Abstract
Dysregulated signal transduction via the notch pathway has been noted in human and mouse medulloblastoma studies. Gamma secretase inhibitors impair notch signaling by preventing the cleavage of transmembrane notch proteins into their active intracellular domain fragments. Previous studies have demonstrated that gamma secretase inhibitor treatment caused apoptosis and impaired medulloblastoma cell engraftment in xenograft systems. In this study, we used in vivo genetic and pharmacologic approaches to quantify the contribution of notch signaling to sonic hedgehog (shh)-activated mouse medulloblastoma models. In contrast to prior in vitro studies, pharmacologic inhibition of notch pathways did not reduce the efficiency of medulloblastoma xenotransplantation nor did systemic therapy impact tumor size, proliferation or apoptosis in genetically engineered mouse medulloblastoma models. The incidence and pathology of medulloblastomas driven by the SmoA1 transgene was unchanged by the bi-allelic absence of Notch1, Notch2 or Hes5 genes. These data demonstrate that notch signaling is not essential for the initiation, engraftment, or maintenance of sonic hedgehog pathway driven medulloblastomas.
Keywords: medulloblastoma, Notch, sonic hedgehog, gamma secretase inhibitor
Medulloblastoma is the most common malignant brain tumor in children. One third of patients do not survive despite aggressive therapies (Gilbertson and Ellison, 2008; Zeltzer et al., 1999). While the remainder of children are cured using a combination of surgery, radiation, and chemotherapy, serious side effects resulting from treatment frequently occur. To identify alternative, less toxic therapies, investigators have focused on signaling pathways involved in brain development and medulloblastoma genesis such as the sonic hedgehog (shh) and notch pathways. Unfortunately, in vitro data from both of these pathways is unreliable because proper functioning of each relies on ligand gradients, cell-cell interactions, and the native microenvironment, all of which are absent or variable in monolayer tissue culture. The current study was conducted to assess the in vivo relevance of notch pathway inhibition in medulloblastoma.
Medulloblastomas are thought to arise from progenitor cells in the cerebellum. Normal cerebellar development requires an intricate signal transduction network that includes shh, notch, wnt, BMP, and PI3K signaling. Disruption of normal signaling is a frequent finding in medulloblastoma (Gilbertson and Ellison, 2008). Shh drives cellular proliferation in the cerebellum, while notch signaling promotes a stem-like state in some cells (Eberhart, 2007). Abnormal activation of the shh pathway is sufficient to induce medulloblastomas in mice as a result of ectopic shh expression, inactivation of the Patched1 (Ptch1) tumor suppressor gene, or expression of an activated form of the Smoothened (Smo) proto-oncogene (Goodrich et al., 1997; Hallahan et al., 2004; Hatton et al., 2008; Weiner et al., 2002). Tumors in these mice display molecular profiles that resemble shh-driven human medulloblastomas (Hallahan et al., 2004; Pomeroy et al., 2002). These mouse models have become the gold standard for in vivo preclinical medulloblastoma studies.
Several lines of evidence have linked notch signaling to medulloblastoma engraftment and progression. Notch pathways are upregulated in medulloblastoma and increased expression of HES1, a target of both the canonical notch pathway and the non-canonical shh pathway, is associated with poor prognosis in medulloblastoma patients (Fan et al., 2004; Hallahan et al., 2004; Ingram et al., 2008). Notch2 and Hes5 are overexpressed in the shh-activated SmoA1 mouse, suggesting that activation of the shh pathway is sufficient to induce notch pathway genes (Hallahan et al., 2004). The gamma secretase inhibitor (GSI) DAPT (N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester) induces apoptotic cell death in medulloblastoma cells in vitro (Hallahan et al., 2004). GSI-18 administered to DAOY cells in vitro inhibits their engraftment as flank xenografts in nude mice, which has been interpreted to indicate that notch signaling is necessary for maintenance of medulloblastoma stem cells (Fan et al., 2006). Taken together, these findings conducted on monolayer medulloblastoma cell cultures suggested a central role for notch signaling in medulloblastoma and led to initiation of a national clinical trial of a GSI in children with medulloblastoma. Unfortunately, the comprehensive studies reported here and in a companion manuscript (Julian et al., “Canonical notch signaling is not required for the growth of hedgehog pathway induced medulloblastoma”) clearly demonstrate that notch signaling is dispensable in medulloblastomas driven by shh in genetically precise mouse models.
