Abstract
Background
Antiarrhythmic action of flecainide is based on sodium channel blockade. Beta1-adrenoceptor (β1AR) activation induces sodium channel inhibition, too. The aim of the present study was to evaluate the impact of different β1AR genotypes on antiarrhythmic action of flecainide in patients with structural heart disease and atrial fibrillation.
Methodology/Principal Findings
In 145 subjects, 87 with atrial fibrillation, genotyping was performed to identify the individual β1AR Arg389Gly and Ser49Gly polymorphism. Resting heart rate during atrial fibrillation and success of flecainide-induced cardioversion were correlated with β1AR genotype. The overall cardioversion rate with flecainide was 39%. The Arg389Arg genotype was associated with the highest cardioversion rate (55.5%; OR 3.30; 95% CI; 1.34–8.13; p = 0.003) compared to patients with Arg389Gly (29.5%; OR 0.44; 95% CI; 0.18–1.06; p = 0.066) and Gly389Gly (14%; OR 0.24; 95% CI 0.03–2.07; p = 0.17) variants. The single Ser49Gly polymorphism did not influence the conversion rate. In combination, patients with Arg389Gly-Ser49Gly genotype displayed the lowest conversion rate with 20.8% (OR 0.31; 95% CI; 0.10–0.93; p = 0.03). In patients with Arg389Arg variants the heart rate during atrial fibrillation was significantly higher (110±2.7 bpm; p = 0.03 vs. other variants) compared to Arg389Gly (104.8±2.4 bpm) and Gly389Gly (96.9±5.8 bpm) carriers. The Arg389Gly-Ser49Gly genotype was more common in patients with atrial fibrillation compared to patients without atrial fibrillation (27.6% vs. 5.2%; HR 6.98; 95% CI; 1.99–24.46; p<0.001).
Conclusions
The β1AR Arg389Arg genotype is associated with increased flecainide potency and higher heart rate during atrial fibrillation. The Arg389Gly-Ser49Gly genotype might be of predictive value for atrial fibrillation.
Introduction
Flecainide is used for cardioversion in patients with recent onset atrial fibrillation (AF). It has been successfully implemented for out-of-hospital use as a pill-in-the-pocket approach in patients without structural heart disease [1], [2]. In patients with organic heart disease our group recently reported that a single dosage of flecainide is not harmful, but less efficient than in healthy subjects [3]. Only few factors which might predict the success of flecainide in acute cardioversion have been identified, yet. The duration of AF and the extent of cardiac remodeling in diseased heart might be such factors.
The antiarrhythmic effect of flecainide is based on Na+ current (INa) inhibition [4]. Experimental data have shown that beta1- adrenergic receptor (β1AR) stimulation causes an inhibition of INa, too [5], [6], [7], [8].
For the β1AR, 12 nucleotide polymorphisms (SNPs) have been identified, but only two have being associated with possibly functional relevance (for review see [9]). At position 389 (P389), the putative stimulatory G-protein coupling domain of β1AR, glycine (Gly389) is substituted by arginine (Arg389). The Arg389 variants exhibited higher basal adenyl cyclase activity than the Gly389 variants [10], [11]. In clinical experiments patients with Arg389 allele exerted augmented cardiac contractile response to catecholamines [12]. The second potential relevant polymorphism is linked to the position 49 (P49) of β1AR, where serine (Ser49) is substituted by glycine (Gly49) [10], [13], [14]. Ser49Gly β1AR is considered to play a modulating role without affecting the agonist binding and basal or maximal stimulated adenyl cyclase activity [10], [15], [16].
In the present study we aimed to determine whether the two SNPs P389 and P49 of β1AR are relevant in predicting AF, antiarrhythmic drug therapy and flecainide-induced cardioversion in patients with recent onset AF.
Methods
Ethics Statement
The study complies with the Declaration of Helsinki, the local ethics committee of the University of Cologne has approved the research protocol. All patients gave their written informed consent.
