Abstract
Background
Pancreatic cancer, sometimes called a “silent killer,” is one of the most aggressive human malignancies, with a very poor prognosis. It is the fourth leading cause of cancer-related morbidity and mortality in the United States.
Methods
A mouse peritoneal model was used to test the ability of un-engineered rat umbilical cord matrix derived stem cells (UCMSCs) to control growth of pancreatic cancer. In vivo results are supported by various in vitro assays such as MTT, direct cell count, [3H] thymidine uptake, and soft agar colony assays.
Results
Co-culture of rat UCMSCs with PAN02 murine pancreatic carcinoma cells (UCMSCs:PAN02, 1:6 and 1:3) caused G0/G1 arrest and significantly attenuated the proliferation of PAN02 tumor cells as monitored by MTT assay, direct cell counts, and [3H] thymidine uptake assay. Rat UCMSCs also significantly reduced PAN02 colony size and number as measured by soft agar colony assay. The in vivo mouse studies showed that rat UCMSCs treatment significantly decreased the peritoneal PAN02 tumor burden 3 weeks after tumor transplantation and increased mouse survival time. Histological study revealed that intraperitoneally administered rat UCMSCs survived for at least 3 weeks, and the majority were found near or inside the tumor.
Discussion
These results indicate that naïve rat UCMSCs alone remarkably attenuate the growth of pancreatic carcinoma cells in vitro and in a mouse peritoneal model. Thus, these studies imply that UCMSCs could be a potential tool for targeted cytotherapy for pancreatic cancer.
Keywords: Colony assay, cytotherapy, pancreatic cancer, PAN02 cells, rat umbilical cord matrix stem cells, xenografts
INTRODUCTION
Pancreatic cancer, sometimes called a “silent killer,” is the fourth leading cause of cancer-related morbidity and mortality in the United States 1. Among all pancreatic cancer, pancreatic ductal adenocarcinoma (PDAC) is the most aggressive and constitutes approximately 90% of all primary malignant tumors arising from the pancreas. Of all gastrointestinal malignancies, pancreatic adenocarcinoma is the second most common cause of death from gastrointestinal cancer 1–3. It is an aggressive malignant cancer with a high metastatic rate and is an almost uniformly lethal disease in humans 1, 4, 5. Despite improvements in surgical and chemotherapeutic approaches during the past decades, pancreatic cancer continues to have a miserable prognosis, with an average overall 5-year survival of <5% 1. To date, surgical resection is the only potential therapeutic option; however, due to the lack of early symptoms, the vast majority of patients present with metastatic disease, rendering their malignancy not curable 3, 6. Accordingly, development of an effective therapeutic strategy is urgent.
It is well known that stem cells have inherent tumoritropic properties 7. Signals that mediate this effect appear to be similar or identical to those that mediate recruitment of stromal or defensive cells in tumors 8–10. There are also a number of reports showing that genetically engineered stem cells efficiently deliver therapeutic proteins to cancer and other sites of inflammation 7, 9, 11–15. Stem cells isolated from the Wharton’s jelly of umbilical cord, termed ‘umbilical cord matrix stem cells’ (UCMSCs), also exhibit inherent tumoritropic properties 11. When these cells are engineered to secrete a cytokine, interferon beta (IFN-β), and are administered intravenously, they can attenuate metastatic breast cancer in a SCID mouse model 11. Recently we found that rat UCMSCs completely abolished the growth of Mat B III cancer cells in vitro and in vivo 16. To further explore the preclinical therapeutic potential of rat UCMSCs, we sought to evaluate their effect on an intraperitoneal PAN02 mouse pancreatic ductal carcinoma model in mice. We used rat UCMSCs, since the isolation of mouse UCMSCs has been problematic due to the small size of preterm mouse umbilical cords. Although rat UCMSCs are xenogeneic to the mouse tissue, they appear to be tolerated by mouse immune surveillance. This is in concurrence with evidence that porcine or human UCMSCs, the ortholog to rat UCMSCs, have been shown to be poorly immunogenic 17, 18 Various in vitro assays such as MTT, direct cell counts, thymidine uptake, and soft agar assay were used. Additionally, the in vivo mouse experiments were carried out to evaluate the intrinsic ability of rat UCMSCs to attenuate pancreatic tumor growth. Here we report that even in trans-species transplantation, rat UCMSCs have exhibited a profound anti-tumor effect on murine pancreatic cancer growth without the mice showing any visible adverse effect from the rat UCMSCs transplantation itself.
MATERIALS AND METHODS
Materials
Propidium iodide and MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, Thiazolyl blue) were purchased from Fisher Scientific (Pittsburgh, PA). RNAse A was purchased from QIAGEN Sciences, Inc. (Germantown, MD). [3H] thymidine was purchased from GE Healthcare Bio-Sciences Corp. (Piscataway, NJ). RPMI-1640, DMEM, insulin-transferrin-selenium-X, penicillin/streptomycin, ALBUMax 1, and 4′-6-Diamidino-2-phenylindole (DAPI) nucleic acid stain were purchased from Invitrogen Corp. (Carlsbad, CA). SP-DiI was purchased from Molecular Probes (Eugene, OR). MCBD 201, dexamethasone, and ascorbic acid 2-phosphate were purchased from Sigma-Aldrich Corp. (St. Louis, MO). Epidermal growth factor (EGF) and platelet derived growth factor-BB (PDGF-BB) were purchased from R&D Systems (Minneapolis, MN). Fetal bovine serum (FBS) was purchased from Atlanta Biologicals Inc. (Lawrenceville, GA). All other chemicals were of analytical grade.
Cell culture
Rat UCMSCs were prepared from E19.5 pregnant rats using the method described previously 16 and were maintained in defined medium, containing a mixture of 56% low glucose DMEM, 37% MCBD 201, 2% FBS, 1x insulin-transferrin-selenium-X, 1x ALBUMax 1, 1x penicillin/streptomycin, 10nM dexamethasone, 100μM ascorbic acid 2-phosphate,10ng/ml EGF, and 10ng/ml PDGF-BB. Rat primary cultured skin fibroblasts were prepared from F344 newborn pup skin using an explant method described previously 19 and were maintained in DMEM containing 10% FBS and 1x penicillin/streptomycin. In general, rat UCMSCs were used within 3 to 20 passages, and rat fibroblasts were used within 3 to 8 passages. The pancreatic ductal adenocarcinoma cell line PAN02 was maintained in RPMI-1640 medium supplemented with 10% FBS and 1x penicillin/streptomycin. All cells were cultured at 37°C in a humidified atmosphere containing 5% CO2.