Results and Discussion
We explored whether notch inhibition prevented medulloblastoma engraftment using the gamma secretase inhibitors DAPT (GSI-IX) and MRK-003 (Merck Research Laboratories), which abrogate signaling via all four mammalian notch genes (Sparey et al., 2005). Gamma secretase inhibitors were originally developed to modulate the production of amyloid-β peptide (Aβ) in the brains of Alzheimer's patients, a process that involves cleavage by the gamma secretase protease. Further analysis of these compounds revealed strong in vivo potency within the brain, demonstrated by a 50% reduction in Aβ peptide and a greater than 2:1 ratio of drug levels between brain and plasma (Lewis et al., 2005), providing further motivation to study the efficacy of these compounds in treating human brain tumor patients. Human DAOY medulloblastoma cells were treated with vehicle, DAPT, or MRK-003 in vitro, and the treated cells were injected subcutaneously in the flanks of nude (nu/nu) mice. We designed our experiment with approximately 90% power to detect at least a 50% difference in tumor incidence (two-sided binomial test with alpha=0.05), based on previous findings that notch pathway inhibition reduced xenograft formation in GSI-18 treated DAOY cells (Fan et al. 2006). After 8 weeks, tumors arose in 16 of 20 vehicle-treated xenografts, 13 of 19 of the DAPT-treated xenografts, and 12 of 20 of the MRK-003 treated xenografts (Figure 1a). Thus, in our experiments transient notch inhibition in vitro does not interfere with engraftment (Figure 1a; DAPT p=0.48, MRK-003 p=0.3) or tumor volume (data not shown) in DAOY medulloblastoma xenografts. Analysis of HES1 mRNA levels in DAOY cells indicated decreased expression in response to MRK-003 treatment (Supplementary Figure 1). It is not fully understood why these engraftment studies failed to recapitulate similar work demonstrating a role for notch in flank xenograft engraftment (Fan et al., 2006), but differences between gamma secretase inhibitors including “off target” activity, differences in DAOY culturing conditions, or statistical differences related to the number of replicates may have played a role.
Figure 1. Gamma secretase inhibitors do not affect the engraftment, maintenance, or progression of medulloblastoma xenografts.
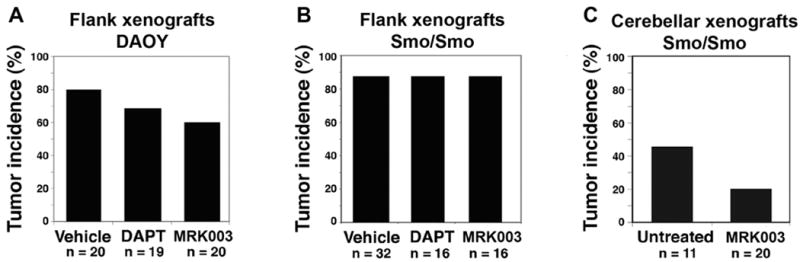
(1a) DAOY cells treated with vehicle, DAPT, or MRK-003 did not significantly differ in their ability to form flank xenografts in nu/nu mice. 1 × 106 human DAOY medulloblastoma cells were seeded into 10 cm plates and incubated for 48 hours with vehicle (0.1% DMSO) or 2 μM MRK-003 (Merck Research Laboratories) or DAPT (GSI-IX, Calbiochem). Five nude mice per arm received four subcutaneous flank injections of 1 × 106 treated cells each mixed 1:1 with Matrigel. Tumors were measured with calipers. Tumors were removed, formalin fixed, paraffin embedded, and stained with hematoxalin and eosin (H&E). (1b) Smo/Smo mice with symptomatic medulloblastomas were then treated systemically with vehicle, DAPT, or MRK-003, removed and placed as flank xenografts. Two Smo/Smo mice with symptomatic tumors per arm were treated once daily for three days with MRK-003 (100 mg/kg/dose oral gavage in 0.5% methylcellulose), DAPT (100 mg/kg/dose intraperitoneally in DMSO), or vehicles (methylcellulose, DMSO). Donor mice were sacrificed one hour after the third dose of drug. Tumors within each group were pooled, then placed as flank xenografts and measured as above. (1c) Similarly, MRK-003 did not affect the incidence of Smo/Smo tumor engraftment in a Smo/Smo cerebellar xenograft system. Eight Smo/Smo mice with symptomatic medulloblastomas were randomized to receive either no treatment (n=3) or two doses of MRK-003 (300 mg/kg/dose in 0.5% methylcellulose by gavage on study days 1 and 4, n = 5). Donors were sacrificed on study day 8. Tumors were triturated, pooled within treatment groups, and resuspended in PBS. 106 tumor cells in 15 μL PBS were stereotactically injected into the right cerebellar hemisphere of recipient mice. Each tumor was transplanted into two nu/nu and two syngeneic C57BL/6 recipients. Asymptomatic mice were observed for six months, while mice with progressive tumors were euthanized. Brains were formalin-fixed, paraffin-embedded, sectioned, stained with hematoxalin and eosin, and scored for the presence or absence of cerebellar tumors. A binomial test (alpha=0.05) had 80% power to detect a 50% decrease in clinical tumor incidence. All p values were calculated using Fisher's exact test.
We then asked whether in vivo notch blockade affected the engraftment, maintenance, or progression of primary Smo/Smo genetically engineered mouse tumors in a flank xenograft system. The Smo/Smo mice possess the ND2:SmoA1 transgene, which contains a mutation in the Smoothened receptor gene that leads to constitutively activated shh pathway signaling within the cerebellum and a high incidence of medulloblastoma (Hatton et al., 2008). Shh pathway activity was previously found to be suppressed when medulloblastomas were cultured in vitro (Sasai et al., 2006) and cell-cell contact is also critical for notch pathway activity (Fortini, 2009), so we chose to determine whether notch pathway inhibition within intact tumors could limit the engraftment of medulloblastoma cells from our Smo/Smo model. Smo/Smo mice with brain tumors were treated with vehicle (n=32), DAPT (n=16), or MRK-003 (n=16) for three days, after which the treated medulloblastoma cells were collected and injected into the flanks of nu/nu mice. GSI dosing was slightly elevated above levels previously shown to inhibit amyloid-beta by 50% in the brains of a genetically engineered Alzheimer's disease model (Lewis et al., 2005), and the study was designed with 90% power to detect a 50% decrease in tumor incidence (two-sided binomial test with alpha=0.05). Six weeks after injections, the tumor incidence was 87.5% in each group (Figure 1b) and tumor volumes were unaffected by GSI (data not shown).
