Abstract

Homoallylation of aldehydes with isoprene and triethyl borane, catalyzed by Ni(acac)2 gave hydroxy alkenes in good yield with excellent regio- and stereoselectivity. Cross metathesis of the hydroxy alkenes with methyl acrylate using second generation Grubbs catalyst and copper(I) iodide afforded α,β-unsaturated esters, which underwent cyclization in the presence of DBU to produce tetrahydrofurans with the correct relative configuration for C1-C9 fragment of amphidinolide C, C2 and F.
The symbiotic marine dinoflagellate Amphidinium sp., isolated from the cells of aceol flatworms Amphiscolops sp., produces a structurally diverse group of macrolide natural products.1 This group of macrolides, named amphidinolides, contains over 30 compounds and the majority of them possess some level of cytotoxicity.
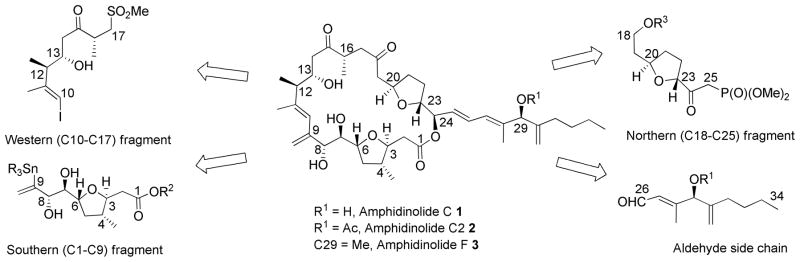
Amphidinolide C (1) (Scheme 1), isolated from the Y-5 strain of Amphidinium sp, is one of the most cytotoxic members of the amphidinolide family.2 Embedded within this macrolide are two 2,5-trans tetrahydrofuran rings and 12 stereocenters. Amphidinolide C was shown to possess cytotoxicity against murine lymphoma L1210 (IC50 0.0058 μg/mL) and human epidermoid carcinoma KB (IC50 0.0046 μg/mL) in vitro. Interestingly, amphidinolide C2 (2) and F (3), which vary only in the structure of the side chain, are close to a thousand fold less active.
Scheme 1.
Retrosynthetic analysis of amphidinolide C
Due to their unique structure and noteworthy biological activity, amphidinolides C (1), C2 (2) and F (3) have become targets for synthesis. The syntheses of several fragments of these molecules have been reported, but they have yet to succumb to total synthesis.3
Our retrosynthetic analysis (Scheme 1) for amphidinolide C divides the molecule into four different subunits; the northern (C18-25), the southern (C1-C9) and the western (C10-C17) subunits, and the side chain (C26-C34). In this report, we describe the synthesis of the tetrahydrofuran (thf) ring of C1-C9 fragment of amphidinolide C, C2 and F.
The C1-C9 fragment of amphidinolide C contains a 2,3,5 trisubstituted tetrahydrofuran with a methyl substituent at the 3 position (4 position in amphidinolide C numbering). The stereochemical relationship between the substituents is 2,5- and 2,3-trans and 3,5-cis. Similar 3-methyl substituted tetrahydrofuran moieties are found in several other natural products, e.g. monensin A,4 amphidinolide T15 and T3,6 tetronasin,7 gambieric acid8 and gymnodimine9.
A particular challenge is finding an efficient method to install the methyl group in an efficient manner. A retrosynthetic analysis (Scheme 2) reveals that unfolding of the tetrahydrofuran ring results in an unsaturated alcohol with 1,3-anti relationship between the methyl and hydroxyl groups. In the forward sense, the tetrahydrofuran can be formed by the addition of the alcohol across the double bond. The cis orientation of R1 and the methyl group in the transition state for cyclization would direct the developing stereocenter at position 2 trans relative to the existing stereocenters (positions 3 and 5), resulting in the required 2,5-trans tetrahydrofuran ring. Substituted 1,3-anti unsaturated alcohols can be prepared very efficiently from appropriately functionalized aldehydes using the highly diastereoselective nickel-catalyzed homoallylation chemistry reported by Tamaru et al.10 Herein, we report the results of the Tamaru reaction with a series of novel electrophiles with specific application to the synthesis of the C1-C6 tetrahydrofuran fragment from amphidiolide C, C2 and F.
Scheme 2.
Retrosynthetic analysis of the C1-C9 fragment
The known homoallylation of benzaldehyde was used to initiate a model study. The reaction of benzaldehyde with isoprene catalyzed by Ni(acac)2 and promoted by triethyl borane yielded homoallyl alcohol 4 with 1,3 anti to syn ratio >15:1 (Scheme 3).10a Palladium catalyzed cyclization/methoxycarbonylation of the hydroxy alkene 4 using modified Semmelhack conditions11 gave the tetrahydrofuran 6 in 68% yield with 2,5-trans to cis ratio 9:1.
