Abstract
Suitable mammalian models for aging with wide range of age-associated pathology are desirable to study molecular mechanisms of human aging. Recent studies have identified that fibroblast growth factor 23 (Fgf-23) null mice and klotho hypomorphs could generate multiple premature aging-like features, including shortened lifespan, infertility, kyphosis, atherosclerosis, extensive soft tissue calcifications, skin atrophy, muscle wasting, T-cell dysregulation, pulmonary emphysema, osteoporosis/osteopenia, abnormal mineral ion metabolism, and impaired vitamin-D homeostasis. The strikingly similar in vivo phenotypes of two separate genetically altered mouse lines implicate that the premature aging-like features may be partly regulated through a common signaling pathway involving both Fgf-23 and klotho; such speculation is experimentally supported by the observation that Fgf-23 requires klotho as a cofactor to exert its functions. Despite about 2,000 fold higher serum levels of Fgf-23 in klotho mutants (compared to wild-type animals), these mice show physical, biochemical and morphological features similar to Fgf-23 null mice, but not as Fgf-23 transgenic mice; these observations suggest that widely encountered premature aging-like features in klotho mutant mice are due to the inability of Fgf-23 to exert its bioactivities in absence of klotho. The results of recent studies showing klotho as a cofactor in Fgf-23 signaling consequently explains that the premature aging-like features in klotho deficient mice is not a primary cause, rather a consequence of lacking Fgf-23 activity. These understandings will help us to redefine the role of klotho as an aging factor.
Keywords: FGF-23, Kotho, Vitamin-D, Calcification, Premature aging
Phosphate homeostasis
Maintaining phosphate homeostasis is of crucial biological importance, as it regulates fundamental cellular functions and skeletal mineralization; it is also an important component of nucleic acids, biologically active signaling proteins, coenzymes, and lipid bilayer of the cell membranes. Ingested phosphate is mostly absorbed in the small intestine and is either incorporated in cells in organic forms, deposited as a component of bone mineral, or eliminated by the kidney; the rate of renal reabsorption and/or excretion is determined by the specific needs of the body.
Roughly 70% of the phosphate is absorbed in the duodenum and jejunum, through a sodium-dependent active transport, a process stimulated by 1,25-dihydroxyvitamin D3 [1,25(OH)2D3]; besides parathyroid hormone (PTH) and low-phosphate diets can also stimulate intestinal absorption of phosphate by exerting effects on vitamin-D. For instance, low extracellular phosphate could stimulate renal activity of 1α-hydroxylase [1α(OH)ase] to increase the synthesis of 1,25(OH)2D3. Kidney is the most important organ that helps in maintaining phosphate homeostasis by controlling urinary phosphate excretion to keep the physiologic balance. About 60–70% of renal reabsorption of phosphate occurs in the proximal tubules via a sodium gradient-dependent process (Gaasbeek and Meinders, 2005; Magagnin et al., 1993; Tenenhouse, 2005). The sodium/phosphate co-transporters (NaPi2a, and NaPi2c), located on the apical brush border membrane of the proximal tubules, contribute to about 85% of the reabsorption. Abundance of NaPi cotransporter proteins determines the rate of active phosphate transport; increased levels of PTH and/or a high-phosphate diet cause an endocytic internalization of the NaPi transporters, and the resultant effect being less reabsorption and an increase in urinary phosphate wasting. In contrary, low levels of PTH and/or a low-phosphate diet cause insertion of the NaPi transporters into the brush border membrane, and thereby increase phosphate uptake (Tenenhouse, 2005; Traebert et al., 2000). The PTH and vitamin-D activities, however, cannot completely explain the complex regulation of the phosphate homeostasis, and the search for phosphatonin (factor responsible for inhibition of phosphate reabsorption) that regulates renal phosphate wasting has led to the identification of fibroblast growth factor 23 (FGF-23) (ADHR_Consortium, 2000; Shimada et al., 2001).
Fibroblast growth factor 23
FGF-23 is a 30 kDa-secreted protein that is processed by a pro-convertase type enzyme into two smaller fragments of approximately 18 kDa (amino fragment) and 12 kDa (carboxy fragment); the exact biological significance of these fragments is not clear, and of intense focus of current research. In contrast to the phosphaturic effects (renal urinary phosphate wasting) of the full-length synthetic FGF-23 protein, the intraperitoneal administration of a synthetic carboxyl terminal fragment of FGF-23 (aa 180–251) or an N-terminal fragment of FGF-23 (25–179) to mice did not produce any such phosphaturic effects (Shimada et al., 2002). Whether in vivo differential processing of the FGF-23 fragments could have diverse biological affects needs to be further investigated. Since the canonical FGFR binding domain is absent in carboxyl terminal fragments of FGF-23, any in vivo response by this fragment would suggest the existence of a novel receptor, in addition to known classic receptors for FGFs.
