Abstract
We investigated axonal plasticity in the bilateral motor cortices in rats after unilateral stroke and bone marrow stromal cell (BMSC) treatment. Rats were subjected to permanent right middle cerebral artery occlusion followed by intravenous administration of phosphate-buffered saline or BMSCs 1 day later. Adhesive-removal test and modified neurologic severity score were performed weekly to monitor limb functional deficit and recovery. Anterograde tracing with biotinylated dextran amine injected into the right motor cortex was used to assess axonal sprouting in the contralateral motor cortex and ipsilateral rostral forelimb area. Animals were killed 28 days after stroke. Progressive functional recovery was significantly enhanced by BMSCs. Compared with normal animals, axonal density in both contralateral motor cortex and ipsilateral rostral forelimb area significantly increased after stroke. Bone marrow stromal cells markedly enhanced such interhemispheric and intracortical connections. However, labeled transcallosal axons in the corpus callosum were not altered with either stroke or treatment. Both interhemispheric and intracortical axonal sprouting were significantly and highly correlated with behavioral outcome after stroke. This study suggests that, after stroke, cortical neurons surviving in the peri-infarct motor cortex undergo axonal sprouting to restore connections between different cerebral areas. Bone marrow stromal cells enhance axonal plasticity, which may underlie neurologic functional improvement.
Keywords: axonal plasticity, bone marrow stromal cell, middle cerebral artery occlusion, rats
Introduction
Stroke is one of the leading causes of long-term disability in adult humans. After stroke, physiologic plasticity of the motor cortical output area occurs in bilateral cerebral hemispheres, and is significantly correlated with clinical improvement of disability and clinical scores (Corbetta et al, 2002; Swayne et al, 2008). Motor mapping studies in animals also show that increase of cortical movement representations contributes to functional recovery after brain injury, including stroke (Ramanathan et al, 2006). There is compelling evidence that axonal sprouting and rewiring in the adult central nervous system underlie functional plasticity and behavioral recovery after ischemic stroke (Nudo, 2007).
The corpus callosum is the largest white matter structure in the human brain, interconnecting almost exclusively cortical neurons in the homologous regions of the two cerebral hemispheres (Innocenti et al, 1986). The transcallosal connection between motor cortices is mainly inhibitory (Ferbert et al, 1992), which has a critical function in motor control (Duque et al, 2007). Stroke leading to damage in the motor area in one hemisphere can result in abnormal disinhibition that impedes functional recovery (Murase et al, 2004). In addition, the corticospinal tract, the major neural pathway for voluntary motor control, originates from both primary motor cortex (M1) and other premotor areas (Nudo and Masterton, 1990). In rodents, both the caudal forelimb area (CFA) and the rostral forelimb area (RFA) are the origin of the corticospinal tract, associated with forelimb movement (Liang et al, 1993). In both primates and rodents, these separate motor subfields are interconnected (Dancause et al, 2006; Rouiller et al, 1993), and are reorganized physiologically and anatomically after a lesion involving the M1, which may contribute to neurologic recovery after stroke (Dancause, 2006; Liu et al, 2008). However, axonal plasticity of such long distance inter- and intracortical connections between the primary motor area and other premotor areas during stroke recovery has not been morphologically verified.
Bone marrow stromal cells (BMSCs), a mixed cell population including stem cells and progenitor cells, are currently a strong candidate for cell-based therapy in stroke (Chopp et al, 2009). Transplanted BMSCs may facilitate endogenous neurorestorative mechanisms, including neuronal remodeling, such as intracortical axonal rearrangement in the peri-infarct parietal cortex (Li et al, 2005), and increase of axonal density in the ischemic striatum (Shen et al, 2007a) and the denervated side of the cervical spinal gray matter (Liu et al, 2008, 2009), to produce therapeutic benefits after ischemic stroke. This study was carried out to clarify the post-ischemic axonal plasticity of interhemispheric and intracortical connections between different motor areas in a rat middle cerebral artery occlusion (MCAo) model treated with BMSCs, using an anterograde neuronal tracer biotinylated dextran amine (BDA).
Materials and methods
All experimental procedures were approved by the Institutional Animal Care and Use Committee of Henry Ford Hospital.
