Abstract
An ataxia-telangiectasia mutated (ATM)-dependent DNA damage signal is amplified through the interaction of various factors, which are recruited to the chromatin regions with DNA double-strand breaks. Spatial and temporal regulation of such factors is analysed by fluorescence microscopy in combination with laser micro-irradiation. Here we describe a novel and simple technique for micro-irradiation that does not require a laser source. Cells were labelled with BrdU for 48–72 h, covered with porous polycarbonate membranes, and exposed to UVC. All BrdU-labelled cells showed localized foci of phosphorylated ATM, phosphorylated histone H2AX, MDC1 and 53BP1 upon irradiation, showing that these foci were induced irrespective of the cell-cycle phase. They were also detectable in nucleotide excision repair-defective XPA cells labelled with BrdU, indicating that the foci did not reflect an excision repair-related process. Furthermore, an ATM-specific inhibitor significantly attenuated the foci formation, and disappearance of the foci was significantly abrogated in non-homologous end-joining-defective cells. Thus, it can be concluded that micro-irradiation generated DNA double-strand breaks in BrdU-sensitized cells. The present technique should accelerate research in the fields of DNA damage response, DNA repair and DNA recombination, as it provides more chances to perform micro-irradiation experiments without any specific equipment.
INTRODUCTION
An ataxia-telangiectasia mutated (ATM)-dependent cellular response to DNA double-strand breaks plays a pivotal role in maintaining genome integrity (1–5). Upon irradiation, autophosphorylation and monomerization of ATM proteins occur, and activated ATM phosphorylates various downstream mediators and effectors, such as histone H2AX, MDC1, 53BP1 and NBS1. A proper ATM-dependent DNA damage response requires amplification of the damage signal by recruiting the factors to the site of chromatin with the aid of histone H2AX phosphorylation (6,7). The recruited factors create discrete foci in the nuclei, which are detectable under fluorescence microscopy (8). These foci are often called ionizing radiation-induced foci (IRIF). The physiological importance of IRIF formation has been demonstrated by various studies, in which the cells lacking IRIF factors display a compromised DNA damage response, as evidenced by deficiencies in cell-cycle arrest and DNA repair (1,2,5,9–11).
Activated ATM mediates the phosphorylation of serine or threonine residues, which create specific docking sites for proteins harbouring FHA and BRCT domains (12). In particular, phosphorylation of histone H2AX at serine 139 is the primary modification, which is essential for persistent recruitment of IRIF factors (13). Furthermore, recruited proteins, including MDC1, NBS1, MRE11 and 53BP1, are also targets for ATM-dependent phosphorylation, which is required for the sequential protein–protein interactions involved in IRIF formation (14–19). Thus, analyses of the dynamics of both recruitment and phosphorylation of the IRIF factors are indispensable for a comprehensive understanding of DNA damage response.
So far, the foci of phosphorylated ATM and its downstream factors have been visualized by fluorometric assays using phospho-specific antibodies. Recruitment of MDC1 and 53BP1 into the foci is also demonstrated with specific antibodies. However, some DNA damage response factors, like Ku and DNA–PKcs proteins, have never been found to form foci after conventional irradiation (20), while phosphorylated DNA–PKcs formed foci (21,22). It can be postulated that the number of such DNA repair proteins locally accumulated at the site of one DNA double-strand break is not sufficient for the detection by immunofluorescence technique. Therefore, to circumvent the problem, localized laser micro-irradiation of subnuclear regions in combination with halogenated thymidine analogues has been developed and applied in recent studies (8,20,23–25).
Since the first study using an ultraviolet A (UVA) laser in combination with BrdU was reported, the UVA lasers of different wavelengths have been used to investigate the dynamics of DNA damage response and repair factors (8,14,20,23–25). Moreover, green and near-infrared lasers, which do not require DNA sensitization, were used in some studies (26–28). Recently, a comparative study discussed mechanisms of DNA damage induction by different laser micro-irradiation systems (29). From these studies, the usefulness of laser micro-irradiation has already been proved. However, to perform these experiments requires the specific devices, which are the laser sources.
While UVA lamps have been used for creating DNA double-strand breaks (30), it is impossible to generate localized DNA damage in subnuclear regions without any focusing units. In this report, we have developed a novel and simple micro-irradiation technique by irradiating cells through micro-pore membranes, whose application has been reported elsewhere (31–33). In addition, we utilized UVC light from a germicidal lamp, because UVC is significantly more effective than UVA on photosensitization of BrdU in DNA (30,34). Cells were labelled with BrdU for 48–72 h, covered by micro-pore membranes, and exposed to UVC light in order to induce photochemical events leading to DNA double-strand break. After 1-h incubation, the cells were fixed and stained with antibody recognizing phosphorylated histone H2AX at serine 139, whose phosphorylation has been treated as a reliable biochemical marker for DNA double-strand breaks (16,35,36). We have successfully created localized DNA double-strand breaks independent of DNA replication and unscheduled DNA synthesis caused by nucleotide excision repair. Activation of ATM and recruitment of DNA damage checkpoint factors including MDC1 and 53BP1 were also observed.
Our novel technique is a simple and undemanding method for generating localized DNA double-strand breaks. It also obviates the need for a true radiation irradiator to generate DNA double-strand breaks. Thus, the method should accelerate research in the fields of DNA damage response, DNA repair and DNA recombination without the need of any specific equipment.
