Abstract
Donor leukocyte infusions (DLI) in the allogeneic hematopoietic transplant setting can provide a clinically relevant boost of immunity to reduce opportunistic infections and to increase graft-versus-leukemia activity. Despite significant advances in applicability, DLI has not been available for single unit recipients of unrelated cord blood transplant (UCBT). Ex-vivo expansion of cord blood T cells can be achieved with IL-2 and CD3/CD28 co-stimulatory beads. However, significant apoptosis occurs in proliferating T cells, diminishing the yield and skewing the CD4/CD8 ratio in the T cell population, jeopardizing the potential efficacy of DLI. In this study, we demonstrate that IL-7 not only reduces apoptosis of activated T lymphocytes and enhances their proliferation, but also promotes functional maturation leading to secretion of interferon-gamma and other key cytokines. Recognizing that infused T lymphocytes will need to meet microbial antigens in secondary lymphoid organs to generate effectors, we also show that expansion with IL-7 promotes the preservation of a polyclonal broad T cell receptor repertoire and a surface phenotype that favors lymph node homing. Expanded lymphocytes lack alloreactivity against recipient and other allogeneic cells indicating a favorable safety profile from graft-versus-host disease. Nevertheless, expanded T cells can be primed subsequently against lymphoid and myeloid leukemia cells to generate tumor-specific cytotoxic T cells. Taken together, our findings offer a major step in fulfilling critical numerical and biological requirements to quickly generate a DLI product ex vivo, using a negligible fraction of a cord blood graft that provides a flexible adoptive immunotherapy platform for both for children and adults.
Introduction
Lymphopenia, in particular affecting T cells is an almost uniform consequence of hematopoietic cell transplantation (HCT) extending sometimes beyond the first year. Adoptive transfer of naturally primed and ex vivo restimulated T lymphocytes in the form of donor leukocyte infusions (DLI) has demonstrated efficacy to prevent/treat EBV-associated lymphomas, post-transplant viral infections (1), and could augment graft versus leukemia (GVL) activity. Despite significant recent advances in the applicability and outcome following unrelated cord blood transplantation (UCBT), currently there is no obviously available post-transplant source for DLI from the transplanted unit. Not only is there a shortage of leukocytes available for DLI, but cord blood (CB) T lymphocytes are also antigen inexperienced/naïve cells. Moreover, multiple placental factors bias against Th1/Tc1 development in utero (2, 3) impairing Th1/Tc1 cytokine production in particular IFNγ (4), cytotoxicity (5), collectively leading to impaired antiviral immunity that leads to significant transplant related mortality in the first 3-6 months after UCBT, reviewed (6).
The tempo of cellular recovery is quite variable after UCBT. Although mitogenic responses may reach normal range in children 6-9 months after UCBT, T cell reconstitution is gradual and typically does not reach age appropriate numbers before 9-12 months. This is in contrast with adults where T cell reconstitution typically extends beyond the first year, presumably as a result from age-dependent decline in pre-transplant thymic function (7). A detailed analysis in a cohort of adults who underwent UCBT for hematological malignancies demonstrated extremely severe T cell lymphopenia that extended throughout the first year (8).
Attaining a vigorous cellular immune profile in particular against common pathogens such as herpes viruses impacts survival after UCBT. Investigators from the Cord Blood Transplantation Study (COBLT) found that development of antigen-specific proliferation in the first 3 years to either CMV, HSV, or VZV led to a lower probability of leukemia relapse and a higher overall survival (9). Robust proliferative T cell responses likely represent a powerful surrogate marker for functional T cell immune reconstitution leading to more effective graft-versus-leukemia (GVL) activity. These data underscore the high clinical relevance to design novel approaches aimed at alleviating post-transplant lymphopenia and the Th1/Tc1 functional deficits displayed by cord blood T cells infused in the graft.
Recently, we and others have demonstrated the feasibility of ex vivo CB T cell expansion (10, 11), drawing from the pioneering work by June et al. (12) utilizing paramagnetic Dynal beads coated with anti-CD3 and anti-CD28 stimulatory antibodies. These artificial antigen presenting cells (APC) simultaneously provide agonistic TCR and co-stimulatory signals triggering sufficient T cell proliferation in vitro to generate clinically relevant DLI products from living donors (13-15). Although in our previous work (10), robust T cell expansion and even partial Th1/Tc1 maturation was evident starting with frozen/thawed CB specimens, significant apoptosis (∼16%) resulted in an inverted CD4/CD8 ratio and diminished yield despite relatively low concentrations of IL-2 in the medium. The high degree of apoptosis was likely the result of activation induced cell death (AICD) following strong TCR signaling on CB T cells as previously described (16). Infusion of overstimulated, apoptosis prone DLI product would likely lead to a narrow T cell repertoire and shortened T cell survival in vivo. Moreover, it could falsely suggest futility of ex vivo expanded DLI strategies.
In the current study, we tested and confirmed our hypotheses, that interleukin-7 (IL-7) acting in concert with a new, clinical grade CD3/CD28 costimulatory bead and IL-2, would not only enhance ex vivo CB T cell proliferation while retaining a broad TCR repertoire as predicted (17), but it would also reduce activation induced cell death.
We also demonstrate that IL-7 permits Th1/Tc1 maturation of the proliferating cord blood T cells to progress until a clinically desired intermediate state while preserving the starting population's naïve surface phenotype conducive to lymph node homing. Absence of cytotoxicity against recipient and other allogeneic cells indicate a favorable safety profile from graft-versus-host disease. Importantly, expanded CD3/CD28-expanded T cells can be subsequently primed against lymphoid and myeloid leukemia cells to generate tumor-specific CTL. Taken together, the presented experimental strategies for the first time fulfill critical numerical and biological requirements to generate a DLI product ex vivo in ∼ 14 days from a negligible (≤3%) fraction of a cord blood graft offering a flexible adoptive immunotherapy platform for pediatric as well as adult clinical trials.
Materials and Methods
Specimens
Frozen umbilical cord blood samples not eligible for clinical use were obtained from research units at the Duke Stem Cell Laboratory. Eligible patients were consented on an IRB-approved protocol to obtain fibroblasts and ≤3% aliquots of their UCB grafts.
T cell enrichment and expansion system
CD3+ T cells were enriched by negative immunomagnetic selection with EasySep® (StemCell Technology) as described (10). Expansion was started at 6-8 ×105 T cells/mL in gas permeable VueLife®Teflon bags (American Fluoroseal, Gaithesburg, MD). T cells were co-cultured with ClinExVivo™ Dynabeads® (Dynal/Invitrogen Corp, Sammamish, WA) at a cell : bead ratio of 3:1 in X Vivo-15 (BioWhittacker, Walkersville, MD), supplemented with 5.5 × 10-5M of BME, 10mM Hepes, 5% PHS (Valley Biomedical, Winchester, VA) and 100u/mL IL-2 (Proleukin, Novartis, Hanover, NJ), ± 10ng/mL of IL-7 (R&D Systems, Minneapolis, MN) as indicated. Medium and cytokines were replenished three times/week to maintain a cell concentration of ∼1 × 106 TNC/mL (10). After 12-14 days, after vigorous agitation, the beads were removed by a Dynal magnet: MPC-2 or Dynal ClinExVivo™ MPC™.
