Abstract
Mouse studies indicate that the synthetic glucocorticoid Dexamethasone (Dex) impairs the proliferation of granule neuron precursors in the cerebellum, which are transformed to medulloblastoma by activation of Sonic hedgehog (Shh) signaling. Here we show that Dex treatment also inhibits Shh-induced tumor growth, enhancing survival of tumor-prone transgenic mice. We found that Nmyc was specifically required in granule cells for Shh-induced tumorigenesis and that Dex acted to reduce Nmyc protein levels. Moreover, we found that Dex-induced destabilization of Nmyc is mediated by activation of GSK3β, which targets Nmyc for proteasomal degradation. Together, our findings show that Dex antagonizes Shh signaling downstream of Smoothened in medulloblastoma.
Keywords: Medulloblastoma, Dexamethasone, Sonic Hedgehog, Nmyc
Introduction
Medulloblastoma is the most common malignant brain tumor in childhood. Despite significant advances in treatment strategies many children die from this tumor due to the lack of therapeutic options that specifically target tumor-initiating and – propagating mechanisms (1). One mechanism known to drive the growth of medulloblastoma in about 25% of human cases is constitutive activation of the Sonic hedgehog-Smoothened (Shh-Smo) signaling pathway in cerebellar granule neuron precursors (CGNP) (2, 3). While in a normal situation, Shh signaling is only active in a precisely defined period of late embryonic and early postnatal cerebellar development, mutations in the Hedgehog pathway suppressor Patched or alterations of other pathway components result in permanent pathway activation and tumor formation (2, 4).
Both in mice and humans, these tumors display expression of the transcription factor Nmyc (3, 5), the stabilization of which prohibits cell cycle exit in normal development and medulloblastoma (5, 6). Inhibition of Hedgehog signaling by small molecule drugs has proved to effectively block the growth of medulloblastoma in mice (7), but significant side effects have been noted with some inhibitors (8).
We have previously shown that the treatment with dexamethasone, a clinically used synthetic glucocorticoid, is able to inhibit Shh-induced CGNP proliferation both in vitro and in vivo at early neonatal stages (P0-P7) of cerebellar development (9). Now, we show that dexamethasone treatment in P21 tumor-prone mice inhibits the growth of Shh-Smo-driven medulloblastoma and significantly prolongs survival. Genetic deletion of Nmyc in Math1-cre:SmoM2-associated medulloblastoma similarly resulted in inhibited tumor growth and prolonged survival. We show that Dex treatment results in decreased Nmyc protein levels and is associated with increased activity of GSK3β, which is know to promote destabilization through terminal phosphorylation of Nmyc in the MB1 domain (10).
Materials and Methods
Animals and animal treatment
All mouse procedures were performed in accordance with National Research Council recommendations. SmoM2Fl/Fl (Gt(ROSA)26Sortm1(Smo/EYFP)Amc/J) mice (11) were obtained from Andrew P. McMahon (Harvard University, Cambridge, MA, USA). Generation and characterization of NmycFl/Fl as well as Math1-cre transgenic animals have been previously described (12, 13). Genotyping was done by PCR analysis using genomic DNA from mouse tail biopsies. Primers have been previously published. Intraperitioneal (i.p.) injection of mice with dexamethasone (3μg/g, Sigma) was performed daily; controls received equivalent volumes of saline vehicle.
Histology, in situ hybridization and immunohistochemistry
For conventional histology and immunohistochemistry, mouse brains were formalin-fixed, paraffin-embedded and cut using standard protocols. For in situ hybridization, brains were perfused and post-fixed overnight with 4% paraformaldehyde/PBS. Tissue was then equilibrated in 0.5M sucrose/PBS (pH7.4), embedded in OCT and cut. In situ hybridization (ISH) on frozen sections was performed as previously published (13). For immunohistochemistry, sections were subjected to heat antigen retrieval at 99°C in 10 mM sodium citrate buffer for all antibodies. Staining was performed using the HRP/DAB based Envision+ staining system (DAKO) according to the manufacturer's specifications. Immunofluorescence stainings were performed using standard conditions with Alexa goat anti-rabbit and Alexa goat anti-mouse secondary antibodies (Molecular probes, #A21121, A21123, A11008, A11010). DAPI was used to visualize cell nuclei. Primary antibodies were NeuN (Chemicon, MAB377), pH3 (#9706, Cell signaling), PCNA (Chemicon, MAB424) and Caspase3 (#9661, Cell signaling).
