Abstract
Borrelia burgdorferi bb0323 encodes an immunogenic protein in mammalian hosts including humans. An analysis of bb0323 expression in vivo showed variable transcription throughout the spirochete infection cycle, with elevated expression during tick-mouse transmission. Deletion of bb0323 in infectious B. burgdorferi did not affect microbial survival in vitro, despite significant alterations in growth kinetics and cell morphology. bb0323 mutants were unable to infect either mice or ticks, and were quickly eliminated from immunocompetent and immunodeficient hosts and the vector, within the first few days of inoculation. Chromosomal complementation of the mutant with native bb0323 and phenotypic analysis in vivo indicated the significant restoration of spirochete virulence and persistence throughout the mouse-tick infection cycle. BB0323 may serve an indispensable physiological function that is more pronounced during microbial persistence and transitions between the host and the vector in vivo. Strategies to interfere with BB0323 function may interrupt the infectious cycle of spirochetes.
Keywords: Lyme disease, Borrelia burgdorferi, microbial pathogenesis
INTRODUCTION
Lyme borreliosis remains an increasing arthropod-borne health threat in humans and animals in many parts of the world [1]. The disease is caused by the tick-borne spirochete Borrelia burgdorferi, which survives in a wide range of vertebrate hosts and, remarkably, in many organ locations within a given host. Clinical complications of Lyme borreliosis result from pathogen-induced host inflammatory responses. While the natural hosts for B. burgdorferi are asymptomatic, a selective set of incidental hosts, including humans, may develop multi-system diseases including skin rashes, arthritis, carditis, and neuropathy [2, 3]. Antibiotic therapy for Lyme disease is usually curative; however, patients with persistent infections often require intense antibiotic treatment and, in some cases, infection continues for months or years after antibiotic therapy. Moreover, a vaccine to prevent the incidence of human Lyme disease is not currently available.
The B. burgdorferi genome contains approximately 1780 genes, many of which encode hypothetical proteins of undefined functions [4, 5] and are differentially produced in the arthropod-mammal infection cycle of the spirochete [6]. The spirochete is known to alter gene expression patterns when exposed to altered growth conditions [7–11] or when encountering a new host environment [12]. Thus, investigators have used gene expression patterns as a surrogate to understand the role of spirochete genes in host infectivity and transmission. Genes that are selectively expressed in specific phases of the B. burgdorferi life cycle may encode proteins of functional significance, as shown by recently-developed genetic studies [13] and identified a handful of B. burgdorferi genes that support B. burgdorferi persistence in a natural infection cycle, such as pncA [14], adeC [15], bmpA/B [16], ospA/B [17], ospC [18, 19] dbpA/B [20, 21], bbk32 [22, 23], bptA [24], bb0365 [25] and bb0690 [26]. B. burgdorferi genes have also been identified that are differentially expressed in vivo, such as ospD, luxS, BbCRASP-2, and chbC, but lack an essential role in the spirochete natural infection cycle [27–31]. Therefore, the development of preventive measures against Lyme disease will be contributed by the continued efforts to identify immunogenic antigens that are essential for microbial virulence and infectivity.
bb0323 encodes a hypothetical lipoprotein [4, 5] that has been identified as a membrane-associated immunogenic protein in mammalian hosts including humans [32, 33]. An earlier study on genome-wide transposon mutagenesis of non-infectious B. burgdorferi yielded a bb0323 disrupted clone with obvious defects in its outer membrane integrity [34]. Based on the studies in immunogenicity and outer membrane localization of BB0323, we assessed the role of BB0323 in the life cycle of spirochetes and have shown that the antigen is essential for B. burgdorferi virulence, persistence and transmission through the enzootic infection cycle of the pathogen.
MATERIALS AND METHODS
Borrelia burgdorferi, ticks and mice
B. burgdorferi infectious isolate, B31-A3 [35], was used throughout this study. Four- to six-week-old C3H/HeN mice and NCr-SCID mice were purchased from the National Institutes of Health. Ixodes scapularis ticks used in this study originated from a colony that is maintained in the laboratory [27]. Animal experiments were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee and Institutional Bio-safety Committee.
PCR
The primers used in specific PCR reactions are indicated in supplementary Table 1. Isolation of RNA and RT-PCR or quantitative RT-PCR (qRT-PCR) analysis was performed as described [27]. bb0323 transcripts were analyzed by qRT-PCR in groups of 5 C3H mice (105 spirochetes/mouse) at 7, 14, 21 or 28 days of infection or in larval and nymphal ticks that fed on 14-day infected mice (20 ticks/mouse) as described [17, 19, 36, 37]. Ticks (whole larva and dissected nymphal gut) were analyzed at days 1, 2 or 3 of feeding or 25 days after feeding. For assessment of bb0323 expression during transmission, infected nymphs were fed on naïve mice (20 ticks/mice, 3 mice/group), and dissected tissues were analyzed at days 1, 2 or 3 of feeding as described [17, 19, 36, 37]. The levels of bb0323 transcript were normalized against flaB transcripts.
Protein expression, preparation of BB0323 antiserum and immunoblotting
The bb0323 gene was cloned into pGEX-6P-1 (Amersham-Pharmacia Biotech) using specific primers (supplementary Table 1) and the recombinant protein without the N-terminal leader sequence was produced in E. coli. Expression, purification and enzymatic cleavage of the glutathione S-transferase (GST) fusion protein were performed as described previously [16]. Generation of polyclonal murine antiserum against recombinant BB0323 (without the GST tag) and assessment of titer and specificity of the antisera, using ELISA and immunoblotting, were performed as described [27].
Preparation of outer membrane vesicles and Proteinase K accessibility assay
Preparation of outer membrane (OM) vesicles, immuno-detection of membrane proteins and proteinase K accessibility assay were performed as described [27, 38] and further detailed in the supplementary text.
Generation of bb0323 mutant and complemented isolates of B. burgdorferi
Genetic manipulation of B. burgdorferi was performed according to published procedures [27] using the primers listed in supplementary Table 1, and further detailed in the supplementary text. One of the bb0323 knockout isolates retaining the same set of plasmids as the wild type isolate [16, 27] was selected for further experiments. Wild type and bb0323 mutants were also processed for transmission electron microscopy as described earlier [36]. Genetic complementation of the bb0323 mutant was achieved by re-insertion of a wild type copy of the bb0323 gene in the B. burgdorferi chromosome, as described [26].
