Abstract
Neurogenesis increases in the adult rodent forebrain subventricular zone (SVZ) after experimental stroke. Newborn neurons migrate to the injured striatum, but few survive long-term and little evidence exists to suggest that they integrate or contribute to functional recovery. One potential strategy to improve stroke recovery is to stimulate neurogenesis and integration of adult-born neurons by using treatments that enhance neurogenesis. We examined the influence of retinoic acid (RA), which stimulates neonatal SVZ and adult hippocampal neurogenesis, and environmental enrichment (EE), which enhances survival of adult-born hippocampal neurons. We hypothesized that the combination of RA and EE would promote survival of adult-generated SVZ-derived neurons and improve functional recovery after stroke. Adult rats underwent middle cerebral artery occlusion, received BrdU on days 5–11 after stroke and were treated with RA/EE, RA alone, EE/vehicle or vehicle alone and were killed 61 days after stroke. Rats underwent repeated MRI and behavioral testing. We found that RA/EE treatment preserved striatal and hemisphere tissue and increased SVZ neurogenesis as demonstrated by Ki67 and doublecortin (DCx) immunolabeling. All treatments influenced the location of BrdU- and DCx-positive cells in the post-stroke striatum. RA/EE increased the number of BrdU/NeuN-positive cells in the injured striatum but did not lead to improvements in behavioral function. These results demonstrate that combined pharmacotherapy and behavioral manipulation enhances post-stroke striatal neurogenesis and decreases infarct volume without promoting detectable functional recovery. Further study of the integration of adult-born neurons in the ischemic striatum is necessary to determine their restorative potential.
Keywords: subventricular zone, neurogenesis, stroke, focal ischemia, regeneration, doublecortin, retinoic acid, environmental enrichment, striatum, neural cell proliferation
Introduction
The capacity of the adult mammalian brain to generate new neurons has been well established over recent years. Neural stem cells from the adult forebrain subventricular zone (SVZ) give rise to olfactory bulb (OB) interneurons while those in the hippocampal dentate gyrus generate new neurons in the granule cell layer (Altman, 1969, Cameron and Gould, 1994, Corotto, et al., 1993, Kaplan and Hinds, 1977, Kuhn, et al., 1996, Lois and Alvarez-Buylla, 1994). Brain insults such as seizure, stroke, and trauma activate neural stem cells to proliferate more rapidly, migrate into injured regions, and form new neurons and glia (Arvidsson, et al., 2002, Goings, et al., 2004, Lichtenwalner and Parent, 2006, Parent, et al., 2002a, Parent, et al., 2002b, Parent, et al., 1997, Zhang, et al., 2001). Stroke increases the production of doublecortin (DCx)- and polysialylated neural cell adhesion molecule (PSA-NCAM)-positive neuroblasts in the SVZ and striatum; however, few cells survive long-term to become functional striatal neurons (Arvidsson, et al., 2002, Ohab, et al., 2006, Parent, et al., 2002b). This latter finding suggests that interventions are necessary to enhance the long-term survival of new neurons after stroke. Moreover, structural and behavioral examination is critical to determine whether the newborn neurons integrate appropriately and contribute to functional recovery after focal ischemic insults.
Many factors regulate neurogenesis during development. The plasticity exhibited by the brain after injury suggests that these factors also may be good candidates for enhancing post-stroke neurogenesis, and one developmental molecule of particular interest in this regard is retinoic acid (RA). During development, RA plays an essential role in anteroposterior patterning of the hindbrain and spinal cord (Liu, et al., 2001, Maden, 2002, Melton, et al., 2004), dorsoventral patterning of spinal cord neurons (Diez del Corral, et al., 2003, Novitch, et al., 2003, Wilson and Maden, 2005) and striatal neuron differentiation (Toresson, et al., 1999, Valdenaire, et al., 1998). RA expression and signaling continues in the postnatal and adult brain. Retinoid binding proteins are expressed in the SVZ-olfactory bulb pathway (Zetterstrom, et al., 1999, Zetterstrom, et al., 1994), RA receptors persist in the olfactory bulb (Krezel, et al., 1999), and the RA synthesizing enzyme RALDH3 is present in the olfactory bulb, rostral migratory stream (RMS), and SVZ (Wagner, et al., 2002). We and others have found that retinoid signaling is a key regulator of postnatal and adult SVZ-olfactory bulb neurogenesis, and that exogenous retinoid administration stimulates this process (Calza, et al., 2003, Haskell and LaMantia, 2005, Wang, et al., 2005). RA signaling also appears necessary for adult hippocampal neurogenesis as depletion of RA in adult mice decreases dentate granule cell differentiation (Jacobs, et al., 2006). These studies led us to investigate whether RA enhances post-stroke striatal neurogenesis.
Environmental enrichment is a commonly used behavioral intervention for rodents and primates after brain injury. Many studies demonstrate the positive effects of environmental enrichment (EE) on functional recovery after injury, although the timing of EE or related interventions is one factor that determines whether it provides beneficial or deleterious effects on recovery or secondary neural degeneration (Bland, et al., 2001, Kleim, et al., 2003, Schallert, et al., 2000, Tillerson, et al., 2001). Other work also shows positive effects on dentate gyrus neurogenesis (Kempermann, et al., 1998, Kempermann, et al., 1997, Komitova, et al., 2002, Nilsson, et al., 1999, Wurm, et al., 2007), in that EE enhances neuron survival; however, few studies have examined the effects of EE on striatal neurogenesis.
Given that RA stimulates forebrain SVZ neurogenesis and EE enhances the survival of some adult-born neurons and improves functional recovery, we hypothesized that combining oral RA-treatment with EE would enhance the generation and long-term survival of SVZ-derived striatal neurons as well as increase functional recovery after stroke. We treated adult rats with RA/EE, RA alone, EE/vehicle or vehicle after inducing focal ischemia, and examined the effects of treatment on SVZ and striatal neurogenesis, stroke volume and sensorimotor function. We found that RA/EE treatment preserved striatal and hemisphere tissue, increased SVZ neurogenesis, influenced the location of newborn cells in the post-stroke striatum, and increased the number of new striatal neurons but did not detectably improve behavioral recovery.
Methods
Transient Middle Cerebral Artery Occlusion
Adult (210–250 gram) male Sprague-Dawley rats (Charles River) were used for all experiments and all procedures were approved by the University of Michigan Committee on Use and Care of Animals. Animals were group housed on a 12-hour reverse light-dark cycle and provided with food and water ad libitum. Rats were anesthetized with a ketamine/xylazine mixture (100mg/kg, intraperitoneal). Body temperature was maintained using a 37°C re-circulating water pad. Transient middle cerebral artery occlusion (tMCAO) was produced for 90 minutes using the external carotid artery insertion method as described previously (Longa, et al., 1989). Briefly, the left common carotid artery was exposed through a midline incision, and the internal and external carotid arteries separated. A 3.0 nylon monofilament with tip rounded by heat was placed in the external carotid artery and advanced through the internal carotid artery until resistance was felt. The monofilament was left in place for 90 minutes and then removed under anesthesia. Animals were observed until they recovered from anesthesia. This protocol produced infarcts involving the striatum and cortex with a mortality rate of approximately 30%.
BrdU labeling
The thymidine analog, bromodeoxyuridine (BrdU, Roche, Indianapolis, IN) was used to label cells in S-phase (Miller and Nowakowski, 1988). Rats received twice daily intraperitoneal (i.p.) injections of BrdU (50mg/kg in sterile PBS, 6 hours apart) on days 5–11 after tMCAO. This timing was chosen based on previous studies showing that SVZ proliferation peaks approximately one week after stroke (Parent, et al., 2002b, Zhang, et al., 2001) and to limit labeling of the earlier post-injury glial response.
MRI
All animals received T2-weighted MRIs on days 7 and 59 post-tMCAO. T2-weighted MRIs were performed using a 7.0 tesla, 18.3 cm horizontal bore magnet as previously described (Chenevert, et al., 1997, Kim, et al., 1995, Moffat, et al., 2006). Serial coronal slices were acquired at 1.5 mm intervals through the rostro-caudal extent of the brain, resulting in 13 slices/animal. Animals were randomly assigned to treatment groups using day 7 MRI. To keep infarct size consistent between groups, we excluded animals with greater than 88% or less than 65% residual hemispheric volume at the level of the striatum (ipsilateral/contralateral hemisphere volume × 100).
