Abstract
The taxanes are among the most important cancer chemotherapy drugs approved for clinical use in the last two decades. Paclitaxel is used as first-line therapy for a variety of cancers, and numerous drug delivery approaches are under investigation to enhance its selectivity and effectiveness against tumors. One strategy is to produce sustained, low drug levels within the tumor to enhance apoptosis and inhibit angiogenesis. The interest in altering drug concentration/time exposure profiles to improve therapeutic outcomes creates the necessity to quantify low concentrations of paclitaxel in cells or tissues. Here, a selective solid phase extraction (SPE) method, coupled with a capillary liquid chromatography-tandem mass spectrometry (μLC-MS/MS) method, was developed to quantify low, therapeutically relevant concentrations of paclitaxel that could not be analyzed using conventional LC-MS/MS. Under optimized SPE wash and elution conditions, paclitaxel was selectively extracted from biological samples, and most matrix components were removed. A 150×0.5 mm ID ODS capillary column was used for μLC separation and the flow rate was 12 μL/min. Sample extracts were focused at the front of the μLC column and then eluted with a gradient. The lower limits of detection and quantification were 5 and 20 pg/mL, respectively, permitting quantification of paclitaxel in small tissue samples or in cultured cells exposed to low drug concentrations. The quantitative linear range was 20–20,000 pg/mL. The ability to quantify these low concentrations of paclitaxel provides an important tool to study the concentration-dependent pharmacological effects of this important drug.
1. Introduction
Paclitaxel and docetaxel (Figure 1) are two clinically-approved taxanes that play an important role in chemotherapy for numerous cancers 1–3. Paclitaxel was the first approved, and is administered as the clinical product Taxol® (Bristol-Myers Squibb). Various drug delivery approaches are under investigation to improve therapeutic outcomes and eliminate the toxicity of Cremophor EL, the formulation vehicle in the Taxol® product. Recent data from a variety of sources suggest that low (nano- to sub-nanomolar) concentrations of paclitaxel can exert important pharmacological effects, such as the inhibition of angiogenesis 4–6. Drug delivery vehicles can enhance site-specific drug delivery, reduce toxicity to normal tissues, and provide a means to alter the concentration/time profile of tumor exposure to drug, thereby achieving sustained intra-tumor drug levels 7–14. Furthermore, although the main clinical application of paclitaxel is cancer chemotherapy, sustained-release coatings containing paclitaxel have been applied to stents used in angioplasty surgery 15–17, with the objective of reducing restenosis by sustained release of low concentrations of paclitaxel.
Figure 1.
Molecular structures of (a) Paclitaxel and (b) Docetaxel
Given that the optimization of paclitaxel therapeutic uses may be achieved by administering low and sustained concentrations of the drug, accurate and sensitive methods are necessary for determining the pharmacokinetics of paclitaxel at ultra-low concentrations. Although many analytical techniques have been published for the quantification of paclitaxel 18–22, most have detection limits of 0.2–1 ng/mL, which is inadequate to study cellular accumulation at low, therapeutically relevant drug concentrations.
The purpose of this work was to develop a highly sensitive and accurate assay to quantify paclitaxel when delivered at ultra-low concentrations to tumor cells in culture; quantification of intracellular drug is essential to enable pharmacodynamic studies that will elucidate the concentration-dependence of its pharmacological effects. This task is challenging because the drug is present at low concentration in a highly complex biological matrix, which necessitates exceptional analytical sensitivity and selectivity. Recently our lab developed a novel analytical strategy for ultra-sensitive quantification of corticosteroids in animal tissue samples 23. A selective solid phase extraction (SPE) method, coupled with a highly-sensitive capillary LC (μLC)-MS/MS analysis procedure, was devised. The purpose of the selective SPE was to eliminate the majority of undesirable compounds from the sample matrix, and enable a high sample loading volume on the μLC-MS/MS without compromising chromatographic performance. Ultra-low detection limits (low pg/mL) in plasma were achieved for several corticosteroids. In the research reported here, we have extended this strategy to permit the quantification of low levels of paclitaxel, a considerably more non-polar compound, in biological matrices.
2. Experimental Methods
2.1 Chemicals and cell line
Paclitaxel (99% purity) was either obtained as a gift from Phytogen Life Sciences Inc, (Vancouver, BC, Canada) or from the National Institutes of Health (Bethesda, MD., USA). Docetaxel (97% purity), which used as the internal standard (I.S.) was obtained as a gift from Rhone Poulenc Inc. (Paris, France). HPLC grade methanol, acetonitrile, and water were obtained from B&J (Muskegon, MI, USA). Phosphoric and formic acid were from Sigma (St Louis, MO, USA). Oasis HLB solid phase extraction cartridges (30 μm particle size, 60 mg sorbent) were obtained from Waters (Milford, MA, USA). The Zorbax SB-C18 capillary HPLC column (5 μm particle size, 150×0.5 mm I.D.) was from Agilent (Santa Clara, CA, USA). A121a human ovarian cancer cells were obtained from Roswell Park Cancer Inst. (Buffalo, NY, USA) and were cultured in RPMI 1640 cell culture medium supplemented with 10% fetal bovine serum (FBS)24.
