Abstract
Memories are usually multidimensional, including contents such as sensory details, motivational state and emotional overtones. Memory contents generally change over time, most often reported as a loss in the specificity of detail. To study the temporal changes in the sensory contents of associative memory without motivational and emotional contents, we induced memory for acoustic frequency by pairing a tone with stimulation of the cholinergic nucleus basalis. Adult male rats were first tested for behavioral responses (disruption of ongoing respiration) to tones (1–15 kHz), yielding pre-training behavioral frequency generalization gradients (BFGG). They next received three days of training consisting of a conditioned stimulus (CS) tone (8.00 kHz, 70 dB, 2 s) either Paired (n = 5) or Unpaired (n = 5) with weak electrical stimulation (~48 μA) of the nucleus basalis (100 Hz, 0.2 s, co-terminating with CS offset). Testing for behavioral memory was performed by obtaining post-training BFGGs at two intervals, 24 and 96 h after training. At 24 h post-training, the Paired group exhibited associative behavioral memory manifested by significantly larger responses to tone than the Unpaired group. However, they exhibited no specificity in memory for the frequency of the tonal CS, as indexed by a flat BFGG. In contrast, after 96 h post-training the Paired group did exhibit specificity of memory as revealed by tuned BFGGs with a peak at the CS-band of frequencies. This increased detail of memory developed due to a loss of response to lower and higher frequency side-bands, without any change in the absolute magnitude of response to CS-band frequencies. These findings indicate that the sensory contents of associative memory can be revealed to become more specific, through temporal consolidation in the absence of non-sensory factors such as motivation and emotion.
Keywords: Acetylcholine, Association, Auditory system, Conditioning, Nucleus basalis
1. Introduction
Memory storage has two essential temporal characteristics: changes in strength and changes in content. An understanding of these qualities is essential to achieve an adequate neurobiological account of mnemonic processes. Changes in memory strength have received extensive study. That post-experiential factors can either increase or decrease memory strength is one of the foundations of contemporary approaches to and understanding of the neural bases of memory (reviewed in McGaugh, 2000). In contrast, the brain mechanisms underlying changes in the actual content of memory have received less attention. This lack of information may reflect, in part, the difficulty in obtaining neural representations of the specific contents of memory. However, recent research on sensory cortical fields may provide a convenient point of entry.
In contrast to traditional conceptions of sensory cortices as simply stimulus analyzers, contemporary neuroscience recognizes that even the primary auditory, somatosensory and visual cortices also have important functions in learning and memory. For example, sensory stimuli that gain behavioral importance through learning receive preferential processing. Most extensively studied in the primary auditory cortex (A1), this generally takes the form of a re-tuning of receptive fields so that more cells respond better to the signal stimulus (Dahmen & King, 2007; Edeline, 2003; Fritz, Elhilali, & Shamma, 2005; Irvine, 2007; Palmer, Nelson, & Lindley, 1998; Rauschecker, 2003; reviewed in Weinberger, 1995; Weinberger, 2007). This specificity of re-tuning, termed “associative representational plasticity”, has led to a reconceptualization of the primary auditory cortex in particular, and to a challenge of the traditional conception of the “sensory–association–motor” organizational schema of the cortex in general (Weinberger, 2008).
Associative representational plasticity provides a means for determining the temporal dynamics of cerebral representations of acquired information by permitting the tracking of learning-induced changes in responses to a stimulus dimension, rather than being restricted to a single signal stimulus, such as a conditioned stimulus (CS). For example, classical aversive conditioning to a tone is accompanied by tuning shifts in the primary auditory cortex (A1) that are directed to the frequency of the CS. However, the temporal evolution of such associative re-tuning depends on the frequency (octave) distance between the pre-training best frequency (BF) of cells; neurons tuned near the CS frequency exhibit immediate tuning shifts to the CS frequency whereas neurons that are spectrally more distant require up to three days to complete the tuning shift (Galván & Weinberger, 2002).
While analysis of representational plasticity provides novel insights into learning-induced neural mechanisms, it remains necessary to obtain comparable behavioral information on the temporal dynamics of the sensory contents of memory. Studied in relative isolation, the contributions of each component of memory content may be elucidated. The multidimensional nature of memory content does complicate inquiry. Thus, memories include not only content about discrete (often conditioned) stimuli, but also, among other items, information concerning motivational state, the hedonic nature of events and emotional reactions to the entire experience. The multidimensionality of natural memory makes it difficult to interpret changes in memory content over time as reflecting only the dynamics of sensory content. However, it appears feasible to induce associative memory in Pavlovian conditioning that is largely or entirely devoid of motivational and emotional content.
We have developed an approach, based on a systems-level model of auditory associative plasticity and behavior, that postulate activation of the cholinergic nucleus basalis as a “final common path” which is sufficient to promote or induce long-term memory. In this schema, the nucleus basalis is thus “downstream” of motivational and emotional systems (Weinberger, 1998; Weinberger et al., 1990b). We have been able to induce auditory behavioral memory by pairing a tone with stimulation of the nucleus basalis (NBstm). (We use the redundant term “behavioral memory” to distinguish it from the all-too-common practice of equating learning-related neural plasticity with “memory”.) Memory induced by pairing a tone with NBstm (hereafter “NB-induced memory”) has major characteristics of “natural memory”. It is associative, highly specific to the CS frequency and can be rapidly- induced (McLin, Miasnikov, & Weinberger, 2002, 2003; Miasnikov, Chen, & Weinberger, 2006). Furthermore, the contents (amount of acoustic detail) of such NB-induced memory can be controlled by the level of NB activation. Weak stimulation (~45.0 μA) produces associative memory lacking frequency specificity whereas moderate stimulation (~65.0 μA) also induces CS-specific behavioral memory (Weinberger, Miasnikov, & Chen, 2006). NB-induced memory is mediated by ACh that engages muscarinic receptors, as it is blocked by post-training administration of scopolamine (Miasnikov, Chen, & Weinberger, 2008b). However, NB-induced associative memory can be induced without the likely engagement of hedonic or motivational content, or emotional reaction thereto, that usually accompanies classical conditioning. Thus, NB stimulation that can induce specific associative memory does not affect behavior in a conditioned place preference (CPP) test; subjects neither prefer nor avoid an area in which they have received memory-inducing stimulation (Miasnikov, Chen, Gross, Poytress, & Weinberger, 2008a).
The present report is concerned with the temporal dynamics of associative memory for acoustic frequency. Rats were trained either with an 8.00 kHz tone either paired with NBstm or tone and NBstm unpaired. They were tested for associativity and specificity of memory 24 and 96 h after training. The present findings indicate that the specificity of the sensory (acoustic) contents of auditory associative memory change systematically over time; the contents become more specific.
2. Materials and methods
The materials and methods were generally the same as those previously reported (Miasnikov et al., 2006; Weinberger et al., 2006), and thus will be described only briefly. All procedures were performed in accordance with the University of California Irvine Animal Research Committee and the NIH Animal Welfare guidelines. During training and testing, subjects were continuously monitored by video cameras.
2.1. Subjects and surgery
The subjects were 10 adult male Sprague–Dawley rats (428 ± 59 g, mean ± SD) housed individually with ad libitum food and water, on a 12/12 h light–dark cycle (lights on at 7:15 AM). Following several days of adaptation to the vivarium, they were handled and learned to sit calmly during attachment of a thermistor assembly and a cable to their skull pedestal. Under general anesthesia (sodium pentobarbital, 40 mg/kg i.p.), a 0.8-mm diameter stainless steel recording epidural screw electrode was inserted over the right primary auditory cortex at the locus showing the largest amplitude evoked potential to a contralateral noise burst. Two screws over the frontal sinus served as reference electrodes. EEG recordings obtained from the auditory cortex were used to monitor arousal state throughout the study, using analyses identical to those previously reported (Weinberger et al., 2006), and to insure that NB stimulation elicited cortical activation, which is an index of the cortical release of acetylcholine during natural behavior and by NB stimulation (Casamenti, Deffenu, Abbamondi, & Pepeu, 1986; Celesia & Jasper, 1966; Duque, Balatoni, Detari, & Zaborszky, 2000; Détári, Rasmusson, & Semba, 1997, 1999; Marrosu et al., 1995). A concentric bipolar stainless steel stimulating electrode was implanted through the contralateral (left) hemisphere (45° angle in the frontal plane at AP −2.2, L 3.2; Paxinos & Watson, 1997), into the right nucleus basalis. The final locus was determined by obtaining 1–5 s of auditory cortical EEG activation to stimulation of the nucleus basalis (NBstm; pairs of 0.2 ms opposite polarity pulses, 100 Hz, 200–300 ms trains; S88 stimulator, PSIU6 isolation units, Grass Instrument Co., Quincy, MA). A dental acrylic pedestal was built with two aluminum hex threaded standoffs embedded therein, and all leads connected to a miniature socket that could be led to a commutator via a multi-conductor cable. Subjects were allowed 1–2 weeks to recover from surgery.
2.2. Stimuli, recording and data analyses
Training and testing took place while each subject was in a box (23 × 23 × 31 cm) supplied with fresh bedding and lined inside with acoustic-damping tile, contained in a double-walled acoustic chamber. Acoustic stimuli were pure tones (1.0–15.0 kHz, 2 s duration, cosine 10 ms rise/fall time [10–90%], 70 dB SPL) produced by Tucker–Davis Technologies (TDT, Alachua, FL) System 3 components and delivered to a calibrated loudspeaker positioned 35 cm above the floor of the box. NBstm current used during training was several times weaker than that used during surgery because the absence of anesthesia greatly reduces the threshold for EEG activation, as described below.
To assess the induction of memory, we measured the disruption of the ongoing pattern of regular respiration to various tones before and after training. The rationale for using respiration to detect learning is that the pairing of a tone with another stimulus constitutes classical (Pavlovian) conditioning. Changes in respiration (and several autonomic measures) are sensitive indices of successful classical conditioning, i.e., they reveal the association between two sequentially-paired stimuli, and are widely employed in the field of learning and memory (reviewed in, e.g., Winters, McCabe, & Schneiderman, 2002). Lacking such sensitive behavioral conditioned responses, it would not be possible to determine if the NB is part of the circuitry underlying auditory memory by using motivationally- neutral NB brain stimulation, because NB stimulation itself does not produce eye-blinks or other somatic unconditioned responses.
