Summary
Iron overload occurs in patients who require regular blood transfusions to correct genetic and acquired anaemias, such as β-thalassaemia major, sickle cell disease, and myelodysplastic syndromes. Although iron overload causes damage in many organs, accumulation of cardiac iron is a leading cause of death in transfused patients with β-thalassaemia major. The symptoms of cardiac iron overload will occur long after the first cardiac iron accumulation, at a point when treatment is more complex than primary prevention would have been. Direct measurement of cardiac iron using T2* magnetic resonance imaging, rather than indirect methods such as measuring serum ferritin levels or liver iron concentration have contributed to earlier recognition of myocardial iron loading and prevention of cardiac toxicity. Cardiac siderosis occurs in all transfusional anaemias, but the relative risk depends upon the underlying disease state, transfusional load, and chelation history. All three available iron chelators can be used to remove cardiac iron, but each has unique physical properties that influence their cardiac efficacy. More prospective trials are needed to assess the effects of single-agent or combination iron chelation therapy on the levels of cardiac iron and cardiac function. Ultimately, iron chelation therapies should be tailored to meet individual patient needs and lifestyle demands.
Keywords: Cardiac iron, Iron chelation therapy, Myelodysplastic syndrome, Sickle cell disease, β-Thalassaemia major
Introduction
Iron overload occurs in patients who are dependent on red blood cell transfusions to correct genetic and acquired anaemias, such as β-thalassaemia major, sickle cell disease, and myelodysplastic syndromes (MDS). Although iron overload causes damage in many organs, accumulation of cardiac iron is the leading cause of death in transfused patients with β-thalassaemia major. 1 With adequate iron chelation therapy, the deleterious effects of cardiac iron overload can be reversed and survival rates in modern cohorts have improved dramatically. 2 Nonetheless, cardiac toxicity remains common and develops only after longstanding cardiac iron deposition, leading to difficult and protracted therapies. Direct cardiac iron measurement using magnetic resonance imaging (MRI), rather than indirect methods such as measuring serum ferritin level or liver iron concentration, allows recognition of preclinical cardiac iron and proactive modifications of iron chelation therapy. Cardiac MRI also provides insights into the mechanisms and kinetics of cardiac iron transport, and serves as a marker of chelation efficacy. This chapter reviews various aspects of cardiac iron overload across a number of transfusion-dependent anaemias and the impact of iron chelation therapy on cardiac iron loading.
Mechanisms of cardiac iron overloading
During the progression of iron overload, iron accumulates in the ventricular wall, the epicardium, the papillary muscles, and the ventricular septum. Grossly visible cardiac iron deposits are associated with cardiac dysfunction and usually with chronic cardiac failure. 3 Iron accumulation occurs initially in the ventricular followed by the atrial myocardium, but remains greater in working than in conducting myocardium. First degree heart block and supraventricular arrhythmias are correlated with the extent of iron deposition in the atrial myocardium. 3
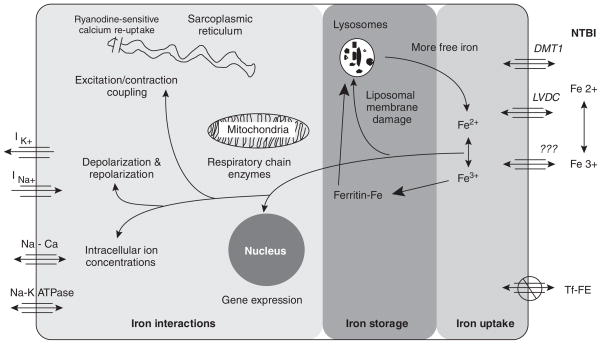
Several transport systems play a role in cardiac iron uptake (Figure 1). 4 To reduce toxicity, circulating iron is bound and transported by transferrin, which is about 30% saturated in normal patients. Under conditions of iron homeostasis, cardiac iron needs are supplied via highly regulated transferrin-mediated uptake mechanisms. During iron overloading, transferrin becomes saturated and toxic non-transferrin-bound iron (NTBI) begins to appear in the circulation. NTBI readily enters cardiomyocytes, predominantly as ferrous iron via L-type Ca2+ (voltage-dependent) channels, raising the levels of labile cardiac iron. 5 Endosome-mediated iron uptake may also take place during iron overload, but the physiology of this mechanism is poorly understood.
Fig. 1.
Scheme showing iron entry, storage, and toxicity in the heart, each represented as separate processes. Reproduced with permission from Wood JC, et al. 4 Ann NY Acad Sci 2005;1054:387–95. © 2005 New York Academy of Sciences.