2.2 Optimization of SPE wash and elution conditions
In order to extract and concentrate paclitaxel and docetaxel while simultaneously reducing the quantity of matrix components derived from cell culture medium or cell lysates, a selective SPE procedure was developed. Cell culture medium was spiked to a final concentration of 2 ng/mL paclitaxel and used for the optimization of SPE washing and elution conditions. Five mL aliquots of cell lysates were spiked to 2 ng/mL paclitaxel and used for the optimization of cell sample analysis. Oasis HLB cartridges were conditioned with 5 mL of methanol followed by equilibration with 5 mL of water. In order to release protein-bound drug, phosphoric acid was added to samples to a final concentration of 3%, 23,25. Two 5 mL aliquots of this solution were loaded onto each of 17 conditioned SPE cartridges. After loading, the cartridges were washed with 10 mL of water and separated into two groups. For Group 1 samples, which were intended for optimization of elution solvent composition and conditions, all cartridges were washed with 5 ml of 5% methanol, and paclitaxel was then eluted from the cartridges with 5 ml of solvent consisting of one of the following methanol concentrations (in water, v/v): 60%, 65%, 70%, 75%, 80%, 85%, 90%, 95% or 100%. For Group 2 samples, which were intended for optimization of Wash-2 solvent composition and conditions, individual cartridges were washed with 5 ml of consisting of one of the following methanol concentrations (in water, v/v): 55%, 60%, 65%, 70%, 75%, 80%, 85%, or 90%, and then paclitaxel was eluted from all cartridges with 5 ml of 100% methanol. After elution, samples were spiked with the internal standard (docetaxel) to a final concentration of 20 ng/mL. The samples were dried under nitrogen, reconstituted with 1 mL of methanol, and again dried under nitrogen. After drying, the samples were reconstituted with 200 μL of 35% methanol, transferred to fresh 1.5 ml polypropylene centrifuge tubes, and centrifuged at 7600g for 20 min to remove any insoluble material. One hundred μL of each sample was transferred to LC-MS/MS sample vials for analysis.
2.3. Cell sample preparation
Replicate cultures of A121a cells were grown in 25 cm2 (T-25) flasks. To initiate drug uptake studies, the cell culture medium was removed and replaced with fresh RPMI 1640 cell culture medium containing 10% FBS and 1 ng/mL paclitaxel. Incubation was continued at 37°C for various intervals. At each time point, the cell culture medium was removed from replicate flasks. The cells were washed rapidly with 3 mL of phosphate buffered saline (PBS) containing 5% FBS, then with an additional 3 mL PBS, and finally incubated in 0.5 mL of 0.05% trypsin containing 1 mM EDTA (ethylenediamine tetraacetate). After 3–4 minutes of incubation at 37°C, the dissociated cells were collected into a tube, and the tissue culture flask was washed with an additional 1.5 mL PBS, which was combined with the cell suspension. From the 2 mL of flask contents collected, a volume of 0.2 mL was removed for cell counting. The cell concentration was typically 5×105/mL. Fifty % methanol in water (v/v) was added to the remaining 1.8 mL of cell suspension to achieve a final volume of 5 mL. The samples were then quickly frozen in liquid nitrogen and thawed in a 37°C water bath. After a second rapid freeze-thaw cycle, the samples were stored at −80°C until analysis.
For analysis, the frozen samples were thawed, phosphoric acid was added to a final concentration of 3%, and samples were vortexed for 30 seconds. The I.S. was spiked into each sample at 600 pg/mL. SPE cartridges were conditioned by washing with 5 mL of methanol and then 5 mL of water, and 5 ml of each sample was loaded. The columns were processed using the optimized wash and elution conditions identified by experiments described above; the final conditions were: a wash with 10 mL of water, followed by a wash with 5 mL of 70% methanol, and then elution of the analyte with 5 mL of 97.5% methanol. The eluate was dried under nitrogen, reconstituted with 1 mL of methanol, and dried again under nitrogen. The samples were reconstituted with 200 μL of 35% methanol, transferred into a fresh 1.5 ml polypropylene centrifuge tube, and centrifuged at 7600g for 20 minutes to eliminate particulates. Because of the high aqueous content of the initial mobile phase used for column loading (see below), reconstitution with mobile phase was less reliable for sample reconstitution (data not shown). The centrifuged sample was transferred into a μLC-MS/MS sample vial for analysis.
