Abstract
Clinical outcomes after intra-synovial flexor tendon repair have been substantially improved over the past two decades through advances in tendon suture techniques and postoperative rehabilitation methods. Nevertheless, complications such as repair site elongation (i.e., gap formation) and rupture continue to occur frequently. Experimental studies have shown that repair site strength fails to increase in the first three weeks following tendon suture. After three weeks, the strength and rigidity of the repair site improves significantly, a process that continues for several months. Formation of a repair site gap during the early rehabilitation period has been shown to considerably delay the accrual of repair site strength over time. Thus, it is of prime importance that the method of tendon suture achieves and maintains a stiff and strong repair site during the early healing interval by maintaining close approximation of the tendon stumps and by stimulating, where possible, the intrinsic repair response. In this review we describe recent efforts to enhance the integrity of the immature repair site. We focus on two major areas of advancement: surgical technique modifications and manipulation of the biologic and biochemical environment.
Keywords: growth factor, canine, suture technique, tendon healing, biomechanics
SURGICAL TECHNICAL MODIFICATIONS FOR ENHANCED FLEXOR TENDON REPAIR
Number of core suture strands
Increasing the number of suture strands crossing the repair site increases strength, stiffness, and resistance to gap formation (1,2). Early methods of repair with two core suture strands (e.g. Kessler, Bunnell, Tajima methods, etc.) coupled with post-operative immobilization led to a decrease in ultimate strength during the first three weeks following tendon suture (3). While subsequent studies showed that the decrease in strength could be partially obviated by early motion exercise, the strength of the repair did not recover beyond the baseline level until three weeks after surgery (3,4). When repairs used multi-strand sutures in conjunction with early motion exercise, not only was there a maintenance of strength values, but under select conditions, modest increases in strength and stiffness were noted in this earliest interval (5). The Winters method (2), which employs a looped suture with two parallel modified-Kessler sutures in an eight-strand construct (Figure 1) has been shown to lead to significantly higher strength and stiffness values compared to four-strand methods, which used only one pass of the modified-Kessler suture (5). The eight-strand suture was also shown to have superior strength and stiffness compared to the six-strand Savage method and compared to the two-strand Tajima and Kessler methods (2). Using a looped suture effectively doubles the number of suture strands crossing the repair site without increasing the number of knots. These findings indicate that a larger number of suture strands (6 to 8) leads to improved mechanical properties following tendon suture.
Figure 1.
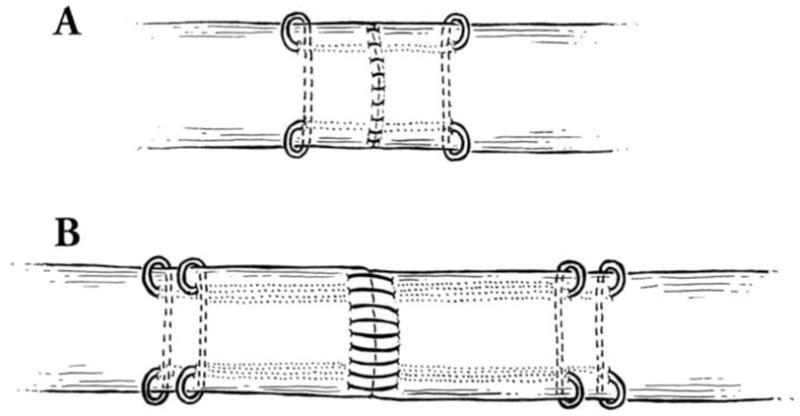
A) The classical (“historical”) approach used a modified Kessler four-strand repair using a double-stranded 4-0 Supramid suture was used for the core suture. The length of the core suture purchase was 0.75 cm. A running unlocked peripheral suture was then placed circumferentially using a 6-0 Prolene suture. The peripheral suture passed through only the epitenon and superficial tendon. B) In the modified eight-strand method, the same double-stranded 4-0 Supramid suture was used for the core suture. The eight-strand repair was composed of two parallel placed modified Kessler four-strand sutures. The length of the core suture purchase was 1.2 cm. A running unlocked peripheral suture was then placed circumferentially using a 5-0 Prolene. This peripheral suture had a purchase of two-millimeter length from the cut tendon edge and two-millimeter depth from the tendon surface.