Recent genetically modified animal studies have provided insights into the role of FGF-23 in regulating phosphate homeostasis. Transgenic mice over-expressing FGF-23 exhibit hypophosphatemia, with no significant changes in serum levels of calcium (Bai et al., 2004; Larsson et al., 2004; Shimada et al., 2004b), while an opposing effect of high serum levels of phosphate, and increased vitamin D activities are documented in Fgf-23 null mice (Shimada et al., 2004a; Sitara et al., 2004). More importantly, the phenotype of Fgf-23 null animals mimics patients with familial tumoral calcinosis (FTC), an autosomal recessive disorder characterized by ectopic calcifications and elevated serum levels of phosphate due to inactivating mutations in the FGF-23 gene (Benet-Pages et al., 2005; Frishberg et al., 2006). Conversely, the phenotype of FGF-23 transgenic animals mimics patients with autosomal dominant hypophosphatemic rickets (ADHR) carrying mutations in the FGF-23 gene that lie within 3 nucleotides of each other in the proprotein convertase cleavage site (ADHR_Consortium, 2000); these mutations prevent proteolytic cleavage of the FGF-23 protein, with net effect being phosphate wasting in the affected patients, perhaps due to enhanced biologic activities of FGF-23. These genetically altered mouse models have clear clinical relevance and provide the in vivo tool to study, in depth, the biology of FGF-23. Available information supports the notion that FGF-23 is the master molecule to regulate phosphate homeostasis.
However, how and where FGF-23 binds to its receptor is of intense focus of research, and preliminary observations suggest that FGF-23 could exert its bioactivities through binding with the known receptors of the FGF family (Yu et al., 2005); recent studies have provided convincing evidence that klotho acts as a cofactor in FGF-23 and receptor binding to induce subsequent intracellular signaling (Kuro-o, 2006; Urakawa et al., 2006).
Klotho
The klotho gene encodes a single-pass transmembrane protein. The extracellular domain of Klotho protein consists of two homologous domains that share sequence homology to the β-glucosidase of bacteria and plants. The klotho gene has selective expression in such tissues as kidney (in the distal convoluted tubules) and brain (in the choroid plexus) (Li et al., 2004). Polymorphisms in the human KLOTHO gene are suggested to correlate with the occurrence of age-related pathologies, including osteoporosis, and coronary artery diseases, and predicted to influence overall survival (Arking et al., 2002). Experimental studies have shown that mice lacking klotho activities produce phenotypes resembling human aging that include muscle and skin atrophy, osteopenia, vascular and soft tissue calcification, pulmonary emphysema, and the resultant effect being short life span (Kuro-o, 2001; Nabeshima, 2002); klotho mutant mice also show biochemical abnormalities that include high serum levels of phosphate, and increased vitamin-D activities. Interestingly, physical, biochemical and morphological features documented in klotho mutant mice completely resemble the features documented in Fgf-23 null mice, raising the possibility of close functional in vivo interactions between these two molecules.
FGF-23, klotho and receptor interactions
Recent studies have shown that both the full length and the extracellular domain of klotho protein are able to bind to various FGF receptors (FGFRs) (Kuro-o, 2006); signal-transducing FGFRs contain an extracellular ligand-binding domain and an intracellular tyrosine kinase domain. In general, in order for FGF to bind to and activate the FGFR system, heparin sulphate proteoglycans or heparin-like molecules are required (Amaya et al., 1991; Mohammadi et al., 2005a; Mohammadi et al., 2005b; Ornitz et al., 1992) FGF-23 is a recently identified member of the FGF family, and is significantly distinct from other FGFs in that it contains a pro-convertase processing site. Whether FGF-23 also needs heparin-like molecules to activate the FGFR system is an intense area of research (Yu et al., 2005), but it is becoming increasingly clear that FGF-23 needs klotho as a cofactor to induce its receptor activities to exert biological effects. Although the extracellular domain of klotho does not directly bind to FGF-23, it enhances FGF-23 binding to its receptor complex with much higher affinity than to FGFR alone (Kuro-o, 2006). Furthermore, FGF-23, in presence of klotho could activate downstream signaling events, as determined by phosphorylation of FGF receptor substrate-2a, extracellular signal-regulated kinase (ERK) and early growth response element-1 (Egr-1) (Kuro-o, 2006; Urakawa et al., 2006). These observations, though preliminary, are validated by two separate groups of investigators (Kuro-o, 2006; Nabeshima, 2006; Urakawa et al., 2006), indicating that klotho indeed acts as a cofactor in FGF23-FGFR interaction and subsequent signaling. One of the likely scenarios is that Klotho regulates FGF-23 signaling through interacting with glycosaminoglycans, a notion that needs experimental validation (Razzaque and Lanske, 2006; Yu et al., 2005).
