB is of interest, at the very least with reference to the characteristics of natural associative memory. If the pre-exposure effect were found with CS/NBstm pre-exposure, then there might be a neural link between a specific brain structure and several important psychological problems.
Group IU received about 200 presentations each of tone and NBstm (i.e., CS and “US”) alone prior to their pairing when the contingencies were reversed in the second phase of the experiment, thus potentially producing LI/LIRR due to pre-exposure to the CS, the “US” or both.
The present study does provide modest support for the pre-exposure effect using tone and NB stimulation. First, the magnitude of CS band conditioned responses was smaller in the IU group than in the IP group, specifically 42% of the latter group (Fig. 3). However, this effect was not statistically significant, therefore it should not be given undue weight. Second, the S–T analysis of response latencies (Fig. 5) demonstrates that the pre-exposure to unpaired stimulation eliminated the early components of conditioned responses following subsequent pairing, delaying associative behavior several seconds (e.g., Fig. 4C and F). The early component is ordinarily well-developed following training in naïve subjects Fig. 4B and E). In addition, the pre-exposure retardation of acquisition was specific to the CS band, a degree of specificity that has not been reported previously. However, whether the current effects are due to latent inhibition or learned irrelevance cannot be determined from the present study.
4.4. Spectro-temporal patterns of associative responses
As noted, CS-specific associative memory developed both in the IP group in initial training and in the IU group after contingency reversal. However, spectro-temporal analyses of associative responses revealed a different latency of associative responses. This associative latency effect would not have been detected had analysis been limited to the 2-s period of tone presentation. Rather, it might have been concluded that pre-exposure of the IU group to the CS and NBstm unpaired had produced little associative response.
These findings demonstrate in general the importance of employing behavioral analyses that are sufficiently sensitive to avoid a Type II error. Thus, while such pre-exposure did not appear to retard subsequent acquisition of processing of frequency information, the significant increase in response latency may indicate the involvement of additional processing resources. Thus, the IU group may have had to overcome presumptive prior learning that the CS predicted nothing, so that suppression of this knowledge may have required an extra step before the new association could become manifest. Such “on-line” behavioral tracking could prove useful in pursuing networks of neurons engaged in the formation of specific memories.
4.5. Future directions for NB-induced associative memory
The current study has demonstrated that specific, associative behavioral memory that is induced by stimulation of the nucleus basalis exhibits experimental extinction. However, it is moot regarding latent inhibition, probably because of the use of physiologically-potent NBstm, which is neither sensory nor motivational in character. These findings raise the issue of how many characteristics of natural memory should be found to also occur for NB-induced memory? For example, should latent inhibition be re-studied by presenting tone alone before pairing it with NBstm? We have explained above our rationale for the current design of maintaining the same overall density of tone and NBstm throughout the study. Innumerable other associative phenomena could be studied as well (Bouton, 2007). At what point is the specter of “diminishing returns” attained? We suggest that this point may now have been reached. NB-induced memory has been shown to possess key attributes of natural associative memory: associativity, specificity, rapid acquisition, consolidation, long-term (days) retention and now experimental extinction.
NB-induced memory also permits a “dissection” of memory processes. It does not involve motivational and emotional processes; thus, aspects of “pure” associative processes can be studied neurobiologically. For example, now that NB-induced memory has been shown to be very similar to natural memory, it would be appropriate to determine the neural effects and the neural networks that enable NBstm to produce associative memory. Another advantage of NB-induced memory is that it provides a way to understand the contents of memory, because the level of activation controls the level of detail that is encoded, stored and retrieved; low levels of activation produces association without frequency specificity (“flat” generalization gradients), whereas moderate levels of activation produce both associativity and specificity (“sharp” generalization gradients with a peak at the CS frequency band) (Weinberger et al., 2006).
Finally, a third line of inquiry that is now possible given the known attributes of NB-induced memory is that of investigating the behavioral use of the embedded information. For example, suppose a subject has “learned” that an 8.0 kHz tone is important because it has been paired with NBstm, although the tone likely signifies importance without serving as a signal for anything. Nonetheless, if 8.0 kHz is now behaviorally pre-potent, then the subject should be more likely to pay attention when it encounters this stimulus in a new setting, in the absence of NBstm. The heightened salience of this tone should have predictable consequences if indeed NB-induced memory involves the encoding of information in the same sense that natural learning does, e.g., the importance of the CS frequency. This information should be capable of facilitating new learning or the solution of a completely novel problem. This outcome would serve as the first validation that specific information can be directly “inserted” into the brain.
