Abstract
To guide the development of tactile speech aids, tactual detection and temporal order discrimination by congenitally deaf and normal-hearing adults have been examined. Tactual detection thresholds for sinusoidal vibrations between 2 and 300 Hz were measured at the left thumb and index finger using an adaptive paradigm. Temporal onset- and offset-order discrimination were tested using stimuli of 50 Hz at the thumb and 250 Hz at the index finger, delivered asynchronously and varied independently in amplitude and duration. Mean detection thresholds for the deaf and normal-hearing groups did not differ significantly at any frequency tested. Temporal onset-order discrimination thresholds varied widely, particularly among congenitally deaf individuals, but no statistically significant difference was found between group means. Both experimental groups exhibited a broad range of discrimination thresholds for temporal offset-order, and mean thresholds did not differ significantly. On the whole, tactual offset-order thresholds were substantially higher than onset-order thresholds. Differences in the relative levels of paired stimuli systematically affected sensitivity to both onset- and offset-orders in most subjects. Differences in the relative durations of paired stimuli had little effect on onset-order discrimination, but had a robust effect on offset-order discrimination thresholds, which was consistent across all subjects.
INTRODUCTION
Recent data indicate that a significant fraction of adult cochlear implant recipients can achieve relatively high levels of acoustic speech reception under favorable listening conditions (e.g., see Tyler et al., 1996; Bassim et al., 2005; Wilson and Dorman, 2008). Furthermore, studies indicate that the most successful child implantees may approach the performance levels of their normal-hearing peers in the areas of speech reception, speech production, and linguistic development (e.g., Ouellet and Cohen, 1999; Uchanski and Geers, 2003; Nicholas and Geers, 2007). However, not all deaf persons are able to be implanted or to achieve benefits from implantation. For example, although cochlear implants are highly beneficial for speech reception in adults with late-onset deafness, their usefulness in cases of pre-lingual deafness is generally limited to those individuals who are implanted in early childhood. In addition, cochlear implantation is precluded in individuals with certain physiological and medical conditions, and some individuals who do receive implants are unsuccessful in their use.
Tactile devices may serve as an alternative to auditory prostheses in individuals who are not good candidates for implantation or who are unsuccessful in the use of a cochlear implant. Additionally, the great majority of deaf persons worldwide live in low-income countries (World Health Organization, 2006) and for them, the cost of implantation is prohibitive. Tactile aids are non-invasive and can be made available at substantially lower cost than cochlear implants. In particular, relatively simple, low-cost tactile devices may provide access to acoustically derived cues that are not available through speechreading alone.
During speech, visual facial cues to the voicing distinction are extremely weak, a major disadvantage to a hearing-impaired individual when relying on lipreading to communicate (Heider and Heider, 1940; Erber, 1974; Walden et al., 1977). Confusions between voiced and unvoiced consonants are common in lipreading, particularly when voicing is the only cue for discriminating between the consonants. In the interest of improving speech reception by deaf individuals, one strategy has been to reintroduce cues to consonant voicing to the lipreader as a vibrotactile temporal cue (Yuan et al., 2004a, 2005b). Such a temporal cue has been demonstrated effective in enabling normal-hearing subjects to make consonant voicing discriminations when lipreading isolated syllables supplemented by tactual cues (Yuan et al., 2005b), but the utility of such tactual cues for deaf subjects has not yet been examined. Utilization of the vibrotactile voicing cue requires a user to discriminate the temporal onset- and offset-orderings of tactual stimuli with asynchronies in the range of 50–200 ms (Yuan et al., 2004a, 2004b). Of the few studies addressing tactual processing in deaf adults, the one most directly relevant to tactual temporal resolution (Heming and Brown, 2005) suggests that early deafness is associated with altered thresholds (or criteria) for reporting that two tactile stimuli have been presented simultaneously. The current study examines tactual temporal resolution in congenitally deaf (CD) and normal-hearing (NH) subjects using objective methods and stimuli that are chosen to approximate those used by Yuan et al. (2005b) to convey consonant voicing as a tactual cue.
Tactual discrimination of temporal order by normal-hearing individuals
Previous studies of temporal order discrimination in the tactual domain have commonly used transient, mechanical, or electrocutaneous stimuli, which do not overlap in time (e.g., Hirsh and Sherrick, 1961; Sherrick, 1970; Marks et al., 1982; Shore et al., 2002; Craig and Baihua, 1990; Craig and Busey, 2003). In normal-hearing adults, temporal order discrimination thresholds at the fingertips using brief vibromechanical stimuli are typically on the order of 20–40 ms. Eberhardt et al. (1994) used more sustained stimuli to examine temporal onset-order thresholds between vibratory and movement stimuli presented to the same finger, and found thresholds in the same range (25–45 ms).
When the paired stimuli delivered to the skin are brief and non-overlapping, judgments of temporal order may be based on several potential cues, including stimulus onsets and offsets, as well as total energy. By contrast, the sustained vibratory stimuli used in the present study allow us to examine onset- and offset-order discrimination capacities independently and to examine the contribution of total stimulus energy as a function of stimulus amplitude and duration.
Yuan and colleagues (Yuan, 2003; Yuan et al., 2004b, 2005a; Yuan and Reed, 2005) examined both temporal onset- and offset-orders using sustained sinusoidal tactile stimuli, varying durations between 50–800 ms and amplitudes between 25–45 dB Sensation Level (SL) relative to the average tactual detection threshold. Stimulus amplitudes and durations were chosen to span the ranges of these values previously observed among the amplitude envelopes of different frequency-filtered speech bands (Yuan et al., 2004a), which are used to modulate 50 and 250 Hz vibrotactile carrier signals in the tactile speech coding scheme implemented by Yuan et al. (2005b) and Yuan and Reed (2005). Stimuli were presented in pairs—one was presented to the left thumb pad at a frequency of 50 Hz, and the other was presented to the left index finger pad at a frequency of 250 Hz—through a multi-finger tactual display. Different pulse rates were used for the two fingers in the interest of encoding location and vibratory frequency redundantly, with the aim of facilitating temporal order judgments (Taylor, 1978). Tactual temporal onset-order thresholds were consistent with those of previous studies, with thresholds averaging 34 ms across four normal-hearing young adults. The relative amplitudes of paired stimuli affected these thresholds far more substantially than the relative durations.
In contrast, offset-order thresholds were roughly four times as large as onset-order thresholds, averaging 142 ms across four normal-hearing young adults (Yuan et al., 2004b; Yuan and Reed, 2005). However, further studies of temporal offset-order discrimination in the tactual domain are lacking, presumably because temporal order has so routinely been examined using brief, non-overlapping stimuli.
Tactual discrimination by deaf individuals
Previous studies of tactual perception in deaf children have suggested some degree of enhanced performance, relative to normal-hearing controls, on a variety of tasks (e.g., Chakravarty, 1968; Schiff and Dytell, 1972), including two-point discrimination and line-orientation discrimination. Cranney and Ashton (1982) reported enhanced tactile spatial discrimination in deaf subjects, relative to normal-hearing controls, in various age groups. Levänen and Hamdorf (2001) tested tactile sensitivity in congenitally deaf adults. They found that deaf subjects performed either comparably to or better than normal-hearing controls on two tasks involving tactual detection of changes in vibratory frequency in the range of 160–250 Hz.
Heming and Brown (2005) examined the perception of simultaneity of two tactile stimuli in subjects who had been profoundly deaf in both ears from before two years of age. Punctate mechanical stimuli were delivered, in pairs, to the pads of the index and middle fingers of either the left or right hand. Subjects were asked to indicate whether the two stimuli were “perceived simultaneously or non-simultaneously.” Thresholds for perceived simultaneity on this task were significantly higher for deaf subjects (84±25 ms) than for age-matched, normal-hearing controls (22±15 ms). These results reflect pooled data from the left and right hands, which were similar to one another in both groups. Importantly, all subjects in this study were between 18 and 32 years of age. By contrast, in a previous study utilizing the same experimental technique, normal-hearing adults over 60 years of age had a mean threshold of 61 ms (Brown and Sainsbury, 2000), suggesting that these simultaneity threshold values have strong dependencies that cannot be related simply to auditory experience. Furthermore, the perceptual judgment of “simultaneity” required of subjects in these studies is entirely subjective—the stimuli are always presented with some amount of asynchrony—and so differences in judgment criteria cannot be separated from the subjects’ actual sensitivity. It should also be noted that normal-hearing subjects in Heming and Brown’s study were instructed in English, while profoundly deaf subjects were instructed in American sign language (ASL). English and ASL are fundamentally different languages, and it is reasonable to consider that semantic factors might contribute to differences in decision criteria adopted by subjects in the two experimental groups. Given the subjective nature of Heming and Brown’s simultaneity protocol, it is conceivable that their ten pre-lingually deaf subjects exhibited response tendencies that reflect social, cultural, and linguistic experiences that differ fundamentally from those of their normal-hearing counterparts.
The present study differs from previous investigations of tactual temporal processing in adults with early-onset deafness in several important ways. First, objective experimental methods are used to assess temporal order resolution, requiring subjects to discriminate well-defined perceptual attributes, largely avoiding any semantic ambiguities and allowing separation of tactual sensitivity from response bias. Previous investigations of tactile temporal resolution have often used brief or punctate stimuli, with which onset and offset cues are not readily distinguishable. By contrast, the present study uses sinusoidal stimuli of various durations and amplitudes, which allows for separate examination of onset and offset cues, and the dependences of each on relative stimulus durations and sensation levels.