Cell culture
Primary cultures from Math1-cre:SmoM2 tumors were established by triturating tumor tissue from 21 day old MB-bearing mice in DMEM and plating the cells on poly-D-lysine-coated dishes. Cells were maintained in DMEM containing 10% FCS (Sigma) and treated with 200 μM dexamethasone and 10μM lactacystin (Calbiochem).
RT-PCR
For RNA extraction, primarily cultured tumor cells were homogenized in TRIzol (Invitrogen) before chloroform was added. After a 3-minute incubation, homogenates were centrifuged at 12,000 g for 15 min. The aqueous phase was collected and precipitated with 350 μl isopropanol. The precipitate was collected by centrifugation at 12,000 g for 15 min and resuspended in 100 μl RNase-free H2O. Before continuing with the RT-PCR, RNA was cleaned up with an RNeasy Kit (Qiagen). RT-PCR was performed on isolated RNAs with an Advantage RT-for-PCR Kit (Clontech) and the following primers: Nmyc forward, 5′-GGGGGCTCAGGCTCT TCGCTTTTG-3′; Nmyc reverse, 5′-CCCGCCGTGGTCTTCCCCTTCC-3′; Gli1 forward, 5′-ACAGCGGGG GCAGAAGTCG-3′; Gli1 reverse, 5′-CCTCAGCCCCAGTATCCCCAGTCG-3′. For β-actin, the Mouse β-actin Control Amplimer Set (Clontech) was used.
Western blotting
To detect proteins by immunoblotting, non-denaturing lysates were prepared from CGNPs as described previously (5). Immunoblots were incubated overnight at 4°C in the following primary antibodies: cyclin D1 (Neomarkers Ab-3); β-tubulin (T4026; Sigma-Aldrich); Gli1 (sc-20687, Santa Cruz); Nmyc (OP13; Calbiochem); PT58Nmyc (AB288842, Abcam); PS54Nmyc (Ab51156, Abcam); GSK3B (610201, BDBiosciences); PSer9GSK3αβ (9331, Cell Signaling); PT216GSK3β(44-604G, Invitrogen). Subsequently, immunoblots were developed using HRP-conjugated anti-rabbit (Pierce) or anti-mouse (Jackson ImmunoResearch) secondary antibodies and ECL reagents (Amersham).
Results and Discussion
Systemic treatment of wild type mice with dexamethasone (Dex) inhibits Shh-induced proliferation of CGNPs (9). In order to investigate whether Dex treatment would have an effect on medulloblastoma (MB), we used an established Math1-cre:SmoM2 mouse model (2). These mice express a mutated Smoothened protein (SmoM2) in Math1-positive cerebellar granule cells and develop medulloblastomas with 100% penetrance and an average survival of 35 days.
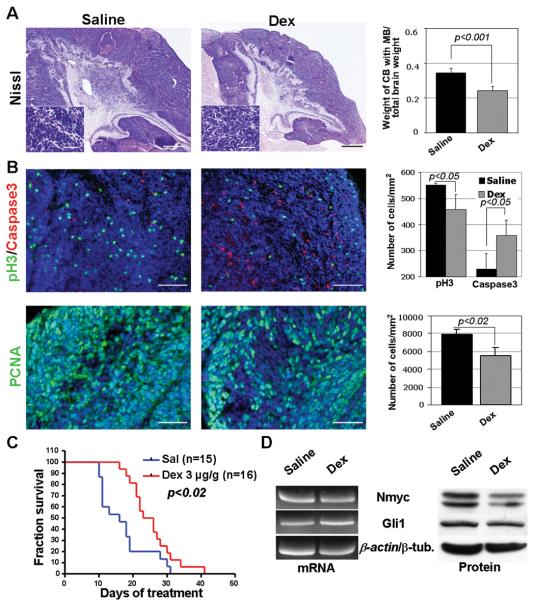
We treated Math1-cre:SmoM2 animals with a daily i. p. dose of 3μg/g Dex. Control animals received saline injections. Treatment was started at P10, a time point by which all CGNP have undergone neoplastic transformation in Math1-cre:SmoM2 animals (2). Macroscopic examination of the brains at day 21 revealed that Dex-treated animals had significantly smaller brains (data not shown), in keeping with previous studies (9). While tumor cell morphology of Dex-treated tumors and tumors from mice that received control injections for 11 days was similar tumors sizes grossly differed between the two groups (Figure 1A). Indeed, as measured by the weight ratio of the cerebellum including medulloblastoma and the total brain, medulloblastomas from Dex-treated animals (n=4) were significantly smaller than controls (n=5, p<0.001). These data indicate that Dex-treatment was able to inhibit the growth of Shh-associated medulloblastoma, and that such inhibition was disproportionate compared to general growth of the brain. Analysis of phosphorylated histone H3, PCNA and cleaved Caspase-3 expression revealed that mitotic activity was significantly reduced, whereas apoptotic activity was significantly enhanced in Dex-treated tumors (p<0.05, p<0.02 and p<0.05, respectively, Figure 1B).