Inside the myocyte, iron is rapidly bound by ferritin and transported to lysosomes for degradation and long-term storage. Iron stored in lysosomes can be visualized by MRI, and this forms the basis of non-invasive monitoring of cardiac iron. When the antioxidant capacity of the cell is exceeded, reactive oxygen species are formed, 6 damaging organelles, interfering with electrical and mechanical processes, and triggering myocyte apoptosis. 5
Measurement of cardiac iron
Preventing cardiac iron overload requires careful monitoring of iron balance as well as of iron levels within the myocardium. The simplest, though indirect, way of assessing cardiac risk is to determine serum ferritin levels. High serum ferritin levels are significantly associated with the subsequent development of cardiac failure and death. 1,2,7–9 Measuring ferritin levels is inexpensive, widely available, and clinically indicated even when resources allow more sophisticated monitoring methods. However, its usefulness in assessing iron overload is limited because the concentration of serum ferritin is influenced by many factors other than iron status, including infection, ineffective erythropoiesis, ascorbate status, and hepatic damage.
Liver iron concentration (LIC) reflects both iron stores and chronic iron balance. 10,11 Markedly elevated LIC is associated with cardiac complications. 8,10 LIC was classically measured by chemical determination in a liver biopsy. In experienced hands, this invasive procedure has a only a small risk of complications, 12 but sampling errors are common, especially in cirrhotic and fibrotic livers. Non-invasive methods such as MRI are gradually replacing liver biopsy for determination of LIC at major thalassaemia centres. 13–15
While both serum ferritin and LIC determinations are essential for safe and successful iron chelation therapy, cardiac iron uptake and toxicity can occur despite apparently adequate total body iron balance. 16–18 Signs of cardiac toxicity as measured by electrocardiography, echocardiography, or radionuclide angiography do not appear until severe cardiac iron deposition has occurred and the process is more difficult to reverse. 4,9 Although cardiac biopsy could potentially measure subclinical levels of iron overload, it is not suitable for routine screening because it is invasive and has unacceptable sampling error. Non-invasive MRI assessment of cardiac iron, however, can reliably identify patients with subclinical cardiac iron concentrations and stratify their risk of subsequent cardiac dysfunction. 4,16 The technique measures the half-life, T2*, of cardiac muscle darkening (with respect to echo time) produced by magnetically active stored cardiac iron. 16 Cardiac T2* correlates inversely with cardiac iron concentration. 19,20
Clinical relevance of cardiac T2* measurements
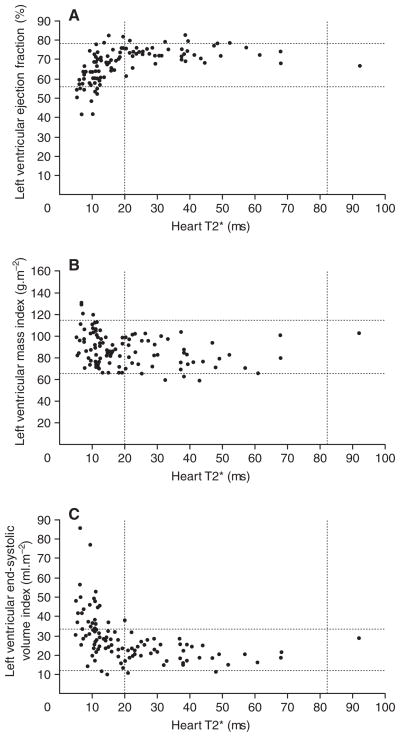
Cardiac T2* predominantly reflects safely stored cardiac iron, explaining how patients can have abnormal cardiac T2* values with normal cardiac function. However, the safely stored iron pool is in dynamic equilibrium with labile myocyte iron, leading to higher incidence/prevalence of cardiac decompensation at low T2* values. 16 In patients with β-thalassaemia major, T2* values below 10 ms predict potentially lethal cardiac iron levels and cardiac dysfunction (Figure 2). 21,22 In addition to predicting cardiac function, cardiac T2* correlates with endothelial dysfunction and arterial stiffness. 21,23 Resulting increases in peripheral vascular resistance decrease left atrial size, left ventricular size, and cardiac output.
Figure 2.