Based on the relevance of temporal order cues in tactual displays of speech as aids to lipreading, the current study was designed to examine the basic ability of deaf and normal-hearing listeners in psychophysical tasks concerned with the discrimination of temporal onset- and offset-orders. We report here the results of three experiments, in which congenitally deaf adults (aged 18–56) and normal-hearing adults (aged 23–58) were asked to make systematic judgments regarding sinusoidal vibrotactile stimuli delivered to the distal glabrous surfaces of the left thumb and index finger. The first experiment measured absolute detection thresholds for sinusoidal vibration at frequencies between 2 and 300 Hz. This frequency range includes stimuli in the kinesthetic, flutter, vibratory, and cutaneous perceptual ranges. In the second experiment, sinusoidal stimuli of 50 and 250 Hz were presented to the thumb and index finger, respectively, and subjects were asked to indicate which of the two stimuli had the earlier onset. The third experiment was similar to the second, except that subjects were asked to indicate which stimulus had the later offset. The sites and frequencies of stimulation in the second and third experiments were chosen in accordance with the tactile speech coding scheme implemented by Yuan et al. (2005b) and Yuan and Reed (2005).
METHODS
Subjects
Fourteen right-handed individuals, ranging in age from 18 to 58 years, served as subjects in this study. Nine of these (five females and four males, ages 18–56) had been profoundly deaf in both ears from birth (Table 1). Their primary native languages included ASL, signed exact English (SEE), Pidgin signed English (PSE), spoken English, cued English, and total communication; several subjects reported more than one. The other five subjects (one female and four males, ages 23–58) had normal hearing (defined as 30 dB HL or better in the frequency range of 250–4000 Hz), and all reported English as their primary native language. Audiometric testing was conducted on each subject prior to participation in this study, and audiometric thresholds are presented in Table 2. All subjects received compensation for their participation.
Table 1.
Information on subjects with congenital deafness. Communication methods are abbreviated as follows: PSE=Pidgin signed English; ASL=American Sign Language; SEE=Signed exact English.
| Subject | Age | Sex | Communication methods | Onset∕etiology | |
|---|---|---|---|---|---|
| Early | Current | ||||
| CD1 | 18 | F | Cued English (w∕some signing) | Cued speech | Congenital∕unknown |
| CD2 | 28 | F | PSE, then ASL | ASL and English | Congenital∕unknown (also has mild CP) |
| CD3 | 33 | M | SEE to pre-teen, then ASL | ASL | Congenital∕unknown |
| CD4 | 42 | F | Oral | ASL | Congenital∕unknown |
| CD5 | 42 | F | Oral to age 19, then ASL | ASL | Congenital∕rubella |
| CD6 | 45 | M | Total communication, ASL | ASL | Congenital∕unknown |
| CD7 | 47 | M | Oral | ASL | Congenital (premature birth) |
| CD8 | 51 | F | Oral to age 18, then total communication | ASL and English | Congenital∕unknown |
| CD9 | 56 | M | ASL | ASL and English | Congenital∕hereditary |
Table 2.
Audiometric thresholds in dB HL.
| Thresholds (dB HL) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subject | Age | 250 Hz | 500 Hz | 1000 Hz | 2000 Hz | 4000 Hz | 8000 Hz | ||||||
| Left | Right | Left | Right | Left | Right | Left | Right | Left | Right | Left | Right | ||
| CD1 | 18 | 90 | 85 | 85 | 80 | 100 | 100 | >120 | 115 | 120 | 120 | >110 | >110 |
| CD2 | 28 | 80 | >90 | 95 | 100 | 100 | 105 | 95 | 100 | 75 | 85 | 90 | 90 |
| CD3 | 33 | >85 | 85 | >90 | >90 | >100 | >100 | >100 | >100 | >95 | >95 | - | - |
| CD4 | 42 | >105 | 100 | 110 | 105 | 110 | 115 | 120 | 115 | >120 | >120 | >110 | >110 |
| CD5 | 42 | 85 | 100 | 105 | 105 | 105 | 110 | 95 | 115 | 95 | >115 | >100 | >100 |
| CD6 | 45 | 75 | 85 | 75 | 80 | 85 | 90 | 85 | 85 | 65 | 70 | 105 | 110 |
| CD7 | 47 | 55 | 50 | 100 | 75 | 95 | 90 | 90 | 85 | 85 | 90 | 80 | 85 |
| CD8 | 51 | 85 | >85 | 90 | >85 | >100 | 90 | >100 | >100 | >95 | >95 | - | - |
| CD9 | 56 | 80 | 85 | 85 | >90 | >100 | >100 | >100 | >100 | >95 | >95 | - | - |
| NH1 | 23 | 0 | 0 | 5 | 5 | 0 | 0 | 10 | 5 | 10 | 5 | 0 | 5 |
| NH2 | 30 | 15 | 10 | 5 | 5 | 0 | 0 | 5 | 10 | 5 | 15 | 15 | 15 |
| NH3 | 35 | 5 | 10 | 0 | 0 | 0 | 10 | 5 | 10 | 15 | 30 | 20 | 20 |
| NH4 | 45 | 5 | 5 | 15 | 5 | 5 | 5 | 15 | 0 | 15 | 10 | 40 | 30 |
| NH5 | 58 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 20 | 10 | 45 | 30 |
Normal-hearing subjects were instructed in the requirements of experimental tasks by means of verbal communication and demonstration. Deaf subjects were instructed through a combination of verbal (lipreading) and written communication, demonstration, and in one case, through an ASL interpreter. Effective communication was easily established with all subjects. At the start of each temporal discrimination experiment, prior to the collection of any data, all subjects demonstrated near-perfect performance in practice trials with the experimental variable set well above threshold, indicating that they understood the requirements of each task.
Apparatus
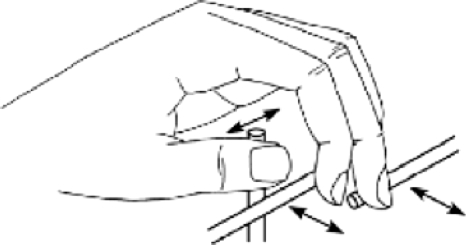
The tactual stimulating device, called the “Tactuator,” is illustrated in Fig. 1. It consists of three rods that lightly contact the thumb, index finger, and middle finger of the left hand, in an arrangement that allows for a natural hand configuration. Each rod is moved independently by a head-positioning motor, which is controlled by a digital signal-processing board through a servomechanism. The apparatus is described in detail by Tan and Rabinowitz (1996) and Yuan (2003). Briefly, it is designed to deliver stimuli with frequencies from dc to around 400 Hz, spanning most of the cumulative receptive range of the four major types of somatosensory afferent receptors, which together mediate kinesthetic, flutter, vibratory, and cutaneous tactual percepts (Bolanowski et al., 1988). Each rod has roughly a 26 mm range of motion, meaning that it can reliably deliver stimuli with amplitudes ranging from absolute threshold (the smallest displacement that can be detected) to about 50 dB above threshold, throughout its useful frequency range. The effect of loading due to light finger contact is minimal (Tan and Rabinowitz, 1996).
Figure 1.
Illustration of Tactuator finger-interface configuration.
Stimuli and Procedures
In all experiments, subjects sat facing a computer screen, which provided visual cues and feedback as appropriate. The subject’s left hand rested on the tactual stimulating device, with the thumb and index finger gently touching the stimulators. The subject’s right hand was situated by a computer keyboard, whereby responses were entered. During testing, both profoundly deaf and normal-hearing subjects wore foam earplugs (approximately 30 dB attenuating) and headphones delivering pink masking noise [roughly 80 dB sound pressure level (SPL)] in order to minimize the possibility of either air- or bone-conducted sounds from the device influencing performance at the highest stimulating levels.
Tactual detection threshold measurements
Sinusoidal vibratory stimuli were delivered to the distal glabrous surface of either the left thumb or the left index finger. Stimuli had 5-ms rise∕fall times and were 500 ms in duration, sufficiently long such that detection threshold level should be independent of duration (Verrillo, 1965). The frequencies examined were 2, 5, 10, 25, 50, 100, 200, 250, and 300 Hz, which span most of the useful perceptual range of human tactual system for sinusoidal vibratory stimuli (Goff, 1967).
Detection thresholds were measured using a two-interval, two-alternative forced choice adaptive procedure. At each finger, the order in which frequencies were tested was randomized. Subjects were asked to indicate in which of two visually cued intervals a tactual stimulus was presented. Trial-by-trial correct-answer feedback was presented visually. Following an initial supra-threshold stimulus, subsequent stimulus presentations were governed by a “two-down, one-up” paradigm, which converges on the stimulus level at which a subject would respond correctly 70.7% of the time (Levitt, 1971). An initial amplitude step-size of 4 dB was decreased to 1 dB following the second reversal in the direction of stimulus amplitude adjustment. Each run was terminated following the tenth reversal of direction, and the detection threshold was defined as the average stimulus level over the final six reversals. At each finger, and for each vibratory frequency, this protocol was repeated twice, and the results of the two runs were averaged.