2.4 μLC-MS/MS
Analysis by μLC-MS/MS was performed using an API 3000 triple-quadrupole mass spectrometer (ABI/Sciex) equipped with a Turboionspray interface. The LC system components were all from Agilent (San Jose, CA, USA), and consisted of a binary capillary pump with a passive microflow rate control system, an online degasser, and a microplate autosampler. The analytical column was an Agilent Zorbax SB (5 μm particle size, 0.5 mm I.D. × 150 mm) C18 capillary column. The injection volume was 8μL, and the flow rate was 12 μL/min. The mobile phases consisted of (A) 30:70 acetonitrile: water with 0.1% (w/v) formic acid, and (B) 90:10 acetonitrile: water with 0.1% (w/v) formic acid. The percent of mobile phase B was held at 30% for the first 2 minutes, and then increased to 100% over 8 minutes. To flush the column after analysis, 100% B was held for 5.5 minutes, and then the column was equilibrated at 30% B for 5 min before the next analysis. Multiple reactions monitoring (MRM) conditions, including m/z of parent/fragment pairs, collision energy, and orifice potential were optimized by direct infusion of solutions containing 1 μg/mL of both the target drug and the internal standard in 0.1% formic acid and 70% methanol. For μLC-MS/MS, the dwell time of each MRM transition was 200 ms, and the pause time for scan parameter changes was 8 ms. The flow rates of nebulizer gas (air) and curtain gas were 1 L/min and 0.6 L/min, respectively. The drying gas was turned off because of the low chromatographic flow rate. The pressure of the collision gas (N2) for collisionally activated dissociation (CAD) was 4.8 mTorr. The ion spray voltage, orifice potential, and ring focus voltage were set at 5000 V, 60 V, and 280 V, respectively.
2.5 Calibration and Method Validation
The method developed was validated separately for two different matrices: cell culture medium and cell lysates. To prepare the cell lysates, A121a human ovarian cancer cells were grown and processed as described above, except no drug was incubated with the cells prior to harvesting. A standard curve was prepared in both sample matrices, and consisted of samples containing 20, 100, 500, 2500 and 20,000 pg/mL paclitaxel. The internal standard (docetaxel) was spiked into all samples at 600 pg/mL. The μLC-MS/MS data was analyzed using Analyst software (ABI, Inc, USA, version 1.4.1). Calibration curves were constructed by taking peak area ratio for the analyte: internal standard and plotting the ratio against analyte concentration. The resulting standard curve was analyzed using weighted (1/x2) least squares regression.
For method validation, a batch of quality control (QC) samples was prepared that contained 80, 400, and 4000 pg/mL drug. This set of standards was compared to the calibration curve at both inter-day and intra-day intervals. Intra-day and inter-day variability was determined by calculating the coefficient of variation (CV%) of the replicate measurements.
3. RESULTS AND DISCUSSION
3.1. SPE Optimization
Preliminary experiments that employed protein precipitation followed by conventional LC-MS/MS analysis 18,20,21,26–28 did not yield the required sensitivity for planned experiments (data not shown). Because ESI/MS constitutes a concentration-dependent detector, low flow capillary LC was selected as a means to increase peak concentration in the detector and thus the analytical sensitivity. However, the loading capacity of μLC columns is also correspondingly lower 29, which to some extent abrogates the advantage of higher sensitivity of μLC-MS/MS. Moreover, a large amount of matrix compounds may be retrieved if a non-selective extraction approach is used for highly complex samples such as those derived from cells, tissues, or biological media. These two factors increase the likelihood that the quantity of compounds in a cell- or tissue-derived sample may exceed the μLC column capacity unless a very low injection volume (Vinj) is used. Our previous work on the ultra-sensitive quantification of corticosteroids in plasma demonstrated that a relatively large Vinj of a biological sample extracted using a generic SPE procedure resulted in seriously deteriorated chromatographic performance, due to column over-capacity caused by co-extracted matrix components 23. To address this problem, a selective SPE procedure was developed to extract the target analytes selectively from the sample and simultaneously removing to the extent possible, either by washing or by retention of the unwanted components on the SPE column during elution 23.
Based on this strategy, we set out to develop a selective SPE approach to concentrate paclitaxel from cellular samples, thereby enabling a relatively large Vinj on the capillary column and increasing sensitivity. The first challenge was to identify optimal wash conditions that would remove interfering matrix components from the SPE cartridge without eluting the drug, and then optimal elution conditions that would recover the target analyte efficiently, yet leave more hydrophobic matrix components on the cartridge. Oasis HLB cartridges were selected, based on a previous investigation of several brands and types 23. Optimization procedures were carried out using culture medium containing 10% FBS and spiked with 2 ng/mL paclitaxel, and then the procedures were validated with cell samples. All samples were mixed with phosphoric acid to a final concentration of 3% to disrupt or inhibit drug-protein binding 23,25, and then loaded on the SPE cartridges in aliquots.