Relationships between myocardial T2* values and parameters of ventricular function: (A) left ventricular ejection fraction, (B) left ventricular mass index, (C) left ventricular end-systolic volume index. The broken lines represent the normal reference ranges for myocardial T2* and parameters of cardiac function. Below a myocardial T2* of 20 ms, there is a progressive and significant decline in left ventricular ejection fraction and an increase in the left ventricular end-systolic volume index and left ventricular mass index. Reproduced with permission from Anderson LJ, et al. 16 Eur Heart J 2001;22:2271–9. By permission of Oxford University Press. © 2001 by the European Society of Cardiology.
The relationship between cardiac T2* values and iron balance is quite complicated because the mechanisms and kinetics of cardiac iron uptake and clearance differ from the liver. Cardiac T2* does not correlate with serum ferritin concentration and LIC in cross sectional analysis, while longitudinal studies continue to imply a causal relationship. 16 Since they reflect different aspects of the iron storage pool, all three parameters (serum ferritin, LIC, and cardiac T2*) should be regularly measured to monitor total body and cardiac iron load.
Cardiac iron overload in different anaemias
Cardiac iron overload can develop in many types of chronically transfused patients. 3 In patients who do not receive iron chelation therapy, cardiac iron overloading begins after as few as 75 units of blood have been transfused. 3,24
β-Thalassaemia major
Iron cardiomyopathy has been studied most intensively in β-thalassaemia major. Cardiac involvement correlates with other extrahepatic iron toxicities, including diabetes and other endocrine complications. 25 Men are more than twice as likely as women to succumb to cardiac iron overloading despite comparable iron exposures and serum ferritin levels. 26 In a careful epidemiological study, lower serum ferritin levels were associated with improved survival, and outcomes continued to improve with continued decrease in serum ferritin levels, without any apparent threshold. 27
Just under half of adult patients with β-thalassaemia major have detectable cardiac iron, though many of them are asymptomatic. 23,27 However, patients with β-thalassaemia major who receive adequate iron chelation therapy do not exhibit cardiac iron loading until the second decade of life, after exposure to a minimum of 35 g of transfusional iron (approximately 175 units of blood). 28 This threshold is slightly lower than the values of 40 g of iron 3 and 44–51 g of iron 10 identified when cardiac death was the end-point.
Myelodysplastic syndromes
Patients with MDS may require transfusion intensities comparable with those required in patients with β-thalassaemia major. Total blood exposure is limited by mortality from the underlying blood disorder in some patients, but patients who are younger, have fewer comorbidities, and lower MDS risk live long enough to potentially develop cardiac toxicity. Patients with MDS who do not receive iron chelation therapy may develop cardiac iron overload after 75–100 units of blood have been transfused. 24
Reported studies of cardiac iron in MDS patients have generally been small and poorly controlled. In a pilot study in 7 patients, cardiac iron overload was seen after 2–4 years of continual transfusion (mean exposure, 206 units), but some patients did not develop cardiac iron overload even after 12 years. 29 Transfusion intensity, i.e. total blood volume transfused per year, and not total blood volume per se, was higher in those patients who developed cardiac iron overload. In two subsequent studies, cardiac iron deposition was absent, with none of 11 patients showing significant cardiac iron loading after transfusions for 1–12 years 30 and none of 10 patients showing cardiac iron overload after 2–6 years of transfusion therapy. 31 However, the transfusion intensity and duration in these studies were significantly less than reported for patients with β-thalassaemia major, and routine chelation was performed in patients with the heaviest transfusional exposures. Until further information from appropriate studies is available, the transfusion threshold guidelines of 75 units of blood in unchelated patients and 175 units in regularly chelated patients should serve as useful indicators for initiating cardiac T2* surveillance in this population.
Rare anaemias
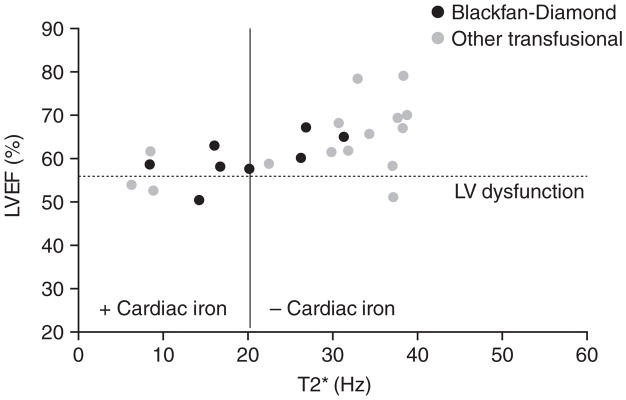
Patients with rare transfusion-dependent anaemias, such as congenital dyserythropoietic anaemia and Blackfan-Diamond syndrome, also develop iron overload-induced cardiomyopathy. These syndromes are rare, and hence prevalence estimates of cardiac iron overload are difficult. Detectable cardiac iron overload is quite common (~70%) in Blackfan-Diamond syndrome and congenital sideroblastic anaemia, and occurs less frequently (~20%) in patients with pyruvate kinase deficiency and survivors of acute myeloid leukaemia. 29 In the author’s institution, the prevalence and severity of cardiac iron loading (assessed by cardiac MRI T2* imaging) and of cardiac dysfunction are similar in the rare anaemias and in β-thalassaemia major (Figure 3). Multicentre trials will be required to stratify cardiac risk more accurately in subpopulations of patients with rare transfusion-dependent anaemias.
