Abstract
Background
Despite setbacks to the approval of new medications for the treatment of irritable bowel syndrome, interim guidelines on endpoints for IBS trials have enhanced interest as new targets for medical therapy are proposed based on novel mechanisms or chemical entities.
Aim
To review the approved lubiprostone, two targets that are not meeting expectations (tachykinins and corticotrophin-releasing hormone), the efficacy and safety of new 5-HT4 agonists, intestinal secretagogues (chloride channel activators, and guanylate cyclase-C agonists), bile acid modulation, anti-inflammatory agents and visceral analgesics.
Methods
Review of selected articles based on PubMed search and clinically relevant information on mechanism of action, safety, pharmacodynamics, and efficacy
Conclusions
The spectrum of peripheral targets of medical therapy address chiefly the bowel dysfunction of IBS, and these effects are associated with pain relief. There are less clear targets related to the abdominal pain or visceral sensation in IBS. The new 5-HT4 agonists are more specific than older agents, and show cardiovascular safety to date. Secretory agents have high specificity, low bioavailability, and efficacy. The potential risks of agents “borrowed” from other indications (like hyperlipidemia, inflammatory bowel disease or somatic pain) deserve further study. There is reason for optimism in medical treatment of IBS.
Keywords: serotonin, bile acid, colesevelam, anti-inflammatory, visceral analgesic
Introduction
Despite claims based on meta-analyses (1) that there are several treatments such as peppermint oil, antidepressants, and probiotics based on Bifidobacteria which are efficacious for the treatment of irritable bowel syndrome (IBS), patients and clinicians question their effectiveness. A recent review assessed the pipeline of medical treatments in IBS, as well as challenges in study design and regulatory guidance (2). Though the list is exhaustive, many candidate medications are still at a very early stage of development. What are realistic receptor targets of medical therapy of IBS? What targets appear to be less promising based on initial experience in humans? To appraise realistic opportunities and to assess whether there is any reason for optimism, this overview focuses on classes of medications or individual medications where there is at least mechanistic proof of concept in humans, and initial evidence of efficacy in clinical trials. There are some targets of therapy that appeared to be very promising, but they have been disappointing, and they are briefly discussed. While some targets for therapy are not necessarily new, there are new approaches which promise to produce safer restoration of normal bowel function by stimulating motility and secretion. There are still no robust targets or proven therapies directed at visceral pain.
Thus, this overview will address the current state of knowledge in efficacy and safety of new 5-HT4 agonists, intestinal secretagogues (chloride channel activators, guanylate cyclase C agonists), bile acid modulation, anti-inflammatory approaches, κ-opioid agonist, pregabalin and gabapentin.
Medications Aimed at Putative Targets that Have not Fulfilled their Promise to Date
There are two targets of medical therapy with potential in IBS which have been less promising in human studies than was predicted based on animal models:
a. Tachykinin antagonists
While NK1 antagonists such as aprepitant and casopitant are effective in chemotherapy-induced emesis or postoperative nausea or vomiting (3-5), there are no reports of pharmacodynamic (6) or clinical efficacy in changing bowel sensation or motility in humans (healthy or IBS). Similarly, the NK3 antagonist class has been tested in pharmacodynamic studies in which it did not significantly alter rectal sensation in humans (7) and in clinical trials (8). The NK2-receptor antagonist, nepadutant (9), reduced contraction frequency and amplitude on migrating motor complexes in the small intestine in healthy controls. It also effectively antagonized the motility-stimulating effects of infused NKA. Effects in clinical trials have not been reported.
b. Corticotrophin releasing hormone I antagonist
CRH stimulates colonic motility in healthy humans (10,11), activates CRH receptor subtypes R1 and R2 on subepithelial mast cells, thereby inducing increased transcellular uptake of protein antigens in human colonic biopsies in Ussing chambers (12,13), and the CRH 1 antagonist reduces sensation (11) as well as the EEG signal in response to rectal distension (14). However, a proof of concept study with a CRF1 antagonist, pexacerafont, and colonic transit and bowel function in IBS-D showed no advantage of pexacerafont over placebo (15). The selective CRF-1 receptor antagonist, GW876008, was shown to attenuate psychological stress-induced rectal hypersensitivity in 9 patients with IBS (16). However, a multicenter, randomized, double blind, placebo controlled, two-period crossover clinical trial of 19 weeks’ duration with the same agent (GW876008) showed no significant difference from placebo in the global improvement scale, daily self-assessment of IBS pain/discomfort or individual lower GI symptoms (17). .
c. 5-HT1A receptor antagonist
A large study of 402 patients explored the efficacy of AZD7371, a 5-HT1A receptor antagonist, which was not more effective than placebo in providing adequate relief from IBS or change in score in validated symptom and quality-of-life questionnaires. Hallucinations were reported by eight patients taking AZD7371, none on placebo (18).
d. Cholecystokinin antagonist
Dexloxiglumide is a competitive antagonist of the CCK1 receptor. CCK is thought to a potential mediator of the colonic contraction in response to feeding that may cause symptoms such as urgency or diarrhea. Initially, studies conducted in patients with female IBS-C patients provided contradictory results with two large phase III trials showing no efficacy (19). This latter was consistent with a pharmacodynamic study in C-IBS (20), which paradoxically showed slowing of ascending colon emptying and no significant effect on overall colonic transit or satisfactory relief of IBS. In a more recent phase III randomized withdrawal study, dexloxiglumide demonstrated sustained relief of symptoms or at least a longer time to loss of therapeutic response in a randomized withdrawal trial in female (not male) IBS patients (21). It is worth noting that among 869 IBS-C patients receiving single-blinded dexloxiglumide, there were 413 responders. The clinical benefit of this class of medication is still unclear
e. Beta-3 adrenergic agonist
Solabegron was proposed after animal studies showed functional receptors on enteric neurons (22). However, a reliable pharmacodynamic assessment in humans failed to show any significant effect on gastrointestinal or colonic transit (23). A clinical trial with this medication has been completed, and a preliminary report shows that solabegron, 200mg, b.i.d., led to a statistically significant increase in the proportion of females (p=0.019) and possibly both genders (p=0.06) achieving adequate relief of IBS pain and discomfort compared to placebo. There were also improvements in pain scores and number of pain-free days, but (consistent with transit results) no significant change in bowel symptoms (24).
What went wrong? Was the problem in the target or the specific medication?
One feature in common with all these classes of medications is that their investigation in humans followed extensive animal toxicology and preclinical effects that appeared robust. However, application of the same principles in human proof of concept studies has been disappointing. There may be several reasons, some of which are reviewed in a scholarly treatise on the topic by Mayer et al. (25); others may be more speculative.
First, there is little evidence that CRF mechanisms are upregulated in the human condition, and the degree of stress experienced by patients attending a clinic is not as severe as that of a rat avoiding water! In addition, there is no evidence that drug alteration of rectal sensory threshold predicts responsiveness in clinical trials in IBS.
Second, the efficacy of neurokinin modulators predicted from animal studies has been uniformly disappointing in larger human studies. It is conceivable that redundant mechanisms in sensation (e.g. norepinephrine, CGRP) or motor function (e.g. acetyl choline) compensate for the specific antagonism of the neurokinin receptors.
Third, the role of 5-HT1A receptors in modulating colonic motor or sensory function suspected from animal models was not confirmed in our study in healthy volunteers (26). Thus, buspirone did not significantly alter colonic compliance, tone, or sensation relative to placebo whereas a positive control (venlafaxine) was effective.