After loading samples, two wash steps were used before elution: Wash-1 consisted of 10 mL water to remove residual proteins and thus protect the cartridges from clogging by protein precipitates that would be produced by exposure to the Wash-2 solvent, which contained high concentrations of methanol. Wash-2 was optimized for retention of the analyte on the SPE cartridge, using the optimization samples denoted Group 2 (cf. Experimental Methods); various methanol-water mixtures of increasing organic solvent strength were tested for their ability to remove less polar matrix components while leaving the target analyte bound. Finally, using the optimization samples denoted Group 1 (cf. Experimental Methods), elution conditions were optimized for maximized yield of the target compound from the cartridge without eluting the more non-polar residual substances. Because comparison of absolute yield from the SPE procedure was of interest (i.e., recovered vs. original), the final eluate, rather than the initial sample, was spiked with the internal standard.
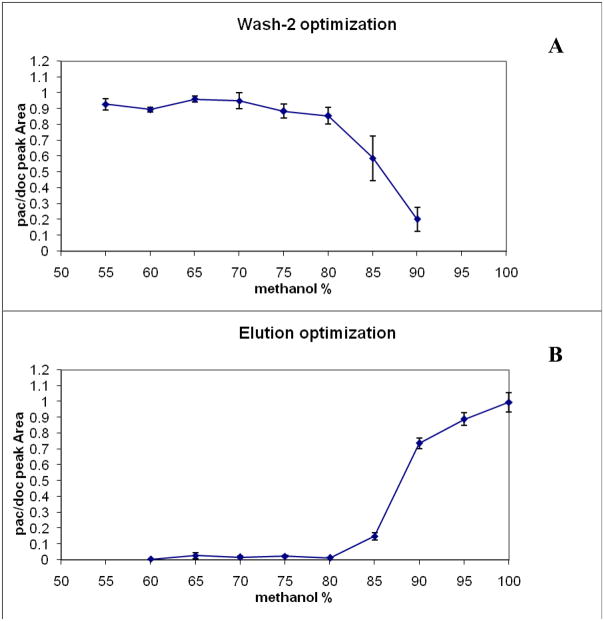
As described in the experimental section, Wash-2 and elution conditions were optimized separately. The results of the two optimizations are shown in Figure 2. From the data shown in Figure 2a, the optimal Wash-2 composition selected was 70% methanol; higher concentrations led to noticeable losses in yield of the analyte. It was reasoned that Wash-2 concentrations lower than 70% methanol would be less effective in eliminating more hydrophobic substances that could remain on the SPE cartridge and then co-elute with paclitaxel. For the elution step, 97.5% methanol was selected. The effect of Wash-2 and elution conditions upon yield of docetaxel (the internal standard) were also investigated and found to be very similar to the conditions optimal for paclitaxel yield (data not shown).
Figure 2. Optimization of the selective SPE conditions.
(A) Optimization of wash-2 solvent composition; (B) optimization of elution solvent composition. Abscissa shows the concentration of methanol in water (vol: vol) used either to wash the SPE cartridge (panel A) or to elute paclitaxel (panel B). Ordinate represents the ratio of the peak area of paclitaxel (PAC) in the eluate compared to the peak area of the internal standard (DOC), which was spiked into the eluate in order to calculate absolute yield.
3.2 μLC-MS/MS optimization
3.2.1 MRM conditions
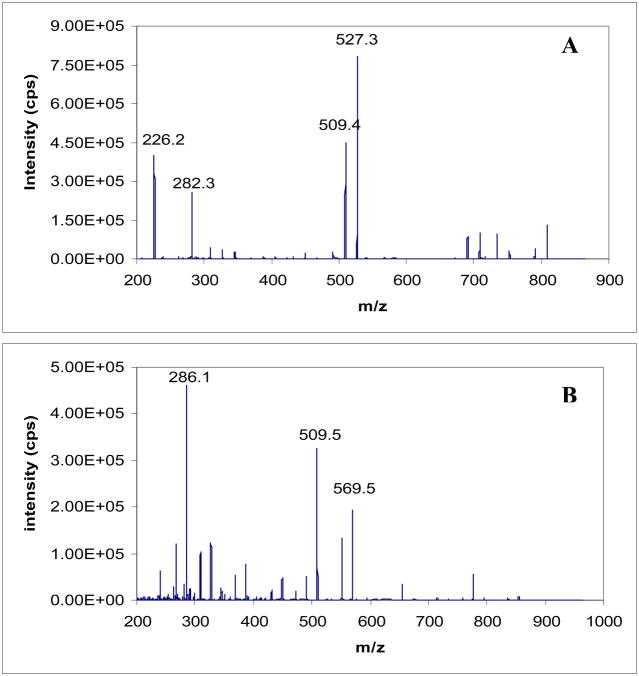
The product ions of paclitaxel and docetaxel were investigated in order to identify appropriate MRM transitions for quantification, and MS/MS conditions were optimized for the highest signal to noise (S/N) ratio for the selected transitions. Figure 3 shows the fragmentation patterns of both compounds. The most abundant ions for each, which were also observed in previous reports 18,20,26–28, were selected for MRM transitions. The optimized conditions for each transition are shown in Table 1.