Why klotho mutants show similar phenotypes as Fgf-23 mutants?
Since both FGF-23 and klotho appear to be in the same signaling cascade, it is not surprising to find out strikingly similar phenotypes in Fgf-23 null and klotho mutant mice; these observations imply the fact that premature aging-like phenotypes in both these genetically altered mouse models are the consequence of the disruption of a common signaling pathway (Razzaque and Lanske, 2006). It is however, interesting to note that despite extremely high serum levels of Fgf-23 (about 2,000 fold higher) in klotho mutants, Fgf-23 is unable to exert its phosphaturic effects in these mice. Both human diseases with increased FGF-23 activity, and experimental studies with over-expression of FGF-23 in transgenic animals have convincingly demonstrated that FGF-23 is a potent phosphaturic factor that induces renal phosphate wasting (ADHR_Consortium, 2000; Bai et al., 2004). However, the lack of phosphaturic activity despite extremely high levels of Fgf-23, signifies that Fgf-23 is unable to exert its physiological functions in the absence of klotho. Hence, it is obvious that the strikingly similar physical, biochemical and morphological phenotypes in the Fgf-23 null mice and klotho mutant mice, that include shortened lifespan, emphysema, infertility (Fig-1), kyphosis, atherosclerosis, extensive soft tissue calcifications, skin atrophy, muscles wasting, T-cell dysregulation, pulmonary emphysema, osteoporosis/osteopenia, abnormal mineral ion metabolism, and impaired vitamin-D homeostasis are due to either absence of Fgf-23 (Fgf-23 null mice) or inability of Fgf-23 to exert its function (klotho mutant mice). It is, therefore, reasonable to suggest that the premature aging-like phenotypes in the klotho mutants are mostly caused by the inability of Fgf-23 to exert its bioactivities.
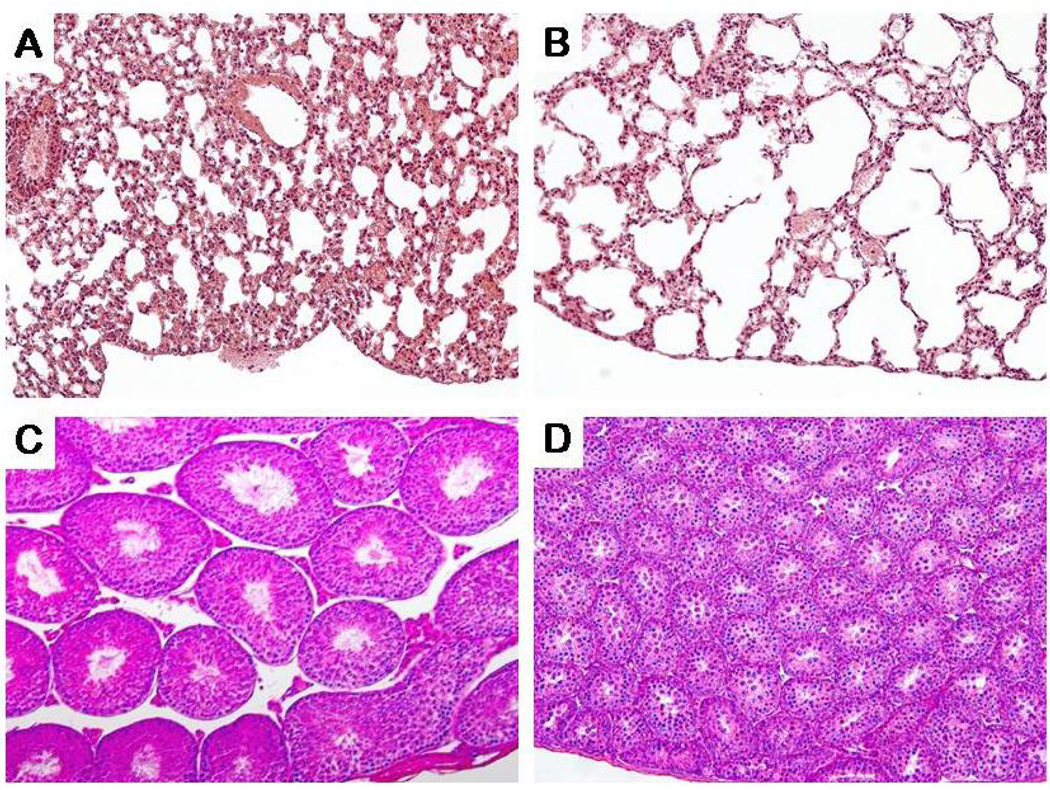
Figure-1. Pulmonary emphysema and testicular atrophy in Fgf-23 null mice.