Material and caliber of core suture
Increasing the strength and stiffness of the suture material has been shown to lead to a decrease in gap formation and to an increase in both ultimate strength and stiffness (6). Increasing the caliber of the core suture material also significantly increases the strength of the repair. Barrie et al (7) showed that the use of 3-0 braided dacron (Ethicon Inc., Somerville, NJ) sutures for tendon repair results in a 2- to 3-fold increase in fatigue strength compared to 4-0 sutures. Taras et al (8) reported significant increases in repair strength when suture caliber was increased from 5-0 to 2-0. The strength of the modified Kessler repairs improved by 167% and the strength of the double-grasping repairs improved by 391% when 2-0 compared to 5-0 suture was used. This finding suggests that high caliber sutures may maximize the strength and stiffness of the repair.
Purchase of core suture
The core suture purchase (i.e., the longitudinal distance from the cut tendon end to the transverse component of the core suture) significantly affects the strength of the repair. An in vitro animal study reported that the resistance to gap formation and the ultimate strength of the repairs increased significantly as the core suture purchase increased (9). A different in vitro animal study examined a range of purchase lengths (0.4 – 1.2 cm) and reported that the force required for gap formation and the ultimate failure load were significantly lower in repairs where 0.4-cm core suture purchase was used compared to those where 1.0-cm purchase was used (10). In this study, the repairs with 1.0 cm core suture purchase failed by suture breakage whereas those with 0.4 cm purchase failed predominantly by suture pullout. The findings in these studies suggest that the optimal purchase length is in the range of 0.7–1.2 cm.
Grasping and locking loop configurations of core suture
Since Pennington’s report described the locking-loop configuration (11), there have been numerous studies that have confirmed the superior tendon-holding power of the locking-loop configuration compared to the grasping- or no-loop configurations (3,7,12). In the locking-loop configuration, the transverse component is passed in front of the longitudinal component, and thus, the suture passes around or “locks” a bundle of tendon fibers. In the grasping-loop configuration, the transverse component passes behind the longitudinal component, and the suture does not pass around or lock a bundle of tendon fibers. The locking configuration has greater tensile strength and greater gap resistance than the grasping configuration both at time zero and during the first three weeks of repair (3). An in vivo animal study reported a decrease of ultimate strength during the first three weeks of repair with both the locking-loop configuration and the grasping-loop configuration (3). Nonetheless, absolute strength was higher in the locking-loop configuration than in the grasping-loop configuration at all time points. An in vitro study reported that repairs with the locking loops demonstrated higher gap resistance than those with no locking loops at low cyclic loads (12). These findings suggest that the locking-loop configuration provides additional gap resistance at the low cyclic loads anticipated during the early postoperative motion exercise period.
Location and number of core suture knots
Placing the suture knots outside the repair site was shown to result in higher tensile strength than placing the knots within the repair site both at time zero and during the first three weeks of repair (13). Tensile strength gradually increases after the initial period of decline, and by six weeks after repair the tensile strength becomes comparable between both methods of knot placement. Despite this, the higher strength of “knots-outside” repairs may result in better repair stability during the early postoperative motion exercise than “knots-inside” repairs. Tying knots in any suture strand weakens the suture material, and knots are always the weakest areas of a suture construct. Therefore, making as few knots as possible and placing them away from the repair site should improve repair strength. Placing knots outside the repair site, however, raises the concern of increasing the tendon gliding resistance and stimulating adhesion formation. It was shown in an in vitro animal study that tendon repairs with knots on both lateral sides of a tendon had significantly increased gliding resistance compared to those with one lateral knot or those with one volar knot (14). Therefore, one knot placed outside the repair site, preferably on the lateral side of the tendon, is considered optimal in terms of both tensile strength and tendon gliding.
Purchase of peripheral suture
Studies using both analytic models and in vitro experiments have demonstrated that increasing the purchase of the peripheral suture (i.e., placing the peripheral suture farther from the cut tendon end and deeper from the tendon surface) significantly increases the tensile strength of tendon repair (15,16). Core sutures augmented with a peripheral suture that was placed deep into the tendon were found to have 80% greater strength than those with a peripheral suture that was placed superficially only through the epitenon and the superficial tendon (15). Similarly, core sutures augmented with a peripheral suture that was placed 2 mm from the cut tendon end were found to be stronger by 37% than those augmented with a 1-mm peripheral suture. Therefore, a peripheral suture should be placed deep from the tendon surface and far from the cut tendon end to enhance repair site strength.
