Abstract
The goal of the present communication was to develop a strategy for detachment of cells and biomaterial constructs from indium tin oxide (ITO) electrodes.
The electrical manipulation of biointerfacial properties has emerged as an important tool for exercising spatiotemporal control over interactions of cells and proteins with surfaces. A number of electrochemical surface manipulation strategies have appeared in the literature.1–5 For example, Mrksich and co-workers synthesized electrochemically active alkanethiols that underwent a redox reaction under applied voltage and became cell adhesive.1 Lahann and co-workers described a different surface switching strategy whereby conformation of the alkanethiol and wettability of gold surfaces could be controlled by applied voltage.4
An alternative to electrochemical modulation of composition or conformation of self-assembled molecules is to remove these molecules altogether. Electrochemical desorption of alkanethiols from gold has been described in the literature6 and has been used previously to control cell spreading from geometric confinements or to position three different cell types on the same surface.7,8 Our group has demonstrated that electrochemical desorption may be used to detach proteins and cells anchored to gold substrates via alkanethiols.9,10 However, cultivation of cells on gold is not very practical due to difficulties with optical characterization.
In contrast, indium tin oxide (ITO) is optically transparent yet conductive material that is well-suited for cell cultivation and observation. Several groups including ours have demonstrated that electrochemical modulation of ITO surfaces may be used to control protein and cell attachment.11 In addition a recent report by Guillaume-Gentil et al. described detachment of cell sheets and polyelectrolyte layers from ITO.12
In the present study we sought to design a strategy for releasing cells from ITO substrates. However, unlike previous reports describing detachment of cells from self-assembled monolayers10,13 and polyelectrolyte layers12 our goal was to investigate the release of three-dimensional biomaterial scaffolds that could be used as vehicles for cell transplantation in later studies. Based on our previous report of reductive desorption of silane molecules from ITO,11 we hypothesized that biomaterials and cells covalently coupled to ITO substrates via a silane layer may be released upon electrochemical disruption of this coupling layer.
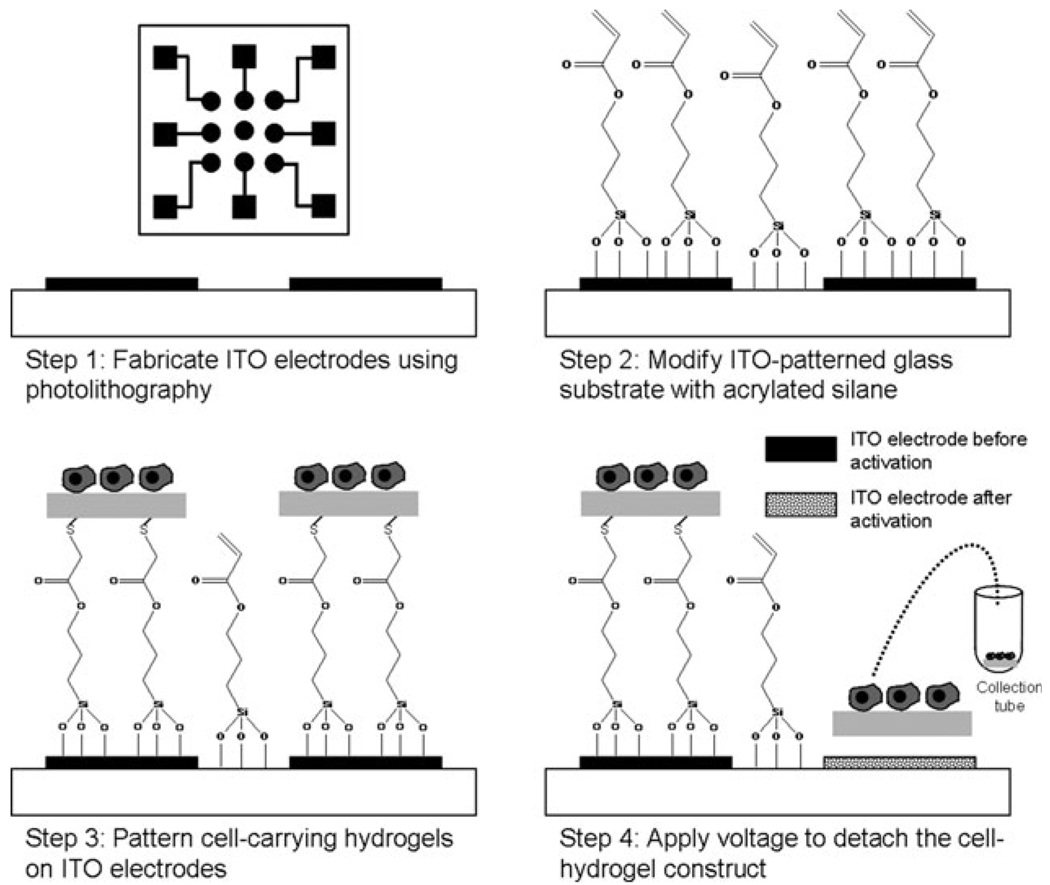
Fig. 1 provides a pictorial description of our strategy for releasing biomaterial structures and cells. As shown in Fig. 1 (step 1), substrates used in this study contained arrays of nine individually addressable ITO electrodes with single electrode diameter of 1.8 mm. The electrode arrays were fabricated on glass using standard photolithography and wet etching protocols described in detail in the ESI†. The use of an electrode array format was highly significant because it permitted both spatial and temporal control over the release of polymer structures and cells.