Subjects
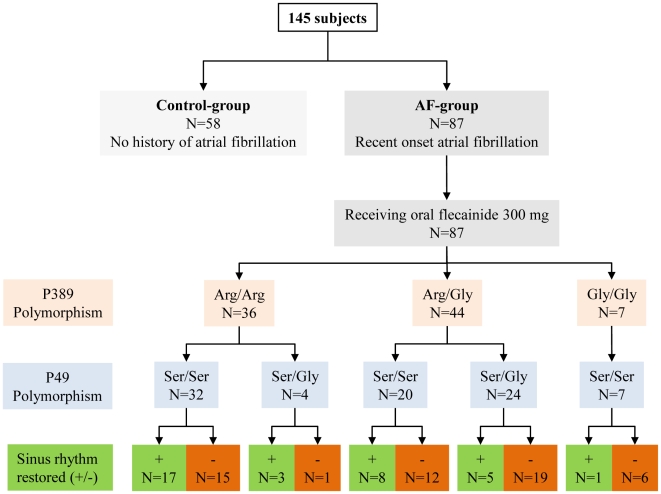
The cohort of 145 subjects was composed of patients with recent onset (<10 days, mean 5.8±0.5 days) AF (AF-group; n = 87) and matched patients without documented and subjective history of AF (control group; n = 58; Figure 1). Patients with AF were at age above 18 years and revealed at least one of the additional comorbidities: coronary heart disease (CHD), dilated cardiomyopathy (DCM) with reduced left ventricular ejection fraction (<50%), heart failure (> NYHA II) or a PROCAM-score above 41 [17]. In all patients with AF transesophageal echocardiography was performed to exclude the presence of intracardiac thrombi. Routine transthoracic echocardiography was performed to measure cardiac dimensions and asses valvular, left and right ventricular function.
Figure 1. Study flow chart.
Allele frequencies of different SNPs at position 389 and 49. The Gly49Gly variant was not present in any patient. All seven patients with Gly389Gly were homozygous for Ser49Ser.
Matched patients in the control group were admitted to our hospital with acute myocardial infarction for percutaneous coronary intervention, without any history of AF.
Sinus rhythm cardioversion
For pharmacological cardioversion patients with AF received oral flecainide 300 mg. A successful cardioversion was defined as sinus rhythm restoration within the first 6 hours after flecainide intake. After 12-h ECG monitoring had confirmed the persistence of a stable sinus rhythm. Patients in which flecainide did not restore sinus rhythm underwent electrical cardioversion (data not shown). At four weeks the persistence of sinus rhythm was controlled.
Genotyping
Genomic DNA was extracted from blood of subjects by standard techniques [18]. Polymorphism detection was performed like previously described [10]. Briefly, DNA was amplified by polymerase chain reaction and subjected to enzymatic restriction analysis by BsmF1 for the position 389 and Eco01091 for the position 49 [10], [12]. Genotyping was performed blindly to all other data.
Statistical analysis
All variables were tested for normal distribution with the Kolmogorov-Smirnov test. Continuous variables are expressed as means ± standard error of the mean. Comparison of 2 means was performed with the t test for normally distributed variables and the Mann-Whitney U test for non-Gaussian variables. Chi-square test was used for nonparametric comparisons. All statistical tests were 2-tailed, and p<0.05 was considered statistically significant.
Results
Patient Characteristics
The mean age of participants with AF was 67.8±1.3 years (71% men). Genotyping revealed a prevalence of homozygous patients for Arg389Arg of 41.4% (n = 36), heterozygous for Arg389Gly of 50.6% (n = 44) and homozygous for Gly389Gly of 8% (n = 7). 59 patients (67.8%) with AF were homozygous for serine at position 49 (Ser49Ser) and 28 patients (32.2%) heterozygous for Ser49Gly. The Gly49Gly genotype was not present in any patient (Figure 1). Baseline characteristics were similar in all variants (Table 1). In particular the calculated PROCAM-score, serum potassium and sodium concentrations were not significantly divergent between different allele carriers. Baseline heart rate during AF was significantly higher in patients with the Arg389Arg genotype with 110.7±2.7 bpm versus 104.8±2.4 bpm in patients with Arg389Gly and 96.9±5.8 bpm in Gly389Gly carriers (p = 0.03). In patients homozygous to Arg389Arg the heart rate was not influenced by Ser49Ser (n = 32; 110.8±3.0 bpm; p = ns) and Ser49Gly (n = 4; 110.0±4.1 bpm; p = ns). The resting heart rate was not modified in the same manner by Ser49Ser or Ser49Gly polymorphism in patients heterozygous for Arg389Gly (Ser49Ser: n = 20; 102.5±2.9 bpm; Ser49Gly: n = 24; 106.7±3.0 bpm; p = ns). In patients homozygous for Gly389Gly only the Ser49Ser variant was present.