Figure 3. Product ions of paclitaxel (A) and docetaxel (B) (internal standard).
The collisionally activated dissociation fragments of (A) Paclitaxel and (B) Docetaxel. The spectra were obtained by product ion scans during direct infusion of the two compounds as 1 μg/mL methanol solutions.
Table 1.
Final MRM conditions for the quantification of paclitaxel or docetaxel
| Compound | Major fragments (m/z) | Transition | Optimal collision energy (eV) | |||
|---|---|---|---|---|---|---|
| paclitaxel | 286.2 | 509.6 | 569.6 | 551.5 | 854.5/286 | 23 |
| docetaxel | 527.4 | 226.1 | 509.3 | 282.2 | 808.6/527 | 15 |
3.2.2 Chromatographic optimization
Both column loading and gradient separation conditions were optimized to increase analytical sensitivity and selectivity. As we observed previously 23, focusing of the selectively-extracted samples during the loading procedure can increase assay sensitivity considerably. Here, in order to increase the injection volume (Vinj) without overloading the column or broadening analyte peaks during chromatographic separation, a two- segment gradient was developed. The “trapping segment”, used for loading the sample, employs a relatively high percentage of aqueous solvent (mobile phase A) for 2 min. During this segment, relatively hydrophobic compounds such as the target compound and the I.S. are focused on the front end of the column. The second, a “separation segment,” employs a series of gradient steps that are selected for separation of paclitaxel and the I.S. (docetaxel) from interfering peaks. The chromatographic conditions finally selected are specified in the Experimental section.
The ability to load higher sample volumes is advantageous, given that it would be difficult to concentrate the eluates from the selective SPE further without compromising quantitative performance. Because the selective SPE greatly reduced the complexity of the cell-derived samples, and the low-organic solvent used for loading in the trapping segment focused the drugs on the front of the column, a relatively large Vinj could be injected on the μLC column. The Vinj suggested by the column manufacturer was 0.2 μL. However, we were able to inject 8 μL of cell lysate-derived, selectively extracted sample onto the 0.5 mm I.D. capillary column without any noticeable peak broadening or peak shape deterioration. We observed that the signal-to-noise ratio increased almost proportionally as the Vinj was increased to the maximum of 8 μL.
3.3 Quantification of paclitaxel in cell culture samples
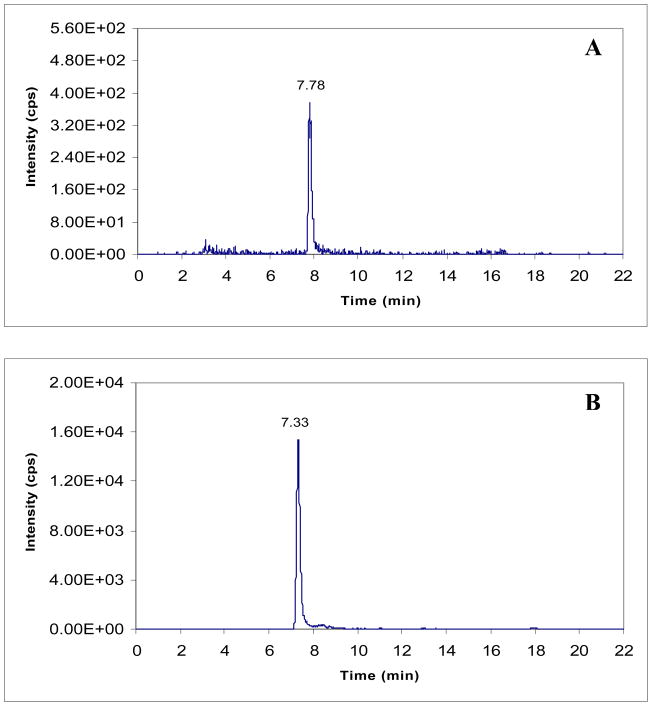
Method validations were carried out in both the cell culture medium and cell lysates. For validation in cell lysates, A121a human ovarian cancer cells were selected because they are a highly sensitive target for paclitaxel-based therapy 24 and have been used for numerous in vivo studies of therapeutic efficacy. The calibration curve for cell lysates showed excellent linearity (r2>0.993) over the paclitaxel concentration range of 20–20,000 pg/mL. The slopes for the calibration curves in cell lysates and culture medium were 1.24 and 1.6, respectively, and the intercepts for cell lysates and culture medium were −0.003 and −0.0002. The CV% for the calibration standards were in the range of 3.8–11%. An ultra-low detection limit of 5 pg/mL was achieved for paclitaxel. The LOQ, which was initially defined as 4 times the detection limit and then validated at this concentration, was 20 pg/mL in cell lysates. This corresponds to 5–8 pg drug/105 cells, depending on the number of cells per mL of harvested suspension. Typical chromatograms for paclitaxel and the I.S. in cell lysates at the LOQ concentration are shown in Figure 4. Accuracies for quantification of drug in cell lysates ranged from 89.8%–106% for QC samples spiked with 3 different drug concentrations (Table 2). Intra-day variations were in the range of 1.5–5.7%, and inter-day variations were approximately 2.4–10.6% (Table 2).