Histological appearances of control (A) and Fgf-23 null mice (B) lungs, showing dilated alveolar spaces, resembling emphysema in Fgf-23 null mice (B). Histological features of testis, obtained from control (C) and Fgf-23 null mice (D). Note that in vivo genomic ablation of the Fgf-23 gene has resulted in severe atrophic changes in the testis, and the resultant effect being infertility. The histological images are taken from 6 weeks old mice. (Magnification: A and B ×20; C and D ×10).
Effects of Fgf-23 or klotho ablation on vitamin-D homeostasis in mice
Both Fgf-23 null and klotho mutant mice have shown to have increased renal expression of the 1α(OH)ase gene, accompanied by elevated serum levels of active vitamin-D, 1,25(OH)2D3 (Shimada et al., 2004a; Sitara et al., 2004; Sitara et al., 2006; Tsujikawa et al., 2003); a significant rescue of premature aging-like features has been achieved by either reducing vitamin-D activities or genetically ablating vitamin-D activities from Fgf-23 null and klotho mutant mice (Razzaque and Lanske, 2006; Tsujikawa et al., 2003). Reducing vitamin-D activities in klotho ablated mice by feeding a vitamin-D deficient diet has resulted, not only in disappearance of ectopic calcifications, but also in gain of fertility, and most importantly prolonged survival; these observations suggest that the premature aging-like features in klotho mutant mice are downstream events resulting from increased activity of vitamin-D (Tsujikawa et al., 2003). In the same line, when vitamin-D activities were genetically ablated from Fgf-23 null mice by deleting the 1α(OH)ase gene (Fgf-23−/−/1α(OH)ase−/− compound mutants), most of the premature aging-like features in Fgf-23 null mice were rescued (Razzaque and Lanske, 2006; Razzaque et al., 2006; Razzaque et al., 2005); the phenotype of Fgf-23−/−/1α(OH)ase−/− double mutants resulted in the disappearance of ectopic calcifications from heart, kidney, and lung (Fig-2); moreover, the generalized atrophic changes in skin, intestine and other organs of Fgf-23 null mice were rescued in Fgf-23−/−/1α(OH)ase−/− double mutants, and the resultant effect being increased overall survival of vitamin-D ablated Fgf-23 null mice. It is, therefore, reasonable to conclude that most of the premature aging-like features in Fgf-23 null and klotho mutant mice are due to hypervitaminosis-D which is most likely the consequence of lack of activity of its counter regulatory hormone, i.e., Fgf-23 (Liu et al., 2006; Razzaque and Lanske, 2006; Razzaque et al., 2006; Saito et al., 2005).
