Abstract
Objective
To determine whether the Canine Brief Pain Inventory (CBPI) can detect changes in dogs with osteoarthritis treated with an NSAID or a placebo.
Design
Double-blind, randomized, placebo-controlled clinical trial.
Animals
70 dogs with osteoarthritis.
Procedures
Owners completed the CBPI on day 0. Dogs received carprofen or a placebo on days 1 through 14. Owners completed the CBPI again on day 14. Pain severity and pain interference scores from the CBPI were calculated, and the change from day 0 to day 14 was assessed within each group and between groups.
Results
No significant differences were detected in median scores for pain severity (3.50 and 3.25 on days 0 and 14, respectively) and pain interference (3.92 and 3.25 on days 0 and 14, respectively) in dogs receiving the placebo. Dogs receiving carprofen had significant changes in median scores for pain severity (4.25 to 2.25 on days 0 and 14, respectively) and pain interference (4.33 to 2.67 on days 0 and 14, respectively). There was a significantly greater improvement in pain severity and pain interference scores in dogs treated with carprofen, compared with improvement in scores for dogs receiving the placebo.
Conclusions and Clinical Relevance
The CBPI was able to detect improvements in pain scores in dogs with osteoarthritis treated with an NSAID or a placebo. These results, in combination with previous reliability and validity testing, support the use of the CBPI to obtain quantifiable assessments from owners regarding the severity and impact of chronic pain and treatment for dogs with osteoarthritis.
The availability of quantitative measures of chronic pain that are valid and reliable in clinical patients is crucial for the development and testing of interventions (eg, drugs or surgical procedures) designed to reduce such pain. Studies designed to test the efficacy of interventions intended to decrease chronic pain in companion dogs with osteoarthritis have relied heavily on a veterinarian's assessment of lameness supported by values generated through the use of force plate gait analysis. When properly collected, gait analysis data offer an objective measure that can be reliably monitored over time; however, it can be an extremely time-consuming process, requires specialized equipment, and relies on relatively strict inclusion criteria. In addition, these measures only evaluate an animal at 1 specific time point and outside of its typical environment, and weight bearing on an affected limb is only 1 part of the much larger picture of chronic pain in companion dogs with osteoarthritis.1–5 A detailed behavior-based assessment of chronic pain performed by the owner is routinely relied on when making clinical decisions and offers the advantages of an extended assessment of a dog in its typical environment by someone who is most knowledgeable about its behavior. Although an owner's detailed assessment can be quite useful, few programs have reported the development of an owner-completed questionnaire for use as an outcome assessment tool in clinical studies.6–13
In response to this need, the CBPI was developed as an owner-completed questionnaire designed to quantify the severity and impact of chronic pain in companion dogs with osteoarthritis.6 The CBPI contains 4 questions pertaining to the severity of pain evident in a dog (the responses for these questions can be used to calculate a mean value that can provide a pain severity score) and 6 questions pertaining to how the pain interferes with the dog's typical activities (the responses to these question can be used to calculate a mean value that can provide a pain interference score).a The CBPI was created by use of standard methods for the stepwise development of a health-assessment questionnaire.14–16 Questions were generated through information gathered from focus groups of owners and an expert panel of veterinarians, and the final questionnaire was subjected to factor-analysis, reliability, and validity testing.6 Although the CBPI had excellent psychometric (reliability and validity) properties, assessment of validity and reliability alone are not sufficient to suggest that a questionnaire would be useful in quantifying the health status of an animal. The questionnaire must also be able to detect changes in the animal's condition that are the result of an intervention (eg, medication or surgery), disease progression, or resolution of the condition.14–16
The ability to detect change is the property of the questionnaire referred to as responsiveness. The objective of the study reported here was to test the responsiveness of the CBPI by determining whether it could detect changes in dogs with osteoarthritis after treatment with an intervention of known efficacy. The hypothesis was that within the context of a double-blind, randomized, placebo-controlled clinical trial, the CBPI could be used to detect substantial improvements in pain severity and pain interference scores in osteoarthritic dogs treated with an FDA-approved NSAID and would detect little improvement in scores for osteoarthritic dogs treated with a placebo.