MCAo Model and BMSC Administration
Adult male Wistar rats (2-month-old, body weight 225 to 250 g) were purchased from Charles River Breeding Company (Wilmington, MA, USA). Permanent focal cerebral ischemia was induced by inserting a 4-0 monofilament nylon suture (18.5 to 19.5 mm, determined by the animal body weight), with its tip rounded by heating near a flame, into the right internal carotid artery to block the origin of the MCAo (Garcia et al, 1993). Bone marrow stromal cells harvested from donor adult male Wistar rats were provided by Theradigm (Baltimore, MD, USA). Briefly, bone marrow from hind leg long bones (femurs and tibiae) was mechanically dissociated and the cells were washed, and then suspended in culture medium. After 3 days, cells tightly adhered to the flasks were considered as BMSCs. Bone marrow stromal cells from passage 5 (P5) were used in this study, which were characterized by expression of mesenchymal cell-surface markers CD29, CD73, and CD90 (>80%), and were free of the hematopoetic cell-surface marker CD34. One day after stroke, animals were randomly selected to receive 3 × 106 BMSCs in 1 mL of phosphate-buffered saline (PBS) or PBS alone injected into a tail vein (n=8 per group). A third group of naive rats without surgery and treatment was used for normal control (n=6).
Behavioral Assessment
The functional deficit and recovery were measured with adhesive-removal test and modified neurologic severity score performed 1 day after MCAo and weekly thereafter. All researchers involved in data collection were masked to the treatment group.
Intracortical Neuronal Labeling
To detect the changes of inter- and intracortical connections originating from the ischemic cortex, we injected BDA into the right cortex to label the cortical neurons 7 days before the kill. Briefly, the animals were placed in a stereotaxic device, and a unilateral craniotomy was performed on the skull overlying the right cerebral cortex. BDA solution (100 nL; a 10% solution dissolved in 0.1 mol/L PBS (pH 7.4), molecular weight 10,000 Da; Molecular Probes, Eugene, OR, USA) was injected through a finely drawn glass capillary into the right CFA (stereotaxic coordinates: 0.5 mm rostral to the bregma, 2.5 mm lateral to the midline, 1.5 mm depth from the surface of the cortex) over a 3-mins time period. The micropipette remained in place for 4 mins after the injection was administered.
Tissue Preparation
After 1 week of BDA injection (4 weeks after stroke), animals were anesthetized with ketamine and perfused transcardially with saline, followed by 4% paraformaldehyde. The entire brain was removed and immersed in 4% paraformaldehyde overnight. The brain tissues were processed for adjacent 100-μm-thick coronal sections using a vibratome. Five sections from the RFA (3.0 to 3.5 mm rostral to the bregma) and 10 sections from the CFA (0 to 1.0 mm rostral to the bregma) were used to detect BDA labeling. Briefly, free-floating sections were incubated with 0.5% H2O2 in 50 mmol/L Tris-buffered saline (pH 7.4) for 20 mins, and washed with Tris-buffered saline containing 0.25% Triton X-100 three times for 30 mins each at room temperature. Then the sections were incubated with avidin–biotin–peroxidase complex (Vector Laboratories, Burlingame, CA, USA) in Tris-buffered saline/Triton X-100 at 4°C for 3 days, and BDA labeling was visualized with 3,3′-diaminobenzidine-nickel (Vector Laboratories) for light microscopy examination.
Data Analysis and Statistics
The behavior outcomes were evaluated by the time needed to successfully remove the adhesive tape from the impaired forepaw and the scores of neurologic severity. Lesion size was measured on a reference brain coronal vibratome section at the bregma level by NIH imaging software (Image J, Bethesda, MD, USA), and presented as a percentage of the lesion area compared with the contralesional hemisphere, as previously reported (Garcia et al, 1993). Digital images were captured using MCID software (Imaging Research, St Catherine's, ON, Canada), through a digital camera (Sony DXC-970MD, Tokyo, Japan) mounted on a microscope (Olympus BX40, Tokyo, Japan). Image J was used for subsequent quantitative measurements of BDA-positive axonal densities in the right RFA, both sides of CFA, and the middle portion of the corpus callosum, shown as percentage of proportional areas. To avoid interanimal variation in the tracing efficiency by a subtle difference of injection volume, we corrected the axonal densities with a quotient coefficient of average BDA-labeling density in the right CFA on 10 sections for each individual animal divided by the total mean density in all animals.
All data are presented as mean±s.d. A value of P<0.05 is taken as significant. A global test using the generalized estimating equation was performed to study the effect of BMSC treatment on functional outcome (Lu et al, 2003). One-way analysis of variance was used to evaluate the lesion size and BDA-labeled axonal densities in the right RFA, left CFA, and the corpus callosum among groups. Correlations between functional scores and index of axonal density were tested by Pearson's correlation coefficients.