MATERIALS AND METHODS
Cell culture and BrdU labelling
Normal human diploid fibroblast-like (HE49) cells (37) and the two primary XP-A fibroblasts (XPEMB-1 and XP2OS) were cultured in MEM supplemented with 10% fetal bovine serum (TRACE Bioscience PTY Ltd., Australia). Chinese hamster ovary (CHO) cells, Ku80-deficient xrs5 cells and XRCC1-deficient EM9 cells were cultured in α-MEM supplemented with 10% fetal bovine serum (TRACE Bioscience PTY Ltd., Australia). XPEMB-1 (JCRB0325) and XP2OS (KURB1007) cells were obtained from Japanese Collection of Research Bioresources (JCRB). Exponentially growing cells were plated onto sterilized 22 × 22 mm cover slips at a density of 5 × 104 cells per slip, and the cells were incubated for overnight at 37°C in a 5% CO2 incubator. For BrdU labelling, the culture medium was replaced by a new medium containing 10 µM BrdU, and they were incubated for further 48–72 h. The ATM kinase activity was inhibited by a specific inhibitor, KU55933 and 40 µM of KU55933 was administrated 30 min before UVC-irradiation. Immediately after irradiation, a fresh medium containing 40 µM of KU55933 was fed, and the cells were cultured at 37°C in a 5% CO2 incubator until they were fixed.
Micro-irradiation
Cells were washed with PBS twice, and three-fourth of the cells were covered by a polycarbonate micro-pore membrane (Isopore membrane, Millipore, Tokyo). Then, cells were exposed to UVC light from germicidal lamp (GL-15, TOSHIBA, Tokyo). Immediately after exposure, a fresh BrdU-free medium was fed, and the cells were cultured at 37°C in a 5% CO2 incubator for various time before fixation. The dose rate was 1 J/m2 per second, which was measured by a UV dosimeter (UVR-1, TOPCON, Tokyo).
Immunofluorescence
Cells cultured on coverslips were fixed with 4% formaldehyde for 10 min, permeabilized with 0.5% Triton X-100 for 5 min, and were washed extensively with phosphate-buffered saline (PBS). Fixation and permeabilization were performed on ice. The primary antibodies were diluted in 100 µl of TBS–DT (20 mM Tris–HCl, pH7.6, 137 mM NaCl, containing 50 mg/ml skim milk and 0.1% Tween-20), and the antibodies were applied on the coverslips. The samples were incubated for 2 h in a humidified CO2 incubator at 37°C. Then, the primary antibodies were washed with PBS, and Alexa488-labelled anti-mouse or Alexa594-labelled anti-rabbit IgG antibodies (Molecular Probes, Inc., OR) were added. The coverslips were incubated for 1 h in a humidified CO2 incubator at 37°C, washed with PBS, and counterstained with 0.1 µg/ml of DAPI. The samples were examined with a F3000B fluorescence microscope (Leica, Tokyo). Digital images were captured and the images were analysed by FW4000 software (Leica, Tokyo). In order to quantify the fluorescence intensity, green dot-like signals were marked, and the sum of the pixel intensity within the marked area was calculated by FW4000 software. The primary antibodies used in this study were mouse anti-phosphorylated histone H2AX at serine 139 monoclonal antibody (clone 2F3, BioLegend, San Diego, CA), rabbit anti-phosphorylated histone H2AX at serine 139 polyclonal antibody (A300-081A, BETHYL, Montgomery, TX), mouse anti-phosphorylated ATM at serine 1981 monoclonal antibody (Clone 10H11.E12, Rockland, Gilbertsville, PA), rabbit anti-MDC1 polyclonal antibody (IHC-00075, BETHYL, Montgomery, TX), rabbit anti-53BP1 polyclonal antibody (A300-272A, BETHYL, Montgomery, TX), mouse anti-RAD51 monoclonal antibody (Clone 14B4, GeneTex, Irvine, CA), mouse anti-(6-4) photoproducts monoclonal antibody (Clone 64M-2, Cosmo Bio, Tokyo), mouse anti-cyclobutane pyirimidine dimers monoclonal antibody (Clone TDM-2, Cosmo Bio, Tokyo), rabbit anti-XRCC-1 polyclonal antibody (NB110-38896, NOVUS, CO), and mouse anti-replication protein A (RPA) monoclonal antibody (Ab-3, Oncogene Research Products, Boston, MA).