Fig. 1.
Selective electrochemical detachment of cell-carrying hydrogels. Step 1: individually addressable ITO electrodes are fabricated on glass using photoresist lithography and wet etching. Inset shows the layout of an array of nine electrodes ITO with leads and contact pads. Step 2: ITO-glass substrates were modified with acrylated silane. Step 3: heparin-based hydrogels and cells were patterned on top of ITO electrodes using a PDMS stencil. Vinyl groups of the silane layer reacted with thiolated heparin by Michael addition, covalently linking heparin gel to the ITO surface. Step 4: applying reductive potential (−1.8 V vs. Ag/AgCl reference for 60 s) to the desired electrode leads to desorption of the underlying silane layer as well as a cell-carrying hydrogel construct.
The arrays of ITO electrodes were modified with 3-(acryloxypropyl) trichlorosilane to introduce vinyl groups onto the surface (see Fig. 1, step 2). This vinyl-terminated silane layer served to anchor biomaterial constructs onto conductive substrates. While the surface manipulation approach described here is applicable to a wide range of biomaterials, we were particularly interested in demonstrating the release of heparin-based hydrogels. These hydrogels, developed by us recently,14 are formed by Michael addition reaction between thiolated heparin (Hep–SH) and the vinyl groups of the diacrylated polyethylene glycol (PEG–DA). Importantly, heparin within the hydrogel retains its bioactivity and interacts with growth factors or matrix proteins containing heparin-binding domains.14–16 Therefore, heparin-based hydrogels provide an excellent matrix for release of biomolecules and for cultivation of cells.17
In order to form heparin hydrogel structures on top of ITO electrodes, a PDMS stencil containing nine through holes (~ 1.3 mm in diameter) was aligned with an electrode array and secured on top of the substrate. The heparin-based hydrogel structures were formed within the reservoirs of the PDMS stencil by adjusting the temperature to 37 °C. Importantly, the Michael addition reaction between thiolated heparin and vinyl groups likely occurred at the ITO–solution interface as well as in the bulk, resulting in effective anchoring of hydrogel structures to the electrodes (see Fig. 1, step 3). The PDMS stencil was removed upon gelation, leaving behind heparin gel structures aligned with the ITO electrodes as shown in Fig. 2A.
Fig. 2.
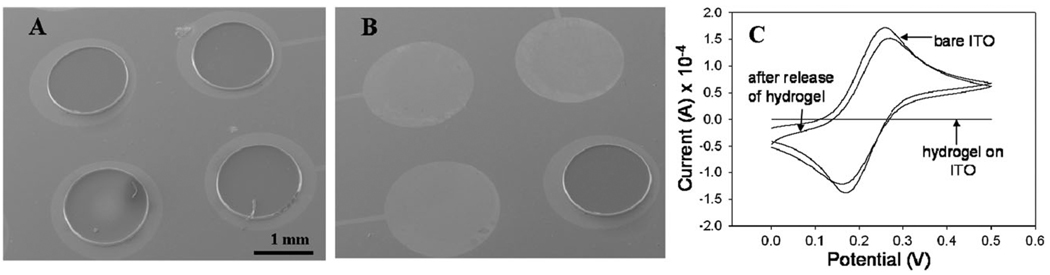
Detachment of hydrogel structures from ITO electrodes. (A) SEM micrograph showing heparin-based hydrogel structures patterned on top of ITO electrodes. (B) Applying reductive potential (−1.8 V for 60 s) to selected electrodes resulted in detachment of hydrogel elements while hydrogel on unactivated electrode (bottom right) remained attached. (C) Ferricyanide cyclic voltammetry was used to verify the assembly and desorption of silane molecules and hydrogel elements. The redox peaks shown with bare ITO electrode disappeared upon silane modification and hydrogel patterning, but reappeared after electrical stimulation of an electrode. The resumption of interfacial electron transfer pointed to removal of the hydrogel/silane layer.