Combined application of technical modifications
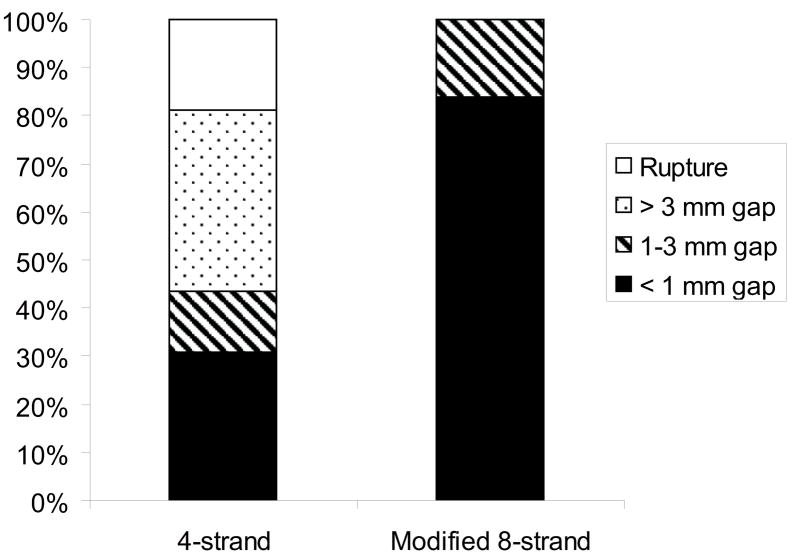
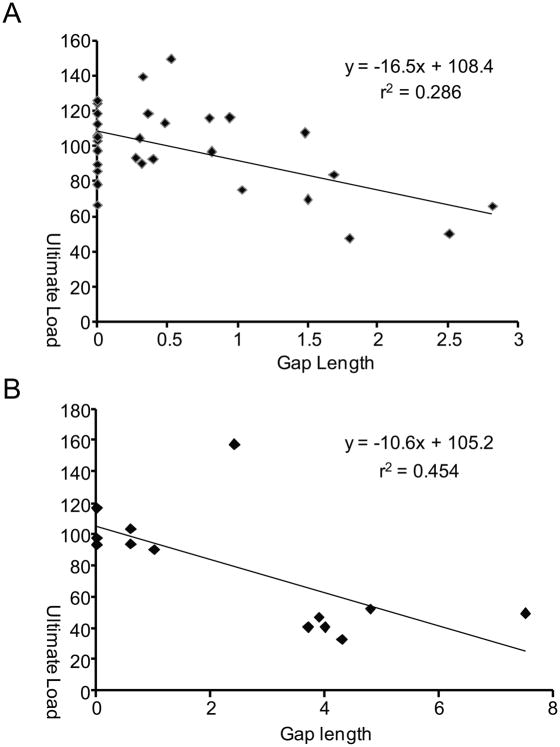
Each of the studies described above investigated individual effects of suture materials or techniques on tendon repair. Little is known, however, about the effects of combined application of these modifications. To this end, canine digital flexor tendons were injured and repaired using five technical modifications (Figure 1, Table 1): 1) Winters’ eight-strand core-suture technique using a looped 4-0 pseudo-monofilament nylon suture (2) (Supramid extra; S. Jackson Inc., Alexandria, VA), 2) Pennington’s locking loops (11), 3) core suture purchase length of 1.2 cm (9), 4) 5-0 Prolene peripheral suture that was placed 2 mm deep and 2 mm far from the cut tendon end, and 5) placement of one suture knot on the lateral side of the tendon. Tensile data for this group were compared to historical data of a previous canine study at time zero and 6 weeks after surgery (5). In that study, the tendon was repaired with the same technique except for the use of a four-strand core suture, no locking loops, 0.75-cm core-suture purchase length, and 6-0 Prolene peripheral suture that was passed through only the epitenon and superficial tendon (Figure 1). At 6 weeks after surgery, none of the 30 tendons repaired with the modified technique were found to have ruptured whereas 19% of tendons repaired with the previous technique were found to have been disrupted (Figure 2). The mean gap length was significantly decreased with the modified technique compared to the previous study (0.61 ± 0.80 mm vs. 2.52 ± 2.40 mm). None of the tendons repaired with the modified technique had a gap larger than three millimeters whereas 38% of the tendons had had a gap larger than three millimeters in the previous study (Figure 2). Ultimate strength was significantly increased compared to the previous study both at time zero (74.5 ± 10.7 N vs. 48.7 ± 7.5 N) and at 6 weeks after surgery (98.3 ± 24.3 N vs. 78.4 ± 37.4 N). Stiffness and elongation were also significantly improved using the modified eight strand methods (Table 2). There was a significant negative correlation between ultimate load and gap length (Figure 3) and between repair stiffness and gap length. These findings demonstrate that combined application of the technical modifications described above leads to a dramatic improvement of tensile strength and gap resistance of the repaired tendon compared to historical methods.