We additionally assessed whether or not the cerebellar microenvironment contributes to notch-mediated tumor engraftment. Medulloblastoma cells were isolated from Smo/Smo mice receiving either MRK-003 or no treatment. Tumor cells were triturated and then stereotactically injected into the cerebella of nude and syngeneic recipient mice. Five of 11 recipients in the control group developed tumors, and 4 out of 20 recipients in the MRK-003 group developed tumors. Tumors arose with approximately equal frequency in nude and syngeneic Smo/Smo tumor transplant recipients. Treatment with MRK-003 did not significantly alter the tumor incidence (Figure 1c, p=0.22; two-sided Fisher's exact test), though there was a trend toward fewer tumors in the MRK-003 treated group. In contrast to previous reports (Fan et al., 2006), we found that pharmacologic notch inhibition does not affect the engraftment, maintenance, or progression of medulloblastoma xenografts.
While xenograft studies are informative, a preferred model system is an autochthonous mouse model in which medulloblastomas arise spontaneously as a result of a single genetic mutation, within the microenvironment of the cerebellum where cell-cell contacts are maintained. Hence, we conducted a trial to determine whether MRK-003 impacts the spontaneous rate of medulloblastoma formation, tumor size, apoptotic rate, or proliferative index in the Smo/Smo mouse model (Hatton et al., 2008). MRK-003 was selected for its ability to penetrate into the brain, with previous studies demonstrating 100mg/kg doses resulting in concentrations above 10uM in the brains of AAP-YAC mice, which were generated on a C57Bl/6 background, as were the Smo/Smo mice (Lewis et al., 2005). Mice were treated for 28 days with either MRK-003 or vehicle during a critical window in which most mice develop tumors, and then sacrificed for histopathological analysis. Given the specified sample sizes, a two-sided binomial test (alpha=0.05) had approximately 80% power to detect a 50% decrease in clinical tumor incidence; however, there was no significant difference in the incidence of clinical or subclinical tumors between vehicle and MRK-003 treated mice (Figure 2a, 57% versus 69%, p=0.7). Similarly, there was no difference in tumor size (Figure 2b), apoptotic rate (Figure 2c), or cellular proliferation (Figure 2d) between vehicle and MRK-003 treated mice.
Figure 2. Inactivation of notch signaling with MRK-003 does not affect Smo/Smo mouse medulloblastoma incidence, tumor size, or apoptotic rate.
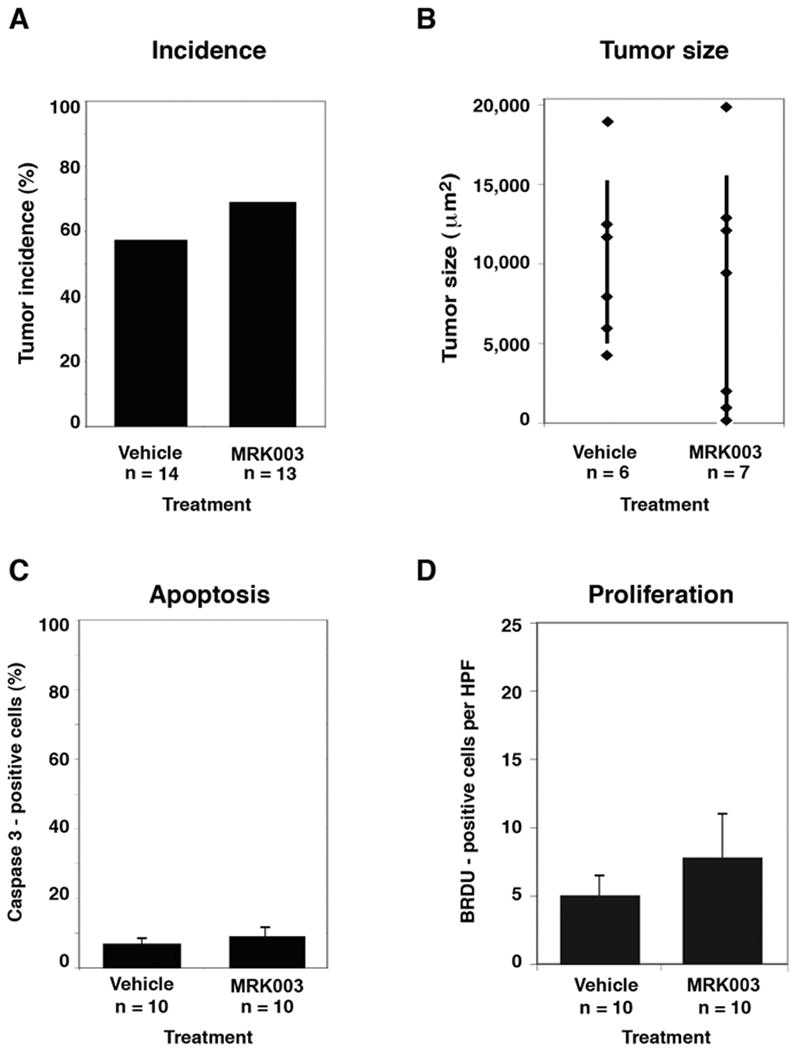
(2a) Histological examination revealed no difference in tumor incidence in Smo/Smo mice treated with vehicle or MRK-003. Smo/Smo mice without clinical evidence of tumors were enrolled at 28 days of age, at which time 90% of mice have subclinical medulloblastomas (Hatton et al., 2008). Smo/Smo mice were randomized to receive either MRK-003 (Merck Research Laboratories, 100 mg/kg/dose) or vehicle (0.5% methylcellulose) by oral gavage. In a dosing schema optimized to limit gastrointestinal toxicity, mice received four week-long cycles of therapy consisting of once daily drug for 3 days, followed by 4 days without drug. (2b) There was no difference in tumor size between vehicle and MRK-003 treated mice. (2c) Mice treated with MRK-003 showed no significant difference in the amount of apoptosis in tumors compared with controls. Quantitative apoptosis was measured by Caspase 3 immunohistochemical staining (Biocare Medical, Concord, CA). Error bars represent average +/- SEM. (2d) No significant differences were detected in the amount of cellular proliferation in MRK-003 treated tumors versus vehicle-treated tumors. On day 28, all mice received one intraperitoneal dose of bromodeoxyuridine (BRDU, 0.1 mg/kg) one hour before sacrifice, in order to mark proliferating cells. Error bars represent average +/- SEM and p values were calculated using Fisher's exact test except for tumor size where p values were calculated with a two-sample t-test.