Acknowledgments
We thank Gabriel K. Hui and Jacquie D. Weinberger for assistance. This study was funded by Award Number R56DC002938 to NMW from the National Institute on Deafness and Other Communication Disorders.
References
- Baker AG. Learned irrelevance and learned helplessness: Rats learn that stimuli, reinforcers, and responses are uncorrelated. Journal of Experimental Psychology: Animal Behavior Processes. 1976;2:130–141. [Google Scholar]
- Baker AG, Mackintosh NJ. Excitatory and inhibitory conditioning following uncorrelated presentations of CS and UCS. Animal Learning & Behavior. 1977;5:315–319. [Google Scholar]
- Bakin JS, Weinberger NM. Induction of a physiological memory in the cerebral cortex by stimulation of the nucleus basalis. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(20):11219–11224. doi: 10.1073/pnas.93.20.11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartus RT, Flicker C, Dean RL, Pontecorvo M, Figueiredo JC, Fisher SK. Selective memory loss following nucleus basalis lesions: Long term behavioral recovery despite persistent cholinergic deficiencies. Pharmacology, Biochemistry, and Behavior. 1985;23(1):125–135. doi: 10.1016/0091-3057(85)90139-x. [DOI] [PubMed] [Google Scholar]
- Bennett CH, Maldonado A, Mackintosh NJ. Learned irrelevance is not the sum of exposure to CS and US. The Quarterly Journal of Experimental Psychology B: Comparative and Physiological Psychology. 1995;48B:117–128. [PubMed] [Google Scholar]
- Bigl V, Woolf NJ, Butcher LL. Cholinergic projections from the basal forebrain to frontal, parietal, temporal, occipital, and cingulate cortices: A combined fluorescent tracer and acetylcholinesterase analysis. Brain Research Bulletin. 1982;8(6):727–749. doi: 10.1016/0361-9230(82)90101-0. [DOI] [PubMed] [Google Scholar]
- Bonardi C, Hall G. Learned irrelevance: No more than the sum of CS and US preexposure effects? Journal of Experimental Psychology: Animal Behavior Processes. 1996;22:183–191. [Google Scholar]
- Bonardi C, Hall G, Ong SY. Analysis of the learned irrelevance effect in appetitive Pavlovian conditioning. The Quarterly Journal of Experimental Psychology B: Comparative and Physiological Psychology. 2005;58B:141–162. doi: 10.1080/02724990444000087. [DOI] [PubMed] [Google Scholar]
- Bonardi C, Ong SY. Learned irrelevance: A contemporary overview. The Quarterly Journal of Experimental Psychology B: Comparative and Physiological Psychology. 2003;56B:80–89. doi: 10.1080/02724990244000188. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Learning and behavior: A contemporary synthesis. Sunderland, MA: Sinauer Associates; 2007. [Google Scholar]
- Cabrera SM, Chavez CM, Corley SR, Kitto MR, Butt AE. Selective lesions of the nucleus basalis magnocellularis impair cognitive flexibility. Behavioral Neuroscience. 2006;120(2):298–306. doi: 10.1037/0735-7044.120.2.298. [DOI] [PubMed] [Google Scholar]
- Cape EG, Jones BE. Differential modulation of high-frequency gamma-electroencephalogram activity and sleep–wake state by noradrenaline and serotonin microinjections into the region of cholinergic basalis neurons. Journal of Neuroscience. 1998;18(7):2653–2666. doi: 10.1523/JNEUROSCI.18-07-02653.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cape EG, Jones BE. Effects of glutamate agonist versus procaine microinjections into the basal forebrain cholinergic cell area upon gamma and theta EEG activity and sleep–wake state. European Journal of Neuroscience. 2000;12(6):2166–2184. doi: 10.1046/j.1460-9568.2000.00099.x. [DOI] [PubMed] [Google Scholar]
- Celesia GG, Jasper HH. Acetylcholine released from cerebral cortex in relation to state of activation. Neurology. 1966;16(11):1053–1063. doi: 10.1212/wnl.16.11.1053. [DOI] [PubMed] [Google Scholar]
- Chapman PF, Steinmetz JE, Thompson RF. Classical conditioning does not occur when direct stimulation of the red nucleus or cerebellar nuclei is the unconditioned stimulus. Brain Research. 1988;442(1):97–104. doi: 10.1016/0006-8993(88)91436-9. [DOI] [PubMed] [Google Scholar]
- Chen G, Yan J. Cholinergic modulation incorporated with a tone presentation induces frequency-specific threshold decreases in the auditory cortex of the mouse. European Journal of Neuroscience. 2007;25(6):1793–1803. doi: 10.1111/j.1460-9568.2007.05432.x. [DOI] [PubMed] [Google Scholar]