The present study builds on our previous observation that applying reductive potential (−1.4 V vs. Ag/AgCl reference, for 60 s) to silane-modified ITO electrodes results in desorption of the silane layer.11 We hypothesized that hydrogels adherent to the electrode surface via silane coupling layer could be detached upon electrochemical desorption this anchoring layer. To test this hypothesis, hydrogel-containing ITO electrodes were stimulated with reductive potential varying from −1.2 V to −1.8 V in 0.2 V increments. The gel detachment was indeed observed and was dependent on the magnitude and time of applied voltage. With −1.4 V, hydrogel was detached in ~8 min and the detachment time was decreased to ~3 min with −1.6 V. Moving to a more negative potential of −1.8 V led to reproducible detachment of hydrogel elements in under 1 min. Therefore, −1.8 V vs. Ag/AgCl reference was chosen as the stimulus in hydrogel and cell detachment studies.
SEM images in Fig. 2A,B highlight our ability to form hydrogel structures on ITO electrodes and to detach these structures upon electrical stimulation. As seen in Fig. 2B, the use of individually addressable electrodes allowed to release biomaterial constructs from specific locations on the surface. Importantly, no hydrogel residues could be observed by SEM after electrical stimulation of the electrodes, pointing to the effective removal of this biomaterial.
Cyclic voltammetry (CV) with ferricyanide serving as redox species was employed to further investigate interfacial properties of the ITO electrodes before and after electrochemical desorption (see Fig. 2C). CV analysis demonstrated disappearance of ferricyanide redox peaks after silane modification and heparin hydrogel immobilization on ITO electrodes. Application of reductive potential resulted in re-appearance of ferricyanide redox peaks that were similar in amplitude to unmodified ITO electrodes. These data suggest that interfacial electron transfer after electrochemical desorption of silane and hydrogel layers was comparable to that of an unmodified ITO electrode. Taken together, SEM and electrochemical analysis of the ITO electrode arrays showed that both heparin hydrogel structures and the underlying vinyl-terminated silane layer were desorbed by electrical stimulation.
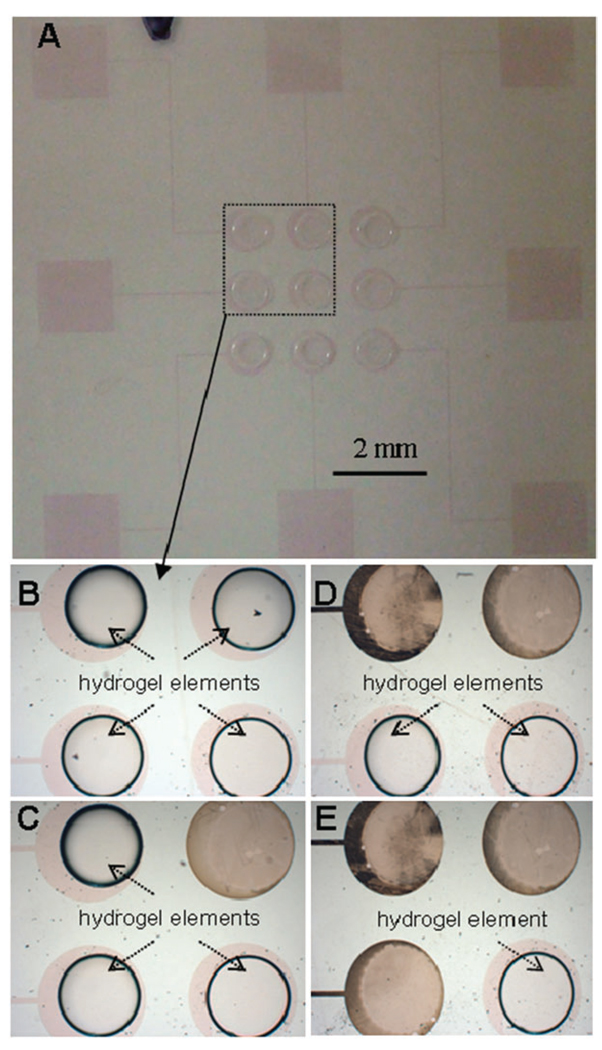
There are multiple scenarios where exercising spatial and temporal control over the release of biomaterials or cells is advantageous. Fig. 3 highlights the spatiotemporal control over the hydrogel release afforded by an array of hydrogel-coated ITO electrodes. Fig. 3A shows an array of nine ITO electrodes with immobilized hydrogel elements. Focusing on four electrodes, Fig. 3B–E depicts actuation of individual electrodes in a counter-clockwise manner and the release of hydrogel structures from activated electrodes.