Immunophenotypic and functional characterization of the CD3/28-expanded T cells
Surface and intracellular immunophenotyping, cytokine secretion, T cell enumeration was performed by 4-color FACS as described (10, 18, 19). Acquisition of >5,000 CD3+ events was performed on FACSCalibur or FACSCanto II (BD Biosciences, San Jose, CA). All antibodies including isotype-specific controls were purchased from BD except anti-Granzyme B was from Serotec (Raleigh, NC). Cytotoxicity was measured against IM9 cell line (20) or recipient fibroblasts by Delfia®EuTDA cytotoxicity assay on a Victor 2 microplate reader (both from PerkinElmer, Boston, MA). Following 7-9 days of pre-stimulation with Mitomycin C-treated targets (Sigma, 100mcg/mL) in the presence of IL-7 (5 ng/mL) and IL-2 (25 U/mL), effectors were washed and cultured in serial dilution with 5000 fresh untreated targets for 2 & 3hr. Percent specific europium chelated ligand (EuTDA) release was calculated: [Experimental release (counts) − Spontaneous release (counts)]/[Maximum release (counts) − Spontaneous release (counts)] ×100. Percent spontaneous release = [(Spontaneous release (counts)-background (counts)]/[Maximum release (counts) − background (counts)] ×100.
TCRVβ spectratyping and DKL analysis was performed as previously published (21).
Leukemia-specific CTL generation from CD3/28-expanded cultures
CD3/28-expanded ‘day 14’ T cells generated from CB grafts were cultured in parallel with 2 different Mitomycin C-treated leukemia cells (IM9 and U937) at a stimulator : responder ratio of 1:10 in 24 well plates (Costar, Corning, NY) at ∼1 × 106 cells/ml. IM9 leukemia cell line represents a lymphoid malignancy (20), while U937 monoblastoid leukemia cells are of myeloid origin (22). CTL were cultured in X Vivo-15 medium (Lonza, MD) supplemented with 5% fetal calf serum (Gibco, Invitrogen), 5ng/mL of IL-7, 5ng/mL IL15, and 10ng/mL IL-12 (all from R&D Systems) for 9-10 days. U837 cells were treated in vitro with IFNγ (R&D Systems) at 500U/ml for ∼48h prior to Mitomycin treatment to enhance their immunogenecity. Cultures were re-stimulated with Mitomycin C-treated leukemia cells twice. First, in the presence of IL-7 and IL-15, and second with IL-15 alone, each for 6-7 days. CTL cultures were refed with medium alone after half the medium was removed the night before culture termination. Washed effectors were tested in Delfia®EuTDA cytotoxicity assay as described above against fresh, unmanipulated BATDA®-loaded targets that included IM9 and U937 leukemia cells and PHA blasts of the CB transplant recipient. Percent specific EuTDA release was calculated as described above.
Analysis of human TRC gene rearrangement
The signal joint TCR excision circles (sjTREC) assay was performed using real-time quantitative polymerase chain reaction (RT-qPCR) by quantifying the episomal circles generated as a by-product of TCRα gene re-arrangement as we previously published (7). For each cell suspension prepared for TREC analysis total nucleated cell count and absolute T cells content was enumerated by Trucount FACS as previously described (10, 18) and thereafter dry pellets were prepared and kept frozen at -80C until batched matched pairs were thawed. TREC content was expressed after adjustment for 105 T cell/sample.
Statistical Analysis
Two sided paired student t-test was employed to compare conditions ± IL-7 and to compare T cell cytotoxicity against the described targets. Statistical significance was set at P values <0.05.
Results
Favorable impact of IL-7 on CB T cell survival, proliferation, and TCR Vβ repertoire during CD3/28 mediated expansion
Purified T cells obtained from frozen/thawed cord blood specimens were split and cultured in parallel with and without IL-7. Matched pair analysis demonstrated significantly more viable T cells when IL-7 was added to IL-2 in the medium leading to an average of 165 fold T cell expansion (Table 1 and Fig. 1A). Following 14 days of expansion, striking dilution of TCR excision circles was noted as the sjTREC content in CD3+ T cells was depleted by ∼ 2log in both culture conditions as compared to the starting population of pre-expansion cord blood T cells (Figure 1B), irrespective of IL-7 exposure. There was no significant difference in Trypan Blue viability between the culture conditions, typically >85% by days 12-14. This was in stark contrast once cellular events at the end of 12-14 days of culture were examined by flow cytometry. Significantly more viable CD45 bright T lymphocytes were identified in cultures supplanted with IL-7 (71% ±10) compared to cultures with IL-2 alone (mean 46% ±15) (Fig. 2A, Table 1). CD3/28-costimulated lymphocytes that have recently undergone apoptosis can be accurately enumerated with the aid of a simple flow cytometry gating strategy (10). Recently apoptotic/dead cells display altered physical properties, as defined by FSC/SSC, additionally stain less intensely with CD45 (and CD3) compared to ‘viable” lymphocytes (Fig 3A). We also tracked T cells that have recently entered the apoptosis pathway, identified/gated in the ‘viable’ CD45/FSC/SSC region by intracellular (ic) expression of activated Caspase-3 (Fig. 2C) and 7-AAD/Annexin stain as well (Fig. 3B). As determined by ic activated Capsase-3 expression, there were significantly fewer T cells undergoing active apoptosis in the presence of IL-7 (median 4% versus 8%) (Table 1, Fig. 3C). The anti-apoptotic effect if IL-7 was evident in both CD4+ and CD8+ subsets (Table 1). To test T cell survival promoting effects of IL-7 beyond the in vitro expansion period, expanded cells were frozen on Day 14 and subsequently thawed and rested for 24h in culture medium devoid of cytokines. Although the rest period in vitro can not mimic the in vivo post-infusion conditions exactly, these experiments demonstrate that T cells post expansion retain the potential to up-regulate IL-7 receptor/CD127 (Fig 3A), and that the majority of IL-7 + IL-2 expanded T cells are still alive after freeze, thaw, and 24 hour culture in medium (Fig 3B). Independent of the described anti-apoptotic effects, IL-7 promoted significantly greater T cell proliferation. About 2/3rd of all T cells were still actively cycling at the termination of the expansion period, as detected by intracellular Ki-67 expression (Fig 2B, Table 1). Since naïve T cells with recent thymic emigrant phenotype (CD28+/CD27+CD45RA+/CD62L+) represent the vast majority of unmanipulated CB T cells, these findings corroborate earlier studies demonstrating the proliferative and anti-apoptotic effects of IL-7 to be operational predominantly in the naïve/CD45RA+ T cell compartment (17, 23). In addition to superior T cell proliferation and reduced apoptosis in IL-7 supplanted conditions, we also found higher TCRVβ diversity per family (p=0.04, n=3) displaying a broad polyclonal spectrum (Fig.4).
Table 1. Surface and intracellular (ic) characterization of the expanded progeny (Mean, +/- SD).