Phenotypic analysis of bb0323 mutant and complemented isolates
Spirochete burdens in mice and ticks were assessed using qRT-PCR analysis of flaB gene and normalized against murine or tick β-actin genes as described [27]. C3H mice were infected with B. burgdorferi (105 spirochetes/mouse), and sacrificed at days 7, 14, 21 and 28 following inoculation. Groups of SCID mice (3 mice/group) were also similarly infected with spirochetes and analyzed at 14 days after infection. The skin, heart and joint were stored in liquid nitrogen, and aliquots of blood and spleen tissues were cultured in BSK medium to test for the presence of viable spirochetes. Pathogen burdens were assessed in ticks (20 ticks/mouse) that fed on infected mice following two weeks of infection as described [16, 19, 25]. Naturally-infected or microinjected nymphs were also generated, and spirochete burdens in dissected gut were determined by confocal microscopy as described [17, 19]. Infected ticks were fed on naïve mice (5 ticks/mice, 6 mice/group) and B. burgdorferi burdens in whole ticks or dissected gut and salivary glands were determined by qRT-PCR at different time points. To assess the capability of spirochetes to transmit from ticks to mice, infected nymphs were allowed to feed to repletion (3 ticks/mouse, 5 mice/group). The engorged ticks were subjected to qRT-PCR analysis to assess the infection. At day 14 following tick feeding, all the mice were sacrificed, and the tissues were isolated and assessed for the spirochete burden by qRT-PCR.
Evaluation of arthritis
B. burgdorferi-infected mice were examined for swelling of the tibiotarsal joints as detailed earlier [16] and further detailed in the supplementary text. Joint sections were assessed for histological parameters of B. burgdorferi-induced inflammation, as described [39, 40].
Bioinformatics and Statistical analysis
Bioinformatic analysis and gene/protein annotations are detailed in the supplementary text. Results are expressed as the mean ± standard error (SEM). The significance of the difference between the mean values of the groups was evaluated by two-tailed Student’s t-test.
RESULTS
Expression of bb0323 throughout the mouse-tick infection cycle of B. burgdorferi
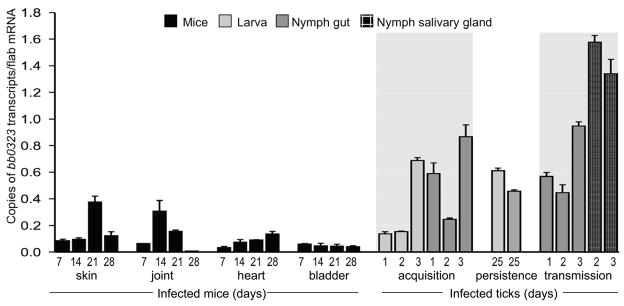
To understand the role of BB0323, we first assessed the temporal and spatial expression of bb0323 in an animal model of B. burgdorferi enzootic cycle. C3H mice were infected with B. burgdorferi and skin, joint, heart and bladder samples were collected at days 7, 14, 21 or 28 following infection. Larval and nymphal ticks were fed on parallel groups of 14-day-infected mice (25 ticks/mouse) and engorged ticks were isolated following 1, 2 or 3 days of feeding. One group of fed intermolt larva was collected at day 25 after feeding, while another group was allowed to molt to nymphs. The unfed infected nymphs were allowed to feed on naïve C3H mice (20 ticks/mice), and their gut and salivary glands were isolated following 1, 2 or 3 days of feeding. Quantitative RT-PCR analysis indicated a lower bb0323 expression in mice, but the gene is highly expressed in infected ticks (figure 1). The highest level of bb0323 expression was noted during transmission of spirochetes from ticks to the murine host. In agreement with an earlier report [32], infected mice developed antibody response against BB0323 (data not shown).
Figure 1. Consistent expression of bb0323 throughout the enzootic life cycle of B. burgdorferi.
Total RNA was isolated from multiple tissues covering the major stages of mouse-tick infection cycle and bb0323 transcripts were measured using quantitative RT-PCR and presented as copies of bb0323 transcript per copy of flaB transcript. Shaded area denotes duration of tick feeding. Error bars represent the mean ± SEM from four quantitative RT-PCR analyses of two independent murine-tick infection experiments.
Generation of bb0323 mutant B. burgdorferi
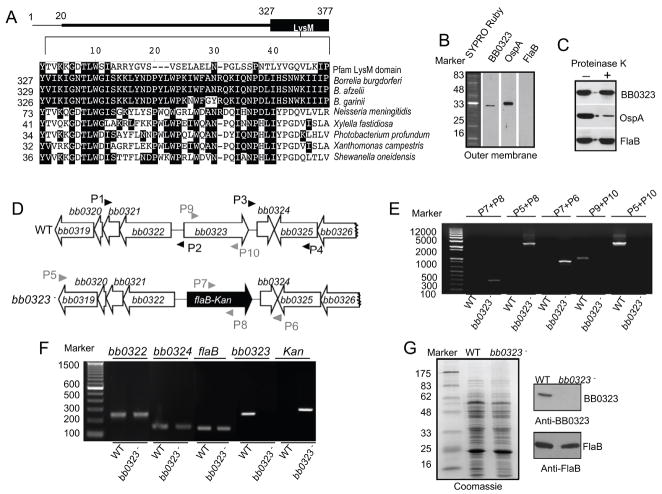
The gene product of B. burgdorferi bb0323 is annotated as LysM (Lysin Motif) domain protein. The location of LysM-like domain in BB0323 and its amino acid sequence similarities (E = 1.8e−04) with a designated LysM domain (Pfam database accession number PF01476) or similar domains in related spirochetes or other bacteria with closest homologies are shown (figure 2A). BB0323 is associated with the spirochete outer membrane (figure 2B), however, proteinase K-mediated digestion of B. burgdorferi surface proteins (figure 2C) and densitometric analysis of the immunoblot (data not shown) indicated that, unlike FlaB, the antigen has a minor (18±1.3% of cellular BB0323 levels) sensitivity to proteinase K treatment. We next created a BB0323-deficient B. burgdorferi for direct assessment of its role in the pathogen life cycle. An infectious B. burgdorferi isolate was used to create an isogenic mutant by the exchange of the bb0323 open reading frame with a kanamycin resistance cassette via homologous recombination (figure 2D). Out of 12 transformed clones that grew in antibiotic-containing media, PCR analysis further selected one clone with the desired chromosomal integration of the antibiotic cassette (figure 2E) and with the same endogenous plasmid profile as the parental isolate (supplementary figure S1A). RT-PCR analysis showed that the mutant failed to produce bb0323 mRNA, and that the mutagenesis did not impose polar effects on the transcription of surrounding genes bb0322 and bb0324 (figure 2F). The protein profile of the bb0323 mutant was similar to that of the wild type spirochete (figure 2G, left panel), and the mutant failed to produce BB0323 protein (figure 2G, upper right panel). Compared to parental isolates, the bb0323 mutant displayed a slower growth rate in vitro and formed unusually large clumps, especially at cell density greater than 107 cells/ml (supplementary figure S1B and S1C). In agreement with an earlier study [34], transmission electron microscopic analysis further confirmed significant morphological deformities of BB0323-deficient spirochetes.