Figure 3.
Relationship between left ventricular ejection fraction and myocardial T2* in patients with thalassaemia and rare anaemias. Dotted line represents reference range for ejection fraction. Wood JC, et al. Unpublished data.
Sickle cell disease
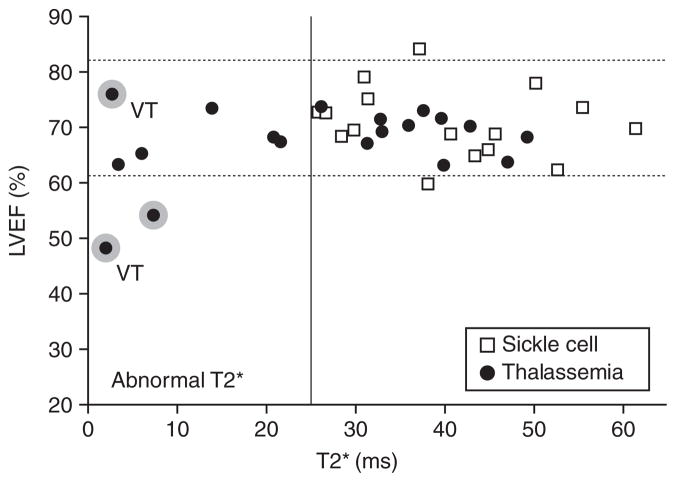
Most patients with sickle cell disease (SCD) do not require routine transfusion therapy. However, about 20% of children with SCD are now being placed on regular transfusion therapy to prevent primary or recurrent neurovascular complications. Hence, these patients develop comparable total body iron burdens to TM patients. As in β-thalassaemia major, cardiac dysfunction occurs in SCD, but is rarely associated with detectable cardiac iron (Figure 4). 27 One reason for this difference may be that transfusions are generally started later in life in SCD, and the total transfusional exposures are thus lower than in β-thalassaemia major for any given patient age. In addition, transferrin saturation and NTBI levels are lower in patients with SCD than in those with β-thalassaemia major. 32,33 Chronically transfused patients with SCD also show signs of increased inflammation, 32 and this may inhibit iron release from the reticuloendothelial system through increased hepcidin 34 or other mediators. Decreased cardiac risk in chronically transfused patients with SCD is concordant with the decreased prevalence of endocrine complications in these patients. 35
Figure 4.
Relationship between left ventricular ejection fraction and myocardial T2* in patients with thalassaemia and sickle cell disease. Dotted lines represent reference range for ejection fraction. Shading round points indicates a need for cardiac medications. Two patients had ventricular tachycardia, indicated by the letters VT. Reproduced with permission from Wood JC, et al. 27 Blood 2004;103:1934–6. © 2004 the American Society of Hematology.
While cardiac iron overloading is relatively rare in patients with SCD receiving regular transfusions, it does occur. Serum ferritin levels are a less reliable indicator of iron overload in SCD than in β-thalassaemia major or MDS 36 because ferritin is an acute-phase reactant. Chronically transfused adolescent and adult SCD patients should undergo regular MRI screening if the facilities are available. Although cardiac iron is seldom elevated in SCD, the prevalence of cardiac dysfunction is relatively high. 37 The pathophysiology of this dysfunction is poorly understood, but is unlikely to result simply from chronic anaemia. Fibrotic changes have been described in a few patients with SCD not receiving transfusions, 37,38 but fibrosis cannot be invoked to explain cardiac dysfunction. Further research is necessary to fully characterize this non-specific, asymptomatic cardiac dysfunction.