Results
Neurologic Functional Assessment and Lesion Size
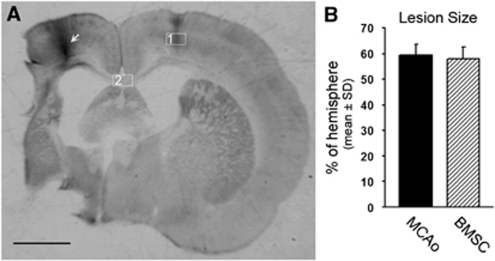
All rats subjected to MCAo exhibited a progressive recovery with time. Bone marrow stromal cell treatment significantly improved the behavioral outcome measured by the adhesive-removal test and modified neurologic severity score (Table 1, P<0.05). However, no significant difference was found on the ischemic lesion, including the supraoptic area, the striatum, and the cortex (Figure 1A) between MCAo control and BMSC treatment groups (Figure 1B; 59.3% versus 57.8%, P=0.48). These data are consistent with previously reported studies (Liu et al, 2008; Lu et al, 2003).
Table 1. Functional outcome in rats after MCAo and BMSC treatment.
| Group | Day 1 | Day 7 | Day 14 | Day 21 | Day 28 |
|---|---|---|---|---|---|
| MCAo | |||||
| Adhesive removal test | 120.0±0 | 103.4±13.4 | 82.0±8.1 | 61.3±13.7 | 41.8±12.4 |
| mNSS | 12.0±0 | 10.3±0.7 | 8.9±0.6 | 7.3±0.7 | 5.6±0.9 |
| BMSC | |||||
| Adhesive removal test | 118.3±4.9 | 92.5±8.3* | 67.8±12.0* | 46.1±9.8† | 30.6±4.5* |
| mNSS | 11.9±0.4 | 10.1±0.4 | 8.1±0.6* | 6.0±0.9† | 4.4±1.1† |
BMSC, bone marrow stromal cell; MCAo, middle cerebral artery occlusion; mNSS, modified neurologic severity score.
Statistical significances were measured using global test.
*P<0.05, †P<0.01.
Figure 1.
Permanent MCAo induced ischemic lesion in the rat brain. (A) A representative rat coronal brain section shows the ischemic lesion 4 weeks after MCAo. An arrow indicates the location of BDA injection in the right cortex. Rectangle fields in the left cortex (1) and corpus callosum (2) indicate the position of the photomicrograph appearing in Figures 2 and 3, respectively. (B) Quantitative data show no statistical difference in the ischemic lesion size between animal groups treated with PBS or BMSCs. Scale bar=2 mm.
Cortical Axonal Connections Between Bilateral Hemispheres
To determine whether neurons in the peri-infarct tissue of the ischemic cortex undergo axonal sprouting toward the homologous area in the intact hemisphere, we injected BDA into the right cortex as shown in Figure 1A. After 7 days of injection, the BDA-positive axons were found in the homologous tissue in the contralateral cortex transported through the corpus callosum (Figure 2A). As measured by the positive staining areas, a significant increase of axonal density in the contralateral cortex was evident in rats 28 days after MCAo (Figures 2C and 2E) compared with that in normal animals (Figures 2B and 2E, P<0.001 versus normal). Furthermore, such axonal plasticity was significantly increased by BMSC treatment 1 day after stroke (Figures 2D and 2E, P<0.001 versus MCAo control).
Figure 2.
Transcallosal axons in the contralateral cortex labeled with BDA intracortical injection. (A) A representative image shows the BDA-positive labeling in a normal rat brain. The BDA solution was injected into the right cortex 7 days before the kill. The BDA-labeled axons in the boxed area in the contralateral CFA were measured as proportional areas, as shown in B for normal, in C for MCAo, and in D for BMSC-treated groups. The axonal density was significantly increased 28 days after MCAo compared with normal animals (C and E). Bone marrow stromal cell administration further enhanced such stroke-induced axonal reorganization (D and E). Scale bar=1 mm in A and 250 μm in B–D.
Axonal Labeling in the Corpus Callosum
To determine whether increased axons in the contralesional left cortex are attributed to axonal regeneration originating from the ischemic cortex, we measured BDA-positive axonal density in the area crossing the midline of the corpus callosum on coronal sections among the normal, MCAo control, and BMSC treatment groups (Figure 3A). Axonal density was essentially unaltered by either MCAo or BMSC treatment (Figure 3B; 3.6% in normal, 3.8% in MCAo, and 3.8% in treatment group, P=0.56). This observation ruled out the possibility of new axonal generation along the corpus callosum between cortical hemispheres.