Figure 4. Chromatograms of (A) paclitaxel and (B) docetaxel in cell lysate at 600 pg/mL.
Chromatograms of (A) paclitaxel in cell lysates at 20 pg/mL and (B) docetaxel in cell lysate at 600 pg/mL
Table 2.
Intra- and inter-day variability of paclitaxel quantificationa
| Spiked level (ng/mL) | Intra-day accuracy (%) | Intra-day precision (CV%) | Inter-day accuracy (%) | Inter-day precision (CV%) |
|---|---|---|---|---|
| Cell Lysate: | ||||
| 0.08 | 89.8 | 4.6 | 101.6 | 10.6 |
| 0.4 | 89.9 | 5.7 | 99.3 | 8.2 |
| 4 | 106.4 | 1.48 | 107.6 | 2.4 |
| Cell Medium: | ||||
| 0.08 | 98.7 | 1.8 | 102.2 | 7.7 |
| 0.4 | 91.3 | 4.2 | 94.5 | 3.0 |
| 4 | 88.2 | 3.3 | 96.0 | 13.8 |
Aliquots of spiked cell lysates or cell culture medium, stored at − 20°C, were analyzed three consecutive times in one day (Intra-day, n=3), and on three different days (Inter-day, n=3).;
For method validation in cell culture medium, the linearity was also excellent (r2>0.986) Accuracies for paclitaxel quantification in cell culture media ranged from 88.2–98.0% for QC samples spiked with 3 different concentrations (Table 2). Intra-day variations were in the range of 1.8–4.2%, and inter-day variations were approximately 3.0–13.0% (Table 2). The accuracies for the LOQ were in the range of 87–111%, and the CV% were in the range of 8–12%. In both the culture media and cell lysates, the stability of the extracted drugs appeared to be acceptable, with <5% loss of paclitaxel from samples after storage for more than 6 weeks at −20°C. No noticeable matrix effects were observed during method validation.
3.4 Quantification of time-depended paclitaxel accumulation in A121a ovarian cancer cells
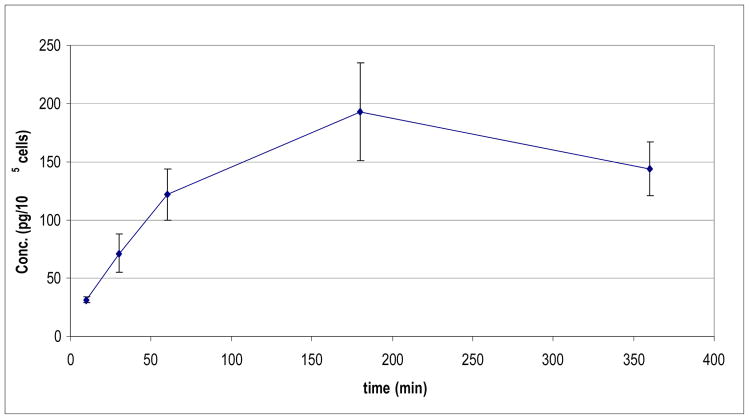
To demonstrate the utility of this method, A121a human ovarian cancer cells were treated with low concentrations of paclitaxel for varying intervals in order to investigate the temporal dependence of cellular paclitaxel accumulation. Our initial results, using conventional LC-MS/MS assays similar to those published, showed that quantification of cellular uptake was feasible when cells were exposed to ≥ 20 ng/mL paclitaxel. However, lower concentrations of drug, which are of interest in the context of investigating pharmacological effects of low-dose paclitaxel (e.g. 1 ng/mL and lower), yielded cellular accumulations below the limit of quantification (data not shown). By employing the selective SPE strategy described here, in conjunction with μLC-MS/MS, we were able to observe the kinetics A121a cell uptake of paclitaxel during incubation with 1 ng/mL drug, with good temporal resolution. The intracellular drug concentration increased with exposure time until a maximum value was achieved after approx 120–180 min (Figure 5). The earliest point at which drug uptake was measured (Figure 5) corresponds to approximately 75 pg/mL in the cell lysate.