Scheme 3.
Synthesis of tetrahydrofuran ring
Alternatively, cross metathesis of the hydroxy alkene 4 with methyl acrylate in the presence of second generation Grubbs catalyst and copper(I) iodide12 gave the α,β-unsaturated methyl ester 5 in 79% yield. Cyclization of 5 with 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU)13 in CH2Cl2 gave 2,5-trans-tetrahydrofuran 6 as a single diastereomer in an excellent 93% yield. Thus, using either method, the required 2,5-trans-tetrahydrofuran with correct relative configuration for the C1-C9 fragment of amphidinolide C was achieved.
The next challenge was to identify a suitable aldehyde that contained, or that had suitable functionality for introducing, the 1,2-anti diol found at positions 7 and 8 of ampidinolide C. Initial efforts focused on the hemiacetal derivatives of erythronolactone.
Homoallylation of the bis tert- butyldimethylsilyl (TBS) protected hemiacetal 8 provided two diastereomers 9a and 9b in 63% combined yield with 1:6 diastereoselectivity (Scheme 4). The diastereomers were separated by silica gel column chromatography. The major diastereomer 9b was crystalline and could be recrystallized to give x-ray quality crystals.
Scheme 4.
Homoallylation of erythrolactol
The crystal structure of major diastereomer 9b (see supporting information) clearly shows that the silyloxy groups at C2 and C3 are anti and the hydroxyl and methyl groups at C4 and C6 are anti. However, the silyloxy at C3 and and the hydroxy at C4 have the wrong relative configuration (anti) and thus the minor isomer 9a has the correct relative (3,4-syn) and absolute configuration required for amphidinolide C.
The observed diastereofacial selectivity was much higher than expected for the homoallylation reaction. In the limited number of chiral aldehydes investigated by Tamaru et al., the diastereofacial selectivity with respect to the aldehyde was typically very low (1:1 to 1.6 :1).10c The formation of the major isomer 9b appears to follow the trend observed by Evans et al. in the aldol reactions of a series of alkoxy and bisalkoxy aldehydes.14 A Cornforth model15 was used to explain stereochemical outcome of the addition of the enolate to the aldehyde.
Undaunted by this setback, the cyclization of both diastereomers 9 was investigated. The Semmelhack palladium-catalyzed cyclization/methoxycarbonylation of major isomer 9b failed to yield the tetrahydrofuran 14b. Therefore, the alternate chain extension and cyclization method was examined. Cross metathesis of both the minor isomer 9a and the major isomer 9b with methyl acrylate gave α,β-unsaturated methyl ester 13a and 13b, respectively (Scheme 5). Cyclization of 13a and 13b using DBU afforded the tetrahydrofurans 14a and 14b. Some migration of silyl group was observed during the cyclization process. The TBS group migrated from the secondary 14 to the primary position 15 (Scheme 5).16
Scheme 5.
Cross metathesis and cyclization
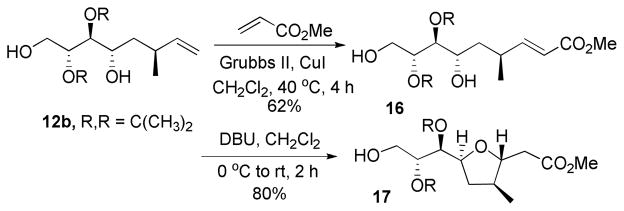
In order to avoid protecting group migration and perhaps effect a more favorable stereochemical outcome, an alternate protecting group was examined. Homoallylation of acetonide protected hemiacetal 11 (Scheme 4) gave a mixture of diastereomers 12a and 12b in a 1:3 ratio. The diastereomers were separable by repeated silica gel column chromatography. Cross metathesis of the major diastereomer 12b with methyl acrylate (Scheme 6) followed by cyclization with DBU gave 17 in 80% yield.
Scheme 6.
Cross metathesis and cyclization
To compare the configuration of the tetrahydrofurans 17 and 14b obtained from the acetonide protected hemiacetal 11 and the TBS protected hemiacetal 8, the protecting groups were removed (Scheme 7). The TBS groups were cleaved using HF/pyridine in MeOH, which provided the triol 18 in 70% yield. The acetonide group was removed using Amberlyst 15 resin in MeOH to give the triol 18 in 60% yield. The 1H and 13C spectra of the triol 18 obtained by deprotection of both 14b and 17 were identical, suggesting that the tetrahydrofuran obtained from both hemiacetals have the same configuration.
Scheme 7.