Cell proliferation assay
The MTT assay was performed to study the effect of rat UCMSCs on PAN02 cell proliferation. In brief, different ratios of rat UCMSCs (500 or 1000 cells/well) and 3000 PAN02 cells (rat UCMSCs: PAN02 = 1:6 and 1:3) in RPMI-1640 were seeded in 96 well plates and cultured for 72 hrs. MTT solution (20 μl of 5 mg/ml) was added after 68 hrs of incubation. Formazan crystals formed were dissolved by adding 100 μl solublization buffer (10% SDS containing 0.01N HCl) and incubating overnight in the incubator. The following day, color developed by the reaction was measured at 550 nm, and background absorbance was measured at 630 nm using the Molecular Devices Spectramax 190 plate reader (Global Medical Instrumentation, Inc. Ramsey, MN).
[3H] thymidine uptake assay
To evaluate cell proliferation by a second method, a [3H] thymidine uptake assay was carried out. In all [3H] thymidine incorporation experiments, rat UCMSCs (1×103 or 2×103/well) were mixed with 6×103 PAN02 cells, directly plated in 24-well culture plates, and cultured in the CO2 incubator for 72 hrs. Cells were pulsed for the last 4 hrs of the treatment time with 1.0 μCi [3H] thymidine per well. The free [3H] thymidine in the medium was washed away with PBS. The cell-incorporated [3H] thymidine was solubilized by adding 0.5N NaOH and counted using a Packard liquid scintillation counter Tri-Carb 2100TR (Perkin Elmer Life Science Boston, MA).
Transwell study
A direct cell count was performed to study the effect of rat UCMSCs on PAN02 cell growth in Transwell culture plates (BD Biosciences, San Jose, CA). In brief, PAN02 cells were seeded with normal growth medium at 5 × 104 cells/well in 6-well plates. After allowing the cancer cells to settle for 1 hr, 8.33×103 and 1.67 × 104 rat UCMSCs were seeded on the cell culture inserts (3.0 μm pore size). After 72 hr co-culture, cells grown in the bottom of the culture dish were collected by trypsinization and counted using a hemocytometer.
Cell cycle analysis
To analyze the effect of rat UCMSCs co-culture on PAN02 cells, cell cycle analysis was carried out using propidium iodide staining. In brief, rat UCMSCs (1×104 or 2×104) were co-cultured in Transwell inserts with 6×104 PAN02 cells in the bottom chamber; cells were allowed to grow for 72 hrs. After incubation, PAN02 cells in the bottom chamber were collected and fixed overnight in 70% pre-chilled ethanol. After collecting the cells by centrifugation, cells were incubated in PBS containing 40 μg/ml propidium iodide and 100 μg/ml RNAse A for 1 hr at room temperature. The fluorescence (excitation at 488 nm and emission at 585/42 nm) of 20,000 cells from each sample was measured with a FACS Calibur flow cytometer (Becton Dickinson, SanJose, CA). Data were analyzed using ModFit software and the results were displayed as histograms.
Colony formation study
A two layer-colony formation assay was carried out as follows: 0.5 ml of 0.9% agarose (Sea Plaque agarose, Cambrex Bio Science Rockland, Inc. Rockland, ME) in defined medium containing 5% FBS was poured into wells of a 12-well tissue culture plate (bottom layer). Different numbers of rat UCMSCs and PAN02 cells (1×104 cells) were suspended in 0.5ml of defined medium containing 5% FBS, 0.04% MatriGel®, and 0.45% agarose and plated on top of the bottom agar layer. The cells were incubated at 37°C with 5% CO2 for growth of colonies. On day 10, colony growth was evaluated by an automated phase contrast microscope equipped with Micro Suite Analysis Suite (Olympus CKX41, Center Valley, PA). Colonies greater than 5,000 μm2 in area were counted using Micro Suite Analysis Suite software.
Animals
Wild-type female C57BL/6 mice were obtained from the Jackson Laboratory (Bar Harbor, ME). All mice were housed in a clean facility and held for 10 days to acclimatize. All animal experiments were done under strict adherence to the Institutional Animal Care and Use Committee protocols set by Kansas State University.
PAN02 tumor transplantation and rat UCMSCs treatment
To develop peritoneal tumors, 1×106 PAN02 tumor cells (100 μl) were transplanted into the peritoneal cavity of mice under light isoflurane anesthesia. Mice receiving PAN02 cells were randomly divided into (1) PBS, (2) rat skin fibroblast or (3) rat UCMSC treatment groups. On days 2 and 4 after PAN02 inoculation, mice received intraperitoneally either 200 μl PBS (PBS group) or 5×105 rat UCMSCs (rat UCMSC group; 20% of cells were stained with SP-DiI fluorescent dye) or rat skin fibroblasts in 200 μl PBS. Three weeks after PAN02 transplantation, all mice were sacrificed, entire tumor masses were collected from the peritoneal cavity, and combined tumor weights were recorded. Changes in the tumor weight compared to vehicle-treated controls were used for determining the effect of rat UCMSCs. All the tumor samples were fixed in 10% formalin-saline
To determine the effect of rat UCMSCs therapy, survival of pancreatic carcinoma graft-bearing mice was monitored after the treatments. Three days after the intraperitoneal inoculation of PAN02 cells (1×106 cells/200μl PBS), mice were intraperitoneally injected with either rat UCMSCs (bolus injection of 5×105cells/200μl PBS) or 200μl PBS. Mice were kept under strict observation and body weights were measured every other day. Mice were eventually euthanized when their body weight loss exceeded 15% of original. Estimated survival times were calculated by the Kaplan-Meier survival estimation method. Significance was analyzed by the log-rank test.