Tactual temporal onset-order discrimination
To examine temporal onset-order discrimination, sinusoidal stimuli were delivered to the left thumb and forefinger during each trial of a one-interval, two-alternative forced choice task, and the subject was asked to indicate which stimulus had the earlier onset. Subjects responded on a computer keyboard and received visual trial-by-trial correct-answer feedback. Subjects were instructed to press “T” if the onset of the stimulus delivered to the thumb preceded that of the stimulus delivered to the index finger, and to press “I” if the index finger stimulus onset arrived earlier. The thumb was stimulated exclusively at 50 Hz and the index finger at 250 Hz. Stimulus durations and amplitudes were roved in a manner that reflects duration and level variations across low- and high-frequency bands of speech; this paradigm also ensured that neither offset asynchrony nor total stimulus energy could provide a reliable cue for onset-order. The duration of each stimulus in a trial pair was varied randomly among a fixed set of seven possible values: 50, 100, 200, 400, 500, 600 and 800 ms. Thus, there were 49 equally likely duration combinations for each stimulus pair. Signal levels at each site varied randomly among a set of five equally likely values. The 50 Hz stimuli to the thumb varied randomly among amplitudes of 26, 31, 36, 41, and 46 dB re 1 μm peak displacement. The 250 Hz stimuli to the index finger varied randomly among amplitudes of 6, 11, 16, 21, and 26 dB re 1 μm peak displacement. These amplitude values were previously selected by Yuan et al. (2005a) to span the range of 25–45 dB above the average detection thresholds of three normal-hearing adults (those thresholds being 1 dB re 1 μm peak for 50 Hz at the left thumb and −19 dB re 1 μm peak for 250 Hz at the left index finger). Beyond about 55 dB SL, vibratory stimuli become unpleasant or painful (Verrillo and Gescheider, 1992).
In the present study, average thresholds across all subjects are approximately 5 dB re 1 μm peak for 50 Hz at the left thumb and −22 dB re 1 μm peak for 250 Hz at the left index finger, and relative to these values, sensation levels ranged from about 21–41 dB for 50 Hz stimuli to the thumb and 28–48 dB for 250 Hz stimuli to the index finger. The stimulus amplitudes chosen by Yuan et al. (2005a) were retained for the sake of consistency, and their reference amplitudes for “sensation level” (dB SL) are retained throughout this text for purposes of clarity. Thus, the five stimulus amplitudes at both the left thumb and index finger are defined as having sensation levels of 25, 30, 35, 40, and 45 dB SL. Since both stimuli in a given trial were varied randomly among five possible amplitudes, there were a total of 25 possible amplitude combinations.
The amplitudes of the 50- and 100-ms duration stimuli were increased by 6 dB and 3 dB, respectively, relative to the other, longer stimuli. This adjustment is intended to compensate for the fact that temporal integration is observed for tactual stimuli of durations up to roughly 200 ms (Verrillo, 1965).
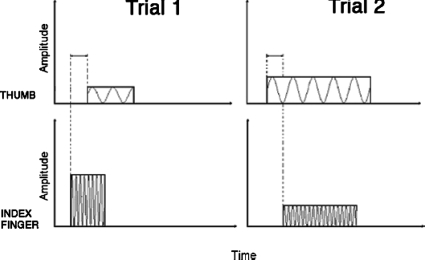
Two trials of the onset-order discrimination experimental paradigm are illustrated in Fig. 2. We define stimulus onset asynchrony (SOA) as the difference in stimulus onset timings (Onsetthumb−Onsetindex). Thus, a negative SOA value (SOA<0) in a given trial indicates that the thumb stimulus onset preceded that of the index finger, whereas a positive SOA value (SOA>0) indicates that the index finger onset preceded that of the thumb. In Fig. 2, “trial 1” has a positive-valued SOA, since the thumb onset time is later than that of the index finger, whereas “trial 2” has a negative-valued SOA—the correct responses would be “I” in the first trial and “T” in the second trial. During any given run, the absolute value of the SOA was kept constant in each trial, and only the order of stimulus onsets (the sign of the SOA) was varied. Three SOA values were chosen for each subject during a pre-testing phase, so as to span the range for which the subject responded below 100% correct but above chance. This protocol reveals the pattern of temporal onset-order discrimination falloff with decreasing |SOA|, and allows us to interpolate performance levels for intervening |SOA| values. All subjects completed eight to ten 50-trial runs at each of the three selected values of |SOA|, which were interleaved randomly.
Figure 2.
Two trials of the temporal onset-order experimental paradigm. Trial 1 has a positive-valued SOA (index onset precedes thumb) and trial 2 has a negative-valued SOA (thumb onset precedes index). The upper traces represent 50-Hz vibration to the thumb, and the lower traces represent 250-Hz vibration to the index finger.
Tactual temporal offset-order discrimination
The temporal offset-order discrimination experiment was nearly identical to the onset-order discrimination experiment, with the exception that the subject was now instructed to indicate which stimulus had a later offset (rather than an earlier onset). Subjects were instructed to press “T” if the offset of the stimulus delivered to the thumb was later than that of the stimulus delivered to the index finger, and to press “I” if the index finger stimulus offset was later. All other aspects of the stimuli and the experimental paradigm were the same as those described above for the onset-order experiment.
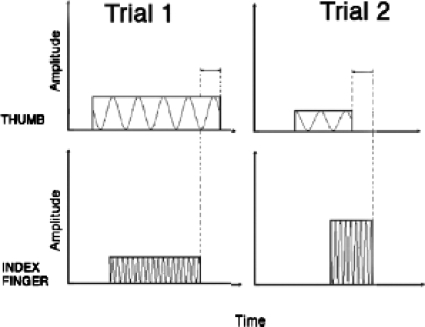
Two trials of the offset-order discrimination experimental paradigm are illustrated in Fig. 3. We define stimulus offset asynchrony (SOFA) as the difference in stimulus offset timings (Offsetthumb−Offsetindex). Thus, a negative-SOFA value (SOFA<0) for a given trial indicates that the index finger stimulus offset follows that of the thumb, whereas a positive-SOFA value (SOFA>0) indicates that the thumb offset follows that of the index finger. In Fig. 3, “trial 1” has a positive-valued SOFA, since the thumb offset time is later than that of the index finger, whereas “trial 2” has a negative-valued SOFA—the correct responses would be “T” in the first trial and “I” in the second trial. As in the onset-order experiment, the absolute value of the SOFA in the offset-order experiment was held constant for each trial of a given run, and only the sign of the SOFA was varied. Three SOFA values were chosen for each subject during a pre-testing phase, so as to span the range for which the subject responded below 100% correct but above chance. All subjects completed eight to ten 50-trial runs at each |SOFA|, which were interleaved randomly.
Figure 3.
Two trials of the temporal offset-order experimental paradigm. Trial 1 has a positive-valued SOFA (thumb offset follows index) and trial 2 has a negative-valued SOFA (index offset follows thumb). The upper traces represent 50-Hz vibration to the thumb, and the lower traces represent 250-Hz vibration to the index finger.
Data analysis
Tactual detection thresholds
The detection threshold in each experimental run was defined as the average stimulus level over the final six reversals of a two-down, one-up adaptive procedure. This protocol was repeated twice for each subject and averaged for each different combination of finger and vibratory frequency. Results were also averaged across subjects within each of the two groups (deaf and normal-hearing).
Tactual temporal onset- and offset-order discriminations
Results from each experimental run of the temporal onset- and offset-order discrimination experiments for each subject were summarized in a 2×2 stimulus-response confusion matrix. The signal detection measures of sensitivity (d′) and bias (β) were calculated (Green and Swets, 1966; Durlach, 1968) and averaged over all runs, sharing a common |SOA| or |SOFA|. Data from the onset- and offset-order experiments were summarized in plots of d′ vs. |SOA| and d′ vs. |SOFA|, respectively, for each subject separately. The discrimination threshold was then estimated as the interpolated value of |SOA| or |SOFA| corresponding to d′=1.
The effects of roving stimulus amplitudes and durations on temporal onset- and offset-order discriminations were also examined. For the analysis of amplitude effects on temporal onset-order discrimination, data are grouped into five categories along the abscissa, according to the difference in sensation level between the earlier- and later-onset stimuli, as described in Table 3. The sensation level of the earlier-onset stimulus is lower than that of the later-onset stimulus by 15–20 dB SL in trials of category 1, and by 5–10 dB SL in those of category 2. In trials of category 3, the two stimuli have approximately equal sensation levels. In trials of categories 4 and 5, the sensation level of the earlier-onset stimulus is larger than that of the later-onset stimulus by 5–10 and 15–20 dB SL, respectively. (Note that this system of categorization does not take into account the identities of the stimulated digits, but only the order of presentation.) For each subject, trials were sorted by amplitude category and |SOA| value, and sensitivity (d′) was calculated from 2×2 confusion matrices for each set of conditions separately.
Table 3.