Treatment
On day 7 post tMCAO, animals were randomly assigned to one of four treatment groups: combined RA-enriched diet and environmental enrichment (RA/EE), RA-enriched diet and standard environment (RA/SE), normal diet and environmental enrichment (NML/EE) or control with normal diet and SE (NML/SE). Day 7 was chosen as this time point is near the peak of stroke-induced SVZ neurogenesis (Parent et al., 2002b). Animals in the RA groups received the RA-enriched diet on days 7–41 post tMCAO to prolong proliferation and enhance survival of newly generated neuroblasts. Animals survived an additional 20 days to ensure that any cells that incorporated BrdU on days 5–11 after tMCAO and differentiated into neurons likely would be fully integrated if they persisted for the subsequent 7–8 weeks. We chose to stop RA treatment on day 41 after tMCAO (a 5-wk course) because longer durations or higher doses of RA treatment caused adverse hindlimb motor effects. Animals in the EE groups were placed into monkey cages containing an assortment of tunnels, ropes and wire mesh for climbing, chew toys, hidden snacks, and a number of other rodent toys during their dark cycle on days 8–27 post tMCAO to promote functional recovery and enhance survival of newly generated neurons. Animals were rotated through 4 different monkey cages, each with a unique set-up. Unlike previous studies, EE rats were not housed continuously in EE cages, but instead were introduced to the EE cages on a gradual basis (1 hr/day for 2 days, 2 hr/day for 2 days, 3 hr/day for remainder) and housed there for a maximum of 3 hours/day. This protocol was chosen to more closely mimic the intermittent nature of human rehabilitative therapy.
Behavioral Testing
Animals underwent a set of sensorimotor tests on day 6 post-tMCAO to determine initial deficits, and the battery was repeated on days 28 and 60 post-tMCAO to measure recovery over time. The same animals were imaged by MRI on days 7 and 59 after tMCAO. All behavioral testing was performed during the dark cycle. Hind-limb deficits were measured on the tapered, ledged beam test as previously described (Zhao, et al., 2005). The number of ipsilateral and contralateral hindlimb “foot faults” was counted on 4 video-recorded trials/animal/time point. Forelimb asymmetry was assessed using the cylinder test as well as vibrissae-evoked placing (Schallert, et al., 2000, Schallert, et al., 2002). For the cylinder test, animals were placed into a plexiglass cylinder and all trials were video-recorded via an angled mirror for blinded analysis. The number of ipsilateral, contralateral, and bilateral forepaw wall contacts for weight support or shifting was counted and the percent use of the affected limb calculated using the formula: [(ipsilateral/total)−(contralateral/total)] × 100% (Woodlee, et al., 2005). Animals underwent 10 trials/side/time point of vibrissae-evoked placing and the number of placings was recorded.
Histopathology
On day 61 post-tMCAO, animals were deeply anesthetized with sodium pentobarbital and perfusion-fixed with saline followed by 4% paraformaldehyde (PFA). Brains were removed and post-fixed in 4% PFA overnight, cryoprotected in 30% sucrose, then frozen in powdered dry ice. Coronal serial sections were cut at 40 µm on a cryostat, collected into tris, then transferred into cryoprotectant and stored at −20°C. To confirm lesion severity from MRI analyses, 4 striatal level sections/animal were mounted onto slides, Nissl-stained using cresyl violet, and bilateral hemisphere, striatal and SVZ areas measured using Image J (see below).
Immunohistochemistry
Diaminobenzidine immunohistochemistry was performed with antibodies to doublecortin (DCx) and BrdU using previously published protocols (Parent, et al., 2002b, Plane, et al., 2004). For BrdU staining, sections were rinsed in Tris, denatured in 2N HCl at 37°C for 30 min, neutralized in 0.1M boric acid for 10 min, rinsed, blocked with 10% normal horse serum (Invitrogen, Carlsbad, CA), and incubated overnight at 4°C with anti-BrdU antibody (1:1000 dilution; mouse monoclonal, Roche Applied Science, Indianapolis, IN). Sections were rinsed, incubated in secondary antibody (1:200 biotinylated horse anti-mouse IgG, Vector Labs) for 1 hour, rinsed, signal amplified with Vector ABC kit, developed with stable diaminobenzidine (Invitrogen), mounted onto slides, dehydrated and cleared in ethanol and xylenes, and coverslipped using Permount (Sigma). DCx immunostaining followed a similar protocol except that sections were put into 1% H2O2 for 30 min instead of 2N HCl and boric acid. Sections were blocked with normal horse or goat serum and incubated either in goat anti-DCx (1:2000, overnight, Santa Cruz) or rabbit anti-DCx antibody (1:1000 for 2 nights, (Parent, et al., 2002b); biotinylated horse anti-goat or goat anti-rabbit IgG secondary antibodies were used. For single label immunofluorescence, sections were washed with Tris-Buffered Saline (TBS), blocked in goat serum, then incubated in anti-Ki67 (1:1000 rabbit, Vector Labs) or anti-PSA-NCAM (1:500 mouse IgM, Chemicon) overnight at 4°C. Sections were washed in TBS, incubated in goat anti-rabbit Alexa 488 or 594, or goat anti-mouse IgM Alexa 488 (1:800, Invitrogen) at room temperature for 1.5 hours. Sections were washed, mounted onto slides and allowed to dry, then coverslipped with an anti-fade medium (Pro-Long, Invitrogen). Double-label immunofluorescence staining was performed as previously described to identify dividing SVZ neuroblasts or newly formed striatal neurons (Parent, et al., 2002b, Plane, et al., 2004). Sections were stained with primary antibodies to Ki67 (1:1000) and PSA-NCAM (1:500), or to BrdU (1:250 rat, Accurate Chemical) and NeuN (1:1000, mouse monoclonal, Chemicon), followed by incubation in species- and isotype-appropriate Alexa 488 and 594 secondary antibodies.
Quantitative Analyses
MRIs were converted to tiff files and imported into NIH Image J for analysis. All images were auto-adjusted for brightness/contrast and intact striatal or hemispheric area measured at the level of the striatum (3 images/animal/timepoint). Residual striatal or hemispheric tissue was outlined and area measured using the freehand tool; stroke volume was calculated as area × slice thickness × number of slices. Area measurements were also obtained from Nissl stained striatal sections (n=4/animal) to confirm MRI findings. Unbiased stereology was performed on every 12th section (n=5 sections) to count striatal BrdU+ cells. Striatal areas were outlined at low magnification and BrdU-positive cells were counted at 100× using the optical fractionator technique (StereoInvestigator; (Plane, et al., 2004, West, et al., 1996). Peri-infarct BrdU labeling was quantified at the level of the anterior striatum in 3 sections/animal. Images of the dorsal and ventral striatum adjacent to the infarct, nearest the level of the dorsal and ventral lateral ventricle, respectively, were captured without knowledge of experimental group using a SPOT-RT digital camera at 40× magnification and all BrdU-positive cells within these fixed areas were counted. DCx immunoreactivity was quantified in the SVZ in 4 equidistant anterior striatal sections/animal. DCx-positive SVZ area and density measurements were obtained using Image J from 10× images of the dorsolateral SVZ. Ki67-positive cells were counted in the lateral SVZ of 3 equidistant striatal sections/animal in 20× images of the lateral ventricle. Immunofluorescence double labeling and confocal microscopy (Zeiss LSM 510) were used to assess co-localization of immunofluorescence for double labeling and to quantify BrdU+ or BrdU/NeuN+ cells (3–6 63× fields/animal). All analyses were performed in a blinded manner.
Statistics
Analysis of variance (ANOVA) with post-hoc t-tests were used to compare differences between treatments for all quantitative analyses. Results were presented as means ± standard error of the mean (SEM) and considered significant when p≤0.05.