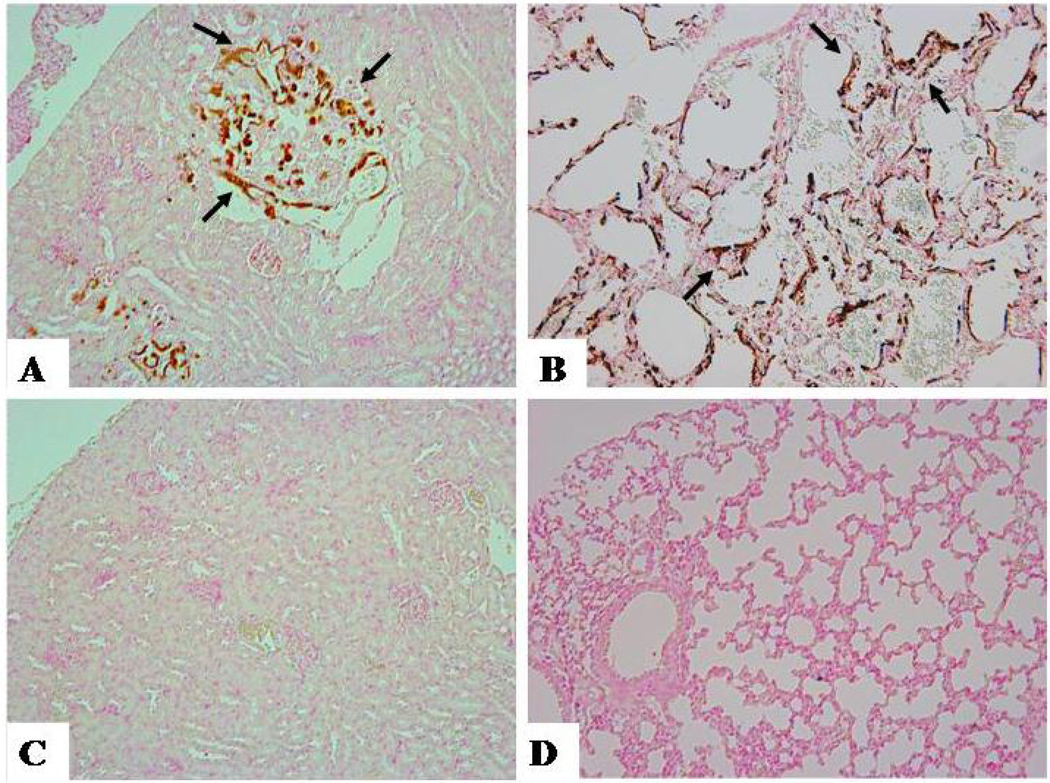
Figure-2. Soft tissue calcification.
von Kossa staining on paraffin sections of the kidney (A, C) and lung (B, D), showing widespread renal (A) and pulmonary (B) calcifications (arrows) in Fgf-23 null mice. Note that in vivo ablation of the 1α-hydroxylase gene from Fgf-23 null mice has eliminated renal (C) and pulmonary (D) calcifications from double null mice (Fgf-23−/−/1α(OH)ase−/−). The histological images are taken from 6 weeks old mice. (Magnification: ×10).
Is klotho an anti-aging factor?
The possible anti-aging effects of klotho were initially suggested from the observation that klotho hypomorph mice develop numerous premature aging-like features; recent studies, however, have clearly shown that premature aging-like phenotypes in klotho ablated mice are caused by abnormal mineral ion homeostasis due to altered regulation of Fgf-23 and vitamin-D. FGF-23 is a counter regulatory molecule of vitamin-D, and loss of Fgf-23 activity results in hypervitaminosis-D in both klotho mutants and Fgf-23 null mice; most of the premature aging-like phenotypes in these mouse strains are caused by the hyperactivity of vitamin-D, as suppression of vitamin-D activities from these mouse models, not only reduces the premature aging-like features, but also extends life span (Razzaque and Lanske, 2006; Razzaque et al., 2006; Tsujikawa et al., 2003). It will be, therefore, interesting to explore the molecular mechanisms of extended survival of klotho over-expressing mice.
Reduced oxidative stress and repressing intracellular signals of insulin and IGF-1 have been suggested to be related to increased survival of klotho transgenic mice (Kurosu et al., 2005). Such speculation is partly based on the observation that compound klotho hypomorphs with insulin receptor substrate-1 deleted mice (kl/IRS-1+/−) could extend life-span, and survived as long as 120 to 130 days after birth (Kurosu et al., 2005). Of relevance, the klotho hypomorphs survive less than 100 days. In the view of the fact that the survival of klotho hypomorphs with reduced vitamin-D activity is actually much longer (more than 180 days without producing any obvious premature aging-like features) (Tsujikawa et al., 2003) than kl/IRS-1+/− mice (Kurosu et al., 2005), the oxidative stress and suppression of insulin signaling cascade, therefore, appear to play a minor role in producing the premature aging-like phenotypes in klotho-deficient mice. Although oxidant stress and IGF-1 activity are known factors that influence the mammalian aging process (Adamo and Farrar, 2006; Edwards et al., 2003; Sonntag et al., 2005; Zha et al., 2006), the involvement of klotho in such regulation requires carefully designed studies.
Again summarizing the available experimental studies, it appears that suppression/elimination of vitamin-D activities from klotho mutant mice, and Fgf-23 null mice could rescue most, if not all, of the premature aging-like phenotypes from these genetically altered mouse lines (Razzaque and Lanske, 2006; Tsujikawa et al., 2003); that include but are not limited to the prevention in the occurrence of atherosclerosis, ectopic calcifications in various soft tissues, osteopenia, skin atrophy, emphysema, and hypogonadism, and the resultant effect being extended survival in both the mutants. Furthermore, altered glucose/insulin homeostasis observed in klotho deficient and Fgf-23 null mice can be markedly improved by reducing vitamin-D activities from these mutants, suggesting that altered glucose/insulin homeostasis in klotho-mutant and Fgf-23 null mice is indeed a secondary effect caused by the increased vitamin-D activities in these mice (Hesse et al., 2006; Tsujikawa et al., 2003). It has, therefore, become increasingly clear that premature aging-like features in klotho mutants are caused by the inability of Fgf-23 to exert its effects, that lead to increased activity of its counter regulatory hormone, 1,25(OH)2D3 (Liu et al., 2006), resulting in altered mineral ion homeostasis to produce most of the premature aging-like features.