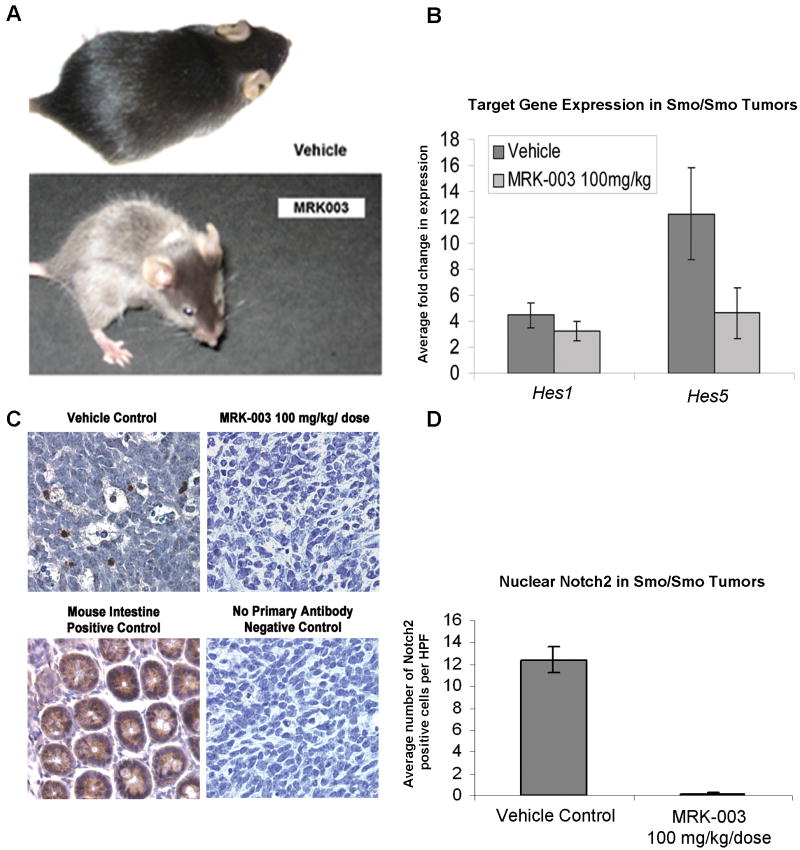
Using GSI dosing levels that were previously shown to have high in vivo efficacy within the brain, we found additional evidence that MRK-003 was clearly hitting target in our in vivo studies. Mice treated with MRK-003 developed gray hair and their whisker color oscillated between black and gray in concordance with each cycle of MRK-003 (Figure 3a), phenotypes previously attributed to notch blockade (Schouwey and Beermann, 2008). In our study, autoregulated Notch1 and Notch2 protein expression were diminished in MRK-003 treated Smo/Smo tumors (Figure 3c-d and data not shown). Additionally, the expression of notch target gene Hes5 was reduced in the tumors from MRK-003 treated mice (Figure 3b, p=0.04). Interestingly, the canonical notch target gene Hes1 was not downregulated by MRK-003 in Smo/Smo tumors (Figures 3b, p=0.17). A likely explanation is that Hes1 is both a canonical notch target and a non-canonical shh target gene, whereas Notch1, Notch2 and Hes5 are targets of notch signaling but perhaps not shh signaling. In the setting of chronic shh pathway activation in the Smo/Smo cerebellum, gamma secretase inhibitor treatment alters Notch1, Notch2 and Hes5 expression but is unable to affect shh-mediated Hes1 expression.
Figure 3. Analysis of MRK-003 on-target effects.