RESULTS
Induction of localized DNA damage with micro-pore membrane
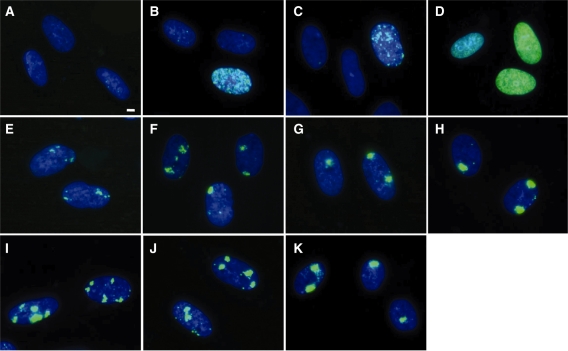
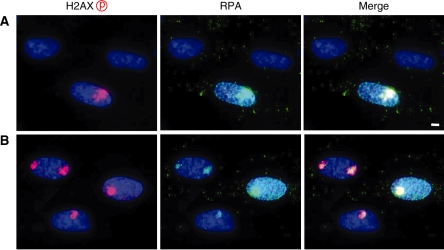
Exponentially growing normal human cells were cultured in the presence of 10 µM BrdU for up to 72 h. While ∼60% of cells were BrdU-positive at 24 h, only one-third of them showed strong BrdU-signal (Supplementary Figure S1 and Supplementary Table S1). In contrast, ∼60 and 90% of cells were labelled with BrdU at 48 and 72 h, respectively, and most of them showed strong BrdU-signal. Therefore, in the following experiments, we incubated cells with BrdU for 48–72 h before UVC irradiation. As shown in Figure 1A, BrdU labelling, by itself, did not cause phosphorylation of histone H2AX. No apparent growth retardation and cell death was induced by prolonged BrdU treatment, however, slight increase of cells with phosphorylated H2AX foci was observed in cells treated for 72 h (Supplementary Figure S2 and Supplementary Table S2). Phosphorylated H2AX foci were colocalized with those of 53BP1. Without BrdU labelling, a diffused phosphorylation signal throughout the nucleus was observed only in the S-phase cells after exposure of cells to 30 J/m2 of UVC without micro-pore membrane (Figure 1B). When the non-labelled cells were covered with micro-pore membrane, UVC irradiation (30 J/m2) induced weak dot-like signals, but these were observed only in RPA-positive S-phase cells (Figures 1C and 2A). BrdU labelling significantly increased the signal intensity of H2AX phosphorylation in all of the BrdU-positive cells exposed to 30 J/m2 of UVC, as shown in Figure 1D. With the porous membrane, such signals were localized within a part of the nucleus, and the nuclear subregions corresponding to the pores developed localized phosphorylation of histone H2AX (Figure 1E–K).
Figure 1.
Induction of DNA double-strand breaks by local micro-irradiation. Exponentially growing normal human diploid cells were incubated for 72 h with (A, D–K) or without (B and C) 10 µM BrdU. After UVC irradiation, cells were incubated for 30 min, fixed with 4% formaldehyde, permeabilized with 0.5% Triton X-100, and incubated with anti-phosphorylated histone H2AX at serine 139 as described in ‘Materials and Methods’ section. The primary antibody was detected by Alexa488-conjugated anti-mouse IgG antibody. (A) Unirradiated cells. (B–D); Cells were exposed to 30 J/m2 of UVC with (C) or without (B and D) 5-µm pore membrane. (E–H); Cells were exposed to various doses of UVC with 5-µm pore membrane. (E) 5 J/m2, (F) 10 J/m2, (G) 20 J/m2, (H) 30 J/m2. (I-K); Cells were exposed to 30 J/m2 of UVC through membranes with different pore sizes. (I) 2-µm pore membrane. (J) 3-µm pore membrane. (K) 5-µm pore membrane. Bar indicates 2 µm.
Figure 2.
Cell-cycle dependent foci formation by UV micro-irradiation. Exponentially growing normal human diploid cells were incubated for 72 h with (B) or without (A) 10 µM BrdU. Cells were washed with PBS and exposed to 30 J/m2 of UVC through 5-µm pore size membrane. Then, they were incubated for 30 min, fixed with 4% formaldehyde, permeabilized with 0.5% Triton X-100, and incubated with rabbit anti-phosphorylated histone H2AX at serine 139 and mouse anti-RPA antibodies, which were detected by Alexa594-conjugated anti-rabbit IgG antibody and Alexa488-conjugated anti-mouse IgG antibody, respectively. Bar indicates 2 µm.
Localized phosphorylation of histone H2AX was intensified by increasing the UVC dose, as shown in Figure 1E–H. Cells exposed to 5 J/m2 developed phosphorylation signals, but they were rather dispersed. The signals became stronger as increasing the UVC dose. Foci induced by 30 J/m2 were dense and showed strong fluorescence, and UVC doses higher than 30 J/m2 showed no significant change in the foci. Therefore, in most of the experiments, the cells were exposed to 30 J/m2. While the number and size of foci varied dependent upon the pore size of the membranes (Figure 1I and K), in most of the experiments, we used membranes with 5 µm pores (Figure 1K), and the average number of fluorescence foci per nucleus was ∼1.4.
DNA replication-independent induction of DNA damage
Since UVC-induced DNA photoproducts arrest DNA replication, which result in the induction of DNA double-strand breaks, cell-cycle dependent foci formation was determined. Because cells were continuously labelled with BrdU, cells in the S phase at the time of examination were visualized by anti-RPA antibody. Without BrdU labelling, UVC-induced foci were detected in 32.7% of cells (Table 1), and all of the foci-positive cells were RPA-positive (Figure 2A), confirming that only cells in the S phase developed foci. In contrast with BrdU labelling, ∼88% of BrdU-positive cells were foci-positive upon UVC irradiation. Among foci-positive cells, 61% of cells were RPA-negative (Table 1 and Figure 2B). These results indicate that BrdU labelling sensitizes cells in every cell-cycle phase. DNA replication-independent foci formation was further confirmed in density-inhibited cells and the G1-phase cells obtained by mitotic shake-off method (Supplementary Table S3). In both cases, most of the foci-positive cells were RPA-negative, indicating that UVC-induced foci were dependent on BrdU labelling but independent of DNA replication.