Figure 5. Paclitaxel uptake by A121a human ovarian cancer cells.
Cells were grown to 90% confluence in 25 cm2 flasks and at time “0” were exposed to 1 ng/ml paclitaxel for varying periods of time. At the times indicated, flasks in triplicate were washed with buffer, and cells were dissociated and counted. Samples were then processed for paclitaxel content as described in Experimental Methods. Drug concentration data (ordinate) is normalized per 105 cells. Maximum uptake of paclitaxel was observed within 180 minutes of drug addition.
To our knowledge, quantification of cellular uptake of paclitaxel has not been performed previously for such low concentrations of drug, due to the lack of analytical sensitivity. Investigation of the literature revealed that the best limit of detection for published LC-MS/MS-based assays was approx. 0.2 ng/mL, and the feasible LOQ was considerably higher. Therefore, the analytical strategy described here offers improvement of the sensitivity for paclitaxel quantification by at least 40 fold, and will enable future detailed studies of cellular uptake of paclitaxel at extremely low, but pharmacologically relevant, concentrations.
4. CONCLUSIONS
The ability to quantify low levels of paclitaxel in cells and bio-fluids is essential for the investigation of the pharmacological effects of ultra-low concentrations of drug. Previous assay limits of quantification were insufficient for this purpose. Using a strategy similar to one we developed for ultra-sensitive quantification of corticosteroids 23, we developed a selective SPE-μLC-MS/MS approach. The selective SPE cleanup procedure concentrates the target analyte while decreasing or eliminating interfering matrix components. In conjunction with a 2-segment LC gradient procedure, the selective SPE enabled a relatively large Vinj to be employed with the highly sensitive μLC-MS/MS. As a result, an ultra-low detection limit of 5 pg/mL was achieved, and the LOQ was 20 pg/mL. Using this method, high sensitivity and excellent reproducibility has been achieved for quantifying low concentrations of paclitaxel in cell culture medium and cell extracts. The data acquired will be used in continuing investigations to develop novel formulations for paclitaxel delivery.
Acknowledgments
This work was supported by grant R01CA107570 from the National Cancer Inst., National Institutes of Health (USA) to RMS, and an MBRS supplement to R01CA107570 for JRG. The LC-MS/MS was obtained through a Shared Instrumentation Grant (S10RR14573) from the National Center for Research Resources, NIH, USA. We thank the Pharmaceutical Sciences Instrumentation Facility at the University at Buffalo (State University of New York, USA) for the use of the liquid chromatography/tandem mass spectrometry systems and Ms. D. Ruszaj for assistance with the analysis.
Abbreviations
- EDTA
ethylenediamine tetraacetate
- FBS
fetal bovine serum
- HPLC
High performance liquid chromatography
- LC-MS/MS
Liquid chromatography/tandem mass spectrometry
- MRM
Multiple reactions monitoring
- PBS
Dulbecco’s Phosphate buffered saline
- SPE
solid phase extraction
- μLC-MS/MS
capillary liquid chromatography-tandem mass spectrometry
References
- 1.Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT. J Am Chem Soc. 1971;93:2325–2327. doi: 10.1021/ja00738a045. [DOI] [PubMed] [Google Scholar]
- 2.Blagosklonny MV, Fojo T. Int J Cancer. 1999;83:151–156. doi: 10.1002/(sici)1097-0215(19991008)83:2<151::aid-ijc1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 3.Miller ML, Ojima I. Chem Rec. 2001;1:195–211. doi: 10.1002/tcr.1008. [DOI] [PubMed] [Google Scholar]
- 4.Pushkarev VM, Starenki DV, Saenko VA, Namba H, Kurebayashi J, Tronko MD, Yamashita S. Endocrinology. 2004;145:3143–3152. doi: 10.1210/en.2004-0127. [DOI] [PubMed] [Google Scholar]
- 5.Kunstfeld R, Wickenhauser G, Michaelis U, Teifel M, Umek W, Naujoks K, Wolff K, Petzelbauer P. J Invest Dermatol. 2003;120:476–482. doi: 10.1046/j.1523-1747.2003.12057.x. [DOI] [PubMed] [Google Scholar]