Figure 2. Construction and analysis of the bb0323 mutant B. burgdorferi.
A, Position and amino acid sequence alignment of putative LysM-like domain of B. burgdorferi BB0323. The upper panel represents BB0323 protein indicating an amino terminal signal peptide (encompassing the first 20 amino acids) and a carboxyl terminal LysM domain. Lower panel shows amino acid sequence alignment of LysM-like domain of BB0323 to a designated LysM domain (Pfam accession number PF01476) or to orthologs in major B. burgdorferi sensu lato isolates and representative non-spirochete proteins with closest homologies. Dashes indicate gaps introduced to optimize alignments and identical amino acids are shaded. The numbers on the left are the positions of the amino acid residues in the proteins, for which corresponding species and annotations are indicated in the supplementary text. B, BB0323 is detectable in the outer membrane. Outer membrane vesicles were separated by sucrose density gradient centrifugation, separated by SDS-PAGE, and stained with SYPRO Ruby or immunoblotted with BB0323, OspA and FlaB antibodies. C, BB0323 is partially sensitive to proteinase K-mediated degradation of B. burgdorferi surface proteins. Viable B. burgdorferi were incubated with (+) or without (−) proteinase K for the degradation of protease-sensitive surface proteins and processed for immunoblot analysis using BB0323 antibodies. B. burgdorferi OspA and FlaB antibodies were utilized as controls for surface-exposed and sub-surface proteins, respectively. D, Schematic drawings of the wild type (WT) and the bb0323 mutant (bb0323−) isolates at the bb0323 locus. Genes bb0319 - bb0326 (white box arrows) and the kanamycin-resistance cassette driven by the B. burgdorferi flaB promoter (flaB-Kan, black box arrow) are indicated. Primers P1–P4 (black arrow-heads) were used to amplify 5′ and 3′ arms for homologous recombination, and regions flanking up- and down-stream of the bb0323 locus were ligated on either side of the flaB-Kan cassette as detailed in the text. E, Integration of the mutagenic construct, flab-Kan, in the intended genomic locus. Primers 5–10 (gray arrow-heads, positions indicated in figure 2D) were used for PCR analysis using isolated DNA from wild type (WT) or mutant B. burgdorferi (bb0323−) and subjected to gel electrophoresis. The combination of primers used for PCR is indicated at the top, and migration of the DNA ladder is shown on the left. F, RT-PCR assessment of bb0323 ablation and the polar effects of mutagenesis. Total RNA was isolated from wild type B. burgdorferi (WT) or bb0323 mutant (bb0323−) converted to cDNA and used to amplify regions within bb0323, flaB, kanamycin and genes surrounding bb0323 locus (bb0322 and bb0324) and visualized on a gel. G, Protein analysis of wild type and mutant isolates. Equal amounts of protein were by SDS-PAGE gel, and either stained with Coomassie blue (left panel) or immunoblotted with BB0323 and FlaB antibodies (right panel). Migration of protein standards is shown to the left in kDa.
BB0323-deficient B. burgdorferi is not infectious to murine host and ticks
To determine whether the lack of BB0323 influences B. burgdorferi infectivity in vivo, groups of 5 C3H/HeN mice were inoculated intradermally with equal numbers of wild type or bb0323 mutant B. burgdorferi (105 spirochetes/mouse). Infection was assessed by quantitative RT-PCR analysis of pathogen burden in skin and blood samples at 3, 5, 7 and 10 days of infection, and by culture of tissue biopsies. The qRT-PCR results indicated that, although wild type spirochetes persisted in mice, bb0323 mutants were undetectable during murine infection. Similarly, wild type spirochetes were isolated by culture of infected spleen and blood; whereas, attempts to isolate viable bb0323 mutants remained unsuccessful (data not shown). Mice infected with wild type B. burgdorferi developed ankle swelling and histological signs of arthritis, which were absent in mice infected with bb0323 mutant B. burgdorferi (data not shown). When Ixodes ticks were allowed to engorge on the mice infected with wild type or bb0323-deficient B. burgdorferi and fed ticks were subjected to qRT-PCR analyses, mutants remained undetectable in ticks. Similar to immunocompetent mice, bb0323 mutants remained non-infectious in severe combined immunodeficient (SCID) mice. Groups of SCID mice (3 animals/group) were infected with 105 B. burgdorferi and infectivity was assessed at 14 days after infection. qRT-PCR analysis of pathogen burdens in the heart, skin, bladder and joint samples, and culture of blood and spleen biopsies, consistently detected the wild type spirochetes; whereas, bb0323 mutants remained undetectable (data not shown). Collectively, these observations suggest that BB0323 is required for survival of B. burgdorferi in the murine host and for acquisition of spirochetes by ticks.