Histopathology
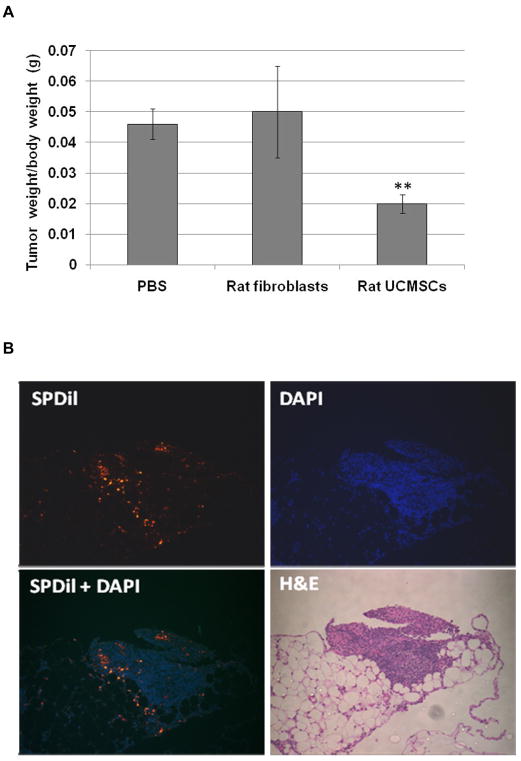
Tumor tissues from the peritoneal cavity were fixed in 10 % formalin-saline and embedded in paraffin, serially sectioned at 5–6μm, and stained with hematoxylin and eosin (H&E) for microscopic examination of tumor morphology. Additional serial sections were counterstained with DAPI nuclear stain and observed under epifluorescence microscopy for tracking the rat UCMSCs.
Statistical analysis
All values are expressed as means ± SE for all in vitro and in vivo experiments except for the mouse survival study. Statistical significance was assessed by Tukey-Kramer Pairwise Comparisons test using KyPlot (Version 2.0 beta 15) statistical software. If not otherwise stated, all experiments reported represent two independent replications performed in triplicate. Statistical significance was set at * p < 0.05; ** p < 0.01; *** p < 0.001. For mouse survival study analysis, Kaplan-Meier’s survival estimation analysis was used. A dead mouse was counted as censored 0, whereas a euthanized mouse with lethal body condition was defined as censored 1. The difference between the estimated survival times of the two groups was evaluated by the log-rank-test. The statistical significance of the log-rank test was considered if p < 0.05 according to chi-square distribution.
RESULTS
Rat UCMSCs inhibit anchorage-dependent and -independent growth of PAN02 cells
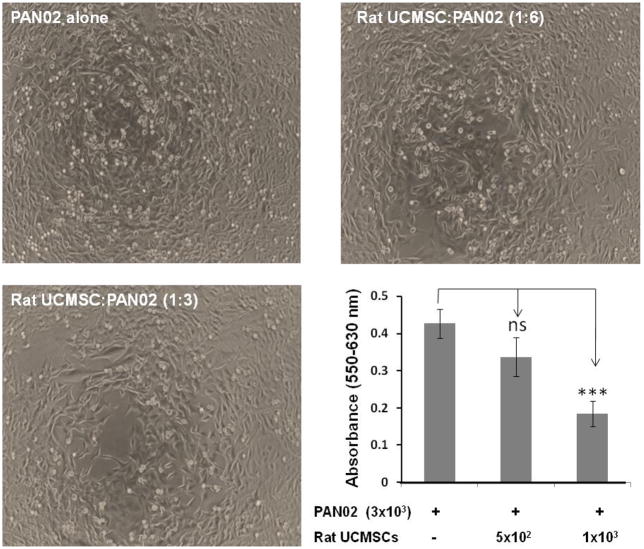
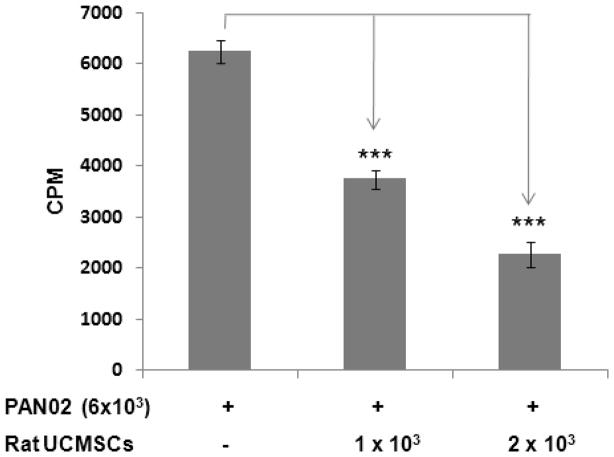
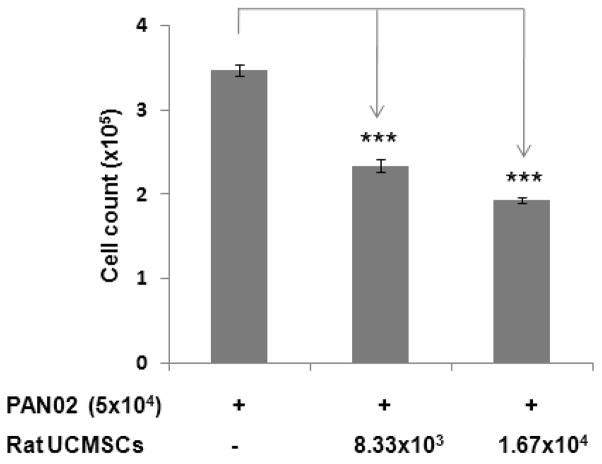
The effect of un-engineered rat UCMSCs on anchorage-dependent growth of PAN02 cells was evaluated by both direct and indirect co-culture. As shown in Fig. 1, direct co-culture of rat UCMSCs with PAN02 cells (ratio, 1:6 or 1:3) markedly decreased the total cell number as measured by MTT assay. Since an increase of DNA synthesis in cells is a good index for cell proliferation, the extent of DNA synthesis was measured by the incorporation of [3H] thymidine. The results revealed that small numbers of rat UCMSCs co-cultured with PAN02 significantly and dose-dependently inhibited DNA synthesis in the PAN02 cells (Fig. 2). In addition to these studies in which both cell types directly contacted each other, an indirect co-culture study was carried out using a Transwell culture system in which rat UCMSCs were cultured in Transwell inserts and PAN02 cells were cultured in the bottom of the culture dish. The effect of this co-culture was assessed by counting the PAN02 cells after 72 hrs co-culture (Fig. 3). The findings of this experiment corroborated the results from the MTT assay (Fig. 1) and thymidine uptake assay (Fig. 2). Although the MTT assay was slightly less sensitive than the other two assays, all three assay results are very similar and clearly indicate that un-engineered rat UCMSCs dose-dependently attenuated the growth of PAN02 cells. This Transwell culture experiment suggests that rat UCMSC-dependent cell growth attenuation may be mediated through a diffusible molecule or molecules produced by rat UCMSCs.