Table 1. Baseline characteristics in patients with different β1AR variants.
| All patients with AF N = 87 | Arg389Arg N = 36 | Arg389Gly N = 44 | Gly389Gly N = 7 | Ser49Ser N = 59 | Ser49Gly N = 28 | |
| Women/Men (%) | 25/62 (29/71) | 8/28 (22/78) | 14/30 (31/69) | 3/4 (43/57) | 18/41 (31/29) | 7/21 (25/75) |
| Age | 67.8±1.3 | 67.1±2.1 | 67.7±1.8 | 72.0±2.6 | 67.1±2.1 | 69.3±3.8 |
| BMI | 26.6±0.4 | 27.2±0.5 | 26.4±0.5 | 24.8±1.0 | 26.4±0.7 | 27.0±0.5 |
| Heart rate per minute | 106.6±1.7 | 110.7±2.7* | 104.8±2.4 | 96.9±5.8 | 107.4±1.8 | 104.9±2.4 |
| PROCAM-Score | 44.4±0.5 | 44.2±0.7 | 44.9±0.8 | 42.8±1.3 | 44.4±0.7 | 44.4±0.5 |
| Hypertension (%) | 47 (54) | 17 (47) | 25 (57) | 5 (71) | 33 (56) | 14 (50) |
| CAD (%) | 32 (37) | 13 (36) | 18 (41) | 1 (14) | 22 (37) | 10 (36) |
| Myocardial infarction (%) | 17 (20) | 9 (25) | 8 (18) | 0 (0) | 13 (22) | 4 (14) |
| LVEF <40% | 22 (25) | 10 (28) | 11 (25) | 1 (14) | 16 (27) | 6 (21) |
| CABG (%) | 7 (8) | 4 (11) | 3 (7) | 0 (0) | 5 (8) | 2 (7) |
| Serum sodium (mmol/l) | 137.8±0.5 | 137.1±0.9 | 138.6±0.5 | 136.5±1.0 | 138.1±0.4 | 137.3±0.5 |
| Serum potassium (mmol/I) | 4.1±0.1 | 4.1±0.1 | 4.1±0.1 | 4.3±0.3 | 4.0±0.1 | 4.3±0.4 |
BMI indicates body mass index, CAD indicates coronary artery disease, LVEF indicates left ventricular ejection fraction, CABG indicates coronary artery bypass grafting.
*p<0.05 vs. other variants.
Echocardiographic assessment revealed similar left atrial and ventricular diameters and left ventricular ejection fraction in all allele groups (Table 2). There was a trend towards larger diastolic interventricular septum diameter (IVSDD) in patients with Arg389Arg alleles (11.4±0.2 mm; Arg389Arg-Ser49Ser: 11.4±0.3 mm; Arg389Arg-Ser49Gly 11.3±0.3 mm) versus Arg389Gly (10.9±0.2 mm; Arg389Gly-Ser49Ser 11.2±3.8 mm; Arg389Gly-Ser49Gly 10.7±0.2 mm) and Gly389Gly alleles (10.1±0.3 mm; p = 0.05 between P389 polymorphisms). The SNP49 variants did not significantly modify the IVSDD. Cardiovascular medication was similar in groups with different polymorphisms (Table 3).
Table 2. Echocardiographic values in patients with different β1AR variants.
| All patients with AF N = 87 | Arg389Arg N = 36 | Arg389Gly N = 44 | Gly389Gly N = 7 | Ser49Ser N = 59 | Ser49Gly N = 28 | |
| LA (mm) | 46.7±0.8 | 47.6±1.5 | 46.0±1.1 | 47.1±1.4 | 47.6±1.0 | 44.7±1.5 |
| LVEDD (mm) | 51.5±0.7 | 51.4±0.9 | 52.0±0.9 | 49.4±2.5 | 50.7±0.7 | 53.3±1.3 |
| IVSDD (mm) | 11.1±0.2 | 11.4±0.2# | 10.9±0.2 | 10.1±0.3 | 11.2±0.2 | 10.8±0.2 |
| LVEF (%) | 56.8±1.5 | 55.6±2.5 | 57.0±2.1 | 62.1±5.0 | 58.6±1.6 | 53.0±3.0 |
LA indicates left atrium, LVEDD indicates left ventricular enddiastolic diameter, IVSDD indicates diastolic interventricular septum diameter, LVEF indicates left ventricular ejection fraction.
Table 3. Cardiovascular medication in patients with different β1AR variants.
| All patients with AF N = 87 | Arg389Arg N = 36 | Arg389Gly N = 44 | Gly389Gly N = 7 | Ser49Ser N = 59 | Ser49Gly N = 28 | |
| Beta-blocker (%) | 79 (91) | 34 (94) | 39 (89) | 6 (86) | 53 (89) | 26 (93) |
| ACE-Inhibitor (%) | 27 (31) | 12 (33) | 13 (30) | 2 (29) | 19 (32) | 8 (29) |
| ARB (%) | 22 (25) | 10 (28) | 9 (20) | 3 (43) | 15 (25) | 7 (25) |
| Statin (%) | 38 (44) | 17 (47) | 19 (43) | 2 (29) | 25 (42) | 13 (46) |
ACE indicates angiotensin converting enzyme, ARB indicates angiotensin receptor blocker.