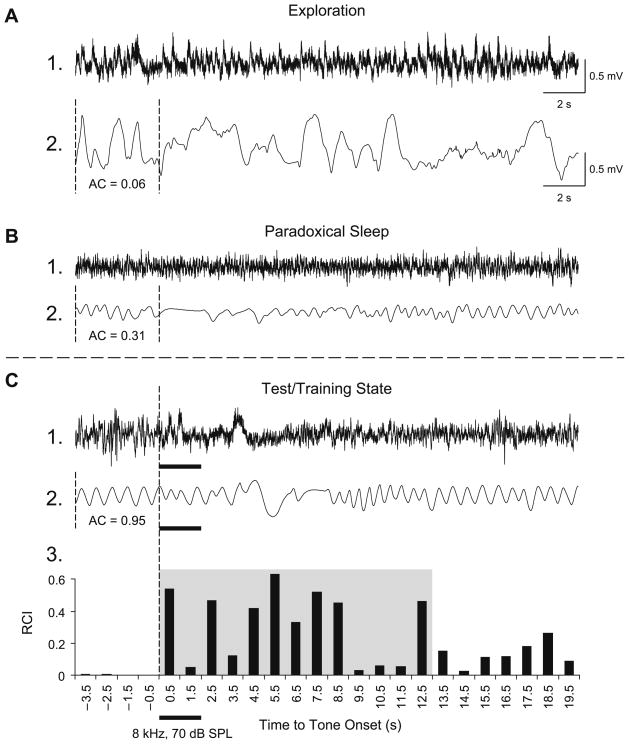
Respiration was detected as breathing-related thermal fluctuations with a glass-encapsulated thermistor attached to a light-weight pedestal-mounted assembly positioned in front of a naris, as described previously (McLin et al., 2002). The amplified output signal was fed to an A–D module, and the autocorrelation function (AC) was calculated on-line. The AC was used to present tones only when the subject was in a quiet behavioral state (Miasnikov et al., 2008b), thus excluding states such as the exploration/grooming and paradoxical (REM) sleep (Fig. 1A and B). Trials meeting the criterion of regular baseline (AC≥0.95) for over 4 s (Fig. 1C) were presented if the scheduled inter-trial interval period had passed (30–180 s). This state control was employed to avoid giving stimuli when very high levels of ACh were being released in the cortex, as during exploration or REM sleep (Giovannini et al., 2001; Jasper & Tessier, 1971; Kametani & Kawamura, 1990; Marrosu et al., 1995) to prevent a ceiling effect, thus promoting a physiologically-effective release of ACh by NBstm.
Fig. 1.
Behavioral state control. Examples of measures of respiration corresponding to major behavioral states: exploration, paradoxical sleep, quiet waking. The pattern of respiration can serve as a reliable marker for each state (Miasnikov et al., 2008b; Weinberger et al., 2006). Shown are the EEGs from the primary auditory cortex (lines 1) and the respiration records (lines 2). (A) During periods of preoccupation with ongoing activity, such as exploration or grooming, while the EEG is fast (A1), respiration is chaotic (A2: AC = 0.06) and the animal may show disproportionate response to tones (startle). (B) During paradoxical (REM) sleep, the EEG is low-voltage fast (B1) and respiration is irregular although less chaotic (B2: AC = 0.31), with many high-frequency shallow breathing movements. (C) During quiet waking, which is the state used for presenting tone during testing and training, the EEG is less desynchronized high-voltage (C1), animals are not moving, respiration is highly regular (C2: AC = 0.95) and the animals are responsive to tones. The respiration autocorrelation function (AC) was continuously calculated on-line over 4-s long epochs. When a randomly-selected inter-trial interval had passed, the software compared the current value of an AC with pre-selected threshold (AC≥0.95) and triggered a stimulus. C3: Quantification of a regular sinusoidal baseline respiration record (first 4 s) disrupted by tone presentation. The “Respiration Change Index” (RCI, Methods) is sensitive to both increases and decreases in signal amplitude and frequency. This example shows a typical response of a Paired animal to the CS tone, recorded while obtaining the behavioral frequency generalization gradient 24 h following completion of the pairing session. The shaded area indicates the first 13-s portion of the respiratory record containing the majority of tone-evoked response. The RCI values found within this epoch were used in the behavior data analysis.
Major evoked changes in respiration occurred within 0.5–12.5 s after tone onset. The collected data were used to calculate a “Respiration Change Index” (RCI), on a second-by-second basis. The index was sensitive to increases and decreases of both frequency and amplitude. RCIs were calculated as: RCIi = (|Posti − Pre|)/(Posti − Pre) where Post and Pre were the values of power of respiration signal (Miasnikov et al. 2006). An RCI value of zero would indicate no change and a value of 1.0 would indicate complete cessation of respiration. An example of a record of the tone-elicited disruption of respiration is provided in Fig. 1C2 and its RCI quantification in Fig. 1C3. Statistical analyses used SPSS v.15 software (SPSS, Chicago, IL).
2.3. Experimental design
The subjects were assigned to two groups, Paired (n = 5) and Unpaired (n = 5). After recovery from surgery, NBstm thresholds were determined while subjects were in a quiet waking state. NBstm was delivered every few minutes at increasing levels starting at ~30 μA (100 Hz bipolar, 200 ms train) until stimulation reliably elicited 1–5 s epoch of cortical activation (decrease in low frequency activity often accompanied by increase in gamma activity). The current levels used in subsequent training with NBstm did not elicit body movements.
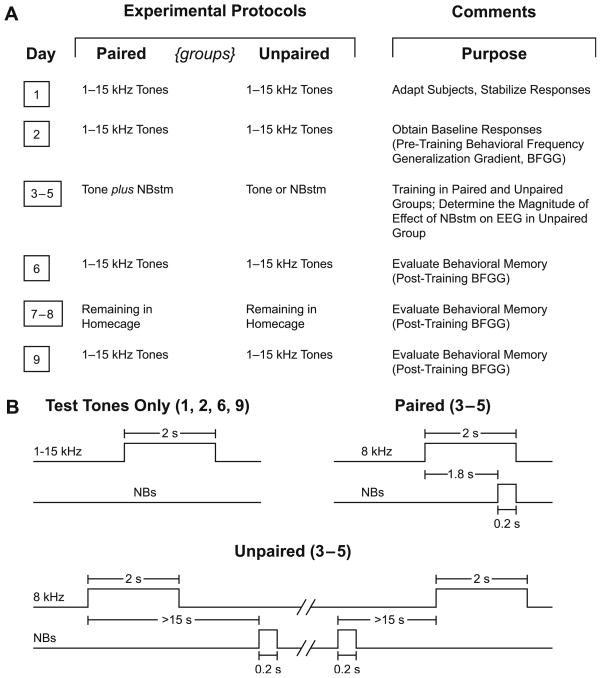
To induce and subsequently evaluate stimulus-specific memory, we used the approach of acquiring behavioral baseline responses to many frequencies, then training with one frequency and later testing the training effects with many frequencies. The protocol required nine consecutive days (Fig. 2A). Days 1–2 obtained the pre-training baseline response to test tones; the Day 1 session was used to acclimatize subjects to the testing environment and thus data from this session were not analyzed. Data from Day 2 constituted the pre-training baseline. Days 3–5 each had one training session, in which a CS tone was paired with NBstm in the Paired group (200 trials per day = 200 tone + 200 NBstm presentations), or the same number of tones and NBstm presented in the Unpaired group. In the Paired group, the CS tone (8.00 kHz, 2 s, 70 dB SPL) was followed by 0.2 s of NBstm (same level as determined post-operatively) that co-terminated with CS offset (i.e., the CS–US interval was 1.8 s). In the Unpaired group, tone and NBstm never overlapped. Inter-trial intervals averaged 80 s (range ~25–150 s) (Fig. 2B). Potential transfer between training and frequency testing sessions was reduced by using different contexts for the two types of session. Thus, animals were delivered to the lab via different circuitous routes and they were trained in the dark (red light) but tested (pre- and post-training) in the light. On Day 6, post-training responses to tones were obtained. The animals were then left for two days (Days 7–8) in their home cages undisturbed and re-tested on Day 9.
Fig. 2.
Experimental design. (A) The six main stages of the experiment used to obtain pre-training and post-training behavioral frequency generalization gradients (BFGG) for the Paired and Unpaired groups. (B) Detailed temporal relationships of stimuli for the various phases of the experiment: delivery of test tones (Days 1, 2, 6 and 9) and tone–NBstm Paired and Unpaired (Days 3–5).
On frequency test days, subjects received random presentation of tones of nine different frequencies (1.00, 2.75, 4.50, 6.25, 8.00, 9.75, 11.50, 13.25, and 15.00 kHz, 70 dB SPL, constrained only by presenting not more than two stimuli of the same frequency in a row) for 200 trials total (Fig. 2B). Intervals between tone presentations averaged 94 s. The initial statistical analyses of respiration responses were based on averaging the data for triplets of frequencies: 1.00–4.50 kHz (lower-band), 6.25–9.75 kHz (middle-band) and 11.50–15.00 kHz (upper-band). The middle frequency band (6.25, 8.00, 9.75 kHz) is referred to as the “CS-band” (Weinberger et al., 2006). The subsequent detailed analysis involved responses to all individual test frequencies as well as certain selected, functionally relevant ones.
