Abstract
Purpose
We hypothesized that the type-1 interferons (IFNs) would play a pivotal role in anti-glioma immunosurveillance through promotion of type-1 adaptive immunity and suppression of immunoregulatory cells.
Experimental Design
We induced de novo gliomas in Ifnar1−/− (deficient for type-1 IFN receptors) or wild type (WT) mice by intracerebroventricuar transfection of NRAS and a short hairpin RNA against P53 using the Sleeping Beauty (SB) transposon system. We analyzed survival of 587 glioma patients for single-nucleotide polymorphisms (SNPs) in type-1 IFN-related genes.
Results
Ifnar1−/− mice exhibited accelerated tumor growth and death. Analyses of brain tumor-infiltrating lymphocytes (BILs) in Ifnar1−/− mice revealed an increase of cells positive for CD11b+Ly6G+ and CD4+FoxP3+, which represent myeloid-derived suppressor cells (MDSCs) and regulatory T-cells (Tregs), respectively, but a decrease of CD8+ CTLs compared with WT mice. Ifnar1−/− mouse-derived glioma tissues exhibited a decrease in mRNA for the CTL-attracting chemokine CXCL10, but an increase of CCL2 and CCL22, both of which are known to attract immunoregulatory cell populations. Dendritic cells (DCs) generated from the bone marrow of Ifnar1−/− mice failed to function as effective antigen-presenting cells (APCs). Moreover, depletion of Ly6G+ cells prolonged the survival of mice with developing gliomas. Human epidemiological studies revealed that SNPs in IFNAR1 and IFNA8 are associated with significantly altered overall survival of patients with WHO grade 2–3 gliomas.
Conclusions
The novel SB-induced murine glioma model led us to discover a pivotal role for the type-1 IFN pathway in anti-glioma immunosurveillance and relevant human SNPs that may represent novel prognostic markers.
Introduction
Gliomas are the most common malignant brain tumors. Despite their dismal prognosis, limited information is available regarding their etiology and the prognostic factors that influence patients’ survival. It is therefore critical to gain a better understanding of the complex biological interactions that regulate glioma development and growth.
Animal models that mimic the complexity of human gliomas would be useful in understanding glioma biology and predicting therapeutic responses. In this regard, a novel SB transposon-mediated de novo murine glioma model has been recently developed in which tumor initiation and progression can be monitored by bioluminescent imaging (1). These murine tumors share many features with the human disease including glial marker expression, pseudopalisading necrosis, and brain invasion. In contrast to traditional transplanted models, these tumors evolve with the host immune system; herein we demonstrate that they are profoundly infiltrated by regulatory immune cells that suppress anti-tumor immunity, which is similar to human gliomas (2). Therefore, this de novo glioma model allows us to address immunological mechanisms of gliomagenesis.
With regard to tumorigenesis, the concept of cancer immunosurveillance has been proposed; that is, the immune system can protect the host against tumor development (3). Among the relevant molecules, type-1 IFNs have been suggested as central coordinators in the dynamic relationship between the host immune system and cancers (4). In particular, hematopoietic cells in the host (rather than tumor cells) have been shown to be the crucial targets of the antitumor activity of endogenous type-1 IFNs (4).
In recent years, a number of SNPs across the genome have been identified in association with cancers (5). With regard to SNPs in immune system modulators, previous epidemiological studies including ours have demonstrated that SNPs in Interleukin (IL)-4R are associated with altered glioma risks (6) and prognosis (7,8). Another recent study has demonstrated that a common variant (V249I) in the chemokine receptor Cx3cr11 is associated with increased survival and reduced tumor infiltration by microglia in glioma patients (9). These studies suggest that cytokines and chemokines may be critically involved in the pathways that regulate glioma development and prognosis.
Based on these findings, we hypothesized that defects in the type-1 IFN pathway would play an important role in the pathogenesis and clinical course of gliomas. In the current study, we show that the Ifnar1-deficiency in mice accelerates gliomagenesis and is associated with an increased infiltration of MDSCs and Tregs along with a decreased infiltration of DCs and CTLs. In particular, DCs derived from Ifnar1−/− mice exhibit altered antigen-presenting functions. Furthermore, monoclonal antibody (mAb)-mediated depletion of MDSCs prolongs the survival of tumor-developing mice, implicating that MDSCs promote glioma development. Moreover, type-1 IFN-inducing treatment delays gliomagenesis in mice in an Ifnar1-dependent manner. Finally, we show significant effects of SNPs in type-1 IFN-related genes (IFNAR1 and IFNA8) on survival of patients with WHO grade 2–3 gliomas. Collectively, these findings indicate a pivotal role of the type-1 IFN pathway in gliomagenesis and support the development of type-1 IFN-based strategies for prevention of malignant transformation in glioma patients.
Materials and Methods
Animals
WT C57BL/6 mice (H-2b) were obtained from Taconic Farms. C57BL/6-background Ifnar1−/− mice were kindly provided by Dr. Murali-Krishna Kaja (University of Washington, WA); they are deficient for α-subunit for type-1 IFN receptor (10). Pmel-1 mice were obtained from The Jackson Laboratory; they are C57BL/6-background and transgenic for an hgp10025–33-specific T cell receptor that cross-reacts to an H-2Db/mgp10025–33 complex (11). Animals were handled in the Animal Facility at the University of Pittsburgh per an Institutional Animal Care and Use Committee-approved protocol.
Antibodies, gp100-specific tetramer, and peptides
The following mAbs were obtained from BD Biosciences: anti-CD11c (HL3), anti-CD4 (VH129.19), anti-CD8 (53-6.7), CD86 (GL1), anti-I-Ab (AF6-120.1), anti-IFN-γ (XMG1.2), anti-IL-4 (11B11), anti-H2-Kb (AF6-88.5), and isotype-matched controls. Anti-CCR7 (4B12) mAb was obtained from BioLegend. The following mAbs were obtained from eBioScience: anti-CD11b (M1/70), anti-FoxP3 (NRRF-30), and anti-Ly6G (RB6-8C5). Anti-CD25 mAb (PC61) was kindly provided by Dr. Masaki Terabe (National Cancer Institute, Bethesda, MD). Control IgG was obtained from Sigma-Aldrich. H-2Db/mgp10025–33 tetramer was obtained from National Institute of Allergy and Infectious Disease Tetramer Facility at Emory University Vaccine Center (Altanta, GA). The following peptides were synthesized in University of Pittsburgh Peptide Synthesis Facility: H-2Db-binding human/mouse gp100 (h/mgp100) 25–33 (KVPRNQDWL), H-2Db-binding Garc177–85 (AALLNKLYA), and H-2Db-binding EphA2671–679 (FSHHNIIRL).