Surface and intracellular phenotyping, PMA/ionomycin induced cytokine secretion was performed as described.(1-3)
| Variable | IL2 Only | IL2 + IL7 | p-value* |
|---|---|---|---|
| Day 12-14 Mean-fold expansion | 48 +/- 21 | 165 +/- 123 | 0.04 |
| %Viable T Lymphocytes among CD45+ events | 46 +/- 15 | 71 +/- 10 | 0.002 |
| %CD4+ | 60 +/- 20 | 65 +/- 16 | 0.26 |
| %CD8+ | 52 +/- 26 | 45 +/- 20 | 0.15 |
| %CD4+/CD8+ | 6 +/- 5 | 3 +/- 3 | 0.43 |
| ic BCL-2/CD3+ (MFI) | 82 +/- 19 | 82 +/- 16 | 0.97 |
| % ic Ki67+/CD3+ “proliferating” | 50 +/- 14 | 65 +/- 11 | 0.003 |
| %ic Ki67+/CD4+ “proliferating” | 50 +/- 15 | 62 +/- 10 | 0.03 |
| %ic Ki67+/CD8+ “proliferating” | 52 +/- 12 | 68 +/- 11 | 0.001 |
| % ic Activated Caspase 3+/CD3+ “apoptotic” | 8 +/- 3 | 4 +/- 2 | 0.011 |
| % ic Activated Caspase 3+/CD4+ “apoptotic” | 7 +/- 3 | 4 +/- 2 | 0.02 |
| % ic Activated Caspase 3+/CD8+ “apoptotic” | 8 +/- 5 | 4 +/- 2 | 0.03 |
| %CD25+/CD3+ | 62 +/- 14 | 63 +/- 17 | 0.95 |
| %CD25+/CD45RO+ | 43 +/- 19 | 25 +/- 28 | 0.08 |
| %CD28+/CD3+ | 89 +/- 5 | 95 +/- 3 | 0.02 |
| %CDRA+/RO- “naïve” | 45 +/- 30 | 78 +/- 28 | 0.06 |
| %CDRA-/RO+ “memory” | 10 +/- 4 | 18 +/- 27 | 0.65 |
| %CDRA+/CD62L+ “phenotypically naive” | 73 +/- 14 | 90 +/- 5 | 0.03 |
| %CDRA+/CD27+/CD8+ “naïve CD8+” | 79 +/- 7 | 90 +/- 4 | 0.003 |
| %CDRA-/CD27+/CD8+ “central memory” | 13 +/- 6 | 4 +/- 2 | 0.002 |
| %CDRA+/CD27-/CD8+ “effector CTL” | 7 +/- 6 | 6 +/- 4 | 0.26 |
| %CD57+/CD28-/CD8+ “effector CTL” | 0 +/- 0 | 0 +/- 0 | 0.35 |
| %HLA-DR+/CD3+ “activated” | 46 +/- 12 | 44 +/- 11 | 0.53 |
| %HLA-DR+/CD4+ “activated” | 45 +/- 12 | 37 +/- 11 | 0.1 |
| %HLA-DR+/CD8+ “activated” | 45 +/- 16 | 53 +/- 15 | 0.003 |
| %NKG2D+/CD3+ | 38 +/- 23 | 27 +/- 20 | 0.03 |
| %NKG2D+/CD137-/CD8+ “resting” | 73 +/- 17 | 62 +/- 26 | 0.05 |
| %NKG2D-/CD137+/CD8+ “activated” | 1 +/- 2 | 0 +/- 0 | 0.32 |
| %NKG2D-/CD137-/CD8+ “anergic” | 24 +/- 16 | 37 +/- 26 | 0.07 |
| %NKG2D+/CD137+/CD8+ “cytotoxic” | 2 +/- 3 | 1 +/- 1 | 0.24 |
| % ic GranzymeA+/CD8+ | 44 +/- 26 | 44 +/- 23 | 0.95 |
| % ic GranzymeB+/CD8+ | 41 +/- 35 | 35 +/- 25 | 0.26 |
| % ic Perforin+/CD8+ | 6 +/- 4 | 4 +/- 2 | 0.10 |
| % ic INFγ+/CD3+ | 20 +/- 8 | 19 +/- 10 | 0.64 |
| % ic TNFα +/CD3+ | 50 +/- 20 | 55 +/- 27 | 0.65 |
| % ic IL2+/CD4+ | 79 +/- 9 | 54 +/- 36 | 0.41 |
| % ic IL4+/CD3+ | 2 +/- 2 | 2 +/- 2 | 0.55 |
| % OX40+/CD4+ | 83 +/- 24 | 78 +/- 21 | 0.79 |
2-tailed, paired Student t-test
MFI: Mean Fluorescence Index
Figure 1. (A) CD3+ T cell expansion is superior in the presence of IL-7.
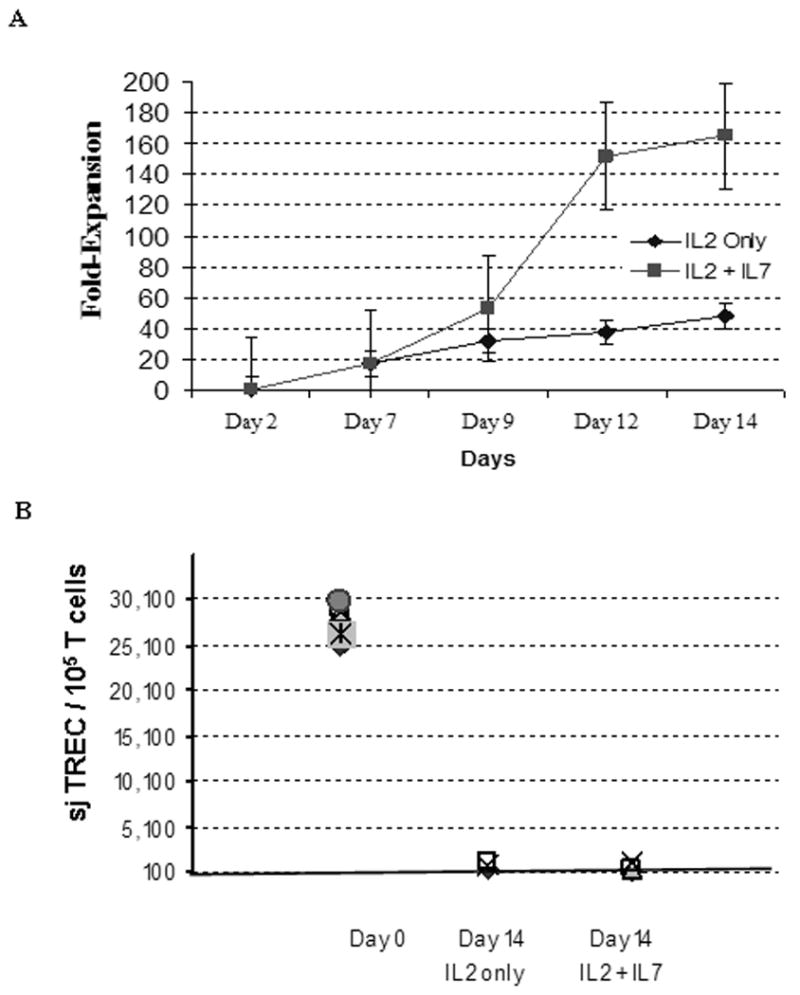
Frozen/thawed cord blood T cells were enriched by negative selection, enumerated by lyse/no wash Multitest T cell® staining in Trucount® tubes (BD) as previously described (18, 50), then split equally into two under identical culture conditions except for the presence of IL-7 as indicated. cell were cultured for 12—14 days with ClinExVivo™ Dynabeads® while medium and cytokines were replenished × 3/week. A 50ul aliquot was removed from the bags at indicated time points and absolute T cells number was enumerated in Trucount® tubes. (B) Irrespective of IL-7 in the culture medium, expansion leads to dilution and near complete loss of sjTREC in day 14 progeny. The signal joint TCR excision circles (sjTREC) were measured before and after expansion, n=4, as published previously (7). For each sample total nucleated cell count and absolute T cells content was enumerated by Trucount FACS method (10, 18). TREC content was expressed after adjustment for 105 T cells/sample.