2.4. Histology
Following the completion of the experiment, an electrolytic lesion (4 ms pulses at 100 Hz, 500 μA for 60 s) was made with bipolar current through the stimulating electrode while the animal was under sodium pentobarbital anesthesia. The animal was then given an overdose of sodium pentobarbital and perfused through the heart with saline followed with 3.7% paraformaldehyde in 0.1 M phosphate buffer (pH 7.3). The brain was removed and the Anterior–Posterior (AP) and Medial–Lateral (ML) coordinates of the recording electrode relative to Bregma and midline, respectively, were precisely measured on the skull from the interior of the calvaria with a caliper at 0.1 mm resolution. The auditory cortex recording site, which had been determined during surgery by click- or noise burst-induced local field potentials, was estimated by projecting the location of the epidural recording electrode onto the underlying cortical surface. The recording site location was subsequently verified by plotting the obtained coordinates of the mentioned cortical projection onto a stereotaxic map of the auditory and surrounding areas of cortex derived from the Paxinos and Watson (1997) atlas. The AP and ML data collected from individual subjects were combined with respect to experimental protocol, and the groups (Paired vs. Unpaired) were compared using t-test to determine whether the location of the recording sites differed.
Following several days of post-fixation in paraformaldehyde solution with 0.8 M sucrose added for subsequent tissue cryo-protection, the brain was blocked and sectioned at 50 μm with a freezing microtome. The sections were mounted onto gelatincoated slides, dried and stained for Nissl substance to recover the electrolytic lesion sites and thus to determine the actual locus of intracranial stimulation. The location of the stimulation site was then projected onto the closest outline of frontal (coronal) sections taken from the Paxinos and Watson (1997) atlas. Based on subcortical structure outlines, the AP, ML, and Dorsal –Ventral (DV) coordinates of the tip of the stimulating electrode were determined and converted into standardized atlas dimensions. The AP, ML and DV data collected from individual subjects were combined with respect to experimental protocol, and the groups (Paired vs. Unpaired) were compared using a t-test to determine whether the location of the sites of stimulation differed.
3. Results
3.1. Location of electrodes
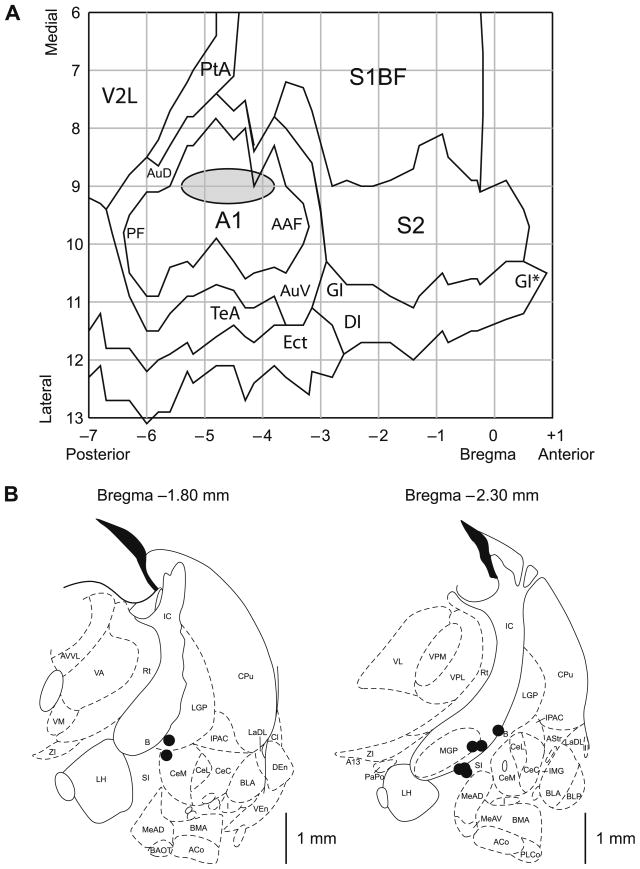
A summary of the location of the recording and stimulating electrodes is presented in Fig. 3. Cortical recording electrodes were all located above the primary auditory cortex, indicating that EEG activation produced by NBstm did occur in A1 (Fig. 3A). The recording sites of the Paired and Unpaired groups were intermingled and did not differ either in the AP (t(6) = 1.06, p > .30, two-tailed t-test) or the ML (t(6) = 0.48, p > .60) dimensions.
Fig. 3.
Location of recording and stimulation sites. (A) The auditory cortex EEG recording locations. The gray oval indicates the location of epidural recordings based on their stereotaxic coordinates using a cortical map derived from Paxinos and Watson (1997). The center of the location area corresponds to the X–Y coordinates of the mean for the group on the flattened standardized cortical surface, and the horizontal and vertical edges of the oval correspond to the ranges from mean − SD to mean + SD for the AP (−4.60 ± 0.78 mm, mean ± SD) and the ML (9.03 ± 0.32 mm) coordinates, respectively. The sites of recording in Paired and Unpaired groups overlapped, did not differ statistically (see Section 3) and thus are shown as a single group. Abbreviations: A1, primary auditory cortex; PF, posterior auditory field; AAF, anterior auditory field; AuD, secondary auditory cortex, dorsal; AuV, secondary auditory cortex, ventral; PtA, parietal association cortex; V2L, secondary visual cortex, lateral area; S1BF, S1 (primary somatosensory) cortex, barrel field; S2, secondary somatosensory cortex; TeA, temporal association cortex; Ect, ectorhinal cortex; DI, dysgranular insular cortex; GI, granular insular cortex; (*), an extension of GI that was arbitrarily cut to fit the diagram. (B) Diagrams of the two coronal sections (AP = −1.8 mm and AP = −2.3 mm) from the atlas showing the NB stimulation sites (Paxinos & Watson, 1997). The electrodes were implanted by a contralateral approach, to avoid damage to ipsilateral structures. The stimulation sites in the Paired and the Unpaired groups were intermingled and did not differ statistically (see Section 3) and thus are shown as a single group. In all animals, stimulation was within the caudal nucleus basalis (ventrolateral internal capsule, ventromedial lateral globus pallidus and nucleus basalis of Meynart) which projects preferentially to the auditory cortex. In general, the coordinates of the area of stimulation, as referenced to the coronal plan, were as follows: AP, −2.18 ± 0.23 mm (mean ± SD); the ML, 3.18 ± 0.26 mm; and the DV, 7.82 ± 0.40 mm. All the located stimulation sites were in the basal forebrain within structures containing corticopetal cholinergic cells (Bigl, Woolf, & Butcher, 1982; Johnston, McKinney, & Coyle, 1979; Mesulam, Mufson, Levey, & Wainer, 1983). Major abbreviations: B, basal nucleus of Meynert; CeM, amygdala central nucleus, medial; CeL, amygdala central nucleus, lateral; CPu, caudate–putamen; IC, internal capsule; IPAC, interstitial nucleus of posterior limb of anterior commissure; LGP, lateral globus pallidus; LH, lateral hypothalamus; MGP, medial globus pallidus; SI, substantia innominata; Rt, reticular thalamic nucleus.
The NB stimulation sites from eight subjects, for which the histology was available, are shown in Fig. 3B. The stimulation sites of the Paired and Unpaired groups were intermingled and did not differ either in the AP (t(6) = 1.73, p > .10, two-tailed t-test), the ML (t(6) = 0.98, p > .35) or the DV (t(6) = 0.08, p > .90) dimensions. For technical reasons we were unable to recover two brains. However, the stimulation was as effective in producing the cortical EEG activation and the stimulation electrodes implants were made in the same manner in those two animals as in the eight subjects presented. There was no difference in NBstm current between the groups (t(8) = 0.76, p > .45; 48 ± 12 μA, mean ± SD for the population).
3.2. Effects of training on NB-induced memory
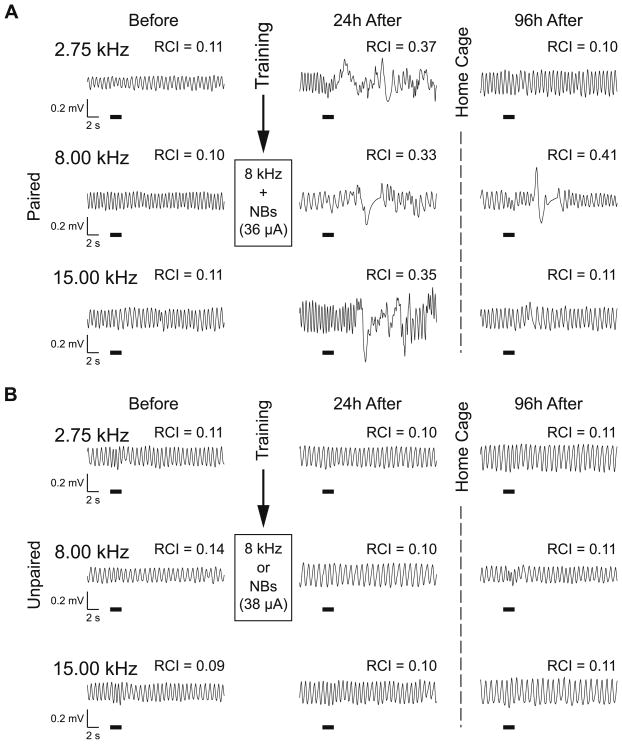
The Paired and Unpaired groups exhibited the same respiratory responses to tones of 1–15 kHz before training. In contrast, 24 h after training, they exhibited differential responses despite the fact that they had been exposed to an identical number of an 8.00 kHz tone and NBstm. Fig. 4 provides examples of respiratory records for a single presentation of three tones for a Paired animal and an Unpaired animal. In this example, both subjects exhibited little response to either the CS tone (8.00 kHz) or a lower (2.75 kHz) or higher (15.00 kHz) tone prior to training. However, after training, the Paired animal displayed a large response to the CS frequency, as well as larger responses to the tones of higher and lower frequencies indicating non-CS-specific response augmentation (Fig. 4A). In contrast, the Unpaired subject did not display post-training responses to the CS frequency or the other frequencies (Fig. 4B). Following several days of no intervention (96 h after the last training day), responses to tone changed. While the Unpaired animal showed little change, the response of the Paired became markedly sharper, being confined to the CS frequency.
Fig. 4.