Figure 3.
Axonal labeling in the corpus callosum. (A) A representative image shows the transcallosal axons in the middle portion of corpus callosum enlarged from the box 2 in Figure 1A. (B) There are no significant differences in axonal quantification among normal animals and ischemic rats with or without BMSC treatment. Scale bar=150 μm.
Intracortical Connections Between the CFA and RFA
To determine axonal plasticity on the intracortical connection between CFA and RFA, we further measured axonal changes in the ipsilesional RFA after stroke and BMSC treatment. Our results indicated a significant increase of axonal density in the RFA traced from CFA in animals after ischemic stroke (Figures 4B and 4D, P<0.001 versus normal), compared with normal rats (Figure 4A). In addition, BMSC treatment further enhanced the axonal plasticity induced by MCAo lesion (Figures 4C and 4D, P<0.01 versus control).
Figure 4.
Coronal sections showing intracortical axonal connections in the right RFA originating from the ipsilateral CFA. In normal rats, few axons were detected in the cortex at the RFA level with BDA injection into the cortex of the CFA (A). Biotinylated dextran amine-positive axons were increased in the ischemic animals treated with PBS (B) and BMSCs (C). Quantitative data showed a significant enhancing effect on both MCAo lesion and BMSC treatment, respectively (D). Insets in A, schematic drawing of the rat brain dorsal view showing the RFA region from which slices were obtained (lines) and BDA-injection position in the CFA (dot). Cross-hatching indicates the ischemic lesion. Scale bar=250 μm.
Relating Axonal Plasticity to Behavioral Outcome
Correlations were examined between axonal density and functional outcome measured 28 days after stroke in adult rats with or without BMSC treatment. Axonal plasticity in both CFA and RFA was highly and significantly correlated with spontaneous functional recovery and recovery enhanced by BMSCs (r>0.61, P<0.05; the complete set of correlation coefficients is given in Table 2).
Table 2. Correlation coefficients between behavioral scores and axonal plasticity at 28 days after MCAo.
| Functional tests | Axonal density in contralesional CFA | Axonal density in ipsilateral RFA |
|---|---|---|
| Adhesive | r=0.89 | r=0.80 |
| removal test | P<0.001 | P<0.001 |
| mNSS | r=0.77 | r=0.61 |
| P<0.001 | P<0.05 |
CFA, caudal forelimb area; mNSS, modified neurologic severity score; RFA, rostral forelimb area.
Discussion
This study was focused on determination of the extent and distribution of axonal sprouting between cortical hemispheres and the CFA and RFA in adult rats after stroke with BMSC treatment. We found that both interhemispheric and intracortical axonal connections in the CFA and RFA originating from the peri-infarct motor cortex were significantly increased 4 weeks after stroke. Bone marrow stromal cell administration further enhanced such axonal plasticity. The structural plasticity was highly correlated with functional recovery. Although other studies have shown axonal sprouting from the contralesional cortex to the ipsilesional striatum and ischemic penumbra (Carmichael and Chesselet, 2002; Napieralski et al, 1996), to our knowledge, this is the first demonstration of axonal connection originating from the lesioned motor cortex to the distant cerebral motor areas that appear to contribute to functional recovery. These data also provide an anatomical mechanism underlying the BMSC-induced functional improvement in ischemic stroke. We have shown that BMSC therapy significantly improves functional outcome after stroke in female young adult (Li et al, 2006) and aged rodents (Shen et al, 2007a, 2007b). Although stroke is primarily a disease of the aged population, as a proof-of-principle study, our finding of axonal plasticity in young adult ischemic rats suggests that neuronal rewiring is a potential treatment target to improve neurologic outcome after ischemic stroke and other central nervous system diseases.