Fourth, if CCK is associated with colonic motor response to feeding, why would it be tested in patients with IBS-C? In addition, dexloxiglumide slowed ascending colon emptying time which might be predicted to aggravate constipation or at least to cause harder stool consistency.
Fifth, in studies of a β3-adrenergic agonist that alters enteric cholinergic neurons may require a baseline measurement of symptoms in detail or an assessment of colonic transit to select patients more likely to respond to this form of therapy.
In summary, it appears that failures might be attributable to insufficient proof of concept in humans before embarking on costly and time-consuming phase IIb or III clinical trials.
Medications that Are Fulfilling their Promise to Date
Table I provides a summary of the classes of medications targeting motor, secretory or sensory mechanisms that appear to be fulfilling their promise to date.
Table I.
Summary of Mechanism of Action and Clinical Efficacy of New or Promising Medications in Treatment of IBS (adapted from ref. 2, Camilleri M, Chang L. Gastroenterology 2008;135:1877-91
| Drug Class | Examples | Rationale or Putative Action | Pharmacodynamic (intestine or colon) | Clinical Efficacy: Phase IIB or III Primary Endpoints | Safety Issues/Comments |
|---|---|---|---|---|---|
| CCK1 antagonist | dexloxiglumide | Competitive antagonist of the CCK1- receptor | Slower ascending colon emptying with no significant effect on overall colonic transit | Two initial IIB or III trials: not efficacious in IBS-C; a randomized withdrawal design trial showed longer time to loss of therapeutic response, longer for dexloxiglumide | |
| NK antagonists | NK1 antagonist, ezlozipant | NK1-receptors’ role in nociception | Reduce the emotional response of IBS patients to rectosigmoid distension | None | |
| NK2-antagonist, nepadutant | NK2-receptors’ influence on smooth muscle contractility | Reduce contraction frequency and amplitude on MMC in SB in healthy males | None | ||
| NK3-antagonist, talnetant | NK3-receptors’ role in nociception | No effect on rectal compliance, sensory thresholds or intensity ratings in healthy controls | Two IIB trials in 1350 IBS patients: no benefit | ||
| 5-HT4-agonists | prucalopride | Stimulate intrinsic cholinergic neurons to enhance motility | Increases SB, colon motility and transit in healthy controls and patients with chronic constipation | IIB and III in CC (thousands of pts): BM frequency and satisfaction with bowel function both improved | Greater selectivity for 5-HT4 than 5-HT1B or hERG channel |
| ATI-7505 | Stimulate intrinsic cholinergic neurons to enhance motility | Increases colon transit in healthy controls | None reported | Greater selectivity for 5-HT4; not metabolized by CTP 3A4 | |
| velusetrag (TD-5108) | Stimulate intrinsic cholinergic neurons to enhance motility | Dose-related increase in SB and colon transit in healthy controls | IIB, dose-ranging study in 401 CC patients increased BM frequency and proportion with adequate relief | Greater selectivity for 5-HT4 | |
| 5-HT3-antagonist | ramosetron | Inhibits secretion, motility, nociception | None reported | IIB, dose-ranging studies document benefit on global relief and bowel function endpoints in IBS-D | Safety concern regarding ischemic colitis with same drug class |
| C1-C2 channel activator | lubiprostone | Increases intestinal water and electrolyte secretion | Accelerates SB and colonic transit in healthy controls | Two phase III in several hundred CC and IBS-C patients: efficacious | Nausea that is usually mild; FDA approved |
| Guanylate cyclase-C agonist | linaclotide | Increases intestinal water and electrolyte secretion | Accelerated ascending colonic transit and altered bowel function in 36 women with IBS-C | IIA and IIB studies in CC or IBS: increased BM frequency | |
| K-opioid agonist | asimadoline | κ-opioid receptors in visceral perception | Reduce sensation in response to colon distensions in the non-noxious range; relax colon tone in healthy controls; increase sensory thresholds in patients with IBS | On-demand dosing not effective in reducing severity of abdominal pain in 100 IBS patients; IIB, dose-ranging study, 596 IBS patients: at least average moderate pain benefit in IBS-D and IBS-A | |
| 2,3-Benzodiazepine modulator | dextofisopam | Potential to reduce stimulation-induced colonic motility and visceral sensitivity | None reported | IIB study in 140 IBS patients: increased number of months of adequate overall relief of IBS symptoms; efficacy lower over time | ? more abdominal pain or headaches vs placebo |
5-HT4 Agonists
Withdrawal of cisapride and tegaserod because of cardiac or potential vascular adverse events requires that any new drugs in this class prove selectivity (27) for the 5-HT4 receptor over other receptors (e.g. 5-HT2B, 5-HT7) and channels (e.g. delayed rectifier potassium channel) and safety through studies of arrhythmogenic potential and effects on QT interval (28). Three 5-HT4 agonists in development are reported to have greater selectivity for 5-HT4 over other receptors, and have advanced to human trials.
a. Prucalopride
is efficacious in accelerating colonic transit (29) and in the treatment of severe chronic constipation unresponsive to laxative treatment (30-32). More recent reports document the continued efficacy in a 2 year open-label study of patients recruited from 7 prior placebo-controlled, randomized, controlled trials. Cardiovascular safety was also reported in 100 elderly constipated patients randomized (25/treatment group) to prucalopride in a dose-escalation study. Vital Signs, ECG, and Holter data in these patients (~80 % of whom had history of or current cardiovascular disease) were not different from placebo, and importantly, no patients had QTcF > 500msec (33,34).
b. ATI-7505
is a benzamide 5-HT4 receptor agonist whose chemical structure includes substituted piperidine-based scaffolds, that are similar to cisapride. ATI-7505 is the pure (3S, 4R) isomer, whereas cisapride is a mixture of (3R, 4S) and (3S, 4R) isomers. It is a full agonist in the GI tract, but a partial agonist in the heart, devoid of other 5-HT receptor activities and negligible inhibitory activity at the hERG channel. The affinity ratio between IKr and 5-HT4 receptors is at least 1000-fold. Its primary metabolite is 100-fold less active than the parent drug at the 5-HT4 receptor and it has no detectable hERG channel inhibitory activity (35). Unlike cisapride, ATI-7505 undergoes hydrolytic esterase metabolism, not oxidative CYP450 metabolism. Therefore drug interactions with agents that affect or are metabolized by CYP 3A4 are unlikely. A dose-response pharmacodynamic study showed ATI-7505 accelerated colonic transit and enhanced evacuation of stool (36). A study in rodents suggests ATI-7505 may alter visceral sensitivity (37). Results from clinical trials and dose-response clarifications are awaited.
c. Velusetrag (TD-5108)
is a potent agonist at human 5-HT4 receptors with high intrinsic activity and selectivity for 5-HT4, >500-fold selective over other 5-HT receptors (including h5-HT2B and h5-HT3A. At 3 μM, TD-5108 had no effect on human ether-à-go-go-related gene encoded K+ channels (38,39). In pharmacodynamic studies, velusetrag dose dependently accelerated colonic transit after single doses; with multiple doses, gastric and colonic transit were accelerated by 15 and 30mg doses (40). Clinical trials demonstrate these doses are also effective in the treatment of chronic constipation (41). To date, placebo controlled trials in 60 healthy volunteers and >400 patients did not identify any cardiac rhythm abnormalities.