Concluding remarks
In this brief article, based on recent studies, we have provided relevant data to explain why we believe that premature aging-like features of klotho mutant mice, a widely known model for aging research, is actually due to the inability of Fgf-23 to exert its function in these mutant mice. Since, klotho acts as a cofactor to propagate Fgf-23 signaling, in klotho mutant mice, despite significantly high levels of Fgf-23, it is unable to exert its function, and therefore exhibits phenotypes that resemble the ones found in Fgf-23 null mice; moreover, lack of Fgf-23 activity in klotho mutant mice eliminates the physiologic counter regulation of Fgf-23 and vitamin-D, resulting in hypervitaminosis-D in these mice to induce premature aging-like features in klotho mutants. We, therefore, speculate that FGF-23, by affecting the activities of vitamin-D might play a major role in the aging process that include but are not limited to senile osteoporosis and vascular calcifications. Finally, in this article, we wanted to highlight two important functional aspects of klotho: 1) is premature aging in klotho mutant mice a primary cause or a secondary consequence? The existing observations clearly suggest that the widespread premature aging-like features of klotho mutant mice are the consequence of resistance to Fgf-23 activity, leading to the vitamin-D hyperactivity. That raises another important question, 2) how klotho might act as an anti-aging molecule, if the premature aging-like features are not the primary cause of its deficiency in klotho mutant mice? Further controlled in vivo studies will explain these complicated, yet clinically important issues of the exact role of klotho in aging.
References
- Adamo ML, Farrar RP. Resistance training, and IGF involvement in the maintenance of muscle mass during the aging process. Ageing Res Rev. 2006;5:310–331. doi: 10.1016/j.arr.2006.05.001. [DOI] [PubMed] [Google Scholar]
- ADHR_Consortium. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. The ADHR Consortium. Nat Genet. 2000;26:345–348. doi: 10.1038/81664. [DOI] [PubMed] [Google Scholar]
- Amaya E, Musci TJ, Kirschner MW. Expression of a dominant negative mutant of the FGF receptor disrupts mesoderm formation in Xenopus embryos. Cell. 1991;66:257–270. doi: 10.1016/0092-8674(91)90616-7. [DOI] [PubMed] [Google Scholar]
- Arking DE, Krebsova A, Macek M, Sr, Macek M, Jr, Arking A, Mian IS, Fried L, Hamosh A, Dey S, McIntosh I, Dietz HC. Association of human aging with a functional variant of klotho. Proc Natl Acad Sci U S A. 2002;99:856–861. doi: 10.1073/pnas.022484299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai X, Miao D, Li J, Goltzman D, Karaplis AC. Transgenic Mice Overexpressing Human Fibroblast Growth Factor 23(R176Q) Delineate a Putative Role for Parathyroid Hormone in Renal Phosphate Wasting Disorders. Endocrinology. 2004;145:5269–5279. doi: 10.1210/en.2004-0233. [DOI] [PubMed] [Google Scholar]
- Benet-Pages A, Orlik P, Strom TM, Lorenz-Depiereux B. An FGF23 missense mutation causes familial tumoral calcinosis with hyperphosphatemia. Hum Mol Genet. 2005;14:385–390. doi: 10.1093/hmg/ddi034. [DOI] [PubMed] [Google Scholar]
- Edwards MG, Sarkar D, Klopp R, Morrow JD, Weindruch R, Prolla TA. Age-related impairment of the transcriptional responses to oxidative stress in the mouse heart. Physiol Genomics. 2003;13:119–127. doi: 10.1152/physiolgenomics.00172.2002. [DOI] [PubMed] [Google Scholar]
- Frishberg Y, Ito N, Rinat C, Yamazaki Y, Feinstein S, Urakawa I, Navon-Elkan P, Becker-Cohen R, Yamashita T, Araya K, Igarashi T, Fujita T, Fukumoto S. Hyperostosis - Hyperphosphatemia Syndrome: A Congenital Disorder of O-Glycosylation Associated with Augmented Processing of Fibroblast Growth Factor 23. J Bone Miner Res. 2006 doi: 10.1359/jbmr.061105. doi: 10.1359/jbmr.061105. [DOI] [PubMed] [Google Scholar]