Effects of paired tone–NBstm training on behavioral memory in Paired and Unpaired groups following training. (A) Examples of respiratory waveforms obtained from a Paired subject. Shown are baseline responses to the CS (8.00 kHz) and a lower (2.75 kHz) and higher (15.00 kHz) frequency during Day 2 (“Before”), Day 6, 24 h post-training (‘24 h After”), and Day 9, 96 h post-training (“96 h After”). RCI values indicate the average over 13 s post-stimulus onset disruption of ongoing respiration. Before training, responses to all three frequencies were minimal (RCI = 0.10–0.11). After training (24 h), the CS frequency produced a large disruption of respiration (RCI = 0.33). The absence of specificity of this example of NB-induced behavioral memory is indicated by the presence of strong responses to the lower (RCI = 0.37) and higher (RCI = 0.35) frequencies. However, 96 h after training, while the CS frequency still produced a large disruption of respiration (RCI = 0.41) the responses to the lower (RCI = 0.10) and higher (RCI = 0.11) frequencies were significantly lower, comparable to pre-training values. (B) Examples of respiratory waveforms from a subject in the Unpaired group. Similar to the Paired group, responses before training were minimal (RCI = 0.09–0.14). In contrast to the Paired group, the response to the CS frequency 24 h after training did not change much (CS RCI = 0.10). The same was true for responses to the lower (RCI = 0.10) and higher (RCI = 0.10) frequencies. Similarly, responses obtained 96 h after training were minimal both at the CS and the lower and higher test frequencies (all RCIs = 0.11). Note that both subjects had comparable level of NBstm (36 μA in Paired group, and 38 μA in Unpaired group) during training. The thick horizontal bars indicate tone presentation.
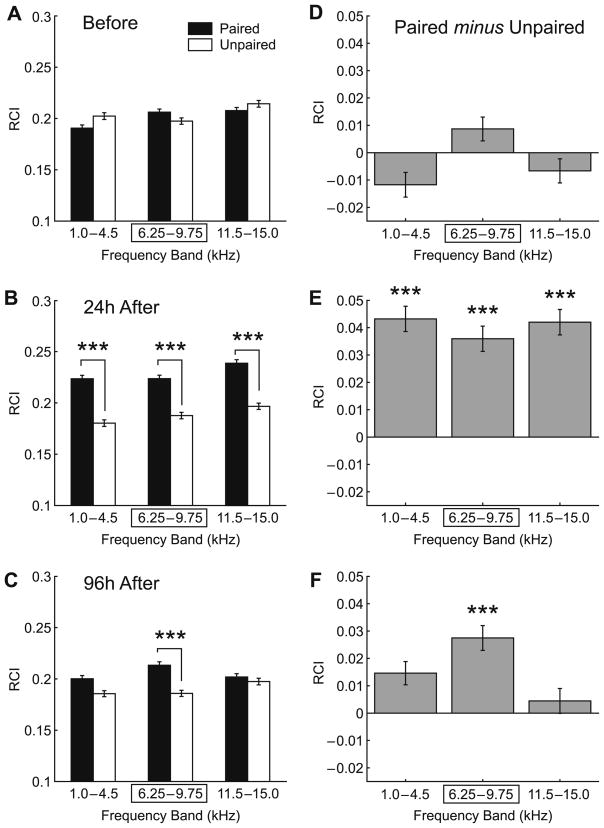
Fig. 5 summarizes the group data. The pre-training behavioral frequency generalization gradients of the Paired and Unpaired groups were similar in all frequency bands, the lower (1.0– 4.5 kHz), the CS (6.25–9.75 kHz) and the higher frequency band (11.5–15.0 kHz). Although animals were differentially sensitive to different frequencies (2-way ANOVA, frequency factor: F(2, 26039) = 11.12, p < .0001), the difference between the Paired and Unpaired groups was not significant (F(1,26039) = 1.59, p > .20), although the group × frequency interaction was significant (F(2,26039) = 5.68, p < .005) (Fig. 5A).
Fig. 5.
Effect of training and retention interval on NB-induced memory. Graph bars show mean ± SE of Respiration Change Index, RCI (Y-axis). (A) Pre-training (‘Before”) frequency generalization gradients to tones in three frequency bands: “Low” (1.0–4.5 kHz), “CS” (6.25–9.75 kHz [framed with rectangle]; the CS was 8.00 kHz) and “High” (11.5–15.0 kHz). There were no significant differences between Paired (black bars) and Unpaired (white bars) groups before training. (B) Post-training BFGGs at 24 h for the Paired and Unpaired groups. Note the significant difference in response in the all frequency bands between the groups indicating associative learning, which was not frequency-specific. (C) Post-training BFGGs at 96 h, for the Paired and Unpaired groups. Note the CS-specificity, due to decreased responses at the low and high-frequency bands, which rendered them no different from the Unpaired group. (D) Baseline between-group BFGG differences (“Paired minus Unpaired”). Note that the Paired group had slightly larger responsiveness to tones within the CS frequency band while the Unpaired group had slightly greater responses to tones within lower and higher frequency bands. There were no significant difference between groups at any frequency band (ANOVA: p > .20, see Section 3 for details). (E) Post-training (24 h) between-group differences. The Paired group had larger responsiveness to tones within each tested frequency band, including the CS-band compared to the Unpaired group. The response differences were significant for the entire distribution (ANOVA: p < .0001), that was attributable to the differences found within each tested frequency band (p < .0001, see Section 3 for details) as measured post hoc. (F) Post-training (96 h) between-group differences. The Paired group had larger responses to tones within each tested frequency band, including the CS-band compared to the Unpaired group. The response differences were significant for the entire distribution (ANOVA: p < .0001). However, in contrast to 24 h post-training the 96 h post-training between-group differences were attributable exclusively to the differences found within the CS frequency band (p < .0001, see Section 3 for details) as measured post hoc. Level of statistical significance: ***p < .005, Tukey’s test.
In contrast, the groups did differ after training (2-way ANOVA, group: F(1, 25987) = 227.70, p < .0001). While the frequency factor remained significant (F(2, 25987) = 12.72, p < .0001), the group × frequency interaction was not significant (F(2, 25987) = 0.70, p > .45). Post hoc tests revealed that the between-group difference was found for all frequency bands (Tukey’s test: CS-band, p < .0001; higher-band, p < .0001; lower-band, p < .0001). That is, the BFGG was essentially flat (Fig. 5B). These findings indicate that the Paired group had acquired an associative memory following tone–NBstm pairing that could be detected 24 h after training. However, this memory was not specific to the CS-band of frequencies.
When animals were re-tested 3 days later (96 h after the completion of the last training session), the groups factor remained significant (F(1, 25909) = 36.64, p < .0001), as did the frequency factor (F(2, 25909) = 3.10, p < .05). However, the group × frequency interaction (F(2, 25909) = 6.69, p < .002) was now significant, indicating a change in the frequency specificity of response. The nature of this frequency-specific effect was revealed in post hoc tests; the between- group difference was limited to the CS frequency band (Tukey’s test: p < .0001) while responses did not differ in lower (p > .05) and higher (p > .99) bands (Fig. 5C). Analysis of between-group differences (Paired minus Unpaired) further illustrates the nature of the differences between the groups. They revealed that while the two groups were matched before the onset of training (Fig. 5D), the differences between the Paired and Unpaired groups increased within all three frequency bands 24 h post-training (Fig. 5E). Furthermore, while remaining all positive the differential responses between the Paired and Unpaired groups were most pronounced at the CS-band 96 h post-training (Fig. 5F).
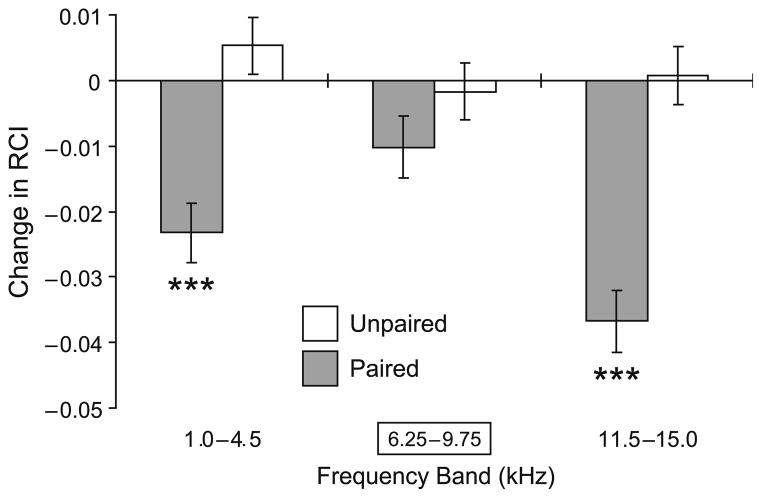
As the changes in BFGGs were most dramatic from 24 to 96 h post-training, we compared the within-group changes for the Paired and Unpaired groups within that time frame Fig. 6). This analysis showed that responses in the Unpaired group did not change significantly. In contrast, the Paired group developed significant loss of response in both the lower (p < .0001) and higher side-band frequencies (p < .0001) while responses within the CS frequency band remained the same (p > .50) as measured 24 h post-training.
Fig. 6.
Comparison of within-group changes (“96 h After minus 24 h After”: post-training 96 h retention test minus post-training 24 h test sessions) in BFGGs. The responses did not change significantly in the Unpaired group (white bars). In contrast, when tested at 96 h, the responses in Paired group (black bars) showed a significant decline (lower-band: p < .0001; higher-band: p < .0001) within the side-bands (relative to the CS), while responses within the CS frequency band remained as potent (p > .50) as measured 24 h post-training. Level of statistical significance: ***p < .005, t-test.
