Abstract
T cell-dependent immune responses generate long-lived plasma cells and memory B cells, both of which express hypermutated antibody (Ab) genes. The relationship between these cell types is not entirely understood. Both appear to emanate from the germinal center (GC) reaction, but it is unclear whether memory cells evolve while obligatorily generating plasma cells by siblings under all circumstances. In the experiments we report, plasma cell development was functionally segregated from memory cell development by a series of closely-spaced injections of Ag delivered during the period of germinal center development. The injection series elevated serum Ab of low affinity, supporting the idea that a strong Ag signal drives plasma cell development. At the same time, the injection series produced a distinct population of affinity/specificity-matured memory B cells that were functionally silent, as manifested by an absence of corresponding serum Ab. These cells could be driven by a final booster injection to develop into antibody forming cells. This recall response required that a rest period precede the final booster injection, but a pause of only 4 days was sufficient. Our results support a model of memory B cell development in which extensive affinity/specificity maturation can take place within a B cell clone under some circumstances in which a concomitant generation of antibody forming cells (AFC) by siblings does not take place.
Keywords: B Lymphocytes, Immunoglobulins, Memory development
Introduction
At least three pathways contribute to Ab formation in T cell-dependent immune responses: a short-term AFC response in extrafollicular regions of secondary lymphoid tissue, a long-lived AFC response in the bone marrow by GC emigrants, and a memory B cell response that provides recall immunity for the life of the animal (1–7). The short-term extrafollicular response occurs within a few days of immunization and is characterized in the spleen by clusters of AFC in the peri-arteriolar-lymphoid sheath (PALS) regions and by antibody products of low affinity, many of which are likely derived from marginal zone B cells and encoded by unmutated variable (V) region genes (8–10). The bulk of this reaction is concluded by 2 weeks, although some plasma cells persist for longer periods (6, 7, 11). Interestingly, this short term AFC component is absent from the A/J immune response to the hapten p-azophenylarsonate (Ars) (12). The reason for this is not known. Perhaps Ars-specific precursors to the early extrafollicular AFC are missing in this strain (12, 13). Alternatively the diazonium bond linking Ars to the carrier protein may be unstable in the extrafollicular environment. Regardless of the reason, the absence of the extrafollicular pathway simplifies serological studies of the long term AFC and memory cell components of humoral immunity.
The long-lived AFC and memory responses are each derived from GC B cells that hypermutate their Ab V region genes (8, 14–21). In a well-defined immune response to the hapten, 4-hydroxy-3-nitrophenyl acetyl (NP), the earliest bone marrow AFC are seen at approximately 2 weeks following immunization (20, 21). Although these early bone marrow AFC carry few somatic mutations, a high frequency of the mutations are those that enhance affinity for NP (20). Tarlinton and colleagues (22) have taken this as an indication that an early strong signal through the B cell receptor (BCR) favors development of GC B cells along the bone marrow AFC pathway. Additional evidence for this idea comes from studies in bcl-2 Tg mice, in which affinity maturation is perturbed in GC but not in bone marrow AFC (23) and from studies in which an increased AFC response was observed following multiple injections of soluble Ag during the primary immune response (24). Most recently, Paus et al. (25) extended this finding using HEL transgenic mice.
The population dynamics behind memory B cell development have proved to be the most difficult to define. Based on genealogical analyses, it is clear that hypermutation in memory precursors occurs over multiple rounds of DNA replication and presumably cell division (26–29). There is also good evidence for competition among developing memory lineage cells, a process that may occur locally between adjacent clones rather than globally among all participating clones (30, 31). But much less is known about how memory lineage B cells in GC sense and respond to variations in Ag dose and the relationship between memory lineage cells and long-term AFC. Smith et al. (20) observed a continuous improvement in the affinity of Ab produced during the primary immune response and continuous seeding of bone marrow AFC by GC B cells. These results imply that AFC and memory B cells may continuously generated by common lineages during a primary immune response. However, Decker et al. (32) used a splenic fragment culture system to show that some memory progenitors mutate their Ab genes and generate higher order memory cells that do not produce AFC siblings until they encounter antigen sometime later. Their results suggest that a pause in antigenic stimulation is required to render some memory cells competent for differentiation into AFC and that a subsequent challenge with Ag after the pause is required to induce differentiation into AFC. To our knowledge, in vivo evidence for this scenario of memory development has not been reported.
To determine whether memory cells can evolve in vivo without secreting long-term AFC generation, we exploited several advantageous features of the anti-Ars immune response. In addition to having no early extrafollicular component, this immune response embodies a memory pathway in which approximately half of the B cell participants express one set of Ab variable (V) gene segments with a common Vκ-Jκ junction and a VHCDR3 that only varies at two boundary codons, mutations excepted (33, 34). A highly-specific anti-clonotypic antibody called mAbE4 can be used to identify this canonical Ab structure. Most importantly, rare mutants of the canonical Ab, acquire specificity for a related hapten sulfanilic acid (Sulf) and can be recruited into the response when Sulf is provided secondarily as an immunogen (35–37). This switch in specificity from Ars to Sulf can be used as a qualitative indicator of “affinity-maturation” and memory B cell development.
Using this model in conjunction with variations in immunogen, dose and tempo, we demonstrate the existence of memory progenitor cells with a stem cell-like property (38). They can be driven by antigen to evolve higher affinity and specificity for Ag without significant differentiation into AFC. We also show that a pause in antigenic stimulation of only 4 days is needed to render memory progenitors competent for AFC differentiation upon a subsequent antigenic challenge. Finally, we show that Ag pulsing can have opposite effects on memory progenitors and AFC with respect to the affinity of the Ab product.
Materials and Methods
Animals and immunization protocols for serological studies
A/J mice were purchased from the Jackson Laboratory and housed in the Biological Resource Center at National Jewish. All animals were used according to an IACUC approved animal protocol. In all cases 6–8 weeks old animals were used, and immunizations were intraperitoneal (i.p.) as specified.
ELISA, europium and radioimmuno assays
In direct binding assays, 96-well trays were coated overnight with Ars5-bovine serum albumin (BSA) or Ars15-BSA (10ug/ml in 100 μl). After washing, plates were incubated with blocking buffer (2% BSA/1% Gelatin/0.05% Tween-20/0.04% Thimerasol in PBS) for 1 h at RT. Serial dilutions of sera and standard anti-Ars antibody, mAb36–65 (17, 39), were incubated for 1 h at RT. The plates were washed and incubated with biotin-rat-anti-mouse-kappa (mAb187.1) (40) (bio-rat-anti-Mκ, 0.5 μg/ml) for 1 h. After washing, the trays were incubated for 30 min. with streptavidin-coupled horseradish peroxidase (SA-HRP, 1μg/ml) (Vector Laboratories, Burlingame, CA) or SA-Europium (0.05 μg/ml) (Wallac, Turku Finland) and developed with 2′2′-azino-bis(3-ethylbenz-thiazoline-6-sulfonic acid) (ABTS) (Sigma, St. Louis MO) or enhancement solution (100 μl, Wallac, Turku Finland). Results were quantified using a Wallac Victor 2 fluorimeter using a photometry setting of 450 nm for ABTS absorbance and excitation and emission settings of 340 nm and 615 nm respectively for Eu fluorescence (41).
Relative affinity of total serum Ab, was determined based on the method of Herzenberg et al. (42). Plates were coated with Ars5-BSA or Ars15-BSA (10 μg/ml, 4 °C overnight), blocked, and incubated with sera at dilutions that gave ~90% of maximum binding and developed as above in the direct binding assay. Dilutions that gave 50% of maximum binding were determined using Microsoft Excel 2001 (Microsoft Corp., Redmond, WA) using linear regression.
To determine relative affinity by hapten inhibition, plates were coated overnight with Ars5-BSA, blocked and incubated with sera at dilutions that afforded ~90% of maximum binding. Sera were mixed with Ars-N-acetyl-L-tyrosine (Ars-Tyr) at concentrations ranging from 10−3 to 10−9 M. Plates were developed with 125I-labeled rat anti-mouse κ (40 ng/ml), and bound radioactivity was measured in a Gamma 5500B counter (Beckman Instruments, Inc, Fullerton, CA). I50 values were calculated using Jmp 2.3.6 (SAS Institute Inc. Cary, NC).
Canonical Ab were quantified in a competition assay (43). Trays were coated with mAbE4 (44) blocked and incubated with serial dilutions of sera or standard mAb36-65 (Id+) pre-mixed with bio-mAb36-65 (17) (0.2 μg/ml). The assays were developed with SA-HRP and ABTS as above.