Fig. 3.
Sequential electrochemical detachment of hydrogels patterned on individually addressable ITO electrodes. (A) A glass substrate with an array of nine hydrogel-patterned ITO electrodes before the detachment process. (B–E) Higher magnification view of four hydrogel-coated electrodes being actuated in a sequential manner. The gel elements were detached in a counter-clockwise direction from upper right-hand corner (C) to lower left-hand corner (E).
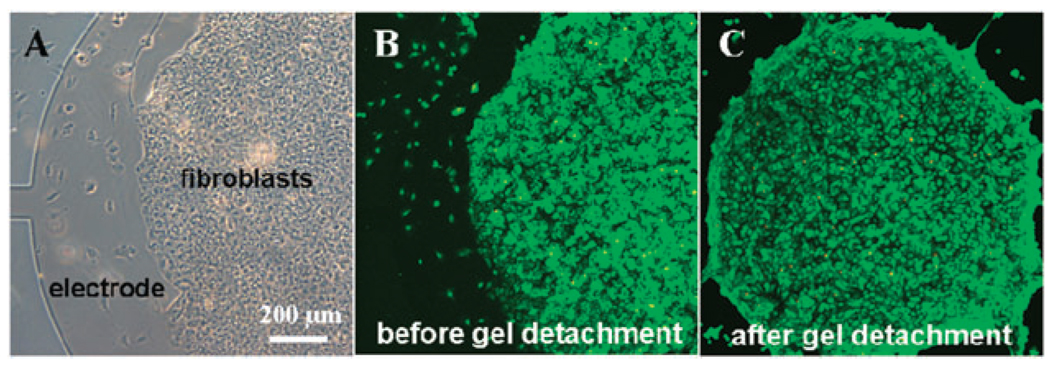
To demonstrate suitability of this surface switching approach for biological applications, mammalian cells (3T3 fibroblasts) were cultured on top of the heparin hydrogel elements as shown in Fig. 4A. To enhance cell attachment, fibronectin was added into the polymer preparation prior to gelation. After 24 h cultivation, cells and hydrogels were detached from the ITO electrodes by applying a reductive potential of −1.8 V vs. Ag/AgCl for 1 min. As highlighted by images in Fig. 4B,C, the applied voltage did not have a detrimental effect on cell viability. The results in Fig. 4 are highly promising as they demonstrate retrieval of viable cells after cultivation on ITO surfaces.
Fig. 4.
Cultivation of cells and release of cell-carrying hydrogels from ITO electrodes. (A) A bright field microscopy image showing fibroblasts adherent on hydrogel elements. The edge of the electrode as well as the lead (connection) are visible on the left side of the image. (B–C) Viability staining of cells adherent on heparin hydrogels before (B) and after (C) electrochemical detachment shows that cells are viable after detachment.
In conclusion, the present communication describes a novel strategy for on-cue detachment of three-dimensional biomaterial constructs from optically transparent ITO electrodes. Electrode array format was employed to exercise spatiotemporal control over the detachment of biomaterial constructs. Importantly, we also demonstrated that mammalian cells remained viable after electrochemical desorption. The cell and biomaterial retrieval approach described here is particularly suited for harvesting cells and/or biopolymers from micropatterned surfaces for either downstream analysis or transplantation. Overall, a novel electrochemical strategy that allows to selectively release small groups of cells incorporated into a biomaterial scaffold will open new opportunities for cell analysis and tissue regeneration.