Results
RA treatment preserves hemisphere volume after stroke
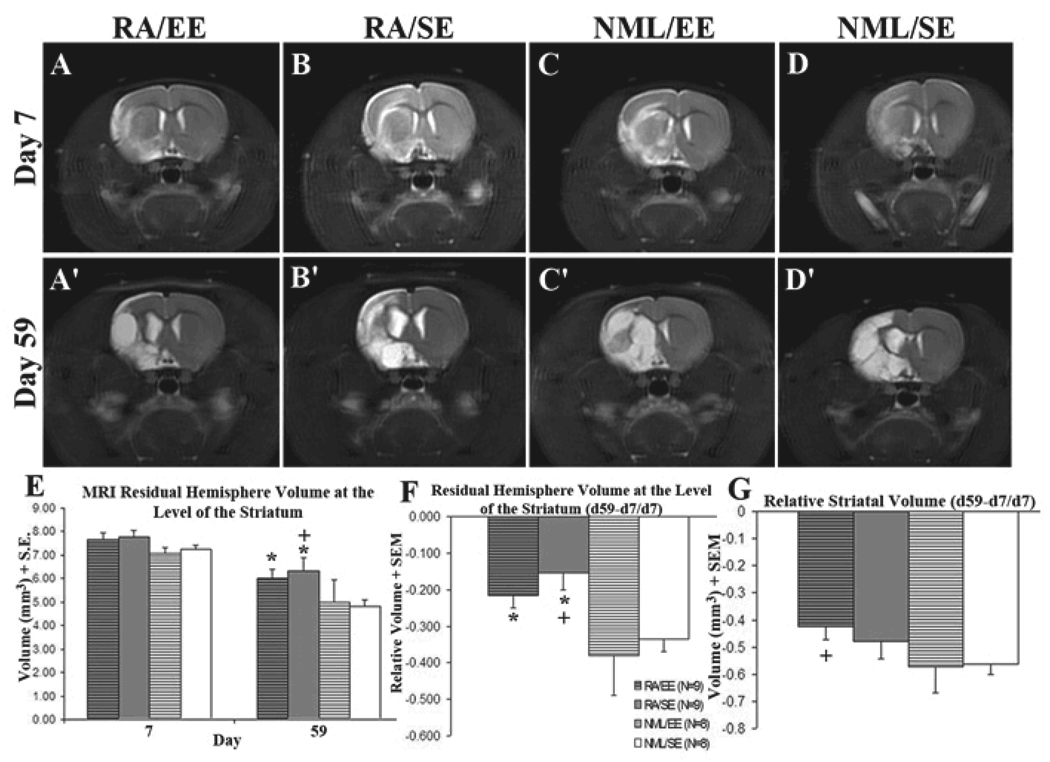
RA is a key regulator of neurogenesis in the postnatal and adult SVZ-OB pathway (Haskell and LaMantia, 2005, Wang, et al., 2005). Because RA stimulates SVZ neurogenesis and EE increases the survival of adult-born neurons in the dentate gyrus, we sought to determine if RA-treatment combined with EE would promote adult SVZ neurogenesis and functional recovery after stroke. To evaluate lesion severity, assign animals to treatment groups, and assess long-term effects of treatment on stroke volume, rats received serial MRIs on days 7 and 59 after tMCAO. Animals were randomly assigned to treatment groups or excluded from the study based on day 7 MRI results. All animals had similar lesion sizes on day 7, prior to treatment (Figure1A–D, E). They underwent repeat MRI on day 59 post-tMCAO to compare treatment effects on stroke volume. Animals that received an RA-enriched diet had more residual hemisphere tissue than those on a standard diet (Figure 1A’–D’, E, p<0.05 RA/EE vs. NML/EE, RA/SE vs. NML/EE or NML/SE). RA-treated animals also had less decline in hemispheric volume over time [(d59−d7)/d7] compared with controls (Figure 1F, p<0.05 RA/EE vs. NML/EE, RA/SE vs. NML/EE or NML/SE). Animals that received both RA and EE also lost significantly less striatal volume over time compared with untreated animals (Figure 1G, p=0.03). These differences could potentially be confounded by differences in nutrition or food consumption due to the RA-enriched diet. However, animals were weighed periodically and there was no difference in weight between animals on normal diet vs. RA-enriched diet (data not shown). We similarly compared day 7 and day 59 MRIs for hemispheric minus striatal volume, a representation mainly of cortical volume at these forebrain levels, and found no significant difference between treatment groups and controls (p>0.10, ANOVA with Dunnett’s post-hoc test). Thus, retinoic acid decreases post-stroke tissue loss assessed by MRI in an area of the rodent brain, the striatum, where stroke-induced neurogenesis is known to occur.
Figure 1. RA preserved hemisphere volume after stroke.
All animals had T2-weighted MRIs on day 7 (A–D) and day 59 (A'–D') after tMCAO and were assigned to treatment groups based on day 7 stroke volume (≥65% normal signal volume vs. intact hemisphere). All groups had similar stroke volumes prior to treatment (E). Rats that received an RA-enriched diet had more residual hemisphere volume at day 59 after MCAO compared with those on a normal diet (E, *p<0.05 vs NML/EE, +p<0.05 vs. NML/SE) as well as a smaller change in hemisphere volume over time (F, *p<0.05 vs NML/EE, +p<0.05 vs. NML/SE). Rats that received an RA-enriched diet and environmental enrichment maintained more striatal volume over time compared with normal controls (G, +p<0.05). All values are expressed as volume (mm3) ± SEM.
RA treatment increases SVZ neurogenesis and alters neuroblast migration 61 days after stroke
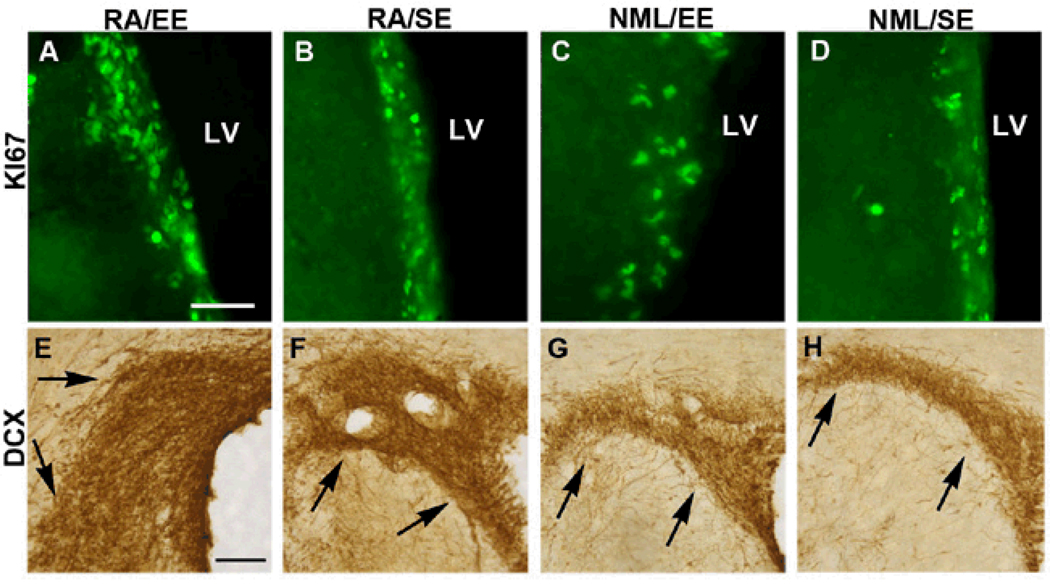
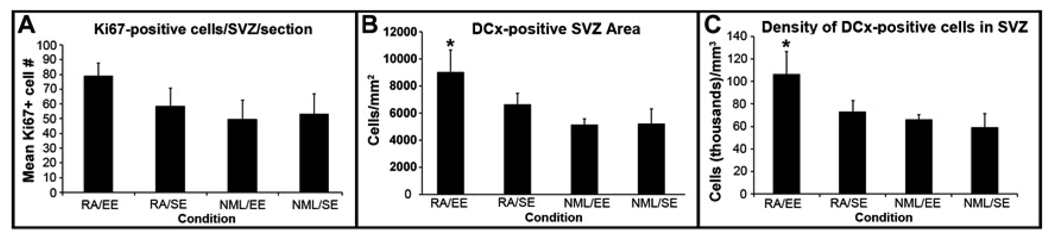
To determine whether our interventions stimulated SVZ neurogenesis as a potential explanation for the preservation of tissue after stroke, we first used Ki67, an endogenous proliferation marker, to assess SVZ cell proliferation 61 days after stroke. As expected, Ki67-positive cells were present in the dorsolateral tail of the SVZ and along the lateral wall of the lateral ventricle 61 days after stroke in the control group; however, rats that received both RA and EE showed a strong trend toward greater numbers of Ki67-positive cells in the SVZ (Figure 2A–D, Figure 3A), although the difference was not statistically significant. We next examined whether the increased cell proliferation was associated with an increase in SVZ neurogenesis. Using DCx immunohistochemistry to identify SVZ neuroblasts, we found that the RA/EE-treated group displayed increased SVZ DCx expression in an expanded SVZ (Figure 2E–H, Figure 3B). The enlarged DCx-immunoreactive SVZ area led us to question whether the increase was simply a result of the cells being more spread out. We therefore measured the density of DCx immunostaining in the SVZ and found a significant increase in the density of DCx-positive cells in the SVZ of RA/EE-treated rats compared with controls (Figure 3C, p<0.05).