A counter-inhibition assay was used to detect canonical antibodies in serum with specificity for Sulf among a mixture including others with specificity for Ars (37). Trays were first coated with mAbE4, blocked as above and incubated for 1 h with competing sera, together with a biotin-labeled antibody called mAb3A4 (70 ng/ml), which has no capacity to bind Ars or Sulf (37, 43) and Ars-Tyr or Sulf-N-acetyl-L-tyrosine (Sulf-Tyr) (2.5 × 10−4 M). Test sera were used at a dilution that inhibited mAbE4 binding to mAb3A4 by 90%. Plates were developed with SA-europium as above. Canonical Ab in sera inhibits the binding of bio-mAb3A4 to mAbE4-coated wells in the absence of hapten, but this is counter-inhibited by hapten if it binds to the serum Ab.
In one experiment (Figure 4B) sera were pooled from 4 control animals which were not given the final booster injection with Sulf-KLH. The pooled sample was affinity purified on a Sulf-BSA sepharose column. Bound antibodies were eluted with 3M KSCN, dialyzed and tested in the counter-inhibition assay.
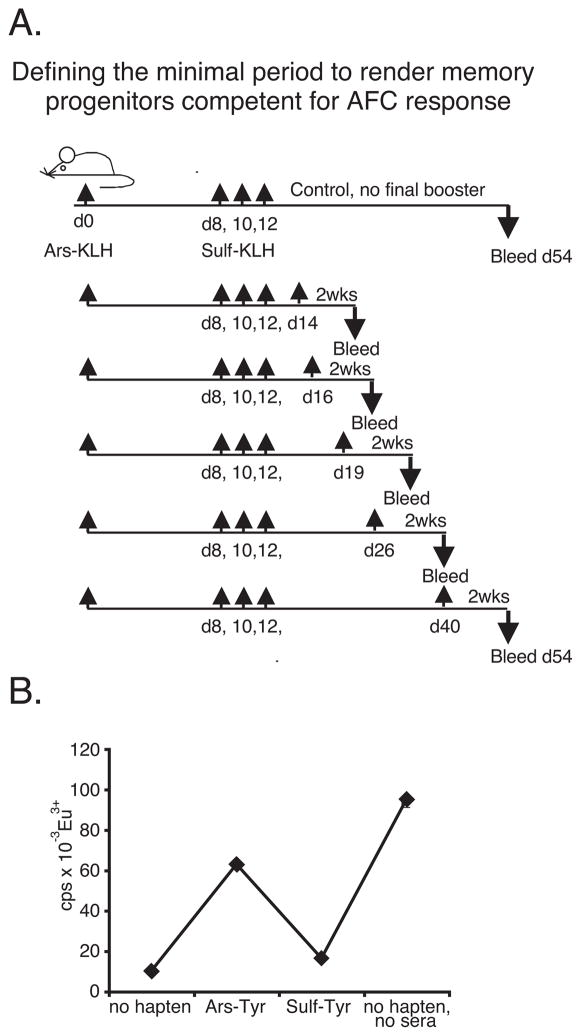
Figure 4.
A, Defining the minimal period of rest required before eliciting a recall response by Sulf-switch mutants. Mice were immunized as shown. Initial injection was with Ars15-KLH (100 μg) i.p. in IFA. Serial injections and booster injections were with 100 μg i.p. in PBS. B, Pooled sera from control group (last row Table IV) were affinity-purified on Sulf-BSA sepharose and tested in the counter-inhibition assay as in Figure 3. Affinity-purified canonical antibody accounted for ~14% of all canonical antibody in the sample, as assessed with mAbE4.
Hybridoma studies
One Ars-5 mouse (45) carrying multiple copies of a canonical VHIdCR-Dfl16.2-JH2-IgM transgene on a C57BL/6 genetic background, was immunized i.p. with 300 μg of Ars15-KLH in CFA. Serial injections with Sulf-KLH i.p. (300 μg) were delivered on days 7, 10, 12, 14, 17, and 20. The mouse was injected i.p. with 100μg Sulf-KLH in IFA at day 45 and bled on day 70. Sera were tested in a counter-inhibition assay for antibodies with bearing the E4 idiotype that bound Sulf-Tyr more strongly than Ars-Tyr. It was given a booster injection with Sulf-KLH (100μg) on day 200 and sacrificed for hybridoma formation with SP2/0 cells on day 203 (46). Selected hybridomas were cloned three times by limiting dilution.
Constructing an unmutated correlate of the Sulf-binding mAb
A 3.7 kb Eco RI- Hind III fragment, containing the assembled heavy chain V gene of mAbR16.7 (origin of the VH transgene in Ars-5 mice) was cloned into a plasmid vector for expression in context of an IgG2b constant gene (47). This genetic construction was transfected into a cell line (36–65κ) expressing an unmutated version of the kappa gene encoding the canonical light chain of the dominant anti-Ars idiotype (34). Secreted antibodies were purified from ascites fluid by affinity chromatography with Ars-BSA-sepharose (43).
V gene sequencing
Canonical light chain V genes were amplified from hybridoma DNA (100 ng) by a nested PCR strategy and directly sequenced, as described (48, 49). cDNA encoding Ig heavy chains was prepared by the method of Chomczynski (50) using primers specific for IgG1: 5′ CCAGGGTCACCATGGAGTTAGT, or IgG2b: 5′ GGATCCAGAGTTCCAAGTCACA. Hybridoma cDNA was initially amplified through 30 cycles using cDNA synthesis primers in conjunction with a primer that hybridized to the leader exon in the VHIdCR gene: 5′GGATGGAGCTTCATCTTTCTCT. A second round of amplification (15 cycles) was performed with nested primers for IgG1: 5′ GAGTTAGTTTGGGCAGCAGATC, or IgG2b: 5′ CAGGCACCCAGAGGTCACGGA, in conjunction with the same 5′ leader primer.
Histology
Spleens were quick-frozen in Tissue-Tek O.C.T. Serial sections of 6–8μm were fixed in acetone and stored at −80 °C. Sections were thawed at RT and blocked with 5% normal goat serum in PBS for 10 min at RT. For immunohistochemistry analyses of germinal centers, frozen sections were incubated with bio-mAbE4, and bio-PNA and developed using Vectastain ABC alkaline phosphatase and Vectastain ABC Elite kits (both Vector Laboratories, Burlingame, CA) followed by BCIP/NBT alkaline phosphatase substrate kit IV or DAB peroxidase substrate kit respectively (both Vector Laboratories, Burlingame, CA) according to the manufacturer’s protocol. Sections were photographed using a Nikon Diaphot coupled to a Photometrix CCD camera and analyzed using IPLab Spectrum Version 3.1a. Germinal center sizes were determined as ‘small’ being <2000 pixels, ‘medium’ 2000–4000 pixels, and ‘large’ >4000 pixels.
For immunofluorescent analyses of germinal centers, spleen sections were preincubated for 30 min with one of two competitors (Ars-BSA or Sulf-BSA at 10 μg/ml) in staining buffer (2% FCS in PBS, 0.1NaN3). Sections were then stained for 30 min with biotinylated or FITC-coupled PNA (Vector Laboratories, Burlingame, CA) and E4 and APC-B220 (clone RA3-6B2, eBioscience, San Diego, CA) in the presence of the competitors. Biotinylated stains were resolved with streptavidin-Cy3. Slides were photographed using the Marianas system (Intelligent Imaging Innovations Inc., Denver, CO) and analyzed using Slidebook 4.0.2. Pictures of an entire section were assembled in a montage. Sections were renormalized keeping the settings for the different channels constant across the sections. Numbers of total and E4+ germinal centers were determined, and the frequency of E4+ germinal centers in each section was calculated. The proportions of E4+ GC binding Ars and Sulf were determined from the frequencies of E4+ GC in 3 consecutive sections, incubated with either no hapten or the competitors Ars-BSA or Sulf-BSA.
Results
Differential effects of serial Ag injections on late primary and secondary immune responses
To determine how repeated antigenic stimulation during an ongoing primary immune response influences development of long-lived plasma cells and memory cells, we took advantage of the observation that the immune response elicited in strain A/J mice by Ars lacks an early extrafollicular AFC component (12). This permitted us to examine the long-term AFC and memory responses within the same animal by assaying serum Ab at different time points following immunization.