β1AR Profile and Flecainide Success
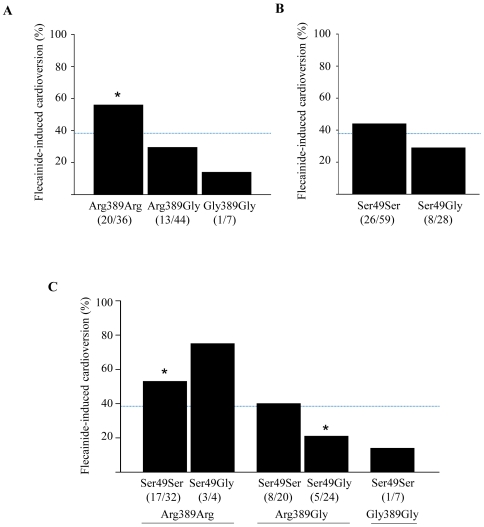
The overall cardioversion success rate after oral flecainide 300 mg was 39% (34/87). The significantly highest rate of sinus rhythm restoration of 55.5% (20/36) was found in patients homozygous to Arg389Arg polymorphism (OR 3.30; 95% CI; 1.34–8.13; p = 0.003; Figure 2).
Figure 2. Success of flecainide-induced cardioversion in different β1AR genotypes, A, P389 polymorphism, B, P49 polymorphism, C, combination genotype of P389 and P49 variants.
Dashed line marks the average cardioversion rate of 39%. *p<0.05 vs. other variants.
In patients with Arg389Gly alleles flecainide was slightly less effective with a cardioversion rate of 29.5% (13/44; OR 0.44; 95% CI; 0.18–1.06; p = 0.066). In the small group of patients with Gly389Gly polymorphism in one of seven patients sinus rhythm could be restored (OR 0.24; 95% CI 0.03–2.07; p = 0.17). The SNP49 variants alone were not associated with the flecainide success in cardioversion. The combination of different SNP389 and SNP49 variants revealed the highest rate for a successful cardioversion with flecainide in patients with the genotype Arg389Arg independently to the coexisting SNP49 variants (Table 4, Figure 2). The lowest chance for a successful cardioversion was associated with the genotype Arg389Gly-Ser49Gly (OR 0.31; 95% CI 0.10–0.93; p = 0.03).
Table 4. Association between different genotypes and the success of flecainide-induced cardioversion.
| Flecainide Success | OR | 95% CI | p | |
| Arg389Arg (n = 36) | 20 (55.5%) | 3.30 | 1.34−8.13 | 0.003 |
| Ser49Ser (n = 32) | 17 (53.1%) | 2.53 | 1.03−6.23 | 0.04 |
| Ser49Gly (n = 4) | 3 (75.0%) | 5.03 | 0.50−50.52 | 0.14 |
| Gly49Gly (n = 0) | - | - | - | - |
| Arg389Gly (n = 44) | 13 (29.5%) | 0.44 | 0.18−1.06 | 0.066 |
| Ser49Ser (n = 20) | 8 (40.0%) | 1.05 | 0.38−2.92 | 0.93 |
| Ser49Gly (n = 24) | 5 (20.8%) | 0.31 | 0.10−0.93 | 0.03 |
| Gly49Gly (n = 0) | - | - | - | - |
| Gly389Gly (n = 7) | 1 (14.3%) | 0.24 | 0.03−2.07 | 0.17 |
| Ser49Ser (n = 7) | 1 (14.3%) | 0.24 | 0.03−2.07 | 0.17 |
| Ser49Gly (n = 0) | - | - | - | - |
| Gly49Gly (n = 0) | - | - | - | - |
Mean heart rate of patients with successful cardioversion was during AF higher versus patients without sinus rhythm restoration, although statistical significance was not reached (108.0±4.9 bpm vs. 99.4±3.3 bpm; p = ns).
All patients in which pharmacological cardioversion failed underwent successful electrical cardioversion. At four weeks follow-up two patients showed a relapse of atrial fibrillation.