Materials and Methods
Sample population
The CBPI was completed by owners of dogs with osteoarthritis. The study was conducted as a single-center, double-blind, randomized, placebo-controlled clinical trial. All owners received a detailed written description of the protocol and provided written informed consent before their dogs were evaluated for inclusion in the study. The protocol was approved by an institutional animal care and use committee.
Inclusion criteria required that all dogs weighed ≥ 8 kg (17.6 lb) and had a medical history, clinical signs, physical examination findings, and radiographic findings consistent with osteoarthritis. Dogs were recruited for the study by use of e-mail, advertising circulars, and newspaper advertisements. Only dogs with newly diagnosed osteoarthritis and in which medical management had not yet been initiated or in which a diagnosis of osteoarthritis had been made sometime in the past but the owners had opted not to medicate those dogs on a regular basis were recruited. Dogs were excluded from the study if they had received NSAIDs within 2 weeks preceding the initial evaluation or glucocorticoids or opioids within 4 weeks preceding the initial evaluation; had clinically important neurologic disease, orthopedic disease (other than osteoarthritis), or any chronic disease for which the dog was receiving daily medication (as determined on the basis of the medical history and results of physical examination); had a history of coagulopathy, unexplained bleeding episodes, or hypersensitivity to NSAIDs; or had clinically relevant abnormalities detected during screening (a CBC and biochemical analysis). Thus, the study population consisted of medium to large-breed dogs with no clinically important abnormalities (other than osteoarthritis) and that were not currently receiving medications.
Estimation of sample size
It was determined that at least 29 dogs would need to be included in each group (carprofen and placebo) to provide 80% power of detecting differences between the groups of ≥ 30% for changes in scores of the CBPI (SD, 40%; P = 0.05). To compensate for protocol deviations and losses to follow-up for an ITT analysis, it was determined that 70 dogs would be randomly assigned to each of the 2 groups (35 dogs/group). This is consistent with the sample size for another study5 in which investigators used a global owner assessment as well as force plate analysis to detect differences in outcome between carprofen- and placebo-treated dogs with osteoarthritis.
Randomization
A simple randomization sequence with 2 potential treatment groups (carprofen or placebo) was generated at an off-site pharmacy. The sequence was concealed so that no members of the research team were aware of the group to which a dog would be allocated as it was evaluated in the screening process. Once screening was completed and a dog was considered eligible for inclusion in the study, a unique study number was assigned in sequence. The study number and body weight of each dog were then provided to the pharmacy. Pharmacy personnel matched the study number with their randomization sequence, formulated the appropriate treatment (carprofen or placebo), and packaged the pills into blister packs for use by the investigators.
Blinding
Pharmacy personnel packaged carprofen or placebo for administration to each dog. Carprofen was administered at a dosage of 4.4 mg/kg (2 mg/lb). Pills containing the placebo were formulated to appear identical to the pills containing carprofen; packaging for both types of pills was identical. Thus, all study personnel and the owners were unaware of the treatment group to which each dog was assigned.
Study design
The CBPI was completed by an owner of each dog that was eligible for inclusion in the study (day 0). The 4 pain severity questions were scored on a scale of 0 (no pain) to 10 (extreme pain). The responses for these questions were averaged to generate the pain severity score. The 6 pain interference questions (ie, how much the pain interfered with the dog's typical function) were scored on a scale of 0 (does not interfere) to 10 (completely interferes). The responses for these questions were averaged to generate the pain interference score. Owners administered the prescribed pills once daily on days 1 through 14. Three additional doses were included in the event that an owner encountered a delay in returning the dog for a follow-up appointment. The same owner who completed the CBPI on day 0 completed a second CBPI on day 14. Scores for days 0 (baseline) and 14 were compared within each group (carprofen and placebo). To determine the ability of the CBPI to detect overall effects of treatment, the change in the scores of the placebo-treated group before and after treatment was compared with the change in the scores of the carprofen-treated group before and after treatment.17
After collection of data on day 14, a 2-week supply of carprofen at a dosage of 4.4 mg/kg was dispensed to each owner for administration to their dogs so that all owners could determine the potential benefits of NSAIDs and could be counseled on continued treatment and follow-up evaluations with their veterinarians.