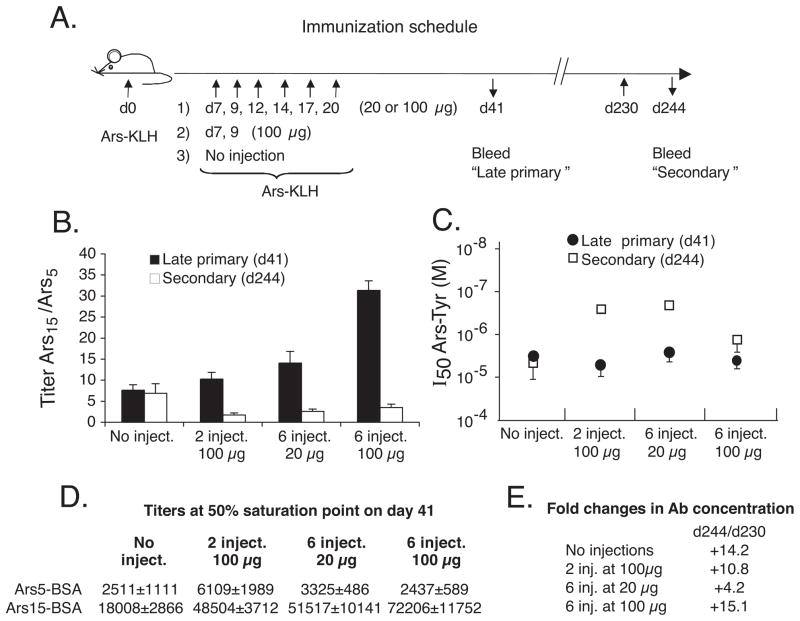
A/J mice were given an initial injection of Ars-KLH in IFA. Groups of these mice then received either none, 2 or 6 injections of Ars-KLH in PBS during the period of germinal center development (49), as illustrated in Figure 1A. The mice were bled at day 41 (late primary response), and the relative quantities of high affinity and low affinity anti-Ars Ab in sera were determined by ELISA using trays coated with Ars5-BSA (bound by high-affinity Ab) or Ars15-BSA (bound by high + low affinity Ab). Figure 1B illustrates the results of this assay, expressed as ratios of Ab titers on the two forms of antigen (solid bars). The titers were indistinguishable in the presence of 2 mercaptoethanol (10 μM), indicating little or no IgM antibody (data not shown). The lack of a strong primary IgM response is not surprising considering the absence of a short term extrafollicular AFC response to Ars in this strain (9, 12). Groups that received any injection series produced a higher proportion of lower affinity antibody at day 41 than the group that did not, and the proportion of low affinity Ab generally increased with the dose of antigen. Moreover the titers of serum antibody increased in groups receiving the soluble injections (Figure 1D). These data are consistent with those of Pulendran et al. (24) in supporting the idea that higher doses of Ag drive lower-affinity GC B cells into the long-term bone marrow AFC pathway.
Figure 1.
Soluble antigen differentially affects primary and secondary immune responses. A, Immunization schedule. The initial immunization was with 100 μg of Ars15-KLH i.p. in IFA (n = 4–5 mice/group). The primary injection series on days 7–20 was in PBS. B, Differential effects of primary injection series on overall affinity of Ab in the late primary and secondary immune responses. Dilution points at 50% of maximum binding were determined and used to calculate the ratios shown. High affinity Ab selectively bind Ars5-BSA Ab, while both high and low affinity Ab bind Ars15-BSA. C, Relative affinities among higher affinity Ab in late primary and secondary immune response sera. Trays were coated with Ars5-BSA. Sera were used a dilution giving 90% of maximal binding on Ars5-BSA. Ordinate indicates Ars-Tyr concentrations at which binding to Ars5-BSA was inhibited by 50% (I50). Each point is average of Log10 I50 for 4–5 mice. Lower concentration indicates higher affinity Ab. Standard errors indicated. D, Anti-Ars titers defined as dilution at 50% of maximal binding on Ars-BSA for day 41 sera. E, Fold increase in anti-Ars titers between days 230 and 244 as assessed on Ars5-BSA.
To examine the effects of the injection series on the memory immune response, mice were rested until day 230 and given a final booster injection of Ars-KLH in PBS. Sera were taken 14 days later and tested for titers of high and low-affinity Ab on Ars5-BSA and Ars15-BSA. Total serum Ab titers rose from 4 to 15-fold between day 230 and 14 days after the booster injection (Figure 1E), indicating that secondary serum Ab included only a minor amount of Ab from the primary response. Results of the inhibition test, illustrated in Figure 1B (open bars), revealed that the injection series did not have the same effect on Ab affinity at this point as it did in the late primary immune response. Instead, the injections resulted in a consistent but modest overall improvement in the affinity of the recall response Ab. Collectively, these results indicate that the injection series had opposite effects on the affinities of the primary long-term AFC response and the memory response. This dichotomy suggested that the injection series might have generated a silent population of high-affinity memory precursor cells that, during their development, did not generate AFC.
Evidence for affinity maturation in a silent memory-precursor population
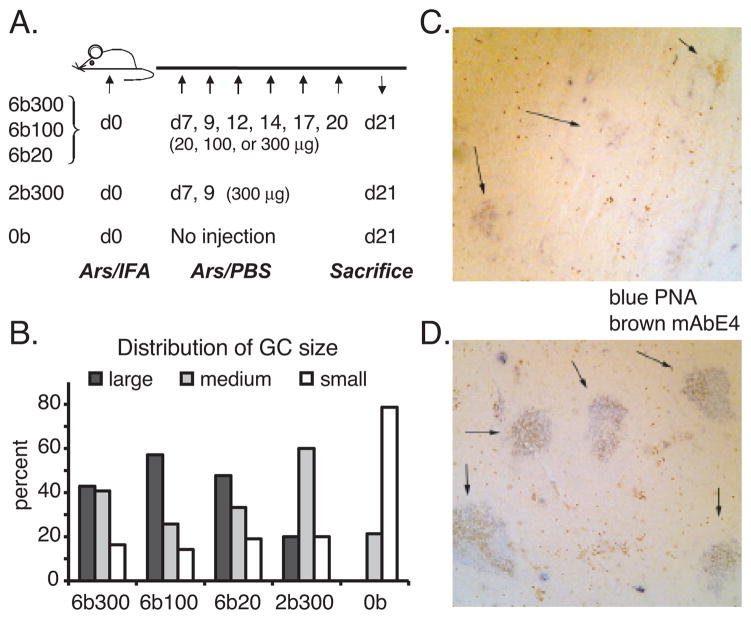
To more conclusively test whether the injection series with Ars-KLH drove the development and affinity maturation of a silent memory population, we determined relative affinities of serum Ab in late primary and secondary response sera, using a hapten-inhibition assay with Ars-Tyr. Importantly, to focus selectively on the highest affinity Ab, this assay was conducted using trays coated with Ars5-BSA. In this test, Ab of higher affinity are inhibited by lower concentrations of Ars-Tyr. The I50 values, shown in Figure 1C, reveal that the injection series resulted in the generation of high affinity Ab in the secondary immune response that was not detected in day 41 sera. This affinity-maturation effect was generally greatest in mice receiving lower doses of Ag. The injection series extended GC longevity, as determined by GC size distributions observed at day 21 (Figure 2). These results support the idea that injection of Ag during the primary immune response drives development and affinity-maturation of a population of memory precursor cells, which do not generate significant long-term AFC along the way. However, following a rest period, these cells are rendered competent to produce a recall AFC response upon a secondary challenge with Ag.
Figure 2.
Injection of soluble antigen extends GC longevity. A, Immunization schedule for the analysis of GC size. Mice received either 6 (300 μg/ml, 100μg/ml or 20 μg/ml), 2 (300 μg/ml), or no injections of indicated quantities of Ars-KLH in PBS. B, Distribution of GC sizes following immunization. Sections of spleens were stained with PNA to identify GC, which were measured (see experimental procedures), enumerated and plotted as % of total for each class size. C, Vanishing GC response on day 21 in mice without soluble antigen injection (0b). Arrows point to residual GCs. D, Prolonged GC reaction on day 21 in mice after 6 injections with 300 μg Ars-KLH (6b300). Spleen section shows large germinal centers (PNA = blue) with anti-idiotypic staining (mAbE4 = brown).
Specificity maturation in a silent memory-precursor population
Up to this point, our interpretations were based solely on quantitative differences in Ab affinity, resulting from different immunization schedules. To qualitatively test the silent memory concept, we exploited a special feature of the anti-Ars immune response. During hypermutation, rare mutants of the predominant canonical Ars clonotype (33, 34) arise that acquire an enhanced affinity for a related hapten, sulfanilic acid (Sulf), while losing or severely reducing their affinity for Ars (35–37). Somatic mutations at VH codon 35 are responsible for this change in antigenic specificity. These mutants can be selected by injecting Sulf-KLH during a primary immune response initiated by Ars-KLH. The initial Ars injection is required because Sulf does not bind to the unmutated canonical BCR with sufficient affinity to drive a primary immune response (35, 51). We used this system to determine whether a Sulf-KLH injection series, delivered during the primary immune response, would select and expand a silent population of Sulf-binding canonical memory precursor B cells.