Figure 2. Flow cytometry profile of the expanded T cell progeny ± IL-7.
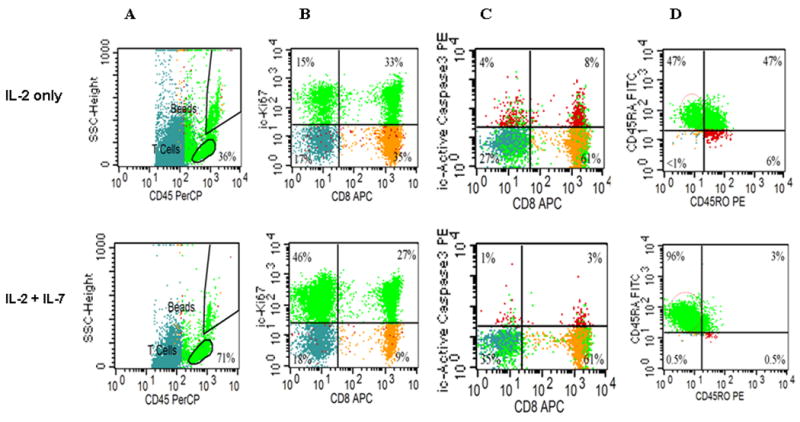
Surface and intracellular (ic) FACS characterization was performed as shown previously (10, 18, 19). The relative size of T cell subsets in each quadrant is expressed as the percentage of total viable T cells, see Table I for p values. (A) CD45-PERCP/SSC defines an unambiguous region of viable cells. All other CD45 dim cells (recently apoptotic) stain also dim for CD3, data not shown. (B) icKI-67 staining (upper quads) identifies more proliferating T cells when expanded with IL-7 than without. (C) When expanded without IL-7 more T cells undergo apoptosis and stain with ic ActiveCasp-3+ even though gated from the viable region of Fig2A. (D) More T cells display the phenotype of ‘naïve/CD45RA+/RO- T cells when expanded with IL-7. Representative of ten experiments.
Figure 3. (A) Kinetic analysis of surface CD25 and CD127 expression.
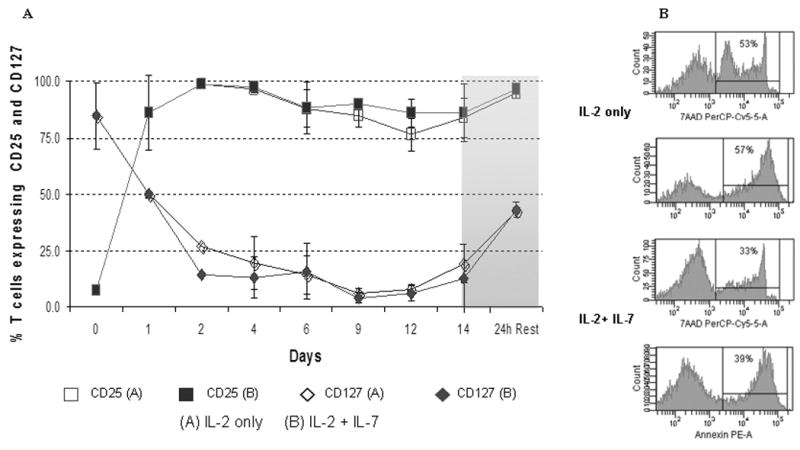
Simultaneous monitoring of IL-2Rα (CD25) and IL-7Rα (CD127) was performed after FACS surface staining and acquisition as described (18, 19) on serial aliquots obtained before (Day 0) and during expansion onn the indicated days. (B) Cell death after 24h of rest in cytokine free medium was assayed and scored by positive staining for Annexin and 7-AAD in parallel after freeze and thaw of expanded day 14 T cells, representative of four experiments.
Figure 4. TCR Vβ repertoire of T cells expanded in the presence of IL-7 display high TCR diversity.

Day 14 progeny of an IL-7 supplanted ClinExVivo™ CD3/28 co-stimulated culture. TCRVβ spectratyping and DKL analysis was performed as we previously published (21). Representative of three experiments.
Limited Th1/Tc1 ‘maturation’ during expansion and low expression levels of 4-1BB/CD137, CD40L, and perforin correlate with absent alloreactivity
Once we have demonstrated the salutary effects of IL-7 on T cell viability, expansion, and overall T cell receptor diversity, we sought to determine its impact on surface and intracellular phenotype and overall T cell function as measured by cytokine secretion profile and cytotoxicity. Despite undergoing several cycles of cell division triggered by IL-2 + IL-7 in concert with TCR and CD28 co-stimulation, significantly more CB T cells retained the naïve starting phenotype, CD45RA+/CD62L+ in the IL-7-containing condition (90% +/- 5%) compared to cells cultured in IL-2 alone (73% +/- 14%, p=0.03) (Fig. 2D). Surface expression of L-selectin (CD62L) is essential for effective T cell homing to secondary lymphoid organs, a desired destination for antigen inexperienced, unprimed adoptive T cell infusions. CCR-7, a chemokine receptor implicated in both the entry and also in the retaining of T cells in lymph nodes, was also expressed on the majority of expanded T cells, data not shown. Interestingly, while the surface of post-expansion T cells appeared identical to unmanipulated fresh cord blood T cells in terms of CD28+/CD27+/CD45RA+/CD62L co-expression, expanded T cells displayed several upregulated surface molecules commonly seen after activation, including CD25, HLA-DR, OX40, see Table 1. However, <10% of cells expressed CD40L, data not shown. Despite the preservation of resting, naïve, ‘RTE-mimicking’ surface phenotype, as indicated by CD28+/CD27+/CD45RA+/CD62L co-expression, CD3/CD28 co-stimulation led to rapid down-regulation of membrane CD127 (IL7Rα) in parallel with surface CD25 (IL2Rα) up-regulation on the very same T cells (Fig 3A). This “receptor switching process’ is not dependent on the presence of IL-7 in our cultures as CD25 and CD127 expression levels were superimposable in the presence and absence of IL-7 (Fig 3A). Moreover, since a near complete reversal between CD25 and CD127 expression has occurred by ∼24-48h of culture (Fig 3A) this phenomenon appears independent of cell division. Numerical T cell expansion does not begin in earnest in the cultures until day-3-4, (Fig 1, and data not shown). Interestingly, when expanded T cells were frozen on Day 14 and subsequently thawed and rested for 24h in culture medium devoid of cytokines, CD127 was re-expressed on nearly half of the viable T cells (Fig 3A). IL-7 receptor re-expression could permit delivery of pro-survival signals to expanded T cells administered by clinical DLI infusions in the lymphopenic post-transplant state where endogenous IL-7 level has been demonstrated to be elevated up to 10-30 pg/ml weeks after transplant (24). Although it is possible that high levels of IL-7 in vivo could induce down-regulation of CD127 in the responding T cells, nevertheless our results suggest that the expanded T cells retain the capacity to re-express CD127 even when rested post-thaw with IL-7 at 15-30 pg/ml, data not shown.