Statistical analysis
An ITT analysis was performed. Once randomly assigned in the study, all dogs were monitored through the conclusion of the study and included in the analysis.18 Descriptive statistics were calculated. Continuous data (age, body weight, and pain severity and pain interference scores of dogs) were expressed as median values and ranges, and categoric data (sex and breed of dogs) were expressed as frequencies for each group. The Wilcoxon signed test was used to compare pain severity and pain interference scores before and after treatment. The Mann-Whitney test was used to compare the change in pain severity and pain interference scores between the carprofen-and placebo-treated dogs. Univariate linear regression analysis was performed to determine factors (age, body weight, sex, breed, and baseline pain severity and pain interference scores) associated with the change in pain severity and pain interference scores. Two-tailed assessments were used for all analyses, and values of P < 0.05 were considered significant.
Multiple linear regression analyses were performed to evaluate the association of treatment (carprofen vs placebo) on the percentage change in pain severity and pain interference scores while controlling for other variables (age, body weight, sex, breed, and baseline pain severity and pain interference scores). Two-way interactions among the main effects were investigated. An interaction term was retained when there was a value of P < 0.05 on the t test for factors with a single degree of freedom (age, body weight, sex, and baseline pain severity and pain interference scores) or on an F test for factors with multiple terms (breed). Variables that were not effect modifiers and that had a value of P < 0.2 on univariate analysis were tested in the model. Variables were retained in the model when the P value for that variable was ≤ 0.05 or when its addition to the model changed a coefficient for the treatment by > 15%. To determine the clinical value of the variable estimates, the covariable-adjusted fitted values for the percentage change in pain severity and pain interference scores were also calculated. Model assumptions were evaluated. Normality of residuals was assessed with a kernel density plot. The constant variance of residuals was evaluated with the Cook-Weisberg test for heteroskedasticity. The variance inflation factor was calculated to test for multicollinearity. Residual scatter-plots were used to evaluate nonlinearity. All analyses were performed by use of a statistical program.b
Results
Animals
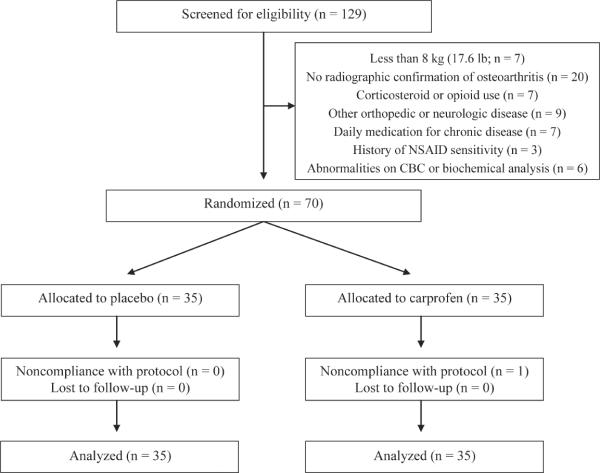
Initially, 129 client-owned dogs were screened to attain 70 dogs eligible for randomization (Figure 1). On the basis of information from the owners and review of the blister packs, all 70 dogs received the prescribed treatment for 14 to 16 days, and all owners completed the CBPI on day 0 and the last day of treatment (days 14 to 16). One dog in the carprofen group ruptured a cranial cruciate ligament during the first week of treatment, and that dog was referred to the orthopedic service at our university veterinary medical teaching hospital for appropriate care, which included surgery and continued treatment with carprofen. In accordance with the ITT analysis, data were collected for that dog and included in the analysis. All other dogs were in complete compliance with the protocol; thus, data for all 70 dogs were included in the analysis.
Figure 1.
Flow diagram of study subjects in the randomized, placebo-controlled clinical trial on response to treatment in dogs with osteoarthritis.
The 35 dogs that received the placebo consisted of 11 females and 24 males. Dogs were between 3 and 14 years of age (median, 9 years) and weighed between 16 and 77 kg (35.2 and 169.4 lb), with a median of 39 kg (85.8 lb). There were 12 mixed-breed dogs, 7 Rottweilers, 3 Doberman Pinschers, and 13 other purebred dogs (1 or 2 dogs in each of 10 other breeds). The 35 dogs that received carprofen consisted of 22 females and 13 males. Dogs were between 3 and 14 years of age (median, 8 years) and weighed between 8 and 76 kg (17.6 and 167.2 lb), with a median of 39 kg. There were 10 mixed-breed dogs, 12 Rottweilers, 3 Labrador Retrievers, and 10 other purebred dogs (1 or 2 dogs in each of 8 other breeds).