Complementation restores the phenotypic defects including virulence and persistence of BB0323-deficient B. burgdorferi throughout the mouse-tick infection cycle
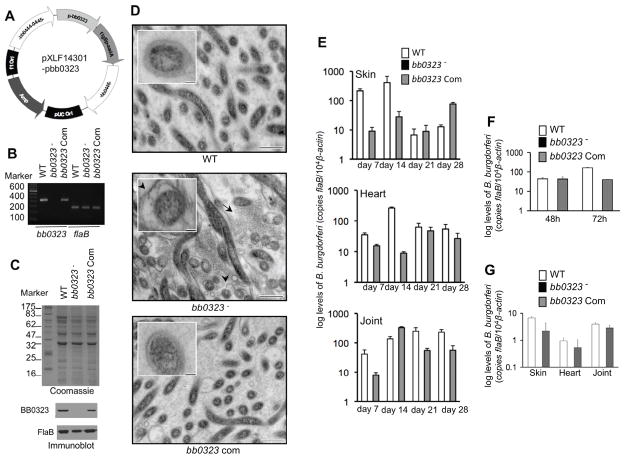
To ascertain that the observed phenotypic defects of bb0323 mutant B. burgdorferi were due to the loss of bb0323 gene rather than the result of anomalous effects of genetic manipulation, we complemented the bb0323 mutant spirochetes with a wild type copy of the bb0323 gene in the chromosome and used this isolate in murine infection studies. To accomplish this, the open reading frame of bb0323, with its upstream promoter, was fused to the streptomycin resistance cassette aadA and inserted into pXLF14301, which carries up- and downstream recombination arms for insertion of a DNA construct into B. burgdorferi chromosome via allelic exchange (figure 3A). The mutant was transformed with the recombinant plasmid pXLF14301-pbb0323, and one of the transformants that produced both bb0323 transcript (figure 3B) and BB0323 protein (figure 3C) was isolated. Quantitative RT-PCR (data not shown) showed that the complemented isolates, however, produced lower (30 ±10%) levels of bb0323 transcripts during growth in vitro, compared to parental isolates. The bb0323-complemented isolate contained a similar set of plasmids as bb0323 mutant or wild type isolates (supplementary figure S1A). Unlike bb0323 mutant, the complemented isolate displayed growth patterns similar to the parental isolate and did not form larger clumps (supplementary figure S1C). Transmission electron microscopic studies further confirmed that while the bb0323 mutant culture is dominated by spirochetes with obvious defects in the organization of outer membrane, presence of numerous vesicular structures or membrane blebs, both wild type and bb0323-complemented spirochetes have normal outer membrane organization without significant presence of membrane blebs (figure 3D).
Figure 3. Complementation of bb0323 mutant B. burgdorferi with bb0323 restores spirochete infectivity throughout an experimental enzootic cycle.
A, Construction of DNA construct (pXLF14301-pbb0323) for chromosomal integration of the bb0323. The open reading frame of bb0323 and its native promoter together with streptomycin resistance gene (aadA) was cloned into pXLF14301, which contains B. burgdorferi inserts for integration of the complemented gene in spirochete chromosome. B, RT-PCR analysis of the bb0323 transcripts. Total RNA was isolated from the wild type (WT), bb0323 mutant (bb0323−) or bb0323-complemented B. burgdorferi (bb0323 Com), converted to cDNA and subjected to PCR analysis. C, Reproduction of BB0323 protein by the complemented B. burgdorferi. Lysates of B. burgdorferi were separated on a SDS-PAGE gel, which was either stained with Coomassie blue (upper panel) or immunoblotted with specific antibodies (lower panels). D, Complementation of bb0323 mutant with bb0323 gene restores ultrastructural defects of mutant cells. B. burgdorferi were grown at a density of 5×107/ml in BSK medium and processed for transmission electron microscopy. Arrows indicate enhanced shedding of vesicular structures or blebs, possibly derived from the outer membrane, in the mutants. Inset shows higher magnification images of a representative single cell indicating altered organization of outer membrane in the bb0323 mutant. Bar = 500 nm (inset Bar = 100 nm). E, Complementation restores spirochete infectivity in mice. Spirochete burden in groups of infected mice (5 animals/group) was analyzed by quantitative RT-PCR at days 7, 14, 21 or 28 by measuring copies of the flaB gene and normalized against mouse β-actin levels. Bars represent the mean ± SEM of relative tissue levels of pathogen from three independent animal infection experiments. F, bb0323-complemented B. burgdorferi are acquired by ticks. Mice were inoculated with spirochetes (5 mice/group) and following two weeks of infection, naïve I. scapularis nymphs (25 ticks/mice) were allowed to feed on mice. Spirochete burdens were assessed in ticks by qRT-PCR, by measuring copies of the B. burgdorferi flaB gene and normalized against tick β-actin levels. Bars represent the mean ± SEM from three independent experiments. G, bb0323-complemented B. burgdorferi persisted through larval-nymphal molt and transmit from feeding ticks to mice. B. burgdorferi-infected nymphs were generated by feeding larva on infected mice, which were allowed to feed to repletion on naïve mice (3 tick/mouse, 5 animal/group). Murine tissues were isolated at day 14 after repletion and the spirochete burdens were assessed by qRT-PCR. Bars represent the mean ± SEM from two independent experiments.
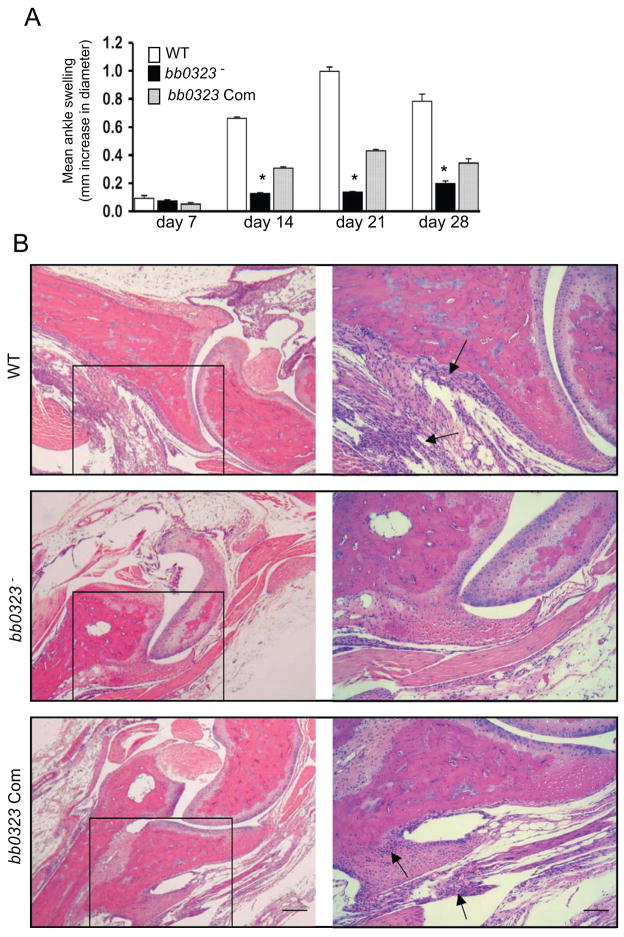
We then examined the virulence and persistence of the bb0323-complemented spirochetes throughout the mouse-tick infection cycle. Groups of 5 C3H/HeN mice were intradermally challenged with the wild type spirochete, bb0323 mutant, or bb0323-complemented B. burgdorferi (105 cells/mouse) and spirochete burden in skin, heart and joint were evaluated at days 7, 14, 21 or 28 of infection. The results showed that while bb0323 mutants remained undetectable, both bb0323-complemented isolates and wild type spirochetes persisted in all murine tissues throughout the infection (figure 3E). When larval and nymphal ticks were allowed to engorge on mice following 14 days of B. burgdorferi infection, only wild type and bb0323-complemented B. burgdorferi were able to migrate to larvae (data not shown) and nymphs (figure 3F). Fed infected larvae were allowed to molt into nymphs in the laboratory and when fed on naïve mice, both wild type and bb0323-complemented B. burgdorferi transmitted to the mice (figure 3G). As expected, both wild type and bb0323-complemented isolates induced significantly higher levels of disease in mice than the bb0323 mutant, as reflected by the development of swelling in the tibiotarsal joints and the histopathological signs of arthritis (figure 4). These experiments conclusively demonstrate that BB0323 is essential for microbial persistence and virulence in mice.