Table 1.
Foci formation in RPA-positive cells
| No. cells counted | No. of cells (%) |
|||
|---|---|---|---|---|
| RPA+ | Foci+ | Foci+/RPA+ | RPA+/Foci+ | |
| Without BrdU 3471 | 1261 (36.3) | 1135 (32.7) | 1135/1261 (90.0) | 1135/1135 (100) |
| With BrdU 3527 | 1352 (38.3) | 3101 (87.9) | 1203/1352 (88.9) | 1203/3101 (38.8) |
Determination of DNA double-strand break induction
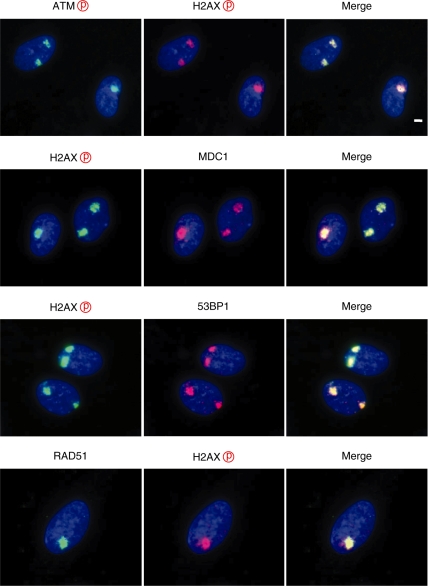
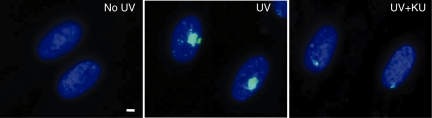
Exposure of BrdU-labelled cells to UVC in combination with micro-pore membrane developed localized foci of phosphorylated histone H2AX. Although several previous studies have shown that phosphorylated histone H2AX foci were reliable surrogate marker for DNA double-strand breaks, it needs to be determined whether or not the phosphorylated histone H2AX foci obtained in this study represent DNA double-strand breaks. In Figure 3, we check colocalization of other DNA damage checkpoint factors that form colocalized foci with those of phosphorylated histone H2AX. We found that phosphorylated ATM foci and the foci of MDC1, 53BP1 and RAD51 were colocalized with those of phosphorylated histone H2AX. Furthermore, the cells treated with a specific ATM inhibitor significantly abrogated the foci formation of phosphorylated histone H2AX (Figure 4), suggesting that the UVC-induced foci contain DNA double-strand breaks.
Figure 3.
Colocalization of DNA damage checkpoint factors. Exponentially growing normal human diploid cells were incubated for 72 h with 10 µM BrdU. Cells were washed with PBS and exposed to 30 J/m2 of UVC through 5-µm pore size membrane. Then, they were incubated for 30 min, fixed with 4% formaldehyde, permeabilized with 0.5% Triton X-100, and incubated with various antibodies as indicated. Bar indicates 2 µm.
Figure 4.
Effect of ATM inhibitor on foci formation by UV micro-irradiation. Exponentially growing normal human diploid cells were incubated for 72 h with 10 µM BrdU. Cells were treated with 40 µM of KU55933 for 30 min before exposure to UVC (UV+KU). They were washed with PBS and exposed to 30 J/m2 of UVC through 5-µm pore size membrane (UV and UV+KU). Then, they were incubated for 30 min, fixed with 4% formaldehyde permeabilized with 0.5% Triton X-100, and incubated with mouse anti-phosphorylated histone H2AX at serine 139. (No UV) Mock irradiated. Bar indicates 2 µm.
Previously, there was a report showing that nucleotide excision repair mediates the phosphorylation of histone H2AX in response to UV irradiation (38). As reported previously (31–33), we confirmed the localized formation of the cyclobutane pyrimidine dimer (CPD) and the (6-4) photoproduct after micro-irradiation (Supplementary Figure S3). Therefore, the involvement of nucleotide excision repair in foci formation was examined by using XPA cells defective in nucleotide excision repair. Without BrdU labelling and micro-pore membrane, XPA cells showed phosphorylated histone H2AX signals only in the S phase; the results were comparable to those obtained in normal human cells. With BrdU labelling and micro-pore membrane, we detected the localized foci, and there was no difference in the frequency of foci-positive cells between normal human diploid cells and two XPA fibroblasts (Table 2). Thus, it was indicated that the observed foci did not reflect nucleotide excision repair but rather reflected subnuclear regions containing DNA double-strand breaks.
Table 2.