CD3/CD28 co-stimulation with ClinExVivo™ Dynabeads® in this series of experiments enhanced in a larger fraction of post-expansion T cells the capacity for intracellular expression of IFNγ, TNFα, and Granzyme B (Table 1) than we previously reported using different artificial-APC beads (10). Nevertheless, despite the potential for an increase in alloreactivity (25) after the more robust expansion in the presence of IL-7, the expanded progeny lacked cytotoxicity against a highly immunogenic (CD40+, CD80+, CD86+) EBV+ allogeneic lymphoblastoid cell line (IM9) (n=7), or recipient fibroblasts (n=2), despite a week long pre-sensitization prior to performing the CTL assay (Fig. 5). Interestingly, absent cytotoxicity coincided with low expression of 4-1BB/CD137, CD40L, and perforin (Table I). Together, these features support a favorable safety profile of ‘day 14” ClinExVivo™ expanded T cells with reduced likelihood for inducing GVHD in vivo upon adoptive transfer.
Figure 5. Absent cytotoxicity of the expanded CB T cells against allogeneic targets irrespective of ± IL-7.
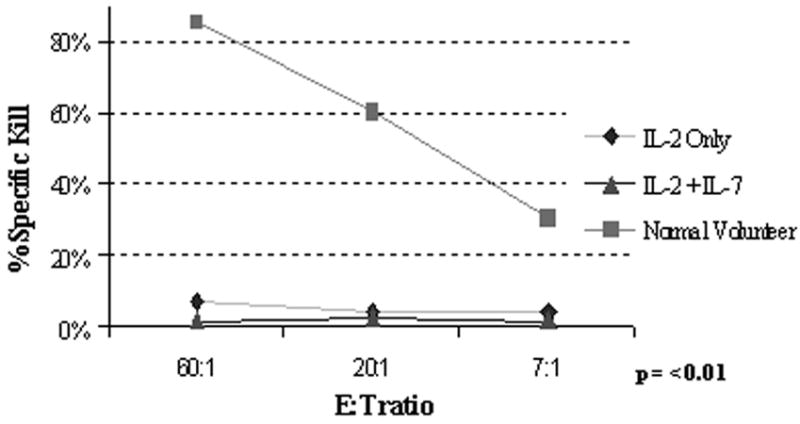
Effector T cells were obtained from PBL of healthy volunteers as positive controls and compared with CD3/28 co-stimulated CB T cells ± IL-7. First, effectors were primed/sensitized against a highly immunogeneic (HLA-DR+, CD40+, CD80+, CD86+) IM9 cell line for 7-9 days at 1:1 to 1:3 responder : stimulator ratio, then re-exposed to fresh BATDA®-loaded IM9 targets at the indicated E:T ratios for 2 & 3h. Europium release was measured by the Delfia® EuTDA cytotoxicity assay (10) and the calculated percent specific cytotoxicity is presented on the Y-axis. Representative of seven experiments.
Ex vivo expanded, CD3/CD28-costimulated cord blood T cells can be primed in vitro against lymphoid and myeloid leukemia
Donor leukocyte infusion with ‘day 14’ ClinExVivo™ +IL-2 +IL-7 expanded T cells generated from the originally infused cord blood graft could alleviate post-transplant lymphopenia and qualitative T cell defects until thymic regeneration could contribute new T cells. However, such DLI would be antigen non-specific and will require microbial and/or tumor antigens to in vivo prime infused T cells in the transplant recipients. In a series of experiments we evaluated the potential of ‘day 14’ CD3/CD28-costimulated/expanded T cells to undergo in vitro priming against specific leukemic targets. In vitro generated tumor-specific CTL responses could be adoptively infused to treat leukemia patients with minimal residual disease and/or relapse. CD3/CD28-expanded ‘day 14’ T cells were stimulated vitro for 3 weeks in parallel cultures with killed, Mitomycin C treated lymphoid leukemia cells (IM9) and IFNγ-treated myeloid leukemia cells (U937). U937 cells by themselves can not induce allogeneic T cell response unless a stimulating anti-CD3 antibody is added to cultures (22). In addition, they do not provide co-stimulation via the CD80/CD86-CD28 pathway, but likely via the ubiquitously expressed CD147, CD98 molecules (22). CTL priming was performed in the decreasing presence of IL-12, IL7, and IL-15 drawing from our published experimental strategy to in vitro prime anti-viral responses from cord blood (26). Robust T cell expansion (195X, ±115, n=4) ensued over the course of ∼ 3 weeks when killed leukemia cells rather than ClinExVivo™ beads served as APC. After the course of two to three repeated stimulations, strong leukemia-specific cytotoxicity was detected in CTL assays, killing the stimulating leukemia cells but not the other leukemia or most importantly cord blood transplant recipient PHA blasts, n=4, p=<0.01, (Fig 6A and Fig 6B). Failure to recognize and kill CB transplant recipient PHA blasts indicates future clinical safety from the potential toxicity of GVHD.
Figure 6. Leukemia-specific CTL can be in vitro primed starting with the CD3/28-expanded CB T cells.
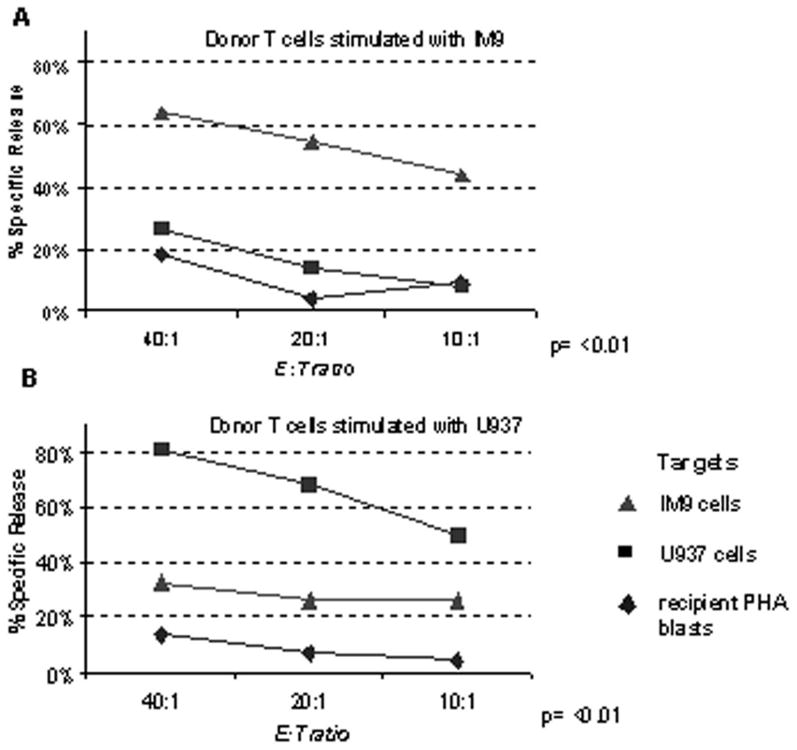
T cells were first CD3/28-expanded in the presence of IL2+ IL-7 over 14 days as described and thereafter were primed/sensitized against 2 killed leukemia cell lines in parallel cultures for 7-9 days at 10:1 responder : stimulator ratio in the presence of IL-12, IL-7, and IL-15. A, CTL primed in vitro with Mitomycin C-treated IM9 cells. B, CTL primed in vitro with IFNγ-treated and Mitomycin C-treated U937 cells. Each CTL culture was re-stimulated 2 more times (1st IL7+ IL-15, thereafter 2nd in IL15 alone) for a total of 3 weeks with their respective killed leukemia cells. Cytotoxicity of washed effectors after 3 weeks in CTL culture was tested against fresh, unmodified, BATDA®-loaded IM9, U937 cells, and recipient PHA blasts at the indicated E:T ratios for 3h, as indicated. Europium release was measured by the Delfia® EuTDA cytotoxicity assay and the calculated percent specific cytotoxicity is presented on the Y-axis. Representative of four experiments.