Change in pain severity score
Pain severity score did not change significantly between days 0 (median, 3.50; range, 0.75 to 7.75) and 14 (median, 3.25; range, 0 to 6.75) in the dogs receiving the placebo. However, dogs receiving carprofen had a significant (P < 0.001) improvement in pain severity score between days 0 (median, 4.25; range, 0.25 to 6.75) and 14 (median, 2.25; range, 0 to 6.00). Carprofen-treated dogs had a significantly (P = 0.005) greater improvement in pain severity score, compared with the difference in pain severity score for the placebo-treated dogs. Linear regression analysis of the percentage change in pain severity score revealed an interaction between treatment group and baseline pain severity score. For dogs receiving the placebo, a higher baseline pain severity score resulted in a greater percentage decrease in pain severity score on day 14. For example, when the baseline pain severity score was between 2 and 3, dogs receiving the placebo had a median increase in pain severity score of 13%, compared with a median decrease in pain severity score of 7% when the baseline pain severity score was between 4 and 5. Univariate analysis revealed 2 factors with a value of P < 0.2 that were tested in the model (body weight [P = 0.16] and sex [P = 0.06]). The P values for each of these variables increased when added to treatment and baseline score in the model; thus, they subsequently were not retained. Variables retained in the model included baseline pain severity score (coefficient of 0.089; 95% CI, 0.020 to 0.157; t = 2.58; P = 0.012), treatment group (coefficient of 0.848; 95% CI, 0.327 to 1.368; t = 3.25; P = 0.002), and the interaction term (coefficient of −0.128; 95% CI, −0.244 to −0.013; t = −2.22; P = 0.030). For the variable treatment group, the baseline comparison was the value for the placebo-treated group. Percentage change in the pain severity score on the basis of adjusted baseline pain severity score revealed that a dog with a baseline pain severity score of 4 treated with the placebo would have an estimated improvement in pain severity score of 3%, compared with an estimated improvement of 37% when treated with carprofen.
Change in pain interference score
Pain interference score did not change significantly between days 0 (median, 3.92; range, 0.50 to 8.50) and 14 (median, 3.25; range, 0 to 7.33) for dogs receiving the placebo. However, dogs receiving carprofen had a significant (P < 0.001) improvement in pain interference score between days 0 (median, 4.33; range, 0.50 to 8.50) and 14 (median, 2.67; range, 0 to 7.50). Carprofen-treated dogs had a significantly (P = 0.037) greater improvement in pain interference score, compared with the difference in pain interference score for the placebo-treated dogs. There were no interactions detected during linear regression analysis. Univariate analysis revealed that only body weight (P = 0.08) had a value of P < 0.2 and was tested in the model with treatment group. In the final model, treatment group and body weight were significantly associated with the percentage change in pain interference score. Variables retained in the model were treatment group (coefficient of 0.251; 95% CI, 0.169 to 0.432; t = 2.76; P = 0.008) and body weight (coefficient of −0.008; 95% CI, −0.016 to −0.001; t = −2.32; P = 0.023). For the variable treatment group, the baseline comparison was the value for the placebo-treated group. Percentage change in interference score adjusted on the basis of body weight revealed that a 40-kg (88-lb) dog treated with placebo would have an estimated improvement in pain interference score of 7%, compared with an estimated improvement of 33% when treated with carprofen.
Discussion
The CBPI detected changes in pain scores that would be expected in the context of a randomized, controlled trial in which there was an active treatment (an NSAID) and a placebo. The carprofen-treated group had a significant improvement in pain scores. If the CBPI were unable to detect changes in dogs with osteoarthritis, the pain scores for these dogs would not have improved even though carprofen is an FDA-approved product for the relief of pain and inflammation associated with osteoarthritis in dogs. In addition, the CBPI was able to detect the slight changes that can be expected in placebo-treated dogs.