- Gaasbeek A, Meinders AE. Hypophosphatemia: an update on its etiology and treatment. Am J Med. 2005;118:1094–1101. doi: 10.1016/j.amjmed.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Hesse M, Frohlich LF, Zeitz U, Lanske B, Erben RG. Ablation of vitamin D signaling rescues bone, mineral, and glucose homeostasis in Fgf-23 deficient mice. Matrix Biology. 2006 doi: 10.1016/j.matbio.2006.10.003. In Press. [DOI] [PubMed] [Google Scholar]
- Kuro-o M. Disease model: human aging. Trends Mol Med. 2001;7:179–181. doi: 10.1016/s1471-4914(01)01921-9. [DOI] [PubMed] [Google Scholar]
- Kuro-o M. Klotho as a regulator of fibroblast growth factor signaling and phosphate/calcium metabolism. Curr Opin Nephrol Hypertens. 2006;15:437–441. doi: 10.1097/01.mnh.0000232885.81142.83. [DOI] [PubMed] [Google Scholar]
- Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuro-o M. Suppression of aging in mice by the hormone Klotho. Science. 2005;309:1829–1833. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson T, Marsell R, Schipani E, Ohlsson C, Ljunggren O, Tenenhouse HS, Juppner H, Jonsson KB. Transgenic mice expressing fibroblast growth factor 23 under the control of the alpha1(I) collagen promoter exhibit growth retardation, osteomalacia, and disturbed phosphate homeostasis. Endocrinology. 2004;145:3087–3094. doi: 10.1210/en.2003-1768. [DOI] [PubMed] [Google Scholar]
- Li SA, Watanabe M, Yamada H, Nagai A, Kinuta M, Takei K. Immunohistochemical localization of Klotho protein in brain, kidney, and reproductive organs of mice. Cell Struct Funct. 2004;29:91–99. doi: 10.1247/csf.29.91. [DOI] [PubMed] [Google Scholar]
- Liu S, Tang W, Zhou J, Stubbs JR, Luo Q, Pi M, Quarles LD. Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. J Am Soc Nephrol. 2006;17:1305–1315. doi: 10.1681/ASN.2005111185. [DOI] [PubMed] [Google Scholar]
- Magagnin S, Werner A, Markovich D, Sorribas V, Stange G, Biber J, Murer H. Expression cloning of human and rat renal cortex Na/Pi cotransport. Proc Natl Acad Sci U S A. 1993;90:5979–5983. doi: 10.1073/pnas.90.13.5979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi M, Olsen SK, Goetz R. A protein canyon in the FGF-FGF receptor dimer selects from an a la carte menu of heparan sulfate motifs. Curr Opin Struct Biol. 2005a;15:506–516. doi: 10.1016/j.sbi.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Mohammadi M, Olsen SK, Ibrahimi OA. Structural basis for fibroblast growth factor receptor activation. Cytokine Growth Factor Rev. 2005b;16:107–137. doi: 10.1016/j.cytogfr.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Nabeshima Y. Klotho: a fundamental regulator of aging. Ageing Res Rev. 2002;1:627–638. doi: 10.1016/s1568-1637(02)00027-2. [DOI] [PubMed] [Google Scholar]
- Nabeshima Y. Toward a better understanding of Klotho. Sci Aging Knowledge Environ. 2006;2006:pe11. doi: 10.1126/sageke.2006.8.pe11. [DOI] [PubMed] [Google Scholar]
- Ornitz DM, Yayon A, Flanagan JG, Svahn CM, Levi E, Leder P. Heparin is required for cell-free binding of basic fibroblast growth factor to a soluble receptor and for mitogenesis in whole cells. Mol Cell Biol. 1992;12:240–247. doi: 10.1128/mcb.12.1.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzaque MS, Lanske B. Hypervitaminosis D and premature aging: lessons learned from Fgf23 and Klotho mutant mice. Trends Mol Med. 2006;12:298–305. doi: 10.1016/j.molmed.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Razzaque MS, Sitara D, Taguchi T, St-Arnaud R, Lanske B. Premature aging-like phenotype in fibroblast growth factor 23 null mice is a vitamin D-mediated process. Faseb J. 2006;20:720–722. doi: 10.1096/fj.05-5432fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzaque MS, St-Arnaud R, Taguchi T, Lanske B. FGF-23, vitamin D and calcification: the unholy triad. Nephrol Dial Transplant. 2005;20:2032–2035. doi: 10.1093/ndt/gfh991. [DOI] [PubMed] [Google Scholar]