Descriptions of categories used in analysis of stimulus amplitude effects on temporal onset- and offset-order discriminations (Fig. 7). The relationships indicated for paired stimuli in each amplitude category reflect frequency-specific sensation levels, rather than absolute amplitude differences.
| Amplitude category | Stimuli differ by(dB SL) | Onset-order experiment | Offset-order experiment | ||
|---|---|---|---|---|---|
| 1 | 15–20 | Earlier-onset stimulus has lower sensation level | Later-offset stimulus has lower sensation level | ||
| 2 | 5–10 | ||||
| 3 | 0 | Stimuli have equal sensation levels | Stimuli have equal sensation levels | ||
| 4 | 5–10 | Earlier-onset stimulus has higher sensation level | Later-offset stimulus has higher sensation level | ||
| 5 | 15–20 | ||||
For the analysis of stimulus duration effects on temporal onset-order discrimination, data are grouped into seven categories according to the difference between the durations of the earlier- and later-onset stimuli, as described in Table 4. In trials of categories 1–3, the earlier-onset stimulus is shorter in duration than the later-onset stimulus. In trials of category 4, the two stimuli have equal durations. In trials of categories 5–7, the earlier-onset stimulus is longer in duration than the later-onset stimulus. (See Table 4 for specific duration difference criteria for each category.)
Table 4.
Descriptions of categories used in analysis of stimulus duration effects on temporal onset- and offset-order discriminations (Fig. 7).
| Duration category | Stimuli differ by(ms) | Onset-order experiment | Offset-order experiment | ||
|---|---|---|---|---|---|
| 1 | 450–750 | Earlier-onset stimulus has shorter duration | Later-offset stimulus has shorter duration | ||
| 2 | 300–400 | ||||
| 3 | 50–200 | ||||
| 4 | 0 | Stimuli have equal durations | Stimuli have equal durations | ||
| 5 | 50–200 | Earlier-onset stimulus has longer duration | Later-offset stimulus has longer duration | ||
| 6 | 300–400 | ||||
| 7 | 450–750 | ||||
For the analysis of stimulus amplitude effects on offset-order discrimination, data are grouped into five categories along the abscissa of each graph, according to the difference in sensation level between the later- and earlier-offset stimuli, as described in Table 3. The sensation level of the later-offset stimulus is lower than that of the earlier-offset stimulus by 15–20 dB SL in trials of category 1, and by 5–10 dB SL in those of category 2. In trials of category 3, the two stimuli have approximately equal sensation levels. In trials of categories 4 and 5, the sensation level of the later-offset stimulus is larger than that of the earlier-offset stimulus by 5–10 and 15–20 dB SL, respectively. For each subject, trials were sorted by amplitude category and |SOFA| value, and sensitivity (d′) was calculated from 2×2 confusion matrices for each set of conditions separately.
For the analysis of stimulus duration effects on offset-order discrimination, data are grouped into seven categories according to the difference between the durations of the later- and earlier-offset stimuli, as described in Table 4. In trials of categories 1–3, the later-offset stimulus is shorter in duration than the earlier-offset stimulus. In trials of category 4, the two stimuli have equal durations. In trials of categories 5–7, the later-offset stimulus is longer in duration than the earlier-offset stimulus. (See Table 4 for specific duration-difference criteria for each category.)
RESULTS
Detection thresholds
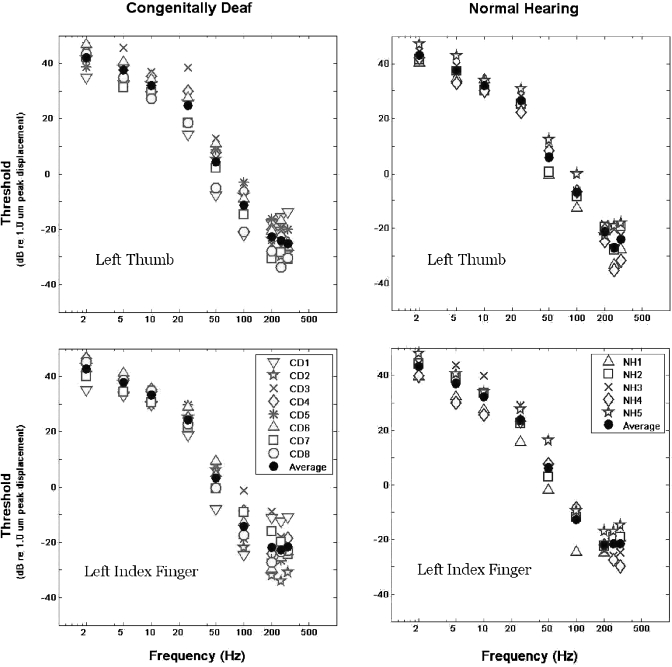
Tactual detection threshold measurements for CD and NH subjects are plotted as a function of stimulation frequency in the left- and right-hand panels in Fig. 4, respectively. The upper panels in Fig. 4 present data for each subject’s left thumb, whereas the lower panels present data for the left index finger. Table 5 provides the mean thresholds and standard deviations of the two subject groups, calculated for each of the vibratory frequencies examined. Stimulus amplitude is reported in dB with respect to a 1 μm peak displacement.
Figure 4.
Tactual detection thresholds (in dB re 1.0 μm peak displacement) are plotted as a function of stimulation frequency. Measurements for congenitally deaf subjects are presented in the two left-hand panels, and those for normal-hearing subjects are presented in the two right-hand panels. Data in the upper panels correspond to the left thumb of each subject, while those in the lower panels correspond to the left index finger.
Table 5.
Mean tactual detection thresholds and standard deviations (in dB re 1 μm peak displacement) for congenitally deaf and normal-hearing subjects across all vibratory frequencies examined, for left thumb and left index finger.
| Frequency | Left thumb | Left index finger | ||||||
|---|---|---|---|---|---|---|---|---|
| Congenitally | Normal | Congenitally | Normal | |||||
| Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | |
| 2 | 42.0 | 3.9 | 43.2 | 2.6 | 42.7 | 3.6 | 43.3 | 3.6 |
| 5 | 37.5 | 4.6 | 37.4 | 4.0 | 37.9 | 2.8 | 37.1 | 5.8 |
| 10 | 32.0 | 3.4 | 31.8 | 1.9 | 33.2 | 2.6 | 32.1 | 5.6 |
| 25 | 24.9 | 7.7 | 26.5 | 3.2 | 24.3 | 3.7 | 23.6 | 5.4 |
| 50 | 4.4 | 7.5 | 5.9 | 5.6 | 3.2 | 5.8 | 6.3 | 6.8 |
| 100 | −11.4 | 7.0 | −6.9 | 4.6 | −14.3 | 7.8 | −12.7 | 6.9 |
| 200 | −22.8 | 5.0 | −21.2 | 2.5 | −21.9 | 8.8 | −22.0 | 3.1 |
| 250 | −24.1 | 6.5 | −27.1 | 7.3 | −22.9 | 6.4 | −21.6 | 4.8 |
| 300 | −25.2 | 5.7 | −23.9 | 5.7 | −21.8 | 5.7 | −21.5 | 5.8 |
Threshold measurements for all subjects exhibit the expected dependence on stimulation frequency. Thresholds at both fingers are lowest in the range of 200–300 Hz, increasing rapidly as frequency decreases below 200 Hz. A two-way analysis of variance (ANOVA) was performed within each frequency, testing for effects of group (deaf, hearing) and site of stimulation (thumb, index finger). No significant effects of either group or stimulation site were observed at any of the nine frequencies examined. The ANOVA for detection threshold measurements at 100 Hz gave the lowest p-values for the null hypotheses on the main effects for group [F(1,24)=1.65, p=0.21], as well as for stimulation site [F(1,24)=2.4, p=0.13].
Stimuli in the temporal order discrimination experiments were restricted to 50 Hz at the left thumb and 250 Hz at the left index finger. Mean (±1 s.d.) detection thresholds for 50 Hz stimuli at the left thumb are 4.4(±7.5) dB re 1 μm for CD subjects and 5.9(±5.6) dB re 1 μm for NH subjects. Mean (±1 s.d.) detection thresholds for 250 Hz stimuli at the left index finger are −22.9 (±6.4) dB re 1 μm for CD subjects and −21.6 (±4.8) dB re 1 μm for NH subjects.
Temporal onset-order discrimination
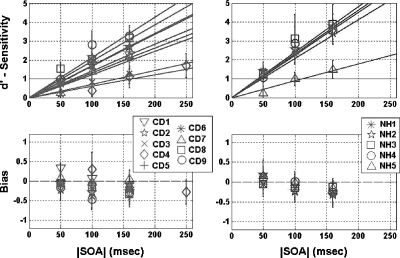
The results of the temporal onset-order discrimination experiment are presented in Fig. 5, and onset-order thresholds (defined as the interpolated value of |SOA| for which d′=1) are summarized in Table 6.
Figure 5.
Mean values of d′ (top) and bias (bottom) are plotted as a function of |SOA| for congenitally deaf (left) and normal-hearing (right) subjects in the temporal onset-order discrimination experiment. Each subject was tested at three |SOA| values. In the top panels, a straight line that crosses the origin has been fit to the data of each subject by linear regression; onset-order discrimination threshold is defined as the value of |SOA| at which this line crosses d′=1. Error bars indicate standard deviation.
Table 6.