Deprotection of TBS and acetonide groups
Next, our attention turned to epoxy aldehydes. Epoxides are versatile functional groups that can be opened with water to reveal a diol or oxidatively cleaved to obtain an aldehyde. As a consequence, epoxycinnamaldehyde 19 was selected as the next substrate for homoallylation. Oxidation of commercially available (2R,3R)-3-phenylglycidol (epoxycinnamyl alcohol)17 gave (2R,3R)-3-phenylglycidal 19 in 73% yield. Homoallylation of 19 gave a separable mixture of diastereomers 20 in a 2.5:1 ratio and 63% combined yield (Scheme 8). The absolute configuration of the diastereomers was determined by the Mosher’s ester method.18 The major isomer 20a possessed the correct configuration for the tetrahydrofuran ring of amphidinolide C.
Scheme 8.
Homoallylation, cross metathesis and cyclization
Cross metathesis of 20a with methyl acrylate (or ethyl acrylate) gave α,β-unsaturated methyl (or ethyl) ester 21a (or 21b) which on cyclization with DBU gave 22a (or 22b) as a single isomer. Cleavage of the epoxide in tetrahydrofuran 22a (or 22b) with NaIO4 in acetonitrile/water (2:1)19 gave aldehyde 23a (or 23b) which could be isolated or subsequently reduced in situ with NaBH4 in MeOH to afford alcohol 24a (or 24b) in 64% overall yield (Scheme 8). The enantiomers of alcohol 24a could be separated by GC on a chiral stationary phase. The alcohol (24a) derived from the nonracemic glycidol had an enantiomeric excess of >95%. The tetrahydrofuran aldehyde 23b and alcohol 24b had 1H and 13C NMR spectra and optical rotation matching those reported by Roush and coworkers.3a
Aldehyde 23b has been elaborated into the C1-C9 fragment of amphidinolide C by Roush and coworkers.3a Therefore, the synthesis of tetrahydrofuran alcohol 24b and aldehyde 23b constitute a formal synthesis of the C1-C9 fragment of amphidinolide C. The aldehyde 23b was prepared in 5 steps from the commercially available phenyl glycidol in 12% overall yield. Roush reported two routes to 23b. The first approach gave 23b in 13 steps in <7% yield (17 from commercial materials) and the second approach gave 23b in 10 from commercial materials in 21% overall yield. We have provided a shorter synthesis, but with a lower overall yield.
Supplementary Material
Acknowledgments
This work was supported by grant number R01-GM076192 from National Institute of General Medical studies. We are also grateful to the National Science Foundation for a grant to purchase the X-ray diffractometer (CHE-9309690). We thank Prof. R. E. K. Winter and Mr. Joe Kramer of the Department of Chemistry and Biochemistry, University of Missouri-St. Louis for mass spectra.
Footnotes
Supporting Information Available: Detailed experimental procedure and spectral data of all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Kobayashi J, Ishibashi M. Chem Rev. 1993;93:1753. [Google Scholar]; (b) Tsuda M, Izui N, Shimbo K, Sato M, Fukushi E, Kawabata J, Kobayashi J. J Org Chem. 2003;68:9109. doi: 10.1021/jo035278z. [DOI] [PubMed] [Google Scholar]; (c) Kobayashi J, Tsuda M. Nat Prod Rep. 2004;21:77. doi: 10.1039/b310427n. [DOI] [PubMed] [Google Scholar]
- 2.(a) Kobayashi J, Ishibashi M, Walchli MR, Nakamura H, Hirata Y, Sasaki T, Ohizumi Y. J Am Chem Soc. 1988;110:490. [Google Scholar]; (b) Kubota T, Tsuda M, Kobayashi J. Org Lett. 2001;3:1363. doi: 10.1021/ol015741z. [DOI] [PubMed] [Google Scholar]