β1AR Polymorphism and Risk of Atrial Fibrillation
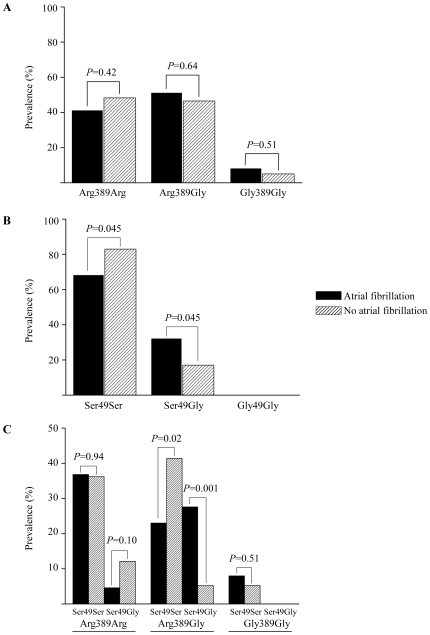
To identify whether the risk for AF is linked to a specific β1AR polymorphism, we compared the relative allele frequencies in patients with AF (AF-group; n = 87) to patients with no history of AF (control-group; n = 58).
This comparison revealed quite similar SNP389 allele distributions in patients with AF versus control-group (Arg389Arg: 41.4% AF-group vs. 48.3% control-group, p = ns; Arg389Gly: 50.6% AF-group vs. 46.6% control-group, p = ns; Gly389Gly: 8.0% AF-group vs. 5.1%, p = ns; Figure 3A). The prevalence of the Ser49Ser polymorphism was tendentiously higher in patients without AF versus those with AF (32.2% vs. 17.2%; p = 0.045; Figure 3B). Conversely, Ser49Gly genotype was slightly more common in patients with than without AF (73.7% vs. 26.3%; p = 0.045). The combination of both SNPs revealed that the prevalence of the Arg389Gly-Ser49Gly genotype was significantly higher in patients with versus without AF (27.6% versus 5.2%; p = 0.001; Figure 3C). Thus, a hazard ratio of 6.98 for AF in patients with the Arg389Gly-Ser49Gly genotype was calculated (95% CI, 1.99–24.46; p<0.001).
Figure 3. Relative allele distribution in patients with and without AF.
A, P389 variants, B, P49 variants, C, combined genotype of P389 and P49 variants.
Discussion
The Arg389Gly polymorphism has been shown to be associated with heart failure, acute myocardial infarction, hypertension and left ventricular remodeling in response to beta- blockade [19], [20], [21].
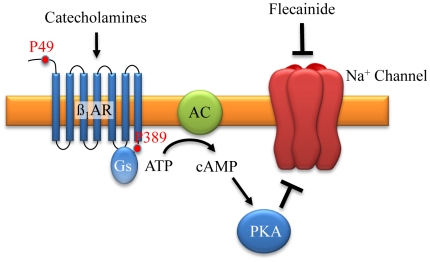
We found that the Arg389Arg polymorphism was strongly associated with a higher efficiency of flecainide action in patients with AF. Reported different affinity of β1AR SNP389 variants to catecholamines may explain the underlying mechanism of this observation [11], [22], [23]. In vitro experiments have demonstrated that the Arg389Arg genotype is a gain-of-function variant with enhanced coupling to Gs protein, following a slightly elevated basal and three- to four-fold higher adenylate cyclase activity than Gly389 variants [10]. Isoproterenol-stimulation enhances Gs protein activation and adrenergic-mediated reduction of sodium current [24]. This accessory reduction of sodium influx may potentiate the flecainide induced-blockade of INa (Figure 4).
Figure 4. Schematic explanation of beta1-adrenoceptor and flecainide interaction.
Localisation of P49 on N-terminus and P389 on C-terminus. β1AR activation results via G protein (Gs) and adenylate cyclase (AC) pathway in increase of cyclic adenosine monophosphate (cAMP) and activation of protein kinase A (PKA). PKA induced phosphorylation inactivates INa channel activity. Flecainide inhibits INa directly.
Compared to more healthy subjects we noticed a poorer cardioversion rate in population with organic heart diseases despite the expected higher susceptibility to flecainide due to elevated catecholamine concentrations in these patients [1], [2], [25], [26]. But contrarily, diseased cardiomyocytes promote AF due to complex electrical and structural remodeling [27], [28], [29], [30]. These changes may outbalance the supposed increased antiarrhythmic effects of flecainide. Chronic treatment with flecainide has been reported to be harmful, particularly in patients with structural heart diseases and myocardial infarction [31]. Higher serum catecholamine concentrations in patients with cardiovascular diseases may have augmented the β1AR-induced INa blockade and the proarrhythmic effects of flecainide, especially in patients with vulnerable myocardium and the Arg389Arg genotype [25], [26], [32], [33], [34], [35].
Newer antiarrhythmic agents like vernakalant and dronedarone with sodium channel blocking attribute may be similarly influenced by β1AR variants [36], [37].