Figure 1.
Direct co-culture of a small number of rat UCMSCs significantly attenuated the growth of PAN02 cells. Rat UCMSCs (500 and1000 cells/well) were co-cultured with 3 × 103 PAN02 (rUCMSC: PAN02 ratio, 1:6 and1:3). After three days of co-culture, the MTT assay was carried out. Pictures show the morphology of PAN02 alone or PAN02 co-cultured with rat UCMSCs for 72 hrs. Histogram shows the summarized results from the MTT assay. The experiment was performed twice with 6 determinations at each point. Results are presented as the mean ± standard error of mean. The ns indicates not significant. *, p≤0.05, **, p<0.001 as compared to the level of control.
Figure 2.
[3H] thymidine-uptake into PAN02 cells was significantly attenuated by co-culture with a small number of rat UCMSCs. Rat UCMSCs (1×103 or 2×103/well) were co-cultured with PAN02 cells (6×103) in a 24-well culture plate. The [3H] thymidine-uptake was evaluated after 72 hrs of co-culture, as described in the Methods section. The experiment was performed twice with quadruplicate determinations. The error bars represent the standard error of the mean of the samples. The ns indicates not significant. *, p≤0.05 as compared to the level of control.
Figure 3.
Indirect co-culture of a small number of rat UCMSCs significantly attenuated the growth of PAN02 cells in Transwell co-culture. In this study, 8.33 × 103 or 1.67 × 104 rat UCMSCs were indirectly co-cultured in Transwell culture dishes with 5 × 104 PAN02 cells. The ratio of the two cell types was 1:6 and 1:3. After three days co-culture, live PAN02 cells were directly counted using a hemocytometer. The experiment was performed twice with triplicate determinations. Results are presented as the mean ± standard error of mean. ***, p<0.001 as compared to the level of control.
Since anchorage-independent growth is a hallmark of tumorigenesis, a soft agar assay was carried out to evaluate the effect of rat UCMSCs on the growth of PAN02 cell colonies. Since the PAN02 cells alone did not make colonies in soft agar, 0.02% MatriGel was added in the upper gel layer when PAN02 cells were seeded. The results showed that the colony size and number of PAN02 cells in soft agar were significantly attenuated when rat UCMSCs were co-cultured - (Fig. 4).
Figure 4.
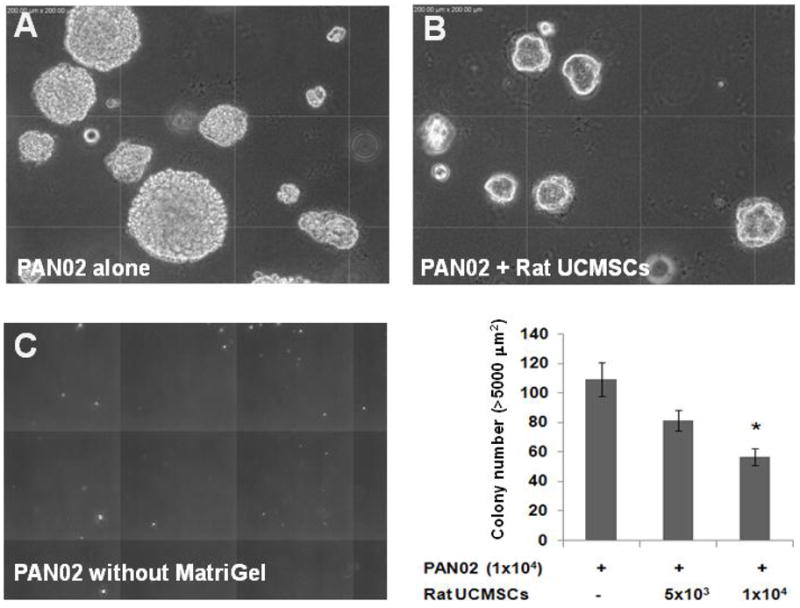
The colony growth of PAN02 pancreatic carcinoma cells was significantly attenuated by co-culture with rat UCMSCs in soft agar. PAN02 cells (1 × 104) were mixed with either 5 × 103 or 1 × 104 rat USMSCs in the 0.45% top agar layer containing 0.04% Matrigel and 10% FBS and seeded on top of the 0.9% bottom agar layer. Colony growth was evaluated 10 days after seeding the cells. The pictures show the morphology of the PAN02 colonies in the presence (A) or absence (C) of 0.02% Matrigel or PAN02 colonies co-cultured with rat UCMSCs in the presence of 0.02% Matrigel (B) in soft agar after 10 days. The bar graph shows the summarized results from the colony assay carried out in the presence of 0.02% Matrigel. The experiment was performed twice with triplicate determinations. Although the total number of PAN02 and rat USMSCs combined was larger than in the control, the colony size and number were significantly smaller than in the control. The error bars represent the standard error of the mean of the samples. *, p≤0.05, as compared to the level of control.
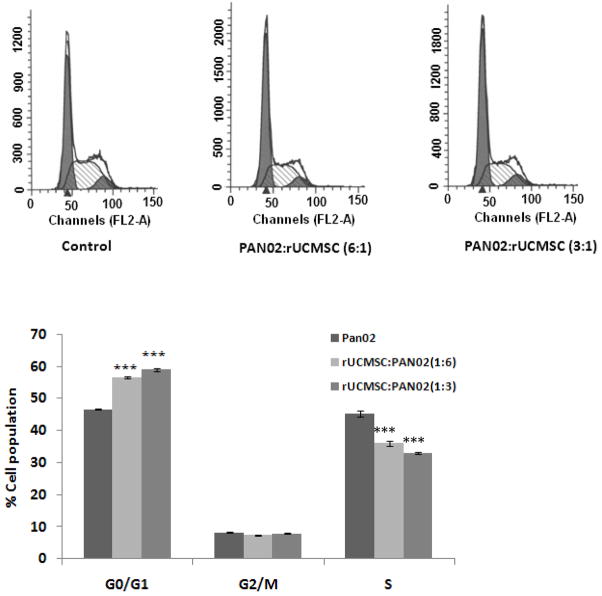
Rat UCMSCs induced G0/G1 cell cycle arrest in PAN02 cells
From direct and indirect co-culture studies, it seems that some diffusible molecules secreted by the rat UCMSCs were probably responsible for the growth attenuation. To find out the mechanism by which rat UCMSCs attenuate the growth of PAN02 cells, cell cycle analysis was carried out after PAN02 cells were co-cultured with rat UCMSCs in Transwell culture dishes. The result shows an increase of the G0/G1 population, indicating that the rat UCMSCs caused G0/G1 arrest of PAN02 cells (Fig. 5).