In this experiment, mice were first injected with Ars-KLH in CFA. This was followed by 3 injections on consecutive days with Sulf-KLH in PBS. In different groups of mice, the Sulf-KLH injections were initiated on different days, starting on day 6 for the first group and on day 15 for the last. A control group received a series of injections with Ars-KLH starting on day 11. Late primary response sera were collected, and the mice were given a booster injection with Sulf-KLH in IFA, both on day 41. Sera sampled on day 57 were used to assess the secondary immune response. A hapten counter-inhibition assay, described in Figure 3 (37), was used to test for the presence of canonical Ab that bound to Sulf with greater affinity than to Ars (switched specificity mutants) as revealed by superior counter-inhibition achieved by Sulf-Tyr relative to Ars-Tyr. The canonical idiotype was identified in this assay with an anti-clonotypic Ab called mAbE4, which is highly-specific for unique features of canonical Ab including the appropriate heavy and light chain V regions and VHCDR3 (44, 52). The results, shown in Table I, revealed that serum Ab with switched antigenic specificity could only be detected in mice that had received the serial injections of Sulf-KLH. The number of mice demonstrating Sulf-switched Ab was highest in those receiving the injections that ended at day 10 or later. Only 1 in 4 mice injected on or before day 9 showed evidence for the switch, possibly reflecting the immaturity of germinal centers at early time points. This result indicated that the Sulf-KLH injections selected and expanded rare mutant B cells producing this Ab of switched antigenic specificity. However, Sulf-switched Ab was only observed in secondary immune response sera. In view of its absence in the late primary sera (day 41), this indicates that Sulf-KLH injection series drove the expansion of mutant Sulf-specific memory progenitors that apparently did not produce significant AFC during their development.
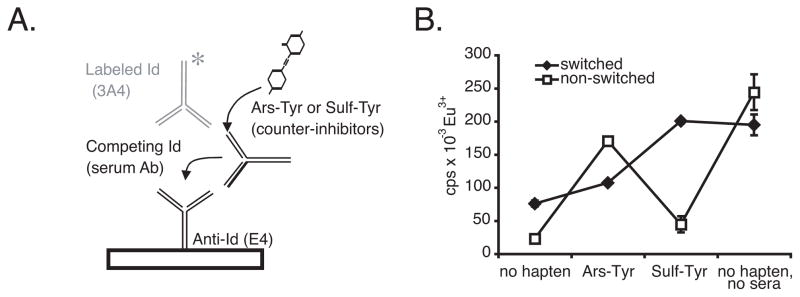
Figure 3.
Counter-inhibition assay used to identify canonical Ab of switched specificity. A, Binding of biotin-labeled Ab 3A4 to anti-Id E4 is inhibited by canonical E4+ Id in serum. If serum Id binds hapten, inhibition is reversed. Note, 3A4 does not bind Ars-Tyr or Sulf-Tyr. B, Representative results for two sera, one of which contains E4+ Id that binds more strongly to Sulf-Tyr than Ars-Tyr (switched specificity, representative of group #5 Table IV), and the other of which behaves conversely (not switched, representative of control group Table IV).
Table I.
Recruiting memory B cells with a new mutation-generated Ag specificity
| Groups | serial injections with 1 | on days 2 | Sulf>Ars d413 | Sulf> Ars d574 | % counter-inhibition by 5 | |
|---|---|---|---|---|---|---|
| Ars-Tyr | Sulf-Tyr | |||||
| #1 | Sulf15-KLH | 6,7,8 | 0/4 | 1/4 | 22 | 25 |
| #2 | " | 7,8,9 | 0/4 | 1/4 | 5 | 22 |
| #3 | " | 8,9,10 | 0/4 | 4/4 | 16 ± 2 | 48 ± 8 |
| #4 | " | 9,10,11 | 0/4 | 3/4 | 8 ± 3 | 44 ± 8 |
| #5 | " | 10,11,12 | 0/4 | 3/4 | 23 ± 12 | 41 ± 6 |
| #6 | " | 11,12,13 | 0/4 | 2/4 | 43 ± 23 | 67 ± 33 |
| #7 | " | 12,13,14 | 0/4 | 3/4 | 14 ± 5 | 55 ± 13 |
| #8 | " | 13,14,15 | 0/4 | 3/4 | 10 ± 3 | 37 ± 12 |
| #9 | " | 14,15,16 | 0/4 | 3/4 | 9 ± 7 | 33 ± 6 |
| #10 | " | 15,16,17 | 0/4 | 3/4 | 13 ± 2 | 26 ± 2 |
| Ctrl. | Ars14-KLH | 11,12,13 | 0/4 | 0/4 | 63 ± 5 | 3 ± 0 |
Groups of A/J mice (n = 4) were immunized with Ars14-KLH in CFA on day 0. Serial injections were given with Ars14-KLH or Sulf15-KLH in PBS on days indicated in 2.
Days of serial injections in PBS.
Number of mice (numerator) out of total at day 41 producing idiotype+ Ab that bound Sulf more strongly than Ars.
Same as 3 except mice were given booster injection on day 41 with Sulf15-KLH in IFA and bled on day 57.
Average percent counter-inhibition (+/− SD) by Ars-Tyr or Sulf-Tyr for experimental mice showing a switched phenotype and for all mice in the control group showing a non-switched phenotype on day 57.
Structural correlates of specificity maturation
To determine the structural consequences of multiple antigen injections during the primary immune response, we produced hybridomas from a mouse that was given a series of six injections with Sulf-KLH during a primary immune response to Ars-KLH (see Materials and Methods). This experiment was performed in an Ars-5 transgenic mouse carrying a mu heavy chain transgene encoding a canonical anti-Ars Ab (45). This animal was used so that we could unambiguously identify somatic mutations in the event that the VH gene was extensively altered. From this animal, we recovered three canonical Sulf-binding Ab reactive with the mAbE4. Sequences of their V genes confirmed that the Ab were encoded by the Tg VH. This was most evident by the presence of 4 somatic mutations that were present in the original transgene. The light chain was encoded by an endogenously-derived canonical Vκ10.1-Jκ1 rearrangement with the invariant junctional Arg codon (43, 53, 54). The Ab were of the IgG class, which is consistent with a transchromosomal recombination event resulting in a switched isotype as described previously in this mouse (37, 45, 55).
The three mAb were very heavily mutated, more so by far than any of the numerous conventionally-elicited canonical anti-Ars Ab examined to date. Their V genes carried between 39 and 53 nucleotide changes, producing between 24 and 32 amino acid replacements (Table II). Notably, all three carried mutations producing amino acid replacements at the critical VH35 codon in CDR-1, which determines Ars/Sulf specificity (35, 36, 56).
Table II.
Amino acid replacements (somatic mutations) in V genes of Sulf-switch mutant canonical mAb.
| S2-2 |
S3-4 |
S10-16 |
|||
|---|---|---|---|---|---|
| VH | Vκ | VH | Vk | VH | Vκ |
| Q5 -> H | D1 -> E | Q5 -> K | M4 -> L | A9 -> T | I2 -> T |
| A14 -> S | S22 -> T | K19 -> Q | T8 -> P | Y27 -> F | A25 -> S |
| T28 -> P | D28 -> G | T28 -> S | V19 -> I | T28 -> S | K39 -> R |
| F29 -> V | S30 -> N | S31 -> N | A25 -> S | S31 -> T | D70 -> H |
| S31 -> N | V44 -> M | N35 -> S | S30 -> N | I34 -> V | S76 -> N |
| Y32 -> F | K45 -> R | G49 -> A | N31 -> S | N35 -> V | I83 -> V |
| I34 -> V | H55 -> Q | N52 -> S | K45 -> R | E46 -> Q | N92 -> Y |
| N35 -> V | G64 -> A | Y57 -> F | G68 -> K | N52 -> S | |
| G42 -> S | Y71 -> F | K59 -> S | S76 -> N | G54 -> E | |
| N52 -> S | Q80 -> P | G66 -> D | N92 -> D | N55 -> G | |
| Y57 -> F | I83 -> F | K67 -> Q | T93 -> A | Y57 -> F | |
| K59 -> E | N92 -> S | V72 -> G | K59 -> E | ||
| G66 -> V | T93 -> A | K74 -> R | E62 -> A | ||
| V72 -> I | M81 -> L | K63 -> R | |||
| K74 -> Q | A97 -> V | K65 -> T | |||
| M81 -> L | F108 -> Y | V72 -> M | |||
| V93 -> I | K74 -> R | ||||
| N100 -> T | |||||
| Q113 -> R | |||||
Bold values represent critical residues conferring change in specificity from Ars to Sulf
To determine how the somatic mutations altered antibody affinity and specificity, we measured their affinities for Ars-Tyr and Sulf-Tyr by fluorescence quenching (57, 58). For comparative purposes, it was necessary to generate an unmutated correlate (mAbT-H12) of the precursor to these antibodies (see Materials and Methods). The T-H12 antibody bound Ars with a modest affinity, but its affinity for Sulf was barely measurable (<1/50th of that for Ars). In contrast, the three monoclonal antibodies had dramatically increased affinities for Sulf that ranged from 375-fold to 1,300-fold over that of mAbT-H12. At the same time their affinities for Ars were reduced from 25 to >100-fold relative to mAbT-H12 (Table III). Thus, somatic mutations in these antibodies produced changes in the ratios of affinities with which the two haptens were bound that ranged from 25,000- to 40,000-fold relative to the precursor antibody in the Ars-5 mouse. Collectively these results reveal the influence of the Sulf-KLH injection series on the evolution and affinity maturation of a silent memory precursor population.