Resting heart rate has been associated with Arg389Gly variants [38], [39]. Concordantly, we observed during AF significantly higher ventricular heart rates in homozygous patients for the arginine allele and the lowest heart rate in patients homozygous for the glycine variant (heart rate Arg389Arg>Arg389Gly>Gly389Gly). This may have clinical consequences and patients with Arg389Arg genotype may need intensified beta-blocker treatment.
The interventricular septum thickness was larger in patients with Arg389Arg than in carriers of other variants. This may be explained due to elevated catecholamine impact on cardiomyocytes in this genotype and enhanced hypertrophy signaling [40]. The β1AR SNP49 polymorphism itself was not associated with functional effects of flecainide. But in combination with the P389 polymorphism flecainide was most effective in Arg389Arg-Ser49Ser genotype and least effective in Arg389Gly-Ser49Gly genotype. This finding is supported by previous reports that the SNP49 variants have not an effect on the adenyl cyclase activity and position 389, but not 49, obviously determines the functional responsiveness of β1AR, and P49 may only play a modulating role [9]. The genotype Arg389Arg-Ser49Gly may need higher flecainide concentrations for successful cardioversion.
In the present study the SNP389 β1AR genotype alone was not, and the Ser49Gly genotype slightly associated with the AF prevalence. But in combination of both SNPs, the genotype Arg389Gly-Ser49Gly was associated with an almost 7-fold higher risk for AF than other genotypes. Despite the limited sample size in our cohort, this finding confirms a recent report that the Ser49Gly variant may be associated with AF [41]. Further studies are needed to figure out the causal explanation for this supposed genetic determination of AF.
The present investigation provides more insight into the potential influences of β1AR polymorphism on antiarrhythmic drug action, which are currently underestimated. Our results indicate that genetic testing might help identifying patients at elevated risk for AF. When this observation is confirmed in larger cohorts, preventive strategies in these patients might be helpful. Further studies with genetic testing may help to identify patients with organic heart diseases in whom a differential antiarrhythmic therapy might be indicated. Newer antiarrhythmic drugs may also interfere with the β1AR polymorphism.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: The authors have no support or funding to report.
References
- 1.Alboni P, Botto GL, Baldi N, Luzi M, Russo V, et al. Outpatient treatment of recent-onset atrial fibrillation with the “pill-in-the-pocket” approach. N Engl J Med. 2004;351:2384–2391. doi: 10.1056/NEJMoa041233. [DOI] [PubMed] [Google Scholar]
- 2.Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation). J Am Coll Cardiol. 2006;48:854–906. doi: 10.1016/j.jacc.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 3.Er F, Aslan O, Caglayan E, Gassanov N, Nia AM, et al. Flecainide for cardioversion in patients at elevated cardiovascular risk and persistent atrial fibrillation: a prospective observational study. Clin Res Cardiol. 2010 doi: 10.1007/s00392-010-0129-7. [DOI] [PubMed] [Google Scholar]
- 4.Roden DM, Woosley RL. Drug therapy. Flecainide. N Engl J Med. 1986;315:36–41. doi: 10.1056/NEJM198607033150106. [DOI] [PubMed] [Google Scholar]
- 5.Ono K, Fozzard HA, Hanck DA. Mechanism of cAMP-dependent modulation of cardiac sodium channel current kinetics. Circ Res. 1993;72:807–815. doi: 10.1161/01.res.72.4.807. [DOI] [PubMed] [Google Scholar]
- 6.Ono K, Kiyosue T, Arita M. Isoproterenol, DBcAMP, and forskolin inhibit cardiac sodium current. Am J Physiol. 1989;256:C1131–1137. doi: 10.1152/ajpcell.1989.256.6.C1131. [DOI] [PubMed] [Google Scholar]
- 7.Schubert B, VanDongen AM, Kirsch GE, Brown AM. Beta-adrenergic inhibition of cardiac sodium channels by dual G-protein pathways. Science. 1989;245:516–519. doi: 10.1126/science.2547248. [DOI] [PubMed] [Google Scholar]