Discussion
Interleukin-7 is a very attractive immune modulator with proposed utility for in vivo administration to alleviate post-transplant lymphopenia, reviewed in (27). It is well accepted, that exogenous IL-7 can increase survival of naïve T cells and promotes homeostatic expansion in particular amongst naive T cells (28-31), reviewed in (17, 23). Large animal models have established that IL-7 alone will not only lead to peripheral expansion of naïve T cells but will also lead to enrichment of antiviral CMV-specific T cells (32), demonstrating the physiological relevance of IL-7Ra expression on both naïve cells and central memory T cells. Recently, the first cohorts of humans received rhIL-7 at the NCI to assess it's safety and effects on T cell homeostasis and function (33, 34). IL-7 induced in vivo T cell cycling, bcl-2 up-regulation, and a sustained increase in peripheral blood and total body T cell mass affecting both CD4+ and CD8+ subsets. Moreover, T cell expansion caused significant broadening of circulating T cell receptor (TCR) repertoire diversity as naive T cells expanded preferentially (34). Homeostatic expansion is the primary mechanism of IL-7 mediated immune-restoration in these studies accompanied by dilution of TCR excision circles (TREC) amongst sorted CD4+ and CD8+ naïve cells (34). Importantly, IL-7 may induce T cell maturation in non-lymphopenic circumstances as demonstrated by another NCI study performed in rhesus macaques (35). In immune-competent macaques IL-7 induced the acquisition of central memory-cell markers in both naive CD8+ and CD4+ T-cell subsets. Moreover, both central memory and effector memory T cells entered cell cycle, demonstrating IL-7s contribution to the maintenance of the entire T-cell pool. Nevertheless, taken together from the human and primate studies, there appears to be a preferential expansion of RTE and naïve T cells thus explaining the broadened TCVB repertoire post-IL-7 therapy and dilution of TREC in the sorted naïve cells.
Despite the consensus on the proliferative and anti-apoptotic effects of IL-7 there have been conflicting reports on the functional changes induced by IL-7. Most studies agree that IL-7 signaling can sensitize naïve CD4+ T cells and prime T cells for IL-2 induced IFNγ production (36-38), however, the variable degree of this functional maturation may have dramatic consequences in an allogeneic milieu where exaggerated T cell activation and alloreactivity could induce and/or potentiate graft versus host disease (GVHD), a continuing barrier to the safety of allogeneic transplantation, reviewed (39). It is still vigorously debated whether donor T cell exposure to IL-7 will worsen GVHD as some murine studies contend (10, 25, 40, 41) or not (42, 43), reviewed (17).
The impact of IL-7 on human cord blood T cells has previously been tested in vitro (28, 44-46), however, these experiments were probing it's effect without concomitant CD3/CD28 co-stimulation and without concurrent exogenous IL-2. Compared to adult T cells, cord blood T cells have been shown to express higher surface levels of IL-7Rα chain matched with lower amounts of the common γc chain. Notably, IL-7 does not alter the surface phenotype of proliferating naïve cells (28, 47). Interestingly, while in adult blood only “true” RTE, namely the CD31+ subset of CD62L+/CD45RA+/CD4+ T cells enter cell cycle in response to IL-7, in cord blood both CD31+ and CD31- cells proliferate vigorously (48).
Our data presented above clearly demonstrate the salutary role of IL-7 on ex vivo cord blood T cell expansion as evidenced by enhanced T cell cycling, reduced apoptosis, and increased TCRVβ diversity per family. Just as important from a clinical immunotherapy perspective, we also demonstrate that the addition of IL-7 to ex vivo expansion cultures emplying artificial CD3/28 APC beads permits the limited acquisition of granzymes, and secretion of Th1/Tc1 cytokines without acquisition of alloreactive cytotoxicity. Significantly, despite T cell maturation and an activated surface phenotype, IL-7 promotes the preservation of a surface phenotype (CD28+/CD27+/CD45RA+/CD62L), typically seen on naïve T cells including RTE. These surface features together with CCR7 expression should facilitate effective T cell homing to secondary lymphoid tissues that will be an essential step for the infused post-expansion T cells before priming could occur by encountering their cognate antigens in the right microenvironment. In parallel, we propose that reduced/absent cytotoxicity against allogeneic cells as a manifestation of residual functional ‘immaturity’ will be an essential parameter in order for clinical DLI products to safely cross the HLA barrier, as opposed to full ‘adult like’ cytotoxicity that may lead to severe alloreactivity/GVHD in the HLA-mismatched, unrelated UCBT setting. The predicted safety of expanded T cell products in moderate doses is supported by an in vivo NOD/SCID-β2m-/-mouse model, where adoptive transfer of CD3/28-costimulated human CB T cells facilitated engraftment in the absence of inducing xenogeneic GVHD (49).
To our knowledge, our study is the first to present a relatively simple, easily reproducible methodology to ex vivo expand cord blood T cells that meet both numerical and critical qualitative biological benchmarks to usher DLI to the clinical UCBT setting. Donor leukocyte infusions with ex vivo expanded T cells from the originally infused graft could alleviate post-transplant lymphopenia and qualitative T cell defects well before thymic regeneration could contribute new T cells. In fact, by starting with ∼2 ×106 total CB T cells, obtainable easily by ‘sacrificing’ 2-3% of a typical UCB graft, ∼100 fold expansion would yield ∼2 × 106cells/kg for patients up to 90 kg. Dose escalation/phase I trials will be started at lower doses in the HLA-mismatched UCBT setting to test safety while testing for augmented cellular immune function in a prophylactic manner for high risk patients such as those with viral reactivation, severe lymphopenia. Significantly, as we have demonstrated with both a lymphoid and a myeloid leukemia cell line model the antigen nonspecific expansion strategy will also lend itself as a starting platform to generate and expand large numbers of tumor-specific CTL available for adoptive transfer to treat residual and/or relapsed leukemia post-transplantation.
Acknowledgments
The authors thank Mark Bonyhadi, Joanne Kurtzberg for helpful comments, Eli Lien for ClinExVivo™ Dynabeads®, Susan Buntz, Jeff Hale, and Melissa Ventevogel for technical assistance, and the staff at the Duke University Stem Cell Laboratory and the Carolinas Cord Blood Bank at Duke University.