Table III.
Affinities of canonical mAb for Ars-Tyr and Sulf-Tyr.
| Ka (M−1) |
||
|---|---|---|
| Sulf |
Ars |
|
| mAb T-H121 | 8.5 × 104 | 5.2 × 106 |
| mAb S2-2 (γ1) | 5.0 × 107 | 1.2 × 105 |
| mAb S3-4 (γ1) | 1.1 × 108 | 2.1 × 105 |
| mAb S10-16 (γ2b) | 3.2 × 107 | <5 × 104 2 |
| mAb 36-653 | < 5 × 104 | 5.9 × 105 |
This mAb precisely represents the unmutated Ars-5 precursor of the others in this table
The limit of sensitivity by the fluorescence quenching method.
An unmutated canonical antibody.
Only a brief pause is required to render memory-precursors competent for recall responses
Up to this point, our results indicated that injections of Ag during the primary immune response drove the development of a memory precursor population that is silent, as assessed by a lack of corresponding serum Ab. After a pause in antigenic stimulation, however, the cells attained competence to differentiate into AFC upon further antigenic challenge. To define the minimal pause required to render the memory precursors competent, we immunized mice with Ars-KLH in IFA and delivered a series of 3 Sulf-KLH injections in PBS on days 8, 10 and 12, as shown in Figure 4A. The mice were rested for various periods of time and then given a final booster injection of Sulf-KLH in PBS. One group did not receive the final booster injection. All mice were bled two weeks following the final booster injection, except for the control, which was bled at a point corresponding to the latest bleed (experimental group #5). Sera were tested in the counter-inhibition assay for the presence of Ab reactive with mAbE4 that bound more strongly to Sulf-Tyr than to Ars-Tyr. The results in Table IV show that, as before, the control mice that did not receive the final booster injection had no detectable canonical Ab of switched antigenic specificity. In contrast, such Ab was observed in 4 of 5 groups of mice that did receive the booster injection. The results show that a rest period of only 4 days was sufficient to render the memory progenitors competent to generate a serum Ab response upon subsequent antigenic challenge.
Table IV.
Determining rest period required for a successful recall response
| Group | serial injections 1 | on days 2 | rest period 3 | Sulf>Ars (14 days post boost)4 | % counter-inhibition by 5 | ||
|---|---|---|---|---|---|---|---|
| Ars-Tyr | Sulf-Tyr | ||||||
| #1 | Sulf15-KLH | 8,10,12 | 2 days | 0/4 | (day 28) | 39 ± 16 | 18 ± 5 |
| #2 | Sulf15-KLH | 8,10,12 | 4 days | 3/3 | (day 30) | 5 ± 2 | 60 ± 7 |
| #3 | Sulf15-KLH | 8,10,12 | 7 days | 4/4 | (day 33) | 11 ± 4 | 55 ± 12 |
| #4 | Sulf15-KLH | 8,10,12 | 14 days | 4/4 | (day 40) | 34 ± 10 | 66 ± 19 |
| #5 | Sulf15-KLH | 8,10,12 | 28 days | 4/4 | (day 54) | 41 ± 21 | 67 ± 9 |
| Ctrl | Sulf15-KLH | 8,10,12 | no final booster | 0/4 | (day 54) | 72 ± 6 | 11 ± 3 |
Groups of A/J mice (n = 4) were immunized with Ars14-KLH in IFA. Sulf15-KLH injections were in PBS
Days of soluble Sulf15-KLH injections.
Days of rest preceding the final boost with Sulf15-KLH in PBS.
Number of mice (numerator) out of total (denominator) producing Ab reactive with anti-Id E4 that bound Sulf-Tyr more strongly than Ars-Tyr in counter-inhibition assay
% counter-inhibition (+/− standard deviation) by Ars-Tyr or Sulf-Tyr 14 days post final booster injection.
As seen in Table IV, Sulf-Tyr afforded some counter-inhibition of sera from the control group (last row) that received the soluble injections of Sulf-KLH but not the final booster injection. For this reason, we examined control sera more closely in order to distinguish Ars-binding canonical Ab that cross-react weakly with Sulf from potentially genuine Sulf-switched canonical Ab. To this end, Sulf-binding antibodies were affinity-purified from the pooled sera of the control group, represented by the last row in Table IV and tested in the counter-inhibition assay. If the small amount of inhibition by Sulf-Tyr (11%) were due to genuine Sulf-switched Ab, as opposed to cross-reactive Ab, then we would expect Sulf-switched Ab to be enriched in the affinity-purified fraction. However, this was not the case. The affinity-purified fraction was again much more strongly inhibited by Ars-Tyr than Sulf-Tyr (Figure 4B). Thus, we cannot detect Sulf-switched Ab in control sera of mice that did not receive a final booster injection, even when the canonical antibodies are affinity-purified on Sulf.
Silent memory progenitors in GC
To look for direct evidence of silent memory progenitor cells, we immunized mice with Ars-KLH in IFA and delivered a series of 6 Sulf-KLH injections (100 μg) according to the protocol shown in Figure 2A. Mice were sacrificed on days 27. An additional group of control animals receiving the Sulf-KLH injection series were given a final booster injection with Sulf-KLH on day 27 and bled on day 41 to confirm the presence of canonical Ab with a switched specificity (data not shown). Consecutive spleen sections were then stained with mAbE4 in the presence or absence of competing antigen in the form of Ars-BSA or Sulf-BSA to look for developing E4+ memory B cells that bound Sulf more strongly than Ars.
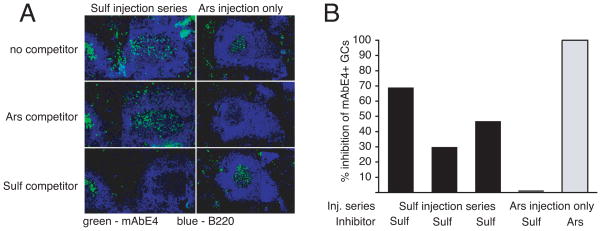
The result of this experiment is illustrated in Figure 5. Sulf-BSA reduced the staining of B cells by mAbE4 in 30% to 70% of all E4+ germinal centers of experimental mice. In contrast, Sulf-BSA did not reduce E4 staining of control GC from an animal immunized only with Ars, even though Ars-BSA was an effective competitor in the same GC. This result demonstrates the physical presence in GC of E4+ Sulf-binding B cells generated by the Sulf-KLH injection series despite an absence of corresponding serum antibody.
Figure 5.
Visualizing Sulf-binding memory B cells. Mice were initially immunized with Ars15-KLH (100 μg) i.p. in IFA. 3 mice were given serial injections of Sulf-KLH on days 7, 9, 12, 14, 17, 20. Mice were sacrificed on day 27. Control mouse (“Ars injection only”) received only the initial injection in IFA and were sacrificed on day 13 at the height of the germinal center response. Consecutive spleen sections were pre-incubated with the indicated competitors (Ars-BSA, Sulf-BSA) or with no competitor, before and during a subsequent stain with fluorescently labeled mAbE4 (green) and anti-B220 (blue). A, Representative example of GC with Sulf-binding E4+ B cells, as revealed by inhibition of mAbE4-staining with Sulf-BSA. B, Percentages of E4+ GC in which a majority of B cells bound Sulf-BSA, as assessed by inhibition of mAbE4 stain.
Discussion
In this report we demonstrated that antigen, injected during the primary immune response, drove the development and affinity/specificity-maturation of a silent pool of memory precursor B cells. Their silence is inferred from a lack of corresponding serum Ab that only appeared if a pause in antigenic stimulation occurred prior to a final antigenic challenge. The pause is apparently required for the memory precursors to develop competency for a secondary AFC response. In the model we examined, a pause of only 4 days was required for the development of competent memory cells.
The interpretation of silent memory derives from two experimental approaches that took advantage of an extensively-studied model immune response to the hapten Ars in strain A mice. First, the absence of an immediate extrafollicular AFC response to Ars immunization permitted us to assay serum Ab from the long-term AFC component and to compare its affinity to that of Ab produced by memory B cells driven in a recall response, all in the same animal (12). Second, rare mutants of a canonical Ars-associated clonotype lose affinity for Ars while acquiring affinity for another hapten, Sulf (35–37, 56). These can be recruited with Sulf, and their serum Ab identified with a monoclonal anti-idiotype. Using this system, we qualitatively assessed the long-term primary AFC response and the memory response for these mutant Ab products with switched antigenic specificity. Results of both approaches led to the same conclusion - that antigenic pulses during the primary immune response drove mutation and clonal selection in memory precursor cells without a detectable AFC response by immediate siblings. This generated memory progenitors with V regions that had undergone affinity/specificity maturation, as revealed by serology and hybridoma sampling studies performed after a final recall challenge and by immunohistology performed on GC immediately following the antigenic pulses. The mutants that we isolated in the form of hybridomas increased their Sulf/Ars affinity ratios by greater than 20,000-fold relative to the starting germline Ab. Although the simplest interpretation of our experiments is that memory B cell development with affinity maturation was achieved during the entire series of soluble Ag injections, it is possible that memory development is inhibited by high concentrations of soluble antigen and only takes place following the last injection, as the concentration of soluble antigen decreases.