Compared with low-molecular-weight BDA (3 kDa), the high-molecular-weight BDA (10 kDa) used in this study is taken up mostly by the somata of the neurons and transported anterogradely to the axon terminals, to yield sensitive and exquisitely detailed labeling of axons and terminals (Reiner et al, 2000). Indeed, only few neurons in the contralateral cortex or ipsilateral RFA were retrogradely labeled to the cell bodies (data not shown). Therefore, the BDA-positive axons in the contralesional cortex or ipsilateral RFA originate from the labeled neurons at the injection site in the ischemic CFA. Thus, our observation of increased axonal density after stroke indicates that neurons residing in the peri-lesion area increase their ability for axonal outgrowth in response to the ischemic lesion and BMSC treatment. However, the central nervous system in adult mammals is a highly inhibitory environment for axonal regeneration. From measurement of axonal density in the corpus callosum, our data showed that neither the ischemic lesion nor BMSC treatment altered the density of BDA-labeled transcallosal axons originating from the peri-infarct cortical area. This suggests that there were no additional axons in the corpus callosum generated through axonal regeneration from the ipsilesional motor cortex. Therefore, the increased axons after stroke likely derived from local axonal sprouting or branching within the contralesional cortex.
Normally, the motor cortices maintain balance through transcallosal inhibition through the interhemispheric projections (Ferbert et al, 1992; Perez and Cohen, 2008). In the early stage after stroke, interhemispheric inhibition is decreased (Shimizu et al, 2002), which leads to increased cortical excitability in the intact hemisphere (Grefkes et al, 2008; Marshall et al, 2000). In addition, an abnormally high interhemispheric inhibition deriving from the contralesional M1 may occur due to the contralesional cortical overactivity, which in turn further inhibits the ipsilesional motor cortex in stroke patients (Duque et al, 2005; Takeuchi et al, 2005), thereby leading to poor motor recovery (Murase et al, 2004). In contrast, decreasing excitability in the contralesional motor cortex by repetitive transcranial magnetic stimulation caused an improved motor performance of the affected hand by releasing the transcallosal inhibition (Takeuchi et al, 2005). Moreover, a progressive rebalancing from early contralesional activity to late ipsilesional activity occurs in the stroke patients with good functional recovery (Marshall et al, 2000). Our finding of increased axonal density in the contralesional cortex in animals suggests an anatomical neuronal substrate of change in transcallosal inhibition in stroke patients. Although the corpus callosum has been thought to be a major pathway for cell migration, this tissue was apparently not amenable to BMSC-enhanced axonal plasticity. Our data show that there are no additional regenerating axons crossing the corpus callosum from the ipsilesional cortex. This is consistent with the hypothesis that the BMSCs acting directly on the cell body of neurons surviving in the peri-infarct cortical area promote neurite outgrowth in the contralesional cortex to extend their innervating arbors, possibly to restore the interhemispheric connections lost after stroke. This may facilitate recovery of the interhemispheric disinhibition derived from the lesioned hemisphere and the overinhibition derived from the contralesional hemisphere, which subsequently enhance the ipsilesional neuronal activity. Our demonstration that axonal plasticity was highly correlated with behavioral outcome in rats after MCAo with BMSC treatment suggests that the increase of the interhemispheric and intracortical connections may, at least in part, contribute to neurologic recovery.
Nonprimary motor areas in the human frontal lobe are connected directly to hand muscles (Teitti et al, 2008). Intracortical excitability has an important function in regulating motor output to facilitate functional recovery during the late stage after stroke (Newton et al, 2006; Swayne et al, 2008). In rat, a focal cortical lesion in the CFA causes a significant expansion in the ipsilateral RFA (Conner et al, 2005). A secondary lesion targeting the RFA completely disrupts functional recovery (Conner et al, 2005). This study provided the first anatomical evidence showing increased axonal connections originating from the lesioned CFA to the distant ipsilateral RFA in response to ischemic stroke and BMSC treatment. This intracortical axonal plasticity may be attributed to localized axonal sprouting from the surviving neurons in the peri-infarct areas extending their innervating arbors, to take over the lost innervation from neurons damaged in the ischemia, perhaps similar to our observations of interhemispheric connections. However, due to the technical limitations we are unable to individually identify the physiologic function of these axons in vivo.