Figure 4. Deficiency of B. burgdorferi bb0323 affects the severity of arthritis in mice.
A, Severity of joint swelling in B. burgdorferi-infected mice. Groups of mice (5 animals/group) were infected with the wild type (WT), bb0323 mutant (bb0323−) or bb0323-complemented B. burgdorferi (bb0323 Com), and assessed for joint swelling. Bars represent the mean ± SEM from three independent infection experiments. Differences in the joint swelling between groups of mice infected with bb0323 mutant and those with the bb0323-complemented isolates were highly significant (*P < 0.01) at all time points, except for day 7. B, Representative demonstration of joint histology in mice infected with wild type or genetically-manipulated B. burgdorferi isolates. Twenty-one days following spirochete infection, tibiotarsal joints were analyzed for histopathology. The left panel indicates low-resolution (40x, bar = 400 μm) joint images. Higher-resolution (200x, bar = 80 μm) images of selected areas from corresponding sections (marked by box) are shown in the right panels.
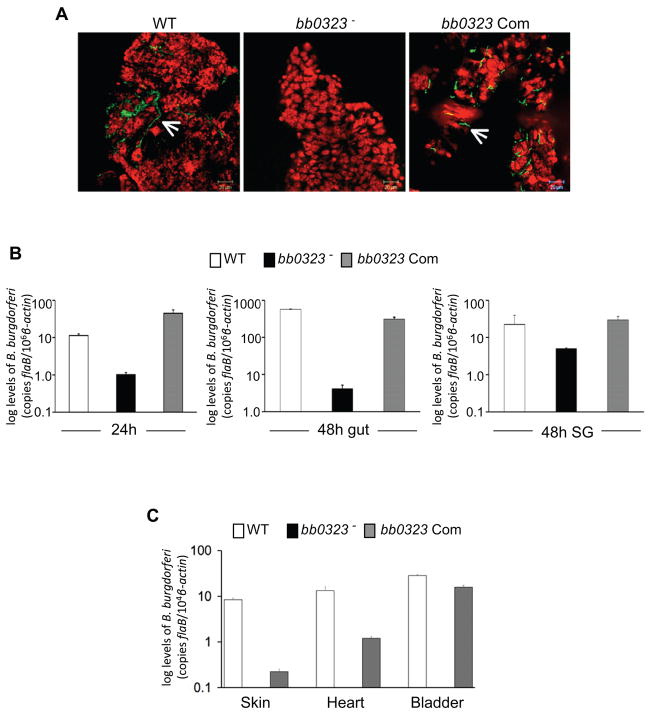
As the bb0323 mutant was unable to infect mice, and thus, cannot be naturally acquired by ticks, the role of this gene product for spirochete life cycle in the vector remained inconclusive. Although bb0323-complemented isolate was acquired by ticks from infected mice (figure 3F), it is possible that bb0323 function may be required for spirochete entry into the vector, but may be redundant for persistence in ticks or transmission back to mice. We, therefore, directly introduced bb0323 mutants into ticks, and studied the ability of the mutants to persist in unfed and fed ticks, and transmit back to naïve mice. A microinjection procedure was used to deliver an equal number of wild type or genetically-manipulated isolates into the tick gut as previously described [17, 19]. Three groups of ticks were infected with the wild type spirochete, bb0323 mutant or bb0323-complemented B. burgdorferi (103 cells/tick). Following infection, one group of ticks was allowed to remain in the unfed condition for 14 days, while a parallel group was allowed to feed on naïve C3H mice (5 ticks/mouse) for 48 hrs. The spirochete burdens in the unfed ticks, the feeding tick gut and the salivary glands were determined by qRT-PCR. Unlike wild type spirochetes and bb0323-complemented isolates, BB0323-deficient spirochetes were unable to survive in unfed ticks (figure 5A). At 24 hours of feeding, the burden of bb0323 mutant into ticks began to decline, and at 48 hours of feeding, the numbers of bb0323 mutants in the gut or salivary gland remained significantly lower, when compared to bb0323-complemented or wild type isolates (figure 5B). Following 14 days of feeding, analysis of mouse infection by culture (data not shown) or qRT-PCR analysis (figure 5C) indicated that bb0323 failed to transmit to mice; whereas, both wild type spirochetes and bb0323-complemented isolates were transmitted to the host. These data establish that BB0323 is necessary for B. burgdorferi persistence in the vector and transmission through feeding ticks to the murine host.
Figure 5. bb0323 is required for B. burgdorferi to persist in ticks and for transmission to naïve host.
A, bb0323 mutants were unable to persist in unfed Ixodes nymphs. Nymphal ticks were microinjected with B. burgdorferi (WT), bb0323 mutant (bb0323−) or bb0323-complemented B. burgdorferi (bb0323 Com), and spirochete burdens in the gut of unfed ticks were analyzed at 14 days after injection. The spirochetes (arrow) were labeled with FITC-labeled goat anti-B. burgdorferi antibody (shown in green), and the nuclei of the gut cells were stained with propidium iodide (shown in red). Images were obtained using a confocal immunofluorescence microscope at 400x magnification, and presented as merge image for clarity. B, Decreased burdens of microinjected bb0323 mutants in feeding ticks. Nymphal ticks were microinjected with B. burgdorferi, as described in figure 5A, and placed on naïve mice 48 hours after injection. Spirochete burden was analyzed by quantitative PCR analysis of whole ticks at 24 hours and dissected gut or salivary glands at 48 hours of feeding by measuring copies of the B. burgdorferi flaB gene, normalized against tick β-actin levels. Bars represent the mean ± SEM of relative tissue levels of B. burgdorferi from three independent animal infection experiments. Differences between bb0323 mutant burdens and those with complemented or wild type isolates were significant at all time points and tissues (P < 0.02 or lower). C, bb0323 mutants were unable to transmit to mice. Nymphal ticks were microinjected as described in figure 5B, and placed on naïve mice following 48 hours after injection. Ticks were allowed to engorge and transmission of B. burgdorferi was assessed by measuring copies of the flaB gene, normalized against mouse β-actin levels, in indicated murine tissues after 14 days of tick feeding. Bars represent the mean ± SEM of relative tissue levels of B. burgdorferi from three independent animal infection experiments.