Iron chelation and its effect on cardiac iron
Iron overload-induced cardiomyopathy is a preventable and treatable condition. Prior to the use of MRI, mild reductions in left ventricular function were used as the basis on which to escalate iron chelation therapy, with complete reversal of cardiomyopathy in all patients who could comply with continuous intravenous deferoxamine therapy. 9 Unfortunately, the process took years and patients who failed to comply for the duration of the treatment died.
Fortunately, MRI assessment of cardiac T2* provides a longer treatment window for iron overload-induced cardiomyopathy, allowing correction of cardiac iron overload through more modest changes in iron chelation therapy. All three available iron chelators –deferasirox, deferoxamine, and deferiprone – can remove cardiac iron, but each has unique biophysical properties that influence the dosages required and the routes of administration. The primary limitation of deferoxamine is its difficulty of administration, which leads to poor compliance. In addition, its very short half-life limits its suppression of NTBI to the hours of drug administration, so that effective cardiac iron chelation requires continuous or near-continuous administration.
The oral chelator deferiprone is a very small molecule (for an iron chelator) and readily enters myocytes and intracellular compartments. Both observational 26,39 and randomized controlled trials 23,40 indicate that deferiprone is an effective cardiac iron chelator when used alone or in combination with deferoxamine. Cardiac iron, cardiac function, and survival all appear to improve with deferiprone use. Its primary limitation has been the rare but potentially lethal complication of agranulocytosis, which necessitates weekly complete blood counts for the duration of therapy. It also has not been approved for use in the USA and Canada.
Reports of the cardiac effects of the most recently approved oral iron chelator, deferasirox are emerging. In vitro demonstrated that intracellular sites of labile cardiac iron accumulation are accessed more rapidly by deferasirox and deferiprone than by deferoxamine. 41 In addition, deferasirox restored the contractility of cultured rat cardiomyocytes after iron loading. All three chelators were able to remove labile iron from plasma. 41 Studies in gerbils also suggest that deferasirox and deferiprone have comparable access to cardiac iron. 42,43
While extensive evidence supports the efficacy of deferasirox in removing iron from the liver, 44–47 data regarding its cardiac efficacy in actual clinical practice are more limited. In a single-institution study of patients with transfusion-dependent β-thalassaemia major and other iron overload conditions, deferasirox improved cardiac T2* by 2.1% per month (P = 0.013), compared with 1.6% per month for deferoxamine (P = 0.11) during a 13 month interval. 48 Comparable changes (2.4% per month) were reported in preliminary sixth month results from the multicenter US04 trial, with 18/20 patients improving cardiac T2*. 49 Both studies were too small to demonstrate any beneficial effects on left ventricular function. In contrast, the larger ESCALATOR trial (252 patients) showed a small but significant increase of 1.7 ±7.8% in LVEF indicating relief of mild, subclinical cardiac dysfunction in patients thought to have normal cardiac function (starting LVEF of 65.0 ±6.9%). 50 Larger open-label trials are nearing completion, randomized trials will be needed to compare cardiac iron chelation efficiencies among the different therapies available.
Conclusions
The rate and mechanism of iron loading are different in transfusion-dependent anaemias from those in hereditary haemochromatosis. Although cardiac iron overload occurs later than hepatic iron overload, it has more serious consequences. 2 Serum ferritin levels and LIC do not correlate well with cardiac iron accumulation; however, cardiac MRI allows preclinical recognition of cardiac iron overload. MRI has also provided fresh insights into the pathology of cardiac iron overload across various anaemias and can be used to monitor cardiac iron overload and cardiac function. All chronically transfused patients are at risk for cardiac iron accumulation after sufficient exposure to transfusional iron, but the risk is modulated by disease state and chelation therapy. All three available iron chelators remove cardiac iron, but each has distinct chemical properties that influence their efficacy. More prospective trials are needed to assess the effects of single-agent or combination iron chelation therapy on cardiac iron levels and cardiac function. Ultimately, iron chelation therapies should be tailored to meet each individual patient’s needs and lifestyle demands, similar to most medical therapies.
Acknowledgments
Medical editorial assistance was provided by Georgina Hutber and funded by Novartis Pharmaceuticals.
Footnotes
Conflict of interest and funding
Dr John Wood has received speaker’s honoraria and research funding from Novartis. Dr Wood has received speaker’s honoraria from Apotex.