Intracerebroventricular DNA injection
The procedure has been described previously (1). In vivo-compatible DNA transfection reagent (In vivo-JetPEI™) was obtained from Polyplus Transfection. The following DNA plasmids were used: pT2/C-Luc//PGK-SB13, pT/CAGGS-NRASV12, and pT2/shP53.
In vivo bioluminescent intensity (BLI) measurement
The procedure has been described previously (12). Luciferin was obtained from Caliper Life Sciences.
Tumor cell culture
YAC1 murine lymphoma cell line was obtained from ATCC. All cells were maintained in a mouse complete medium consisting of RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum, 100 units/ml penicillin, 100 mg/ml streptomycin, and 10 μM L-glutamine in a humidified incubator in 5% CO2 at 37°C. All of the reagents described here were obtained from Invitrogen Life Technologies.
Intracranial cell injection and BIL isolation
The procedure has been described previously (13).
Preparation of CTLs and DCs
The procedure has been described previously (13). The following recombinant murine cytokines were obtained from R&D Systems: granulocyte/macrophage colony-stimulating factor, rmIFN-α, and rmIL-12. Recombinant human IL-2 (rhIL-2) was obtained from PeproTech. Lipopolysaccaride (LPS) was obtained from Sigma-Aldrich. We used 3×106 CTLs or 1×106 DCs.
Quantitative real-time polymerase chain reaction (RT-PCR)
The procedure has been described previously (14). The primers and probes for the following genes were obtained from Applied Biosystems: Ccl2 (Mm00441242_m1), Ccl22 (Mm00436439_m1), Cxcl10 (Mm99999072_m1), Gp100 (Mm00498996_m1), Epha2 (Mm00438726_m1), and Trp2 (Mm00441984_m1).
Cytokine and chemokine release assay
The ELISA kits were obtained as follows: mIFN-γ from BD Biosciences; mIL-12p70 from eBioscience; mCXCL10 and mCCL22 from R&D Systems. All assays were conducted according to the manufacturers’ instructions.
In vivo CTL proliferation assay and cytolytic assay
The procedure has been described previously (13). Carboxyfluorescein diacetate succinimidyl ester (CFSE) was obtained from Invitrogen.
mAb-mediated cell depletion assay
The protocol was established on the basis of previous studies (15, 16). Mice with developing gliomas received intraperitoneal (i.p.) injections of anti-Ly6G mAb (clone RB6-8C5, 0.25 mg/dose) on days 21, 23, 25, and 27 after tumor induction. Some mice received i.p. injections of anti-CD25 mAb (clone PC61, 0.25 mg/dose) on days 21 and 24 after tumor induction.
Administraion of polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose (poly-ICLC)
Poly-ICLC was kindly provided by Oncovir (Washington, D.C.). Mice with developing gliomas received intramuscular (i.m.) injections of either poly-ICLC (2.5 mg/kg/dose) or mock PBS starting on days 21 and 24 after tumor induction, and weekly thereafter.
Human subjects
The population used in this study has been described previously (17). Adults (aged 18 and older) newly diagnosed with a glioma (ICD-O-3 codes 9380-9481) in Harris County, TX were recruited between 2001 and 2006. The original study population (n=761) was restricted to non-Hispanic whites with WHO grade 2–4 gliomas (n=587) for the genetic analyses. Blood samples for DNA extraction were collected from the cases before initiation of chemotherapy or radiation therapy. The male to female ratio was 1.4:1. Treatment and survival data were collected from medical record review for all cases. The study was approved by the institutional review boards of all participating institutions, and written informed consent was obtained from each participant.
SNP selection and genotyping
Given our interest in type-1 IFNs, SNPs present on the Illumina Human 610 Quad SNP Chip in the following genes were selectively addressed: IFNAR1, IFNAR2, IFNA1, IFNA2, IFNA4, IFNA5, IFNA6, IFNA7, IFNA8, IFNA13, IFNA14, IFNA16, and IFNA21. DNA samples were excluded if less than 95% of loci were successfully genotyped. For all SNPs utilized in this analysis, ≥95% of the samples was genotyped successfully and each SNP had a GenCall score of ≥0.25. In addition, duplicate samples were used for quality control; >99% of all genotyping results were concordant between original and duplicate samples.
Statistical analyses
In mouse studies, the statistical significance of differences between two groups was determined by Student’s t-test; one-way analysis of variance with Holm’s post hoc test was conducted for a multiple group comparison. Log-rank test was used to determine significant differences in survival curves among groups. All mouse data were analyzed by R Environment version 2.10.1. In human studies, survival time was calculated beginning at the date of hospital registration. Log-rank test was used to determine significant differences in survival curves stratified by genotype. The proportional hazards assumption for each model was tested using log-log plots; there was no evidence that the proportional hazards assumption was violated for any of the models. All human data were analyzed by SAS version 9.1. P<0.05 was considered to be statistically significant.
Results
Ifnar1−/− mice exhibit accelerated tumor growth and death following SB-based induction of gliomas
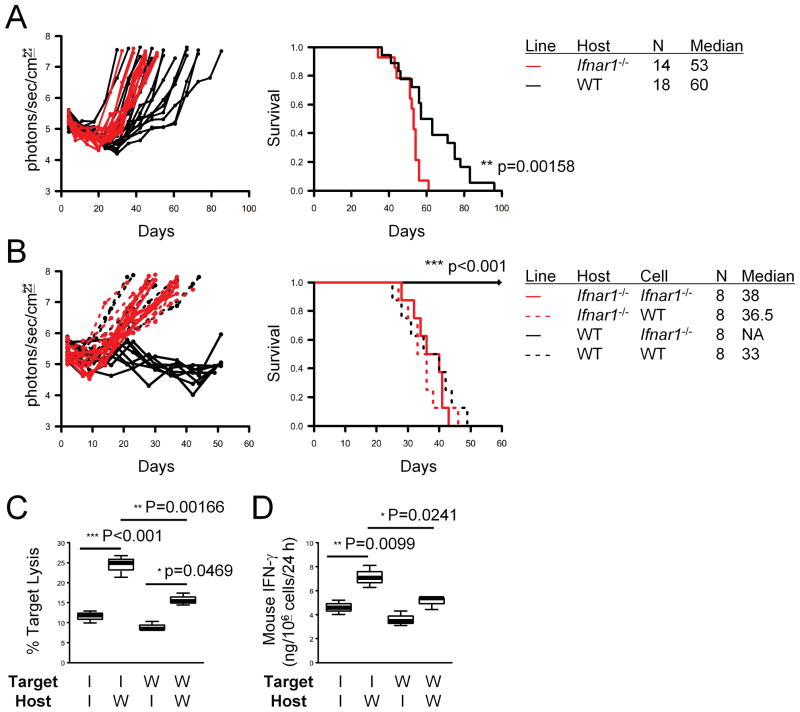
We first evaluated the role of type-1 IFN pathway on development of gliomas induced by SB-mediated transfection of NRAS and shp53 (silencing RNA against P53) in Ifnar1−/− or WT mice. The induced tumors exhibited characteristics of human gliomas such as pseudopalisading necrosis and invasion (Supplementary Fig. 1). Ifnar1−/− mice exhibited accelerated tumor growth (Fig. 1A, left panel) and death (right panel) compared with WT mice.