- Cruikshank SJ, Edeline JM, Weinberger NM. Stimulation at a site of auditory–somatosensory convergence in the medial geniculate nucleus is an effective unconditioned stimulus for fear conditioning. Behavioral Neuroscience. 1992;106(3):471–483. doi: 10.1037//0735-7044.106.3.471. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW, Evenden JL, Marston HM, Jones GH, Sirkiä TE. The effects of excitotoxic lesions of the substantia innominata, ventral and dorsal globus pallidus on the acquisition and retention of a conditional visual discrimination: Implications for cholinergic hypotheses of learning and memory. Neuroscience. 1987;22(2):441–469. doi: 10.1016/0306-4522(87)90346-0. [DOI] [PubMed] [Google Scholar]
- Fisher A, Brandeis R, Chapman S, Pittel Z, Michaelson DM. M1 muscarinic agonist treatment reverses cognitive and cholinergic impairments of apolipoprotein E-deficient mice. Journal of Neurochemistry. 1998;70(5):1991–1997. doi: 10.1046/j.1471-4159.1998.70051991.x. [DOI] [PubMed] [Google Scholar]
- Giovannini MG, Rakovska A, Benton RS, Pazzagli M, Bianchi L, Pepeu G. Effects of novelty and habituation on acetylcholine, GABA, and glutamate release from the frontal cortex and hippocampus of freely moving rats. Neuroscience. 2001;106(1):43–53. doi: 10.1016/s0306-4522(01)00266-4. [DOI] [PubMed] [Google Scholar]
- Heffner HE, Heffner RS, Contos C, Ott T. Audiogram of the hooded Norway rat. Hearing Research. 1994;73(2):244–247. doi: 10.1016/0378-5955(94)90240-2. [DOI] [PubMed] [Google Scholar]
- Introini-Collison IB, McGaugh JL. Modulation of memory by post-training epinephrine: Involvement of cholinergic mechanisms. Psychopharmacology. 1988;94(3):379–385. doi: 10.1007/BF00174693. [DOI] [PubMed] [Google Scholar]
- Jasper HH, Tessier J. Acetylcholine liberation from cerebral cortex during paradoxical (REM) sleep. Science. 1971;172(983):601–602. doi: 10.1126/science.172.3983.601. [DOI] [PubMed] [Google Scholar]
- Johnston MV, McKinney M, Coyle JT. Evidence for a cholinergic projection to neocortex from neurons in basal forebrain. Proceedings of the National Academy of Sciences of the United States of America. 1979;76(10):5392–5396. doi: 10.1073/pnas.76.10.5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kametani H, Kawamura H. Alterations in acetylcholine release in the rat hippocampus during sleep–wakefulness detected by intracerebral dialysis. Life Sciences. 1990;47(5):421–426. doi: 10.1016/0024-3205(90)90300-g. [DOI] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM. Cortical map reorganization enabled by nucleus basalis activity. Science. 1998;279(5357):1714–1718. doi: 10.1126/science.279.5357.1714. [DOI] [PubMed] [Google Scholar]
- Konorski J. Integrative activity of the brain. Chicago, USA: University of Chicago Press; 1967. [Google Scholar]
- Kremer EF. Truly random and traditional control procedures in CER conditioning in the rat. Journal of Comparative and Physiological Psychology. 1971;76:441–448. doi: 10.1037/h0031398. [DOI] [PubMed] [Google Scholar]
- Lennartz RC, Weinberger NM. Analysis of response systems in Pavlovian conditioning reveals rapidly versus slowly acquired conditioned responses: Support for two factors, implications for behavior and neurobiology. Psychobiology. 1992;20(2):93–119. [Google Scholar]
- Lubow RE, Moore AU. Latent inhibition: The effect of nonreinforced pre-exposure to the conditional stimulus. Journal of Comparative and Physiological Psychology. 1959;52(4):415–419. doi: 10.1037/h0046700. [DOI] [PubMed] [Google Scholar]
- Luiten PG, Gaykema RP, Traber J, Spencer DG., Jr Cortical projection patterns of magnocellular basal nucleus subdivisions as revealed by anterogradely transported Phaseolus vulgaris leucoagglutinin. Brain Research. 1987;413(2):229–250. doi: 10.1016/0006-8993(87)91014-6. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Dalley JW, Morrison CH, Everitt BJ, Robbins TW. Selective behavioral and neurochemical effects of cholinergic lesions produced by intrabasalis infusions of 192 IgG-saporin on attentional performance in five-choice serial reaction time task. The Journal of Neuroscience. 2002;22(5):1905–1913. doi: 10.1523/JNEUROSCI.22-05-01905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackintosh NJ. Stimulus selection: Learning to ignore stimuli that predict no change in reinforcement. In: Hinde RA, Stevenson-Hinde J, editors. Constraints on learning. New York: Academic Press; 1973. pp. 75–100. [Google Scholar]