We found that injecting low to modest doses of antigen during the primary response resulted in an overall reduction in Ab affinity for primary response antibodies. A similar effect of soluble Ag was reported by Nossal et al. (59), who found that it enhanced the magnitude of the splenic AFC precursor frequency when injected from 8 to 10 days following an initial immunization. These and related data support a model in which GC cells receiving the strongest signals through the BCR develop along the AFC pathway, while those that receive weaker signals continue to mutate their Ig V genes and develop along the memory pathway in GC (22).
Although critical transcription factors that differentially guide memory versus plasma cell differentiation have been defined, the cellular events that induce expression of these in vivo are not very well understood. In their analysis of the immune response to a multideterminant synthetic polypeptide, Press and Giorgetti reported evidence of silent memory B cell development to a particular side chain determinant (60). They proposed a model in which B cell clones that possessed low affinity receptors could receive an adequate signal for memory development but inadequate with respect to AFC differentiation because low affinity receptors gathered insufficient antigen to recruit the necessary quantity or quality of T cell help. A simpler model is that AFC differentiation is favored by a strong B cell-intrinsic signal initiated by highly aggregated BCR. Both models invoke BCR aggregation, one directly and the other indirectly (for antigen uptake and processing), as key determinants in the fate of antigen-stimulated B cells.
In our studies, the relative degree to which the BCR is aggregated could explain why soluble injections of Ars-KLH induced differentiation of the primary Ars-specific B cells into AFC, while soluble injections of Sulf-KLH did not induce differentiation of Sulf-switch mutants. In the first instance, it was the low-affinity component of the anti-Ars response that was enhanced. In the second case, high-affinity, Sulf-binding canonical mutants remained silent. If soluble antigen reaches the follicles as reported by Pape et al. (61), high-affinity Sulf-switch clones will engage disproportionally more BCR in a monovalent nonstimulatory manner relative to low-affinity primary Ars-specific clones. This “prozone” effect would limit both BCR-initiated signaling and antigen-presentation to T cells and thereby favor development of memory cells according to both variations of the hypothesis. In the extreme case, very high doses of soluble antigen could lead to B cell death for lack of adequate BCR aggregation. This is consistent with the observation that high doses of soluble antigen increase the frequency of apoptotic B cells in GC, and in extreme cases induce GC dissolution. Notably, in studies of antigen-induced B cell death, considerably more soluble antigen (milligrams) was injected than in the work reported here (62–64). In addition, the BCR in these studies have much higher affinities than those of primary anti-Ars B cells (58).
The short duration of the pause in antigenic delivery required for memory B cell development was unexpected. While the mechanism by which this pause promotes competence in memory B cells could involve follicular T helper cells, cytokines or other B cell extrinsic factors, the short period of the pause suggests that the mechanism may be intrinsic to the B cell. According to the preceding argument, if the period following the last injection of soluble antigen provides for Ag clearance by mechanisms that do not involve the BCR, we would not necessarily expect B cells to differentiate into plasma cells during this period. However, the rest period may allow for antigen clearance and for accumulation of secreted antibody by primary AFC, such that upon a later injection with the same antigen dose, a higher degree of BCR aggregation is attained due to partial blockade or removal by secreted antibody. We recognize that more complex models are possible, and propose this one only because of its minimalist nature.
The idea that B cell memory is stratified into tiers that are generated upon successive challenges with antigen was proposed by Decker et al. (32), who used a limiting-dilution splenic focus assay to assess memory development in vitro. They provided evidence that each pulse of antigen produced both AFC and memory cells that underwent further rounds of hypermutation. In vivo, the long-term AFC response may be considered one tier of memory since it emanates from the GC reaction. The silent memory progenitor cells would constitute a second tier. However, whether hypermutation and affinity maturation occur again in memory B cells that are participating in a true secondary immune response is still unresolved. While it is clear that secondary antigenic challenge drives memory cells to undergo massive proliferation and differentiation into AFC in the red pulp of the spleen and the medullary cords of lymph nodes without hypermutation (65, 66), a secondary antigenic challenge also induces GC reactions. So it is conceivable that these GC arise from memory B cells that mutate and develop into another tier of memory during the recall response.
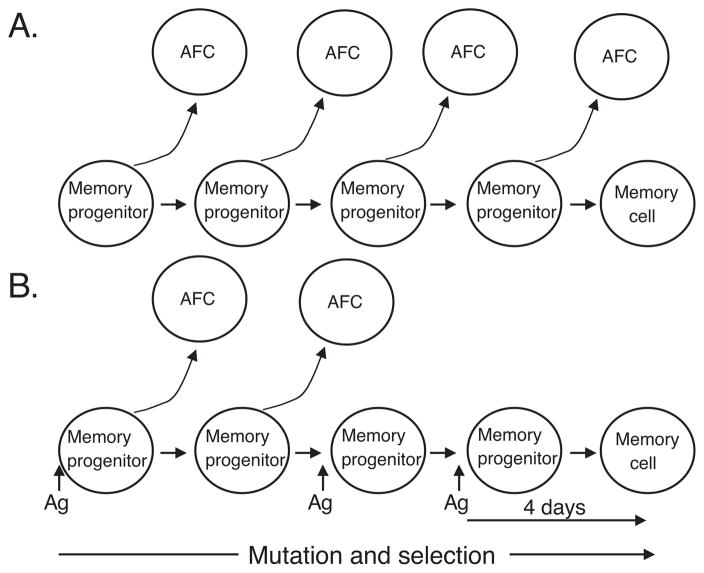
It is noteworthy that in our study there was a lack of affinity improvement in the recall response of mice that did not receive the Ars-KLH injection series (Figure 1C). In these mice, Ab of the late primary response sera (day 41) achieved affinities that were indistinguishable from those of secondary response sera (day 244). This result stands in contrast to the affinity maturation seen in mice that received the primary injection series. At the same, time it is consistent with a report by Takahashi et al. (21), who argued that the germinal center reaction continuously seeds the primary AFC reaction, which consequently undergoes continuous affinity maturation. We can envision two explanations for the lack of further affinity improvement in Ab of the recall response in mice that did not receive the injection series (Figure 6). One possibility is that silent memory cells may occur routinely, even when the concentration of Ag is not increased artificially by further injections or naturally during an actual infection. According to this scenario, the failure to observe affinity maturation in mice not receiving the injection series could be due to a limited duration of Ag stimulation by the particular antigen that we used, Ars-KLH. In preceding studies and unpublished work, we noted that GC development peaks already by day 14 in mice that receive only one antigen injection (49). In contrast, the duration of GC reactions are extended in mice that receive the Ars-KLH injection series (Figure 2). Additional Ars-KLH injections delivered during the primary immune response may promote exaggerated affinity maturation in memory precursors by extending the life of the germinal center reaction and the period of Ag-driven selection. Alternatively it is possible that developing memory cells must sense an increase in Ag during an established primary immune response in order to develop along the silent memory pathway i.e. without significant differentiation of siblings into AFC. That is, an increase in Ag concentration may signal a novel developmental pathway.
Figure 6.
Models of memory cell and AFC generation. A, Continuous generation of AFC and memory cells from a common clone during affinity maturation in GC. This model is consistent with results from mice given a single injection of antigen. B, Extensive affinity maturation in memory progenitor cells without AFC formation. This model is consistent with data from mice that received serial injections of antigen during the primary immune response. A pause in antigenic stimulation is required for memory progenitors to finalize their development into competent memory cells.
The biological significance of silent memory is unknown, and it may be difficult to define in the context of natural infectious agents. However it is interesting that the longevity of murine GC reactions against vesicular stomatitis virus is much greater than it is to a simple hapten protein conjugate delivered in Freund’s adjuvant (67). In this sense, delivering an Ag injection series may produce an immune response that more closely resembles a natural scenario where the concentration of Ag may rise and fall during infection. One possible advantage of silent memory is that it may inhibit the evolution of microbial resistance during infection. Thus, even if a microorganism mutated an epitope that was initially targeted by Ab secreted early in the immune response, the high affinity antibody produced by reactivation of formerly silent memory cells might bind well enough to eliminate the infection.
Acknowledgments
We thank Drs. Jacqueline Sharon and Behnaz Parhami-Seren for providing the IgG2b vector.
Non-standard abbreviations
- Ars p
azophenylarsonate
- Sulf p
azophenylsulphonate
Footnotes
This work was supported by NIH grants: AI033613 and AI048108.
Publisher's Disclaimer: This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI), publisher of The JI, holds the copyright to this manuscript. This version of the manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence, it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the U.S. National Institutes of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org.