Stimulus onset-order (|SOA|) and offset-order (|SOFA|) sensitivity thresholds (interpolated at d′=1) for congenitally deaf and normal-hearing subjects.
| Group | Subject | Age | Onset-order threshold (ms) | Offset-order threshold (ms) |
|---|---|---|---|---|
| Congenitally deaf | CD1 | 18 | 70 | 132 |
| CD2 | 28 | 59 | 92 | |
| CD3 | 33 | 138 | 183 | |
| CD4 | 42 | 165 | 276 | |
| CD5 | 42 | 59 | - | |
| CD6 | 45 | 76 | 129 | |
| CD7 | 47 | 82 | 83 | |
| CD8 | 51 | 51 | 75 | |
| CD9 | 56 | 47 | 142 | |
| Mean±s.d. | 83±41 | 139±66 | ||
| Normal-hearing | NH1 | 23 | 48 | 145 |
| NH2 | 30 | 41 | 90 | |
| NH3 | 35 | 44 | 83 | |
| NH4 | 45 | 43 | 65 | |
| NH5 | 58 | 112 | 221 | |
| Mean±s.d. | 58±31 | 121±63 | ||
In the top panels in Fig. 5, each data point indicates the mean and standard deviation of d′ in runs with a given |SOA|, plotted for each subject individually. All subjects were tested at |SOA| values of 50, 100, and 160 ms, with the exception of CD4, who was tested at |SOA| values of 100, 160, and 250 ms. A straight line, crossing the origin, was fit to the data of each subject by linear regression. We define the discrimination threshold as the value of |SOA| at which this line crosses d′=1.
As indicated in Table 6, onset-order thresholds among CD subjects range from 47 to 165 ms, and the mean threshold is 83 ms (± 41 ms s.d.). Among NH subjects, onset-order thresholds range from 41 to 112 ms, with a mean of 58 ms (± 31 ms s.d.). A two-way ANOVA was performed to compare threshold means with respect to hearing status and age (subjects were categorized as either over or under age 40). No significant effects were demonstrated for either group [F(1,10)=1.06, p=0.33] or age [F(1,10)=0.09, p=0.76].
The corresponding bias (β) values are plotted in the bottom panels in Fig. 5; each data point represents the mean bias over all runs of the same |SOA| for a given subject. Positive bias indicates a tendency to respond that the stimulus at the thumb had an earlier onset. For both CD and NH subjects, bias is generally negligible. In no case did |β| exceed 0.5.
Temporal offset-order discrimination
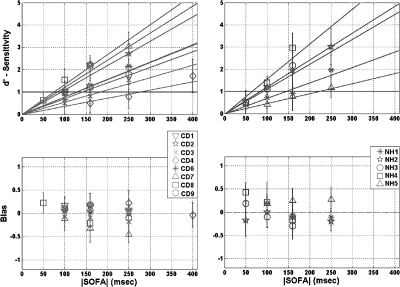
The results of the temporal offset-order discrimination experiment are presented in Fig. 6, and offset-order thresholds (defined as the interpolated |SOFA| at which d′=1) are recorded in the rightmost column of Table 6.
Figure 6.
Mean values of d′ (top) and bias (bottom) are plotted as a function of |SOFA| for congenitally deaf (left) and normal-hearing (right) subjects in the temporal offset-order discrimination experiment. Each subject was tested at three |SOFA| values, except NH2, who was tested at four. In the top panels, a straight line that crosses the origin has been fit to the data of each subject by linear regression; offset-order discrimination threshold is defined as the value of |SOFA| at which this line crosses d′=1. Error bars indicate standard deviation.
In the top panels in Fig. 6, each data point indicates the mean and standard deviation of d′ in runs with the corresponding |SOFA|, for a given subject. Most subjects were tested with |SOFA| values of either 50, 100, and 160 ms, or 100, 160, and 250 ms; the only exception was subject CD4, who was tested with 160, 250, and 400 ms |SOFA| values. A straight line, crossing the origin, was fit to the data of each subject by linear regression, and the discrimination threshold is defined as the value of |SOFA| at which this line crosses d′=1.
Thresholds range from 75 to 276 ms among CD subjects, and the mean threshold is 139 ms (± 66 ms s.d.). Among NH subjects, thresholds range from 65 to 221 ms, with a mean of 121 ms (± 63 ms s.d.). A two-way ANOVA was performed to compare threshold means with respect to hearing status and age (subjects were categorized as either over or under age 40). No significant effects were demonstrated for either group [F(1,9)=0.12, p=0.74] or age [F(1,9)=0.19, p=0.67].
The corresponding β values are shown in the bottom panels in Fig. 6; each data point indicates the mean bias over all runs of the same |SOFA| for a given subject. Positive bias indicates a tendency to respond that the stimulus at the thumb had a later offset. For CD and NH subjects, bias is minimal overall. In no case did |β| exceed 0.5.
Effects of relative level and duration on temporal resolution
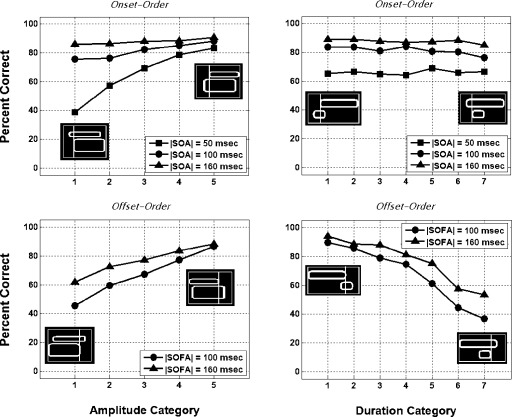
Figure 7 provides an overall impression of the impact of level and duration differences among paired stimuli on temporal order discrimination performance. Data are averaged across the 12 subjects (both congenitally deaf and normal-hearing) who were tested at the three |SOA| values of 50, 100, and 160 ms, and the two |SOFA| values of 100 and 160 ms. The top left panel in Fig. 7 presents average onset-order thresholds plotted as a function of “amplitude category.” As described in Table 3, the sensation level of the earlier-onset stimulus relative to the later-onset stimulus is lowest in trials of amplitude category 1 and highest in trials of amplitude category 5 (whereas the paired stimuli have comparable sensation levels in amplitude category 3 trials). The largest difference between amplitude categories is observed for the 50-ms |SOA|, the smallest onset-asynchrony tested. As a general trend among both deaf and normal-hearing subjects, performance tends to improve as the relative level of the earlier-onset stimulus increases with respect to that of the later-onset stimulus (from left to right along the abscissa).
Figure 7.
Percent correct performance plotted as a function of amplitude category (left panels) and duration category (right panels) in the tactual temporal onset-order and offset-order experiments (top and bottom panels, respectively). Data are averaged across the 12 deaf and normal-hearing subjects who were tested with SOA values of 50, 100, and 160 ms, and SOFA values of 100 and 160 ms. In each panel, illustrative icons depict either the relative level or duration relationships characteristic of paired stimuli in the lowest and highest numbered categories, in conjunction with indicators of their relative temporal onset or offset ordering. The precise relationships characteristic of each amplitude and duration category are described in Tables 3, 4, respectively.
Average offset-order thresholds for stimulus offset-asynchronies of 50 and 100 ms are plotted as a function of amplitude category in the bottom left panel in Fig. 7. The sensation level of the later-offset stimulus relative to the earlier-offset stimulus is lowest in trials of amplitude category 1 and highest in trials of amplitude category 5 (as described in Table 3). Stimulus offset-order discrimination performance tended to improve as the relative level of the later-offset stimulus increased with respect to that of the earlier-offset stimulus (from left to right along the abscissa). This trend was observed consistently across all subjects, at all offset-order asynchrony values tested, although performance varied most substantially across amplitude categories in trials where the |SOFA| value was lowest, and performance was thus poorest overall.
In the top right panel in Fig. 7, average onset-order thresholds are plotted as a function of “duration category.” As described in Table 4, the duration of the earlier-onset stimulus relative to the later-onset stimulus is smallest in trials of duration category 1 and largest in trials of duration category 7 (whereas the paired stimuli have comparable durations in category 4 trials). Duration differences among paired stimuli are seen to have little, if any, effect on performance at any of the three |SOA| values.
The bottom right panel of Fig. 7 presents average offset-order thresholds plotted as a function of duration category. For the offset-order discrimination experiment, trials of duration category 1 are those in which the later-offset stimulus is shortest in duration relative to the earlier-offset stimulus—with increasing duration category number, the duration of the later-offset stimulus increases relative to that of the earlier-offset stimulus (as described in Table 4). In contrast to the findings of the onset-order discrimination experiment, relative stimulus duration has a very consistent and substantial effect on the discrimination of offset-order in normal-hearing and congenitally deaf subjects alike. As observed in the figure, subjects generally perform best in trials of low-number duration categories, for which the duration of the later-offset stimulus is substantially less than that of the earlier-offset stimulus. Moving right along the abscissa, performance progressively worsens as the duration of the later-offset stimulus becomes increasingly long relative to that of the earlier-offset stimulus. This performance pattern was consistent across all subjects and |SOFA| values examined.
DISCUSSION
Tactual detection
We examined tactual detection thresholds at the left thumb and index finger using sinusoidal stimuli at nine frequencies between 2 and 300 Hz. We found no significant differences, at any frequency, between the mean detection threshold of CD and NH subjects. Overall, our measurements are consistent with those reported in previous tactual sensitivity studies that used comparable experimental conditions (e.g., Yuan, 2003; Tan, 1996; Rabinowitz et al., 1987; Lamoré et al., 1986).