Figure 1. Ifnar1−/− mice exhibit accelerated tumor growth and death following SB-based induction of gliomas.
(A) CNS gliomas were induced in C57BL/6-background Ifnar1−/− (red lines) or WT (black lines) neonatal mice by intraventricular transfection of the following plasmids: pT2/C-Luc//PGK-SB1.a3 (0.2 μg), pT/CAG-NRas (0.4 μg), and pT/shp53 (0.4 μg). Tumor growth (left) and symptom-free survival (right) were monitored. P-values are based on log-rank test. (B) Glioma cells derived from Ifnar1−/− (solid lines) or WT (dashed lines) mice were injected into the basal ganglia of Ifnar1−/− (red lines) or WT (black lines) host mice (1 × 105/mouse). Tumor growth (left) and symptom-free survival (right) were monitored. (C) 51Cr release assay was performed to evaluate cytolytic ability of cervical LN cells obtained from the mice bearing Ifnar1−/− cells (solid lines in B) against the Ifnar1−/− and WT cell lines. (D) ELISA was performed to evaluate the amount of mIFN-γ secretion by the CTLs used in (C) per the last 24 hrs. (C and D) I: Ifnar1−/−; W: WT. Lines within boxes denote means; box upper and lower bounds indicate SD; whiskers indicate minimum and maximum values. P-values are based on Holm’s post hoc test.
To characterize the induced gliomas further, we isolated tumor cells from the tumor tissues of Ifnar1−/− or WT mice, and established a total of 41 cell lines in vitro (WT: 19; Ifnar1−/− : 22). All cell lines exhibited spindle-shape morphology and grew at a comparable rate (data not shown). Ifnar1−/− and WT tumors expressed similar levels of the GAAs gp100 (Fig. 2D) and EphA2 in vivo (Supplementary Fig. 2A) as well as MHC class I and II in vitro (Supplementary Fig. 2B). To determine their tumorigenicity in vivo, we injected three cell lines established from each of Ifnar1−/− and WT mice into the brains of Ifnar1−/− and WT mice. Data in Fig. 1B represent experiments with one cell line from each genotype; we observed similar results in all cell lines (data not shown). Ifnar1−/− glioma cells failed to grow in WT hosts (Fig. 1B, black solid lines), while these cells grew aggressively in Ifnar1−/− hosts (red solid lines). In contrast, WT mouse-derived glioma cells grew aggressively both in Ifnar1−/− and WT hosts (dashed lines). These data suggest that the environment of Ifnar1−/− hosts allowed the growth of gliomas that would not have grown in the immunologically competent microenvironment of the central nervous system (CNS).
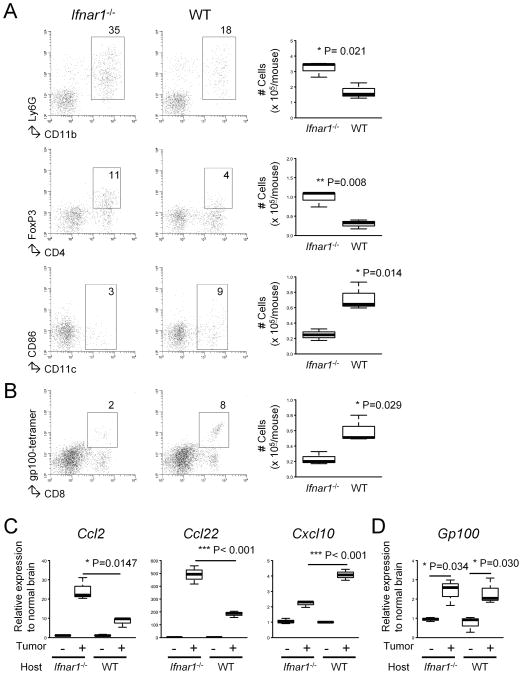
Figure 2. Ifnar1−/− mice demonstrate increased tumor-infiltration of CD11b+Ly6G+ and CD4+FoxP3+ cells but decreased Tc1 effector cells and CD11c+ DCs.
(A and B) The mice bearing SB-induced tumors were sacrificed at around days 50–60, and BILs were isolated based on similar tumor size observed by BLI. BILs obtained from 3 mice in a given group were pooled and then evaluated by flow cytometry for the following subpopulations: CD11b+Ly6G+, CD4+FoxP3+, CD11c+CD86+ (A) and CD8+gp100 tetramer+ (B). Numbers in dot plots indicate percentage of gated subpopulations in leukocyte-gated BILs. Absolute numbers are depicted in the rightmost panels. P-values are based on Student’s t-test. (C and D) Total RNA was extracted from each mouse brain (3 mice/group). Quantitative RT-PCR was performed to evaluate the mRNA expression levels of the following molecules: Ccl2, Ccl22, Cxcl10 (C) and Gp100 (D).
We next sought to determine whether the observed rejection of Ifnar1−/− glioma cells in WT hosts involved CTL-mediated recognition. To this end, we evaluated CTL responses mounted in cervical lymph nodes (LNs) of mice that had received inoculation of Ifnar1−/− or WT glioma cells in the brain. CTLs isolated from Ifnar1−/− hosts exhibited reduced tumoricidal function against the inoculated cell lines compared with WT hosts (Fig. 1C). Moreover, consistent with the Fig. 1B, the WT hosts that had received Ifnar1−/− tumor cells demonstrated the highest levels of CTL activity against the same Ifnar1−/− glioma cells (Fig. 1C). Additional experiments showed minimum lysis of YAC1 target cells, supporting that these responses were not merely antigen-independent natural killer cell responses (Supplementary Fig. 3). In accordance with the CTL data, LN cells derived from WT mice bearing Ifnar1−/− tumors produced the highest level of IFN-γ among the groups (Fig. 1D). Taken together, these data suggest that the Ifnar1−/− tumor cells are more immunogenic than the WT tumor cells in their ability to induce CTL responses.