- 8.Schubert B, Vandongen AM, Kirsch GE, Brown AM. Inhibition of cardiac Na+ currents by isoproterenol. Am J Physiol. 1990;258:H977–982. doi: 10.1152/ajpheart.1990.258.4.H977. [DOI] [PubMed] [Google Scholar]
- 9.Leineweber K, Heusch G. Beta 1- and beta 2-adrenoceptor polymorphisms and cardiovascular diseases. Br J Pharmacol. 2009;158:61–69. doi: 10.1111/j.1476-5381.2009.00187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mason DA, Moore JD, Green SA, Liggett SB. A gain-of-function polymorphism in a G-protein coupling domain of the human beta1-adrenergic receptor. J Biol Chem. 1999;274:12670–12674. doi: 10.1074/jbc.274.18.12670. [DOI] [PubMed] [Google Scholar]
- 11.Joseph SS, Lynham JA, Grace AA, Colledge WH, Kaumann AJ. Markedly reduced effects of (-)-isoprenaline but not of (-)-CGP12177 and unchanged affinity of beta-blockers at Gly389-beta1-adrenoceptors compared to Arg389-beta1-adrenoceptors. Br J Pharmacol. 2004;142:51–56. doi: 10.1038/sj.bjp.0705753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.La Rosee K, Huntgeburth M, Rosenkranz S, Bohm M, Schnabel P. The Arg389Gly beta1-adrenoceptor gene polymorphism determines contractile response to catecholamines. Pharmacogenetics. 2004;14:711–716. doi: 10.1097/00008571-200411000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Tesson F, Charron P, Peuchmaurd M, Nicaud V, Cambien F, et al. Characterization of a unique genetic variant in the beta1-adrenoceptor gene and evaluation of its role in idiopathic dilated cardiomyopathy. CARDIGENE Group. J Mol Cell Cardiol. 1999;31:1025–1032. doi: 10.1006/jmcc.1999.0947. [DOI] [PubMed] [Google Scholar]
- 14.Maqbool A, Hall AS, Ball SG, Balmforth AJ. Common polymorphisms of beta1-adrenoceptor: identification and rapid screening assay. Lancet. 1999;353:897. doi: 10.1016/s0140-6736(99)00549-8. [DOI] [PubMed] [Google Scholar]
- 15.Sandilands A, Yeo G, Brown MJ, O'Shaughnessy KM. Functional responses of human beta1 adrenoceptors with defined haplotypes for the common 389R>G and 49S>G polymorphisms. Pharmacogenetics. 2004;14:343–349. doi: 10.1097/00008571-200406000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Westerlund H, Kivimaki M, Singh-Manoux A, Melchior M, Ferrie JE, et al. Self-rated health before and after retirement in France (GAZEL): a cohort study. Lancet. 2009;374:1889–1896. doi: 10.1016/S0140-6736(09)61570-1. [DOI] [PubMed] [Google Scholar]
- 17.Assmann G, Cullen P, Schulte H. Simple scoring scheme for calculating the risk of acute coronary events based on the 10-year follow-up of the prospective cardiovascular Munster (PROCAM) study. Circulation. 2002;105:310–315. doi: 10.1161/hc0302.102575. [DOI] [PubMed] [Google Scholar]
- 18.Jones AS, Walker RT. Isolation and analysis of the deoxyribonucleic acid of Mycoplasma mycoides var. Capri. Nature. 1963;198:588–589. doi: 10.1038/198588a0. [DOI] [PubMed] [Google Scholar]
- 19.Iwai C, Akita H, Kanazawa K, Shiga N, Terashima M, et al. Arg389Gly polymorphism of the human beta1-adrenergic receptor in patients with nonfatal acute myocardial infarction. Am Heart J. 2003;146:106–109. doi: 10.1016/S0002-8703(03)00110-8. [DOI] [PubMed] [Google Scholar]
- 20.Shioji K, Kokubo Y, Mannami T, Inamoto N, Morisaki H, et al. Association between hypertension and the alpha-adducin, beta1-adrenoreceptor, and G-protein beta3 subunit genes in the Japanese population; the Suita study. Hypertens Res. 2004;27:31–37. doi: 10.1291/hypres.27.31. [DOI] [PubMed] [Google Scholar]
- 21.Bengtsson K, Melander O, Orho-Melander M, Lindblad U, Ranstam J, et al. Polymorphism in the beta(1)-adrenergic receptor gene and hypertension. Circulation. 2001;104:187–190. doi: 10.1161/01.cir.104.2.187. [DOI] [PubMed] [Google Scholar]
- 22.Liggett SB, Mialet-Perez J, Thaneemit-Chen S, Weber SA, Greene SM, et al. A polymorphism within a conserved beta(1)-adrenergic receptor motif alters cardiac function and beta-blocker response in human heart failure. Proc Natl Acad Sci U S A. 2006;103:11288–11293. doi: 10.1073/pnas.0509937103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mialet Perez J, Rathz DA, Petrashevskaya NN, Hahn HS, Wagoner LE, et al. Beta 1-adrenergic receptor polymorphisms confer differential function and predisposition to heart failure. Nat Med. 2003;9:1300–1305. doi: 10.1038/nm930. [DOI] [PubMed] [Google Scholar]