Figure 5.
Co-culture of a small number of rat UCMSCs with PAN02 cells in Transwell culture plates significantly increased G0/G1 populations in PAN02 cells as compared with un-treated PAN02cells alone. In the co-culture study, 1 × 104 or 2 × 104 rat UCMSCs were indirectly co-cultured in a Transwell culture plate with 6 × 104 PAN02 cells. After three days co-culture, PAN02 cells were collected from the bottom chamber by a light trypsinization, fixed with 70% ethanol, stained with propidium iodide as described in Materials and Methods, and subjected to flow cytometry. Panel A represents the flow cytometry analysis and panel B represents the histogram pattern of different phases of the cell cycle. Bar graphs indicate the mean ± standard error of mean two independent triplicate determinations. ***, p≤0.001 as compared to the level of untreated control.
Rat UCMSCs significantly attenuate the growth of PAN02 grafts in a syngeneic peritoneal tumor model
Since all in vitro cell culture-based studies indicated that a relatively small number of un-engineered rat UCMSCs can effectively attenuate the growth of murine pancreatic cancer cells, the in vivo effect of rat UCMSCs was examined using a syngeneic mouse peritoneal tumor graft model. This mouse model is meaningful since the peritoneal cavity is the one of the major sites of metastasis in pancreatic cancer 20. A single intraperitoneal inoculation of one million PAN02 cells caused effective tumor growth. Clinical symptoms of tumor growth in the peritoneal cavity, such as palpable abnormal lumps and rough hair texture, became obvious approximately three weeks after tumor cell inoculation. Tumors were primarily detected on the omentum and at the hepatic portal region. Occasionally, tumors grew on the diaphragm when tumor growth was very fast. However, PAN02 tumors did not grow on the surface of the liver, spleen, kidney, pancreas, or serous membrane. As shown in Fig. 6A, rat UCMSC therapy (0.5 million cells/injection) at two days and four days after primary tumor inoculation significantly decreased the tumor burden determined as whole tumor weight in the peritoneal cavity as compared to rat skin fibroblasts (0.5 million cells/injection, two intraperitoneal injections) or PBS injected groups. Furthermore, histological analysis of serial sections of peritoneal tumors revealed engraftment of rat UCMSCs in close proximity to or within tumor tissues of tumor bearing mice that received SP-DiI labeled rat UCMSCs (Fig. 6B). It is noteworthy that a majority of rat UCMSCs were detected only in tumor areas, but not in fat tissues or on intraperitoneal organ surfaces. These results suggest that intraperitoneally administered rat UCMSCs homed primarily to pancreatic tumor tissues in the peritoneal cavity, thus attenuating tumor growth.
Figure 6.
Rat UCMSC therapy (a half million cells/injection, two intraperitoneal injections with one day interval) significantly attenuated the growth of PAN02 tumors as compared to PBS- or normal skin fibroblast-treated tumors in a syngeneic mouse model. All PAN02 tumors in the peritoneal cavity, primarily located in the hepatic portal region and omentum, were carefully dissected and their weights measured. Omentum, composed of fat and a thin membrane, was removed from un-inoculated healthy mice and used for the normalization as a control omentum weight. B. Histological analysis of the localization of transplanted rat UCMSCs in intraperitoneal tumors. PAN02 syngeneic grafts in the peritoneal cavity were dissected, fixed, and thin sectioned as described in the Materials and Methods section. H and E staining was used to examine overall tumor morphology. Unstained sections were utilized for the detection of SP-DiI labeled rat UCMSCs after DAPI nuclear counterstaining. In this analysis, SP-DiI labeled rat UCMSCs show bright reddish yellow in a dark background. The error bars represent the standard error of the mean of the samples. ** p≤0.01 as compared to the level of PBS-treated controls.
Rat UCMSC therapy enhanced the survival of PAN02 graft bearing mice
Since rat UCMSC therapy significantly attenuated the growth of PAN02 grafts, the efficacy of a single treatment with rat UCMSCs on the survival of PAN02 graft bearing mice was evaluated. Mice (n=16/group) were inoculated with one million PAN02 cells and a half million rat UCMSCs were given three days later. All PBS-injected control mice died within 45 days, whereas approximately 57 % of the treated mice were still alive at that time. A quarter of rat UCMSC treated mice (4/16 mice) survived until 100 days after the PAN02 cell inoculation, Two out of 16 mice were still alive without any problems and without macroscopic tumors when the experiment was closed 170 days after tumor cell inoculation (Fig. 7). Physical examination of mice by their activity and body weight revealed that rat UCMSC-treated mice were much healthier and more active than those treated with PBS (data not shown). However, 14 mice eventually exhibited complications associated with excessive tumor growth, such as malnutrition, accumulation of ascites fluid, bile excretion problems due to the occlusion of the common bile duct, kidney failure due to the occlusion of the ureters, etc. Mice showing such symptoms were sacrificed according to the Institutional Animal Care and Use Committee protocol as set by Kansas State University. These results clearly indicate that treatment with a single injection of rat UCMSCs can increase the life span of tumor bearing mice.
Figure 7.