- 6.Ng SS, Figg WD, Sparreboom A. Cancer Res. 2004;64:821–824. doi: 10.1158/0008-5472.can-03-3391. [DOI] [PubMed] [Google Scholar]
- 7.Ganesh T, Yang C, Norris A, Glass T, Bane S, Ravindra R, Banerjee A, Metaferia B, Thomas SL, Giannakakou P, Alcaraz AA, Lakdawala AS, Snyder JP, Kingston DG. J Med Chem. 2007;50:713–725. doi: 10.1021/jm061071x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gelderblom H, Verweij J, van Zomeren DM, Buijs D, Ouwens L, Nooter K, Stoter G, Sparreboom A. Clin Cancer Res. 2002;8:1237–1241. [PubMed] [Google Scholar]
- 9.Fetterly GJ, Straubinger RM. AAPS PharmSci. 2003;5:E32. doi: 10.1208/ps050432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma US, Balasubramanian SV, Straubinger RM. J Pharm Sci. 1995;84:1223–1230. doi: 10.1002/jps.2600841015. [DOI] [PubMed] [Google Scholar]
- 11.Sharma A, Straubinger RM. Pharm Res. 1994;11:889–896. doi: 10.1023/a:1018994111594. [DOI] [PubMed] [Google Scholar]
- 12.Straubinger RM, Sharma A, Murray M, Mayhew E. J Natl Cancer Inst Monogr. 1993:69–78. [PubMed] [Google Scholar]
- 13.Hofheinz RD, Gnad-Vogt SU, Beyer U, Hochhaus A. Anticancer Drugs. 2005;16:691–707. doi: 10.1097/01.cad.0000167902.53039.5a. [DOI] [PubMed] [Google Scholar]
- 14.Gradishar WJ, Tjulandin S, Davidson N, Shaw H, Desai N, Bhar P, Hawkins M, O’Shaughnessy J. J Clin Oncol. 2005;23:7794–7803. doi: 10.1200/JCO.2005.04.937. [DOI] [PubMed] [Google Scholar]
- 15.Pasquier E, Honore S, Pourroy B, Jordan MA, Lehmann M, Briand C, Braguer D. Cancer Res. 2005;65:2433–2440. doi: 10.1158/0008-5472.CAN-04-2624. [DOI] [PubMed] [Google Scholar]
- 16.Doven O, Ozcan TI, Cicek D, Camsari A, Akkus N, Aytacoglu BN, Ozeren M, Camdeviren H, Cin VG. Int Heart J. 2006;47:1–12. doi: 10.1536/ihj.47.1. [DOI] [PubMed] [Google Scholar]
- 17.Stone GW, Ellis SG, Cox DA, Hermiller J, O’Shaughnessy C, Mann JT, Turco M, Caputo R, Bergin P, Greenberg J, Popma JJ, Russell ME. N Engl J Med. 2004;350:221–231. doi: 10.1056/NEJMoa032441. [DOI] [PubMed] [Google Scholar]
- 18.Guo W, Johnson JL, Khan S, Ahmad A, Ahmad I. Anal Biochem. 2005;336:213–220. doi: 10.1016/j.ab.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 19.Sparreboom A, van Zuylen L, Brouwer E, Loos WJ, de Bruijn P, Gelderblom H, Pillay M, Nooter K, Stoter G, Verweij J. Cancer Res. 1999;59:1454–1457. [PubMed] [Google Scholar]
- 20.Parise RA, Ramanathan RK, Zamboni WC, Egorin MJ. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;783:231–236. doi: 10.1016/s1570-0232(02)00659-1. [DOI] [PubMed] [Google Scholar]
- 21.Hou W, Watters JW, McLeod HL. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;804:263–267. doi: 10.1016/j.jchromb.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 22.Gardner ER, Liau CT, Chu ZE, Figg WD, Sparreboom A. Rapid Commun Mass Spectrom. 2006;20:2170–2174. doi: 10.1002/rcm.2577. [DOI] [PubMed] [Google Scholar]
- 23.Qu J, Qu Y, Straubinger RM. Anal Chem. 2007;79:3786–3793. doi: 10.1021/ac062184r. [DOI] [PubMed] [Google Scholar]
- 24.Sharma A, Straubinger RM, Ojima I, Bernacki RJ. J Pharm Sci. 1995;84:1400–1404. doi: 10.1002/jps.2600841204. [DOI] [PubMed] [Google Scholar]
- 25.Samtani MN, Lohle M, Grant A, Nathanielsz PW, Jusko WJ. Drug Metab Dispos. 2005;33:1124–1130. doi: 10.1124/dmd.105.004309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grozav AG, Hutson TE, Zhou X, Bukowski RM, Ganapathi R, Xu Y. J Pharm Biomed Anal. 2004;36:125–131. doi: 10.1016/j.jpba.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 27.Wang LZ, Goh BC, Grigg ME, Lee SC, Khoo YM, Lee HS. Rapid Commun Mass Spectrom. 2003;17:1548–1552. doi: 10.1002/rcm.1091. [DOI] [PubMed] [Google Scholar]
- 28.Gustafson DL, Long ME, Zirrolli JA, Duncan MW, Holden SN, Pierson AS, Eckhardt SG. Cancer Chemother Pharmacol. 2003;52:159–166. doi: 10.1007/s00280-003-0622-z. [DOI] [PubMed] [Google Scholar]
- 29.Vissers JPC, Claessens HA, Cramers CA. J Chromatogr A. 1997;779:1–28. [Google Scholar]