Grant Support: This work was supported by R01-CA132110 (PS). Immunoscope assays were performed in the Immune Reconstitution and Biomarker Analysis Shared Resource (Duke Human Vaccine Institute, GDS) which is housed in the Regional Biocontainment Laboratory at Duke (UC6 AI58607) and partially supported by the Duke Center for Translational Research (P30 AI51445).
Footnotes
Contribution: PSz designed all aspects of the research, analyzed data and wrote the paper; CD performed experiments, analyzed data; LM performed experiments, analyzed data; DJ performed experiments, analyzed data, GS analyzed data and wrote the paper.
Conflict-of interest disclosure: The authors declare no competing financial interests.
References
- 1.Fujita Y, Rooney CM, Heslop HE. Adoptive cellular immunotherapy for viral diseases. Bone Marrow Transplant. 2008;41(2):193–8. doi: 10.1038/sj.bmt.1705906. [DOI] [PubMed] [Google Scholar]
- 2.Goodwin VJ, Sato TA, Mitchell MD, Keelan JA. Anti-inflammatory effects of interleukin-4, interleukin-10, and transforming growth factor-beta on human placental cells in vitro. Am J Reprod Immunol. 1998;40(5):319–25. doi: 10.1111/j.1600-0897.1998.tb00060.x. [DOI] [PubMed] [Google Scholar]
- 3.Marchant A, Goldman M. T cell-mediated immune responses in human newborns: ready to learn? Clin Exp Immunol. 2005;141(1):10–8. doi: 10.1111/j.1365-2249.2005.02799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chalmers IM, Janossy G, Contreras M, Navarrete C. Intracellular cytokine profile of cord and adult blood lymphocytes. Blood. 1998;92(1):11–8. [PubMed] [Google Scholar]
- 5.Broxmeyer HE, American Association of Blood Banks . Cord blood : biology, immunology, and clinical transplantation. Bethesda, Md: AABB Press; 2004. [Google Scholar]
- 6.Szabolcs P, Niedzwiecki D. Immune reconstitution after unrelated cord blood transplantation. Cytotherapy. 2007;9(2):111–22. doi: 10.1016/j.bbmt.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klein AK, Patel DD, Gooding ME, et al. T-Cell recovery in adults and children following umbilical cord blood transplantation. Biol Blood Marrow Transplant. 2001;7(8):454–66. doi: 10.1016/s1083-8791(01)80013-6. [DOI] [PubMed] [Google Scholar]
- 8.Komanduri KV, St John LS, de Lima M, et al. Delayed immune reconstitution after cord blood transplantation is characterized by impaired thymopoiesis and late memory T-cell skewing. Blood. 2007;110(13):4543–51. doi: 10.1182/blood-2007-05-092130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parkman R, Cohen G, Carter SL, et al. Successful immune reconstitution decreases leukemic relapse and improves survival in recipients of unrelated cord blood transplantation. Biol Blood Marrow Transplant. 2006;12(9):919–27. doi: 10.1016/j.bbmt.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 10.Mazur MA, Davis CC, Szabolcs P. Ex vivo expansion and Th1/Tc1 maturation of umbilical cord blood T cells by CD3/CD28 costimulation. Biol Blood Marrow Transplant. 2008;14(10):1190–6. doi: 10.1016/j.bbmt.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parmar S, Robinson SN, Komanduri K, et al. Ex vivo expanded umbilical cord blood T cells maintain naive phenotype and TCR diversity. Cytotherapy. 2006;8(2):149–57. doi: 10.1080/14653240600620812. [DOI] [PubMed] [Google Scholar]
- 12.June CH, Ledbetter JA, Linsley PS, Thompson CB. Role of the CD28 receptor in T-cell activation. Immunol Today. 1990;11(6):211–6. doi: 10.1016/0167-5699(90)90085-n. [DOI] [PubMed] [Google Scholar]
- 13.Levine BL, Bernstein WB, Aronson NE, et al. Adoptive transfer of costimulated CD4+ T cells induces expansion of peripheral T cells and decreased CCR5 expression in HIV infection. Nat Med. 2002;8(1):47–53. doi: 10.1038/nm0102-47. [DOI] [PubMed] [Google Scholar]
- 14.Laport GG, Levine BL, Stadtmauer EA, et al. Adoptive transfer of costimulated T cells induces lymphocytosis in patients with relapsed/refractory non-Hodgkin lymphoma following CD34+-selected hematopoietic cell transplantation. Blood. 2003;102(6):2004–13. doi: 10.1182/blood-2003-01-0095. [DOI] [PubMed] [Google Scholar]
- 15.Porter DL, Levine BL, Bunin N, et al. A phase 1 trial of donor lymphocyte infusions expanded and activated ex vivo via CD3/CD28 costimulation. Blood. 2006;107(4):1325–31. doi: 10.1182/blood-2005-08-3373. [DOI] [PubMed] [Google Scholar]
- 16.Hagihara M, Chargui J, Gansuvd B, et al. Umbilical cord blood T lymphocytes are induced to apoptosis after being allo-primed in vitro. Bone Marrow Transplant. 1999;24(11):1229–33. doi: 10.1038/sj.bmt.1702050. [DOI] [PubMed] [Google Scholar]
- 17.Snyder KM, Mackall CL, Fry TJ. IL-7 in allogeneic transplant: clinical promise and potential pitfalls. Leuk Lymphoma. 2006;47(7):1222–8. doi: 10.1080/10428190600555876. [DOI] [PubMed] [Google Scholar]
- 18.Szabolcs P, Park KD, Reese M, Marti L, Broadwater G, Kurtzberg J. Coexistent naive phenotype and higher cycling rate of cord blood T cells as compared to adult peripheral blood. Exp Hematol. 2003;31(8):708–14. doi: 10.1016/s0301-472x(03)00160-7. [DOI] [PubMed] [Google Scholar]
- 19.Szabolcs P, Park KD, Marti L, et al. Superior depletion of alloreactive T cells from peripheral blood stem cell and umbilical cord blood grafts by the combined use of trimetrexate and interleukin-2 immunotoxin. Biol Blood Marrow Transplant. 2004;10(11):772–83. doi: 10.1016/j.bbmt.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Harris DT, LoCascio J, Besencon FJ. Analysis of the alloreactive capacity of human umbilical cord blood: implications for graft-versus-host disease. Bone Marrow Transplant. 1994;14(4):545–53. [PubMed] [Google Scholar]
- 21.Kepler TB, He M, Tomfohr JK, Devlin BH, Sarzotti M, Markert ML. Statistical analysis of antigen receptor spectratype data. Bioinformatics. 2005;21(16):3394–400. doi: 10.1093/bioinformatics/bti539. [DOI] [PubMed] [Google Scholar]
- 22.Stonehouse TJ, Woodhead VE, Herridge PS, et al. Molecular characterization of U937-dependent T-cell co-stimulation. Immunology. 1999;96(1):35–47. doi: 10.1046/j.1365-2567.1999.00670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Surh CD, Boyman O, Purton JF, Sprent J. Homeostasis of memory T cells. Immunol Rev. 2006;211:154–63. doi: 10.1111/j.0105-2896.2006.00401.x. [DOI] [PubMed] [Google Scholar]
- 24.Bolotin E, Annett G, Parkman R, Weinberg K. Serum levels of IL-7 in bone marrow transplant recipients: relationship to clinical characteristics and lymphocyte count. Bone Marrow Transplant. 1999;23(8):783–8. doi: 10.1038/sj.bmt.1701655. [DOI] [PubMed] [Google Scholar]