Novel 5-HT3 Antagonist
Prior studies had shown that this class of drugs inhibits colonic secretion and motility, and visceral sensation through central and peripheral mechanisms. In experimental animal models, ramosetron inhibited stress or exogenous CRF-induced water secretion and acceleration of colonic transit, as well as colonic nociception in stress models (42,43). In a double-blind, placebo-controlled, parallel-group study of 418 IBS-D patients, the 5-HT3 receptor antagonist, ramosetron, 5- and 10-μg once daily, increased monthly responder rates of 'Patient-reported global assessment of relief of IBS symptoms’ compared to placebo; the benefit was similar in males and females (44). In a second study, ramosetron, 5 μg once daily, was effective and well tolerated in the treatment of abdominal pain, discomfort and bowel habits in IBS-D patients (45).
Inhibition of Serotonin Synthesis
Given the concerns that 5-HT3 antagonists may be associated with ischemic colitis, and evidence that plasma serotonin or jejunal mucosal serotonin content are increased in patients with diarrhea-predominant IBS (46,47), there are novel approaches to reduce serotonergic stimulation by inhibition of the synthesis of the biogenic amine.
The novel oral tryptophan hydroxylase (TPH) inhibitor, LX1031, resulted in significant reductions in urinary 5-HIAA and was well tolerated in total daily doses up to 4 g over 14 days. These data indicate successful pharmacological inhibition of the target enzyme in the GI tract (48); clinical trials are in progress.
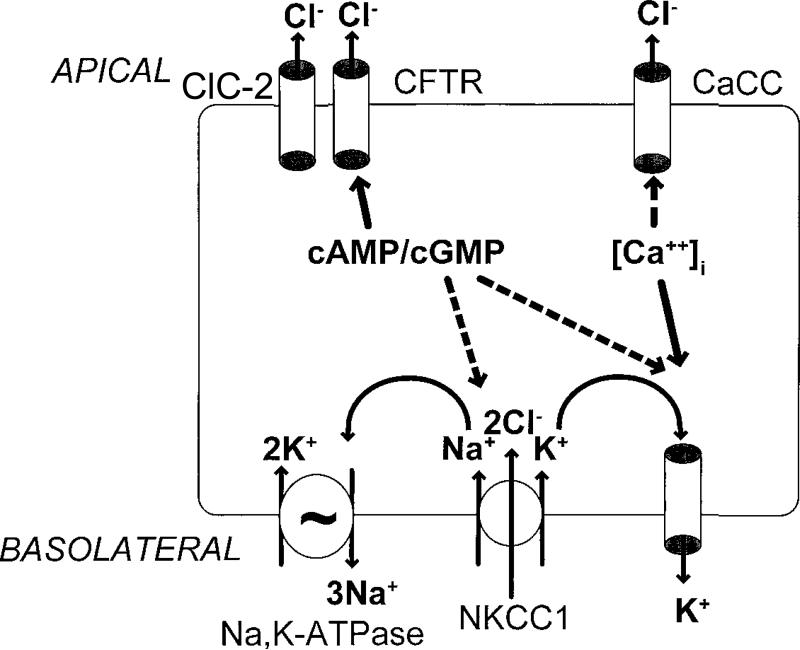
Chloride Secretion
Epithelial chloride transport induces fluid secretion: the main fluxes of chloride [Figure 1 (49)] are the basolateral Na+-K+-2Cl co-transporter, which brings chloride into the enterocytes, aided by the Na pump, and K channel which transport the cations out through the basolateral membrane. In the apical membrane, the cystic fibrosis trans-membrane regulator (CFTR) and ClC-2 Cl- channels in the apical membrane allow Cl- exit, and this induces fluid secretion.
Figure 1.
Chloride transport is also pivotal in the regulation of fluid secretion in the gastrointestinal tract by organs that drain into the intestine. This model of the chloride secretory mechanism in intestinal epithelial cells shows that secretion can be stimulated by increases in intracellular messengers, either cyclic nucleotides (cAMP/cGMP) or cytosolic calcium ([Ca2`]i). Major targets for regulation of secretion include channels in the apical membrane: CFTR, cystic fibrosis transmembrane conductance regulator; CaCC, calcium-activated chloride channel and ClC-2 (chloride channel type 2). Activation of these channels by the intracellular messengers is indicated with solid arrows, with additional postulated sites of action indicated with broken arrows. The identity of basolateral potassium channel(s) involved in either cyclic nucleotide- or calcium-mediated chloride secretion is unknown. Ion channels in the basolateral membrane serve to deliver chloride into the enterocytes, while ensuring that the obligatorily co-transported cations are extruded by energy-dependent (e.g. ATP) mechanisms. These transporters are particularly needed to re-cycle potassium ions. They include: NKCC1 (sodium/potassium/2 chloride co-transporter type 1), IK, intermediate conductance potassium channel; K-cAMP channel, putative potassium channel regulated by cAMP and KCNQ1/KCNE3 heteromeric K+ channels (not shown). Adapted from ref. 49, Barrett KE, Keely SJ. Chloride secretion by the intestinal epithelium: molecular basis and regulatory aspects. Annu Rev Physiol 2000;62:535-72.
Currently, there are three targets of medical therapy related to chloride channels.
a. Crofelemer
is an antagonist at the CFTR; it may antagonize bowel dysfunction in IBS-D (50) based on a single clinical trial.
b. ClC-2 and ClC-3 are expressed in most cells
Lubiprostone is a a bicyclic fatty acid chloride channel activator in a drug class called prostones that induces chloride secretion by enterocytes and colonocytes (51); there are contradictory data in the literature regarding the activation of smooth muscle or activation of prostaglandin receptors by lubiprostone (52,53). In humans, lubiprostone induces acceleration of colonic transit (54). Clinical trials show lubiprostone at 24 μg b.i.d is effective in the treatment of constipation (55); the dose approved for IBS-C is 8 μg b.i.d. (56), which is associated with lower prevalence of nausea. A human study assessed the effects of lubiprostone on colonic motility; minor changes in colonic tone and compliance were observed which are not likely to account for the clinical effects of lubiprostone; hence, the secretory effects appear to be the main mechanism responsible for the effects on constipation (57). In addition, the study showed no significant effect on colonic sensation thresholds or ratings.
c. Guanylate cyclase C (GC-C)
is the principal receptor for heat-stable enterotoxins (STa), major causes in E. coli-induced secretory diarrhea. GC-C is enriched in intestinal epithelium, and has 2 endogenous ligands in the form of small cysteine-rich peptides guanylin and uroguanylin, released in an auto- or paracrine fashion into the intestinal lumen. Guanylin and uroguanylin are also endocrine hormones in gut-kidney communication, regulating ion transport in extra-intestinal epithelia (58).
Linaclotide is a synthetic guanylate cyclase C agonist, which is a 14 amino acid peptide that activates the membrane GC-C on intestinal epithelial cells; highest levels of GC-C expression occur on the luminal surface of the epithelial cells. Linaclotide increases intestinal fluid secretion and increases intestinal transit. Linaclotide also decreases visceral pain in rats, and the effects are attributed to the effects of cGMP (59). In humans, there was no evidence of systemic exposure to linaclotide or its 13-amino acid active metabolite, MM-419447, after oral administration. Linaclotide accelerated colonic transit, loosened loose consistency and increased bowel frequency in patients with chronic constipation (60). Beneficial effects were replicated in a phase 2a RCT in chronic constipation (61) and in a phase 2b RCT in IBS-C (62).