(3a) Smo/Smo mice maintained on a C57Bl/6 background that received 100 mg/kg/dose of MRK-003 by oral gavage developed gray hair and striped whiskers, while mice treated with enteral vehicle remained black. Hair graying is a phenotype caused by notch inhibition (Schouwey and Beermann, 2008). (3b) The canonical notch target gene Hes5 was downregulated by MRK-003 in Smo/Smo cerebellar tumors. Total RNA was extracted from cells using the Qiagen RNeasy Plus Kit and converted to cDNA using the ABI Taqman Reverse Transcription kit (Applied Biosystems (ABI)). Quantitative Real Time PCR was set up using ABI Taqman Master Mix and run on the Applied Biosystems 7900HT Real-Time PCR (384-well qPCR) System. Taqman primers (ABI) for mouse Hes1, Hes5 and Gapdh controls were used. Data was analyzed using SDS 2.3 software (ABI). All conditions were run in triplicate and normalized to mouse Gapdh controls. Expression of both MRK-003 treated (n=4) and vehicle control (n=8) samples were normalized to wild-type cerebella (n=3). Error bars represent average +/- SEM and p values were generated using a t-test. (3c) Notch2 immunohistochemistry demonstrates abundant Notch2 protein in vehicle-treated Smo/Smo medulloblastomas and in mouse intestine positive control, diminished Notch2 in MRK-003 treated Smo/Smo mice, and no staining in a negative control that lacked treatment with primary antibody. (3d) Cerebellar tumor sections were stained with an antibody recognizing Notch2 (Santa Cruz Biotechnology) and five high power fields (HPF) were scored for each sample. Bars represent the average number of Notch2 positive cells per HPF, and error bars represent the standard error. A two-tailed t-test was used to determine p values.
Taken together, these data uphold MRK-003 as an effective agent to inhibit notch signaling in the brain. However, in the context of chronic shh pathway activation in the Smo/Smo cerebellum, the effect of notch pathway inhibition may be limited on preventing tumor growth.
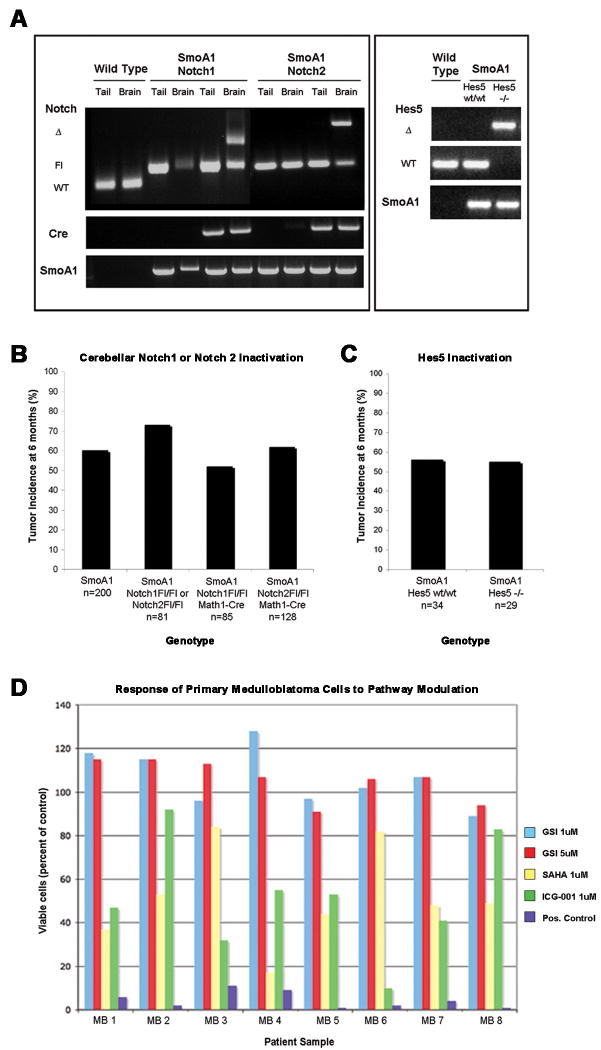
In parallel to the experiments above, we utilized a genetic approach to determine whether the inactivation of notch genes or their targets throughout embryonic and postnatal life affects medulloblastoma formation in SmoA1 mice. Because of the complex genotypes involved, over 4,000 mice were required to generate mice with the medulloblastoma-inducing ND2:SmoA1 transgene onto backgrounds that conditionally lacked Notch1, Notch2 or Hes5 (McCright et al., 2006; Radtke et al., 1999). In this comprehensive study, there was a trend towards decreased tumor incidence in SmoA1 mice conditionally null for Notch1 (52%, n=85; Figure 4b) compared to SmoA1 mice that expressed both copies of all notch genes (64%, n=281), although this trend did not reach significance (p=0.06). No significant difference was observed in tumor incidence in SmoA1 mice conditionally null for Notch2 (62%, n=128, p=0.66; Figure 4b). Given the specified sample sizes, a two-sided binomial test (alpha=0.05) had greater than 85% power to detect a 25% change in tumor incidence and a two-tailed Fisher's exact test was used to calculate p values. Additionally, there was no difference in medulloblastoma incidence or progression when the SmoA1 transgene was expressed in the absence of Hes5 (p=1.0; Figure 4c). We examined the time to tumor onset and evaluated the pathology from SmoA1 mice conditionally null for Notch1 or Notch2 (Supplementary Figure 3). We found that there was a minor delay in tumor onset in SmoA1 mice conditionally null for Notch1 (an average 142 days before tumors became symptomatic versus an average of 138 days in the control group; p=0.002; Supplementary Figure 3a), but did not see a significant decrease in the severity of tumor pathology (p=0.68; Supplementary Figure 3b-c). Tumor onset occurred slightly earlier in SmoA1 mice conditionally null for Notch2 (an average 127 days of age before tumors became symptomatic), but this difference was not significant (p=0.35; Supplementary Figure 3a), nor did the earlier onset correlate with an increase in the severity of tumor pathology in mice conditionally null for Notch2 (p=0.64; Supplementary Figure 3b-c). Additionally, no significant differences were found between the average time to tumor onset in SmoA1 mice that were wild-type (average time to tumor onset 135 days) versus null for Hes5 (average time to tumor onset 133 days; p=0.93, data not shown). A two-tailed logrank test was used to generate p values for time to tumor onset and a two-sided Fisher's exact test was used to calculate p values for the incidence of aggressive tumor phenotypes in compound mutant mice compared to controls. No gross birth defects or other developmental abnormalities were detected in compound transgenic mice.