Figure 1.
Medulloblastoma in 21 days old Math1-cre:SmoM2 mice that were treated with 3μg/g dexamethasone (n=4) from day 10 on had similar morphology than controls (n=5), but were significantly smaller in size (p<0.001). (B) Analysis of phosphorylated histone H3 (pH3), PCNA and caspase 3 revealed significantly less mitotic activity but increased apoptosis in dexamethasone-treated animals. (C) Survival of treated animals was significantly enhanced as compared to controls (p<0.02). (D) RT-PCR analysis from tumor tissue showing similar levels of Nmyc, Gli1 and β-actin mRNA. Western blotting showed posttranslational changes in treated animals with unchanged levels of Gli1 and reduced levels of Nmyc proteins. CB, cerebellum; MB, medulloblastoma. Scale bar is 1 mm for A and 100 μm for insets in A and for B.
Next, we analyzed whether Dex-treatment was associated with an altered survival of Math1-cre:SmoM2 animals and found that Dex-treated mice (n=16) survived significantly longer than saline treated mice (n=15, p<0.02, Figure 1C). In particular, Dex-treated animals showed an average survival of 25 days after the treatment start, whereas saline-treated mice already died after an average of 16 days.
To further decipher the mechanisms of Dex-induced inhibition of tumor growth we asked whether the transcription of Shh-target genes in CGNP were affected. Semi-quantitative RT-PCR analysis did not reveal any differences in mRNA levels of Nmyc or Gli1 (Figure 1D). In contrast, Nmyc protein levels were reduced in tumors from Dex-treated animals by almost 30%, whereas Gli protein levels were unchanged. These results indicate that treatment of medulloblastoma with Dex does not regulate the transcription of primary target genes, Gli and Nmyc. It has, however, a significant impact on Nmyc protein levels. Although these findings are in agreement with previous findings in developing CGNP (9), we note that the dose of Dex required to achieve these effects in transformed CGNP of medulloblastoma is 30-fold higher. This difference presumably reflects the strong activation effects of SmoM2.
Previous data have shown that Nmyc and Gli proteins play critical, and possibly overlapping, roles in driving the proliferation of cerebellar granule cell precursors (4, 5). In order to assess a specific requirement for Nmyc in CGNP per se, we generated Math1-cre:NmycFl/FlSmoM2 mice and assessed survival versus Math1-cre:SmoM2 tumor-prone animals.