There is increasing evidence of axonal plasticity in the adult central nervous system to rewire the denervated tissue that leads to spontaneous, however, limited functional recovery after stroke (Nudo, 2007). In the initial week after stroke, functional recovery may be attributable to the resolution of brain edema, absorption of damaged tissue, or reperfusion of the ischemic penumbra. Our previous study suggests that in rodents, functional recovery is highly correlated with corticospinal tract axonal plasticity at 4 weeks, but not 1 week after MCAo (Liu et al, 2009). Ischemic lesion induces axonal sprouting and promotes waves of specific cellular and molecular events (Carmichael, 2006) that could lead to partial functional recovery. Bone marrow stromal cells transplanted into ischemic brain secrete an array of neurotrophins and growth factors, including brain-derived neurotrophic factor (Dormady et al, 2001; Li et al, 2002) and vascular endothelial growth factor (Chen et al, 2003; Hamano et al, 2000). Most importantly, BMSCs can activate brain parenchymal cells, especially glial cells, which further produce neurotrophins, growth factors, and other restorative agents to amplify brain plasticity and subsequently to improve in neurologic function after stroke (see a review by Chopp et al (2009)). We have shown that after a vascular administration in a rodent model of stroke, most BMSCs localize to the ischemic boundary zone (Shen et al, 2007b). Therefore, the axonal sprouting ability of neurons surviving in the peri-infarct cortical area may be effectively enhanced by such neurotrophic factors. Our data also suggest that BMSCs reduce neurocan, an axonal extension inhibitory molecule, in the glial scar, a primarily astrocytic structure bordering the infarct tissue that inhibits axonal regeneration after stroke (Shen et al, 2008). Thus, BMSC-mediated reduction of this and possibly other inhibitory molecules may contribute to observed axonal sprouting. This study provides a direct morphologic evidence of axonal plasticity to reveal potential mechanisms of underlying spontaneous recovery in rodent experimental stroke and BMSC treatment. However, the detailed molecular mechanisms on the spatiotemporal interaction between the BMSCs and the neurons and/or glial cells warrant further investigation.
Summary
This study shows reactive inter- and intracortical axonal plasticity originating from the ipsilesional motor cortex in adult rats after stroke and treatment with BMSCs, which may underlie both spontaneous and BMSC-amplified functional recovery from stroke.
The authors declare no conflict of interest.
References
- Carmichael ST. Cellular and molecular mechanisms of neural repair after stroke: making waves. Ann Neurol. 2006;59:735–742. doi: 10.1002/ana.20845. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Chesselet MF. Synchronous neuronal activity is a signal for axonal sprouting after cortical lesions in the adult. J Neurosci. 2002;22:6062–6070. doi: 10.1523/JNEUROSCI.22-14-06062.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Zhang ZG, Li Y, Wang L, Xu YX, Gautam SC, Lu M, Zhu Z, Chopp M. Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circ Res. 2003;92:692–699. doi: 10.1161/01.RES.0000063425.51108.8D. [DOI] [PubMed] [Google Scholar]
- Chopp M, Li Y, Zhang ZG. Mechanisms underlying improved recovery of neurological function after stroke in the rodent after treatment with neurorestorative cell-based therapies. Stroke. 2009;40:S143–S145. doi: 10.1161/STROKEAHA.108.533141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner JM, Chiba AA, Tuszynski MH. The basal forebrain cholinergic system is essential for cortical plasticity and functional recovery following brain injury. Neuron. 2005;46:173–179. doi: 10.1016/j.neuron.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Burton H, Sinclair RJ, Conturo TE, Akbudak E, McDonald JW. Functional reorganization and stability of somatosensory-motor cortical topography in a tetraplegic subject with late recovery. Proc Natl Acad Sci USA. 2002;99:17066–17071. doi: 10.1073/pnas.262669099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancause N. Vicarious function of remote cortex following stroke: recent evidence from human and animal studies. Neuroscientist. 2006;12:489–499. doi: 10.1177/1073858406292782. [DOI] [PubMed] [Google Scholar]