- Mackintosh NJ. The psychology of animal learning. New York: Academic Press; 1974. [Google Scholar]
- Mandel RJ, Gage FH, Thal LJ. Spatial learning in rats: Correlation with cortical choline acetyltransferase and improvement with NGF following NBM damage. Experimental Neurology. 1989;104(3):208–217. doi: 10.1016/0014-4886(89)90031-9. [DOI] [PubMed] [Google Scholar]
- Marrosu F, Portas C, Mascia MS, Casu MA, Fà M, Giagheddu M, et al. Microdialysis measurement of cortical and hippocampal acetylcholine release during sleep–wake cycle in freely moving cats. Brain Research. 1995;671(2):329–332. doi: 10.1016/0006-8993(94)01399-3. [DOI] [PubMed] [Google Scholar]
- McLin DE, 3rd, Miasnikov AA, Weinberger NM. Induction of behavioral associative memory by stimulation of the nucleus basalis. Proceedings of the National Academy of Sciences of the United States of America. 2002a;99(6):4002–4007. doi: 10.1073/pnas.062057099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLin DE, III, Miasnikov AA, Weinberger NM. The effects of electrical stimulation of the nucleus basalis on the electroencephalogram, heart rate, and respiration. Behavioral Neuroscience. 2002b;116(5):795–806. [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Levey AI, Wainer BH. Cholinergic innervation of cortex by the basal forebrain: Cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. Journal of Comparative Neurology. 1983;214(2):170–197. doi: 10.1002/cne.902140206. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Wainer BH, Levey AI. Central cholinergic pathways in the rat: An overview based on an alternative nomenclature (Ch1–Ch6) Neuroscience. 1983;10(4):1185–1201. doi: 10.1016/0306-4522(83)90108-2. [DOI] [PubMed] [Google Scholar]
- Metherate R, Cox CL, Ashe JH. Cellular bases of neocortical activation: Modulation of neural oscillations by the nucleus basalis and endogenous acetylcholine. Journal of Neuroscience. 1992;12(12):4701–4711. doi: 10.1523/JNEUROSCI.12-12-04701.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miasnikov AA, Chen JC, Weinberger NM. Rapid induction of specific associative behavioral memory by stimulation of the nucleus basalis in the rat. Neurobiology of Learning and Memory. 2006;86(1):47–65. doi: 10.1016/j.nlm.2005.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miasnikov AA, Chen JC, Gross N, Poytress BS, Weinberger NM. Motivationally neutral stimulation of the nucleus basalis induces specific behavioral memory. Neurobiology of Learning and Memory. 2008a;90(1):125–137. doi: 10.1016/j.nlm.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miasnikov AA, Chen JC, Weinberger NM. Specific auditory memory induced by nucleus basalis stimulation depends on intrinsic acetylcholine. Neurobiology of Learning and Memory. 2008b;90(2):443–454. doi: 10.1016/j.nlm.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moucha R, Pandya PK, Engineer ND, Rathbun DL, Kilgard MP. Background sounds contribute to spectrotemporal plasticity in primary auditory cortex. Experimental Brain Research. 2005;162(4):417–427. doi: 10.1007/s00221-004-2098-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 3. San Diego: Academic Press; 1997. [DOI] [PubMed] [Google Scholar]
- Phillis JW. Acetylcholine release from the cerebral cortex: Its role in cortical arousal. Brain Research. 1968;7(3):378–389. doi: 10.1016/0006-8993(68)90004-8. [DOI] [PubMed] [Google Scholar]
- Phillis JW, Chong GC. Acetylcholine release from the cerebral and cerebellar cortices: Its role in cortical arousal. Nature. 1965;207(5003):1253–1255. doi: 10.1038/2071253a0. [DOI] [PubMed] [Google Scholar]
- Rasmusson DD, Clow K, Szerb JC. Frequency-dependent increase in cortical acetylcholine release evoked by stimulation of the nucleus basalis magnocellularis in the rat. Brain Research. 1992;594(1):150–154. doi: 10.1016/0006-8993(92)91041-c. [DOI] [PubMed] [Google Scholar]