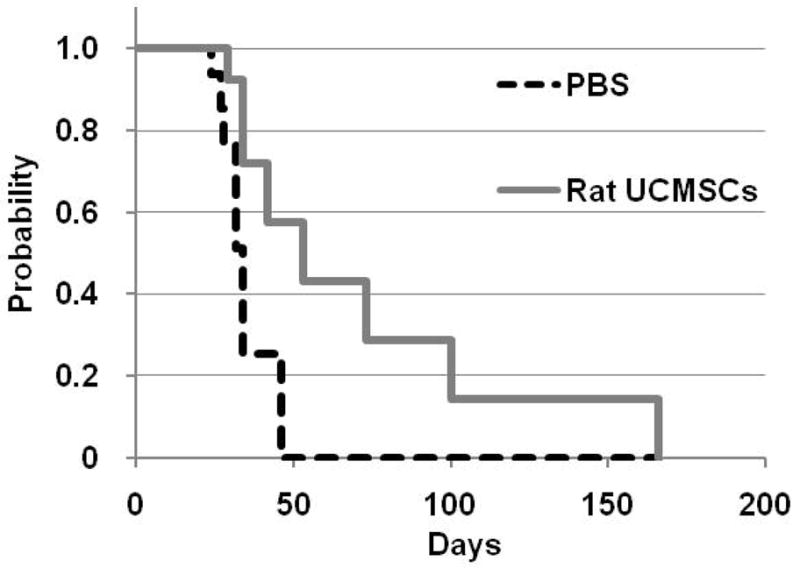
Intraperitoneal administration of rat UCMSCs increased the survival of PAN02 tumor-bearing mice. Survival curves of the PAN02 pancreatic carcinoma bearing mice treated with rat UCMSCs were compared with untreated control group (n=16/group). 3 days after the intraperitoneal inoculation of PAN02 cells (one million cells/200μl PBS), mice were injected intraperitoneally with rat UCMSCs (half million cells/200μl PBS) or 200μl PBS. Estimation of survival times were calculated by the Kaplan-Meier survival estimation method. Significance was analyzed by log-rank test. The survival times of rat USMSCs treated mice was prolonged as compared to the PBS treated group (x2 = 2.47, P = 0.12).
DISCUSSION
The present study shows for the first time that un-engineered naive rat UCMSCs have the potential to eliminate rapidly growing pancreatic tumors in the mouse peritoneal cavity. In addition, we describe here the following important new findings: 1) a relatively small number of rat UCMSCs significantly attenuated proliferation of PAN02 pancreatic ductal carcinoma cells when they were co-cultured; 2) in vitro three dimensional PAN02 colony formation is markedly attenuated when rat UCMSCs were co-cultured; 3) rat UCMSCs caused G0/G1 arrest, and this may be a possible mechanism of the tumor growth attenuation; 4) rat UCMSC therapy significantly decreased the tumor burden in the peritoneal cavity; 5) rat UCMSCs administered intraperitoneally ‘home’ to or near the tumors; 6) naive rat UCMSCs increased the overall survival of PAN02 tumor-bearing mice.
Attenuation of tumor cell growth by rat UCMSCs appears to have both contact-independent and -dependent components. Interestingly, Khakoo et al. showed that bone marrow mesenchymal stem cells could only mediate their effect on Kaposi sarcoma by contact with the tumor cells in vitro 21. Here we showed that in co-culture assays (Figs. 1 and 2); rat UCMSCs co-cultured with PAN02 cells significantly attenuate PAN02 cell proliferation. Since the number of rat UCMSCs was significantly smaller than PAN02 cells (1:6 or 1:3), this rat UCMSC-dependent effect is not strictly contact-mediated; it is likely that some factor(s) independent of cell-to-cell contact is involved. In the Transwell co-culture study, in which PAN02 cells were not in direct contact with rat UCMSCs, the PAN02 cell growth inhibition by rat UCMSCs (Fig. 3) implies involvement of a diffusible factor or factors secreted by the stem cells. This is in good agreement with previous studies from our laboratory in which rat UCMSCs attenuate growth of rat mammary carcinoma cells, apparently through diffusible molecules 16. This factor may be associated with the regulation of the cell cycle, since the present study clearly indicated that rat UCMSCs induced G0/G1 arrest in PAN02 cells when they were co-cultured in a Transwell culture system in which the two cell types do not directly contact each other. Furthermore, the anti-tumor effect shown here may also be associated with pro-apoptotic factors produced by naive rat UCMSCs. In this regard, it is noteworthy to cite Friedman’s report 22 that human UCMSCs secrete significant amounts of cytokines that are associated with anti-tumor effects, including transforming growth factor beta (TGF-β) and leukemia inhibitory factor (LIF), as well as small amounts of tumor necrosis factor alpha (TNFα), interferons alpha and gamma, and interleukin-1a (IL-1a). In future studies, a high priority will be placed on identifying specific genes or gene products responsible for the molecular mechanism by which un-engineered rat UCMSCs powerfully attenuate pancreatic cancer cell growth. Based on the present study, cell cycle regulation- and/or apoptosis-associated genes may be good candidates. However, other mechanisms, such as a stimulation of the host immune system, may also be involved in tumor growth attenuation in vivo. This possibility should also be explored in the future.
The environment in the host’s body, including the immune system, may also play a role in the variation observed in our mouse survival studies. Although the PAN02 cells used were of similar passage numbers, and rat UCMSCs isolated from the same isolate were used in the survival study, there was wide variation of the length of survival in the mouse survival study (Fig. 7). In this study, a small number of UCMSC-treated mice died within a short period of time, but some lived for a long time even though all were treated with rat UCMSCs. Two mice showed complete tumor regression and no sign of recurrence. This large variation in survival time may suggest that host body responses to the transplanted UCMSCs may play a critical role in attenuating cancer cell growth. Therefore, host body responses should be explored thoroughly in the future.
The ability of naive UCMSCs to eliminate pancreatic carcinomas is a distinct advantage, since any manipulation causing the cells to express an exogenous gene could alter them in some way that would potentially make them less safe as transplantable cells. However, since rat UCMSCs are not directly applicable to human therapy, key mechanism(s) by which rat UCMSCs exhibit their powerful anti-tumor effect should be identified; this mechanism may be applied to human UCMSCs for future human application. Therefore, it will be worthwhile to identify which genes or gene products make rat UCMSCs so powerful for attenuation of pancreatic cancer growth. It will also be valuable to evaluate the tumor cell cytotoxicity of human UCMSCs and to compare their cytotoxicity-associated genes with those of rat UCMSCs. If human UCMSCs are as potent as rat UCMSCs, human UCMSCs will potentially be utilized for human cancer therapy. If human UCMSCs are not as potent as rat UCMSCs, their cytotoxicity to cancer cells may be enhanced by manipulation of their gene expression based on the study of rat UCMSC-dependent cytotoxicity against pancreatic cancer.