Figure 2. RA/EE increased SVZ cell proliferation at 61d post-stroke.
Ki67 labeling was performed to identify proliferating cells at the time of sacrifice. All animals had Ki67+ cells present in the dorsolateral tail of the SVZ as well as along the lateral wall of the lateral ventricle. Animals that received the RA-enriched diet combined with EE had the most Ki67+ cells present in the lateral SVZ (A) and animals that received only the RA-enriched diet also displayed increased Ki67-immunoreactivity (B) compared with EE-only (C) and untreated rats (D). The enlarged SVZ contains numerous DCx+ neuroblasts and increased DCx immunoreactivity in RA/EE (E), RA alone (F) and EE alone (G), groups compared to controls (H) 61 days after MCAO. LV=lateral ventricle, shown at right-hand side in all panels. Scale bars: 50µm in A, 100 µm in E.
Figure 3. Quantification of increased SVZ neurogenesis at 61d post-stroke.
RA/EE-treatment increased the average number of Ki67+ cells/section in the lateral portion of the SVZ (A) compared with controls. The RA/EE group also showed a significantly larger DCx+ dorsolateral SVZ area compared to EE-alone or untreated rats (B, *p=0.02). The enlarged DCx+ SVZ of rats in the RA/EE group also contained significantly more densely packed neuroblasts than that of the EE alone group or controls (C, *p<0.05).
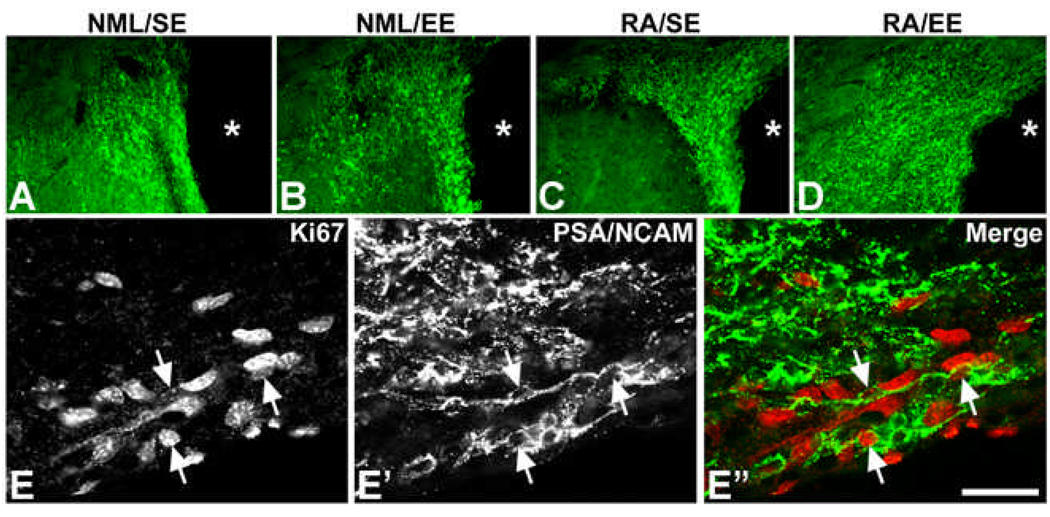
The increase in DCx expression in the neurogenic SVZ strongly suggested that RA/EE treatment increased numbers after stroke. To provide additional evidence of increased neurogenesis, we immunostained SVZ sections for another neuroblast marker, PSA-NCAM, and found that SVZ PSA-NCAM expression similarly was increased 61 days after focal ischemia in the RA/EE group (Figure 4A–D). Double labeling for Ki-67 and PSA-NCAM showed that some of the PSA-NCAM-immunoreactive cells continued to proliferate, further supporting ongoing SVZ neurogenesis (Figure 4 E–E”). Together, these findings indicate that combined RA and EE treatment increases SVZ neurogenesis in the chronic phase after stroke.
Figure 4. SVZ PSA-NCAM expression and neuroblast proliferation after stroke.
Immunostaining for PSA-NCAM at 61 days after focal ischemia showed enhanced PSA-NCAM immunoreactivity maximally in the RA/EE group (D) and to a lesser extent the RA alone (RA/SE) group (C) compared to EE alone (B) or normal diet and no enrichment (NML/SE) control groups (A). Asterisks denote the lateral ventricle. Panels E–E” show double labeling for Ki67 (E) and PSA-NCAM (E’) in a rat from the RA/EE group. Arrows denote double-labeled cells. Scale bar: 75 µm for A–D; 25 µm for E–E”.
Previous studies showed that SVZ neuroblasts migrate to the injured striatum within the first few weeks after stroke (Arvidsson, et al., 2002, Parent, et al., 2002b) and continue to do so chronically (Thored, et al., 2006). To examine whether the prolonged increase in SVZ neurogenesis after RA/EE treatment corresponded with increased striatal neurogenesis after stroke, we injected adult rats with BrdU on days 5–11 after tMCAO and assessed BrdU labeling on day 61 after tMCAO. Unbiased stereology was performed on BrdU-immunostained sections to determine the number of cells generated after stroke that persisted in the ipsilateral striatum 61 days after tMCAO. There was no difference in the total number of striatal BrdU-positive cells between groups (Table 1); however, more BrdU-labeled cells appeared close to the infarct border in the three treatment groups (Figure 5A–E). This observation led us to quantify BrdU-positive cell numbers in the peri-infarct area of the striatum. Images of the dorsal and ventral peri-infarct striatal areas were captured and BrdU-immunoreactive cells counted for each area (Figure 5A). We found, on average, more BrdU-positive cells in the peri-infarct regions in all 3 treatment groups (Figure 5B–E; Table 1: 1272±350 cells for RA/EE, 1146±239 for RA/SE, 1276±253 for NML/EE) compared to controls (999±101 cells); however, the differences were not statistically significant due to the large variability between animals (Table 1).
Table 1.
Striatal BrdU-positive cell analysis. All values are ± SEM. % BrdU/NeuN= (#BrdU/NeuN+/total BrdU+) × 100.
| BrdU Stereology |
Total Peri- infarct BrdU |
% BrdU/ NeuN |
|
|---|---|---|---|
| RA/EE | 711648±114529 | 1272.13±350.74 | 20.97±3.57 |
| RA/SE | 669720±132478 | 1146.75±239.37 | 15.72±2.47 |
| NML/EE | 651623±57937 | 1276.33±253.13 | 15.63±2.87 |
| NML/SE | 632630±97387 | 999.00±101.19 | 15.10±2.90 |
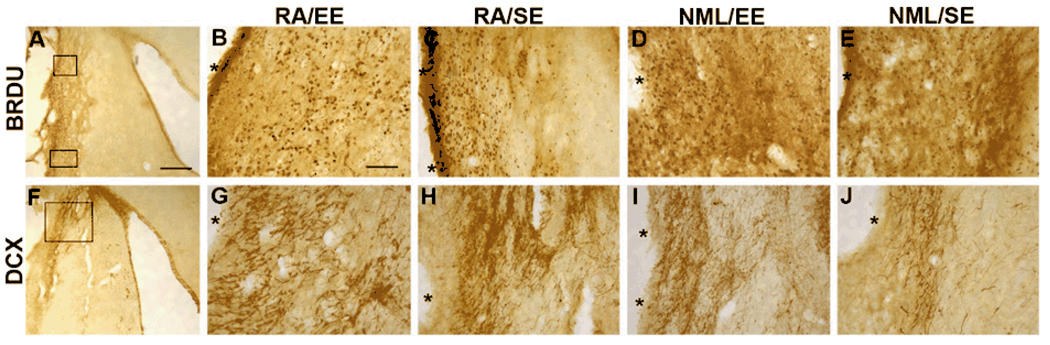
Figure 5. RA/EE increased striatal peri-infarct BrdU and DCx.
Rats were injected with BrdU on days 5–11 after tMCAO. Analysis of peri-infarct BrdU was performed as described in the methods section. BrdU+ cells in the peri-infarct striatum were counted in dorsal and ventral areas as shown (A). BrdU+ cells appeared more numerous within the peri-infarct region of RA/EE (B), RA/SE (C) and NML/EE (D) rats compared with untreated rats (E). The distribution of DCx expression in the injured striatum differs as well. The image in F is from a representative RA/EE rat, showing DCx expression extending from the dorsolateral tail of the SVZ, laterally to the infarct border and deep into the ventral striatum. DCx+ neuroblasts appear more dense and farther from the ventricle in the lesioned striatum in RA/EE (G) and RA/SE (H) than in NML/EE (I) or untreated rats (J). *denotes infarct border. Scale bar in A = 400 µm, in B = 100µm.