Foci formation in XPA fibroblast cells
| Cells | Total no. cells counted | No. of foci-positive cells (%) |
|---|---|---|
| HE49 | 3121 | 2835 (90.8) |
| XP2OS | 3397 | 2805 (82.6) |
| XPEMB-1 | 3019 | 2759 (91.4) |
Repair of BrdU/UVC-induced DNA double-strand breaks
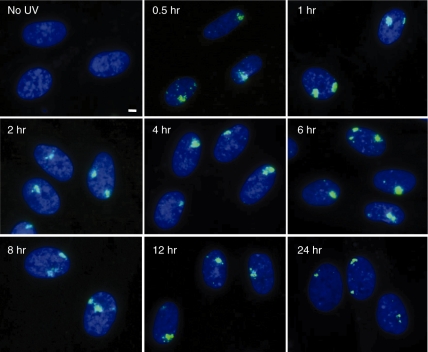
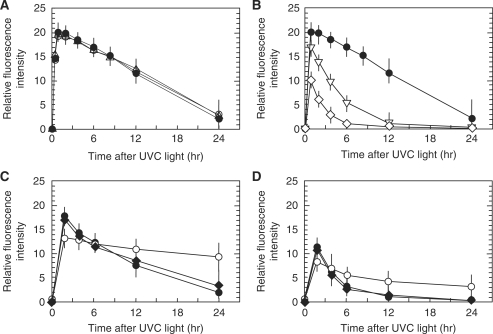
Repair of DNA double-strand breaks by UVC in combination with BrdU labelling was examined. While phosphorylation of histone H2AX could be detected a few minutes after exposure (Supplementary Figure S4), foci corresponding to pore size became clear by 0.5 h after exposure, and the maximum fluorescence was observed 1 h after UVC irradiation (Figure 5). Then, the foci intensity gradually decreased concomitant with the decrease in size. Because the localized foci comprised numerous tiny foci, multiple DNA double-strand breaks are included in the localized foci. As it was not possible to count the exact number of foci in order to evaluate DNA repair, we used the fluorescence intensity of the localized foci, which could be equivalent to the total number of tiny foci consisting of localized foci (Figure 6). The maximum fluorescence intensity was observed 1 h after UVC-irradiation, and according to the total fluorescence intensity, the amount of DNA double-strand breaks in one localized focus induced by 30 J/m2 of UVC was estimated to be equivalent to that induced by 2 Gy of X-rays. The fluorescence intensity was then decreased, and we found that the kinetics was almost the same between normal human cells and the two primary XPA cells (Figure 6A). Because the repair kinetics in cells exposed to 30 J/m2 was much slower than expected, it was also examined in cells exposed to 10 or 20 J/m2 of UVC. As shown in Figure 6B, repair kinetics was dose-dependent, and the intensity returned to the control level much faster in cells exposed to lower doses of UVC. The repair kinetics was also examined in CHO, xrs5 and EM9 cells exposed to 10 or 20 J/m2 of UVC (Figure 6C and D). Ku80-null xrs5 cells are hypersensitive to DNA double-strand breaks because of its defect in non-homologous end-joining repair, while EM9 cells are defective in single-strand break repair. As expected, the foci were efficiently repaired in CHO cells, whereas it was significantly compromised in xrs5 cells. EM9 cells showed little or no defect in foci repair.
Figure 5.
Time-dependent foci formation after UV micro-irradiation. Exponentially growing normal human diploid cells were incubated for 72 h with 10-µM BrdU. They were washed with PBS, exposed to 30 J/m2 of UVC through 5-µm pore size membrane, and incubated for various time after exposure as indicated. Then, they were fixed with 4% formaldehyde, permeabilized with 0.5% Triton X-100, and incubated with mouse anti-phosphorylated histone H2AX at serine 139. Bar indicates 2 µm.
Figure 6.
Repair of DNA double-strand breaks induced by UV micro-irradiation. (A) Exponentially growing normal human diploid cells (filled circle) and primary XPA fibroblasts (open circle; XPEMB-1, open triangle; XP2OS) were incubated for 72 h with 10 µM BrdU. They were washed with PBS, exposed to 30 J/m2 of UVC through 5 µm pore size membrane, and incubated for various time after exposure. (B) Exponentially growing normal human diploid cells were incubated for 72 h with 10 µM BrdU. They were washed with PBS, exposed to 10 J/m2 (open diamond), 20 J/m2 (open inverted triangle), or 30 J/m2 (filled circle) of UVC through 5-µm pore size membrane, and incubated for various time after exposure. (C) Exponentially growing CHO (filled circle), xrs5 (open circle) and EM9 (filled diamond) cells were incubated for 48 h with 10 µM BrdU. They were washed with PBS, exposed to 20 J/m2 of UVC through 5-µm pore size membrane, and incubated for various time after exposure. (D) Exponentially growing CHO (filled circle), xrs5 (open circle) and EM9 (filled diamond) cells were incubated for 48 h with 10 µM BrdU. They were washed with PBS, exposed to 10 J/m2 of UVC through 5 µm pore size membrane, and incubated for various time after exposure. The cells were fixed with 4% formaldehyde, permeabilized with 0.5% Triton X-100, and incubated with mouse anti-phosphorylated histone H2AX at serine 139. Digital images were captured, green dot-like signals were marked, and the sum of the pixel intensity within the marked area was measured by FW4000 software. Average fluorescence intensity was obtained from 500 dot-like signals, and average fluorescence intensity at each time point was divided by that of the mock irradiated cells to calculate relative fluorescence intensity.
DISCUSSION
DNA double-strand breaks are the lesions that show detrimental effects on cells exposed to ionizing radiation. Therefore, cells have evolved sophisticated systems for recognizing and responding to DNA double-strand breaks. ATM plays a central role in the DNA damage response, and its phosphorylation of various downstream mediators and effectors is crucial for the cellular response to DNA double-strand breaks (1–5). Phosphorylation-dependent recruitment of DNA damage checkpoint factors is indispensable for proper transduction of DNA damage signals, and it is the reason why so many studies have focused on the spatiotemporal dynamics of DNA damage checkpoint factors (6–11).