References
- 1.MacLennan IC, Gray D. Antigen-driven selection of virgin and memory B cells. Immunol Rev. 1986;91:61–85. doi: 10.1111/j.1600-065x.1986.tb01484.x. [DOI] [PubMed] [Google Scholar]
- 2.Vieira P, Rajewsky K. Persistence of memory B cells in mice deprived of T cell help. Int Immunol. 1990;2:487–494. doi: 10.1093/intimm/2.6.487. [DOI] [PubMed] [Google Scholar]
- 3.Manz RA, Thiel A, Radbruch A. Lifetime of plasma cells in the bone marrow. Nature. 1997;388:133–134. doi: 10.1038/40540. [DOI] [PubMed] [Google Scholar]
- 4.Slifka MK, Ahmed R. Long-lived plasma cells: a mechanism for maintaining persistent antibody production. Curr Opin Immunol. 1998;10:252–258. doi: 10.1016/s0952-7915(98)80162-3. [DOI] [PubMed] [Google Scholar]
- 5.Tarlinton D. Germinal centers: form and function. Curr Opin Immunol. 1998;10:245–251. doi: 10.1016/s0952-7915(98)80161-1. [DOI] [PubMed] [Google Scholar]
- 6.Smith KG, Hewitson TD, Nossal GJ, Tarlinton DM. The phenotype and fate of the antibody-forming cells of the splenic foci. Eur J Immunol. 1996;26:444–448. doi: 10.1002/eji.1830260226. [DOI] [PubMed] [Google Scholar]
- 7.Jacob J, Kassir R, Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. I. The architecture and dynamics of responding cell populations. J Exp Med. 1991;173:1165–1175. doi: 10.1084/jem.173.5.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacob J, Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. II. A common clonal origin for periarteriolar lymphoid sheath-associated foci and germinal centers. J Exp Med. 1992;176:679–687. doi: 10.1084/jem.176.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song H, Cerny J. Functional heterogeneity of marginal zone B cells revealed by their ability to generate both early antibody-forming cells and germinal centers with hypermutation and memory in response to a T-dependent antigen. J Exp Med. 2003;198:1923–1935. doi: 10.1084/jem.20031498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lopes-Carvalho T, Kearney JF. Development and selection of marginal zone B cells. Immunol Rev. 2004;197:192–205. doi: 10.1111/j.0105-2896.2004.0112.x. [DOI] [PubMed] [Google Scholar]
- 11.Sze DM, Toellner KM, Garcia de Vinuesa C, Taylor DR, MacLennan IC. Intrinsic constraint on plasmablast growth and extrinsic limits of plasma cell survival. J Exp Med. 2000;192:813–821. doi: 10.1084/jem.192.6.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vora KA, Tumas-Brundage KM, Manser T. A periarteriolar lymphoid sheath-associated B cell focus response is not observed during the development of the anti-arsonate germinal center reaction. J Immunol. 1998;160:728–733. [PubMed] [Google Scholar]
- 13.Linton PL, Decker DJ, Klinman NR. Primary antibody-forming cells and secondary B cells are generated from separate precursor cell subpopulations. Cell. 1989;59:1049–1059. doi: 10.1016/0092-8674(89)90761-7. [DOI] [PubMed] [Google Scholar]
- 14.Weigert MG, I, Cesari M, Yonkovich SJ, Cohn M. Variability in the lambda light chain sequences of mouse antibody. Nature. 1970;228:1045–1047. doi: 10.1038/2281045a0. [DOI] [PubMed] [Google Scholar]
- 15.Bothwell AL, Paskind M, Reth M, Imanishi-Kari T, Rajewsky K, Baltimore D. Heavy chain variable region contribution to the NPb family of antibodies: somatic mutation evident in a gamma 2a variable region. Cell. 1981;24:625–637. doi: 10.1016/0092-8674(81)90089-1. [DOI] [PubMed] [Google Scholar]
- 16.Griffiths GM, Berek C, Kaartinen M, Milstein C. Somatic mutation and the maturation of immune response to 2-phenyl oxazolone. Nature. 1984;312:271–275. doi: 10.1038/312271a0. [DOI] [PubMed] [Google Scholar]
- 17.Siekevitz M, Huang SY, Gefter ML. The genetic basis of antibody production: a single heavy chain variable region gene encodes all molecules bearing the dominant anti-arsonate idiotype in the strain A mouse. Eur J Immunol. 1983;13:123–132. doi: 10.1002/eji.1830130207. [DOI] [PubMed] [Google Scholar]
- 18.Selsing E, Storb U. Somatic mutation of immunoglobulin light-chain variable-region genes. Cell. 1981;25:47–58. doi: 10.1016/0092-8674(81)90230-0. [DOI] [PubMed] [Google Scholar]
- 19.Gearhart PJ, Bogenhagen DF. Clusters of point mutations are found exclusively around rearranged antibody variable genes. Proc Natl Acad Sci U S A. 1983;80:3439–3443. doi: 10.1073/pnas.80.11.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith KG, Light A, Nossal GJ, Tarlinton DM. The extent of affinity maturation differs between the memory and antibody-forming cell compartments in the primary immune response. Embo J. 1997;16:2996–3006. doi: 10.1093/emboj/16.11.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takahashi Y, Dutta PR, Cerasoli DM, Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. V. Affinity maturation develops in two stages of clonal selection. J Exp Med. 1998;187:885–895. doi: 10.1084/jem.187.6.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tarlinton DM, Smith KG. Dissecting affinity maturation: a model explaining selection of antibody-forming cells and memory B cells in the germinal centre. Immunol Today. 2000;21:436–441. doi: 10.1016/s0167-5699(00)01687-x. [DOI] [PubMed] [Google Scholar]
- 23.Smith KG, Light A, O’Reilly LA, Ang SM, Strasser A, Tarlinton D. bcl-2 transgene expression inhibits apoptosis in the germinal center and reveals differences in the selection of memory B cells and bone marrow antibody-forming cells. J Exp Med. 2000;191:475–484. doi: 10.1084/jem.191.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pulendran B, Smith KG, Nossal GJ. Soluble antigen can impede affinity maturation and the germinal center reaction but enhance extrafollicular immunoglobulin production. J Immunol. 1995;155:1141–1150. [PubMed] [Google Scholar]
- 25.Paus D, Phan TG, Chan TD, Gardam S, Basten A, Brink R. Antigen recognition strength regulates the choice between extrafollicular plasma cell and germinal center B cell differentiation. J Exp Med. 2006;203:1081–1091. doi: 10.1084/jem.20060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clarke SH, Huppi K, Ruezinsky D, Staudt L, Gerhard W, Weigert M. Inter- and intraclonal diversity in the antibody response to influenza hemagglutinin. J Exp Med. 1985;161:687–704. doi: 10.1084/jem.161.4.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Claflin JL, Berry J, Flaherty D, Dunnick W. Somatic evolution of diversity among anti-phosphocholine antibodies induced with Proteus morganii. J Immunol. 1987;138:3060–3068. [PubMed] [Google Scholar]
- 28.Manser T. Evolution of antibody structure during the immune response. The differentiative potential of a single B lymphocyte. J Exp Med. 1989;170:1211–1230. doi: 10.1084/jem.170.4.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacob J, Przylepa J, Miller C, Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. III. The kinetics of V region mutation and selection in germinal center B cells. J Exp Med. 1993;178:1293–1307. doi: 10.1084/jem.178.4.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vora KA, Manser T. Altering the antibody repertoire via transgene homologous recombination: evidence for global and clone-autonomous regulation of antigen-driven B cell differentiation. J Exp Med. 1995;181:271–281. doi: 10.1084/jem.181.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dal Porto JM, Haberman AM, Shlomchik MJ, Kelsoe G. Antigen drives very low affinity B cells to become plasmacytes and enter germinal centers. J Immunol. 1998;161:5373–5381. [PubMed] [Google Scholar]
- 32.Decker DJ, Linton PJ, Zaharevitz S, Biery M, Gingeras TR, Klinman NR. Defining subsets of naive and memory B cells based on the ability of their progeny to somatically mutate in vitro. Immunity. 1995;2:195–203. doi: 10.1016/s1074-7613(95)80092-1. [DOI] [PubMed] [Google Scholar]
- 33.Manser T, Wysocki LJ, Margolies MN, Gefter ML. Evolution of antibody variable region structure during the immune response. Immunol Rev. 1987;96:141–162. doi: 10.1111/j.1600-065x.1987.tb00513.x. [DOI] [PubMed] [Google Scholar]