It is likely that the improvements detected in the placebo-treated group were attributable to regression to the mean, which is a ubiquitous phenomenon that can be evident whenever there are repeated measurements on the same subject.19,20 Relatively high (or low) values are likely to be followed by less extreme values nearer the subject's mean because of the natural variation in the pain and functionality of dogs with osteoarthritis. They will oscillate between good days and bad days, depending on things such as weather and amount of activity. These oscillations may influence the time at which owners seek treatment for their pets. For example, they may be more likely to seek treatment when an animal is having a period of several bad days. These dogs would then score higher on the CBPI at the baseline assessment than they would during a period of typical days. As these dogs progress through the natural variability of the clinical syndrome, they will regress back to the mean value for discomfort and functionality, and their scores on the CBPI will improve despite the fact that no intervention was initiated. This improvement in a placebo-treated group was verified with gait analysis in a similar study5 in which investigators compared carprofen to a placebo in a randomized, controlled trial in dogs with osteoarthritis. Regression to the mean can also explain the fact that dogs in the placebo-treated group with the highest baseline pain severity scores had the greatest improvement in those scores after treatment with the placebo. In addition, some improvement in the placebo-treated dogs could have been attributable to the owners' perception of their dogs' condition being biased by the fact that they were giving the dog a pill every day. An owner wants to see improvement in their dog's condition, and this desire combined with the action of administering a pill to the dog every day may lead them to believe that there has been improvement, when perhaps there really has been no such improvement.
The fact that dogs in a control group can have improvement with no intervention and that not all of the improvement in dogs in an active treatment group is necessarily attributable to the drug makes the use of a control group crucial to the assessment of intervention efficacy. Without having the randomly allocated, blindly assessed control group, there would be no way to determine how much of the change detected in the carprofen group was simply a result of the natural variability of the disease.20 The fact that the CBPI was able to detect improvements in pain scores in both groups as well as the difference in the improvement between the groups indicates that the CBPI was able to detect the true effect of the drug and not just placebo effects.
Although the use of a placebo-treated group reinforces the ability of the CBPI to detect improvements attributable to the intervention alone, it also has an impact on the ability to generalize the results. We chose to recruit dogs with osteoarthritis that were not currently being treated because use of dogs receiving anti-inflammatory or analgesic medications would have required a washout period during which the NSAIDs were not administered prior to inclusion in the study. In addition to the ethical issues surrounding that type of study design, there would likely have been a number of dogs that would have been in noncompliance with the protocol because they would have required their original treatment regimen to maintain a reasonable degree of comfort and functionality. We chose to minimize losses during follow-up monitoring and protocol deviations, which therefore maximized the internal validity of our results, by including only untreated dogs in the study.
Results of the study reported here can be generalized for dogs with a wide range of age (3 to 14 years), body weight (8 to 77 kg), baseline pain severity score (0.25 to 7.75), and baseline pain interference score (0.50 to 8.50). Although dogs represented 17 breeds in the study, 19 of 70 (27%) were Rottweilers, which reflected the population of orthopedic patients in our veterinary medical teaching hospital. This may not represent the breed distribution at other veterinary hospitals or practices. Although there was a relatively even number of males and females in the study, it is interesting that they were not evenly distributed between the 2 groups. There were more males in the placebo-treated group and more females in the carprofen-treated group. Pain severity and pain interference scores for the carprofen group at baseline were also higher than scores at baseline for the placebo-treated group. Although the goal of randomization would be to evenly distribute dogs between the 2 groups, it is not always successful, particularly when sample sizes are small. Because we used a concealed randomization technique, we know that the unequal distribution by sex and baseline pain scores resulted purely by chance,21 and we controlled for any significant effects of sex and baseline pain scores in the linear regression analysis.
It was interesting that body weight did not significantly impact the improvement in pain severity score but did have a significant impact on the improvement in pain interference score. Whereas the improvement in pain severity score afforded by treatment was consistent across the range of body weights, larger dogs had smaller improvements in pain interference scores, compared with improvements for smaller dogs. One explanation for this would be that smaller dogs were better able to respond to an improvement in pain severity with an improvement in function, compared with the response for larger dogs. For example, with the same percentage improvement in pain severity score, it would be easier for a smaller dog to transition from a lying position to a standing position, compared with that for a larger dog. Body weight can influence degenerative joint disease by affecting the stresses on joints,22–25 and smaller dogs may be better able to compensate for orthopedic disease, compared with the ability for larger dogs; thus, this result was not surprising. Further study of the differential impact of chronic pain on function in larger versus smaller dogs could lead to a better understanding of how to best manage dogs of different sizes to improve their function.