Table 1.
Comparison of suture repair techniques between the previous and current studies
| Historical 4-strand | Modified 8-strand | ||
|---|---|---|---|
| Core suture | Suture material | Double-stranded 4-0 Supramid | Double-stranded 4-0 Supramid |
| Number of suture strands crossing the repair and configuration | 4-strand modified Kessler configuration | 8-strand modified Kessler configuration | |
| Length of suture purchase | 0.75 cm | 1.2 cm | |
| Peripheral suture | Suture material | 6-0 Prolene | 5-0 Prolene |
| Depth and Length of suture purchase | Passed through only the epitenon and superficial tendon | 2 mm long from the cut tendon edge and 2 mm deep from the tendon surface | |
Figure 2.
The distribution of gap lengths was significantly different when comparing the historical 4-strand method to the modified 8-strand method (p < 0.0001, Chi-Squared test).
Table 2.
Tensile properties at time zero and 42 days after repair.
| Time zero | 4-strand (n=16) | Modified 8-strand (n=9) | |
| Repair-site stiffness (N/mm) | 15.4 ± 5.7 | 24.5 ± 6.4 | 0.005* |
| Elongation at 20 N (mm) | 1.70 ± 0.38 | 1.53 ± 0.39 | 0.038† |
| Elongation at failure (mm) | 4.74 ± 1.71 | 5.22 ± 2.31 | 0.318* |
| 42 days after repair | 4-strand (n=13) | Modified 8-strand (n=30) | p value |
| Repair-site stiffness (N/mm) | 95.6 ± 70.7 | 83.5 ± 29.2 | 0.944* |
| Elongation at 20 N (mm) | 0.69 ± 0.40 | 0.48 ± 0.25 | 0.038† |
| Elongation at failure (mm) | 1.48 ± 0.80 | 1.47 ± 0.83 | 0.979* |
p values from Mann-Whitney U tests
p values from unpaired t-tests
Figure 3.
Scatter plots and regression lines are shown for ultimate load vs. gap length for the historical 4 strand method (A) and for the modified 8-strand method (B). There was a significant negative correlation between ultimate load and the gap length regardless of the techniques used.
BIOLOGIC MODIFICATIONS FOR ENHANCED FLEXOR TENDON REPAIR
Although surgeons have achieved substantial gains in tendon repair outcomes by modifying surgical technique, a considerable rate of complications still remains (e.g., repair-site gapping, suture pull-out, tendon rupture, and adhesion formation). Flexor tendon healing relies on intrinsic tendon fibroblast activity arising from the epitenon and the endotenon and on an extrinsic inflammatory response originating from the tendon sheath. This intrinsic and extrinsic cellular response, while assisting in healing, also drives adhesion formation. In order to address these complications, investigators have sought to manipulate the biologic environment with targeted efforts to aid the healing response.
Biochemical modifications of the tendon surface
One approach for improving healing has involved biochemical modification of the tendon surface. Initial reports attempting to use hyaluronic acid to reduce adhesions and improve tendon gliding were inconclusive. Recent reports, however, show a clear beneficial role for hyaluronic acid in flexor tendon healing. Canine peroneous longus tendons were soaked in saline and hyaluronic acid (HA) (17). The HA soaked tendons displayed decreased resistance to gliding. A third group of tendons were modified with hyaluronic acid through carbodiimide derivatization (cd-HA). This group displayed the largest decrease in excursion resistance, suggesting that tendons treated with exogenous cd-HA may display improved HA retention, and therefore better gliding mechanics following repair. These in vitro studies were validated in an in vivo canine model in which repaired, cd-HA treated flexor tendons, had gross and histological evidence of improved healing, both of the tendon and its sheath, with decreased inflammation (18).
The combination of HA and dipalmitoyl phosphatidylcholine, a phospholipid implicated in lubrication of synovial joints, was tested by Moro-oka et al (19). These investigators found that although the HA-phospholipid combination did not decrease the coefficient of friction for tendon gliding, it did contribute to fewer adhesions in an in vivo rabbit model. It was hypothesized that the dipalmitoyl phosphatidylcholine adsorbed to tendon surfaces, acting as a boundary lubricating film.