Medications “Borrowed” from other Indications
Bile Acid Modulation
Up to 95% of bile acids secreted into bile are actively reabsorbed in the terminal ileum. Inadequate absorption of bile in the distal small intestine increases its delivery into the cecum and colon, where bile acids may cause secretory diarrhea (63). Prior studies have shown that both conjugated and non-conjugated bile acids induce secretion in the human colon (64,65). There is also evidence that the di-α-hydroxy bile salt chenodeoxycholate (CDC) increases colonic motility (66,67), though the effective dose is unclear. Ileocolonic delivery of CDC results in accelerated colonic transit and increased stool frequency and consistency in healthy volunteers (68). This ileocolonic delivery is effective at relatively low doses of 0.5 to 1.0g/day, whereas effect of higher doses (2.25g/day) of oral CDC were of variable efficacy in chronic constipation (69). There is considerable information about CDC safety from its use in the dissolution of gallstones. Thus, doses of 7-20 mg CDCA per kg body weight−1 day−1) were not hepatotoxic in humans (70).
Conversely, bile acid binding with cholestyramine is effective for the treatment of diarrhea related to bile acid malabsorption (71), but compliance with cholestyramine is poor due to unpalatability, gritty texture and gastrointestinal side effects, which limit its long term use despite formulations sweetened with sucrose or aspartame. A newer bile acid binder, colesevelam hydrochloride, is better tolerated, has fewer gastrointestinal side effects and is easily administered (72). Initially approved as a lipid-lowering agent, colesevelam is now also approved as an adjunct therapy to improve glycemic control in patients with type 2 diabetes mellitus (73,74). Colesevelam slows colonic transit in a subset of patients with IBS-D (Odunsi and Camilleri unpublished observations). Formal clinical trials are required to determine the role of bile acid binding in chronic diarrhea and IBS-D especially since it has been suggested that up to 70% of patients with chronic watery diarrhea have bile acid malabsorption (75), and 20% of unselected IBS-D patients had serological evidence of bile acid malabsorption (76).
Anti-inflammatory Approaches
There is evidence of a low grade inflammation in IBS, involving mast cells in some patients, particularly in those with post-infectious etiology (77). Three anti-inflammatory targets are being targeted in lower functional gastrointestinal disorders.
a. Sodium cromoglycate
Jejunal fluid and a biopsy were obtained in health and IBS, 6 months after random allocation to no treatment (IBS, n=7) or oral DSCG, 200 mg/8h, (IBS-DSCG, n=11). BMs, stool consistency and abdominal pain were monitored. Mast cell degranulation was assessed by luminal tryptase release and transmission electron microscopy. Mast cell-mediated transcriptional restoration of relevant immuno-modulatory genes in mucosal inflammation with cromoglycate results in clinical improvement of bowel function in IBS-D (78).
b. Ketotifen
a mast cell stabilizer, has been shown to significantly improve gastric emptying after abdominal surgery, and it may play a role in therapy for post-operative ileus, which is associated experimentally with mast cell degranulation (79). In a preliminary report of 60 IBS patients, it was claimed that ketotifen but not placebo increased the threshold for discomfort in visceral hypersensitive IBS patients (n=30); however, the post-treatment thresholds in the two treatment arms were not compared and do not appear to be significant. This effect on rectal sensory thresholds was not observed in normosensitive IBS patients (n=30). Importantly, the percentage of patients reporting severe abdominal pain was significantly decreased by ketotifen (30% to 7% p=0.031), but not by placebo (32% to 29%, NS); similarly, severe complaints of abdominal bloating, flatulence, diarrhea and incomplete evacuation were significantly reduced and quality of life subscales of sleep, diet and sexual functioning improved with ketotifen compared to placebo. Interestingly, spontaneous release of tryptase from IBS patients’ rectal mucosal biopsies was lower from that of healthy volunteers [in contrast to prior studies (80)], and ketotifen treatment of IBS patients did not affect the release of tryptase (81). In summary, these provocative data support the need for further studies; an additional precaution to avoid unblinding of patients would be to use a control medication that induces some sedation or drowsiness.
c. Mesalazine
reduces total immunocytes, mast cells, as well as mucosal IL-1β (pg/ml), histamine (ng/ml) and tryptase (ng/ml) in patients with IBS. This was associated with improved well-being and satisfaction with treatment but the study was too small to demonstrate significance in bowel and pain symptoms (82).
Visceral Sensation as a Target
The κ-opioid agonist, asimadoline, has been extensively studied with pharmacodynamic trials showing reduced pain sensation at relatively low levels of colonic distension (83), and clinical trials that suggest adequate relief especially in patients with IBS-D and at least moderate pain during the run-in period (84). Intermittent use of asimadoline during attacks of IBS pain was not an effective therapy (85).
Gabapentin, a 3-alkylated analogue of γ-amino butyric acid (GABA), has been shown to reduce elements of central sensitization in human experimental hyperalgesia. Forty patients with IBS-D were randomized for 5-day treatment with gabapentin, 300 mg/day and then 600 mg/day, which increased rectal sensory thresholds through attenuating rectal sensitivity to distension and enhancing rectal compliance (86). Pregabalin has been tested in IBS patients in pharmacodynamic study (87), but no clinical trials are reported to date.
New Centrally Acting Agents in IBS
Benzodiazepine Receptor Modulator
Dextofisopam binds to 2,3-benzodiazepine receptors located in subcortical and hypothalamic regions. In animal models, dextofisopam reduced stimulation-induced colonic motility and visceral sensitivity. A 12-week, placebo-controlled, phase II study in patients with diarrhea-predominant or alternating-IBS showed more months with adequate relief in the dextofisopam compared to the placebo group (88). The most prominent effects were observed within the first 4 weeks of treatment, and included an improved consistency and frequency of bowel movements. The drug was well tolerated; however, the significance of the 12% of the patients experiencing worse abdominal pain (versus 4% with placebo) requires further study.
Anti-Psychotic Agent
Quetiapine is an atypical antipsychotic agent that ameliorates anxiety and sleep disturbances, augments the effect of antidepressants, and provides an independent analgesic effect. In a medical records study of patients with severe refractory functional gastrointestinal disorders, Grover et al. (89) reported that among the 11 of 21 patients still on the medication at follow up, 6 demonstrated global relief of symptoms, and 9 were satisfied with treatment. The remaining 10 of 21 discontinued therapy because of somnolence and lack of GI benefits. This is a very specific group of patients with known psychiatric diagnoses, including 14 anxiety, 3 post-traumatic stress disorder and 5 depression, and other centrally acting medications. The dose escalation, unblinded treatment, and adverse effects also may have influenced the reporting of benefit. This approach requires further blinded studies before it can be endorsed. In addition, physicians without experience with this class of agents should probably avoid using such medications because of significant risk of central side effects.
Conclusions
The peripheral targets of medical therapy address the bowel dysfunction of IBS and this is often associated with pain relief. There are less clear targets related to the abdominal pain or visceral sensation in IBS. In recent years, the field of IBS therapy has been characterized by initial promise and subsequent disappointment due to withdrawal or restrictive use of new medications. However, there is reason to be optimistic. Representatives of the Food and Drug Administration have proposed interim endpoints for use in trials of IBS drugs that are based on clinically valid concepts (90). This has provided much appreciated guidance. The new 5-HT4 agonists are more specific and continue to show cardiovascular safety. Secretory agents have high specificity and low bioavailability. The potential risks of agents “borrowed” from other indications (like hyperlipidemia, inflammatory bowel disease or somatic pain) are known. With the convergence of greater understanding of pathophysiological mechanisms in IBS (91), relevant targets for medical therapy, a pipeline of medications that appear safe, and clarity on trial endpoints, there is reason to believe that new medications will relieve patients with IBS.