Although conventional irradiation has revealed the formation of IRIF, which represent the accumulation of such factors, it could not be applied to factors whose molecular numbers were not sufficient for detection by the immunofluorescence method. Thus, several micro-irradiation techniques, which create localized DNA double-strand breaks in a defined area, have been developed to circumvent this problem (8,14,20–28). Various laser micro-irradiation systems have been applied to investigate the dynamics of DNA damage response and repair factors. While these micro-irradiation systems are very useful for studying the spatiotemporal dynamics of DNA damage repair and checkpoint factors, they require laser sources. Therefore, in the present study we have developed a novel system for micro-irradiation using conventional UVC lamps.
The induction of DNA double-strand breaks by UVC irradiation in combination with halogenated nucleotide analogues such as BrdU was described several decades ago (34). More recently, exposure of BrdU-labelled mammalian cells to UVA in the presence of Hoechst dye has been described (30). The mechanism of sensitization of cells to UV light by BrdU substitution was investigated, and it was postulated that the primary photochemical reaction dissociates BrdU to 5-uracilyl free radical and the bromine free radical, in which the former creates a single-strand break in the cis-strand and the latter creates a single-strand break in the complementary strand (34). Thus, it is expected that BrdU in both strands are not always necessary for creating DNA double-strand breaks. In fact, cells incubated with BrdU for 24 h were already predisposed to UV-induced DNA double-strand breaks and they could induce localized damage, although the frequency of cells with foci was lower than cells treated for 48 or 72 h. Because the doubling time of the cells used in this study is approximately 24 h, BrdU is incorporated into a single-strand only, confirming that photochemical dissociation of BrdU to 5-uracilyl free radicals and the bromine free radicals could be the primary cause leading to DNA double-strand breaks. This conclusion was further supported by the experiment, in which l-ascorbic acid phosphate magnesium salt (APM), a potent radical scavenger, decreased UVC-induced foci formation (Supplementary Table S1).
Because UVC is more effective than UVB or UVA lights in photo-activating BrdU (34), we applied UVC light from a conventional germicidal lamp. It is well established that exposure of cells to UVC light induces photoproducts including CPDs and (6-4) photoproducts (Supplementary Figure 3S) (39–46). It was reported that such UV-induced DNA damage resulted in DNA replication arrest in the S phase, by which ATR-dependent phosphorylation of histone H2AX was stimulated (47–49). Furthermore, another study demonstrated that nucleotide excision repair, which is involved in the repair of UV-induced DNA damage, induced phosphorylation of histone H2AX in the G1 phase (38). Therefore, it needed to be determined whether or not the localized foci of phosphorylation of histone H2AX reflected DNA double-strand breaks caused by UVC irradiation in combination with BrdU. We first confirmed that the localized foci were solely induced in cells labelled with BrdU. While we detected BrdU-independent foci in S-phase cells (Figures 1 and 2), there was no localized foci in G1 and G2 cells without BrdU labelling. In addition, as shown in Figure 3, all of the well-known DNA damage checkpoint factors, which accumulated in the chromatin regions containing DNA double-strand breaks, were recruited to the foci of phosphorylated histone H2AX. It was also confirmed that the foci formation depended on ATM activity, which is activated in response to DNA double-strand breaks (50). The localized foci were formed independently of nucleotide excision repair, since foci formation was completely normal in the two primary XPA cells that lack nucleotide excision repair (Table 1). Furthermore, we found that disappearance of the foci was significantly abrogated in NHEJ-deficient cells but not in single-strand break repair-deficient cells (Figure 6C). Considering these results together, it is quite reasonable to conclude that the localized phosphorylated histone H2AX foci observed in this study represent DNA double-strand break formation.
Although conventional whole-cell UVC irradiation with above 20 J/m2 induced apoptotic response in unlabelled cells, apoptosis-related DNA fragmentation became obvious at least 18 h after exposure and apoptosis-positive cells were not so frequent (Supplementary Table S4). In contrast, the localized foci are detectable even 0.5 h after micro-irradiation and approximately 90% of cells were foci-positive. Furthermore, our time course experiments in Figure 5 demonstrated that no apoptotic DNA fragmentation was observed in cells micro-irradiated in combination with BrdU labelling. Therefore, we concluded that the observed signals do not reflect apoptotic response.
As shown in Figure 6B, we noticed that disappearance of foci was delayed in cells exposed to 30 J/m2 of UVC. According to our estimation, the number of DNA double-strand breaks per spot induced by 30 J/m2 of UVC was equivalent to that induced by 2 Gy of X-rays. Therefore, it was highly possible that nuclear sub-region exposed to micro-irradiation induced clustered DNA damage. In fact, we observed that the localized foci started from the cluster of tiny foci (Supplementary Figure S4). Then, each focus grew in size that finally made localized and discrete foci at 0.5 h after exposure. Such clustered DNA double-strand breaks were often reported in cells receiving high-LET radiation (51,52). It should be mentioned that clustered damage was refractory to DNA repair but the repair kinetics did not show such a long delay as observed in the present study (53,54). Therefore, it was suggested that micro-irradiation with higher UVC doses created densely clustered damage or compound damage at localized chromatin regions. In fact, repair of foci was much efficient in cells when they were exposed to lower UVC doses. Thus, different UVC doses could regulate the density of DNA double-strand breaks in a defined area.