- Rasmusson D, Szerb JC. Acetylcholine release from visual and sensorimotor cortices of conditioned rabbits: The effects of sensory cuing and patterns of responding. Brain Research. 1976;104(2):243–259. doi: 10.1016/0006-8993(76)90617-x. [DOI] [PubMed] [Google Scholar]
- Russell RW, Escobar ML, Booth RA, Bermúdez-Rattoni F. Accelerating behavioral recovery after cortical lesions. II. In vivo evidence for cholinergic involvement. Behavioral and Neural Biology. 1994;61(1):81–92. doi: 10.1016/s0163-1047(05)80047-0. [DOI] [PubMed] [Google Scholar]
- Rye DB, Wainer BH, Mesulam M-M, Mufson EJ, Saper CB. Cortical projections arising from the basal forebrain: A study of cholinergic and noncholinergic components employing combined retrograde tracing and immunohistochemical localization of choline acetyltransferase. Neuroscience. 1984;13(3):627–643. doi: 10.1016/0306-4522(84)90083-6. [DOI] [PubMed] [Google Scholar]
- Ryle G. The concept of mind. New York: Barnes and Noble; 1949. [Google Scholar]
- Sarter M, Bruno JP. Cognitive functions of cortical acetylcholine: Toward a unifying hypothesis. Brain Research Reviews. 1997;23(1–2):28–46. doi: 10.1016/s0165-0173(96)00009-4. [DOI] [PubMed] [Google Scholar]
- Sarter M, Bruno JP, Givens B. Attentional functions of cortical cholinergic inputs: What does it mean for learning and memory? Neurobiology of Learning and Memory. 2003;80(3):245–256. doi: 10.1016/s1074-7427(03)00070-4. [DOI] [PubMed] [Google Scholar]
- Steinmetz JE, Lavond DG, Thompson RF. Classical conditioning in rabbits using pontine nucleus stimulation as a conditioned stimulus and inferior olive stimulation as an unconditioned stimulus. Synapse. 1989;3(3):225–233. doi: 10.1002/syn.890030308. [DOI] [PubMed] [Google Scholar]
- Stratton LO, Petrinovich L. Post-trial injections of an anti-cholinesterase drug and maze learning in two strains of rats. Psychopharmacologia. 1963;5(1):47–54. doi: 10.1007/BF00405574. [DOI] [PubMed] [Google Scholar]
- Weinberger NM. Physiological memory in primary auditory cortex: Characteristics and mechanisms. Neurobiology of Learning and Memory. 1998;70(1–2):226–251. doi: 10.1006/nlme.1998.3850. [DOI] [PubMed] [Google Scholar]
- Weinberger NM. Associative representational plasticity in the auditory cortex: A synthesis of two disciplines. Learning and Memory. 2007;14(1–2):1–16. doi: 10.1101/lm.421807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger NM, Ashe JH, Metherate R, McKenna TM, Diamond DM, Bakin JS, et al. Neural adaptive information processing: A preliminary model of receptive-field plasticity in auditory cortex during Pavlovian conditioning. In: Gabriel M, Moore J, editors. Learning and computational neuroscience: Foundations of adaptive networks. Cambridge, MA: MIT Press; 1990. pp. 91–138. [Google Scholar]
- Weinberger NM, Miasnikov AA, Chen JC. The level of cholinergic nucleus basalis activation controls the specificity of auditory associative memory. Neurobiology of Learning and Memory. 2006;86(3):270–285. doi: 10.1016/j.nlm.2006.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger NM, Miasnikov AA, Chen JC. Sensory memory consolidation observed: Increased specificity of detail over days. Neurobiology of Learning and Memory. doi: 10.1016/j.nlm.2008.10.012. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters RW, McCabe PM, Schneiderman N. Functional utility and neurobiology of conditioned autonomic responses. In: Moore JW, editor. A neuroscientist’s guide to classical conditioning. New York: Springer-Verlag; 2002. pp. 46–85. [Google Scholar]
- Zhang Y, Hakes JJ, Bonfield SP, Yan J. Corticofugal feedback for auditory midbrain plasticity elicited by tones and electrical stimulation of basal forebrain in mice. European Journal of Neuroscience. 2005;22(4):871–879. doi: 10.1111/j.1460-9568.2005.04276.x. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Hamilton SE, Nathanson NM, Yan J. Decreased input-specific plasticity of the auditory cortex in mice lacking M1 muscarinic acetylcholine receptors. Cerebral Cortex. 2006;16(9):1258–1265. doi: 10.1093/cercor/bhj067. [DOI] [PubMed] [Google Scholar]