We next examined the location of migrating neuroblasts at 2 months after stroke. DCx immunoreactivity was assessed in the peri-infarct region at the level of the dorsal striatum (Figure 5F). We found that, compared to controls, more DCx-positive cells appeared in the injured striatum of all three treatment groups (Figure 5G–J). DCx-positive neuroblasts were dispersed throughout the injured striatum, but more neuroblasts were found closer to the infarct in the three treatment groups, particularly in the RA/EE group than in controls (Figure 5G–J). Together, these results suggest that RA and EE treatment promote migration of new cells to the peri-infarct striatum.
Combined treatment with RA and EE increases striatal neurogenesis
To determine whether RA and EE treatment yielded more surviving striatal neurons generated after stroke, double-labeling was performed for BrdU and NeuN or Map2 and confocal thin optical z-slices collected from several locations in the ipsilateral striatum. Animals received i.p. injections of BrdU on days 5–11 after MCAO to label proliferating cells and were killed on day 61 post-tMCAO to assess the long-term survival of labeled cells. Rats that received both RA and EE showed a 35% increase in the proportion of BrdU-labeled cells that co-expressed NeuN in the striatum (21±5.7% ) 61 days after tMCAO (Table 1, Figure 6A–D) compared with untreated animals (15.5±3.3% of BrdU+ were BrdU/NeuN double-labeled; Table 1, Figure 6E–H). RA- or EE-alone did not increase the proportion of BrdU/NeuN double-labeled cells in the injured striatum (15.7±2.5% and 15.6±2.9%, respectively). No BrdU/NeuN double-labeled cells were found in peri-infarct cortical regions where many BrdU-positive, presumably glial or endothelial, cells were located (data not shown).
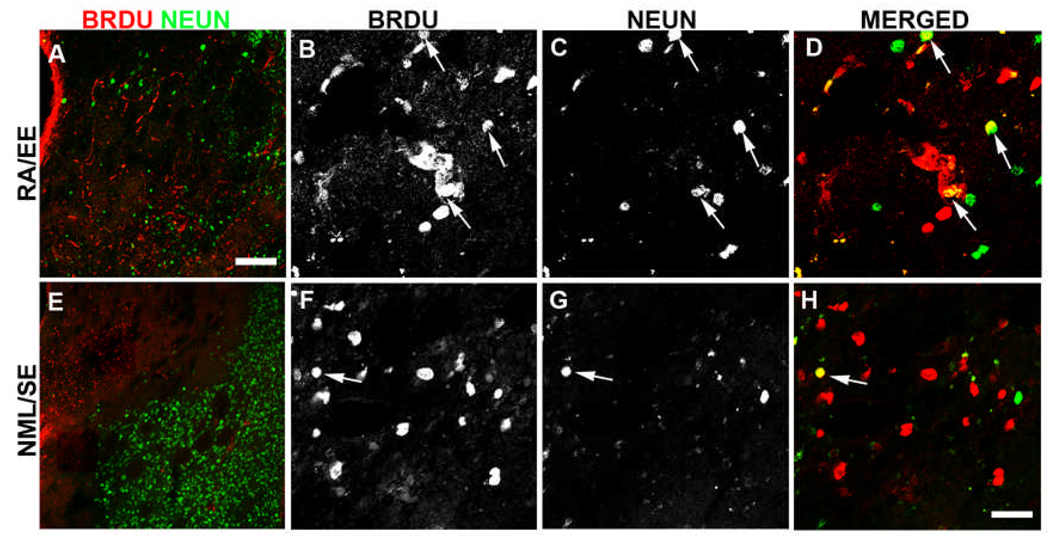
Figure 6. RA/EE enhanced the formation of new neurons in the injured striatum.
Rats were injected with BrdU on days 5–11 and killed 61 days after tMCAO. Double-label immunofluorescence staining for BrdU and NeuN identified new neurons born during this time of peak proliferation after tMCAO. There was a distinct border between BrdU+ cells and healthy neurons in the injured striatum of most rats, regardless of treatment, making it difficult to locate double-labeled cells (A, E). The injured striatum of RA/EE rats contained the most BrdU/NeuN double-labeled cells (arrows, B–D). Of note, very few BrdU/NeuN double-labeled cells were strongly BrdU+, rather most contained strong NeuN (C) and lighter BrdU immunoreactivity (B,D), suggesting that these cells divided numerous times. Similarly, untreated rats typically displayed strong NeuN staining (G) with more diluted BrdU (F) in the same cell (H, arrow), however, fewer double-labeled cells were found in these animals. Scale bar in A = 150µm, in I = 25µm.
Enriched environment may transiently improve functional recovery
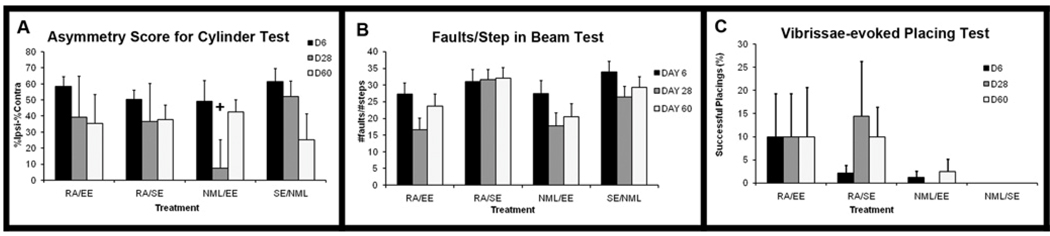
Behavioral testing was performed on day 6 after tMCAO to determine baseline deficits and was repeated on days 28 and 60 to assess functional improvements over time. All groups had similar and severe behavioral deficits on day 6 after injury on all 3 sensorimotor tests and showed some improvement in the cylinder and beam tests by day 28 (Figure 7A–B) but not in the vibrissae-evoked placing test (Figure 7C). The EE-alone group appeared to show the most improvement, however, and this trended toward significance on the cylinder test (Figure 7A, repeated measures ANOVA, p=0.06). The enrichment was stopped on day 27 and the probable beneficial effect was no longer apparent by day 60 (Figure 7A). This transient improvement and subsequent loss is likely due to the halting of EE-treatment on day 27 but suggests that short durations of EE transiently promote functional recovery. Of note, when higher doses of RA were given to animals in pilot studies they often caused weakness of hindlimbs and, to a lesser extent, forelimbs, suggesting the possibility of adverse RA effects that may have obscured the detection of functional benefit.
Figure 7. EE transiently improves functional recovery after tMCAO.
Rats were behavior tested 6 days after tMCAO to establish baseline deficits and again on days 28 and 60 after tMCAO to assess recovery. Forelimb asymmetry was assessed using the cylinder test and vibrissae-evoked placing while hindlimb asymmetry was assessed on the ledged, tapered beam. All rats displayed similar deficits on day 6 in the cylinder test (A, black bars) and beam test (B, black bars). Normal behavior in uninjured rats is 0. However, only a few animals had successful placing and in only about 10% of all trials in the vibrissae-evoked placing test (C, black bars). By day 28, all groups showed some improvement on the cylinder test (A, gray bars) but only the NML/EE group trended toward improvement (+p=0.06 vs. NML/SE). All groups except RA/SE showed some improvement on the beam test but none were significantly different (B, gray bars). RA/SE showed no improvement on the beam test (B, gray bars) but showed a slight but insignificant improvement on the vibrissae-evoked placing test (C, gray bars) while other groups showed no improvement on this test. By day 60, all groups except NML/EE displayed similar deficits on the cylinder test as were seen on day 28 (A, white bars). Interestingly, the NML/EE group had more severe deficits on the cylinder test at day 60 than at day 28 (A, white bars), suggesting that the improvements seen during the course of treatment were lost one month after treatment halted. All groups remained the same on day 60 as they were on day 28 in the beam test (B, white bars) and vibrissae-evoked placing (C, white bars).
Discussion
Retinoic acid influences patterning and neuronal differentiation in the developing brain (Maden, 2007) and is an essential component of early neuronal differentiation and cell survival in the adult mouse dentate gyrus (Jacobs, et al., 2006). Few studies have examined the effects of retinoic acid in stroke-induced neurogenesis and no studies have evaluated the combined effects of a pro-neurogenic factor and environmental enrichment on stroke-induced neurogenesis and functional recovery. The goal of this study was to determine whether the combined treatments improve both functional recovery and promote neurogenesis in the striatum after tMCAO. We found that RA/EE treatment decreased the loss of brain tissue months after stroke, predominantly in the striatum, increased neuroblast production in the SVZ and striatum, and likely influenced neuroblast migration to injury. This treatment, however, did not lead to significant improvements in functional recovery.