3.3. Temporal pattern of behavioral responses
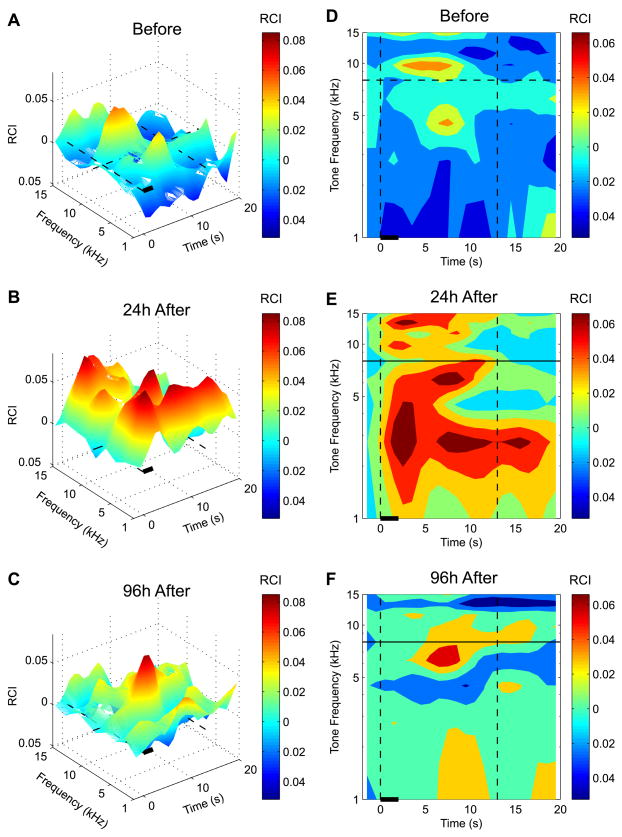
Given the increase in memory specificity for the CS-band over a consolidation period of days, we asked whether any other aspects of behavior also had changed. The recording of respiration responses does provide a spectro-temporal profile of behavior because it yields detailed information not only at a given point in time or brief period, such as during the 2 s tone period, but also for many seconds thereafter, in contrast to most other behavioral measures. This is relevant because behavior that occurs following a stimulus may also be indicative of mnemonic processes. To visualize the over-all spectro-temporal (S–T) pattern of differences between groups, we subtracted the S–T pattern of the Unpaired group from the S–T pattern of the Paired group; this yielded the associative differences between groups for the 24 and 96 h retention periods as well as relevant baseline data. The findings are shown in Fig. 7, in two displays: 3-D differences in magnitude of S–T patterns (Fig. 7A–C) and 2-dimension differences divided into quintiles of response differences (Fig. 7D–F). Although such S–T group differences are not statistically evaluated, the striking changes in pattern over days are sufficiently novel to warrant consideration.
Fig. 7.
Spectro-temporal (S–T) response characteristics of differences between the two groups (Paired minus Unpaired groups), yielding associative effects after training. A and D, baseline; B and E, 24 h post-training; C and F, 96 h post-training. The plots represent original 3-D response surfaces (A–C) and 2-D response maps (D–F) in quintile format (each color represents the area containing 20% of responses measured according to their amplitudes ranked top to bottom). Note that to provide clear visual representations of S–T patterns, we applied a smoothing function: RCIt = (RCIt−2 + 2 × RCIt−1 + 3 × RCIt + 2 × RCIt+1 + RCIt+2)/9 where RCIt was the response amplitude measured at the moment of time “t” with 1 – s resolution (Methods). The smoothing did not shift positions of peaks and/or valleys but did cause the spread of data at temporal extremes for up to 2 s in both (backward and forward) directions along the time dimension. This response spillover over the time axis explains why the increased (E) or the reduced (F) responses to certain tones “invade” the pre-stimulus space. However, the smoothing function was used only for illustrative purposes as all the statistical analyses were conducted on raw unsmoothed data. Note the dramatic effects of conditioning, revealing a broad S–T increase in response (Paired > Unpaired) at 24 h post-training. A second marked change in the S–T profile is evident at 96 h post-training; the increased associative responses are now concentrated at the CS frequency and occur maximally between 4 and 10 s after tone onset. Flanking “inhibition” can also be seen at frequencies higher and lower than the CS-band.
It is obvious that the consolidation of memory from 24 to 96 h is not restricted to the frequency domain (i.e., increased specificity to the CS-band) but also occurs in the temporal domain. Thus, associative behavioral differences 24 h after training extended over many seconds, as well as across all frequencies (compare Fig. 7A and D with Fig. 7B and E, respectively). In fact, at the 24 h retention period, associative differences could occur to some frequencies over the entire 20 s period analyzed. The absence of CS-specificity is underscored by the fact that the duration of responses was not even maximal in the CS frequency band (Fig. 7B and E). In contrast, at the 96 h retention period, the associative differences are far more circumscribed. Not only is the spectral response difference largely confined to the CS-band frequencies, but the latency of the maximal response difference occurs only between 5 and 10 s after tone onset. The change in response latency is dramatic; associative differences to tones 24 h after training began soon after tone onset (Fig. 7E), whereas their earliest appearance at 96 h was at about 4 s, in the CS-band (Fig. 7F). Moreover, side-band “inhibition” is evident at 96 h, i.e., responses to lower and higher- band frequencies were actually smaller in the Paired group than in the Unpaired group. This may be the first evidence of “behavioral lateral inhibition” due to associative processes.
3.4. Relationship of associativity and frequency specificity
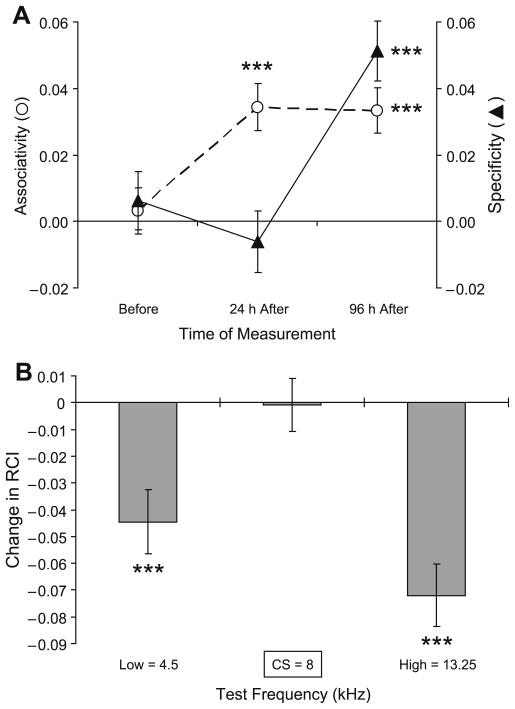
The relationship between associativity and frequency specificity of NB-induced memory can best be appreciated by comparing their quantification across days. To accomplish this, we constructed indices of associativity and specificity, as follows. We concentrated on three frequencies, the CS frequency (8.00 kHz) and the low and high frequencies that showed the largest changes (within the 0–13 s time window represented the majority of responses, Methods), which were 4.5 and 13.25 kHz. The Associativity Index measures the values of differential responses (in units of RCI) at the CS frequency (Paired8 kHz−Unpaired8 kHz = {P−Up}8 kHz). The Specificity Index measures the difference between responses to the CS frequency and the averaged side-band responses, notably, to the frequencies showing maximal post-training (at 96 h) decrement ({P−Up}8 kHz−[{P−Up}4.5 kHz + {P−Up}13.25 kHz]/2). Statistical analysis (t-test) then allowed determining whether the measured parameters were different from zero (where zero stands for “No Associativity” in the Associativity Index, and “No Specificity” in the Specificity Index).
During the baseline, pre-training period, one would expect neither associativity nor CS-specificity, and neither were found (Associativity: t(1507) = 0.45, p > .50); CS-Specificity; t(1507) = 0.71, p > .20). In contrast, 24 h post-training, there was a significant associative effect (t(1507) = 4.84, p < .0001), but not a CS-specific change (t(1507) = 0.66, p > .50). At 96 h post-training, the associative effect was retained (t(1507) = 4.87, p < .0001). In addition, a CS-specific effect was now present (t(1507) = 5.67, p < .0001) (Fig. 8A). Thus, associativity developed within 24 h of training whereas additional consolidation time (between 24 and 96 h) was required for the development of specificity.
Fig. 8.
Quantification of the evolution of behavioral associative responses following tone–NBstm pairing. (A) Relationship between associativity and CS-specificity. (See text for details of respective index formulas.) The statistical analysis (t-test) determines whether the measured parameters were different from zero (where zero stands for “No Associativity” and “No Specificity”). The analysis shows that the baseline responses (“Before”) were both non-associative (t = 0.45, p > .50) and non-specific (t = 0.71, p > .20). At 24 h post-training, animals’ responses to tones were associative (t(1507) = 4.84, p < .0001) but not CS-specific (t(1507) = 0.66, p > .50). In contrast, at the 96 h retention test the responses, in addition to maintaining associativity (t(1507) = 4.87, p < .0001), also exhibited significant CS-specificity (t(1507) = 5.67, p < .0001). (B) The between-sessions (24 h vs. 96 h post-training) analysis of the post-training evolution of associative behavioral responses over time. The bars represent differential (Paired minus Unpaired) responses recorded 24 h post-training subtracted from differential responses obtained 96 h post-training. The differential nature of measures assures that the results pertain to associative effects as non-associative controls (responses in Unpaired group) are taken into account. The plot shows that rise of specificity in conditioned behavior demonstrated in (A), is attributable to a suppression of responses to non-CS frequencies (lower frequency: 4.5 kHz = 0.83 octaves, p < .0001; higher frequency: 13.25 kHz = 0.73 octaves, p < .0001) that surround the CS at close proximity. Level of statistical significance: ***p < .005, t-test.
A between-sessions (24 h vs. 96 h post-training) analysis confirmed initial observations regarding the nature of processes responsible for the evolution of associative increased specificity over days (Fig. 8B). The rise of specificity in conditioned behavior is attributable to a suppression of responses to non-CS frequencies that surround the CS at close proximity (4.5 kHz = 0.83 octaves, and 13.25 kHz = 0.73 octaves relative to the 8.00 kHz CS; p < .001).
4. Discussion
4.1. Summary and validity of the findings
This experiment used NBstm to induce associative behavioral memory in a situation that appears to be devoid of hedonic and motivational factors. The major findings are that associativity and specificity of learning exhibited different temporal dynamics. Indeed, the content of memory could be seen to change over time in a particular way. Associativity was evident at the first post-training retention period of 24 h; the Paired group exhibited significantly greater behavioral responses (interruption of ongoing regular respiration) than the Unpaired group. However, specificity was absent. That is, the frequency generalization gradient was essentially flat, i.e., there was no significant difference in response to the CS-band frequencies vs. lower or higher-band frequencies. In contrast, the situation was different at the 96 h retention test. While associativity was maintained, CS-specific memory also had developed, as indicated by the sharpened frequency generalization gradient, maximal at the CS frequency band. Particularly noteworthy, the development of specificity of memory contents was caused not by a selective increase in response to the CS-band frequencies, but by a significant decrease in response to the lower and higher side-band frequencies. (Figs. 5 and 6).