Lubricin, also known as superficial zone protein, has recently been considered as an alternative adjunct for tendon gliding modification. This protein, absent in genetic syndromes characterized by increased intrasynovial tendon adhesions, is thought to be integral to natural surface boundary lubrication. A study by Taguchi et al tested lubricin’s synergistic effect with HA when added to cd-gelatin and cd-HA-gelatin treatments in intrasynovial tendons (20). These lubricin regimens were compared against saline and cd-HA-gelatin without lubricin. After surgical repair and 1000 simulated flexion/extension cycles, the cd-HA-gelatin treatment, supplemented with lubricin, displayed a smaller increase in excursion resistance than cd-HA-gelatin treatments. These studies demonstrate that biochemical modification of the tendon surface has great potential for improving intrasynovial tendon healing by reducing adhesion formation and improving tendon gliding properties.
Exogenous growth factor administration
Modification of the biological environment also holds great promise for improving flexor tendon healing. Numerous groups have applied growth factors such as bFGF, TGF-β1, and PDGF-BB, to repaired tendons in an attempt to increase collagen production and hence repair site strength. In vitro studies have revealed that flexor tendon fibroblasts exposed to both bFGF and PDGF-BB increased their mitogenic activity and their collagen production by several-fold (21). When combined, the growth factors displayed synergistic mitogenic effects, with further increased cell replication. PDGF-BB, when studied in vivo, has also been shown to increase cell proliferation and collagen production in canine models (22,23). Similarly, in situ injection of exogenous bFGF resulted in increases in cell proliferation and matrix synthesis in a rat patellar tendon injury model (24).
It is clear that the potential utility of growth factors to clinically augment tendon repair cannot be exploited through mere haphazard administration; local concentration, timing, half-life, and growth factor synergy all play important roles in the natural biology and must be considered in the development of therapies. Because of early inconsistent results with simple bolus administration, approaches incorporating controlled release kinetics and sustained delivery systems have recently been employed. Sakiyama-Elbert et al. developed a fibrin/heparin based delivery system to control the release of any heparin binding growth factor (e.g., bFGF, PDGF-BB) (25). By varying the various components of the delivery system, the growth factor release kinetics could be controlled for tendon repair (22,26).
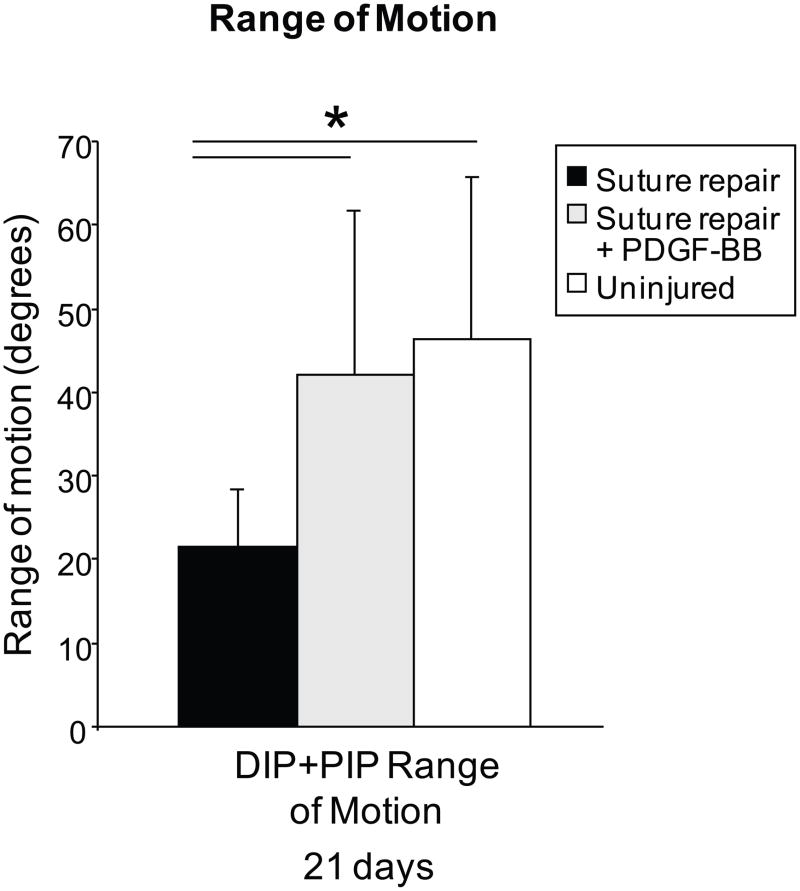
This fibrin/heparin delivery system was employed in a number of in vivo canine flexor tendon studies. Delivery of PDGF-BB resulted in increased fibroblast numbers, fibroblast proliferation, collagen type I deposition, and collagen cross-links at the site of tendon repair (22). These data suggest that the sustained delivery of PDGF-BB may be superior to bolus administration, leading to an early acceleration of the cellular processes involved in tendon healing. Though some studies have shown unwanted effects with due to growth factors, such as increases in adhesions with TGF-β1 (27), the tendons in this study did not show evidence of early adhesion formation, nor was there evidence of increased cell replication in the epitenon. On the contrary, tendon gliding was enhanced by PDGF-BB administration, presumably due to increased production of molecules important for gliding (e.g., hyaluronic acid, lubricin) (Figure 4) (23,28). PDGF-BB treated digits displayed significantly improved joint motion, increased total arc of motion, and greater excursion values than untreated repairs. However, while range of motion was improved, PDGF-BB did not significantly improve the strength of the repair at 3 or 6week timepoints. In summary, the use of growth factors such as PDGF-BB and bFGF holds great promise for improving flexor tendon healing. However, more study is necessary to test different growth factors, alone and in combination, and to optimize growth factor dosage and release kinetics in order to achieve improvements in both tendon function (e.g., gliding) and repair-site mechanical properties (e.g., failure load).