Figure 4. Conditional inactivation of Notch1, Notch2 or Hes5 in the cerebellum does not affect the incidence of medulloblastoma in SmoA1 mice.
(4a) Genomic DNA was isolated from both the tail and from whole cerebellar extracts from wild type mice and from SmoA1, Notch1Fl/Fl and SmoA1, Notch2Fl/Fl animals (Cre positive and Cre negative) and genotyping carried out as previously described (Hallahan et al., 2004, McCright et al., 2006; Radtke et al., 1999). Genotyping primers distinguished between the endogenous (WT), floxed (Fl) and null (Δ) Notch alleles, and the null alleles were present only within the cerebellum of SmoA1, Cre+, Notch1Fl/Fl and SmoA1, Cre+, Notch2Fl/Fl animals, and not in the tail DNA samples. The Hes5 deletion was not conditional, and thus representative genotyping results are shown for genomic DNA isolated from toe snips of wild type mice and from SmoA1, Hes5wt/wt and SmoA1, Hes5-/- mice. (4b) There was no significant difference in medulloblastoma incidence when the SmoA1 transgene was expressed in the absence of Notch1 (52%, n=85, p=0.06) or Notch2 (62%, n=128, p=0.66) compared to mice that expressed both copies of all Notch genes (64%, n=281 (combined columns 1 and 2)). (4c) There was no significant difference in medulloblastoma incidence between SmoA1 that were wild type (56%, n=34) versus null for Hes5 (55%, n=29, p=1.0). The study in Figure 4c was designed to detect a 50% decrease in tumor incidence with greater than 95% power (2-sided binomial test with alpha=0.05); and this power was increased for the SmoA1, Notch1 and SmoA1, Notch2 compound mutant mice with greater than 95% power to detect a 35% decrease in tumor incidence (2-sided, with alpha=0.05). All p values were generated using Fisher's exact test. SmoA1 mice lacking Hes5 were created by crossing SmoA1 mice with Hes5 null mice until homozygous deletion of Hes5 was achieved (Cau et al., 2000; Hallahan et al., 2004). SmoA1 mice lacking cerebellar Notch1 or Notch2 were generated by Cre-lox inactivation via crosses of Smo/Smo, Notch1Fl/Fl, Notch2Fl/Fl, and MATH1-Cre mice using a complex breeding strategy, which is summarized in Supplementary Figure 2. (4c) Response of primary human medulloblastoma cultures to inhibition of notch signaling by MRK-003 (GSI); the histone deacetylase inhibitor suberoylanilide hydroxamic acid (SAHA; vorinostat); the Wnt pathway inhibitor ICG-001; or a positive control of 10uM cisplatin, 10uM etoposide and 25uM cyclosporin A. Surgical specimens from children with medulloblastomas were incubated with drugs at the designated final concentration for 72 hours and assayed by the ViaLyte cell viability assay. All data is compared to cells grown in 0.1% DMSO, which is the vehicle used in all other conditions.
To establish the relevance of these findings to human medulloblastoma in the preclinical setting, we evaluated MRK-003's activity on primary medulloblastoma cells cultured ex vivo for 72 hours in the presence or absence of drug. In all eight cases tested, MRK-003 did not influence proliferation or cell death at 1 or 5 micromolar concentrations (Figure 4d).
In summary, we have shown that notch inhibition does not alter the incidence, engraftment, or maintenance of shh-activated medulloblastoma. Previous studies using human medulloblastoma cell lines have suggested that notch signaling is required for maintaining subpopulations of progenitor-like cells potentially capable of re-populating tumors after initial therapy (Fan et al., 2006), and that notch pathway inhibition can limit tumor cell growth (Fan et al., 2006; Hallahan et al., 2004). Studies conducted on the notch pathway in cultured cells are sometimes unreliable because the dissociation constant of the drug (low nanomolar) is poorly matched with the concentration required to see an effect (micromolar). This raises the possibility of “off target” effects being responsible for tumor cell response. Furthermore, the notch pathway utilizes physical interactions between cells for signaling and these interactions are altered or lost in culture. For these reasons, we attempted to replicate and extend previous studies of anti-notch therapies by using in vivo medulloblastoma models, where cell-cell contacts and signaling networks are maintained. While dysregulated notch signaling is present in some human and mouse medulloblastomas, our study indicates that notch signaling is not an essential component in the mechanism of medulloblastoma genesis or progression in shh-driven tumors. Our findings were supported in a similar study by our colleagues, who demonstrated that the activation of shh signaling could still drive medulloblastoma formation despite the loss of all downstream notch pathway signaling induced by conditional RBP-J deletion (Julian et al., “Canonical notch signaling is not required for the growth of hedgehog pathway induced medulloblastoma”). Together, these findings challenge the paradigm that notch signaling is crucial in shh-activated medulloblastoma.