While the findings indicate that the specificity of memory can increase during the days after associativity has formed, other interpretations should be considered. First, it might be thought that the findings are not due to associative processes. However, the responses of the control and paired groups to tones prior to training did not differ whereas they did so after training. Thus, the findings required pairing of tone with NBstm. Second, one could argue that the failure of the control group to develop CS-specific memory reflects the use of less potent stimulation of the nucleus basalis. However, there was neither significant difference between groups in the location of stimulation sites within the nucleus basalis nor in the level of NBstm current.
Third, it might be thought that NB stimulation actually is motivationally significant. For example, substance P (SP) injected into the NB can be rewarding as assessed in a conditioned place-preference task (CPP) (Holzhäuer-Oitzl, Hasenöhrl, & Huston, 1988; Huston, Hasenöhrl, Boix, Gergardt, & Schwarting, 1993). Given these findings, we also assessed the effects of NBstim in a similar CPP task. We used the same stimulation parameters that were sufficient to induce specific behavioral associative memory. However, there was no evidence of any preference for the place in which stimulation had been administered; subjects neither preferred nor avoided stimulation loci within the apparatus (Miasnikov, Chen, Gross, Poytress, & Weinberger, 2008a). Thus, under the conditions of the present experiment, it is extremely unlikely that NBstm was either rewarding or punishing.
Fourth, the current experimental design involved obtaining behavioral assessments of NB-induce memory within each subject at two time points, 24 and 96 h post-training. Therefore, the first assessment might have infkuenced behavior at the second test period. This sequential, within-subjects design was chosen because it can yield the most direct evidence of post-training time-dependent changes in the specificity of memory. The issue is whether the use of this design can explain the increased specificity of NB-induced memory found in this study. This might be a problem if memory became less-specific or weaker over time, because one might presume that the 24 h test produced some extinction. However, memory did not exhibit such a pattern but itself showed specific changes in the form of maintained magnitude of response to CS-band frequencies while responses to higher and lower side-band frequencies decreased. Additionally, the Unpaired group received test stimuli identical to the Paired group, both at 24 and 96 h post-training. However, in contrast to the Paired group, it showed no change in response between these two time points. Therefore, the frequency-specific changes in memory observed in the Paired group between 24 and 96 h are attributable to the pairing of the CS and NBstm rather than to the use of sequential tests.
Fifth, it could be argued that the change in behavior observed in the Paired group following training does not index genuine memory. This issue has been addressed previously (McLin et al., 2002, 2003; Miasnikov et al., 2006, 2008b). Briefly, evaluation of this argument requires that there be accepted criteria for genuine (i.e., natural) memory, against which NB-induced associative changes in behavior can be assessed. A systematic change in behavior due to associative processes is a major criterion for classical (Pavlovian) conditioning, which is the appropriate domain for this study. As noted above, this criterion has been satisfied. In addition to associativity, NB-induced memory is specific, rapidly acquired, is retained over days and exhibits behavioral generalization gradients similar to those found with natural memory (McLin et al., 2002, 2003; Miasnikov et al., 2006; Weinberger et al., 2006; see also Mackintosh, 1974; Mostofsky, 1965). Thus, NB-induced associative memory has characteristics that are well within the domain of natural associative memory.
However, we do not hold that NB-induced memory is identical to natural memory in all regards. As explained in the Introduction, a rationale for its use is to provide a simplified model system for associative memory, one that apparently lacks hedonic, motivational and emotional components of natural associative memory, e.g., situations involving an appetitive or aversive unconditioned stimulus. It is this simplification of the learning situation, which we believe commends the use of NB-induced memory to understand the salient sensory content of associative memory. The issue is not whether NB-induced memory is identical to natural memory but rather whether it is useful for understanding fundamental processes in natural memory.
4.2. Dynamics of content in sensory memory consolidation
The use of frequency generalization gradients revealed not only increased specificity over days but also served as a means of determining the spectro-temporal features of the dynamics of sensory memory. The frequency generalization gradient could have become sharper in several ways. For example, responses to the CS-band could have increased with a smaller increase or no changes in responses to the lower and higher side-bands. On the other hand, the sharpening could have occurred because there was no change in responses to the CS-band but a decrease in response to the side-band frequencies, and indeed this is what happened.
This profile of change in the contents of auditory memory suggests that the enhancement of detail with temporal consolidation reflects forgetting of the side-band frequencies because it is unclear how a selective difficulty in retrieval could explain the findings. Thus, retrieval deficits often involve the absence of a relevant retrieval cue. However, the post-training testing procedure consisted of multiple presentations of all frequencies. Therefore, the actual frequencies that could have elicited behavioral response at the lower and higher side-bands were present as potential retrieval cues during testing.
Analysis of the temporal profile of the respiratory response across the ~20 s of behavioral recording on tone test trials provided another level of insight into the dynamics of temporal consolidation (Fig. 7). The goal of these analyses was to visualize any associative effects in the spectro-temporal domain. This was accomplished by computing the difference between the Paired and the Unpaired groups (Paired minus Unpaired).
At the 24 h retention period, there was an associative lack not only of the details of acoustic frequency but also of specificity of the temporal expression of behavior. That is, tones across frequency elicited associative responses across ~20 s (Fig. 7B and E). In contrast, at the 96 h retention period, in the absence of any further training, associative effects were not only far more confined to CS-band frequencies, but they were also limited to long latency responses of ~5–15 s, with maximal responses of ~5–10 s (Fig. 7C and F). If the behavioral analyses at the 96 h retention test had been restricted to the 2-s period of tone presentation, then one would have concluded that the Paired group did not exhibit greater responses than the Unpaired group at any frequency. The marked spectro-temporal “shrinkage” (increased specificity) of associative behavior at longer latencies would have been missed.
These observations have at least two implications. First, that behavioral analyses of memory, whether NB-induced or natural, ought to be sufficiently sensitive and detailed so that Type II errors (i.e., false negative conclusions) can be largely eliminated. They further indicate that a comprehensive analysis of behavior in an extended temporal domain may yield previously unsuspected information about memory consolidation. Second, the very long latency of associative response in the Paired group at 96 h suggests that the processing of frequency information after temporal consolidation is much more complex and extended the processing taking place shortly after initial learning. Remarkably, this seems to be the most parsimonious interpretation of an increase of ~4–5 s in associative response latency despite the use of both a highly sensitive behavioral measure (disruption of highly regular, respiration) and an apparently very simple perceptual decision (discrimination of pure-tone frequency). These findings strongly suggest that sensory memory consolidation is achieved at the expense of additional processing resources, and thus the retrieval of memory at 96 h post-training involves additional neurobiological substrates. Therefore, conceptualizations of neurobiological processes in memory consolidation may be informed not only by the fact of increased detail of contents per se, but also by the possibility that different cognitive computations are involved at different retrieval episodes as a function of time after initial learning.
4.3. Relevance for cholinergic mechanisms in learning and memory
The cholinergic system long has been implicated in learning and memory (e.g., reviewed in Deutsch, 1971; Flood, Landry, & Jarvik, 1981; Power, Vazdarjanova, & McGaugh, 2003). Furthermore, stimulation of the nucleus basalis and the release of ACh in the cortex can induce specific associative physiological plasticity. For example, pairing a sound with stimulation of the nucleus basalis induces frequency-specific shifts of neural tuning in the primary auditory cortex (Bakin & Weinberger, 1996; Kilgard et al., 2001) that have major characteristics of neural processes underlying memory. Such shifts are associative, highly specific rapidly-induced, discriminative, consolidate (i.e., become stronger and more specific without additional training) and are retained for at least 24 h (Bakin & Weinberger, 1996; Bjordahl, Dimyan, & Weinberger, 1998; Dimyan & Weinberger, 1999). NB-induced plasticity is not caused by some unknown non-cholinergic mechanism as it requires the engagement of muscarinic receptors (mAChRs) in the auditory cortex (Miasnikov, McLin, & Weinberger, 2001). Most relevant for the current findings, and as noted in the Introduction and above, tone paired with NBstm can induce behavioral memory that has major attributes of natural memory: associativity, specificity, rapid development, retention and normal generalization gradients (reviewed in Weinberger, 2007).
Both the neurophysiological studies and the NB-memory induction studies were undertaken to test a model of natural auditory associative learning, which posits a “final common path” for the long-term, specific storage of information via activation of the nucleus basalis, its release of ACh in the auditory cortex and the subsequent engagement of cholinergic receptors in the auditory cortex (Weinberger et al., 1990a, 1990b). This model is based on three, rapidly successive “convergences”. The first is the convergence of auditory system responses to an acoustic CS with neural responses to a natural unconditioned stimulus (US), such as shock, in the magnocellular medial geniculate nucleus (MGm). The second is the convergence of neural responses to the CS from the lemniscal ventral medial geniculate nucleus (MGv) with plasticity induced in the MGm on preceding trials, in the primary auditory cortex. The third is the convergence of acetylcholine released by the nucleus basalis with the current and/or recent traces of the MGv and MGm input to the auditory cortex. (The MGm projections to the cortex are especially rapid as they are conveyed by giant axons [Winer, Larue, & Huang, 1999].) This “medial geniculate/nucleus basalis” model (MG/NB) hypothesizes that the properly-timed engagement of AChRs in the auditory cortex is sufficient to “convert” short-term plasticity produced by the first two convergences into long-term associative representational plasticity underlying, at least in part, behavioral auditory associative memory. The MG/NB model has successfully predicted many neurophysiological and behavioral findings (reviewed in Weinberger, 1998; Weinberger & Bakin, 1998).