- Saito H, Maeda A, Ohtomo S, Hirata M, Kusano K, Kato S, Ogata E, Segawa H, Miyamoto K, Fukushima N. Circulating FGF-23 is regulated by 1alpha,25-dihydroxyvitamin D3 and phosphorus in vivo. J Biol Chem. 2005;280:2543–2549. doi: 10.1074/jbc.M408903200. [DOI] [PubMed] [Google Scholar]
- Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, Fukumoto S, Tomizuka K, Yamashita T. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest. 2004a;113:561–568. doi: 10.1172/JCI19081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T, Mizutani S, Muto T, Yoneya T, Hino R, Takeda S, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci U S A. 2001;98:6500–6505. doi: 10.1073/pnas.101545198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T, Muto T, Urakawa I, Yoneya T, Yamazaki Y, Okawa K, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T. Mutant FGF-23 responsible for autosomal dominant hypophosphatemic rickets is resistant to proteolytic cleavage and causes hypophosphatemia in vivo. Endocrinology. 2002;143:3179–3182. doi: 10.1210/endo.143.8.8795. [DOI] [PubMed] [Google Scholar]
- Shimada T, Urakawa I, Yamazaki Y, Hasegawa H, Hino R, Yoneya T, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T. FGF-23 transgenic mice demonstrate hypophosphatemic rickets with reduced expression of sodium phosphate cotransporter type IIa. Biochem Biophys Res Commun. 2004b;314:409–414. doi: 10.1016/j.bbrc.2003.12.102. [DOI] [PubMed] [Google Scholar]
- Sitara D, Razzaque MS, Hesse M, Yoganathan S, Taguchi T, Erben RG, H, J. A.-P, Lanske B. Homozygous ablation of fibroblast growth factor-23 results in hyperphosphatemia and impaired skeletogenesis, and reverses hypophosphatemia in Phex-deficient mice. Matrix Biol. 2004;23:421–432. doi: 10.1016/j.matbio.2004.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitara D, Razzaque MS, St-Arnaud R, Huang W, Taguchi T, Erben RG, Lanske B. Genetic Ablation of Vitamin D Activation Pathway Reverses Biochemical and Skeletal Anomalies in Fgf-23-Null Animals. Am J Pathol. 2006;169:2161–2170. doi: 10.2353/ajpath.2006.060329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag WE, Ramsey M, Carter CS. Growth hormone and insulin-like growth factor-1 (IGF-1) and their influence on cognitive aging. Ageing Res Rev. 2005;4:195–212. doi: 10.1016/j.arr.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Tenenhouse HS. Regulation of phosphorus homeostasis by the type iia na/phosphate cotransporter. Annu Rev Nutr. 2005;25:197–214. doi: 10.1146/annurev.nutr.25.050304.092642. [DOI] [PubMed] [Google Scholar]
- Traebert M, Volkl H, Biber J, Murer H, Kaissling B. Luminal and contraluminal action of 1–34 and 3-34 PTH peptides on renal type IIa Na-P(i) cotransporter. Am J Physiol Renal Physiol. 2000;278:F792–F798. doi: 10.1152/ajprenal.2000.278.5.F792. [DOI] [PubMed] [Google Scholar]
- Tsujikawa H, Kurotaki Y, Fujimori T, Fukuda K, Nabeshima Y. Klotho, a gene related to a syndrome resembling human premature aging, functions in a negative regulatory circuit of vitamin D endocrine system. Mol Endocrinol. 2003;17:2393–2403. doi: 10.1210/me.2003-0048. [DOI] [PubMed] [Google Scholar]
- Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444:770–774. doi: 10.1038/nature05315. [DOI] [PubMed] [Google Scholar]
- Yu X, Ibrahimi OA, Goetz R, Zhang F, Davis SI, Garringer HJ, Linhardt RJ, Ornitz DM, Mohammadi M, White KE. Analysis of the biochemical mechanisms for the endocrine actions of fibroblast growth factor-23. Endocrinology. 2005;146:4647–4656. doi: 10.1210/en.2005-0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zha Y, Le VT, Higami Y, Shimokawa I, Taguchi T, Razzaque MS. Lifelong suppression of growth hormone-insulin-like growth factor I activity in genetically altered rats could prevent age-related renal damage. Endocrinology. 2006;147:5690–5698. doi: 10.1210/en.2006-0302. [DOI] [PubMed] [Google Scholar]