In retrospect, it is not surprising that the in vivo studies reported here and in the accompanying manuscript conflicted with the previous in vitro results that we and others previously reported. In our previous study of DAPT activity on medulloblastoma cells in culture, the observed response to DAPT treatment was apoptotic cell death. If the notch pathway was indeed working specifically to maintain a subpopulation of cancer stem cells, then one would expect that the response to a GSI would be premature differentiation of cells in the progenitor pool rather than widespread cell death (Fortini, 2009). It is possible that the DAPT results were a result of “off target” drug activity or toxicity or that the notch pathway is important for medulloblastoma cell survival in monolayer cultures, but is not relevant in vivo. Another possibility is that the critical targets of the notch pathway are maintained in vivo despite suppression of the notch pathway. For example, Hes1 was shown to be a non-canonical target of the shh pathway (Ingram et al., 2008; Solecki et al., 2001; Wall et al., 2009). Because the shh pathway is overexpressed in the SmoA1 mice, it is possible that persistent activation of the shh pathway provided redundant activation of key target genes that are components of the notch and shh pathways. This protection would be absent in cell cultures because the shh pathway is inactivated in cells after a short period of in vitro tissue culture conditions (Sasai et al., 2006).
More difficult to understand is the difference between our results that showed GSI treatment did not inhibit the ability of DAOY cells to form xenografts and previous results that showed GSI-mediated suppression of DAOY engraftment (Fan et al., 2006). One possibility is that the GSI used in the previous study had off-target activity and the cells lost tumor initiating potential due to a notch-unrelated mechanism of action. The previous study also used four replicates per condition compared to 20 in the current study. The number of replicates is important for interpretation of results in a model in which engraftment has typically been reported as 50-75%. Alternatively, the DAOY cells in the two labs may have diverged from one another in a manner that rendered notch pathway activity only variably important. This possibility is supported by the observation that HES1 was reduced by 20% in our cells and by 75% in the previous study (Fan et al., 2006). Whatever the case, the absence of MRK-003-induced effects on Smo/Smo tumor engraftment or SmoA1 autochthonous tumor formation or progression coupled with unaltered medulloblastoma incidence in SmoA1 mice that lacked Notch1, Notch2, or Hes5 indicate that the notch pathway is not essential for shh-driven medulloblastoma genesis or maintenance. This interpretation is supported by the accompanying article that evaluates medulloblastoma formation in the absence of RBP-J, which is a convergence point of all notch pathways (Julian et al., “Canonical notch signaling is not required for the growth of hedgehog pathway induced medulloblastoma”).
While these data suggest that gamma secretase inhibitors may not improve outcomes for the 15-30% of medulloblastoma patients with shh-driven tumors, it is not yet known whether anti-notch therapy will improve outcomes for children with other medulloblastoma subtypes. Examining human medulloblastomas with known molecular profiles in an in vivo setting would thus be an ideal system for comparing whether combinations of targeted therapies may synergistically eliminate both tumor initiating cells and the remaining cells that comprise the bulk of the tumor. Alternatively, less frequent but substantially more concentrated doses of anti-notch therapy might be necessary for completely eliminating sub-populations of tumor initiating cells, and such a strategy could be used as adjuvant therapy in concert with standard regimens.
Supplementary Material
Acknowledgments
We are grateful to Dr. Francois Guillemot for providing the Hes5-null mice, to Freddy Radtke for the notch1 conditional knockout mice, to Thomas Gridley for the notch2 conditional knockout mice, and to Dr. Irwin Bernstein for providing the 18G anti-Notch antibody and advice. The gamma secretase inhibitor MRK-003 was provided by Merck Research Laboratories, which also provided partial funding for the studies. This work was supported by NIH grants 5R01CA112350-04, 5R01CA114567-04, 5T32CA009351 and 5K12CA076930.
Footnotes
Conflict of Interest: Merck Research Laboratories provided the gamma secretase inhibitor MRK-003 and partial funding for the studies, but did not have a role in designing the studies or collecting or analyzing the resulting data. Merck Research Laboratories reviewed the manuscript prior to its submission to Oncogene and stated no conflict in including all data and results for publication.