Ifnar1−/− mice exhibit increased tumor-infiltration of CD11b+Ly6G+ and CD4+FoxP3+ cells but decreased Tc1 effector cells and CD11c+ DCs
We next evaluated whether the defect of the type-1 IFN pathway would impact the immunological microenvironment of de novo gliomas by flow cytometric analyses of BILs. Gliomas induced in Ifnar1−/− mice were infiltrated with remarkably increased numbers of CD11b+Ly6G+ cells (35% vs 18% of leukocyte-gated cells) and CD4+FoxP3+ cells (11% vs 4%), which are considered to be MDSCs and Tregs, respectively (Fig. 2A). Although the small numbers of these cells obtained from small brain tumors in mice did not allow functional analyses of these cells ex vivo, these data suggest that the type-1 IFN pathway plays a critical role in limiting the accumulation of MDSCs and Tregs in the glioma microenvironment. To determine the effects of host type-1 IFN pathway on the recruitment of type-1 effector CTLs (Tc1) into the SB-induced tumors (which express gp100; Fig. 2D), ex vivo activated gp100-reactive Tc1 derived from Pmel-1 mice (13,18) were intravenously (i.v.) transferred into Ifnar1−/− or WT mice bearing established SB-induced gliomas (Fig. 2B). On the fifth day following the i.v. transfer, Ifnar1−/− mice demonstrated a lesser degree of tumor-infiltration by CD8+gp100-tetramer+ Tc1s (2%) compared with WT mice (8%) with SB-induced tumors.
We hypothesized that the observed alterations of BIL populations in Ifnar1−/− mice would be at least partially associated with changes in chemokine profiles. This hypothesis was supported by a recent study demonstrating that IFN-α down-regulates CCL22, a Treg-attracting chemokine (14). In addition, CCL2 has been suggested to be the principal chemokine for MDSC migration in gliomas (19) and Tregs (20). On the other hand, CXCL10 represents type-1 effector CTL-attracting chemokines (13,18,21). We therefore performed quantitative RT-PCR to evaluate the mRNA expression levels of Ccl2, Ccl22, and Cxcl10 in the brain hemisphere bearing SB-induced gliomas (Fig. 2C). There was a significant up-regulation of Ccl2 (295%) and Ccl22 (267%), while Cxcl10 was reduced (54%), in Ifnar1−/− mice compared with WT mice.
Ifnar1−/− DCs fail to induce glioma-associated antigen (GAA)-reactive CTLs in vivo
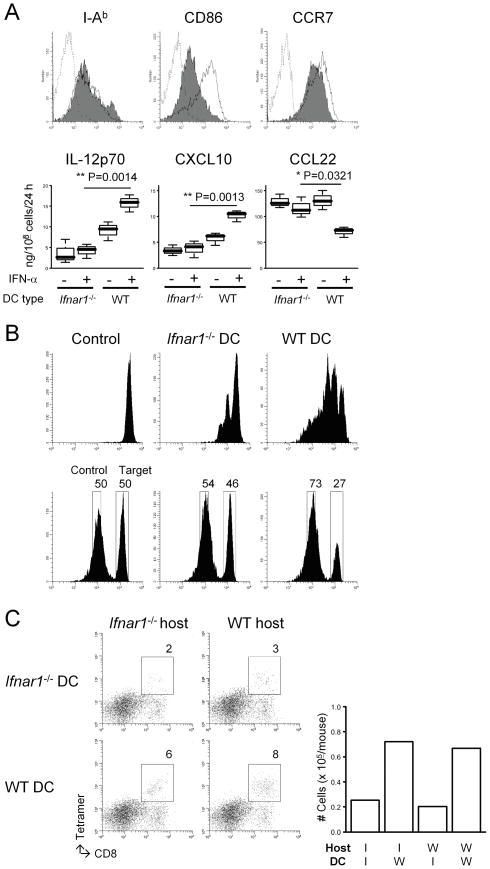
Among a variety of host immune cells that respond to type-1 IFNs, we directed our primary focus on APCs, such as DCs. We generated DCs from bone marrow cells derived from either Ifnar1−/− or WT mice. When the DCs were matured with LPS, Ifnar1−/− DCs exhibited lower levels of the co-stimulatory molecule CD86 compared with WT DCs, while other maturation markers were expressed at similar levels on both DC types (Fig. 3A, upper panels). Ifnar1−/− DCs failed to up-regulate IL-12p70 and CXCL10 production levels in response to IFN- α stimulation compared with WT DCs (Fig. 3A, lower panels). IFN-α stimulation down-regulated CCL22 production levels by WT DC, but not by Ifnar1−/− DCs. These data suggest that the absence of the type-1 IFN signaling induces a substantial shift of the chemokine production profile in APCs.
Figure 3. Ifnar1−/− DCs demonstrate defects in their APC function and the ability to attract CTLs in the glioma sites.
(A) Bone marrow-derived mature DCs were prepared as described in Materials and Methods. (Upper panels) Flow cytometry was performed to evaluate the following surface markers: I-Ab (an MHC class II), CD86, and CCR7. Histograms represent the following DC types: Ifnar1−/− (shaded), WT (open), and WT DCs stained with isotype control IgG (dashed line). (Lower panels) Immature DCs were matured with 250 ng/ml LPS in the presence or absence of 10 ng/ml rmIFN-α. ELISA was performed to evaluate the production levels of the following molecules per the last 24 hours: mIL-12p70, mCXCL10, and mCCL22. (B) WT mice received s.c. immunization with either Ifnar1−/− or WT DCs loaded with 5 μg/ml hgp10025–33 (upper) or Garc177–85 (lower) peptide on Day 0. Control mice received PBS. (Upper panels) On day -1, mice received an i.v. injection of 5 × 106 naive Pmel-I CD8+ T cells labeled with CFSE. On day 6, inguinal LN cells were collected to evaluate the proliferation of CSFE-labeled gp100-reactive cells by flow cytometry. (Lower panels) On day 6, the Garc177–85-specific cytolytic ability of effector T cells was evaluated by in vivo cytolytic assay as described in Materials and Methods. (C) Mice with developing gliomas received intratumoral injections of DCs derived from Ifnar1−/− or WT mice (1 × 105/mouse) on day 45 after birth, and i.v. Tc1 infusions on the same day (3 × 106/mouse). Mice were then sacrificed on day 5 following the DC injection for flow-cytometric evaluation of Tc1 infiltration into the tumor sites. Numbers in dot plots indicate percentage of CD8+gp100-teramer+ cells in lymphocyte-gated BILs. Absolute numbers are depicted in the rightmost panel.
To evaluate the antigen-presenting function of Ifnar1−/− vs WT DCs in vivo, these DCs were loaded with synthetic hgp100 25–33 peptide and injected into Ifnar1−/− mice subcutaneously (s.c.) as vaccines. The vaccination with WT DCs efficiently induced the proliferation (Fig. 3B, upper panels) and cytolytic ability (Fig. 3B, lower panels) of antigen-specific CTLs in vivo, while Ifnar1−/− DCs failed to promote these responses. These data suggest the critical roles of type-1 IFN pathway in the function of APCs.