DISCUSSION
Certain B. burgdorferi genes encoding membrane antigens alter their expression in new host or vector environments and may be important for pathogen persistence [12, 25, 30, 41]. bb0323 was previously identified as a differentially-regulated spirochete gene, since its expression induced in spirochetes cultured in environmental parameters that partially mimic fed ticks, such as 37°C and pH 6.8, compared to the expression in unfed tick-like conditions, such as 23°C and pH 7.5 [10]. Here, we show that bb0323 is variably expressed throughout the spirochete infectious cycle and is indispensable for membrane organization and pathogen survival in vivo, regardless of vector and host environments. Such consistent requirement of BB0323 is in contrast with other regulated membrane antigens, which are either necessary in specific phases of the spirochete life cycle [16, 17] or functionally redundant [27, 29, 30]. With the sole exception of a cellular enzyme, nicotinamidase (PncA) [14], BB0323 is possibly the only other antigen required for persistence and transmission of spirochetes throughout the enzootic infection cycle.
BB0323 is highly conserved in the B. burgdorferi sensu lato complex that causes human infection. BB0323 is immunogenic in mammals, including humans, and identified as a membrane-associated antigen [32, 33]. Deficiency of BB0323 was previously shown to impose severe clumping of cells and the disintegration of outer membranes, often resulting in enormous blebs and few single cells, suggesting the involvement of BB0323 in the fission process [34]. Our data support these observations; however, the specific role of BB0323 in spirochete biology remains unknown. The antigen has an estimated molecular mass of 44 kDa; however, the native protein migrates on SDS-PAGE gels as a 30-kDa protein, which may be the result of aberrant migration of the highly-basic BB0323 or possible proteolytic processing of the immature protein [42]. A major clue to the BB0323 function is the presence of a single LysM (Lysine Motif)-like domain in the carboxyl terminus. LysM domains are found in a variety of prokaryotic and eukaryotic proteins and typically consists of 44–65 amino acids, occurring in single or multiple tandem copies with a poorly conserved central region and relatively high conservation over the first 16 and last 10 amino acids [43]. The BB0323 LysM domain, however, contains poor sequence identity to proteins with defined functions [43], and functions of few proteins with relatively close homologies, including the closest, an outer membrane protein of Neisseria meningitidis (Figure 2A), are unknown. In bacteria, LysM domains generally bind peptidoglycan and are found either in various types of enzymes associated with cell wall degradation or in surface receptors involved in host-pathogen interaction [43] contributing to microbial virulence [44]. bb0323 mutants display dramatic alterations in their cell shape and organization of membranes, which are structurally supported by peptidoglycan [45]. Major fractions of cellular BB0323 remain as a subsurface antigen, and if localized in the periplasm, BB0323 could interact with peptidoglycan via LysM domain, potentially contributing to the proper organization of the spirochete membrane. Our findings that BB0323 is also detectable in the outer membrane with limited yet detectable surface exposure is puzzling and appears to contradict the existence of a functional LysM domain, as peptidoglycan is not present in the borrelial outer membrane [13]. However, BB0323 might have dual roles in spirochete membrane organization and host-pathogen interaction, as shown for other LysM domain-containing proteins, such as Staphylococcus aureus autolysin Aaa [46], which possesses bacteriolytic and host adhesion properties. Nevertheless, as bb0323 deletion imposes significant growth and morphological defects of spirochetes in vitro [34] and results in elimination of spirochetes within the first few days of host or vector infection, this chromosomally-encoded antigen must be involved in a crucial physiological function that is most pronounced during pathogen survival in vivo. Understanding of and interference with the function of B. burgdorferi antigens that are essential for microbial virulence and persistence through the natural infection cycle of the pathogen could, potentially, contribute to the development of effective therapeutic strategies to combat Lyme borreliosis.
Supplementary Material
A, bb0323 mutant (bb0323−) and bb0323-complemented (bb0323 Com) B. burgdorferi contained the same set of endogenous plasmid as retained by the isogenic wild type spirochete. DNA was isolated from B. burgdorferi isolates and used in PCR analysis for detection of endogenous plasmids using specific primer sets as detailed in the text. B, bb0323 mutant B. burgdorferi forms large clumps when grown at late log phase. Spirochetes were grown at a high cell density (5× 107 cells/ml) and an aliquot of viable spirochete culture was imaged using dark-field microscopy. The major fraction of bb0323 mutant B. burgdorferi was visible as large clumps along with occasional free spirochetes (arrows), while both wild type and bb0323-complemented isolates grow as free spirochetes. Bar = 10 μm. C, Growth curves for the wild type and genetically-manipulated spirochetes. Spirochetes were diluted to a density of 105 cells/ml, grown at 33°C in BSK-H medium, and counted under a dark-field microscope every 12 hours using a Petroff-Hausser cell counter. Note that bb0323 mutants grow as single cells until late growth phases (indicated by arrows) where they form large clumps. Differences in the numbers of bb0323 mutant with the bb0323-complemented isolates or wild type B. burgdorferi were significant at all times of growth, including the first time point of assessment at day 0.5 time point (P < 0.05).
Acknowledgments
This work was supported in part by funding from the American Heart Association, Arthritis Foundation, and the National Institutes of Health (AR055323 and AI076684).
We thank Patricia Rosa for providing us the B. burgdorferi lp28-1-Gm clone. We sincerely thank Carolyn Marks, Adam Coleman, Kamoltip Promnares, Deborah Shroder and John Anderson for their assistance with this study.
Footnotes
The authors declare that they have no competing financial interests.