The improvement in pain scores that we identified was consistent with the improvement hypothesized, despite the fact that there was 1 dog in the carprofen group that was not in compliance with the protocol. During the first week of treatment, that dog ruptured a cranial cruciate ligament, and it underwent surgery during the second week of the study. To retain the control of selection bias afforded by randomization, an ITT analysis was planned; therefore, the owner of that dog completed the CBPI as scheduled, and those data were included in the analysis.18 Because of the cruciate rupture and repair, the pain severity and pain interference scores for that dog were higher on day 14 than they were on day 0. This is the opposite of what would be expected for a dog in the carprofen group and, in effect, diluted the positive effect of carprofen for that group. Because we planned for potential protocol deviations when calculating the sample size, we were still able to detect a significant improvement in pain scores in the carprofen-treated group, and a significant difference between the carprofen and placebo-treated groups could still be detected despite this protocol deviation. It was not necessary to create postrandomization selection bias by excluding dogs from the analysis.18
We determined that the CBPI could detect improvements in pain scores that would be expected in osteoarthritic dogs treated with an NSAID or a placebo. In addition the CBPI was able to detect significant differences between the 2 groups. These results, which confirm the responsiveness of the CBPI to changes and its ability to differentiate between groups, in combination with previous results of reliability and validity testing,6 support its use to obtain quantifiable assessments from owners regarding the severity and impact of chronic pain and treatment for dogs with osteoarthritis.
Acknowledgments
Supported by the National Institutes of Health (grant No. 1-K08-DA-017720-02).
The authors thank Molly Love for technical assistance.
Abbreviations
- CBPI
Canine Brief Pain Inventory
- CI
Confidence interval
- ITT
Intention to treat
Footnotes
The CBPI can be downloaded from www.CanineBPI.com.
Stata, version 8, StataCorp, College Station, Tex.
References
- 1.Innes JF, Fuller CJ, Grover ER, et al. Randomised, double-blind, placebo-controlled parallel group study of P54FP for the treatment of dogs with osteoarthritis. Vet Rec. 2003;152:457–460. doi: 10.1136/vr.152.15.457. [DOI] [PubMed] [Google Scholar]
- 2.Lipscomb VJ, AliAbadi FS, Lees P, et al. Clinical efficacy and pharmacokinetics of carprofen in the treatment of dogs with osteoarthritis. Vet Rec. 2002;150:684–689. doi: 10.1136/vr.150.22.684. [DOI] [PubMed] [Google Scholar]
- 3.Moreau M, Dupuis J, Bonneau NH, et al. Clinical evaluation of a nutraceutical, carprofen and meloxicam for the treatment of dogs with osteoarthritis. Vet Rec. 2003;152:323–329. doi: 10.1136/vr.152.11.323. [DOI] [PubMed] [Google Scholar]
- 4.Moreau M, Dupuis J, Bonneau NH, et al. Clinical evaluation of a powder of quality elk velvet antler for the treatment of osteoarthrosis in dogs. Can Vet J. 2004;45:133–139. [PMC free article] [PubMed] [Google Scholar]
- 5.Vasseur PB, Johnson AL, Budsberg SC, et al. Randomized, controlled trial of the efficacy of carprofen, a nonsteroidal anti-inflammatory drug, in the treatment of osteoarthritis in dogs. J Am Vet Med Assoc. 1995;206:807–811. [PubMed] [Google Scholar]
- 6.Brown DC, Boston RC, Coyne JC, et al. Development and psychometric testing of an instrument designed to measure chronic pain in dogs with osteoarthritis. Am J Vet Res. 2007;68:631–637. doi: 10.2460/ajvr.68.6.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown DC, Coyne JC, Boston R, et al. A novel approach to the use of animals in studies of pain: validation of the Canine Brief Pain Inventory in canine bone cancer. Pain Med. 2008;9 doi: 10.1111/j.1526-4637.2008.00513.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wiseman ML, Nolan AM, Reid J, et al. Preliminary study on owner-reported behaviour changes associated with chronic pain in dogs. Vet Rec. 2001;149:423–424. doi: 10.1136/vr.149.14.423. [DOI] [PubMed] [Google Scholar]