Our data in Fig. 2B demonstrated diminished tumor-infiltration of gp100-reactive CTLs in Ifnar1−/− mice. We then evaluated whether supplementation of WT DCs in the microenvironment of Ifnar1−/− glioma would recover the trafficking of i.v. infused effector Tc1 cells (Fig. 3C). Intratumoral injections of WT DCs, but not Ifnar1−/− DCs, recovered the efficient trafficking of Tc1 cells to the gliomas in Ifnar1−/− mice (6% vs 2%), indicating that the type-1 IFN pathway in APCs is particularly important for attraction of CTLs in the glioma microenvironment regardless of IFN signaling in cells that are not APCs. Taken together, these data implicate the type-1 IFN pathway as important for attracting effector Tc1 cells and reducing immunoregulatory cells in the glioma microenvironment.
MDSCs play a significant role in the described phenomena
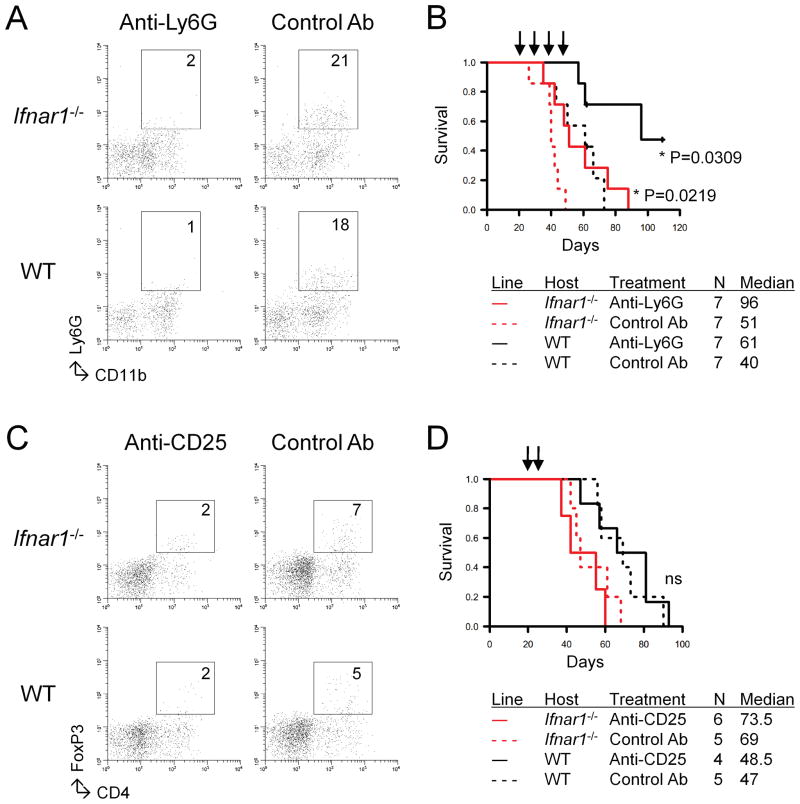
Based on the data shown in Fig. 2 and previous reports on Tregs [reviewed in (22)], we hypothesized that MDSC and Tregs would play a substantial role in promotion of gliomagenesis. To address this, we depleted these cell populations by systemic administration of mAbs. Depletion of Ly6G+ cells reduced the infiltration of CD11b+Ly6G+ cells in tumor sites (Fig. 4A) and prolonged the survival of both Ifnar1−/− and WT mice (Fig. 4B). In contrast, when anti-CD25 mAb was used for depletion of Tregs (23), the recipient mice did not show any prolongation of survival in response to the treatment (Fig. 4C), although the treatment reduced the number of CD4+FoxP3+ cells in the tumor site (Fig. 4D). Taken together, the data suggest that MDSCs, but not Tregs, played a significant role in promotion of gliomagenesis in this murine model.
Figure 4. mAb-mediated depletion of Ly6G+ cells, but not CD25+ cells, inhibit the growth of SB-induced gliomas.
(A and B) Ifnar1−/− or WT mice with developing gliomas received i.p. injections of anti-Ly6G mAb (RB6-8C5; 0.25 mg/dose) or control IgG on days 21, 23, 25, and 27 after tumor induction. (A) The mice were sacrificed, and BILs were isolated for CD11b+Ly6G+ subpopulations. (B) Symptom-free survival was monitored. (C and D) Ifnar1−/− or WT mice with developing gliomas received i.p. injections of anti-CD25 mAb (PC61; 0.25 mg/dose) or control IgG on days 21 and 24 after tumor induction. (C) The mice were sacrificed, and BILs were isolated for CD4+FoxP3+ subpopulations. (D) Symptom-free survival was monitored.
Treatment with poly-ICLC prolongs the survival of mice bearing SB-induced gliomas in an Ifnar1-dependent manner
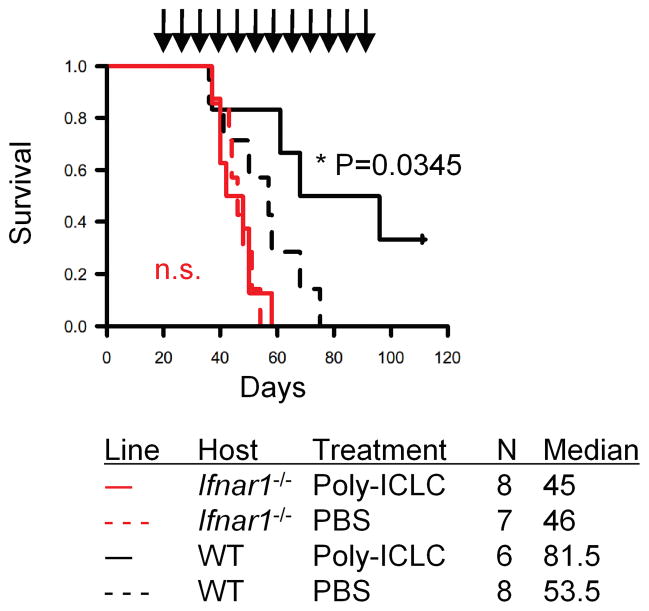
The data described above suggest pivotal roles of the host type-1 IFN pathway in delaying and/or reducing development of SB-induced gliomas. We next sought to determine whether augmentation of the type-1 IFN pathway in WT mice compared with Ifnar1−/− mice would also delay or prevent the growth of SB-induced gliomas by i.m. administration of poly-ICLC as a potent type-1 IFN-inducer (24). Treatment of SB-induced mice significantly prolonged the survival of mice in an Ifnar1-dependent manner, with 2 of 6 treated WT mice still alive on day 100 after birth (Fig. 5), while none of control WT mice treated with PBS lived longer than 75 days.
Figure 5. Treatment with poly-ICLC prolongs the survival of mice bearing SB-induced gliomas in an Ifnar1-dependent manner.
Mice with developing gliomas received i.m. injections of either poly-ICLC (2.5 mg/kg/dose) or mock PBS starting on days 21 and 24 after birth, and weekly thereafter. Symptom-free survival was monitored.