Acknowledgements
The excellent secretarial support of Mrs. Cindy Stanislav is gratefully acknowledged. Dr. Camilleri is supported by grant RO1-DK-54681 from National Institutes of Health.
Footnotes
Disclosure: Mayo Clinic has filed a provisional patent application (inventors: M. Camilleri, D. Burton) related to ileocolonic delivery of chenodeoxycholic acid: No. 61/143,727.
References
- 1.Brandt LJ, Chey WD, Foxx-Orenstein AE, et al. An evidence-based systematic review on the management of irritable bowel syndrome. Am J Gastroenterol. 2009;10:S1–S35. doi: 10.1038/ajg.2008.122. [DOI] [PubMed] [Google Scholar]
- 2.Camilleri M, Chang L. Challenges to the therapeutic pipeline for irritable bowel syndrome: end points and regulatory hurdles. Gastroenterology. 2008;135:1877–1891. doi: 10.1053/j.gastro.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Warr D. The neurokinin1 receptor antagonist aprepitant as an antiemetic for moderately emetogenic chemotherapy. Expert Opin Pharmacother. 2006;7:1653–1658. doi: 10.1517/14656566.7.12.1653. [DOI] [PubMed] [Google Scholar]
- 4.Diemunsch P, Joshi GP, Brichant JF. Neurokinin-1 receptor antagonists in the prevention of postoperative nausea and vomiting. Br J Anaesth. 2009;103:7–13. doi: 10.1093/bja/aep125. [DOI] [PubMed] [Google Scholar]
- 5.Grunberg SM, Rolski J, Strausz J, et al. Efficacy and safety of casopitant mesylate, a neurokinin 1 (NK1)-receptor antagonist, in prevention of chemotherapy-induced nausea and vomiting in patients receiving cisplatin-based highly emetogenic chemotherapy: a randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:549–558. doi: 10.1016/S1470-2045(09)70109-3. [DOI] [PubMed] [Google Scholar]
- 6.Oh-Young L, Manakata J, Naliboff B. A double-blind, parallel group pilot study of the effects of CJ-11974 and placebo on perceptual and emotional responses to rectosigmoid distension in IBS patients. Gastroenterology. 2000;118:A846. [Google Scholar]
- 7.Houghton LA, Cremonini F, Camilleri M, et al. Effect of the NK(3) receptor antagonist, talnetant, on rectal sensory function and compliance in healthy humans. Neurogastroenterol Motil. 2007;19:732–743. doi: 10.1111/j.1365-2982.2007.00934.x. [DOI] [PubMed] [Google Scholar]
- 8.Dukes GE, Dewit OE, Sanger GJ, et al. Lack of effect of the NH3 receptor antagonist, talnetant SB223412, on symptoms of IBS: results of 2 randomized, double-blind, placebo-controlled dose-ranging trials. Gastroenterology. 2007;132:A60. [Google Scholar]
- 9.Lordal M, Navalesi G, Theodorsson E, Maggi CA, Hellstrom PM. A novel tachykinin NK2 receptor antagonist prevents motility-stimulating effects of neurokinin A in small intestine. Br J Pharmacol. 2001;134:215–223. doi: 10.1038/sj.bjp.0704217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fukudo S, Nomura T, Hongo M. Impact of corticotropin-releasing hormone on gastrointestinal motility and adrenocorticotropic hormone in normal controls and patients with irritable bowel syndrome. Gut. 1998;42:845–849. doi: 10.1136/gut.42.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sagami Y, Shimada Y, Tayama J, et al. Effect of a corticotropin releasing hormone receptor antagonist on colonic sensory and motor function in patients with irritable bowel syndrome. Gut. 2004;53:958–964. doi: 10.1136/gut.2003.018911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wallon C, Söderholm JD. Corticotropin-releasing hormone and mast cells in the regulation of mucosal barrier function in the human colon. Ann N Y Acad Sci. 2009;1165:206–210. doi: 10.1111/j.1749-6632.2009.04030.x. [DOI] [PubMed] [Google Scholar]
- 13.Wallon C, Yang PC, Keita AV, et al. Corticotropin-releasing hormone (CRH) regulates macromolecular permeability via mast cells in normal human colonic biopsies in vitro. Gut. 2008;57:50–58. doi: 10.1136/gut.2006.117549. [DOI] [PubMed] [Google Scholar]
- 14.Tayama J, Sagami Y, Shimada Y, Hongo M, Fukudo S. Effect of alpha-helical CRH on quantitative electro-encephalogram in patients with irritable bowel syndrome. Neurogastroenterol Motil. 2007;19:471–483. doi: 10.1111/j.1365-2982.2007.00903.x. [DOI] [PubMed] [Google Scholar]
- 15.Sweetser S, Camilleri M, Linker Nord SJ, et al. Do corticotropin releasing factor-1 receptors influence colonic transit and bowel function in women with irritable bowel syndrome? Am J Physiol. 2009;296:G1299–G1306. doi: 10.1152/ajpgi.00011.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thoua NM, Hobson AR, Dukes GE, Kelleher D, Hicks K, Boardley R, Raeburn A, Emmanuel A. The selective CRF-1 receptor antagonist GW876008 attenuates stress induced rectal hypersensitivity in patients with irritable bowel syndrome (IBS). Neurogastroenterol Motil. 2009;21(Suppl.):85. (abstract) [Google Scholar]
- 17.Duke GE, Mayer EA, Kelleher DL, Hicks KJ, Boardley RL, Alpers DH. A randomised, double blind, placebo (PLA) controlled, crossover study to evaluate the efficacy and safety of the corticotrophin releasing factor 1 (CRF1) receptor antagonist (RA) GW876008 in irritable bowel syndrome (IBS) patients. Neurogastroenterol Motil. 2009;21(Suppl.):84. (abstract) [Google Scholar]
- 18.Drossman DA, Danilewitz M, Naesdal J, Hwang C, Adler J, Silberg DG. Randomized, double-blind, placebo-controlled trial of the 5-HT1A receptor antagonist AZD7371 tartrate monohydrate (robalzotan tartrate monohydrate) in patients with irritable bowel syndrome. Am J Gastroenterol. 2008;103:2562–2569. doi: 10.1111/j.1572-0241.2008.02115.x. [DOI] [PubMed] [Google Scholar]
- 19.Pharmabiz. com. Forest to discontinue development in U.S. of dexloxiglumide for irritable bowel syndrome. Pharmabiz.com 2004;October: http://www.pharmabiz.com/article/detnews.asp?articleid=18255§ionid=14)
- 20.Cremonini F, Camilleri M, McKinzie S, et al. Effect of CCK-1 antagonist, dexloxiglumide, in female patients with irritable bowel syndrome: a pharmacodynamic and pharmacogenomic study. Am J Gastroenterol. 2005;100:652–663. doi: 10.1111/j.1572-0241.2005.41081.x. [DOI] [PubMed] [Google Scholar]
- 21.Whorwell PJ, Pace F, D'Amato M, et al. A phase III, 6 month, double-blind, placebo-controlled, randomized withdrawal trial of the selective CCK 1 antagonist dexloxiglumide in constipation-predominant IBS: the Darwin study. Gastroenterology. 2008;134:A-157. (abstract) [Google Scholar]