The homing ability of stem cells has previously been exploited for drug delivery and targeted gene delivery9, 11–13, 21, 23. The homing of stem cells to tumors and other areas of inflammation is well established 8–11, 24. It appears to be mediated by chemokines secreted by the tumors or their associated stroma 25–27. Potential chemokines could include growth factors such as platelet derived growth factor (PDGF) family members and epidermal growth factor (EGF) 28 in addition to classical chemo-attractants. In the present study, fluorescence microscopic analysis of intraperitoneal tumors revealed that most of the intraperitoneally administered rat UCMSCs are located near or within the tumors one week after the administration of UCMSCs. Few SP-DiI-labeled UCMSCs were detected in fat or membranous tissues in omentum (Fig. 6B). These results suggest that the majority of UCMSCs have effectively migrated to tumor tissues, perhaps under the influence of the chemokines secreted by the tumors or their stroma. However, clarification of this tumor tissue-targeted homing mechanism awaits another study.
In this study we have used rat origin UCMSCs for a mouse cancer model and they have shown a very strong effect. Whether the rat UCMSC-dependent anti-tumorigenic effect is partially due to the xenotransplantation is unclear. However, since rat skin fibroblasts did not show any therapeutic effect (Fig. 6A), and since rat UCMSCs were extremely potent in cell growth attenuation in simple in vitro cell culture studies where no immune components were involved, it is likely that their in vivo effect is also independent from nonspecific immune surveillance induced by xenotransplantation. In support of this, pathological analysis did not show lymphocyte infiltration in tumor tissues (data not shown). In addition, our previous finding that human UCMSCs are poorly immunogenic18 also indirectly supports the above speculation.
Although a specific factor or factors attenuating pancreatic carcinoma cell growth in vitro and in vivo has not been identified in the present study, a few studies indicate involvement of cytokines in umbilical cord blood-derived MSC-dependent growth attenuation in glioma cells 29 and bone marrow-derived cell-dependent cell death in multiple carcinoma cells 30. The basic principle of these studies is similar to that of the present study. Although these studies indicate the importance of cytokines in adult stem cell-dependent growth attenuation in multiple cancer cells, no single cytokine plays the key role in stem cell induced cancer cell death. Apparently, the type of cytokine important in cancer cell death depends on both the stem cell and target cancer cell type. Accordingly, it appears that identification of a key factor or factors in un-engineered rat UCMSC-dependent growth attenuation of pancreatic cancer cells will require a whole cytokine-wide search.
Among many tissue-originated multipotent stem cells, naive UCMSCs have many potential advantages for cytotherapy. These include their abundance, lack of CD34 and CD45 expression 17, low immunogenicity 17, 18, and the simplicity of the methods for harvest and in vitro expansion 15, 31, 32. These properties argue for their development as therapeutic tools or agents because they can potentially be used for allogeneic transplantation. Thus, the findings described here show that UCMSCs may represent a new therapeutic modality for the treatment of cancer and will have important implications for patients with pancreatic cancer and other types of cancer.
Acknowledgments
This work was supported by the Kansas State University (KSU) Terry C. Johnson Center for Basic Cancer Research, KSU Targeted Excellence research grant, Kansas State Legislative Appropriation, KSU College of Veterinary Medicine Dean’s Fund, and NIH grants P20 RR017686, P20 RR01556, and R21 CA135599.
ABBREVIATIONS
- PDAC
Pancreatic ductal adenocarcinoma
- UCMSCs
umbilical cord matrix stem cells
- MTT
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- SP-DiI
a sulfonated derivative of dialkyl indol
- DAPI
4′-6-Diamidino-2-phenylindole
- H&E
hematoxylin and eosin
- FBS
Fetal bovine serum
- PDGF
platelet derived growth factor
- PDGF-BB
platelet derived growth factor-BB
- EGF
Epidermal growth factor
- TGF-β
transforming growth factor beta
- LIF
leukemia inhibitory factor
- TNF-α
tumor necrosis factor alpha/IL-1a, interleukin-1a
- IFN-β
interferon beta
Footnotes
DISCLOSURE OF CONFLICT OF INTEREST
The authors have no conflict of interest.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer Statistics, 2009. CA Cancer J Clin. 2009 doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N, Depinho RA. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 2006;20:1218–49. doi: 10.1101/gad.1415606. [DOI] [PubMed] [Google Scholar]
- 3.Warshaw AL, Fernandez-del Castillo C. Pancreatic carcinoma. N Engl J Med. 1992;326:455–65. doi: 10.1056/NEJM199202133260706. [DOI] [PubMed] [Google Scholar]
- 4.Keleg S, Buchler P, Ludwig R, Buchler MW, Friess H. Invasion and metastasis in pancreatic cancer. Mol Cancer. 2003;2:14. doi: 10.1186/1476-4598-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McKenna S, Eatock M. The medical management of pancreatic cancer: a review. Oncologist. 2003;8:149–60. doi: 10.1634/theoncologist.8-2-149. [DOI] [PubMed] [Google Scholar]
- 6.Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004;363:1049–57. doi: 10.1016/S0140-6736(04)15841-8. [DOI] [PubMed] [Google Scholar]
- 7.Corsten MF, Shah K. Therapeutic stem-cells for cancer treatment: hopes and hurdles in tactical warfare. Lancet Oncol. 2008;9:376–84. doi: 10.1016/S1470-2045(08)70099-8. [DOI] [PubMed] [Google Scholar]
- 8.Aboody KS, Brown A, Rainov NG, Bower KA, Liu S, Yang W, Small JE, Herrlinger U, Ourednik V, Black PM, Breakefield XO, Snyder EY. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natl Acad Sci U S A. 2000;97:12846–51. doi: 10.1073/pnas.97.23.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakamizo A, Marini F, Amano T, Khan A, Studeny M, Gumin J, Chen J, Hentschel S, Vecil G, Dembinski J, Andreeff M, Lang FF. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005;65:3307–18. doi: 10.1158/0008-5472.CAN-04-1874. [DOI] [PubMed] [Google Scholar]