References
- 1.Olivieri NF, Nathan DG, MacMillan JH, et al. Survival in medically treated patients with homozygous beta-thalassemia. N Engl J Med. 1994;331:574–578. doi: 10.1056/NEJM199409013310903. [DOI] [PubMed] [Google Scholar]
- 2.Borgna-Pignatti C, Rugolotto S, De SP, et al. Survival and complications in patients with thalassemia major treated with transfusion and deferoxamine. Haematologica. 2004;89:1187–1193. [PubMed] [Google Scholar]
- 3.Buja LM, Roberts WC. Iron in the heart. Etiology and clinical significance. Am J Med. 1971;51:209–221. doi: 10.1016/0002-9343(71)90240-3. [DOI] [PubMed] [Google Scholar]
- 4.Wood JC, Enriquez C, Ghugre N, et al. Physiology and pathophysiology of iron cardiomyopathy in thalassemia. Ann NY Acad Sci. 2005;1054:386–395. doi: 10.1196/annals.1345.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oudit GY, Trivieri MG, Khaper N, et al. Role of L-type Ca2+ channels in iron transport and iron-overloaded cardiomyopathy. J Mol Med. 2006;84:349–364. doi: 10.1007/s00109-005-0029-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartfay WJ, Bartfay E. Iron-overload cardiomyopathy: evidence for a free radical-mediated mechanism of injury and dysfunction in a murine model. Biol Res Nurs. 2000;2:49–59. doi: 10.1177/109980040000200106. [DOI] [PubMed] [Google Scholar]
- 7.Gabutti V, Piga A. Results of long-term iron-chelating therapy. Acta Haematol. 1996;95:26–36. doi: 10.1159/000203853. [DOI] [PubMed] [Google Scholar]
- 8.Telfer PT, Prestcott E, Holden S, et al. Hepatic iron concentration combined with long-term monitoring of serum ferritin to predict complications of iron overload in thalassaemia major. Br J Haematol. 2000;110:971–977. doi: 10.1046/j.1365-2141.2000.02298.x. [DOI] [PubMed] [Google Scholar]
- 9.Davis BA, O’Sullivan C, Jarritt PH, et al. Value of sequential monitoring of left ventricular ejection fraction in the management of thalassemia major. Blood. 2004;104:263–269. doi: 10.1182/blood-2003-08-2841. [DOI] [PubMed] [Google Scholar]
- 10.Brittenham GM, Griffith PM, Nienhuis AW, et al. Efficacy of deferoxamine in preventing complications of iron overload in patients with thalassaemia major. N Engl J Med. 1994;331:567–573. doi: 10.1056/NEJM199409013310902. [DOI] [PubMed] [Google Scholar]
- 11.Angelucci E, Brittenham GM, McLaren CE, et al. Hepatic iron concentration and total body iron stores in thalassemia major. N Engl J Med. 2000;343:327–331. doi: 10.1056/NEJM200008033430503. [DOI] [PubMed] [Google Scholar]
- 12.Angelucci E, Baronciani D, Lucarelli G, et al. Needle liver biopsy in thalassaemia: analyses of diagnostic accuracy and safety in 1184 consecutive biopsies. Br J Haematol. 1995;89:757–761. doi: 10.1111/j.1365-2141.1995.tb08412.x. [DOI] [PubMed] [Google Scholar]
- 13.St Pierre TG, Clark PR, Chua-Anusorn W, et al. Noninvasive measurement and imaging of liver iron concentrations using proton magnetic resonance. Blood. 2005;105:855–861. doi: 10.1182/blood-2004-01-0177. [DOI] [PubMed] [Google Scholar]
- 14.Wood JC. Diagnosis and management of transfusion iron overload: the role of imaging. Am J Hematol. 2007;82(12 Suppl):1132–1135. doi: 10.1002/ajh.21099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wood JC, Ghugre N. Magnetic resonance imaging assessment of excess iron in thalassemia, sickle cell disease and other iron overload diseases. Hemoglobin. 2008;32:85–96. doi: 10.1080/03630260701699912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson LJ, Holden S, Davis B, et al. Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur Heart J. 2001;22:2171–2179. doi: 10.1053/euhj.2001.2822. [DOI] [PubMed] [Google Scholar]
- 17.Porter JB. Practical management of iron overload. Br J Haematol. 2001;115:239–252. doi: 10.1046/j.1365-2141.2001.03195.x. [DOI] [PubMed] [Google Scholar]