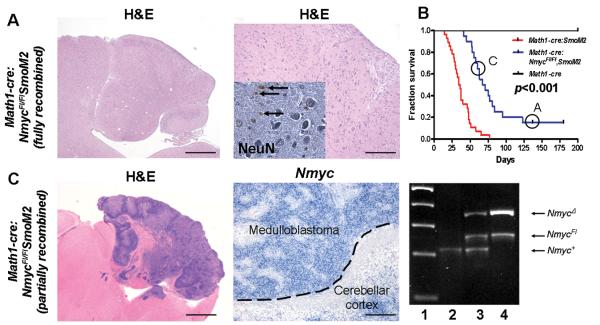
As shown (Figure 2A), 15% of the Math1-cre:NmycFl/FlSmoM2 mice displayed cerebella with a dramatically altered morphology and a nearly complete depletion of granule neurons. Most importantly, they did not develop medulloblastoma, indicating that SmoM2-induced medulloblastomas require Nmyc function in CGNPs. Interestingly, in the 85% of Math1-cre:NmycFl/FlSmoM2 mice that died from medulloblastoma (Figure 2B), we observed expression of full-length Nmyc (Figure 2C). In order to see whether this was caused by an incomplete genomic recombination of the Nmyc allele, we analyzed genomic DNA from tumors of different genotypes using primers picking up wildtype sequences, floxed sequences and deleted Nmyc sequences. As indicated in Figure 2C, a substantial part of the Math1-cre:NmycFl/FlSmoM2 tumors retained the floxed allele due to insufficient recombination, a phenomenon that is well known for the conditional knockout of genes that are required for tumor growth (14). Our results are in agreement with previously described interactions of Shh and Myc family members (15) as well as on Nmyc requirements during cerebellar development (12) and in medulloblastoma (16), but make two novel points. First, we used Math1 promoter sequences, which are more specific for granule cell precursors than the Nestin (which is expressed in multipotent precursors (17)) or NeuroD2 sequences (which is expressed in CGNP and molecular layer interneurons (18)). Second, because our system combines Nmyc deletion and SmoM2 activation simultaneously, our data indicate a cell-autonomous requirement for Nmyc in CGNPs.
Figure 2.
Nmyc is required in medulloblastoma derived from Math1-cre:SmoM2 mice. (A) Sagittal cerebellar section from a 137 days old Math1-cre:NmycFl/FlSmoM2 mouse with a highly disorganized cerebellar cortex. High power magnifications proved absence of a regular internal granular layer and diffusely distributed Purkinje neurons. Note that only single granule neurons are detectable by antibodies against NeuN (inset, arrows). (B) Kaplan Meier analysis revealed significantly prolonged survival of Math1-cre:NmycFl/FlSmoM2 mice (n= 20) as compared to controls (n=28, p<0.001). Circles indicate survival of animals shown in A and C. (C) Math1-cre:NmycFl/FlSmoM2 animals that died from medulloblastoma showing expression of full-length Nmyc as revealed by in-situ hybridizations. Transcription of Nmyc was possible due to incomplete genetic recombination which was demonstrated by PCR on genomic tumor DNA. Lane 1, marker; lane 2, Math1-cre:SmoM2 tumor; lane 3, Math1-cre:NmycFl/+ SmoM2 tumor; lane 4, Math1-cre:NmycFl/FlSmoM2 tumor. Scale bar is 1 mm for left image in A, 2 mm for left image in B, 200 μm for right images in A and B and 60 μm for the inset.
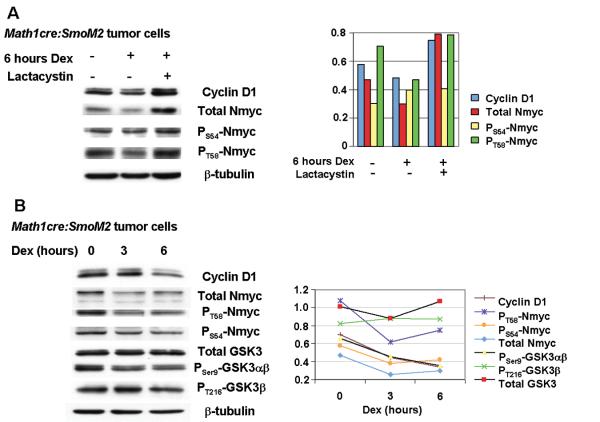
The results above suggested the possibility that the reduced levels of Nmyc proteins in medulloblastoma after Dex-treatment (Figure 1D) could account for reduced tumor growth and enhanced survival of Math1-cre:SmoM2. During normal CGNP development, Nmyc protein destabilization is mediated by a CDK1/cyclinB complex that phosphorylates Nmyc at S54 followed by terminal phosphorylation of T58 by GSK3β (10, 19). Studies in other systems indicate that glucocorticoids can activate GSK3β activity (20). We therefore investigated phosphorylation of Nmyc in primary Math1-cre:SmoM2 tumor cultures treated with Dex +/- lactacystin, a proteasome inhibitor that blocks Nmyc degradation and permits the detection of altered levels of unstable/phosphorylated Nmyc (21). As shown in Figure 3A, levels of PS54Nmyc remain almost unchanged after treatment with Dex and lactacystin. However, the levels of total Nmyc and PT58Nmyc were significantly increased. These findings suggested that Dex treatment increased phosphorylation of Nmyc at T58, which promoted proteosome-mediated degradation. Because GSK3β phosphorylates Nmyc at T58, we assessed GSK3β activation in Dex-treated MB cells. As shown in Figure 3B, levels of PSer9GSK3αβ, but not PT216GSK3β are decreased, indicating an increased activity of GSK3β. We conclude that dexamethasone may activate GSK3β, which, in turn, promotes the phosphorylation of Nmyc at T58 and degradation of Nmyc proteins. Together, these findings suggest that glucocorticoid (GC) signaling inhibits Shh signaling in MB by promoting degradation of Nmyc.