- 25.Chung B, Dudl E, Toyama A, Barsky L, Weinberg KI. Importance of interleukin-7 in the development of experimental graft-versus-host disease. Biol Blood Marrow Transplant. 2008;14(1):16–27. doi: 10.1016/j.bbmt.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 26.Park KD, Marti L, Kurtzberg J, Szabolcs P. In vitro priming and expansion of cytomegalovirus-specific Th1 and Tc1 T cells from naive cord blood lymphocytes. Blood. 2006;108(5):1770–3. doi: 10.1182/blood-2005-10-006536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van den Brink MR, Alpdogan O, Boyd RL. Strategies to enhance T-cell reconstitution in immunocompromised patients. Nature reviews. 2004;4(11):856–67. doi: 10.1038/nri1484. [DOI] [PubMed] [Google Scholar]
- 28.Soares MV, Borthwick NJ, Maini MK, Janossy G, Salmon M, Akbar AN. IL-7-dependent extrathymic expansion of CD45RA+ T cells enables preservation of a naive repertoire. J Immunol. 1998;161(11):5909–17. [PubMed] [Google Scholar]
- 29.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1(5):426–32. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 30.Tan JT, Dudl E, LeRoy E, et al. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc Natl Acad Sci U S A. 2001;98(15):8732–7. doi: 10.1073/pnas.161126098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mackall CL, Fry TJ, Bare C, Morgan P, Galbraith A, Gress RE. IL-7 increases both thymic-dependent and thymic-independent T-cell regeneration after bone marrow transplantation. Blood. 2001;97(5):1491–7. doi: 10.1182/blood.v97.5.1491. [DOI] [PubMed] [Google Scholar]
- 32.Lu H, Zhao Z, Kalina T, et al. Interleukin-7 improves reconstitution of antiviral CD4 T cells. Clin Immunol. 2005;114(1):30–41. doi: 10.1016/j.clim.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 33.Rosenberg SA, Sportes C, Ahmadzadeh M, et al. IL-7 administration to humans leads to expansion of CD8+ and CD4+ cells but a relative decrease of CD4+ T-regulatory cells. J Immunother. 2006;29(3):313–9. doi: 10.1097/01.cji.0000210386.55951.c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sportes C, Hakim FT, Memon SA, et al. Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets. J Exp Med. 2008;205(7):1701–14. doi: 10.1084/jem.20071681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moniuszko M, Fry T, Tsai WP, et al. Recombinant interleukin-7 induces proliferation of naive macaque CD4+ and CD8+ T cells in vivo. J Virol. 2004;78(18):9740–9. doi: 10.1128/JVI.78.18.9740-9749.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Webb LM, Foxwell BM, Feldmann M. Interleukin-7 activates human naive CD4+ cells and primes for interleukin-4 production. Eur J Immunol. 1997;27(3):633–40. doi: 10.1002/eji.1830270309. [DOI] [PubMed] [Google Scholar]
- 37.Fukui T, Katamura K, Abe N, et al. IL-7 induces proliferation, variable cytokine-producing ability and IL-2 responsiveness in naive CD4+ T-cells from human cord blood. Immunol Lett. 1997;59(1):21–8. doi: 10.1016/s0165-2478(97)00093-x. [DOI] [PubMed] [Google Scholar]
- 38.Managlia EZ, Landay A, Al-Harthi L. Interleukin-7 signalling is sufficient to phenotypically and functionally prime human CD4 naive T cells. Immunology. 2005;114(3):322–35. doi: 10.1111/j.1365-2567.2004.02089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reddy P, Arora M, Guimond M, Mackall CL. GVHD: a continuing barrier to the safety of allogeneic transplantation. Biol Blood Marrow Transplant. 2009;15(1 Suppl):162–8. doi: 10.1016/j.bbmt.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sinha ML, Fry TJ, Fowler DH, Miller G, Mackall CL. Interleukin 7 worsens graft-versus-host disease. Blood. 2002;100(7):2642–9. doi: 10.1182/blood-2002-04-1082. [DOI] [PubMed] [Google Scholar]
- 41.Chung B, Dudl EP, Min D, Barsky L, Smiley N, Weinberg KI. Prevention of graft-versus-host disease by anti IL-7Ralpha antibody. Blood. 2007;110(8):2803–10. doi: 10.1182/blood-2006-11-055673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alpdogan O, Schmaltz C, Muriglan SJ, et al. Administration of interleukin-7 after allogeneic bone marrow transplantation improves immune reconstitution without aggravating graft-versus-host disease. Blood. 2001;98(7):2256–65. doi: 10.1182/blood.v98.7.2256. [DOI] [PubMed] [Google Scholar]
- 43.Alpdogan O, Muriglan SJ, Eng JM, et al. IL-7 enhances peripheral T cell reconstitution after allogeneic hematopoietic stem cell transplantation. J Clin Invest. 2003;112(7):1095–107. doi: 10.1172/JCI17865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dardalhon V, Jaleco S, Kinet S, et al. IL-7 differentially regulates cell cycle progression and HIV-1-based vector infection in neonatal and adult CD4+ T cells. Proc Natl Acad Sci U S A. 2001;98(16):9277–82. doi: 10.1073/pnas.161272698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jaleco S, Swainson L, Dardalhon V, Burjanadze M, Kinet S, Taylor N. Homeostasis of naive and memory CD4+ T cells: IL-2 and IL-7 differentially regulate the balance between proliferation and Fas-mediated apoptosis. J Immunol. 2003;171(1):61–8. doi: 10.4049/jimmunol.171.1.61. [DOI] [PubMed] [Google Scholar]
- 46.Vakkila J, Aysto S, Saarinen-Pihkala UM, Sariola H. Naive CD4+ T cells can be sensitized with IL-7. Scand J Immunol. 2001;54(5):501–5. doi: 10.1046/j.1365-3083.2001.01001.x. [DOI] [PubMed] [Google Scholar]
- 47.Dardalhon V, Jaleco S, Rebouissou C, et al. Highly efficient gene transfer in naive human T cells with a murine leukemia virus-based vector. Blood. 2000;96(3):885–93. [PubMed] [Google Scholar]
- 48.Azevedo RI, Soares MV, Barata JT, et al. IL-7 sustains CD31 expression in human naive CD4+ T cells and preferentially expands the CD31+ subset in a PI3K-dependent manner. Blood. 2009;113(13):2999–3007. doi: 10.1182/blood-2008-07-166223. [DOI] [PubMed] [Google Scholar]
- 49.Hexner EO, Danet-Desnoyers GA, Zhang Y, et al. Umbilical cord blood xenografts in immunodeficient mice reveal that T cells enhance hematopoietic engraftment beyond overcoming immune barriers by stimulating stem cell differentiation. Biol Blood Marrow Transplant. 2007;13(10):1135–44. doi: 10.1016/j.bbmt.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 50.Szabolcs P, Park KD, Reese M, Marti L, Broadwater G, Kurtzberg J. Absolute values of dendritic cell subsets in bone marrow, cord blood, and peripheral blood enumerated by a novel method. Stem Cells. 2003;21(3):296–303. doi: 10.1634/stemcells.21-3-296. [DOI] [PubMed] [Google Scholar]