Acknowledgments
This work was partially supported by the World Class University (WCU) program at GIST through a grant provided by the Ministry of Education, Science and Technology (MEST) of Korea (Project No. R31-20008-000-10026-0) and by the Research Center for Biomolecular Nanotechnology, GIST. This was also supported in part by UC Davis start-up funds. SSS acknowledges support from Grant Number T32-GM08799 from NIGMS-NIH.
Footnotes
Electronic supplementary information (ESI) available: Materials, methods used for fabricating ITO electrodes, patterning of heparin hydrogels and cells, and for detaching of hydrogels and cells.
Notes and references
- 1.Yousaf MN, Houseman BT, Mrksich M. Proc. Natl. Acad. Sci. U. S. A. 2001;98:5992–5996. doi: 10.1073/pnas.101112898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yeo WS, Mrksich M. Langmuir. 2006;22:10816–10820. doi: 10.1021/la061212y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yeo WS, Yousaf MN, Mrksich M. J. Am. Chem. Soc. 2003;125:14994–14995. doi: 10.1021/ja038265b. [DOI] [PubMed] [Google Scholar]
- 4.Lahann J, Mitragotri S, Tran TN, Kaido H, Sundaram J, Choi IS, Hoffer S, Somorjai GA, Langer R. Science. 2003;299:371–374. doi: 10.1126/science.1078933. [DOI] [PubMed] [Google Scholar]
- 5.Chan EWL, Park S, Yousaf MN. Angew. Chem., Int. Ed. 2008;47:6267–6271. doi: 10.1002/anie.200800166. [DOI] [PubMed] [Google Scholar]
- 6.Widrig CA, Chung C, Porter MD. J. Electroanal. Chem. 1991;310:335. [Google Scholar]
- 7.Jiang X, Ferrigno R, Mrksich M, Whitesides GM. J. Am. Chem. Soc. 2003;125:2366–2367. doi: 10.1021/ja029485c. [DOI] [PubMed] [Google Scholar]
- 8.Li Y, Yuan B, Ji H, Han D, Chen SQ, Tian F, Jiang XY. Angew. Chem., Int. Ed. 2007;46:1094–1096. doi: 10.1002/anie.200603844. [DOI] [PubMed] [Google Scholar]
- 9.Balasubramanian SG, Revzin A, Simonian AL. Electroanalysis. 2006;18:1885. [Google Scholar]
- 10.Zhu H, Yan J, Revzin A. Colloids Surf., B. 2008;54:250–258. [Google Scholar]
- 11.Shah SS, Lee JY, Verkhoturov S, Tuleuova N, Schweikert EA, Revzin A. Langmuir. 2008;24:6837–6844. doi: 10.1021/la800231e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guillaume-Gentil O, Akiyama Y, Schuler M, Tang C, Textor M, Yamato M, Okano T, Voros J. Adv. Mater. 2008;20(3):560–565. [Google Scholar]
- 13.Inaba R, Khademhosseini A, Suzuki H, Fukuda J. Biomaterials. 2009;30(21):3573–3579. doi: 10.1016/j.biomaterials.2009.03.045. [DOI] [PubMed] [Google Scholar]
- 14.Tae G, Kim YJ, Choi WI, Kim M, Stayton PS, Hoffman AS. Biomacromolecules. 2007;8:1979–1986. doi: 10.1021/bm0701189. [DOI] [PubMed] [Google Scholar]
- 15.McGonigle JS, Tae G, Stayton PS, Hoffman AS, Scatena M. J. Biomater. Sci., Polym. Ed. 2008;19:1021–1034. doi: 10.1163/156856208784909381. [DOI] [PubMed] [Google Scholar]
- 16.Il Choi W, Kim M, Tae G, Kim YH. Biomacromolecules. 2008;9:1698–1704. doi: 10.1021/bm701391b. [DOI] [PubMed] [Google Scholar]
- 17.Kim M, Shin Y, Hong B, Kim YJ, Chun JS, Tae G, Kim YH. Tissue Eng. 2009 doi: 10.1089/ten.TEC.2008.0548. DOI: 10.1089/ten.tec.2008.0548. [DOI] [PubMed] [Google Scholar]