Figure 4.
Total range of motion (i.e., rotation of the proximal interphalangeal joint plus rotation of the distal interphalangeal joint) was significantly higher in the group treated with PDGF-BB group compared with repair alone (* p < 0.05).
Gene and cell therapy approaches
Controlled growth factor delivery may be limited by the finite amounts of growth factor that can be loaded, implanted, and delivered at the time of repair. Other investigators have employed genetic engineering techniques in attempts to more permanently bolster the delivery of growth factors to the site of interest (29). Tang et al. showed that tendon fibroblasts could be transfected with a cloned bFGF gene sequence through an adeno-associated virus-2 vector (29). The successful incorporation of the bFGF gene into the lacerated ends of surgically repaired leghorn chicken flexor tendons resulted in increased local bFGF expression. The bFGF-transformed tendons displayed increased tensile strength at 2 weeks and 4 weeks.
BMP-12, a growth and differentiation factor implicated in tenogenesis, was recently used to enhance tendon healing (30). Chicken tendon fibroblasts transfected with BMP-12 displayed increased collagen synthesis compared to controls. These genetically modified tendon fibroblasts produced 30% more collagen than control fibroblasts. The efficacy of this approach was examined in a flexor tendon injury model in leghorn chickens. A solution of Adv-BMP-12 vectors was injected into repaired tendons. Adv-beta-galactosidase vector injections were used as controls. Though both tendons had similar gross appearance at 2 weeks and 4 weeks, and similar biomechanical profiles at 2 weeks, the BMP-12 transduced tendons displayed a 2-fold increase in tensile strength at 4 weeks compared to the control tendons. These data suggest that increasing local BMP-12 levels can enhance flexor tendon healing. More recently, it was shown that Adv-BMP-12 transduced rat muscle cells implanted into an experimental rat Achilles tendon injury model can enhance healing (31). Repair site tensile strength was increased and there was histologic evidence of enhanced remodeling in treated repairs compared to control repairs.
CONCLUSIONS
The recent findings described in this review demonstrate that surgical technique and biologic modifications can dramatically improve outcomes after intrasynovial tendon repair compared to historical methods. Surgical repair technique should include the following: 1) eight core suture strands with a high caliber suture material, 2) a purchase length of approximately 1.2 cm, 3) a locking-loop configuration with the knot placed outside of the repair site, and 4) a peripheral suture placed deep into the tendon and far from the cut tendon end. While biologic and biochemical modifications have shown great promise in animal models, they have not yet been tested clinically. Nevertheless, future repair site modifications will likely include: 1) biochemical tendon surface treatments to enhance gliding and suppress adhesion formation, and 2) local controlled delivery of growth factors to stimulate extracellular matrix formation and prevent adhesion formation.