References
- Cau E, Gradwohl G, Casarosa S, Kageyama R, Guillemot F. Hes genes regulate sequential stages of neurogenesis in the olfactory epithelium. Development. 2000;127:2323–32. doi: 10.1242/dev.127.11.2323. [DOI] [PubMed] [Google Scholar]
- Eberhart CG. In search of the medulloblast: neural stem cells and embryonal brain tumors. Neurosurg Clin N Am. 2007;18:59–69. viii–ix. doi: 10.1016/j.nec.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Fan X, Matsui W, Khaki L, Stearns D, Chun J, Li YM, et al. Notch pathway inhibition depletes stem-like cells and blocks engraftment in embryonal brain tumors. Cancer Res. 2006;66:7445–52. doi: 10.1158/0008-5472.CAN-06-0858. [DOI] [PubMed] [Google Scholar]
- Fan X, Mikolaenko I, Elhassan I, Ni X, Wang Y, Ball D, et al. Notch1 and notch2 have opposite effects on embryonal brain tumor growth. Cancer Res. 2004;64:7787–93. doi: 10.1158/0008-5472.CAN-04-1446. [DOI] [PubMed] [Google Scholar]
- Fortini ME. Notch signaling: the core pathway and its posttranslational regulation. Dev Cell. 2009;16:633–47. doi: 10.1016/j.devcel.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Gilbertson RJ, Ellison DW. The origins of medulloblastoma subtypes. Annu Rev Pathol. 2008;3:341–65. doi: 10.1146/annurev.pathmechdis.3.121806.151518. [DOI] [PubMed] [Google Scholar]
- Goodrich LV, Milenkovic L, Higgins KM, Scott MP. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science. 1997;277:1109–13. doi: 10.1126/science.277.5329.1109. [DOI] [PubMed] [Google Scholar]
- Hallahan AR, Pritchard JI, Hansen S, Benson M, Stoeck J, Hatton BA, et al. The SmoA1 mouse model reveals that notch signaling is critical for the growth and survival of sonic hedgehog-induced medulloblastomas. Cancer Res. 2004;64:7794–800. doi: 10.1158/0008-5472.CAN-04-1813. [DOI] [PubMed] [Google Scholar]
- Hatton BA, Villavicencio EH, Tsuchiya KD, Pritchard JI, Ditzler S, Pullar B, et al. The Smo/Smo model: hedgehog-induced medulloblastoma with 90% incidence and leptomeningeal spread. Cancer Res. 2008;68:1768–76. doi: 10.1158/0008-5472.CAN-07-5092. [DOI] [PubMed] [Google Scholar]
- Ingram WJ, McCue KI, Tran TH, Hallahan AR, Wainwright BJ. Sonic Hedgehog regulates Hes1 through a novel mechanism that is independent of canonical Notch pathway signalling. Oncogene. 2008;27:1489–500. doi: 10.1038/sj.onc.1210767. [DOI] [PubMed] [Google Scholar]
- Lewis SJ, Smith AL, Neduvelil JG, Stevenson GI, Lindon MJ, Jones AB, et al. A novel series of potent gamma-secretase inhibitors based on a benzobicyclo[4.2.1]nonane core. Bioorg Med Chem Lett. 2005;15:373–8. doi: 10.1016/j.bmcl.2004.10.062. [DOI] [PubMed] [Google Scholar]
- McCright B, Lozier J, Gridley T. Generation of new Notch2 mutant alleles. Genesis. 2006;44:29–33. doi: 10.1002/gene.20181. [DOI] [PubMed] [Google Scholar]
- Pomeroy SL, Tamayo P, Gaasenbeek M, Sturla LM, Angelo M, McLaughlin ME, et al. Prediction of central nervous system embryonal tumour outcome based on gene expression. Nature. 2002;415:436–42. doi: 10.1038/415436a. [DOI] [PubMed] [Google Scholar]
- Radtke F, Wilson A, Stark G, Bauer M, van Meerwijk J, MacDonald HR, et al. Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity. 1999;10:547–58. doi: 10.1016/s1074-7613(00)80054-0. [DOI] [PubMed] [Google Scholar]
- Sasai K, Romer JT, Lee Y, Finkelstein D, Fuller C, McKinnon PJ, et al. Shh pathway activity is down-regulated in cultured medulloblastoma cells: implications for preclinical studies. Cancer Res. 2006;66:4215–22. doi: 10.1158/0008-5472.CAN-05-4505. [DOI] [PubMed] [Google Scholar]
- Schouwey K, Beermann F. The Notch pathway: hair graying and pigment cell homeostasis. Histol Histopathol. 2008;23:609–19. doi: 10.14670/HH-23.609. [DOI] [PubMed] [Google Scholar]
- Solecki DJ, Liu XL, Tomoda T, Fang Y, Hatten ME. Activated Notch2 signaling inhibits differentiation of cerebellar granule neuron precursors by maintaining proliferation. Neuron. 2001;31:557–68. doi: 10.1016/s0896-6273(01)00395-6. [DOI] [PubMed] [Google Scholar]
- Sparey T, Beher D, Best J, Biba M, Castro JL, Clarke E, et al. Cyclic sulfamide gamma-secretase inhibitors. Bioorg Med Chem Lett. 2005;15:4212–6. doi: 10.1016/j.bmcl.2005.06.084. [DOI] [PubMed] [Google Scholar]
- Wall DS, Mears AJ, McNeill B, Mazerolle C, Thurig S, Wang Y, et al. Progenitor cell proliferation in the retina is dependent on Notch-independent Sonic hedgehog/Hes1 activity. J Cell Biol. 2009;184:101–12. doi: 10.1083/jcb.200805155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner HL, Bakst R, Hurlbert MS, Ruggiero J, Ahn E, Lee WS, et al. Induction of medulloblastomas in mice by sonic hedgehog, independent of Gli1. Cancer Res. 2002;62:6385–9. [PubMed] [Google Scholar]
- Zeltzer PM, Boyett JM, Finlay JL, Albright AL, Rorke LB, Milstein JM, et al. Metastasis stage, adjuvant treatment, and residual tumor are prognostic factors for medulloblastoma in children: conclusions from the Children's Cancer Group 921 randomized phase III study. J Clin Oncol. 1999;17:832–45. doi: 10.1200/JCO.1999.17.3.832. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.