Recently, different types of micro-irradiation systems have been developed (55). The most specialized technology is undoubtedly focal irradiation of cell nuclei by soft X-rays, charged particles or heavy ions (56–59). However, a very limited number of laboratories have access to this technology. Lately, microscope systems equipped with laser beam sources have been applied for this purpose, and several laboratories have been able to introduce the micro-irradiation systems. However, there remain some difficulties in their availability for individual experiments. Here, our simple system needs no special facilities or costly equipment, and enables micro-irradiation experiments without any specific knowledge of laser devises. The disadvantage is that UVC irradiation by itself creates UV-induced DNA damage, such as CPDs and (6-4) photoproducts. However, simple experiments, in which cells are exposed to UVC without BrdU labelling, can separate the effects of UV-induced DNA damage from those induced by DNA double-strand breaks. It has been pointed out that similar problems exist in other micro-irradiation systems (55). For example, several studies have shown that UVA lasers with BrdU labelling not only cause DNA double-strand breaks but also induce UV-induced DNA damage (28,29). Furthermore, single-strand breaks and oxidative base modification were generated concurrently with this DNA damage (Supplementary Figure S3) (24,28,29). As a result, the factors involved in the recognition and repair of such damage are recruited to the sites of local irradiation (60–63). Therefore, in any experiments, such combined induction of different types of DNA damage should always be considered in drawing a conclusion.
The micro-irradiation described here is a novel method and simple to use. However, a couple of points regarding the experimental conditions should be considered before the experiments are replicated. One is about the UVC dose for micro-irradiation. Although in the current conditions we never detected the signal of nucleotide excision repair-coupled phosphorylation of histone H2AX in the G1 or G2 phase (Figure 1B and C), micro-irradiation of cells with 100 J/m2 of UVC or 40 J/m2 UVC exposure to quiescent cells reported to visualize phosphorylation signals without BrdU-labelling (38,64). Therefore, optimization of the irradiation protocol has to be done carefully before the experiments are started. A previous study using micro-pore membrane reported the localized formation of UV-induced DNA damage (31–33). The number of damage foci per nucleus was increased as the membrane pore size decreased. This is because the frequency of pores varies depending on the pore size. We also observed pore size-dependent increased numbers of foci per nucleus, as shown in Figure 1I and K. However, membranes with less than 2 µm pore size did not create discrete foci but induce rather diffused foci. Therefore, membranes with a pore size more than 3 µm are recommended for local irradiation. The third point is about the optimal concentration of BrdU. In this study, we used 10 µM of BrdU, but in the literature, concentrations between 0.4 and 10 µM of BrdU have been used. The growth of our cells did not show any adverse effect with 10 µM of BrdU, but cells with different thymidine pool sizes may respond differently. Therefore, the optimal concentration of BrdU should be determined carefully. It was also presumed that prolonged treatment of cells with BrdU may cause adverse effects. In fact, BrdU treatment slightly increased spontaneous frequency of foci (Supplementary Figure S2 and Supplementary Table S2). Thus, the condition of BrdU treatment has to be carefully determined before experimentations
There are urgent needs to study the spatiotemporal dynamics of proteins involved in DNA damage response and DNA repair. Our novel technique is a simple and undemanding micro-irradiation method, which does not require laser sources. It also obviates the need for a true radiation irradiator to generate DNA double-strand breaks. Although the experimental conditions have to be optimized cautiously with verifying the formation of DNA double-strand breaks, our novel technique should accelerate research in the fields of DNA damage response, DNA repair and DNA recombination without the need to use any specific equipment.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
Funding for open access charge: Global Center Of Excellence (GCOE) Program from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Conflict of interest statement. None declared.
Supplementary Material
REFERENCES
- 1.Shiloh Y. ATM and related proteins kinases: safeguarding genome integrity. Nat. Rev. Cancer. 2003;3:155–168. doi: 10.1038/nrc1011. [DOI] [PubMed] [Google Scholar]
- 2.Kitagawa R, Kastan MB. The ATM-dependent DNA damage signaling pathway. Cold Spring Harb. Symp. Quant. Biol. 2005;70:99–109. doi: 10.1101/sqb.2005.70.002. [DOI] [PubMed] [Google Scholar]
- 3.Bartek J, Bartkova J, Lukas J. DNA damage signalling guards against activated oncogenes and tumour progression. Oncogene. 2007;26:7773–7779. doi: 10.1038/sj.onc.1210881. [DOI] [PubMed] [Google Scholar]