- 22.Cellek S, Thangiah R, Bassil AK, Campbell CA, Gray KM, Stretton JL, Lalude O, Vivekanandan S, Wheeldon A, Winchester WJ, Sanger GJ, Schemann M, Lee K. Demonstration of functional neuronal beta3-adrenoceptors within the enteric nervous system. Gastroenterology. 2007;133:175–183. doi: 10.1053/j.gastro.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 23.Grudell AB, Camilleri M, Jensen KL, Foxx-Orenstein AE, Burton DD, Ryks MD, Baxter KL, Cox DS, Dukes GE, Kelleher DL, Zinsmeister AR. Dose-response effect of a beta3-adrenergic receptor agonist, solabegron, on gastrointestinal transit, bowel function, and somatostatin levels in health. Am J Physiol. 2008;294:G1114–G1119. doi: 10.1152/ajpgi.00051.2008. [DOI] [PubMed] [Google Scholar]
- 24.Kelleher DL, Hicks KJ, Cox DS, Williamson RR, Alpers DH, Dukes GE. Randomized, double-blind, placebo (PLA)-controlled, crossover study to evaluate efficacy and safety of the beta 3-adrenergic receptor agonist solabegron (SOL) in patients with irritable bowel syndrome (IBS). Neurogastroenterol Motil. 2008;20(Suppl. 1)):131–132. (abstract) [Google Scholar]
- 25.Mayer EA, Bradesi S, Chang L, Spiegel BM, Bueller JA, Naliboff BD. Functional GI disorders: from animal models to drug development. Gut. 2008;57:384–404. doi: 10.1136/gut.2006.101675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chial HJ, Camilleri M, Ferber I, Delgado-Aros S, Burton D, McKinzie S, Zinsmeister AR. Effects of venlafaxine, buspirone, and placebo on colonic sensorimotor functions in healthy humans. Clin Gastroenterol Hepatol. 2003;1:211–218. doi: 10.1053/jcgh.2003.50031. [DOI] [PubMed] [Google Scholar]
- 27.De Maeyer JH, Lefebvre RA, Schuurkes JA. 5-HT4 receptor agonists: similar but not the same. Neurogastroenterol Motil. 2008;20:99–112. doi: 10.1111/j.1365-2982.2007.01059.x. [DOI] [PubMed] [Google Scholar]
- 28.Morganroth J, Brozovich FV, McDonald JT, Jacobs RA. Variability of the QT measurement in healthy men, with implications for selection of an abnormal QT value to predict drug toxicity and proarrhythmia. Am J Cardiol. 1991;67:774–776. doi: 10.1016/0002-9149(91)90541-r. [DOI] [PubMed] [Google Scholar]
- 29.Bouras EP, Camilleri M, Burton DD, et al. Prucalopride accelerates gastrointestinal and colonic transit in patients with constipation without a rectal evacuation disorder. Gastroenterology. 2001;120:354–360. doi: 10.1053/gast.2001.21166. [DOI] [PubMed] [Google Scholar]
- 30.Camilleri M, Kerstens R, Rykx A, Vandeplassche L. A placebo-controlled trial of prucalopride for severe chronic constipation. N Engl J Med. 2008;358:2344–2354. doi: 10.1056/NEJMoa0800670. [DOI] [PubMed] [Google Scholar]
- 31.Quigley EM, Vandeplassche L, Kerstens R, Ausma J. Clinical trial: the efficacy, impact on quality of life, and safety and tolerability of prucalopride in severe chronic constipation--a 12-week, randomized, double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2009;29:315–328. doi: 10.1111/j.1365-2036.2008.03884.x. [DOI] [PubMed] [Google Scholar]
- 32.Tack J, van Outryve M, Beyens G, Kerstens R, Vandeplassche L. Prucalopride (Resolor) in the treatment of severe chronic constipation in patients dissatisfied with laxatives. Gut. 2009;58:357–365. doi: 10.1136/gut.2008.162404. [DOI] [PubMed] [Google Scholar]
- 33.Camilleri M, Kerstens R, Beyens G, Robinson P, Vandeplassche L. A double-blind, placebo controlled trial to evaluate the safety and tolerability of prucalopride oral solution in constipated elderly patients living in a nursing facility. Gastroenterology. 2009;136(Suppl 1):240. [Google Scholar]
- 34.Camilleri M, Beyens G, Kerstens R, Vandeplassche L. Long-term follow up of safety and satisfaction with bowel function in response to oral prucalopride in patients with chronic constipation. Gastroenterology. 2009;136(Suppl 1):160. [Google Scholar]
- 35.Dennis D, Palme M, Irwin I, Druzgala P, Teichman S. ATI-7505 is a novel, selective 5HT(4) receptor agonist that causes gastrointestinal prokinetic activity in dogs. Gastroenterology. 2004;126:A641. [Google Scholar]
- 36.Camilleri M, Vazquez-Roque MI, Burton D, et al. Pharmacodynamic effects of a novel prokinetic 5-HT receptor agonist, ATI-7505, in humans. Neurogastroenterol Motil. 2007;19:30–38. doi: 10.1111/j.1365-2982.2006.00865.x. [DOI] [PubMed] [Google Scholar]
- 37.Johnson AC, Tyler KR, Palme M, Greenwood-Van Meerveld B. Efficacy of ATI-7505, a novel highly selective 5-HT4 receptor agonist, in a rodent model. Gastroenterology. 2009;136(Suppl 1):158. [Google Scholar]
- 38.Smith JA, Beattie DT, Marquess D, et al. The in vitro pharmacological profile of TD-5108, a selective 5-HT(4) receptor agonist with high intrinsic activity. Naunyn Schmiedebergs Arch Pharmacol. 2008;378:125–137. doi: 10.1007/s00210-008-0282-y. [DOI] [PubMed] [Google Scholar]
- 39.Beattie DT, Armstrong SR, Shaw JP, et al. The in vivo gastrointestinal activity of TD-5108, a selective 5-HT(4) receptor agonist with high intrinsic activity. Naunyn Schmiedebergs Arch Pharmacol. 2008;378:139–147. doi: 10.1007/s00210-008-0281-z. [DOI] [PubMed] [Google Scholar]
- 40.Camilleri M, Manini M, McKinzie S, et al. Dose-related effects of TD-5108, a selective 5-HT4 receptor agonist with high intrinsic activity, on gastrointestinal (GI) and colonic transit in healthy volunteers. Neurogastroenterol Motil. 2008;20(Suppl. 2):6. [Google Scholar]
- 41.Goldberg MR, Li Y-P, Pitzer K, et al. TD-5108, a selective 5-HT4 agonist, is consistently better than placebo regardless of response definition in patients with chronic constipation. Gastroenterology. 2008;133:A547. [Google Scholar]
- 42.Hirata T, Funatsu T, Keto Y, Nakata M, Sasamata M. Pharmacological profile of ramosetron, a novel therapeutic agent for IBS. Inflammopharmacology. 2007;15:5–9. doi: 10.1007/s10787-006-1537-1. [DOI] [PubMed] [Google Scholar]
- 43.Hirata T, Keto Y, Nakata M, Takeuchi A, Funatsu T, Akuzawa S, Sasamata M, Miyata K. Effects of serotonin 5-HT3 receptor antagonists on stress-induced colonic hyperalgesia and diarrhoea in rats: a comparative study with opioid receptor agonists, a muscarinic receptor antagonist and a synthetic polymer. Neurogastroenterol Motil. 2008;20:557–565. doi: 10.1111/j.1365-2982.2007.01069.x. [DOI] [PubMed] [Google Scholar]