Very few studies have examined changes in stroke size after tMCAO within animals. Using MRI, we were able to compare initial stroke volume, prior to treatment, with stroke volume after treatment in the same animal to more accurately determine beneficial or deleterious effects of treatment. This led us to the novel finding that RA-treatment preserves brain tissue. Only one previous study found that pre-treatment with 9-cis RA, but not all-trans RA, decreased infarct size after MCAO (Harvey, et al., 2004); unlike our study, however, this work only examined the acute phase of cerebral ischemia and not regeneration. In our study the brain tissue preservation that resulted from RA-treatment was very unlikely to be a neuroprotective effect given that the treatment was not started until 8 days after stroke, although we cannot exclude an influence on very delayed cell death.
A number of potential explanations exist for the lack of a functional correlate to the tissue preservation. We found that total brain volume was preserved (data not shown), as was hemisphere volume at the level of the striatum. Striatal volume was also preserved, although to a lesser extent than hemisphere volume, leading to the possibility that RA-treatment preserved tissue in brain regions not involved in the sensorimotor tasks used to measure functional recovery. Many animals had already sustained 25–30% damage by day 7 after tMCAO so perhaps the strokes were too large and functional deficits too severe to see any modest effects of RA on functional recovery. Timing and dosage of RA is also important, as too much retinoic acid reduces cell proliferation in the mouse SVZ and dentate gyrus, reduces hippocampal neurogenesis (Crandall, et al., 2004) and causes changes in bone density (Rohde and DeLuca, 2003). In pilot studies, we observed a number of uninjured adult rats with impaired hindlimb use when given higher doses or longer courses of RA, which recovered after removal of the RA-enriched diet. These observations led us to cut the dosage in half and maintain treatment for only 5 weeks for the current study, although 3 rats (out of 18) still displayed transient ipsilesional limb impairments with the regimen we used.
Another possibility is that the adult-born neurons did not effectively integrate after stroke. Such integration is likely necessary to restore function and has not yet been unequivocally demonstrated for stroke-induced striatal neurogenesis. However, following small focal cortical infarcts a combination of epidermal growth factor and erythropoietin delivered intraventricularly one week after injury enhanced SVZ cell proliferation, neuronal migration, cortical tissue generation and functional benefit (Kolb, et al., 2007). Removal of the new tissue reversed, though not immediately, the functional benefit. Regimens that initiate RA or EE treatment at later timepoints or for longer durations at lower doses also should be explored for their potential to improve the survival and integration of striatal neurons generated after stroke.
We found increased SVZ neurogenesis 61 days after tMCAO in RA/EE-treated rats, as shown by Ki67, DCx and PSA-NCAM immunolabeling. Most studies assessed cell proliferation more acutely after injury and found increased neurogenesis in the SVZ in the first few weeks after MCAO (Arvidsson, et al., 2002, Jin, et al., 2001, Parent, et al., 2002b, Zhang, et al., 2001), although a persistent increase in basal stroke-induced neurogenesis has been reported by one group (Thored, et al., 2006). Our data suggest that RA further increases SVZ neurogenesis in the chronic stages after stroke. Contrary to our findings, a recent study found that RA-treatment after photothrombotic stroke decreased BrdU and DCx in the SVZ (Jung, et al., 2007). This study assessed only acute (7 days after injury) proliferation effects, which may be one reason for the disparities with our findings. Further, they used photothrombosis to induce cerebral infarction, resulting in an isolated cortical infarct which differs from the tMCAO model in which rats have both striatal and cortical injury, and this larger injury may influence SVZ neurogenesis in different ways (Kolb, et al., 2007). Additionally, i.p. injections of RA were administered for the first week after stroke, which differs substantially from our 5 week treatment of diet-enriched RA which began one week after tMCAO.
Our data also suggested that RA/EE treatment increased striatal neuroblast numbers and promoted new cells to migrate farther from the SVZ. Numerous DCx-positive neuroblasts and BrdU-positive cells were located near the infarct border in RA/EE treated rats, whereas these cells tended to be located closer to the dorsolateral tail of the SVZ in untreated rats. Previously our lab found that RA influenced the migration of SVZ neuroblasts in the SVZ-olfactory bulb pathway of the early postnatal brain (Wang, et al., 2005), and the results from this study suggest that RA and EE may promote the migration of newly formed SVZ cells towards the injured striatum or promote their survival near the infarct border. RA/EE treatment also appeared to increase striatal neurogenesis after tMCAO, as shown by an increase in the number of BrdU/NeuN double-labeled cells. This slight increase in new neuron survival may be a future therapeutic target to enhance neurogenesis. As shown in this study, increased neurogenesis does not necessarily mean improved function, as we saw no significant behavioral recovery in RA/EE-treated animals compared with untreated rats. In order to determine if neurogenesis is in fact necessary for functional recovery, further studies in which neurogenesis is eliminated or reversibly inhibited are needed. One would expect to see behavioral outcomes worsened by blocking neurogenesis if it is in fact important for functional recovery.
Most studies that evaluate the effects of EE on neurogenesis continuously house the animals in EE and have focused on the dentate gyrus, finding that EE enhances DG cell survival. We found that the short duration of EE used in this study seems to provide a transient improvement in sensorimotor function. Using only up to a maximum of 3 hours of EE per day, we saw a trend toward improvement in forelimb placing at the end of the treatment period. This improvement was lost, however, when the animals were tested again one month after “therapy” halted, suggesting that perhaps short durations of EE over a longer period of time may result in prolonged functional recovery. Similar results were found in a recent study with mice evaluating the effects of environmental enrichment for 3 hr/day for 2 weeks after MCAO (Nygren and Wieloch, 2005). Interestingly, mice that were housed in continuous EE showed improved motor function but also had increased mortality; mice housed in EE for 3 hrs/day for 2 weeks, and then in standard cages for 2 weeks showed a loss of the motor function that they had recovered. These investigators also found that 4 weeks of 3 hr/day EE produced more long-lasting functional recovery. These results support our findings and the notion that a longer treatment period of short duration EE may provide substantial functional recovery. Despite this potential recovery, EE alone did not yield an increase in post-stroke neurogenesis, suggesting a dissociation between these two processes similar to that found for hippocampal-dependent memory effects of EE (Meshi, et al., 2006). Other studies that evaluated the effects of EE on post-stroke neurogenesis found conflicting effects. After cortical stroke and EE, BrdU and Ki67 labeling increased bilaterally in the rat SVZ (Komitova, et al., 2005) and BrdU incorporation increased in the peri-infarct cortex although double-labeling revealed that most of these cells were glia (Komitova, et al., 2006). Another study from the same group found that post-stroke EE increased BrdU and DCx labeling in the SVZ 5 weeks after stroke (Komitova, et al., 2005), while others found that striatal lesioning induced by quinolinic acid or 6-hydroxydopamine plus EE increased striatal DCx expression and enhanced neuroblast migration into the striatum (Urakawa, et al., 2007). In mice, EE increased SVZ progenitor numbers after stroke (Hicks, et al., 2007, Nygren, et al., 2006) but decreased the migration of DCx-positive cells into the striatum (Nygren, et al., 2006). Together with our results, these studies suggest that species, type of injury, and duration of EE differentially regulate SVZ and striatal neurogenesis.
Few neurons typically survive long-term after stroke (Arvidsson, et al., 2002, Parent, et al., 2002b) and these data suggest that the combination of RA and EE may provide a first step in promoting their survival. Future studies are needed to determine the most beneficial timing and dose of RA-treatment, such as whether a lower dose or longer delay prior to RA treatment may provide similar increases in neurogenesis without potential deleterious effects. Similarly, more work is required to determine if a longer timeframe of short-duration EE treatment, possibly including a tapering-off of the treatment, would be more beneficial to cell survival and functional recovery after stroke.
Acknowledgments
This work was supported by NIH grants HD044775, HD-02-03 and NS042345, and a predoctoral training fellowship from the American Heart Association. The authors thank Drs. Richard Keep and Faye Silverstein for critical discussions, and Mr. Vincent Alessi for technical assistance.