- 18.Wood JC. Magnetic resonance imaging measurement of iron overload. Curr Opin Hematol. 2007;14:183–190. doi: 10.1097/MOH.0b013e3280d2b76b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wood JC, Otto-Duessel M, Aguilar M, et al. Cardiac iron determines cardiac T2*, T2, and T1 in the gerbil model of iron cardiomyopathy. Circulation. 2005;112:535–545. doi: 10.1161/CIRCULATIONAHA.104.504415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghugre NR, Enriquez CM, Gonzalez I, et al. MRI detects myocardial iron in the human heart. Magn Reson Med. 2006;56:681–686. doi: 10.1002/mrm.20981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheung YF, Chan GC, Ha SY. Effect of deferasirox (ICL670) on arterial function in patients with beta-thalassaemia major. Br J Haematol. 2008;141:728–733. doi: 10.1111/j.1365-2141.2008.07092.x. [DOI] [PubMed] [Google Scholar]
- 22.Li W, Coates T, Wood JC. Atrial dysfunction as a marker of iron cardiotoxicity in thalassemia major. Haematologica. 2008;93:311–312. doi: 10.3324/haematol.11958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanner MA, Galanello R, Dessi C, et al. A randomized, placebo-controlled, double-blind trial of the effect of combined therapy with deferoxamine and deferiprone on myocardial iron in thalassaemia major using cardiovascular magnetic resonance. Circulation. 2007;115:1876–1884. doi: 10.1161/CIRCULATIONAHA.106.648790. [DOI] [PubMed] [Google Scholar]
- 24.Jensen PD, Jensen FT, Christensen T, et al. Evaluation of myocardial iron by magnetic resonance imaging during iron chelation therapy with deferrioxamine: indication of close relation between myocardial iron content and chelatable iron pool. Blood. 2003;101:4632–4639. doi: 10.1182/blood-2002-09-2754. [DOI] [PubMed] [Google Scholar]
- 25.AI Schafer A, Cheron RG, Dluhy R, et al. Clinical consequences of acquired transfusional iron overload in adults. N Engl J Med. 1981;304:319–324. doi: 10.1056/NEJM198102053040603. [DOI] [PubMed] [Google Scholar]
- 26.Borgna-Pignatti C, Cappellini MD, De Stefano P, et al. Cardiac morbidity and mortality in desferrioxamine or deferiprone treated patients with thalassaemia major. Blood. 2006;107:3733–3737. doi: 10.1182/blood-2005-07-2933. [DOI] [PubMed] [Google Scholar]
- 27.Wood JC, Tyszka JM, Carson S, et al. Myocardial iron loading in transfusion-dependent thalassemia and sickle cell disease. Blood. 2004;103:1934–1936. doi: 10.1182/blood-2003-06-1919. [DOI] [PubMed] [Google Scholar]
- 28.Wood JC, Origa R, Agus A, et al. Onset of cardiac iron loading in pediatric patients with thalassemia major. Haematologica. 2008;93:917–920. doi: 10.3324/haematol.12513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glanville J, Eleftheriou P, Porter JB. MRI evidence of cardiac iron accumulation in myelodysplasia and unusual anaemias. Blood. 2006;108 abstract 1553. [Google Scholar]
- 30.Chacko J, Pennell DJ, Tanner MA, et al. Myocardial iron loading by magnetic resonance imaging T2* in good prognostic myelodysplastic syndrome patients on long-term blood transfusions. Br J Haematol. 2007;138:587–593. doi: 10.1111/j.1365-2141.2007.06695.x. [DOI] [PubMed] [Google Scholar]
- 31.Konen E, Ghoti H, Goitein O, et al. No evidence for myocardial iron overload in multitransfused patients with myelodysplastic syndrome using cardiac magnetic resonance T2* technique. Am J Hematol. 2007;82:1013–1016. doi: 10.1002/ajh.20980. [DOI] [PubMed] [Google Scholar]
- 32.Walter PB, Fung EB, Killilea DW, et al. Oxidative stress and inflammation in iron-overloaded patients with beta-thalassaemia or sickle cell disease. Br J Haematol. 2006;135:254–263. doi: 10.1111/j.1365-2141.2006.06277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shah FT, Westwood MA, Evans PJ, et al. Discordance in MRI assessment in iron distribution and plasma NTBI between transfusionally iron loaded adults with sickle cell and thalassemia. Blood. 2002;100 abstract 668a. [Google Scholar]
- 34.Papanikolaou G, Tzilianos M, Christakis JI, et al. Hepcidin in iron overload disorders. Blood. 2005;105:4103–4105. doi: 10.1182/blood-2004-12-4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fung EB, Harmatz PR, Lee PD, et al. Increased prevalence of iron-overload associated endocrinopathy in thalassaemia versus sickle-cell disease. Br J Haematol. 2006;135:574–582. doi: 10.1111/j.1365-2141.2006.06332.x. [DOI] [PubMed] [Google Scholar]