Figure 3.
Dexamethasone treatment of medulloblastoma cells results in proteasome-mediated destabilization of Nmyc and enhanced GSK3ß activity. (A) Dexamethasone treatment reduced levels of Nmyc in Math1-cre:SmoM2 medulloblastoma cells in vitro. Addition of the proteasome inhibitor, lactacystin, resulted in accumulation of total N-myc. PT58Nmyc levels -but not PS54Nmyc levels - were increased after dexamethasone treatment relative to β-tubulin loading controls. Histograms on the right delineate densitometric quantification. (B) Reduced Cyclin D1 and total Nmyc levels were detected after 3 and 6 hours of dexamethasone treatment. Levels of phosphorylated Nmyc were decreased, suggesting rapid Nmyc turnover after dexamethasone treatment. Consistent with this, levels of PSer9GSK3ß are decreased, suggesting increased activity of GSK3ß within this time course. Densitometric quantification is given on the right.
Acknowledgements
We acknowledge Sovann Kaing and Mike Wong for excellent technical support. We thank Paul S. Knoepfler and Robert N. Eisenmann for providing NmycFl/Fl mice. V.M.H. thanks the Netherlands Organization for Scientific Research (NWO) for a TALENT-stipend. This work was supported by grants from the NIH (NS047527, to D.H.R.), the March of Dimes Foundation (to D.H.R.), the Pediatric Brain Tumor Foundation (to D.H.R.) and from the Wilhelm-Sander-Stiftung (to U.S.). U.S. is a member of the Max-Eder-junior-research-program of the German Cancer Aid, D.H.R is a HHMI Investigator.
References
- 1.Gilbertson RJ. Medulloblastoma: signalling a change in treatment. Lancet Oncol. 2004;5:209–18. doi: 10.1016/S1470-2045(04)01424-X. [DOI] [PubMed] [Google Scholar]
- 2.Schüller U, Heine VM, Mao J, et al. Acquisition of granule neuron precursor identity is a critical determinant of progenitor cell competence to form Shh-induced medulloblastoma. Cancer Cell. 2008;14:123–34. doi: 10.1016/j.ccr.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kool M, Koster J, Bunt J, et al. Integrated genomics identifies five medulloblastoma subtypes with distinct genetic profiles, pathway signatures and clinicopathological features. PLoS One. 2008;3:e3088. doi: 10.1371/journal.pone.0003088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wechsler-Reya R, Scott MP. The developmental biology of brain tumors. Annu Rev Neurosci. 2001;24:385–428. doi: 10.1146/annurev.neuro.24.1.385. [DOI] [PubMed] [Google Scholar]
- 5.Kenney AM, Cole MD, Rowitch DH. Nmyc upregulation by sonic hedgehog signaling promotes proliferation in developing cerebellar granule neuron precursors. Development. 2003;130:15–28. doi: 10.1242/dev.00182. [DOI] [PubMed] [Google Scholar]
- 6.Thomas WD, Chen J, Gao YR, et al. Patched1 deletion increases N-Myc protein stability as a mechanism of medulloblastoma initiation and progression. Oncogene. 2009;28:1605–15. doi: 10.1038/onc.2009.3. [DOI] [PubMed] [Google Scholar]