- 9.Wiseman-Orr ML, Nolan AM, Reid J, et al. Development of a questionnaire to measure the effects of chronic pain on health-related quality of life in dogs. Am J Vet Res. 2004;65:1077–1084. doi: 10.2460/ajvr.2004.65.1077. [DOI] [PubMed] [Google Scholar]
- 10.Wiseman-Orr ML, Scott EM, Reid J, et al. Validation of a structured questionnaire as an instrument to measure chronic pain in dogs on the basis of effects on health-related quality of life. Am J Vet Res. 2006;67:1826–1836. doi: 10.2460/ajvr.67.11.1826. [DOI] [PubMed] [Google Scholar]
- 11.Segurson SA, Serpell JA, Hart BL. Evaluation of a behavioral assessment questionnaire for use in the characterization of behavioral problems of dogs relinquished to animal shelters. J Am Vet Med Assoc. 2005;227:1755–1761. doi: 10.2460/javma.2005.227.1755. [DOI] [PubMed] [Google Scholar]
- 12.Hsu Y, Serpell J. Development and validation of a questionnaire for measuring behavior and temperament traits in pet dogs. J Am Vet Med Assoc. 2003;223:1293–1300. doi: 10.2460/javma.2003.223.1293. [DOI] [PubMed] [Google Scholar]
- 13.Hudson JT, Slater MR, Taylor L, et al. Assessing repeatability and validity of a visual analogue scale questionnaire for use in assessing pain and lameness in dogs. Am J Vet Res. 2004;65:1634–1643. doi: 10.2460/ajvr.2004.65.1634. [DOI] [PubMed] [Google Scholar]
- 14.McDowell I, Newell C. Measuring health: a guide to rating scales and questionnaires. 2nd ed. Oxford University Press; New York: 1996. The theoretical and technical foundations of health measurement; pp. 10–42. [Google Scholar]
- 15.Streiner DL, Norman GR. Health measurement scales: a practical guide to their development and use. 2nd ed. Oxford University Press; New York: 1995. pp. 1–143. [Google Scholar]
- 16.Sudman S, Bradburn NM. Asking questions. A practical guide to questionnaire design. Jossey-Bass Inc; San Francisco: 1982. Questionnaires from start to finish; pp. 281–288. [Google Scholar]
- 17.Francom SF, Chuang-Stein C, Landis JR. A log-linear model for ordinal data to characterize differential change among treatments. Stat Med. 1989;8:571–582. doi: 10.1002/sim.4780080506. [DOI] [PubMed] [Google Scholar]
- 18.Brown DC. Sources and handling of losses to follow-up in parallel-group randomized clinical trials in dogs and cats: 63 trials (2000–2005) Am J Vet Res. 2007;68:694–698. doi: 10.2460/ajvr.68.7.694. [DOI] [PubMed] [Google Scholar]
- 19.Senn SJ, Brown RA. Estimating treatment effects in clinical trials subject to regression to the mean. Biometrics. 1985;41:555–560. [PubMed] [Google Scholar]
- 20.Barnett AG, van der Pols JC, Dobson AJ. Regression to the mean: what it is and how to deal with it. Int J Epidemiol. 2005;34:215–220. doi: 10.1093/ije/dyh299. [DOI] [PubMed] [Google Scholar]
- 21.Brown DC. Control of selection bias in parallel-group controlled clinical trials in dogs and cats: 97 trials (2000–2005) J Am Vet Med Assoc. 2006;229:990–993. doi: 10.2460/javma.229.6.990. [DOI] [PubMed] [Google Scholar]
- 22.Impellizeri JA, Tetrick MA, Muir P. Effect of weight reduction on clinical signs of lameness in dogs with hip osteoarthritis. J Am Vet Med Assoc. 2000;216:1089–1091. doi: 10.2460/javma.2000.216.1089. [DOI] [PubMed] [Google Scholar]
- 23.Kealy RD, Lawler DF, Ballam JM, et al. Five-year longitudinal study on limited food consumption and development of osteoarthritis in coxofemoral joints of dogs. J Am Vet Med Assoc. 1997;210:222–225. [PubMed] [Google Scholar]
- 24.Richardson DC, Schoenherr WD, Zicker SC. Nutritional management of osteoarthritis. Vet Clin North Am Small Anim Pract. 1997;27:883–911. doi: 10.1016/s0195-5616(97)50085-4. [DOI] [PubMed] [Google Scholar]
- 25.Vasseur P. Clinical results following nonoperative management for rupture of the cranial cruciate ligament in dogs. Vet Surg. 1984;13:243–246. [Google Scholar]