- 36.Smiley D, Dagogo-Jack S, Umpierrez G. Therapy insight: metabolic and endocrine disorders in sickle cell disease. Nat Clin Pract Endocrinol Metab. 2008;4:102–109. doi: 10.1038/ncpendmet0702. [DOI] [PubMed] [Google Scholar]
- 37.Raman SV, Simonetti OP, Cataland SR, et al. Myocardial ischemia and right ventricular dysfunction in adult patients with sickle cell disease. Haematologica. 2006;91:1329–1335. [PubMed] [Google Scholar]
- 38.Westwood MA, Shah F, Anderson LJ, et al. Myocardial tissue characterization and the role of chronic anemia in sickle cell cardiomyopathy. J Magn Reson Imaging. 2007;26:564–568. doi: 10.1002/jmri.21018. [DOI] [PubMed] [Google Scholar]
- 39.Anderson LJ, Wonke B, Prescott E, et al. Comparison of effects of oral deferiprone and subcutaneous desferrioxamine on myocardial iron concentrations and ventricular function in beta-thalassaemia. Lancet. 2002;360:516–520. doi: 10.1016/s0140-6736(02)09740-4. [DOI] [PubMed] [Google Scholar]
- 40.Pennell DJ, Berdoukas V, Karagiorga M, et al. Randomized controlled trial of deferiprone or deferoxamine in beta-thalassemia major patients with asymptomatic myocardial siderosis. Blood. 2006;107:3738–3744. doi: 10.1182/blood-2005-07-2948. [DOI] [PubMed] [Google Scholar]
- 41.Glickstein H, El RB, Link G, et al. Action of chelators in iron-loaded cardiac cells: accessibility to intracellular labile iron and functional consequences. Blood. 2006;108:3195–3203. doi: 10.1182/blood-2006-05-020867. [DOI] [PubMed] [Google Scholar]
- 42.Wood JC, Otto-Duessel M, Gonzalez I, et al. Deferasirox and deferiprone remove cardiac iron in the iron-overloaded gerbil. Transl Res. 2006;148:272–280. doi: 10.1016/j.trsl.2006.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Otto-Duessel M, Aguilar M, Nick H, et al. Comparison of twice-daily vs once-daily deferasirox dosing in a gerbil model of iron cardiomyopathy. Exp Hematol. 2007;35:1069–1073. doi: 10.1016/j.exphem.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vichinsky E, Onyekwere O, Porter J, et al. A randomised comparison of deferasirox versus deferoxamine for the treatment of transfusional iron overload in sickle cell disease. Br J Haematol. 2007;136:501–508. doi: 10.1111/j.1365-2141.2006.06455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Piga A, Galanello R, Forni GL, et al. Randomized phase II trial of deferasirox (Exjade, ICL670), a once-daily, orally-administered iron chelator, in comparison to deferoxamine in thalassemia patients with transfusional iron overload. Haematologica. 2006;91:873–880. [PubMed] [Google Scholar]
- 46.Cappellini MD, Cohen A, Piga A, et al. A phase 3 study of deferasirox (ICL670), a once-daily oral iron chelator, in patients with beta-thalassemia. Blood. 2006;107:3455–3462. doi: 10.1182/blood-2005-08-3430. [DOI] [PubMed] [Google Scholar]
- 47.Porter J, Galanello R, Saglio G, et al. Relative response of patients with myelodysplastic syndromes and other transfusion-dependent anaemias to deferasirox (ICL670): a 1-yr prospective study. Eur J Haematol. 2008;80:168–176. doi: 10.1111/j.1600-0609.2007.00985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Porter JB, Tanner MA, Pennell DJ, et al. Improved myocardial T2* in transfusion dependent anemias receiving ICL670 (deferasirox) Blood. 2005;106 abstract 3600. [Google Scholar]
- 49.Wood J, Thompson AA, Paley C, et al. MRI t2* demonstrates reduced cardiac iron burden following moderate-to high-dose deferasirox treatment in chronically transfused beta-thalassemia patients. Haematologica. 2008;93(1) abstract 0844. [Google Scholar]
- 50.Taher A, Al Jefri A, Elalfy MS, et al. Oral deferasirox (Exjade®, ICL670) is effective, with a clinically manageable safety profile, in pediatric β-thalassemia patients with high iron burden. Blood. 2007;110 abstract 2279. [Google Scholar]