- Dancause N, Barbay S, Frost SB, Plautz EJ, Stowe AM, Friel KM, Nudo RJ. Ipsilateral connections of the ventral premotor cortex in a new world primate. J Comp Neurol. 2006;495:374–390. doi: 10.1002/cne.20875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dormady SP, Bashayan O, Dougherty R, Zhang XM, Basch RS. Immortalized multipotential mesenchymal cells and the hematopoietic microenvironment. J Hematother Stem Cell Res. 2001;10:125–140. doi: 10.1089/152581601750098372. [DOI] [PubMed] [Google Scholar]
- Duque J, Hummel F, Celnik P, Murase N, Mazzocchio R, Cohen LG. Transcallosal inhibition in chronic subcortical stroke. Neuroimage. 2005;28:940–946. doi: 10.1016/j.neuroimage.2005.06.033. [DOI] [PubMed] [Google Scholar]
- Duque J, Murase N, Celnik P, Hummel F, Harris-Love M, Mazzocchio R, Olivier E, Cohen LG. Intermanual differences in movement-related interhemispheric inhibition. J Cogn Neurosci. 2007;19:204–213. doi: 10.1162/jocn.2007.19.2.204. [DOI] [PubMed] [Google Scholar]
- Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD. Interhemispheric inhibition of the human motor cortex. J Physiol. 1992;453:525–546. doi: 10.1113/jphysiol.1992.sp019243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia JH, Yoshida Y, Chen H, Li Y, Zhang ZG, Lian J, Chen S, Chopp M. Progression from ischemic injury to infarct following middle cerebral artery occlusion in the rat. Am J Pathol. 1993;142:623–635. [PMC free article] [PubMed] [Google Scholar]
- Grefkes C, Nowak DA, Eickhoff SB, Dafotakis M, Kust J, Karbe H, Fink GR. Cortical connectivity after subcortical stroke assessed with functional magnetic resonance imaging. Ann Neurol. 2008;63:236–246. doi: 10.1002/ana.21228. [DOI] [PubMed] [Google Scholar]
- Hamano K, Li TS, Kobayashi T, Kobayashi S, Matsuzaki M, Esato K. Angiogenesis induced by the implantation of self-bone marrow cells: a new material for therapeutic angiogenesis. Cell Transplant. 2000;9:439–443. doi: 10.1177/096368970000900315. [DOI] [PubMed] [Google Scholar]
- Innocenti GM, Clarke S, Kraftsik R. Interchange of callosal and association projections in the developing visual cortex. J Neurosci. 1986;6:1384–1409. doi: 10.1523/JNEUROSCI.06-05-01384.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Chen J, Chen XG, Wang L, Gautam SC, Xu YX, Katakowski M, Zhang LJ, Lu M, Janakiraman N, Chopp M. Human marrow stromal cell therapy for stroke in rat: neurotrophins and functional recovery. Neurology. 2002;59:514–523. doi: 10.1212/wnl.59.4.514. [DOI] [PubMed] [Google Scholar]
- Li Y, Chen J, Zhang CL, Wang L, Lu D, Katakowski M, Gao Q, Shen LH, Zhang J, Lu M, Chopp M. Gliosis and brain remodeling after treatment of stroke in rats with marrow stromal cells. Glia. 2005;49:407–417. doi: 10.1002/glia.20126. [DOI] [PubMed] [Google Scholar]
- Li Y, McIntosh K, Chen J, Zhang C, Gao Q, Borneman J, Raginski K, Mitchell J, Shen L, Zhang J, Lu D, Chopp M. Allogeneic bone marrow stromal cells promote glial-axonal remodeling without immunologic sensitization after stroke in rats. Exp Neurol. 2006;198:313–325. doi: 10.1016/j.expneurol.2005.11.029. [DOI] [PubMed] [Google Scholar]
- Liang F, Rouiller EM, Wiesendanger M. Modulation of sustained electromyographic activity by single intracortical microstimuli: comparison of two forelimb motor cortical areas of the rat. Somatosens Mot Res. 1993;10:51–61. doi: 10.3109/08990229309028823. [DOI] [PubMed] [Google Scholar]
- Liu Z, Li Y, Zhang X, Savant-Bhonsale S, Chopp M. Contralesional axonal remodeling of the corticospinal system in adult rats following stroke and bone marrow stromal cell treatment. Stroke. 2008;39:2571–2577. doi: 10.1161/STROKEAHA.107.511659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Zhang RL, Li Y, Cui Y, Chopp M. Remodeling of the corticospinal innervation and spontaneous behavioral recovery after ischemic stroke in adult mice. Stroke. 2009;40:2546–2551. doi: 10.1161/STROKEAHA.109.547265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M, Chen J, Lu D, Yi L, Mahmood A, Chopp M. Global test statistics for treatment effect of stroke and traumatic brain injury in rats with administration of bone marrow stromal cells. J Neurosci Methods. 2003;128:183–190. doi: 10.1016/s0165-0270(03)00188-2. [DOI] [PubMed] [Google Scholar]