Subjects in this study were free at all times to adjust the amount of pressure applied to the tactual stimulators by each finger. The contact area between each finger and the corresponding stimulator bar is approximately 0.5–1 cm2, depending on finger size and positioning. Subjects were instructed regarding hand placement and told to touch the bars lightly, but any effects on threshold measurements resulting from the amount of skin indentation may have varied among subjects and between experimental sessions. Researchers who have examined tactile detection thresholds on the hand using a static indentation at the contact surface (e.g., Bolanowski et al., 1988; Gescheider et al., 2002) have observed a 10–20 dB reduction in thresholds at low frequencies (in the range of 1–25 Hz), whereas thresholds in the range of 50–300 Hz were elevated by about 10 dB, relative to studies that did not impose a static indentation. Our detection threshold measurements at both low and high frequencies, however, are more consistent with previous studies in which static indentation was not employed (e.g., Lamoré et al., 1986; Rabinowitz et al., 1987).
Tactual temporal order discrimination
Tactual onset-order discrimination thresholds, as listed in Table 6, varied widely among both CD and NH adults in this study. Onset-order discrimination thresholds in the CD group ranged from 47 to 165 ms (a factor of 3.5), and those in the NH group ranged from 41 to 112 ms (a factor of 2.7). The highest tactual temporal onset-order discrimination thresholds reported here exceed those typically observed among normal-hearing subjects (Sherrick, 1970; Eberhardt et al., 1994; Yuan et al., 2005a). In particular, Yuan et al. (2005a), who measured onset-order thresholds in four normal-hearing adults (aged 21–32), used the same experimental paradigm as was used in the present study, and reported |SOA| thresholds ranging from 18 to 42 ms. Analysis of variance revealed no statistically significant difference between mean tactual onset-order thresholds of subjects grouped either by age or hearing status.
Several previous studies have demonstrated altered tactual temporal processing ability with aging (e.g., Van Doren et al., 1990; Gescheider et al., 1992). Elevated thresholds for judgments of simultaneity were previously observed in adults over 60 years of age (Brown and Sainsbury, 2000), suggesting a correlation between age and temporal resolution. The current results, however, do not support a hypothesis of age-related elevations in onset- or offset-order thresholds, at least over the age range of 18–58 years examined here. Correlation analysis gives coefficients r=0.133 (p=0.65) for onset-order threshold vs. age and r=0.137 (p=0.66) for offset-order threshold vs. age.
Tactual offset-order discrimination thresholds for both CD and NH adults, as listed in Table 6, span an even wider range of values than onset-order thresholds. Offset-order discrimination thresholds in the CD group ranged from 75 to 276 ms (a factor of 3.7), and those in the NH group ranged from 65 to 221 ms (a factor of 3.4). These threshold ranges are consistent with previously observed offset-order thresholds in normal-hearing subjects (Yuan et al., 2004b; Yuan and Reed, 2005).
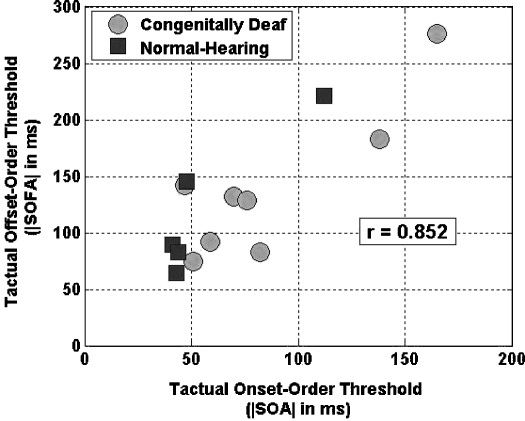
Figure 8 shows tactual offset-order thresholds plotted as a function of corresponding tactual onset-order thresholds for each subject who participated in both temporal order discrimination experiments. For a given subject, offset-order threshold is generally substantially larger than onset-order threshold (although note that the two thresholds are about equal for subject CD7). Overall, subjects’ offset-order discrimination thresholds are highly correlated with their respective onset-order thresholds (correlation coefficient r=0.852, p<0.0005).
Figure 8.
For each subject who participated in both temporal order discrimination experiments, tactual offset-order thresholds are plotted as a function of corresponding tactual onset-order thresholds (Correlation coefficient r=0.852, p<0.0005).
In the auditory system, temporal offset-order discrimination thresholds for pairs of tonal stimuli are typically as low as (or lower than) onset-order thresholds (Pastore, 1983). Substantially higher offset-order thresholds observed for paired vibrotactile stimuli in this study may reflect, at least in part, the characteristically broad spatiotemporal integration patterns of the Pacinian system (which is engaged by moderate-level 50-Hz as well as 250-Hz sinusoidal vibrations). Interactions among vibrotactile stimuli that activate Pacinian receptors at two distinct skin locations have most commonly been investigated as masking phenomena. For example, Sherrick (1964) reported an increase of almost 30 dB in the detection threshold for a 350-ms pulsed 150-Hz vibrotactile signal at the right index finger when presented simultaneously with a 350-ms pulsed 150-Hz vibrotactile masker of 30 dB SL at the right little finger. Verrillo et al. (1983) observed substantial masking of a 300-ms, 300-Hz test stimulus at the index fingertip when it was temporally centered within a 730-ms, 300-Hz masking stimulus at the ipsilateral thenar eminence. They further measured vibration at the index fingertip due to physical transmission of masking vibration from the thenar site, and thereby established that even their maximum intensity masker produced only subthreshold vibration at the fingertip surface (suggesting that the underlying process is likely more central). Given the marked ability of such stimuli to interfere with one another’s detection, it seems reasonable that two partially overlapping, Pacinian-range, vibrotactile stimuli delivered to adjacent digits might interact in such a manner as to obscure their relative temporal order. The fact that discrimination thresholds for offset-order tend to exceed those for onset-order suggests that the effects of vibrotactile forward masking extend over a longer time period than those of backward masking. Such a temporal asymmetry of vibrotactile forward and backward masking has previously been characterized as a “persistence” of tactual stimuli following their offset (Craig and Evans, 1987).
Heming and Brown (2005) previously examined thresholds for perceived simultaneity of two punctate stimuli delivered to separate fingers, among adults with early hearing loss as well as normal-hearing adults. The present study differs from theirs in several key respects. First, we used an objective experimental protocol in which subjects indicate the perceived ordering of stimuli, which enables us to apply signal detection theory to distinguish sensitivity from response bias. Second, we used pairs of sinusoidal stimuli, which were roved independently in amplitude and duration, thereby allowing us to assess the impact of these stimulus parameters on temporal resolution. Third, our use of sinusoidal (rather than punctate) stimuli has enabled us to examine separately the discrimination of relative onset and offset times.
Heming and Brown reported mean simultaneity thresholds of 84 ms (s.d.=25) and 22 ms (s.d.=15) for their deaf and normal-hearing subject groups, respectively. These results were interpreted as evidence of compromised tactile temporal processing in adults with early hearing loss. The results of the current study show a similar trend for larger mean thresholds for CD compared to NH subjects, but this trend does not reach significance. Tactual temporal onset-order thresholds among individual CD subjects in the current study varied substantially, some being within the typical range for most normal-hearing subjects. Moreover, tactual temporal offset-order thresholds in the current study were comparable between the CD and NH groups.
The specific neural processing requirements of a temporal order discrimination task are likely somewhat different from those of an asynchrony detection task, which may, to some extent, account for disparities between our findings and those of Heming and Brown (2005). It has been shown, for example, that perceptual learning following multi-hour training on either an auditory temporal onset-order discrimination task or an auditory asynchrony detection task does not generalize from one to the other (Mossbridge et al., 2006). Moreover, the same study found no benefit to auditory temporal offset-order discrimination following training on either the onset-order or asynchrony task. It is thus likely that no single psychophysical metric can adequately support generalizations about sensory temporal processing in adults with early hearing loss.
Amplitude and duration effects
In the temporal onset- and offset-order discrimination experiments, the amplitudes and durations of paired stimuli were roved independently, spanning value ranges corresponding to those observed among the amplitude envelopes of different frequency-filtered bands of speech (Yuan et al., 2004a). Thus, the experimental conditions employed in the present study roughly simulate the temporal stimulation patterns exhibited by vibrotactile carrier signals when they are modulated by filtered speech envelopes, as in the tactile speech coding scheme implemented by Yuan et al. (2005b) and Yuan and Reed (2005). We report here that the relative levels of two vibrotactile stimuli exert substantial influence over discrimination of both onset and offset asynchronies. Generally, as the sensation level of the stimulus at one site increases relative to the other, subjects are more likely to perceive that stimulus as having an earlier onset than the other stimulus in the SOA discrimination experiment, or as having a later offset in the SOFA discrimination experiment.
By contrast, differences in relative stimulus duration predominantly affect offset-order discrimination performance only. Specifically, when one stimulus in a pair is substantially shorter in duration than the other, subjects are more likely to perceive that stimulus as having a later offset. Consider that, in the SOA experiment, an increase in relative stimulus duration results in the longer-duration stimulus extending even farther in time beyond the shorter duration stimulus. In this case, any influence on onset-order discrimination would likely occur via a backward masking mechanism. In the SOFA experiment, as the difference between the two stimulus durations increases, the longer duration stimulus tends to extend earlier in time relative to the shorter duration stimulus, in which case, the effect of forward masking on offset-order discrimination might be expected to increase. Thus, the selective effect of stimulus duration discrepancy on offset-order sensitivity suggests that increased stimulus duration may elicit forward masking more effectively than backward masking in the Pacinian system. This explanation is consistent with the notion of a temporal asymmetry of vibrotactile forward and backward masking, which we have proposed to underlie the tendency for offset-order discrimination thresholds to exceed those for onset-order discrimination.