References
- Altman J. Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J Comp Neurol. 1969;137:433–457. doi: 10.1002/cne.901370404. [DOI] [PubMed] [Google Scholar]
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- Bland ST, Pillai RN, Aronowski J, Grotta JC, Schallert T. Early overuse and disuse of the affected forelimb after moderately severe intraluminal suture occlusion of the middle cerebral artery in rats. Behav Brain Res. 2001;126:33–41. doi: 10.1016/s0166-4328(01)00243-1. [DOI] [PubMed] [Google Scholar]
- Calza L, Giuliani A, Fernandez M, Pirondi S, D'Intino G, Aloe L, Giardino L. Neural stem cells and cholinergic neurons: regulation by immunolesion and treatment with mitogens, retinoic acid, and nerve growth factor. Proc Natl Acad Sci U S A. 2003;100:7325–7330. doi: 10.1073/pnas.1132092100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron HA, Gould E. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience. 1994;61:203–209. doi: 10.1016/0306-4522(94)90224-0. [DOI] [PubMed] [Google Scholar]
- Chenevert TL, McKeever PE, Ross BD. Monitoring early response of experimental brain tumors to therapy using diffusion magnetic resonance imaging. Clin Cancer Res. 1997;3:1457–1466. [PubMed] [Google Scholar]
- Corotto FS, Henegar JA, Maruniak JA. Neurogenesis persists in the subependymal layer of the adult mouse brain. Neurosci Lett. 1993;149:111–114. doi: 10.1016/0304-3940(93)90748-a. [DOI] [PubMed] [Google Scholar]
- Crandall J, Sakai Y, Zhang J, Koul O, Mineur Y, Crusio WE, McCaffery P. 13-cis-retinoic acid suppresses hippocampal cell division and hippocampal-dependent learning in mice. Proc Natl Acad Sci U S A. 2004;101:5111–5116. doi: 10.1073/pnas.0306336101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez del Corral R, Olivera-Martinez I, Goriely A, Gale E, Maden M, Storey K. Opposing FGF and retinoid pathways control ventral neural pattern, neuronal differentiation, and segmentation during body axis extension. Neuron. 2003;40:65–79. doi: 10.1016/s0896-6273(03)00565-8. [DOI] [PubMed] [Google Scholar]
- Goings GE, Sahni V, Szele FG. Migration patterns of subventricular zone cells in adult mice change after cerebral cortex injury. Brain Res. 2004;996:213–226. doi: 10.1016/j.brainres.2003.10.034. [DOI] [PubMed] [Google Scholar]
- Harvey BK, Shen H, Chen GJ, Yoshida Y, Wang Y. Midkine and retinoic acid reduce cerebral infarction induced by middle cerebral artery ligation in rats. Neurosci Lett. 2004;369:138–141. doi: 10.1016/j.neulet.2004.07.086. [DOI] [PubMed] [Google Scholar]
- Haskell GT, LaMantia AS. Retinoic acid signaling identifies a distinct precursor population in the developing and adult forebrain. J Neurosci. 2005;25:7636–7647. doi: 10.1523/JNEUROSCI.0485-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks AU, Hewlett K, Windle V, Chernenko G, Ploughman M, Jolkkonen J, Weiss S, Corbett D. Enriched environment enhances transplanted subventricular zone stem cell migration and functional recovery after stroke. Neuroscience. 2007;146:31–40. doi: 10.1016/j.neuroscience.2007.01.020. [DOI] [PubMed] [Google Scholar]
- Jacobs S, Lie DC, DeCicco KL, Shi Y, DeLuca LM, Gage FH, Evans RM. Retinoic acid is required early during adult neurogenesis in the dentate gyrus. Proc Natl Acad Sci U S A. 2006;103:3902–3907. doi: 10.1073/pnas.0511294103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Minami M, Lan JQ, Mao XO, Batteur S, Simon RP, Greenberg DA. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc.Natl.Acad.Sci.U.S.A. 2001;98:4710–4715. doi: 10.1073/pnas.081011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung DS, Baek SY, Park KH, Chung YI, Kim HJ, Kim CD, Cho MK, Han ME, Park KP, Kim BS, Kim JB, Oh SO. Effects of retinoic acid on ischemic brain injury-induced neurogenesis. Exp Mol Med. 2007;39:304–315. doi: 10.1038/emm.2007.34. [DOI] [PubMed] [Google Scholar]
- Kaplan MS, Hinds JW. Neurogenesis in the adult rat: electron microscopic analysis of light radioautographs. Science. 1977;197:1092–1094. doi: 10.1126/science.887941. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Brandon EP, Gage FH. Environmental stimulation of 129/SvJ mice causes increased cell proliferation and neurogenesis in the adult dentate gyrus. Curr Biol. 1998;8:939–942. doi: 10.1016/s0960-9822(07)00377-6. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Kim B, Chenevert TL, Ross BD. Growth kinetics and treatment response of the intracerebral rat 9L brain tumor model: a quantitative in vivo study using magnetic resonance imaging. Clin Cancer Res. 1995;1:643–650. [PubMed] [Google Scholar]
- Kleim JA, Jones TA, Schallert T. Motor enrichment and the induction of plasticity before or after brain injury. Neurochem Res. 2003;28:1757–1769. doi: 10.1023/a:1026025408742. [DOI] [PubMed] [Google Scholar]
- Kolb B, Morshead C, Gonzalez C, Kim M, Gregg C, Shingo T, Weiss S. Growth factor-stimulated generation of new cortical tissue and functional recovery after stroke damage to the motor cortex of rats. J Cereb Blood Flow Metab. 2007;27:983–997. doi: 10.1038/sj.jcbfm.9600402. [DOI] [PubMed] [Google Scholar]
- Komitova M, Mattsson B, Johansson BB, Eriksson PS. Enriched environment increases neural stem/progenitor cell proliferation and neurogenesis in the subventricular zone of stroke-lesioned adult rats. Stroke. 2005;36:1278–1282. doi: 10.1161/01.STR.0000166197.94147.59. [DOI] [PubMed] [Google Scholar]
- Komitova M, Perfilieva E, Mattsson B, Eriksson PS, Johansson BB. Effects of cortical ischemia and postischemic environmental enrichment on hippocampal cell genesis and differentiation in the adult rat. J Cereb Blood Flow Metab. 2002;22:852–860. doi: 10.1097/00004647-200207000-00010. [DOI] [PubMed] [Google Scholar]
- Komitova M, Perfilieva E, Mattsson B, Eriksson PS, Johansson BB. Enriched environment after focal cortical ischemia enhances the generation of astroglia and NG2 positive polydendrocytes in adult rat neocortex. Exp Neurol. 2006;199:113–121. doi: 10.1016/j.expneurol.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Komitova M, Zhao LR, Gido G, Johansson BB, Eriksson P. Postischemic exercise attenuates whereas enriched environment has certain enhancing effects on lesion-induced subventricular zone activation in the adult rat. Eur J Neurosci. 2005;21:2397–2405. doi: 10.1111/j.1460-9568.2005.04072.x. [DOI] [PubMed] [Google Scholar]
- Krezel W, Kastner P, Chambon P. Differential expression of retinoid receptors in the adult mouse central nervous system. Neuroscience. 1999;89:1291–1300. doi: 10.1016/s0306-4522(98)00342-x. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenwalner RJ, Parent JM. Adult neurogenesis and the ischemic forebrain. J Cereb Blood Flow Metab. 2006;26:1–20. doi: 10.1038/sj.jcbfm.9600170. [DOI] [PubMed] [Google Scholar]
- Liu JP, Laufer E, Jessell TM. Assigning the positional identity of spinal motor neurons: rostrocaudal patterning of Hox-c expression by FGFs, Gdf11, and retinoids. Neuron. 2001;32:997–1012. doi: 10.1016/s0896-6273(01)00544-x. [DOI] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- Maden M. Retinoid signalling in the development of the central nervous system. Nat Rev Neurosci. 2002;3:843–853. doi: 10.1038/nrn963. [DOI] [PubMed] [Google Scholar]
- Maden M. Retinoic acid in the development, regeneration and maintenance of the nervous system. Nat Rev Neurosci. 2007;8:755–765. doi: 10.1038/nrn2212. [DOI] [PubMed] [Google Scholar]
- Melton KR, Iulianella A, Trainor PA. Gene expression and regulation of hindbrain and spinal cord development. Front Biosci. 2004;9:117–138. doi: 10.2741/1202. [DOI] [PubMed] [Google Scholar]
- Meshi D, Drew MR, Saxe M, Ansorge MS, David D, Santarelli L, Malapani C, Moore H, Hen R. Hippocampal neurogenesis is not required for behavioral effects of environmental enrichment. Nat Neurosci. 2006;9:729–731. doi: 10.1038/nn1696. [DOI] [PubMed] [Google Scholar]