Acknowledgments
This study was supported by grants from the National Institutes of Health (AR033097 & EB004347).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Viinikainen A, Goransson H, Huovinen K, Kellomaki M, Rokkanen P. A comparative analysis of the biomechanical behaviour of five flexor tendon core sutures. Journal of hand surgery (Edinburgh, Scotland) 2004;29:536–543. doi: 10.1016/j.jhsb.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 2.Winters SC, Gelberman RH, Woo SL, Chan SS, Grewal R, Seiler JG., 3rd The effects of multiple-strand suture methods on the strength and excursion of repaired intrasynovial flexor tendons: a biomechanical study in dogs. The Journal of hand surgery. 1998;23:97–104. doi: 10.1016/s0363-5023(98)80096-8. [DOI] [PubMed] [Google Scholar]
- 3.Hatanaka H, Zhang J, Manske PR. An in vivo study of locking and grasping techniques using a passive mobilization protocol in experimental animals. J Hand Surg Am. 2000;25:260–269. doi: 10.1053/jhsu.2000.jhsu25a0260. [DOI] [PubMed] [Google Scholar]
- 4.Aoki M, Kubota H, Pruitt DL, Manske PR. Biomechanical and histologic characteristics of canine flexor tendon repair using early postoperative mobilization. J Hand Surg Am. 1997;22:107–114. doi: 10.1016/S0363-5023(05)80189-3. [DOI] [PubMed] [Google Scholar]
- 5.Boyer MI, Gelberman RH, Burns ME, Dinopoulos H, Hofem R, Silva MJ. Intrasynovial flexor tendon repair. An experimental study comparing low and high levels of in vivo force during rehabilitation in canines. J Bone Joint Surg Am. 2001;83-A:891–899. [PubMed] [Google Scholar]
- 6.Lawrence TM, Davis TR. A biomechanical analysis of suture materials and their influence on a four-strand flexor tendon repair. J Hand Surg Am. 2005;30:836–841. doi: 10.1016/j.jhsa.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 7.Barrie KA, Tomak SL, Cholewicki J, Merrell GA, Wolfe SW. Effect of suture locking and suture caliber on fatigue strength of flexor tendon repairs. J Hand Surg Am. 2001;26:340–346. doi: 10.1053/jhsu.2001.22926. [DOI] [PubMed] [Google Scholar]
- 8.Taras JS, Raphael JS, Marczyk SC, Bauerle WB. Evaluation of suture caliber in flexor tendon repair. J Hand Surg Am. 2001;26:1100–1104. doi: 10.1053/jhsu.2001.28946. [DOI] [PubMed] [Google Scholar]
- 9.Tang JB, Zhang Y, Cao Y, Xie RG. Core suture purchase affects strength of tendon repairs. The Journal of hand surgery. 2005;30:1262–1266. doi: 10.1016/j.jhsa.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 10.Cao Y, Zhu B, Xie RG, Tang JB. Influence of core suture purchase length on strength of four-strand tendon repairs. The Journal of hand surgery. 2006;31:107–112. doi: 10.1016/j.jhsa.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Pennington DG. The locking loop tendon suture. Plast Reconstr Surg. 1979;63:648–652. doi: 10.1097/00006534-197905000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Barrie KA, Tomak SL, Cholewicki J, Wolfe SW. The role of multiple strands and locking sutures on gap formation of flexor tendon repairs during cyclical loading. The Journal of hand surgery. 2000;25:714–720. doi: 10.1053/jhsu.2000.9414. [DOI] [PubMed] [Google Scholar]
- 13.Pruitt DL, Aoki M, Manske PR. Effect of suture knot location on tensile strength after flexor tendon repair. J Hand Surg Am. 1996;21:969–973. doi: 10.1016/S0363-5023(96)80301-7. [DOI] [PubMed] [Google Scholar]
- 14.Momose T, Amadio PC, Zhao C, Zobitz ME, An KN. The effect of knot location, suture material, and suture size on the gliding resistance of flexor tendons. J Biomed Mater Res. 2000;53:806–811. doi: 10.1002/1097-4636(2000)53:6<806::aid-jbm23>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 15.Diao E, Hariharan JS, Soejima O, Lotz JC. Effect of peripheral suture depth on strength of tendon repairs. The Journal of hand surgery. 1996;21:234–239. doi: 10.1016/S0363-5023(96)80106-7. [DOI] [PubMed] [Google Scholar]
- 16.Lotz JC, Hariharan JS, Diao E. Analytic model to predict the strength of tendon repairs. J Orthop Res. 1998;16:399–405. doi: 10.1002/jor.1100160402. [DOI] [PubMed] [Google Scholar]