- 24.Cragun KT, Johnson SB, Packer DL. Beta-adrenergic augmentation of flecainide-induced conduction slowing in canine Purkinje fibers. Circulation. 1997;96:2701–2708. doi: 10.1161/01.cir.96.8.2701. [DOI] [PubMed] [Google Scholar]
- 25.Raab W. Key position of catecholamines in functional and degenerative cardiovascular pathology. Am J Cardiol. 1960;5:571–578. doi: 10.1016/0002-9149(60)90121-1. [DOI] [PubMed] [Google Scholar]
- 26.Slavikova J, Kuncova J, Topolcan O. Plasma catecholamines and ischemic heart disease. Clin Cardiol. 2007;30:326–330. doi: 10.1002/clc.20099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 28.Jalife J, Berenfeld O, Mansour M. Mother rotors and fibrillatory conduction: a mechanism of atrial fibrillation. Cardiovasc Res. 2002;54:204–216. doi: 10.1016/s0008-6363(02)00223-7. [DOI] [PubMed] [Google Scholar]
- 29.Peters NS, Schilling RJ, Kanagaratnam P, Markides V. Atrial fibrillation: strategies to control, combat, and cure. Lancet. 2002;359:593–603. doi: 10.1016/S0140-6736(02)07748-6. [DOI] [PubMed] [Google Scholar]
- 30.Everett THt, Olgin JE. Atrial fibrosis and the mechanisms of atrial fibrillation. Heart Rhythm. 2007;4:S24–27. doi: 10.1016/j.hrthm.2006.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Echt DS, Liebson PR, Mitchell LB, Peters RW, Obias-Manno D, et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med. 1991;324:781–788. doi: 10.1056/NEJM199103213241201. [DOI] [PubMed] [Google Scholar]
- 32.Packer M. The neurohormonal hypothesis: a theory to explain the mechanism of disease progression in heart failure. J Am Coll Cardiol. 1992;20:248–254. doi: 10.1016/0735-1097(92)90167-l. [DOI] [PubMed] [Google Scholar]
- 33.Cohn JN, Levine TB, Olivari MT, Garberg V, Lura D, et al. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med. 1984;311:819–823. doi: 10.1056/NEJM198409273111303. [DOI] [PubMed] [Google Scholar]
- 34.Brophy JM, Joseph L, Rouleau JL. Beta-blockers in congestive heart failure. A Bayesian meta-analysis. Ann Intern Med. 2001;134:550–560. doi: 10.7326/0003-4819-134-7-200104030-00008. [DOI] [PubMed] [Google Scholar]
- 35.Foody JM, Farrell MH, Krumholz HM. beta-Blocker therapy in heart failure: scientific review. JAMA. 2002;287:883–889. doi: 10.1001/jama.287.7.883. [DOI] [PubMed] [Google Scholar]
- 36.Roy D, Pratt CM, Torp-Pedersen C, Wyse DG, Toft E, et al. Vernakalant hydrochloride for rapid conversion of atrial fibrillation: a phase 3, randomized, placebo-controlled trial. Circulation. 2008;117:1518–1525. doi: 10.1161/CIRCULATIONAHA.107.723866. [DOI] [PubMed] [Google Scholar]
- 37.Lalevee N, Nargeot J, Barrere-Lemaire S, Gautier P, Richard S. Effects of amiodarone and dronedarone on voltage-dependent sodium current in human cardiomyocytes. J Cardiovasc Electrophysiol. 2003;14:885–890. doi: 10.1046/j.1540-8167.2003.03064.x. [DOI] [PubMed] [Google Scholar]
- 38.Humma LM, Puckett BJ, Richardson HE, Terra SG, Andrisin TE, et al. Effects of beta1-adrenoceptor genetic polymorphisms on resting hemodynamics in patients undergoing diagnostic testing for ischemia. Am J Cardiol. 2001;88:1034–1037. doi: 10.1016/s0002-9149(01)01986-5. [DOI] [PubMed] [Google Scholar]
- 39.Ranade K, Jorgenson E, Sheu WH, Pei D, Hsiung CA, et al. A polymorphism in the beta1 adrenergic receptor is associated with resting heart rate. Am J Hum Genet. 2002;70:935–942. doi: 10.1086/339621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fu C, Wang H, Wang S, Shi Y, Zhou X, et al. Association of beta 1-adrenergic receptor gene polymorphisms with left ventricular hypertrophy in human essential hypertension. Clin Biochem. 2008;41:773–778. doi: 10.1016/j.clinbiochem.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 41.Nicoulina S, Shulman V, Shesternya P, Chernova A, Salmina A, et al. Association of ADRB1 gene polymorphism with atrial fibrillation. Genet Test Mol Biomarkers. 2010;14:249–253. doi: 10.1089/gtmb.2009.0100. [DOI] [PubMed] [Google Scholar]