Just as we have observed, Yuan et al. (2005a) found that onset-order discrimination was substantially affected by the relative levels of paired stimuli, whereas the effect of roving stimulus durations was nominal. As in the current study, Yuan and colleagues adjusted the amplitudes of the two shortest-duration stimuli (see Sec. 2), and they postulated that these amplitude adjustments may have contributed to the negligible effects of roving stimulus duration on discrimination thresholds. This seems like a reasonable explanation, in light of the amplitude-dependence of thresholds in the onset-order discrimination experiment. The upper left-hand plot in Fig. 7 indicates that, particularly for the lowest |SOA| value examined, performance is better when the amplitude of the earlier-onset stimulus is larger relative to the amplitude of the later-onset stimulus (toward the right on the abscissa). Now consider the likely effects on the upper right-hand plot of Fig. 7 when we raise the amplitudes of all 50- and 100-ms stimuli (by 6 and 3 dB, respectively). Specifically, in duration category 1, the brief stimuli with increased amplitudes will always have the earlier onset, and in duration category 7, they will always have the later onset. Thus, the amplitude adjustment would tend to improve performance in category 1 trials, in which the earlier onset stimulus would otherwise be less salient. In category 7 trials, the adjustment would tend to undermine performance, in which the longer, earlier-onset stimuli might otherwise mask the shorter stimulus more effectively.
The same amplitude adjustments are applied to the two shortest-duration stimuli in the offset-order discrimination experiment, in which the effects of roving stimulus duration on thresholds are quite substantial. In this case, it is likely that the adjustments made to 50- and 100-ms stimulus amplitudes have favored the apparent dependence of performance on relative stimulus durations. In the bottom left-hand plot in Fig. 7, we have noted that subjects perform increasingly well as the amplitude of the later-offset stimulus increases relative to the earlier-offset stimulus (toward the right on the abscissa). In the bottom right-hand plot, the brief, amplitude-adjusted stimuli will always have the later offset in duration category 1 trials and the earlier offset in duration category 7 trials. As a result, subjects most likely perform better in category 1 trials and worse in category 7 trials than they would without the amplitude adjustment.
Implications for tactile displays of speech
A significant motivation for the present study was to guide the development of tactual speech aids for the deaf. In particular, discriminating between voiced and unvoiced consonants (e.g., ∕b∕ and ∕p∕) poses a major challenge to deaf individuals when communicating via lipreading. Yuan et al. (2004a) developed an acoustic cue comparable to voice-onset timing, which they have called “envelope-onset asynchrony” (EOA). The EOA cue is derived from the difference in onset-timing between the envelopes of a 3000 Hz high-pass filtered band and a 350 Hz low-pass filtered band (Yuan et al., 2004a). Analyzing isolated, spoken pairs of CVCs with initial consonants that differ only in the feature voicing, they showed that the amplitude envelopes of the two filtered bands for a voiced initial consonant tend to have similar onsets, separated by several tens of milliseconds at most, whereas for unvoiced initial consonants, the onset of the high frequency envelope generally precedes that of the low frequency envelope by considerably longer durations. EOAs are consistently on the order of 50–200 ms larger for an unvoiced initial consonant than for its voiced counterpart. A comparable relationship between the voicing of final consonants and the envelope-offset asynchrony (EOFA) has also been observed (Yuan et al., 2004b; Yuan and Reed, 2005). EOFA values for paired voiced and unvoiced final consonants typically differ by 200–300 ms.
Yuan and colleagues further established that normal-hearing individuals could exploit vibrotactile presentation of amplitude envelope information from the two speech bands to discriminate EOA and EOFA cues, and thereby distinguish between voiced-unvoiced consonant pairs in both initial (Yuan et al., 2005b) and final (Yuan et al., 2004b; Yuan and Reed, 2005) positions.
The present study sought to evaluate possible limits of cross-channel tactual temporal processing among pre-lingually deaf individuals, in order to determine the need for corresponding constraints on the tactual encoding of acoustic signals by a tactile speech aid. The results suggest that most congenitally deaf participants in this study should have sufficient temporal resolution to take advantage of tactually presented EOA and EOFA cues to supplement lipreading. Moreover, the powerful influence of relative stimulus levels on temporal order perception across skin loci in both the SOA and SOFA experiments has underscored the importance of amplitude range compression in the vibrotactile transduction of an acoustic speech signal. More generally, the present study contributes fundamentally to our understanding of sensory processing in persons with early-onset deafness.
Future research will examine the abilities of pre-lingually deaf individuals to discriminate the voicing distinction in speech sounds using the tactual EOA∕EOFA scheme, and to integrate these tactual cues into a more comprehensive tactual speech representation.
CONCLUDING REMARKS
Tactual detection thresholds are comparable among congenitally deaf and normal-hearing adults for sinusoidal stimuli ranging in frequency between 2 and 300 Hz.
Tactual onset-order discrimination thresholds for congenitally deaf and normal-hearing subjects averaged 83 and 58 ms, respectively; the difference is not statistically significant.
Tactual offset-order discrimination thresholds for congenitally deaf and normal-hearing subjects averaged 139 and 121 ms, respectively; the difference is not statistically significant.
Tactual offset-order discrimination thresholds were, on average, roughly 1.8 times larger than tactual onset-order discrimination threshold, unlike in audition, where offset-order thresholds have been found to be comparable to, if not lower than, onset-order thresholds.
Level differences between paired stimuli play a consistent role in the tactual discrimination of both onset- and offset-orders, for subjects in both groups.
Duration differences play a consistent role in the tactual discrimination of offset-order, but not onset-order, for subjects in both groups.
Discrimination thresholds for tactual offset-order are generally larger than those for tactual onset-order, and individual subjects’ performance on the two tasks is correlated (r=0.852).
Tactual temporal resolution among congenitally deaf subjects is sufficient for utilizing a tactually encoded temporal speech cue, for displaying crucial voicing information that is not available through lipreading.
Future research will examine tactually supplemented speech discrimination by congenitally deaf individuals.
ACKNOWLEDGMENTS
This research was supported by grants from the National Institutes of Health (Grant Nos. 5T32-DC000038, R01-DC000117, and R01-DC00126). The authors would like to thank Nat Durlach, Ruth Litovsky and two anonymous reviewers for their helpful suggestions in revising an earlier version of the manuscript.
References
- Bassim, M. K., Buss, E., Clark, M. S., Kolln, K. A., Pillsbury, C. H., Pillsbury, H. C.III, and Buchman, C. A. (2005). “MED-EL Combi40 + cochlear implantation in adults,” Laryngoscope 115, 1568–1573. 10.1097/01.mlg.0000171023.72680.95 [DOI] [PubMed] [Google Scholar]
- Bolanowski, S. J., Gescheider, G. A., Verrillo, R. T., and Checkosky, C. M. (1988). “Four channels mediate the mechanical aspects of touch,” J. Acoust. Soc. Am. 84, 1680–1694. 10.1121/1.397184 [DOI] [PubMed] [Google Scholar]
- Brown, L. N., and Sainsbury, R. S. (2000). “Hemispheric equivalence and age-related differences in judgments of simultaneity to somatosensory stimuli,” J. Clin. Exp. Neuropsychol 22, 587–98. 10.1076/1380-3395(200010)22:5;1-9;FT587 [DOI] [PubMed] [Google Scholar]
- Chakravarty, A. (1968). “Influence of tactual sensitivity on tactual localization, particularly of deaf children,” J. Gen. Psychol. 78, 219–221. [DOI] [PubMed] [Google Scholar]
- Craig, J. C., and Baihua, X. (1990). “Temporal order and tactile patterns,” Percept. Psychophys. 47, 22–34. [DOI] [PubMed] [Google Scholar]
- Craig, J. C., and Busey, T. A. (2003). “The effect of motion on tactile and visual temporal order judgments,” Percept. Psychophys. 65, 81–94. [DOI] [PubMed] [Google Scholar]
- Craig, J. C., and Evans, P. M. (1987). “Vibrotactile masking and the persistence of tactual features,” Percept. Psychophys. 42, 309–317. [DOI] [PubMed] [Google Scholar]
- Cranney, J., and Ashton, R. A. (1982). “Tactile spatial ability: Lateralized performance of deaf and hearing age groups,” J. Exp. Child Psychol. 34, 123–134. 10.1016/0022-0965(82)90035-2 [DOI] [PubMed] [Google Scholar]
- Durlach, N. I., (1968). A decision model for psychophysics, (MIT, Cambridge, MA: ). [Google Scholar]
- Eberhardt, S. P., Bernstein, L. E., Barac-Cikoja, D., Coulter, D. C., and Jordan, J. (1994). “Inducing dynamic haptic perception by the hand: System description and some results,” in Proceedings of the ASME Dynamic Systems and Control Division, New York, Vol. 1, pp. 345–351.