SNPs in type-1 IFN-related genes correlate with overall survival of glioma patients
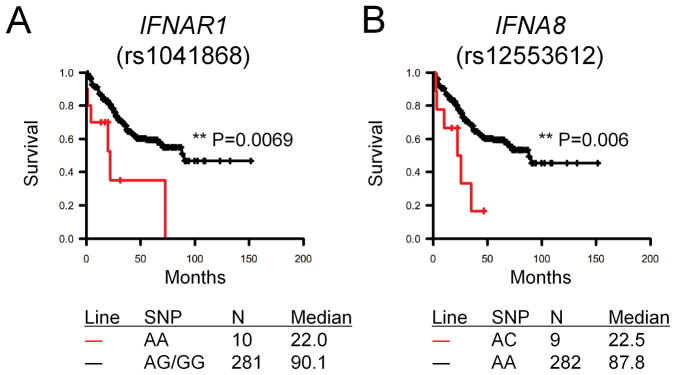
We performed a survival analysis in a population of 587 patients with WHO grade 2–4 gliomas (Supplementary Table 1) for whom genotype data were available to begin to understand the impact of type-1 IFN-related genes on prognosis. Cox regression analyses demonstrated no significant association of IFN-related SNPs with the survival of patients with grade 4 glioblastoma multiforme (GBM; data not shown). However, when the grade 2–3 gliomas were analyzed, we found that patients with the AA genotype of IFNAR1 rs1041868 have significantly poorer overall survival (HR=3.6; 95% CI=1.5–8.3) than those with the AG or GG genotypes. Similarly, patients with the AC genotype of IFNA8 rs12553612 were more likely to die than those with the AA genotype (HR=2.5; 95% CI=1.1–5.9). Furthermore, Kaplan-Meier survival curves indicated that median survival for patients with the IFNAR1 rs1041868 AA genotype was 24 months compared with 90 months for patients with the AG/GG genotypes; the same effect was seen for the IFNA8 rs12553612 AC genotype compared with the AA genotype (Fig. 6). Adjustment of the models by age at diagnosis, extent of surgery, and chemotherapy use did not affect the point estimates (data not shown). It was unlikely that these factors would confound the association between genotype and survival time. We also examined the effect of having one or both of these significant SNPs. However, only one patient carried both variant SNPs; we were therefore unable to examine the joint effect. None of the other SNPs examined were significantly associated with overall survival (data not shown). We further confirmed that each of the genotype groups included patients from both histologic groups, indicating that the observed effects are because of the genotypes but not because the groups with worse survival (AA in rs1041868 and AC in rs12553612) were composed of those with worse histology (grade 3 glioma). Taken together, these data indicate that type-1 IFNs play an important role in gliomagenesis in mice and may present valuable prognostic factors in humans.
Figure 6. Association of SNPs in IFN-related genes and the survival of patients with WHO grade 2–3 gliomas.
Overall survival was evaluated among 304 glioma patients with grade 2–3 gliomas by genotype for SNPs in IFN-related genes. (A) Patients with AA genotype (red line) for IFNAR1 rs1041868 exhibited a significantly shorter survival than those with the AG/GG genotypes (black line). (B) Patients with AC genotype (red line) for IFNA8 rs12553612 exhibited a significantly shorter survival than those with the AA genotype (black line).
Discussion
This is one of the first reports documenting the status of immune cell infiltration and chemokine expression in the microenvironment of de novo murine gliomas. This model allowed us to determine the role of endogenous type-1 IFNs in glioma development in mice and identify type-1 IFN SNPs that are associated with altered prognosis of glioma patients. Such interconnected studies that bridge de novo murine glioma models and human epidemiological data will help us further elucidate novel factors contributing to risk and prognosis for this highly fatal disease, and to develop possible prevention strategies.
A critical role of type-1 IFNs in cancer development has been shown previously in methylcholanthrene-induced skin tumor model (25), where the authors primarily focused on the immunoediting process of cancers. In particular, they demonstrated that tumors arising in the absence of type-1 IFN responsiveness are more immunogenic than tumors arising in immunocompetent mice. We observed similar findings (Fig. 1). As Ifnar1−/− and WT cell lines showed no significant differences in expression of GAAs and MHC, further studies are warranted to delineate the factors that underlie the unique immunogenicity of tumors arising in Ifnar1−/− hosts. Nevertheless, in our current study, we demonstrated specific changes in immune cell populations and chemokine productions in the glioma microenvironment (Fig. 2). In particular, our data suggest that responsiveness of DCs to type-1 IFNs affects their antigen-presenting function (e.g. changes in CD86 expression) as well as the chemokine production profiles in the tumor microenvironment (Fig. 3), which may be responsible for the changes in the percentages and absolute numbers of infiltrating MDSCs and Tregs. We also evaluated the significance of MDSCs in glioma development by mAb-mediated depletion (Fig. 4A and 4B). Taken together, the novel de novo glioma model provided us with unique opportunities to identify the roles of type-1 IFN pathway in anti-glioma immunosurveillance.
A variety of strategies have been described to deplete MDSCs, such as gemcitabine (26) and sunitinib (27). We used anti-Ly6G mAb (RB6-8C5) to deplete Ly6G+ CD11b+ cells (Fig. 4A and 4B) because anti-Ly6G mAb reacts relatively specifically to MDSCs, sparing effector immune cell populations, such as CTLs. RB6-8C5 was originally developed as a mAb recognizing a surface antigen, granulocyte receptor 1 (Gr-1) (28), which is a member of the Ly6 gene family. Then, Ly6G, a granulocyte surface marker, was found to be the major antigen detected by RB6-8C5 (29). Although Ly6G is expressed on neutrophils, DCs, and subsets of monocytes, macrophages, and lymphocytes (15), we have found that systemic administration of the RB6-8C5 did not significantly affect the numbers of these other immune cell populations (data not shown).
We used the anti-CD25 mAb (PC61) to deplete Tregs (Fig. 4C and 4D). This strategy is widely accepted and clinically applied as Daclizumab (30), and our regimen is based on a previous study employing a similar dose and schedule (16). Although there is a theoretical concern is that this strategy might deplete activated T cells that express CD25 (31), it is also known that expression of CD25 is transient following activation (32). Indeed, we observed no significant effect of anti-CD25 mAb on CD8+ T cell subpopulations in BILs (data not shown). Therefore, this does not appear to be a significant concern in most cancer studies.