- 34.Wysocki LJ, Gridley T, Huang S, Grandea AG, 3rd, Gefter ML. Single germline VH and V kappa genes encode predominating antibody variable regions elicited in strain A mice by immunization with p-azophenylarsonate. J Exp Med. 1987;166:1–11. doi: 10.1084/jem.166.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ellenberger J, Creadon G, Zhang X, Wysocki LJ. Recruiting memory B cells with changed antigenic specificity. J Immunol. 1993;151:5272–5281. [PubMed] [Google Scholar]
- 36.Kussie PH, Parhami-Seren B, Wysocki LJ, Margolies MN. A single engineered amino acid substitution changes antibody fine specificity. J Immunol. 1994;152:146–152. [PubMed] [Google Scholar]
- 37.Jena PK, Smith DS, Zhang X, Aviszus K, Durdik JM, Wysocki LJ. Somatic translocation and differential expression of Ig mu transgene copies implicate a role for the Igh locus in memory B cell development. Mol Immunol. 2003;39:885–897. doi: 10.1016/s0161-5890(03)00006-3. [DOI] [PubMed] [Google Scholar]
- 38.Fearon DT, Manders P, Wagner SD. Arrested differentiation, the self-renewing memory lymphocyte, and vaccination. Science. 2001;293:248–250. doi: 10.1126/science.1062589. [DOI] [PubMed] [Google Scholar]
- 39.Marshak-Rothstein A, Siekevitz M, Margolies MN, Mudgett-Hunter M, Gefter ML. Hybridoma proteins expressing the predominant idiotype of the antiazophenylarsonate response of A/J mice. Proc Natl Acad Sci U S A. 1980;77:1120–1124. doi: 10.1073/pnas.77.2.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yelton DE, Desaymard C, Scharff MD. Use of monoclonal anti-mouse immunoglobulin to detect mouse antibodies. Hybridoma. 1981;1:5–11. doi: 10.1089/hyb.1.1981.1.5. [DOI] [PubMed] [Google Scholar]
- 41.Snyder CM, Zhang X, Wysocki LJ. Negligible class II MHC presentation of B cell receptor-derived peptides by high density resting B cells. J Immunol. 2002;168:3865–3873. doi: 10.4049/jimmunol.168.8.3865. [DOI] [PubMed] [Google Scholar]
- 42.Herzenberg LA, Black SJ, Tokuhisa T. Memory B cells at successive stages of differentiation. Affinity maturation and the role of IgD receptors. J Exp Med. 1980;151:1071–1087. doi: 10.1084/jem.151.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wysocki LJ, V, Sato L. The strain A anti-p-azophenylarsonate major cross-reactive idiotypic family includes members with no reactivity toward p-azophenylarsonate. Eur J Immunol. 1981;11:832–839. doi: 10.1002/eji.1830111016. [DOI] [PubMed] [Google Scholar]
- 44.Leo O, Slaoui M, Marvel J, Milner EC, Hiernaux J, Moser M, Capra JD, Urbain J. Idiotypic analysis of polyclonal and monoclonal anti-p-azophenylarsonate antibodies of BALB/c mice expressing the major cross-reactive idiotype of the A/J strain. J Immunol. 1985;134:1734–1739. [PubMed] [Google Scholar]
- 45.Durdik J, Gerstein RM, Rath S, Robbins PF, Nisonoff A, Selsing E. Isotype switching by a microinjected mu immunoglobulin heavy chain gene in transgenic mice. Proc Natl Acad Sci U S A. 1989;86:2346–2350. doi: 10.1073/pnas.86.7.2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shulman M, Wilde CD, Kohler G. A better cell line for making hybridomas secreting specific antibodies. Nature. 1978;276:269–270. doi: 10.1038/276269a0. [DOI] [PubMed] [Google Scholar]
- 47.Sharon J, Gefter ML, Wysocki LJ, Margolies MN. Recurrent somatic mutations in mouse antibodies to p-azophenylarsonate increase affinity for hapten. J Immunol. 1989;142:596–601. [PubMed] [Google Scholar]
- 48.Liu AH, Creadon G, Wysocki LJ. Sequencing heavy- and light-chain variable genes of single B-hybridoma cells by total enzymatic amplification. Proc Natl Acad Sci U S A. 1992;89:7610–7614. doi: 10.1073/pnas.89.16.7610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu AH, Jena PK, Wysocki LJ. Tracing the development of single memory-lineage B cells in a highly defined immune response. J Exp Med. 1996;183:2053–2063. doi: 10.1084/jem.183.5.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 51.Fish S, Zenowich E, Fleming M, Manser T. Molecular analysis of original antigenic sin. I. Clonal selection, somatic mutation, and isotype switching during a memory B cell response. J Exp Med. 1989;170:1191–1209. doi: 10.1084/jem.170.4.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Manser T, Gefter ML. The molecular evolution of the immune response: idiotope-specific suppression indicates that B cells express germ-line-encoded V genes prior to antigenic stimulation. Eur J Immunol. 1986;16:1439–1444. doi: 10.1002/eji.1830161120. [DOI] [PubMed] [Google Scholar]
- 53.Sanz I, Capra JD. V kappa and J kappa gene segments of A/J Ars-A antibodies: somatic recombination generates the essential arginine at the junction of the variable and joining regions. Proc Natl Acad Sci U S A. 1987;84:1085–1089. doi: 10.1073/pnas.84.4.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim SO, Sanz I, Williams C, Capra JD, Gottlieb PD. Polymorphism in V kappa 10 genes encoding L chains of antibodies bearing the Ars-A and A48 cross-reactive idiotypes. Immunogenetics. 1991;34:231–241. doi: 10.1007/BF00215258. [DOI] [PubMed] [Google Scholar]
- 55.Gerstein RM, Frankel WN, Hsieh CL, Durdik JM, Rath S, Coffin JM, Nisonoff A, Selsing E. Isotype switching of an immunoglobulin heavy chain transgene occurs by DNA recombination between different chromosomes. Cell. 1990;63:537–548. doi: 10.1016/0092-8674(90)90450-s. [DOI] [PubMed] [Google Scholar]
- 56.Wong YW, Gill DS, Parhami-Seren B, Short MK, Sompuram SR, Margolies MN. Structural requirements for a specificity switch and for maintenance of affinity using mutational analysis of a phage-displayed anti-arsonate antibody of Fab heavy chain first complementarity-determining region. J Immunol. 1998;160:5990–5997. [PubMed] [Google Scholar]
- 57.Rothstein TL, Gefter ML. Affinity analysis of idiotype-positive and idiotype-negative Ars-binding hybridoma proteins and Ars-immune sera. Mol Immunol. 1983;20:161–168. doi: 10.1016/0161-5890(83)90127-x. [DOI] [PubMed] [Google Scholar]
- 58.Wysocki L, Manser T, Gefter ML. Somatic evolution of variable region structures during an immune response. Proc Natl Acad Sci U S A. 1986;83:1847–1851. doi: 10.1073/pnas.83.6.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nossal GJ, Karvelas M, Pulendran B. Soluble antigen profoundly reduces memory B-cell numbers even when given after challenge immunization. Proc Natl Acad Sci U S A. 1993;90:3088–3092. doi: 10.1073/pnas.90.7.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Press JL, Giorgetti CA. Molecular and kinetic analysis of an epitope-specific shift in the B cell memory response to a multideterminant antigen. J Immunol. 1993;151:1998–2013. [PubMed] [Google Scholar]
- 61.Pape KA, Catron DM, Itano AA, Jenkins MK. The humoral immune response is initiated in lymph nodes by B cells that acquire soluble antigen directly in the follicles. Immunity. 2007;26:491–502. doi: 10.1016/j.immuni.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 62.Pulendran B, Kannourakis G, Nouri S, Smith KG, Nossal GJ. Soluble antigen can cause enhanced apoptosis of germinal-centre B cells. Nature. 1995;375:331–334. doi: 10.1038/375331a0. [DOI] [PubMed] [Google Scholar]
- 63.Han S, Zheng B, Dal Porto J, Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. IV. Affinity-dependent, antigen-driven B cell apoptosis in germinal centers as a mechanism for maintaining self-tolerance. J Exp Med. 1995;182:1635–1644. doi: 10.1084/jem.182.6.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shokat KM, Goodnow CC. Antigen-induced B-cell death and elimination during germinal-centre immune responses. Nature. 1995;375:334–338. doi: 10.1038/375334a0. [DOI] [PubMed] [Google Scholar]
- 65.Liu YJ, Zhang J, Lane PJ, Chan EY, MacLennan IC. Sites of specific B cell activation in primary and secondary responses to T cell-dependent and T cell-independent antigens. Eur J Immunol. 1991;21:2951–2962. doi: 10.1002/eji.1830211209. [DOI] [PubMed] [Google Scholar]
- 66.Vora KA, Tumas-Brundage K, Manser T. Contrasting the in situ behavior of a memory B cell clone during primary and secondary immune responses. J Immunol. 1999;163:4315–4327. [PubMed] [Google Scholar]
- 67.Bachmann MF, Odermatt B, Hengartner H, Zinkernagel RM. Induction of long-lived germinal centers associated with persisting antigen after viral infection. J Exp Med. 1996;183:2259–2269. doi: 10.1084/jem.183.5.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]