- Miller MW, Nowakowski RS. Use of bromodeoxyuridine-immunohistochemistry to examine the proliferation, migration and time of origin of cells in the central nervous system. Brain Res. 1988;457:44–52. doi: 10.1016/0006-8993(88)90055-8. [DOI] [PubMed] [Google Scholar]
- Moffat BA, Chenevert TL, Meyer CR, McKeever PE, Hall DE, Hoff BA, Johnson TD, Rehemtulla A, Ross BD. The functional diffusion map: an imaging biomarker for the early prediction of cancer treatment outcome. Neoplasia. 2006;8:259–267. doi: 10.1593/neo.05844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson M, Perfilieva E, Johansson U, Orwar O, Eriksson PS. Enriched environment increases neurogenesis in the adult rat dentate gyrus and improves spatial memory. J Neurobiol. 1999;39:569–578. doi: 10.1002/(sici)1097-4695(19990615)39:4<569::aid-neu10>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Novitch BG, Wichterle H, Jessell TM, Sockanathan S. A requirement for retinoic acid-mediated transcriptional activation in ventral neural patterning and motor neuron specification. Neuron. 2003;40:81–95. doi: 10.1016/j.neuron.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Nygren J, Wieloch T. Enriched environment enhances recovery of motor function after focal ischemia in mice, and downregulates the transcription factor NGFI-A. J Cereb Blood Flow Metab. 2005;25:1625–1633. doi: 10.1038/sj.jcbfm.9600157. [DOI] [PubMed] [Google Scholar]
- Nygren J, Wieloch T, Pesic J, Brundin P, Deierborg T. Enriched environment attenuates cell genesis in subventricular zone after focal ischemia in mice and decreases migration of newborn cells to the striatum. Stroke. 2006;37:2824–2829. doi: 10.1161/01.STR.0000244769.39952.90. [DOI] [PubMed] [Google Scholar]
- Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. J Neurosci. 2006;26:13007–13016. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent JM, Valentin VV, Lowenstein DH. Prolonged seizures increase proliferating neuroblasts in the adult rat subventricular zone-olfactory bulb pathway. J Neurosci. 2002a;22:3174–3188. doi: 10.1523/JNEUROSCI.22-08-03174.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent JM, Vexler ZS, Gong C, Derugin N, Ferriero DM. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol. 2002b;52:802–813. doi: 10.1002/ana.10393. [DOI] [PubMed] [Google Scholar]
- Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, Lowenstein DH. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci. 1997;17:3727–3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plane JM, Liu R, Wang TW, Silverstein FS, Parent JM. Neonatal hypoxic-ischemic injury increases forebrain subventricular zone neurogenesis in the mouse. Neurobiol Dis. 2004;16:585–595. doi: 10.1016/j.nbd.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Rohde CM, DeLuca H. Bone resorption activity of all-trans retinoic acid is independent of vitamin D in rats. J Nutr. 2003;133:777–783. doi: 10.1093/jn/133.3.777. [DOI] [PubMed] [Google Scholar]
- Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology. 2000;39:777–787. doi: 10.1016/s0028-3908(00)00005-8. [DOI] [PubMed] [Google Scholar]
- Schallert T, Leasure JL, Kolb B. Experience-associated structural events, subependymal cellular proliferative activity, and functional recovery after injury to the central nervous system. J Cereb Blood Flow Metab. 2000;20:1513–1528. doi: 10.1097/00004647-200011000-00001. [DOI] [PubMed] [Google Scholar]
- Schallert T, Woodlee MT, Fleming SM. Disentangling multiple types of recovery from brain injury. Stuttgart: Medpharm Scientific Publishers; 2002. [Google Scholar]
- Thored P, Arvidsson A, Cacci E, Ahlenius H, Kallur T, Darsalia V, Ekdahl CT, Kokaia Z, Lindvall O. Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem Cells. 2006;24:739–747. doi: 10.1634/stemcells.2005-0281. [DOI] [PubMed] [Google Scholar]
- Tillerson JL, Cohen AD, Philhower J, Miller GW, Zigmond MJ, Schallert T. Forced limb-use effects on the behavioral and neurochemical effects of 6-hydroxydopamine. J Neurosci. 2001;21:4427–4435. doi: 10.1523/JNEUROSCI.21-12-04427.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toresson H, Mata de Urquiza A, Fagerstrom C, Perlmann T, Campbell K. Retinoids are produced by glia in the lateral ganglionic eminence and regulate striatal neuron differentiation. Development. 1999;126:1317–1326. doi: 10.1242/dev.126.6.1317. [DOI] [PubMed] [Google Scholar]
- Urakawa S, Hida H, Masuda T, Misumi S, Kim TS, Nishino H. Environmental enrichment brings a beneficial effect on beam walking and enhances the migration of doublecortin-positive cells following striatal lesions in rats. Neuroscience. 2007;144:920–933. doi: 10.1016/j.neuroscience.2006.10.038. [DOI] [PubMed] [Google Scholar]
- Valdenaire O, Maus-Moatti M, Vincent JD, Mallet J, Vernier P. Retinoic acid regulates the developmental expression of dopamine D2 receptor in rat striatal primary cultures. J Neurochem. 1998;71:929–936. doi: 10.1046/j.1471-4159.1998.71030929.x. [DOI] [PubMed] [Google Scholar]
- Wagner E, Luo T, Drager UC. Retinoic acid synthesis in the postnatal mouse brain marks distinct developmental stages and functional systems. Cereb Cortex. 2002;12:1244–1253. doi: 10.1093/cercor/12.12.1244. [DOI] [PubMed] [Google Scholar]
- Wang TW, Zhang H, Parent JM. Retinoic acid regulates postnatal neurogenesis in the murine subventricular zone-olfactory bulb pathway. Development. 2005;132:2721–2732. doi: 10.1242/dev.01867. [DOI] [PubMed] [Google Scholar]
- West MJ, Ostergaard K, Andreassen OA, Finsen B. Estimation of the number of somatostatin neurons in the striatum: an in situ hybridization study using the optical fractionator method. J Comp Neurol. 1996;370:11–22. doi: 10.1002/(SICI)1096-9861(19960617)370:1<11::AID-CNE2>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Wilson L, Maden M. The mechanisms of dorsoventral patterning in the vertebrate neural tube. Dev Biol. 2005;282:1–13. doi: 10.1016/j.ydbio.2005.02.027. [DOI] [PubMed] [Google Scholar]
- Woodlee MT, Asseo-Garcia AM, Zhao X, Liu SJ, Jones TA, Schallert T. Testing forelimb placing "across the midline" reveals distinct, lesion-dependent patterns of recovery in rats. Exp Neurol. 2005;191:310–317. doi: 10.1016/j.expneurol.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Wurm F, Keiner S, Kunze A, Witte OW, Redecker C. Effects of skilled forelimb training on hippocampal neurogenesis and spatial learning after focal cortical infarcts in the adult rat brain. Stroke. 2007;38:2833–2840. doi: 10.1161/STROKEAHA.107.485524. [DOI] [PubMed] [Google Scholar]
- Zetterstrom RH, Lindqvist E, Mata de Urquiza A, Tomac A, Eriksson U, Perlmann T, Olson L. Role of retinoids in the CNS: differential expression of retinoid binding proteins and receptors and evidence for presence of retinoic acid. Eur J Neurosci. 1999;11:407–416. doi: 10.1046/j.1460-9568.1999.00444.x. [DOI] [PubMed] [Google Scholar]
- Zetterstrom RH, Simon A, Giacobini MM, Eriksson U, Olson L. Localization of cellular retinoid-binding proteins suggests specific roles for retinoids in the adult central nervous system. Neuroscience. 1994;62:899–918. doi: 10.1016/0306-4522(94)90482-0. [DOI] [PubMed] [Google Scholar]
- Zhang RL, Zhang ZG, Zhang L, Chopp M. Proliferation and differentiation of progenitor cells in the cortex and the subventricular zone in the adult rat after focal cerebral ischemia. Neuroscience. 2001;105:33–41. doi: 10.1016/s0306-4522(01)00117-8. [DOI] [PubMed] [Google Scholar]
- Zhao CS, Puurunen K, Schallert T, Sivenius J, Jolkkonen J. Effect of cholinergic medication, before and after focal photothrombotic ischemic cortical injury, on histological and functional outcome in aged and young adult rats. Behav Brain Res. 2005;156:85–94. doi: 10.1016/j.bbr.2004.05.011. [DOI] [PubMed] [Google Scholar]