- 44.Matsueda K, Harasawa S, Hongo M, Hiwatashi N, Sasaki D. A phase II trial of the novel serotonin type 3 receptor antagonist ramosetron in Japanese male and female patients with diarrhea-predominant irritable bowel syndrome. Digestion. 2008;77:225–235. doi: 10.1159/000150632. [DOI] [PubMed] [Google Scholar]
- 45.Matsueda K, Harasawa S, Hongo M, Hiwatashi N, Sasaki D. A randomized, double-blind, placebo-controlled clinical trial of the effectiveness of the novel serotonin type 3 receptor antagonist ramosetron in both male and female Japanese patients with diarrhea-predominant irritable bowel syndrome. Scand J Gastroenterol. 2008;43:1202–1211. doi: 10.1080/00365520802240255. [DOI] [PubMed] [Google Scholar]
- 46.Atkinson W, Lockhart S, Whorwell PJ, Keevil B, Houghton LA. Altered 5-hydroxytryptamine signaling in patients with constipation- and diarrhea-predominant irritable bowel syndrome. Gastroenterology. 2006;130:34–43. doi: 10.1053/j.gastro.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 47.Kerckhoffs AP, Ter Linde JJ, Akkermans LM, Samsom M. Trypsinogen IV, serotonin transporter transcript levels and serotonin content are increased in small intestine of irritable bowel syndrome patients. Neurogastroenterol Motil. 2008;20:900–907. doi: 10.1111/j.1365-2982.2008.01100.x. [DOI] [PubMed] [Google Scholar]
- 48.Brown P, Jackson J, Shi Z-C, et al. LX1031: a new approach for managing irritable bowel syndrome (IBS). Gastroenterology. 2009;136(Suppl 1):237. [Google Scholar]
- 49.Barrett KE, Keely SJ. Chloride secretion by the intestinal epithelium: molecular basis and regulatory aspects. Annu Rev Physiol. 2000;62:535–572. doi: 10.1146/annurev.physiol.62.1.535. [DOI] [PubMed] [Google Scholar]
- 50.Mangel AW, Chaturvedi P. Evaluation of crofelemer in the treatment of diarrhea-predominant irritable bowel syndrome patients. Digestion. 2008;78:180–186. doi: 10.1159/000185719. [DOI] [PubMed] [Google Scholar]
- 51.Cuppoletti J, Malinowska DH, Tewari KP, et al. SPI-0211 activates T84 cell chloride transport and recombinant human ClC-2 chloride currents. Am J Physiol. 2004;287:C1173–C1183. doi: 10.1152/ajpcell.00528.2003. [DOI] [PubMed] [Google Scholar]
- 52.Bassil AK, Borman RA, Jarvie EM, et al. Activation of prostaglandin EP receptors by lubiprostone in rat and human stomach and colon. Br J Pharmacol. 2008;154:126–135. doi: 10.1038/bjp.2008.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cuppoletti J, Malinowska DH, Chakrabarti J, Ueno R. Effects of lubiprostone on human uterine smooth muscle cells. Prostaglandins Other Lipid Mediat. 2008;86:56–60. doi: 10.1016/j.prostaglandins.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 54.Camilleri M, Bharucha AE, Ueno R, et al. Effect of a selective chloride channel activator, lubiprostone, on gastrointestinal transit, gastric sensory, and motor functions in healthy volunteers. Am J Physiol. 2006;290:G942–G947. doi: 10.1152/ajpgi.00264.2005. [DOI] [PubMed] [Google Scholar]
- 55.Johanson JF, Drossman DA, Panas R, Wahle A, Ueno R. Clinical trial: phase 2 study of lubiprostone for irritable bowel syndrome with constipation. Aliment Pharmacol Ther. 2008;27:685–696. doi: 10.1111/j.1365-2036.2008.03629.x. [DOI] [PubMed] [Google Scholar]
- 56.Drossman DA, Chey WD, Johanson JF, et al. Clinical trial: lubiprostone in patients with constipation-associated irritable bowel syndrome--results of two randomized, placebo-controlled studies. Aliment Pharmacol Ther. 2009;29:329–341. doi: 10.1111/j.1365-2036.2008.03881.x. [DOI] [PubMed] [Google Scholar]
- 57.Sweetser S, Busciglio IA, Camilleri M, et al. Effect of a chloride channel activator, lubiprostone, on colonic sensory and motor functions in healthy subjects. Am J Physiol. 2009;296:G295–G301. doi: 10.1152/ajpgi.90558.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vaandrager AB. Structure and function of the heat-stable enterotoxin receptor/guanylyl cyclase C. Mol Cell Biochem. 2002;230:73–83. [PubMed] [Google Scholar]
- 59.Bueno L, Beaufrand C, Mahajan-Miklos S, Bryant AP, Currie MG. Antinociceptive actions of MD-1100, a novel therapeutic agent for C-IBS, in animal models of visceral pain. Am J Gastroenterol. 2004;99:S283. [Google Scholar]
- 60.Andresen V, Camilleri M, Busciglio IA, et al. Effect of 5 days linaclotide on transit and bowel function in females with constipation-predominant irritable bowel syndrome. Gastroenterology. 2007;133:761–768. doi: 10.1053/j.gastro.2007.06.067. [DOI] [PubMed] [Google Scholar]
- 61.Johnston JM, Kurtz CB, Drossman DA, et al. Pilot study on the effect of linaclotide in patients with chronic constipation. Am J Gastroenterol. 2009;104:125–132. doi: 10.1038/ajg.2008.59. [DOI] [PubMed] [Google Scholar]
- 62.Johnston J, MacDougall J, Lavins B, et al. Linaclotide significantly improved abdominal pain, constipation and global assessments in adults with irritable bowel syndrome with constipation: results form a large twelve-week, randomized, double-blind, placebo-controlled study. Am J Gastroenterol. 2008;103:S460–S461. [Google Scholar]
- 63.Hofmann AF. The continuing importance of bile acids in liver and intestinal disease. Arch Intern Med. 1999;159:2647–2658. doi: 10.1001/archinte.159.22.2647. [DOI] [PubMed] [Google Scholar]
- 64.Mekhjian HS, Phillips SF, Hofmann AF. Colonic secretion of water and electrolytes induced by bile acids: perfusion studies in man. J Clin Invest. 1971;50:1569–1577. doi: 10.1172/JCI106644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wingate DL, Phillips SF, Hofmann AF. Effect of glycine-conjugated bile acids with and without lecithin on water and glucose absorption in perfused human jejunum. J Clin Invest. 1973;52:1230–1236. doi: 10.1172/JCI107290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kirwan WO, Smith AN, Mitchell WD, Falconer JD, Eastwood MA. Bile acids and colonic motility in the rabbit and the human. Gut. 1975;16:894–902. doi: 10.1136/gut.16.11.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bampton PA, Dinning PG, Kennedy ML, Lubowski DZ, Cook IJ. The proximal colonic motor response to rectal mechanical and chemical stimulation. Am J Physiol. 2002;282:G443–G449. doi: 10.1152/ajpgi.00194.2001. [DOI] [PubMed] [Google Scholar]
- 68.Odunsi ST, Camilleri M, Busciglio IA, et al. Effects of chenodeoxycholic acid on gastrointestinal and colonic transit and bowel function in healthy volunteers. Gastroenterology. 2009;136(Suppl 1):T1247. [Google Scholar]