- Erber, N. P. (1974). “Visual perception of speech by deaf children—recent developments and continuing needs,” J. Acoust. Soc. Am. , 39, 178–185. [DOI] [PubMed] [Google Scholar]
- Gescheider, G. A., Bolanowski, S. J., Pope, J., and Verrillo, R. T. (2002). “A four-channel analysis of the tactile sensitivity of the fingertip: Frequency selectivity, spatial summation, and temporal summation,” Somatosens Mot Res. , 19, 114–124. 10.1080/08990220220131505 [DOI] [PubMed] [Google Scholar]
- Gescheider, G. A., Valetutti, A. A., Padula, M. C., and Verrillo, R. T. (1992). “Vibrotactile forward masking as a function of age,” J. Acoust. Soc. Am. 91, 1690–1696. 10.1121/1.402448 [DOI] [PubMed] [Google Scholar]
- Goff, G. D. (1967). “Differential discrimination of frequency of cutaneous mechanical vibration,” J. Exp. Psychol. 74, 294–299. 10.1037/h0024561 [DOI] [PubMed] [Google Scholar]
- Green, D. M., and Swets, J. A. (1966). Signal Detection Theory and Psychophysics (Wiley, New York: ). [Google Scholar]
- Heider, F., and Heider, G. M. (1940). “An experimental investigation of lipreading,” Psychol. Monogr. , 52, 124–153. [Google Scholar]
- Heming, J. E., and Brown, L. N. (2005). “Sensory temporal processing in adults with early hearing loss,” Brain Cogn 59, 173–182. 10.1016/j.bandc.2005.05.012 [DOI] [PubMed] [Google Scholar]
- Hirsh, I. J., and Sherrick, C. E. (1961). “Perceived order in different sensory modalities,” J. Exp. Psychol. 62, 423–432. 10.1037/h0045283 [DOI] [PubMed] [Google Scholar]
- Lamoré, P. J. J., Muijser, H., and Keemink, C. J. (1986). “Envelope detection of amplitude modulated high-frequency sinusoidal signals by skin mechanoreceptors,” J. Acoust. Soc. Am. 79, 1082–1085. 10.1121/1.393380 [DOI] [PubMed] [Google Scholar]
- Levänen, S., and Hamdorf, D. (2001). “Feeling vibrations: Enhanced tactile sensitivity in congenitally deaf humans,” Neurosci. Lett. 301, 75–77. 10.1016/S0304-3940(01)01597-X [DOI] [PubMed] [Google Scholar]
- Levitt, H. (1971). “Transformed up-down procedures in psychoacoustics,” J. Acoust. Soc. Am. 49, 467–477. 10.1121/1.1912375 [DOI] [PubMed] [Google Scholar]
- Marks, L. E., Girvin, J. P., O’Keefe, M. D., Ning, P., Quest, D. O., Antunes, J. L., and Dobelle, W. H. (1982). “Electrocutaneous stimulations III: The perception of temporal order,” Percept. Psychophys. 32, 537–541. [DOI] [PubMed] [Google Scholar]
- Mossbridge, J. A., Fitzgerald, M. B., O’Connor, E. S., and Wright, B. A. (2006). “Perceptual-learning evidence for separate processing of asynchrony and order tasks,” J. Neurosci. 26, 12708–12716. 10.1523/JNEUROSCI.2254-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas, J. G., and Geers, A. E. (2007). “Will they catch up? The role of age at cochlear implantation in the spoken language development of children with severe to profound hearing loss,” J. Speech Lang. Hear. Res. 50, 1048–1062. 10.1044/1092-4388(2007/073) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellet, C., and Cohen, H. (1999). “Speech and language development following cochlear implantation,” J. Neurolinguist. 12, 271–288. 10.1016/S0911-6044(99)00018-4 [DOI] [Google Scholar]
- Pastore, R. E. (1983). “Temporal order judgment of auditory stimulus offset,” Percept. Psychophys. 33, 54–62. [DOI] [PubMed] [Google Scholar]
- Rabinowitz, W. M., Houtsma, A. J. M., Durlach, N. I., and Delhorne, L. A. (1987). “Multidimensional tactile displays-identification of vibratory intensity, frequency, and contactor area,” J. Acoust. Soc. Am. 82, 1243–1252. 10.1121/1.395260 [DOI] [PubMed] [Google Scholar]
- Schiff, W., and Dytell, R. S. (1972). “Deaf and hearing children’s performance on a tactual perception battery,” Percept. Mot. Skills 35, 683–706. [DOI] [PubMed] [Google Scholar]
- Sherrick, C. E. (1964). “Effects of double simultaneous stimulation of the skin,” Am. J. Psychol. 77, 42–53. 10.2307/1419270 [DOI] [PubMed] [Google Scholar]
- Sherrick, C. E. (1970). “Temporal ordering of events in haptic space,” IEEE Trans. Man Mach. Syst. MMS–11, 25–28. 10.1109/TMMS.1970.299957 [DOI] [Google Scholar]
- Shore, D. I., Spry, E., and Spence, C. (2002). “Confusing the mind by crossing the hands,” Brain Res. Cognit. Brain Res. 14, 153–163. 10.1016/S0926-6410(02)00070-8 [DOI] [PubMed] [Google Scholar]
- Tan, H. Z. (1996). “Information transmission with a multi-finger tactual display,” Ph.D. thesis, Massachusetts Institute of Technology, Cambridge, MA. [Google Scholar]
- Tan, H. Z., and Rabinowitz, W. M. (1996). “A new multi-finger tactual display,” in Proceedings of the Dynamic Systems and Control Division, Vol. 58, pp. 515–522.
- Taylor, B. (1978). “Dimensional redundancy in the processing of vibrotactile temporal order,” Ph.D. thesis, Princeton University, Princeton, NJ. [Google Scholar]
- Tyler, R. S., Gantz, B. J., Woodworth, G. G., Pakinson, A. J., Lowder, M. W., and Schum, L. K. (1996). “Initial independent results with the Clarion cochlear implant,” Ear Hear. 17, 528–536. 10.1097/00003446-199617031-00005 [DOI] [PubMed] [Google Scholar]
- Uchanski, R. M., and Geers, A. E. (2003). “Acoustic characteristics of the speech of young cochlear implant users: A comparison with normal-hearing age-mates,” Ear Hear. 24, 90S–105S. 10.1097/01.AUD.0000051744.24290.C1 [DOI] [PubMed] [Google Scholar]
- Van Doren, C. L., Gescheider, G. A., and Verrillo, R. T. (1990). “Vibrotactile temporal gap detection as a function of age,” J. Acoust. Soc. Am. 87, 2201–2206. 10.1121/1.399187 [DOI] [PubMed] [Google Scholar]
- Verrillo, R. T. (1965). “Temporal summation in vibrotactile sensitivity,” J. Acoust. Soc. Am. 37, 843–846. 10.1121/1.1909458 [DOI] [PubMed] [Google Scholar]
- Verrillo, R. T., and Gescheider, G. A. (1992). “Perception via the sense of touch,” in Tactile Aids for the Hearing Impaired, edited by Summers I. R., (Whurr Publishers, London: ), pp. 1–36. [Google Scholar]
- Verrillo, R. T., Gescheider, G. A., Calman, B. G., and Van Doren, C. L. (1983). “Vibrotactile masking: Effects of one and two-site stimulation,” Percept. Psychophys. 33, 379–387. [DOI] [PubMed] [Google Scholar]
- Walden, B. E., Prosek, R. A., Montgomery, A. A., Scherr, C. K., and Jones, C. J. (1977). “Effects of training on visual recognition of consonants,” J. Speech Hear. Res. , 20, 130–145. [DOI] [PubMed] [Google Scholar]
- Wilson, B. S., and Dorman, M. F. (2008). “Cochlear implants: Current designs and future possibilities,” J. Rehabil. Res. Dev. 45, 695–730. 10.1682/JRRD.2007.10.0173 [DOI] [PubMed] [Google Scholar]
- World Health Organization (2006). “Deafness and hearing impairment,” Fact Sheet No. 300, March 2006. available at http://www.who.int/mediacentre/factsheets/fs300/en/ (Last viewed: 2/16/10).
- Yuan, H. F. (2003). “Tactual display of consonant voicing to supplement lipreading,” Ph.D. thesis, Massachusetts Institute of Technology, Cambridge, MA. [Google Scholar]
- Yuan, H. F., and Reed, C. M. (2005). “A tactual display of envelope-offset asynchrony as a cue to voicing of consonants in the final position,” in Proceedings of the Eighth International Sensory Aids Conference, Portland, ME, May 12.
- Yuan, H. F., Reed, C. M., and Durlach, N. I. (2004a). “Envelope-onset asynchrony as a cue to voicing in initial English consonants,” J. Acoust. Soc. Am. 116, 3156–3167. 10.1121/1.1804626 [DOI] [PubMed] [Google Scholar]
- Yuan, H. F., Reed, C. M., and Durlach, N. I. (2004b). “Envelope offset asynchrony as a cue to voicing in final English consonants (A),” J. Acoust. Soc. Am. 116, 2628–2629. [DOI] [PubMed] [Google Scholar]
- Yuan, H. F., Reed, C. M., and Durlach, N. I. (2005a). “Temporal onset-order discrimination through the tactual sense,” J. Acoust. Soc. Am. 117, 3139–3148. 10.1121/1.1869692 [DOI] [PubMed] [Google Scholar]
- Yuan, H. F., Reed, C. M., and Durlach, N. I. (2005b). “Tactual display of consonant voicing as a supplement to lipreading,” J. Acoust. Soc. Am. 118, 1003–1015. 10.1121/1.1945787 [DOI] [PubMed] [Google Scholar]