References
- 1.Piesman J, Eisen L. Prevention of Tick-Borne Diseases. Annu Rev Entomol. 2008;53:323–343. doi: 10.1146/annurev.ento.53.103106.093429. [DOI] [PubMed] [Google Scholar]
- 2.Barthold SW, DeSouza M, Fikrig E, Persing DH. Lyme borreliosis in the laboratory mouse. In: Schuster SE, editor. Lyme disease. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1992. pp. 223–242. [Google Scholar]
- 3.Steere AC, Glickstein L. Elucidation of Lyme arthritis. Nat Rev Immunol. 2004;4:143–52. doi: 10.1038/nri1267. [DOI] [PubMed] [Google Scholar]
- 4.Casjens S, Palmer N, van Vugt R, et al. A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs in an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol Microbiol. 2000;35:490–516. doi: 10.1046/j.1365-2958.2000.01698.x. [DOI] [PubMed] [Google Scholar]
- 5.Fraser CM, Casjens S, Huang WM, et al. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature. 1997;390:580–6. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- 6.de Silva AM, Fikrig E. Arthropod- and host-specific gene expression by Borrelia burgdorferi. J Clin Invest. 1997;99:377–9. doi: 10.1172/JCI119169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brooks CS, Hefty PS, Jolliff SE, Akins DR. Global analysis of Borrelia burgdorferi genes regulated by mammalian host-specific signals. Infect Immun. 2003;71:3371–83. doi: 10.1128/IAI.71.6.3371-3383.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caimano MJ, Iyer R, Eggers CH, et al. Analysis of the RpoS regulon in Borrelia burgdorferi in response to mammalian host signals provides insight into RpoS function during the enzootic cycle. Mol Microbiol. 2007;65:1193–217. doi: 10.1111/j.1365-2958.2007.05860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ojaimi C, Brooks C, Akins D, et al. Borrelia burgdorferi gene expression profiling with membrane-based arrays. Methods Enzymol. 2002;358:165–77. doi: 10.1016/s0076-6879(02)58088-5. [DOI] [PubMed] [Google Scholar]
- 10.Revel AT, Talaat AM, Norgard MV. DNA microarray analysis of differential gene expression in Borrelia burgdorferi, the Lyme disease spirochete. Proc Natl Acad Sci U S A. 2002;99:1562–7. doi: 10.1073/pnas.032667699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tokarz R, Anderton JM, Katona LI, Benach JL. Combined effects of blood and temperature shift on Borrelia burgdorferi gene expression as determined by whole genome DNA array. Infect Immun. 2004;72:5419–32. doi: 10.1128/IAI.72.9.5419-5432.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liang FT, Nelson FK, Fikrig E. Molecular adaptation of Borrelia burgdorferi in the murine host. J Exp Med. 2002;196:275–80. doi: 10.1084/jem.20020770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosa PA, Tilly K, Stewart PE. The burgeoning molecular genetics of the Lyme disease spirochaete. Nat Rev Microbiol. 2005;3:129–43. doi: 10.1038/nrmicro1086. [DOI] [PubMed] [Google Scholar]
- 14.Purser JE, Lawrenz MB, Caimano MJ, Howell JK, Radolf JD, Norris SJ. A plasmid-encoded nicotinamidase (PncA) is essential for infectivity of Borrelia burgdorferi in a mammalian host. Mol Microbiol. 2003;48:753–64. doi: 10.1046/j.1365-2958.2003.03452.x. [DOI] [PubMed] [Google Scholar]
- 15.Jewett MW, Lawrence K, Bestor AC, et al. The critical role of the linear plasmid lp36 in the infectious cycle of Borrelia burgdorferi. Mol Microbiol. 2007;64:1358–74. doi: 10.1111/j.1365-2958.2007.05746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pal U, Wang P, Bao F, et al. Borrelia burgdorferi basic membrane proteins A and B participate in the genesis of Lyme arthritis. J Exp Med. 2008;205:133–41. doi: 10.1084/jem.20070962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang XF, Pal U, Alani SM, Fikrig E, Norgard MV. Essential role for OspA/B in the life cycle of the Lyme disease spirochete. J Exp Med. 2004;199:641–8. doi: 10.1084/jem.20031960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grimm D, Tilly K, Byram R, et al. Outer-surface protein C of the Lyme disease spirochete: a protein induced in ticks for infection of mammals. Proc Natl Acad Sci U S A. 2004;101:3142–7. doi: 10.1073/pnas.0306845101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pal U, Yang X, Chen M, et al. OspC facilitates Borrelia burgdorferi invasion of Ixodes scapularis salivary glands. J Clin Invest. 2004;113:220–30. doi: 10.1172/JCI19894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi Y, Xu Q, McShan K, Liang FT. Both decorin-binding proteins A and B are critical for the overall virulence of Borrelia burgdorferi. Infect Immun. 2008;76:1239–46. doi: 10.1128/IAI.00897-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blevins JS, Hagman KE, Norgard MV. Assessment of decorin-binding protein A to the infectivity of Borrelia burgdorferi in the murine models of needle and tick infection. BMC Microbiol. 2008;8:82. doi: 10.1186/1471-2180-8-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seshu J, Esteve-Gassent MD, Labandeira-Rey M, et al. Inactivation of the fibronectin-binding adhesin gene bbk32 significantly attenuates the infectivity potential of Borrelia burgdorferi. Mol Microbiol. 2006;59:1591–601. doi: 10.1111/j.1365-2958.2005.05042.x. [DOI] [PubMed] [Google Scholar]
- 23.Li X, Liu X, Beck DS, Kantor FS, Fikrig E. Borrelia burgdorferi lacking BBK32, a fibronectin-binding protein, retains full pathogenicity. Infect Immun. 2006;74:3305–13. doi: 10.1128/IAI.02035-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Revel AT, Blevins JS, Almazan C, et al. bptA (bbe16) is essential for the persistence of the Lyme disease spirochete, Borrelia burgdorferi, in its natural tick vector. Proc Natl Acad Sci U S A. 2005;102:6972–7. doi: 10.1073/pnas.0502565102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pal U, Dai J, Li X, et al. A Differential Role for BB0365 in the Persistence of Borrelia burgdorferi in Mice and Ticks. J Infect Dis. 2008;197:148–155. doi: 10.1086/523764. [DOI] [PubMed] [Google Scholar]
- 26.Li X, Pal U, Ramamoorthi N, et al. The Lyme disease agent Borrelia burgdorferi requires BB0690, a Dps homologue, to persist within ticks. Mol Microbiol. 2007;63(3):694–710. doi: 10.1111/j.1365-2958.2006.05550.x. [DOI] [PubMed] [Google Scholar]