The use of tone paired with NB stimulation, as in the present study, avoids the biologically-significant unconditioned stimulus component and thus bypasses the first two convergences because there is no shock (or similar motivational) US that would ordinarily activate the MGm. Thus, NBstm appears to induce specific associative long-term (96 h) memory without the need to proceed through all three stages that are thought to occur in natural memory. The present findings are not only consistent with the model but provide an additional level of understanding by addressing the temporal dynamics of cholinergically-related auditory memory. We previously found that the amount of detail in memory 24 h after training could be controlled by the level of NBstm; weak stimulation (~45 μA) yielded a flat generalization gradient while moderate stimulation (~65 μA) induced CS-specific memory (Weinberger et al., 2006). We used weak stimulation in this study to produce a flat gradient (no specificity), so that any increase in specificity over days could be readily detected, as indeed it was.
Taken together, the findings suggest that during natural learning, the details of the contents of associative memory will emerge when the NB is engaged, either immediately if NB activation is moderate or strong or later if NB activation is weaker. This is consistent with the finding that more stressful experiences, which should produce higher NB activation, can yield memories that are richer in detail (Laney, Campbell, Heuer, & Reisberg, 2004; reviewed in, e.g., Christianson, 1992; Pitman, 1989). However, if learning involves weak NB/cholinergic activation, then while initial memory may appear to initially lack details, such specificity can emerge through temporal consolidation.
These alternative “routes to specific memory” parallel electrophysiological profiles reported for receptive field plasticity in the auditory cortex. Cells whose pre-training tuning was near the CS frequency developed a complete shift of tuning to the CS frequency immediately after classical conditioning (tone–shock pairing). However, cells whose pre-training frequency tuning was far from the CS frequency (in octave distance) required 3 days to completely shift to the CS frequency (Galván & Weinberger, 2002). The convergence of the current findings with neural plasticity that develops in the auditory cortex during natural learning provides additional evidence that the activation of the nucleus basalis during natural learning is sufficient for the establishment of associative memory.
Although the mechanisms of increased specificity over time of NB-induced memory remain to be studied, a related neurophysiological phenomenon has been reported. Rasmusson and Dykes discovered “long-term cholinergic enhancement” (LTCE) in the somatosensory cortex of the cat (Rasmusson & Dykes, 1988). Following pairing of a tactile stimulus with stimulation of the NB, evoked responses exhibited a gradual rise in the size of somatosensory cortical local field potentials (LFPs) over several hours, the longest time-point investigated (Verdier & Dykes, 2001). Such temporal dynamics in LTCE might contribute to the gradual increase in specificity of memory by increasing the synaptic strength in sensory (auditory) specific corticopetal pathways.
4.4. Relationship to prior studies of temporal changes in memory content
A widely accepted conceptualization of the contents of memory distinguishes between details and gist. The former are generally viewed as item-specific features while the latter are seen as categorical or general features of memory. Many studies of human memory have reported that the details are forgotten faster, or are more difficult to retrieve, or both, than the gist (reviewed in, e.g., Koriat, Goldsmith, & Pansky, 2000). Within the study of animal memory, there is ample evidence that stimulus generalization gradients tend to become “flatter” over time. This dynamic is considered to reflect a tendency to lose, or be less able to retrieve, the details of the particular value of the training stimulus (Riccio, Ackil, & Burch-Vernon, 1992). For example, within Pavlovian conditioning, memory of the specific CS frequency would become functionally less accessible, due to either or both forgetting and retrieval problems, while memory that tones in general predict the unconditioned stimulus would be intact.
The current findings pertaining to consolidation of associative sensory memory in the absence of a motivational unconditioned stimulus, provide a different profile of the post-training dynamics of memory content. The generalization gradient was flat 24 h after training but became more specific as revealed at the 96 h retention test. Had there been either forgetting or difficulty in retrieving the CS frequency information, the generalization gradient could not have become sharper.
This discordance with previous findings raises the issue of whether the use of tone paired with NBstm to induce associative memory is merely a demonstration that the brain can be forced to perform in abnormal ways. That is always a possibility for any invasive methodology, whether it be by brain stimulation, brain lesion, pharmacological intervention or genetic manipulation. Alternatively, the findings may be surprising because this appears to be the first use of “induced” memory to elucidate fundamental mnemonic processes. Therefore, the present findings of increased detail of memory via temporal consolidation may prove revealing. Determination of the full course of events will require sampling at both intervals between 24 and 96 h and also thereafter, until the expected loss of specific detail is obtained. But the current findings do suggest that details of sensory content are present in the brain even when they may not be evident in retention tests of natural memory.
Why might associative “sensory memory” largely or entirely devoid of motivational and emotional processes exhibit the temporal dynamics observed in this study? Although no definitive answers can be given at this early stage of inquiry, it may be helpful to consider the question instead from the aspect of natural memory: “Why does natural associative memory not display such increased precision of detail?” At least two possibilities come to mind. First, in some cases the flattening of the generalization gradient over time may reflect, in part, an insufficiently sensitive analysis of behavior. As discussed above, restriction of analysis to the period of the tone signal would have led to a false negative conclusion, i.e., no difference between groups at the 96 h retention interval (Fig. 7C and F). Second, the presence of motivational and emotional processes in original learning may involve post-training retrieval deficits. Thus, retention tests do not include a repeated instantiation of the actual reinforcement that was present during initial training. For example, even if subjects are tested in an environment in which they had received nociceptive stimulation, still the latter is not presented during the retention test. Therefore, some rather prominent cues are likely to be absent during retention tests. Such an interpretation is consistent with enhanced retrieval by the use of a “reminder” shock during the investigation of reinstatement following extinction (see also Bouton, Nelson, & Rosas, 1999). Whatever the ultimate explanation of the current findings, they may serve to promote an enlarged conceptualization about the temporal dynamics of memory content.
Acknowledgments
We thank Nataliya Gross, Gabriel K. Hui, Jacquie D. Weinberger and Steven Clifford (CED) for assistance. This study was funded by the NIDCD/NIH, DC-02938.
References
- Bakin JS, Weinberger NM. Induction of a physiological memory in the cerebral cortex by stimulation of the nucleus basalis. Proceedings of the national academy of sciences of the United States of America. 1996;93(20):11219–11224. doi: 10.1073/pnas.93.20.11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigl V, Woolf NJ, Butcher LL. Cholinergic projections from the basal forebrain to frontal, parietal, temporal, occipital, and cingulate cortices: A combined fluorescent tracer and acetylcholinesterase analysis. Brain Research Bulletin. 1982;8(6):727–749. doi: 10.1016/0361-9230(82)90101-0. [DOI] [PubMed] [Google Scholar]
- Bjordahl TS, Dimyan MA, Weinberger NM. Induction of long-term receptive field plasticity in the auditory cortex of the waking guinea pig by stimulation of the nucleus basalis. Behavioral Neuroscience. 1998;112(3):467–479. doi: 10.1037//0735-7044.112.3.467. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Nelson JB, Rosas JM. Stimulus generalization, context change, and forgetting. Psychological Bulletin. 1999;125(2):171–186. doi: 10.1037/0033-2909.125.2.171. [DOI] [PubMed] [Google Scholar]
- Casamenti F, Deffenu G, Abbamondi AL, Pepeu G. Changes in cortical acetylcholine output induced by modulation of the nucleus basalis. Brain Research Bulletin. 1986;16(5):689–695. doi: 10.1016/0361-9230(86)90140-1. [DOI] [PubMed] [Google Scholar]
- Celesia GG, Jasper HH. Acetylcholine released from cerebral cortex in relation to state of activation. Neurology. 1966;16(11):1053–1063. doi: 10.1212/wnl.16.11.1053. [DOI] [PubMed] [Google Scholar]
- Christianson SA. Emotional stress and eyewitness memory: A critical review. Psychological Bulletin. 1992;112(2):284–309. doi: 10.1037/0033-2909.112.2.284. [DOI] [PubMed] [Google Scholar]
- Dahmen JC, King AJ. Learning to hear: Plasticity of auditory cortical processing. Current Opinion in Neurobiology. 2007;17(4):456–464. doi: 10.1016/j.conb.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Détári L, Rasmusson DD, Semba K. Phasic relationship between the activity of basal forebrain neurons and cortical EEG in urethane-anesthetized rat. Brain Research. 1997;759(1):112–121. doi: 10.1016/s0006-8993(97)00252-7. [DOI] [PubMed] [Google Scholar]
- Détári L, Rasmusson DD, Semba K. The role of basal forebrain neurons in tonic and phasic activation of the cerebral cortex. Progress in Neurobiology. 1999;58(3):249–277. doi: 10.1016/s0301-0082(98)00084-7. [DOI] [PubMed] [Google Scholar]
- Deutsch JA. The cholinergic synapse and the site of memory. Science. 1971;174(11):788–794. doi: 10.1126/science.174.4011.788. [DOI] [PubMed] [Google Scholar]
- Dimyan MA, Weinberger NM. Basal forebrain stimulation induces discriminative receptive field plasticity in the auditory cortex. Behavioral Neuroscience. 1999;113(4):691–702. doi: 10.1037//0735-7044.113.4.691. [DOI] [PubMed] [Google Scholar]