We demonstrated that SNPs in IFNAR1 and IFNA8 have significant associations with overall survival in patients with the grade 2–3 gliomas (Fig. 6). It is ultimately important to determine whether these SNPs have a significant impact on the biological function of type-1 IFN pathways. The IFNAR1 rs1041868 is located in intron 10, and IFNA8 rs12553612 is located in the 5′ untranslated region of the IFNA8 gene. While the location of these SNPs does not suggest putative functionality, further studies are warranted to determine whether these SNPs impact gene expressions. In addition, these SNPs could be in linkage disequilibrium with as yet unidentified functional SNPs, which could be further elucidated in future studies. Previous studies have shown a significant immunological impact of several SNPs in other IFN-related genes, such as ones in Toll-Like Receptor (TLR) 3 (33,34) and TLR4 (35). Therefore, if the SNPs in IFNAR1 and IFNA8 indeed affect the biological function of the gene products, it will substantiate that these SNPs alter the status of immunological surveillance and could influence the prognosis of glioma patients. Such observations will also prompt us to evaluate whether these SNPs dictate altered risks for occurrence of glioma in general populations.
Despite the significant effects of these SNPs in patient with the grade 2–3 gliomas (Fig. 6), these did not demonstrate a significant association with survival in GBM patients. This may imply that the profound immune suppression and aggressive growth of GBM may overshadow the potential role of type-1 IFN pathway. In humans, grade 2–3 gliomas are considered to represent pre-malignant tumors prior to their transformation into secondary GBMs (36). In our mouse model, SB-induced gliomas appear relatively dormant at days 20–30 after birth (Fig. 1) and therefore may simulate the grade 2–3 gliomas before they start growing more aggressively. We also observed that poly-ICLC treatment started on day 21 after birth leads to therapeutic effects in a host type-1 IFN-dependent manner (Fig. 5). Taken together, these findings suggest that IFN-based therapy may be most appreciated in the lower-grade gliomas rather than GBMs. In addition, since IFN-α treatment failed in a phase III trial for patients with high-grade gliomas due to dose-limiting toxicity (37), toxicity would be a major concern if type-1 IFNs were used in patients particularly with low-grade gliomas. On the other hand, poly-ICLC has been shown to be safe and well-tolerated in glioma patients (38–40) and thus seems to be more suitable for treatment of glioma patients.
Due to the low minor allele frequencies in the SNPs of interest, we analyzed the grade 2–3 gliomas as a group with more similar survival separately from the grade 4 GBMs to have adequate power for the analysis. Future analyses, on a larger number of patients, could determine if further differences exist between patients with grade 2 versus grade 3 gliomas. If the functional significance of the SNPs reported here can be identified, such information may be used to select patients who are likely to benefit from future immunotherapy trials. In summary, the current study indicates a pivotal role of type-1 IFN pathway in gliomagenesis and supports the development of type-1 IFN-based strategies for immunological prevention of gliomas.
Translational Relevance.
Although gliomas are the most common type of primarymalignant brain tumors in adults, only limited information is availableregarding their etiology and prognostic factors that influence patient survival. In this study, using a novel murine glioma model (where de novo gliomas can be induced in mice using the Sleeping Beauty transposon system), we demonstrate that the absence of type-1 IFN signaling causes dynamic changes in the immunological microenvironment in murine gliomas, thereby accelerating the tumor growth. In addition, we show that SNPs in IFNAR1 and IFNA8 are associated with glioma patients’ survival. Cumulatively, these findings imply that active collaboration using the novel murine glioma model and human genetics may foster the discovery of novel risk and/or prognostic factors for gliomas, thereby leading to the development of effective immunotherapeutic strategies and ways to reduce occurrence of gliomas.
Supplementary Material
Acknowledgments
We thank Dr. Wendy Fellows-Mayle for her technical assistance.
Financial Support: the National Institute of Health (NIH; 1R01NS055140, 2P01NS40923, and 1P01CA132714) to HO; Pittsburgh Foundation (D2008-0433) to MF; NIH/National Institute of Neurological Disorders and Stroke (1R21NS055738), American Cancer Society (RSG-09-189-01-LIB), the Randy Shaver Cancer Research and Community Fund, and the Children’s Cancer Research Fund to JRO; the NIH/National Research Service Award (1F31NS067937-01) to SAD; the NIH (K07CA131505) to MES; the NIH (1R01CA119215 and 1R01CA070917) and the Wellcome Trust to MLB.
References
- 1.Wiesner SM, Decker SA, Larson JD, et al. De novo induction of genetically engineered brain tumors in mice using plasmid DNA. Cancer Res. 2009;69:431–9. doi: 10.1158/0008-5472.CAN-08-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parney IF, Waldron JS, Parsa AT. Flow cytometry and in vitro analysis of human glioma-associated macrophages. Laboratory investigation. J Neurosurg. 2009;110:572–82. doi: 10.3171/2008.7.JNS08475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–60. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- 4.Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol. 2006;6:836–48. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- 5.Pharoah PD, Dunning AM, Ponder BA, Easton DF. Association studies for finding cancer-susceptibility genetic variants. Nat Rev Cancer. 2004;4:850–60. doi: 10.1038/nrc1476. [DOI] [PubMed] [Google Scholar]
- 6.Schwartzbaum J, Ahlbom A, Malmer B, et al. Polymorphisms associated with asthma are inversely related to glioblastoma multiforme. Cancer Res. 2005;65:6459–65. doi: 10.1158/0008-5472.CAN-04-3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scheurer ME, Amirian E, Cao Y, et al. Polymorphisms in the interleukin-4 receptor gene are associated with better survival in patients with glioblastoma. Clin Cancer Res. 2008;14:6640–6. doi: 10.1158/1078-0432.CCR-07-4681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wrensch M, Wiencke JK, Wiemels J, et al. Serum IgE, tumor epidermal growth factor receptor expression, and inherited polymorphisms associated with glioma survival. Cancer Res. 2006;66:4531–41. doi: 10.1158/0008-5472.CAN-05-4032. [DOI] [PubMed] [Google Scholar]
- 9.Rodero M, Marie Y, Coudert M, et al. Polymorphism in the microglial cell-mobilizing CX3CR1 gene is associated with survival in patients with glioblastoma. J Clin Oncol. 2008;26:5957–64. doi: 10.1200/JCO.2008.17.2833. [DOI] [PubMed] [Google Scholar]
- 10.de Weerd NA, Samarajiwa SA, Hertzog PJ. Type I interferon receptors: biochemistry and biological functions. J Biol Chem. 2007;282:20053–7. doi: 10.1074/jbc.R700006200. [DOI] [PubMed] [Google Scholar]