- 10.Rachakatla RS, Marini F, Weiss ML, Tamura M, Troyer D. Development of human umbilical cord matrix stem cell-based gene therapy for experimental lung tumors. Cancer Gene Ther. 2007;14:828–35. doi: 10.1038/sj.cgt.7701077. [DOI] [PubMed] [Google Scholar]
- 11.Studeny M, Marini FC, Champlin RE, Zompetta C, Fidler IJ, Andreeff M. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res. 2002;62:3603–8. [PubMed] [Google Scholar]
- 12.Studeny M, Marini FC, Dembinski JL, Zompetta C, Cabreira-Hansen M, Bekele BN, Champlin RE, Andreeff M. Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J Natl Cancer Inst. 2004;96:1593–603. doi: 10.1093/jnci/djh299. [DOI] [PubMed] [Google Scholar]
- 13.Aboody KS, Najbauer J, Schmidt NO, Yang W, Wu JK, Zhuge Y, Przylecki W, Carroll R, Black PM, Perides G. Targeting of melanoma brain metastases using engineered neural stem/progenitor cells. Neuro Oncol. 2006;8:119–26. doi: 10.1215/15228517-2005-012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar S, Chanda D, Ponnazhagan S. Therapeutic potential of genetically modified mesenchymal stem cells. Gene Ther. 2008;15:711–5. doi: 10.1038/gt.2008.35. [DOI] [PubMed] [Google Scholar]
- 15.Mitchell KE, Weiss ML, Mitchell BM, Martin P, Davis D, Morales L, Helwig B, Beerenstrauch M, Abou-Easa K, Hildreth T, Troyer D, Medicetty S. Matrix cells from Wharton’s jelly form neurons and glia. Stem Cells. 2003;21:50–60. doi: 10.1634/stemcells.21-1-50. [DOI] [PubMed] [Google Scholar]
- 16.Ganta C, Chiyo D, Ayuzawa R, Rachakatla R, Pyle M, Andrews G, Weiss M, Tamura M, Troyer D. Rat umbilical cord stem cells completely abolish rat mammary carcinomas with no evidence of metastasis or recurrence 100 days post-tumor cell inoculation. Cancer Res. 2009;69:1815–20. doi: 10.1158/0008-5472.CAN-08-2750. [DOI] [PubMed] [Google Scholar]
- 17.Cho PS, Messina DJ, Hirsh EL, Chi N, Goldman SN, Lo DP, Harris IR, Popma SH, Sachs DH, Huang CA. Immunogenicity of umbilical cord tissue derived cells. Blood. 2008;111:430–8. doi: 10.1182/blood-2007-03-078774. [DOI] [PubMed] [Google Scholar]
- 18.Weiss ML, Anderson C, Medicetty S, Seshareddy KB, Weiss RJ, VanderWerff I, Troyer D, McIntosh KR. Immune properties of human umbilical cord Wharton’s jelly-derived cells. Stem Cells. 2008;26:2865–74. doi: 10.1634/stemcells.2007-1028. [DOI] [PubMed] [Google Scholar]
- 19.Prowse KR, Greider CW. Developmental and tissue-specific regulation of mouse telomerase and telomere length. Proc Natl Acad Sci U S A. 1995;92:481–22. doi: 10.1073/pnas.92.11.4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang L, Hwang R, Pandit L, Gordon EM, Anderson WF, Parekh D. Gene therapy of metastatic pancreas cancer with intraperitoneal injections of concentrated retroviral herpes simplex thymidine kinase vector supernatant and ganciclovir. Ann Surg. 1996;224:405. doi: 10.1097/00000658-199609000-00017. discussion 14–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khakoo AY, Pati S, Anderson SA, Reid W, Elshal MF, Rovira, Nguyen AT, Malide D, Combs CA, Hall G, Zhang J, Raffeld M, Rogers TB, Stetler-Stevenson W, Frank JA, Reitz M, Finkel T. Human mesenchymal stem cells exert potent antitumorigenic effects in a model of Kaposi’s sarcoma. J Exp Med. 2006;203:1235–47. doi: 10.1084/jem.20051921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friedman R, Betancur M, Boissel L, Tuncer H, Cetrulo C, Klingemann H. Umbilical cord mesenchymal stem cells: adjuvants for human cell transplantation. Biol Blood Marrow Transplant. 2007;13:1477–86. doi: 10.1016/j.bbmt.2007.08.048. [DOI] [PubMed] [Google Scholar]
- 23.Aboody KS, Najbauer J, Danks MK. Stem and progenitor cell-mediated tumor selective gene therapy. Gene Ther. 2008;15:739–52. doi: 10.1038/gt.2008.41. [DOI] [PubMed] [Google Scholar]
- 24.Hall B, Andreeff M, Marini F. The participation of mesenchymal stem cells in tumor stroma formation and their application as targeted-gene delivery vehicles. Handb Exp Pharmacol. 2007:263–83. doi: 10.1007/978-3-540-68976-8_12. [DOI] [PubMed] [Google Scholar]
- 25.Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, Verastegui E, Zlotnik A. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–6. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 26.Lee BC, Lee TH, Avraham S, Avraham HK. Involvement of the chemokine receptor CXCR4 and its ligand stromal cell-derived factor 1alpha in breast cancer cell migration through human brain microvascular endothelial cells. Mol Cancer Res. 2004;2:327–38. [PubMed] [Google Scholar]
- 27.Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–63. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 28.Arbab AS, Janic B, Knight RA, Anderson SA, Pawelczyk E, Rad AM, Read EJ, Pandit SD, Frank JA. Detection of migration of locally implanted AC133+ stem cells by cellular magnetic resonance imaging with histological findings. Faseb J. 2008;22:3234–46. doi: 10.1096/fj.07-105676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang SG, Jeun SS, Lim JY, Kim SM, Yang YS, Oh WI, Huh PW, Park CK. Cytotoxicity of human umbilical cord blood-derived mesenchymal stem cells against human malignant glioma cells. Childs Nerv Syst. 2008;24:293–302. doi: 10.1007/s00381-007-0515-2. [DOI] [PubMed] [Google Scholar]
- 30.Larmonier N, Ghiringhelli F, Larmonier CB, Moutet M, Fromentin A, Baulot E, Solary E, Bonnotte B, Martin F. Freshly isolated bone marrow cells induce death of various carcinoma cell lines. Int J Cancer. 2003;107:747–56. doi: 10.1002/ijc.11463. [DOI] [PubMed] [Google Scholar]
- 31.Weiss ML, Medicetty S, Bledsoe AR, Rachakatla RS, Choi M, Merchav S, Luo Y, Rao MS, Velagaleti G, Troyer D. Human umbilical cord matrix stem cells: preliminary characterization and effect of transplantation in a rodent model of Parkinson’s disease. Stem Cells. 2006;24:781–92. doi: 10.1634/stemcells.2005-0330. [DOI] [PubMed] [Google Scholar]
- 32.Weiss ML, Troyer DL. Stem cells in the umbilical cord. Stem Cell Rev. 2006;2:155–62. doi: 10.1007/s12015-006-0022-y. [DOI] [PMC free article] [PubMed] [Google Scholar]