- 3.(a) Bates RH, Shotwell JB, Roush WR. Org Lett. 2008;10:4343. doi: 10.1021/ol801852j. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Shotwell JB, Roush WR. Org Lett. 2004;6:3865. doi: 10.1021/ol048381z. [DOI] [PubMed] [Google Scholar]; (c) Kubota T, Tsuda M, Kobayashi J. Tetrahedron. 2003;59:1613. [Google Scholar]; (d) Armstrong A, Pyrkotis C. Tetrahedron Lett. 2009;50:3325. [Google Scholar]; (e) Mohapatra DK, Dasari PK, Rahaman H, Pal R. Tetrahedron Lett. 2009;50:6276. [Google Scholar]; (f) Mohapatra DK, Rahaman H, Chorghade MS, Gurjar MK. Synlett. 2007;4:567. [Google Scholar]; (g) Mahapatra S, Carter RG. Org Biomol Chem. 2009;7:4582. doi: 10.1039/b916744g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haney ME, Hoehn MM. Antimicrob Agents Chemother. 1968;7:349. doi: 10.1128/AAC.7.3.349. [DOI] [PubMed] [Google Scholar]
- 5.Tsuda M, Endo T, Kobayashi J. J Org Chem. 2000;65:1349. doi: 10.1021/jo991393r. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi J, Kubota T, Endo T, Tsuda M. J Org Chem. 2001;66:134. doi: 10.1021/jo005607c. [DOI] [PubMed] [Google Scholar]
- 7.Davies DH, Snape EW, Suter PJ, King TJ, Falshaw CP. J Chem Soc Chem Comm. 1981;1073 [Google Scholar]
- 8.Nagai H, Torigoe K, Satake M, Murata M, Yasumoto T, Hirota H. J Am Chem Soc. 1992;114:1102. [Google Scholar]
- 9.Seki T, Satake M, Mackenzie L, Kaspar HF, Yasumoto T. Tetrahedron Lett. 1995;36:7093. [Google Scholar]
- 10.(a) Kimura M, Ezoe A, Shibata K, Tamaru Y. J Am Chem Soc. 1998;120:4033. [Google Scholar]; (b) Tamaru Y, Kimura M, Fujimatsu H, Ezoe A, Shibata K, Shimizu M, Matsumoto S. Angew Chem Int Ed. 1999;30:397. doi: 10.1002/(SICI)1521-3773(19990201)38:3<397::AID-ANIE397>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]; (c) Kimura M, Ezoe A, Mori M, Iwata K, Tamaru Y. J Am Chem Soc. 2006;128:8559. doi: 10.1021/ja0608904. [DOI] [PubMed] [Google Scholar]; (d) Kimura M, Ezoe A, Tanaka S, Tamaru Y. Angew Chem Int Ed. 2001;40:3600. doi: 10.1002/1521-3773(20011001)40:19<3600::aid-anie3600>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 11.(a) Semmelhack MF, Zhang N. J Org Chem. 1989;54:4483. [Google Scholar]; (b) Semmelhack MF, Bodurow C. J Am Chem Soc. 1984;106:1496. [Google Scholar]
- 12.(a) Chatterjee AK, Choi TL, Sanders DP, Grubbs RH. J Am Chem Soc. 2003;125:11360. doi: 10.1021/ja0214882. [DOI] [PubMed] [Google Scholar]; (b) Rivard M, Blechert S. Eur J Org Chem. 2003:2225. [Google Scholar]; (c) He A, Yan B, Thanavaro A, Spillling CD, Rath NP. J Org Chem. 2004;69:8643. doi: 10.1021/jo0490090. [DOI] [PubMed] [Google Scholar]
- 13.Abbineni C, Sasmal PK, Mukkanti K, Iqbal J. Tetrahedron Lett. 2007;48:4259. [Google Scholar]
- 14.Evans DA, Cee VJ, Siska SJ. J Am Chem Soc. 2006;128:9433. doi: 10.1021/ja061010o. [DOI] [PubMed] [Google Scholar]
- 15.(a) Cornforth JW, Cornforth RH, Mathew KK. J Chem Sec. 1959:112. [Google Scholar]; (b) Burgi HB, Dunitz JD, Shefter E. J Am Chem Soc. 1973;95:5065. [Google Scholar]; (c) Burgi HB, Dunitz JD, Lehn JM, Wipff G. Tetrahedron. 1974;30:1563. [Google Scholar]
- 15.(a) Ogilvie KK, Entwistle DW. Carbohydr Res. 1981;89:203. [Google Scholar]; (b) Mulzer J, Schollhorn B. Angew Chem Int Ed. 1990;29:431. [Google Scholar]; (c) Wuts PGM, Bigelow SS. J Org Chem. 1988;53:5023. [Google Scholar]; (d) Crich D, Ritchie TJ. Carbohydr Res. 1990;197:324. ref. therein. [Google Scholar]
- 16.Chow KYK, Bode JW. J Am Chem Soc. 2004;126:8126. doi: 10.1021/ja047407e. [DOI] [PubMed] [Google Scholar]
- 17.(a) Mosher HS, Dale JA. J Am Chem Soc. 1973;95:512. [Google Scholar]; (b) Kubota T, Tsuda M, Kobayashi Y. Tetrahedron. 2003;59:1613. [Google Scholar]; (c) Hammerschmidt F, Li YF. Tetrahedron. 1994;50:10253. [Google Scholar]
- 18.Binder CM, Dixon DD, Almaraz E, Tius MA, Singaram B. Tetrahedron Lett. 2008;49:2764. doi: 10.1016/j.tetlet.2008.02.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.