- 11.Overwijk WW, Tsung A, Irvine KR, et al. gp100/pmel 17 is a murine tumor rejection antigen: induction of “self”-reactive, tumoricidal T cells using high-affinity, altered peptide ligand. J Exp Med. 1998;188:277–86. doi: 10.1084/jem.188.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohlfest JR, Demorest ZL, Motooka Y, et al. Combinatorial antiangiogenic gene therapy by nonviral gene transfer using the sleeping beauty transposon causes tumor regression and improves survival in mice bearing intracranial human glioblastoma. Mol Ther. 2005;12:778–88. doi: 10.1016/j.ymthe.2005.07.689. [DOI] [PubMed] [Google Scholar]
- 13.Fujita M, Zhu X, Ueda R, et al. Effective immunotherapy against murine gliomas using type 1 polarizing dendritic cells--significant roles of CXCL10. Cancer Res. 2009;69:1587–95. doi: 10.1158/0008-5472.CAN-08-2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muthuswamy R, Urban J, Lee JJ, Reinhart TA, Bartlett D, Kalinski P. Ability of mature dendritic cells to interact with regulatory T cells is imprinted during maturation. Cancer Res. 2008;68:5972–8. doi: 10.1158/0008-5472.CAN-07-6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J Leukoc Biol. 2008;83:64–70. doi: 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- 16.Terabe M, Ambrosino E, Takaku S, et al. Synergistic enhancement of CD8+ T cell-mediated tumor vaccine efficacy by an anti-transforming growth factor-beta monoclonal antibody. Clin Cancer Res. 2009;15:6560–9. doi: 10.1158/1078-0432.CCR-09-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okcu MF, Selvan M, Wang LE, et al. Glutathione S-transferase polymorphisms and survival in primary malignant glioma. Clin Cancer Res. 2004;10:2618–25. doi: 10.1158/1078-0432.ccr-03-0053. [DOI] [PubMed] [Google Scholar]
- 18.Fujita M, Zhu X, Sasaki K, et al. Inhibition of STAT3 promotes the efficacy of adoptive transfer therapy using type-1 CTLs by modulation of the immunological microenvironment in a murine intracranial glioma. J Immunol. 2008;180:2089–98. doi: 10.4049/jimmunol.180.4.2089. [DOI] [PubMed] [Google Scholar]
- 19.Huang B, Lei Z, Zhao J, et al. CCL2/CCR2 pathway mediates recruitment of myeloid suppressor cells to cancers. Cancer Lett. 2007;252:86–92. doi: 10.1016/j.canlet.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 20.Jordan JT, Sun W, Hussain SF, DeAngulo G, Prabhu SS, Heimberger AB. Preferential migration of regulatory T cells mediated by glioma-secreted chemokines can be blocked with chemotherapy. Cancer Immunol Immunother. 2008;57:123–31. doi: 10.1007/s00262-007-0336-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishimura F, Dusak JE, Eguchi J, et al. Adoptive transfer of type 1 CTL mediates effective anti-central nervous system tumor response: critical roles of IFN-inducible protein-10. Cancer Res. 2006;66:4478–87. doi: 10.1158/0008-5472.CAN-05-3825. [DOI] [PubMed] [Google Scholar]
- 22.Okada H, Kohanbash G, Zhu X, et al. Immunotherapeutic approaches for glioma. Crit Rev Immunol. 2009;29:1–42. doi: 10.1615/critrevimmunol.v29.i1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 24.Zhu X, Nishimura F, Sasaki K, et al. Toll like receptor-3 ligand poly-ICLC promotes the efficacy of peripheral vaccinations with tumor antigen-derived peptide epitopes in murine CNS tumor models. J Transl Med. 2007;5:10. doi: 10.1186/1479-5876-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dunn GP, Bruce AT, Sheehan KC, et al. A critical function for type I interferons in cancer immunoediting. Nat Immunol. 2005;6:722–9. doi: 10.1038/ni1213. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res. 2005;11:6713–21. doi: 10.1158/1078-0432.CCR-05-0883. [DOI] [PubMed] [Google Scholar]
- 27.Ko JS, Zea AH, Rini BI, et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res. 2009;15:2148–57. doi: 10.1158/1078-0432.CCR-08-1332. [DOI] [PubMed] [Google Scholar]
- 28.Tepper RI, Coffman RL, Leder P. An eosinophil-dependent mechanism for the antitumor effect of interleukin-4. Science. 1992;257:548–51. doi: 10.1126/science.1636093. [DOI] [PubMed] [Google Scholar]
- 29.Fleming TJ, Fleming ML, Malek TR. Selective expression of Ly-6G on myeloid lineage cells in mouse bone marrow. RB6–8C5 mAb to granulocyte-differentiation antigen (Gr-1) detects members of the Ly-6 family. J Immunol. 1993;151:2399–408. [PubMed] [Google Scholar]
- 30.Rech AJ, Vonderheide RH. Clinical use of anti-CD25 antibody daclizumab to enhance immune responses to tumor antigen vaccination by targeting regulatory T cells. Ann N Y Acad Sci. 2009;1174:99–106. doi: 10.1111/j.1749-6632.2009.04939.x. [DOI] [PubMed] [Google Scholar]
- 31.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64. [PubMed] [Google Scholar]
- 32.Malek TR. The biology of interleukin-2. Annu Rev Immunol. 2008;26:453–79. doi: 10.1146/annurev.immunol.26.021607.090357. [DOI] [PubMed] [Google Scholar]
- 33.Dhiman N, Ovsyannikova IG, Vierkant RA, et al. Associations between SNPs in toll-like receptors and related intracellular signaling molecules and immune responses to measles vaccine: preliminary results. Vaccine. 2008;26:1731–6. doi: 10.1016/j.vaccine.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang Z, Stratton C, Francis PJ, et al. Toll-like receptor 3 and geographic atrophy in age-related macular degeneration. N Engl J Med. 2008;359:1456–63. doi: 10.1056/NEJMoa0802437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Apetoh L, Ghiringhelli F, Tesniere A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–9. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 36.Purow B, Schiff D. Advances in the genetics of glioblastoma: are we reaching critical mass? Nat Rev Neurol. 2009;5:419–26. doi: 10.1038/nrneurol.2009.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buckner JC, Schomberg PJ, McGinnis WL, et al. A phase III study of radiation therapy plus carmustine with or without recombinant interferon-alpha in the treatment of patients with newly diagnosed high-grade glioma. Cancer. 2001;92:420–33. doi: 10.1002/1097-0142(20010715)92:2<420::aid-cncr1338>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 38.Salazar AM, Levy HB, Ondra S, et al. Long-term treatment of malignant gliomas with intramuscularly administered polyinosinic-polycytidylic acid stabilized with polylysine and carboxymethylcellulose: an open pilot study. Neurosurgery. 1996;38:1096–103. [PubMed] [Google Scholar]
- 39.Butowski N, Chang SM, Junck L, et al. A phase II clinical trial of poly-ICLC with radiation for adult patients with newly diagnosed supratentorial glioblastoma: a North American Brain Tumor Consortium (NABTC01–05) J Neurooncol. 2009;91:175–82. doi: 10.1007/s11060-008-9693-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Butowski N, Lamborn KR, Lee BL, et al. A North American brain tumor consortium phase II study of poly-ICLC for adult patients with recurrent anaplastic gliomas. J Neurooncol. 2009;91:183–9. doi: 10.1007/s11060-008-9705-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.