- Marshall RS, Perera GM, Lazar RM, Krakauer JW, Constantine RC, DeLaPaz RL. Evolution of cortical activation during recovery from corticospinal tract infarction. Stroke. 2000;31:656–661. doi: 10.1161/01.str.31.3.656. [DOI] [PubMed] [Google Scholar]
- Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol. 2004;55:400–409. doi: 10.1002/ana.10848. [DOI] [PubMed] [Google Scholar]
- Napieralski JA, Butler AK, Chesselet MF. Anatomical and functional evidence for lesion-specific sprouting of corticostriatal input in the adult rat. J Comp Neurol. 1996;373:484–497. doi: 10.1002/(SICI)1096-9861(19960930)373:4<484::AID-CNE2>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Newton JM, Ward NS, Parker GJ, Deichmann R, Alexander DC, Friston KJ, Frackowiak RS. Non-invasive mapping of corticofugal fibres from multiple motor areas—relevance to stroke recovery. Brain. 2006;129:1844–1858. doi: 10.1093/brain/awl106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudo RJ. Postinfarct cortical plasticity and behavioral recovery. Stroke. 2007;38:840–845. doi: 10.1161/01.STR.0000247943.12887.d2. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Masterton RB. Descending pathways to the spinal cord, III: sites of origin of the corticospinal tract. J Comp Neurol. 1990;296:559–583. doi: 10.1002/cne.902960405. [DOI] [PubMed] [Google Scholar]
- Perez MA, Cohen LG. Mechanisms underlying functional changes in the primary motor cortex ipsilateral to an active hand. J Neurosci. 2008;28:5631–5640. doi: 10.1523/JNEUROSCI.0093-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan D, Conner JM, Tuszynski MH. A form of motor cortical plasticity that correlates with recovery of function after brain injury. Proc Natl Acad Sci USA. 2006;103:11370–11375. doi: 10.1073/pnas.0601065103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner A, Veenman CL, Medina L, Jiao Y, Del Mar N, Honig MG. Pathway tracing using biotinylated dextran amines. J Neurosci Methods. 2000;103:23–37. doi: 10.1016/s0165-0270(00)00293-4. [DOI] [PubMed] [Google Scholar]
- Rouiller EM, Moret V, Liang F. Comparison of the connectional properties of the two forelimb areas of the rat sensorimotor cortex: support for the presence of a premotor or supplementary motor cortical area. Somatosens Mot Res. 1993;10:269–289. doi: 10.3109/08990229309028837. [DOI] [PubMed] [Google Scholar]
- Shen LH, Li Y, Chen J, Cui Y, Zhang C, Kapke A, Lu M, Savant-Bhonsale S, Chopp M. One-year follow-up after bone marrow stromal cell treatment in middle-aged female rats with stroke. Stroke. 2007a;38:2150–2156. doi: 10.1161/STROKEAHA.106.481218. [DOI] [PubMed] [Google Scholar]
- Shen LH, Li Y, Chen J, Zacharek A, Gao Q, Kapke A, Lu M, Raginski K, Vanguri P, Smith A, Chopp M. Therapeutic benefit of bone marrow stromal cells administered 1 month after stroke. J Cereb Blood Flow Metab. 2007b;27:6–13. doi: 10.1038/sj.jcbfm.9600311. [DOI] [PubMed] [Google Scholar]
- Shen LH, Li Y, Gao Q, Savant-Bhonsale S, Chopp M. Down-regulation of neurocan expression in reactive astrocytes promotes axonal regeneration and facilitates the neurorestorative effects of bone marrow stromal cells in the ischemic rat brain. Glia. 2008;56:1747–1754. doi: 10.1002/glia.20722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T, Hosaki A, Hino T, Sato M, Komori T, Hirai S, Rossini PM. Motor cortical disinhibition in the unaffected hemisphere after unilateral cortical stroke. Brain. 2002;125:1896–1907. doi: 10.1093/brain/awf183. [DOI] [PubMed] [Google Scholar]
- Swayne OB, Rothwell JC, Ward NS, Greenwood RJ. Stages of motor output reorganization after hemispheric stroke suggested by longitudinal studies of cortical physiology. Cereb Cortex. 2008;18:1909–1922. doi: 10.1093/cercor/bhm218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi N, Chuma T, Matsuo Y, Watanabe I, Ikoma K. Repetitive transcranial magnetic stimulation of contralesional primary motor cortex improves hand function after stroke. Stroke. 2005;36:2681–2686. doi: 10.1161/01.STR.0000189658.51972.34. [DOI] [PubMed] [Google Scholar]
- Teitti S, Maatta S, Saisanen L, Kononen M, Vanninen R, Hannula H, Mervaala E, Karhu J. Non-primary motor areas in the human frontal lobe are connected directly to hand muscles. Neuroimage. 2008;40:1243–1250. doi: 10.1016/j.neuroimage.2007.12.065. [DOI] [PubMed] [Google Scholar]