- 27.Coleman AS, Yang X, Kumar M, et al. Borrelia burgdorferi complement regulator-acquiring surface protein 2 does not contribute to complement resistance or host infectivity. PLoS ONE. 2008;3:3010e. doi: 10.1371/journal.pone.0003010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hubner A, Revel AT, Nolen DM, Hagman KE, Norgard MV. Expression of a luxS gene is not required for Borrelia burgdorferi infection of mice via needle inoculation. Infect Immun. 2003;71:2892–6. doi: 10.1128/IAI.71.5.2892-2896.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li X, Neelakanta G, Liu X, et al. Role of outer surface protein D in the Borrelia burgdorferi life cycle. Infect Immun. 2007;75:4237–44. doi: 10.1128/IAI.00632-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stewart PE, Bestor A, Cullen JN, Rosa PA. A tightly regulated surface protein of Borrelia burgdorferi is not essential to the mouse-tick infectious cycle. Infect Immun. 2008;76:1970–8. doi: 10.1128/IAI.00714-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tilly K, Grimm D, Bueschel DM, Krum JG, Rosa P. Infectious cycle analysis of a Borrelia burgdorferi mutant defective in transport of chitobiose, a tick cuticle component. Vector Borne Zoonotic Dis. 2004;4:159–68. doi: 10.1089/1530366041210738. [DOI] [PubMed] [Google Scholar]
- 32.Nowalk AJ, Gilmore RD, Jr, Carroll JA. Serologic proteome analysis of Borrelia burgdorferi membrane-associated proteins. Infect Immun. 2006;74:3864–73. doi: 10.1128/IAI.00189-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nowalk AJ, Nolder C, Clifton DR, Carroll JA. Comparative proteome analysis of subcellular fractions from Borrelia burgdorferi by NEPHGE and IPG. Proteomics. 2006;6:2121–34. doi: 10.1002/pmic.200500187. [DOI] [PubMed] [Google Scholar]
- 34.Stewart PE, Hoff J, Fischer E, Krum JG, Rosa PA. Genome-wide transposon mutagenesis of Borrelia burgdorferi for identification of phenotypic mutants. Appl Environ Microbiol. 2004;70:5973–9. doi: 10.1128/AEM.70.10.5973-5979.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elias AF, Stewart PE, Grimm D, et al. Clonal polymorphism of Borrelia burgdorferi strain B31 MI: implications for mutagenesis in an infectious strain background. Infect Immun. 2002;70:2139–50. doi: 10.1128/IAI.70.4.2139-2150.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pal U, Li X, Wang T, et al. TROSPA, an Ixodes scapularis receptor for Borrelia burgdorferi. Cell. 2004;119:457–68. doi: 10.1016/j.cell.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 37.Pal U, Montgomery RR, Lusitani D, et al. Inhibition of Borrelia burgdorferi-tick Interactions in vivo by outer surface protein A antibody. J Immunol. 2001;166:7398–403. doi: 10.4049/jimmunol.166.12.7398. [DOI] [PubMed] [Google Scholar]
- 38.Skare JT, Shang ES, Foley DM, et al. Virulent strain associated outer membrane proteins of Borrelia burgdorferi. J Clin Invest. 1995;96:2380–92. doi: 10.1172/JCI118295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barthold SW, Hodzic E, Tunev S, Feng S. Antibody-mediated disease remission in the mouse model of Lyme borreliosis. Infect Immun. 2006;74:4817–25. doi: 10.1128/IAI.00469-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang X, Ma Y, Weis JH, Zachary JF, Kirschning CJ, Weis JJ. Relative contributions of innate and acquired host responses to bacterial control and arthritis development in Lyme disease. Infect Immun. 2005;73:657–60. doi: 10.1128/IAI.73.1.657-660.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schwan TG. Temporal regulation of outer surface proteins of the Lyme-disease spirochaete Borrelia burgdorferi. Biochem Soc Trans. 2003;31:108–12. doi: 10.1042/bst0310108. [DOI] [PubMed] [Google Scholar]
- 42.Ostberg Y, Carroll JA, Pinne M, Krum JG, Rosa P, Bergstrom S. Pleiotropic effects of inactivating a carboxyl-terminal protease, CtpA, in Borrelia burgdorferi. J Bacteriol. 2004;186:2074–84. doi: 10.1128/JB.186.7.2074-2084.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buist G, Steen A, Kok J, Kuipers OP. LysM, a widely distributed protein motif for binding to (peptido)glycans. Mol Microbiol. 2008;68:838–47. doi: 10.1111/j.1365-2958.2008.06211.x. [DOI] [PubMed] [Google Scholar]
- 44.Bolton MD, van Esse HP, Vossen JH, et al. The novel Cladosporium fulvum lysin motif effector Ecp6 is a virulence factor with orthologues in other fungal species. Mol Microbiol. 2008;69:119–36. doi: 10.1111/j.1365-2958.2008.06270.x. [DOI] [PubMed] [Google Scholar]
- 45.Motaleb MA, Corum L, Bono JL, et al. Borrelia burgdorferi periplasmic flagella have both skeletal and motility functions. Proc Natl Acad Sci U S A. 2000;97:10899–904. doi: 10.1073/pnas.200221797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heilmann C, Hartleib J, Hussain MS, Peters G. The multifunctional Staphylococcus aureus autolysin Aaa mediates adherence to immobilized fibrinogen and fibronectin. Infect Immun. 2005;73:4793–802. doi: 10.1128/IAI.73.8.4793-4802.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A, bb0323 mutant (bb0323−) and bb0323-complemented (bb0323 Com) B. burgdorferi contained the same set of endogenous plasmid as retained by the isogenic wild type spirochete. DNA was isolated from B. burgdorferi isolates and used in PCR analysis for detection of endogenous plasmids using specific primer sets as detailed in the text. B, bb0323 mutant B. burgdorferi forms large clumps when grown at late log phase. Spirochetes were grown at a high cell density (5× 107 cells/ml) and an aliquot of viable spirochete culture was imaged using dark-field microscopy. The major fraction of bb0323 mutant B. burgdorferi was visible as large clumps along with occasional free spirochetes (arrows), while both wild type and bb0323-complemented isolates grow as free spirochetes. Bar = 10 μm. C, Growth curves for the wild type and genetically-manipulated spirochetes. Spirochetes were diluted to a density of 105 cells/ml, grown at 33°C in BSK-H medium, and counted under a dark-field microscope every 12 hours using a Petroff-Hausser cell counter. Note that bb0323 mutants grow as single cells until late growth phases (indicated by arrows) where they form large clumps. Differences in the numbers of bb0323 mutant with the bb0323-complemented isolates or wild type B. burgdorferi were significant at all times of growth, including the first time point of assessment at day 0.5 time point (P < 0.05).