- 69.Bazzoli F, Malavolti M, Petronelli A, Barbara L, Roda E. Treatment of constipation with chenodeoxycholic acid. J Int Med Res. 1983;11:120–123. doi: 10.1177/030006058301100211. [DOI] [PubMed] [Google Scholar]
- 70.Bell GD, Mok HY, Thwe M, et al. Liver structure and function in cholelithiasis: effect of chenodeoxycholic acid. Gut. 1974;15:165–172. doi: 10.1136/gut.15.3.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sinha L, Liston R, Testa HJ, Moriarty KJ. Idiopathic bile acid malabsorption: qualitative and quantitative clinical features and response to cholestyramine. Aliment Pharmacol Ther. 1998;12:839–844. doi: 10.1046/j.1365-2036.1998.00388.x. [DOI] [PubMed] [Google Scholar]
- 72.Davidson MH, Dillon MA, Gordon B, et al. Colesevelam hydrochloride (cholestagel): a new, potent bile acid sequestrant associated with a low incidence of gastrointestinal side effects. Arch Intern Med. 1999;159:1893–1900. doi: 10.1001/archinte.159.16.1893. [DOI] [PubMed] [Google Scholar]
- 73.Goldberg RB, Fonseca VA, Truitt KE, Jones MR. Efficacy and safety of colesevelam in patients with type 2 diabetes mellitus and inadequate glycemic control receiving insulin-based therapy. Arch Intern Med. 2008;168:1531–1540. doi: 10.1001/archinte.168.14.1531. [DOI] [PubMed] [Google Scholar]
- 74.Fonseca VA, Rosenstock J, Wang AC, Truitt KE, Jones MR. Colesevelam HCl improves glycemic control and reduces LDL cholesterol in patients with inadequately controlled type 2 diabetes on sulfonylurea-based therapy. Diabetes Care. 2008;31:1479–1484. doi: 10.2337/dc08-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fernández-Bañares F, Esteve M, Salas A, et al. Systematic evaluation of the causes of chronic watery diarrhea with functional characteristics. Am J Gastroenterol. 2007;102:2520–2528. doi: 10.1111/j.1572-0241.2007.01438.x. [DOI] [PubMed] [Google Scholar]
- 76.Camilleri M, Nadeau A, Tremaine WJ, et al. Measurement of serum 7alpha-hydroxy-4-cholesten-3-one (or 7alphaC4), a surrogate test for bile acid malabsorption in health, ileal disease and irritable bowel syndrome using liquid chromatography-tandem mass spectrometry. Neurogastroenterol Motil. 2009 Mar 13; doi: 10.1111/j.1365-2982.2009.01288.x. [Epub ahead of print]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Spiller R, Garsed K. Postinfectious irritable bowel syndrome. Gastroenterology. 2009;136:1979–1988. doi: 10.1053/j.gastro.2009.02.074. [DOI] [PubMed] [Google Scholar]
- 78.Lobo B, Vicario M, Martinez C, et al. Clinical benefit in IBS after disodium cromoglycate involves mast cell-mediated recovery of healthy-like innate immunity genes expression profile in the jejunal mucosa. Gastroenterology. 2009;136(Suppl 1):156. [Google Scholar]
- 79.The FO, Buist MR, Lei A, Bennink RJ, Hofland J, van den Wijngaard RM, de Jonge WJ, Boeckxstaens GE. The role of mast cell stabilization in treatment of postoperative ileus: a pilot study. Am J Gastroenterol. 2009 Jun 2; doi: 10.1038/ajg.2009.268. [Epub ahead of print]) [DOI] [PubMed] [Google Scholar]
- 80.Barbara G, Stanghellini V, De Giorgio R, Cremon C, Cottrell GS, Santini D, Pasquinelli G, Morselli-Labate AM, Grady EF, Bunnett NW, Collins SM, Corinaldesi R. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126:693–702. doi: 10.1053/j.gastro.2003.11.055. [DOI] [PubMed] [Google Scholar]
- 81.Klooker TK, Koopman KE, Heide S, Wijngaard RM, Boeckxstaens GE. Treatment with the mast cell stabilizer ketotifen decreases visceral hypersensitivity and improves intestinal symptoms in IBS patients.. Proceedings of the United European Gastroenterology Federation (UEGF) Meeting; 2008.p. OP397. [Google Scholar]
- 82.Corinaldesi R, Stanghellini V, Cremon C, et al. Effect of mesalazine on mucosal immune biomarkers in irritable bowel syndrome: a randomized controlled proof of concept study. Aliment Pharmacol Ther. 2009 May 12; doi: 10.1111/j.1365-2036.2009.04041.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 83.Delgado-Aros S, Chial HJ, Camilleri M, et al. Effects of a kappa opioid agonist, asimadoline, on satiation and gastrointestinal motor and sensory functions in humans. Am J Physiol. 2003;284:G558–G566. doi: 10.1152/ajpgi.00360.2002. [DOI] [PubMed] [Google Scholar]
- 84.Mangel AW, Bornstein JD, Hamm LR, et al. Clinical trial: asimadoline in the treatment of patients with irritable bowel syndrome. Aliment Pharmacol Ther. 2008;28:239–249. doi: 10.1111/j.1365-2036.2008.03730.x. [DOI] [PubMed] [Google Scholar]
- 85.Szarka LA, Camilleri M, Burton D, et al. Efficacy of on-demand asimadoline, a peripheral kappa-opioid agonist, in females with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2007;5:1268–1275. doi: 10.1016/j.cgh.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee KJ, Kim JH, Cho SW. Gabapentin reduces rectal mechanosensitivity and increases rectal compliance in patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2005;22:981–988. doi: 10.1111/j.1365-2036.2005.02685.x. [DOI] [PubMed] [Google Scholar]
- 87.Houghton LA, Fell C, Whorwell PJ, et al. Effect of a second-generation alpha2delta ligand (pregabalin) on visceral sensation in hypersensitive patients with irritable bowel syndrome. Gut. 2007;56:1218–1225. doi: 10.1136/gut.2006.110858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Leventer SM, Raudibaugh K, Frissora CL, et al. Clinical trial: dextofisopam in the treatment of patients with diarrhoea-predominant or alternating irritable bowel syndrome. Aliment Pharmacol Ther. 2008;27:197–206. doi: 10.1111/j.1365-2036.2007.03566.x. [DOI] [PubMed] [Google Scholar]
- 89.Grover M, Dorn SD, Weinland SR, Dalton CB, Gaynes BN, Drossman DA. Atypical antipsychotic quetiapine in the management of severe refractory functional gastrointestinal disorders. Dig Dis Sci. 2009;54:1284–1291. doi: 10.1007/s10620-009-0723-6. [DOI] [PubMed] [Google Scholar]
- 90.Talley NJ. Green light from the FDA for new drug development in irritable bowel syndrome and functional dyspepsia. Am J Gastroenterol. 2009;104:1339–1341. doi: 10.1038/ajg.2009.295. [DOI] [PubMed] [Google Scholar]
- 91.Camilleri M, McKinzie S, Busciglio I, et al. Prospective study of motor, sensory, psychologic, and autonomic functions in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2008;6:772–78. doi: 10.1016/j.cgh.2008.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]