- 17.Sun YL, Yang C, Amadio PC, Zhao C, Zobitz ME, An KN. Reducing friction by chemically modifying the surface of extrasynovial tendon grafts. J Orthop Res. 2004;22:984–989. doi: 10.1016/j.orthres.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 18.Zhao C, Sun YL, Amadio PC, Tanaka T, Ettema AM, An KN. Surface treatment of flexor tendon autografts with carbodiimide-derivatized hyaluronic Acid. An in vivo canine model. J Bone Joint Surg Am. 2006;88:2181–2191. doi: 10.2106/JBJS.E.00871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moro-oka T, Miura H, Mawatari T, Kawano T, Nakanishi Y, Higaki H, et al. Mixture of hyaluronic acid and phospholipid prevents adhesion formation on the injured flexor tendon in rabbits. J Orthop Res. 2000;18:835–840. doi: 10.1002/jor.1100180523. [DOI] [PubMed] [Google Scholar]
- 20.Taguchi M, Sun YL, Zhao C, Zobitz ME, Cha CJ, Jay GD, et al. Lubricin surface modification improves extrasynovial tendon gliding in a canine model in vitro. J Bone Joint Surg Am. 2008;90:129–135. doi: 10.2106/JBJS.G.00045. [DOI] [PubMed] [Google Scholar]
- 21.Thomopoulos S, Harwood FL, Silva MJ, Amiel D, Gelberman RH. Effect of several growth factors on canine flexor tendon fibroblast proliferation and collagen synthesis in vitro. J Hand Surg [Am] 2005;30:441–447. doi: 10.1016/j.jhsa.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 22.Thomopoulos S, Zaegel M, Das R, Harwood FL, Silva MJ, Amiel D, et al. PDGF-BB released in tendon repair using a novel delivery system promotes cell proliferation and collagen remodeling. J Orthop Res. 2007;25:1358–1368. doi: 10.1002/jor.20444. [DOI] [PubMed] [Google Scholar]
- 23.Thomopoulos S, Das R, Silva MJ, Sakiyama-Elbert S, Harwood FL, Zampiakis E, et al. Enhanced flexor tendon healing through controlled delivery of PDGF-BB. J Orthop Res. 2009 doi: 10.1002/jor.20875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan BP, Fu S, Qin L, Lee K, Rolf CG, Chan K. Effects of basic fibroblast growth factor (bFGF) on early stages of tendon healing: a rat patellar tendon model. Acta Orthopaedica Scandinavica. 2000;71:513–518. doi: 10.1080/000164700317381234. [DOI] [PubMed] [Google Scholar]
- 25.Sakiyama-Elbert SE, Hubbell JA. Development of fibrin derivatives for controlled release of heparin-binding growth factors. Journal of Controlled Release. 2000;65:389–402. doi: 10.1016/s0168-3659(99)00221-7. [DOI] [PubMed] [Google Scholar]
- 26.Sakiyama-Elbert SE, Das R, Gelberman RH, Harwood F, Amiel D, Thomopoulos S. Controlled-release kinetics and biologic activity of platelet-derived growth factor-BB for use in flexor tendon repair. J Hand Surg [Am] 2008;33:1548–1557. doi: 10.1016/j.jhsa.2008.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang J, Thunder R, Most D, Longaker MT, Lineaweaver WC. Studies in flexor tendon wound healing: neutralizing antibody to TGF-beta1 increases postoperative range of motion. Plastic & Reconstructive Surgery. 2000;105:148–155. doi: 10.1097/00006534-200001000-00025. [DOI] [PubMed] [Google Scholar]
- 28.Gelberman RH, Thomopoulos S, Sakiyama-Elbert SE, Das R, Silva MJ. The early effects of sustained platelet-derived growth factor administration on the functional and structural properties of repaired intrasynovial flexor tendons: an in vivo biomechanic study at 3 weeks in canines. J Hand Surg [Am] 2007;32:373–379. doi: 10.1016/j.jhsa.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 29.Tang JB, Cao Y, Zhu B, Xin KQ, Wang XT, Liu PY. Adeno-associated virus-2-mediated bFGF gene transfer to digital flexor tendons significantly increases healing strength. an in vivo study . J Bone Joint Surg Am. 2008;90:1078–1089. doi: 10.2106/JBJS.F.01188. [DOI] [PubMed] [Google Scholar]
- 30.Lou J, Tu Y, Burns M, Silva MJ, Manske P. BMP-12 gene transfer augmentation of lacerated tendon repair. Journal of Orthopaedic Research. 2001;19:1199–1202. doi: 10.1016/S0736-0266(01)00042-0. [DOI] [PubMed] [Google Scholar]
- 31.Majewski M, Betz O, Ochsner PE, Liu F, Porter RM, Evans CH. Ex vivo adenoviral transfer of bone morphogenetic protein 12 (BMP-12) cDNA improves Achilles tendon healing in a rat model. Gene Ther. 2008;15:1139–1146. doi: 10.1038/gt.2008.48. [DOI] [PubMed] [Google Scholar]