- 7.Romer JT, Kimura H, Magdaleno S, et al. Suppression of the Shh pathway using a small molecule inhibitor eliminates medulloblastoma in Ptc1(+/−)p53(−/−) mice. Cancer Cell. 2004;6:229–40. doi: 10.1016/j.ccr.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 8.Kimura H, Ng JM, Curran T. Transient inhibition of the Hedgehog pathway in young mice causes permanent defects in bone structure. Cancer Cell. 2008;13:249–60. doi: 10.1016/j.ccr.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 9.Heine VM, Rowitch DH. Hedgehog signaling has a protective effect in glucocorticoid-induced mouse neonatal brain injury through an 11betaHSD2-dependent mechanism. J Clin Invest. 2009;119:267–77. doi: 10.1172/JCI36376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kenney AM, Widlund HR, Rowitch DH. Hedgehog and PI-3 kinase signaling converge on Nmyc1 to promote cell cycle progression in cerebellar neuronal precursors. Development. 2004;131:217–28. doi: 10.1242/dev.00891. [DOI] [PubMed] [Google Scholar]
- 11.Mao J, Ligon KL, Rakhlin EY, et al. A novel somatic mouse model to survey tumorigenic potential applied to the Hedgehog pathway. Cancer Res. 2006;66:10171–8. doi: 10.1158/0008-5472.CAN-06-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knoepfler PS, Cheng PF, Eisenman RN. N-myc is essential during neurogenesis for the rapid expansion of progenitor cell populations and the inhibition of neuronal differentiation. Genes and Development. 2002;16:2699–712. doi: 10.1101/gad.1021202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schüller U, Zhao Q, Godinho SA, et al. Forkhead transcription factor FoxM1 regulates mitotic entry and prevents spindle defects in cerebellar granule neuron precursors. Mol Cell Biol. 2007;27:8259–70. doi: 10.1128/MCB.00707-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim IM, Ackerson T, Ramakrishna S, et al. The Forkhead Box m1 transcription factor stimulates the proliferation of tumor cells during development of lung cancer. Cancer Res. 2006;66:2153–61. doi: 10.1158/0008-5472.CAN-05-3003. [DOI] [PubMed] [Google Scholar]
- 15.Rao G, Pedone CA, Coffin CM, Holland EC, Fults DW. c-Myc enhances sonic hedgehog-induced medulloblastoma formation from nestin-expressing neural progenitors in mice. Neoplasia. 2003;5:198–204. doi: 10.1016/S1476-5586(03)80052-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hatton BA, Knoepfler PS, Kenney AM, et al. N-myc is an essential downstream effector of Shh signaling during both normal and neoplastic cerebellar growth. Cancer Res. 2006;66:8655–61. doi: 10.1158/0008-5472.CAN-06-1621. [DOI] [PubMed] [Google Scholar]
- 17.Tronche F, Kellendonk C, Kretz O, et al. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet. 1999;23:99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- 18.Lin CH, Stoeck J, Ravanpay AC, Guillemot F, Tapscott SJ, Olson JM. Regulation of neuroD2 expression in mouse brain. Dev Biol. 2004;265:234–45. doi: 10.1016/j.ydbio.2003.08.027. [DOI] [PubMed] [Google Scholar]
- 19.Knoepfler PS, Kenney AM. Neural precursor cycling at sonic speed: N-Myc pedals, GSK-3 brakes. Cell Cycle. 2006;5:47–52. doi: 10.4161/cc.5.1.2292. [DOI] [PubMed] [Google Scholar]
- 20.Nuutinen U, Ropponen A, Suoranta S, et al. Dexamethasone-induced apoptosis and up-regulation of Bim is dependent on glycogen synthase kinase-3. Leuk Res. 2009;33:1714–7. doi: 10.1016/j.leukres.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Bonvini P, Nguyen P, Trepel J, Neckers LM. In vivo degradation of N-myc in neuroblastoma cells is mediated by the 26S proteasome. Oncogene. 1998;16:1131–9. doi: 10.1038/sj.onc.1201625. [DOI] [PubMed] [Google Scholar]