- Duque A, Balatoni B, Detari L, Zaborszky L. EEG correlation of the discharge properties of identified neurons in the basal forebrain. Journal of Neurophysiology. 2000;84(3):1627–1635. doi: 10.1152/jn.2000.84.3.1627. [DOI] [PubMed] [Google Scholar]
- Edeline JM. The thalamo-cortical auditory receptive fields: Regulation by the states of vigilance, learning and the neuromodulatory systems. Experimental Brain Research. 2003;153(4):554–572. doi: 10.1007/s00221-003-1608-0. [DOI] [PubMed] [Google Scholar]
- Flood JF, Landry DW, Jarvik ME. Cholinergic receptor interactions and their effects on long-term memory processing. Brain Research. 1981;215(1–2):177–185. doi: 10.1016/0006-8993(81)90500-x. [DOI] [PubMed] [Google Scholar]
- Fritz J, Elhilali M, Shamma S. Active listening: Task-dependent plasticity of spectrotemporal receptive fields in primary auditory cortex. Hearing Research. 2005;206(1–2):159–176. doi: 10.1016/j.heares.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Galván VV, Weinberger NM. Long-term consolidation and retention of learning-induced tuning plasticity in the auditory cortex of the guinea pig. Neurobiology of Learning and Memory. 2002;77(1):78–108. doi: 10.1006/nlme.2001.4044. [DOI] [PubMed] [Google Scholar]
- Giovannini MG, Rakovska A, Benton RS, Pazzagli M, Bianchi L, Pepeu G. Effects of novelty and habituation on acetylcholine, GABA, and glutamate release from the frontal cortex and hippocampus of freely moving rats. Neuroscience. 2001;106(1):43–53. doi: 10.1016/s0306-4522(01)00266-4. [DOI] [PubMed] [Google Scholar]
- Holzhäuer-Oitzl MS, Hasenöhrl RU, Huston JP. Reinforcing properties of substance P in the region of the nucleus basalis magnocellularis in rats. Neuropharmacology. 1988;27(7):749–756. doi: 10.1016/0028-3908(88)90085-8. [DOI] [PubMed] [Google Scholar]
- Huston JP, Hasenöhrl RU, Boix F, Gergardt P, Schwarting RKW. Sequence-specific effects of neurokinin substance P on memory, reinforcement, and brain dopamine activity. Psychopharmacology. 1993;112(2–3):147–162. doi: 10.1007/BF02244906. [DOI] [PubMed] [Google Scholar]
- Irvine DR. Auditory cortical plasticity: Does it provide evidence for cognitive processing in the auditory cortex? Hearing Research. 2007;229(1–2):158–170. doi: 10.1016/j.heares.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasper HH, Tessier J. Acetylcholine liberation from cerebral cortex during paradoxical (REM) sleep. Science. 1971;172(983):601–602. doi: 10.1126/science.172.3983.601. [DOI] [PubMed] [Google Scholar]
- Johnston MV, McKinney M, Coyle JT. Evidence for a cholinergic projection to neocortex from neurons in basal forebrain. Proceedings of the national academy of sciences of the United States of America. 1979;76(10):5392–5396. doi: 10.1073/pnas.76.10.5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kametani H, Kawamura H. Alterations in acetylcholine release in the rat hippocampus during sleep–wakefulness detected by intracerebral dialysis. Life Sciences. 1990;47(5):421–426. doi: 10.1016/0024-3205(90)90300-g. [DOI] [PubMed] [Google Scholar]
- Kilgard MP, Pandya PK, Vazquez J, Gehi A, Schreiner CE, Merzenich MM. Sensory input directs spatial and temporal plasticity in primary auditory cortex. Journal of Neurophysiology. 2001;86(1):326–338. doi: 10.1152/jn.2001.86.1.326. [DOI] [PubMed] [Google Scholar]
- Koriat A, Goldsmith M, Pansky A. Toward a psychology of memory accuracy. Annual Review of Psychology. 2000;51:481–537. doi: 10.1146/annurev.psych.51.1.481. [DOI] [PubMed] [Google Scholar]
- Laney C, Campbell HV, Heuer F, Reisberg D. Memory for thematically arousing events. Memory and Cognition. 2004;32(7):1149–1159. doi: 10.3758/bf03196888. [DOI] [PubMed] [Google Scholar]
- Mackintosh NJ. The psychology of animal learning. New York: Academic Press; 1974. [Google Scholar]
- Marrosu F, Portas C, Mascia MS, Casu MA, Fà M, Giagheddu M, et al. Microdialysis measurement of cortical and hippocampal acetylcholine release during sleep–wake cycle in freely moving cats. Brain Research. 1995;671(2):329–332. doi: 10.1016/0006-8993(94)01399-3. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Memory—A century of consolidation. Science. 2000;287(5451):248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- McLin DE, 3rd, Miasnikov AA, Weinberger NM. Induction of behavioral associative memory by stimulation of the nucleus basalis. Proceedings of the national academy of sciences of the United States of America. 2002;99(6):4002–4007. doi: 10.1073/pnas.062057099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLin DE, 3rd, Miasnikov AA, Weinberger NM. CS-specific gamma, theta, and alpha EEG activity detected in stimulus generalization following induction of behavioral memory by stimulation of the nucleus basalis. Neurobiology of Learning and Memory. 2003;79(2):152–176. doi: 10.1016/s1074-7427(02)00009-6. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Levey AI, Wainer BH. Cholinergic innervation of cortex by the basal forebrain: Cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. Journal of Comparative Neurology. 1983;214(2):170–197. doi: 10.1002/cne.902140206. [DOI] [PubMed] [Google Scholar]
- Miasnikov AA, Chen JC, Gross N, Poytress BS, Weinberger NM. Motivationally neutral stimulation of the nucleus basalis induces specific behavioral memory. Neurobiology of Learning and Memory. 2008a;90(1):125–137. doi: 10.1016/j.nlm.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miasnikov AA, Chen JC, Weinberger NM. Rapid induction of specific associative behavioral memory by stimulation of the nucleus basalis in the rat. Neurobiology of Learning and Memory. 2006;86(1):47–65. doi: 10.1016/j.nlm.2005.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miasnikov AA, Chen JC, Weinberger NM. Specific auditory memory induced by nucleus basalis stimulation depends on intrinsic acetylcholine. Neurobiology of Learning and Memory. 2008b;90(2):443–454. doi: 10.1016/j.nlm.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miasnikov AA, McLin D, 3rd, Weinberger NM. Muscarinic dependence of nucleus basalis induced conditioned receptive field plasticity. NeuroReport. 2001;12(7):1537–1542. doi: 10.1097/00001756-200105250-00047. [DOI] [PubMed] [Google Scholar]
- Mostofsky DI, editor. Stimulus generalization. Stanford, CA: Stanford University Press; 1965. [Google Scholar]
- Palmer CV, Nelson CT, Lindley GA., 4th The functionally and physiologically plastic adult auditory system. Journal of the Acoustical Society of America. 1998;103(4):1705–1721. doi: 10.1121/1.421050. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 3. San Diego: Academic Press; 1997. [DOI] [PubMed] [Google Scholar]
- Pitman RK. Post-traumatic stress disorder, hormones, and memory. Biological Psychiatry. 1989;26(3):221–223. doi: 10.1016/0006-3223(89)90033-4. [DOI] [PubMed] [Google Scholar]
- Power AE, Vazdarjanova A, McGaugh JL. Muscarinic cholinergic influences in memory consolidation. Neurobiology of Learning and Memory. 2003;80(3):178–193. doi: 10.1016/s1074-7427(03)00086-8. [DOI] [PubMed] [Google Scholar]
- Rasmusson DD, Dykes RW. Long-term enhancement of evoked potentials in cat somatosensory cortex produced by co-activation of the basal forebrain and cutaneous receptors. Experimental Brain Research. 1988;70(2):276–286. doi: 10.1007/BF00248353. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP. Functional organization and plasticity of auditory cortex. In: Peretz I, Zatorre RJ, editors. The cognitive neuroscience of music. Vol. 23. New York: Oxford University Press; 2003. pp. 357–365. [Google Scholar]
- Riccio DC, Ackil J, Burch-Vernon A. Forgetting of stimulus attributes: Methodological implications for assessing associative phenomena. Psychological Bulletin. 1992;112(3):433–445. doi: 10.1037/0033-2909.112.3.433. [DOI] [PubMed] [Google Scholar]
- Verdier D, Dykes RW. Long-term cholinergic enhancement of evoked potentials in rat hindlimb somatosensory cortex displays characteristics of long-term potentiation. Experimental Brain Research. 2001;137(1):71–82. doi: 10.1007/s002210000646. [DOI] [PubMed] [Google Scholar]
- Weinberger NM. Dynamic regulation of receptive fields and maps in the adult sensory cortex. Annual Review of Neuroscience. 1995;18:129–158. doi: 10.1146/annurev.ne.18.030195.001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger NM. Physiological memory in primary auditory cortex: Characteristics and mechanisms. Neurobiology of Learning and Memory. 1998;70(1–2):226–251. doi: 10.1006/nlme.1998.3850. [DOI] [PubMed] [Google Scholar]
- Weinberger NM. Associative representational plasticity in the auditory cortex: A synthesis of two disciplines. Learning and Memory. 2007;14(1–2):1–16. doi: 10.1101/lm.421807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger NM. Cortical plasticity in associative learning and memory. In: Byrne JH, editor. Cognitive psychology of memory (Learning and memory: A comprehensive reference. Chap. 3.11 Vol. 3. Oxford: Elsevier; 2008. pp. 187–218. [Google Scholar]
- Weinberger NM, Ashe JH, Metherate R, McKenna TM, Diamond DM, Bakin J. Retuning auditory cortex by learning: A preliminary model of receptive field plasticity. Concepts in Neuroscience. 1990a;1(1):91–132. [Google Scholar]
- Weinberger NM, Ashe JH, Metherate R, McKenna TM, Diamond DM, Bakin JS, et al. Neural adaptive information processing: A preliminary model of receptive-field plasticity in auditory cortex during Pavlovian conditioning. In: Gabriel M, Moore J, editors. Learning and computational neuroscience. Foundations of adaptive networks. Vol. 3. Cambridge, MA: MIT Press; 1990b. pp. 91–138. [Google Scholar]
- Weinberger NM, Bakin JS. Learning-induced physiological memory in adult primary auditory cortex: Receptive fields plasticity, model, and mechanisms. Audiology and Neuro-otology. 1998;3(2–3):145–167. doi: 10.1159/000013787. [DOI] [PubMed] [Google Scholar]
- Weinberger NM, Miasnikov AA, Chen JC. The level of cholinergic nucleus basalis activation controls the specificity of auditory associative memory. Neurobiology of Learning and Memory. 2006;86(3):270–285. doi: 10.1016/j.nlm.2006.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer JA, Larue DT, Huang CL. Two systems of giant axon terminals in the cat medial geniculate body: Convergence of cortical and GABAergic inputs. Journal of Comparative Neurology. 1999;413(2):181–197. [PubMed] [Google Scholar]
- Winters RW, McCabe PM, Schneiderman N. Functional utility and neurobiology of conditioned autonomic responses. In: Moore JW, editor. A neuroscientist’s guide to classical conditioning. New York: Springer; 2002. pp. 46–85. [Google Scholar]