- 4.Harper JW, Elledge SJ. The DNA damage response: ten years after. Mol. Cell. 2007;28:739–745. doi: 10.1016/j.molcel.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 5.Lavin MF. Ataxia-telangiectasia: from a rare disorder to a paradigm for cell signalling and cancer. Nat. Rev. Mol. Cell Biol. 2008;9:759–769. doi: 10.1038/nrm2514. [DOI] [PubMed] [Google Scholar]
- 6.Lou Z, Minter-Dykhouse K, Franco S, Gostissa M, Rivera MA, Celeste A, Manis JP, van Deursen J, Nussenzweig A, Paull TT, et al. MDC1 maintains genomic stability by participating in the amplification of ATM-dependent DNA damage signals. Mol. Cell. 2006;21:187–200. doi: 10.1016/j.molcel.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 7.Yamauchi M, Oka Y, Yamamoto M, Niimura K, Uchida M, Kodama S, Watanabe M, Sekine I, Yamashita S, Suzuki K. Growth of persistent foci of DNA damage checkpoint factors is essential for amplification of G1 checkpoint signaling. DNA Repair. 2008;7:405–417. doi: 10.1016/j.dnarep.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 8.Paull TT, Rogakou EP, Yamazaki V, Kirchgessner CU, Gellert M, Bonner WM. A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr. Biol. 2000;10:886–895. doi: 10.1016/s0960-9822(00)00610-2. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi J, Antoccia A, Tauchi H, Matsuura S, Komatsu K. NBS1 and its functional role in the DNA damage response. DNA Repair. 2004;3:855–861. doi: 10.1016/j.dnarep.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 10.Taylor AM, Groom A, Byrd PJ. Ataxia-telangiectasia-like disorder (ATLD)-its clinical presentation and molecular basis. DNA Repair. 2004;3:1219–1225. doi: 10.1016/j.dnarep.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 11.Difilippantonio S, Nussenzweig A. The NBS1-ATM connection revisited. Cell Cycle. 2007;6:2366–2370. doi: 10.4161/cc.6.19.4758. [DOI] [PubMed] [Google Scholar]
- 12.Matsuoka S, Ballif BA, Smogorzewska A, McDonald ER, 3rd, Hurov KE, Luo J, Bakakarski CE, Zhao Z, Solimini N, Lerenthal Y, et al. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science. 2007;316:1160–1166. doi: 10.1126/science.1140321. [DOI] [PubMed] [Google Scholar]
- 13.Bonner WM, Redon CE, Dickey JS, Nakamura AJ, Sedelnikova OA, Solier S, Pommier Y. GammaH2AX and cancer. Nat. Rev. Cancer. 2008;8:957–967. doi: 10.1038/nrc2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lukas C, Bartek J, Lukas J. Imaging of protein movement induced by chromosomal breakage: tiny ‘local' lesions pose great ‘global’ challenges. Chromosoma. 2005;114:146–154. doi: 10.1007/s00412-005-0011-y. [DOI] [PubMed] [Google Scholar]
- 15.Stucki M, Jackson SP. gammaH2AX and MDC1: anchoring the DNA-damage-response machinery to broken chromosomes. DNA Repair. 2006;5:534–543. doi: 10.1016/j.dnarep.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 16.Kinner A, Wu W, Staudt C, Iliakis G. Gamma-H2AX in recognition and signalling of DNA double-strand breaks in the context of chromatin. Nucleic Acids Res. 2008;36:5678–5694. doi: 10.1093/nar/gkn550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.FitzGerald JE, Grenon M, Lowndes NF. 53BP1: function and mechanism of focal recruitment. Biochem. Soc. Trans. 2009;37:897–904. doi: 10.1042/BST0370897. [DOI] [PubMed] [Google Scholar]
- 18.Misteli T, Soutoglou E. The emerging role of nuclear architechture in DNA repair and genome maintenance. Nat. Rev. Mol. Cell Biol. 2009;10:243–254. doi: 10.1038/nrm2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Attikum H, Gasser SM. Crosstalk between modifications during the DNA damage response. Trends. Cell Biol. 2009;19:207–217. doi: 10.1016/j.tcb.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 20.Bekker-Jensen S, Lukas C, Kitagawa R, Melander F, Kastan MB, Bartek J, Lukas J. Spatial organization of the mammalian genome surveillance machinery in response to DNA strand breaks. J. Cell Biol. 2006;173:195–206. doi: 10.1083/jcb.200510130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan DW, Chen BPC, Prithivirajsingh S, Kurimasa A, Story MD, Qin J, Chen DJ. Autophosphorylation of the DNA-dependent protein kinase catalytic subunit is required for rejoining of DNA double-strand breaks. Genes Dev. 2002;16:2333–2338. doi: 10.1101/gad.1015202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen BPC, Chang DW, Kobayashi J, Burma S, Asaithamby A, Mortomi-Yano K, Botvinick E, Qin J, Chen DJ. Cell cycle dependence of DNA-dependent protein kinase phosphorylation in response to DNA double strand breaks. J. Biol. Chem. 2005;280:14709–14715. doi: 10.1074/jbc.M408827200. [DOI] [PubMed] [Google Scholar]
- 23.Rogakou EP, Boon C, Redon C, Bonner WM. Megabase chromatin domains involved in DNA double-strand breaks in vivo. J. Cell Biol. 1999;146:905–915. doi: 10.1083/jcb.146.5.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tashiro S, Walter J, Shinohara A, Kamada N, Cremer T. Rad51 accumulation at sites of DNA damage and in postreplicative chromatin. J. Cell Biol. 2000;150:283–291. doi: 10.1083/jcb.150.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lukas C, Falck J, Bartkova J, Bartek J, Lukas J. Distinct spatiotemporal dynamics of mammalian checkpoint regulators induced by DNA damage. Nat. Cell Biol. 2003;5:255–260. doi: 10.1038/ncb945. [DOI] [PubMed] [Google Scholar]
- 26.Kim JS, Krasieva TB, LaMorte VJ, Taylor AMR, Yokomori K. Specific recruitment of human cohesin to laser-induced DNA damage. J. Biol. Chem. 2002;277:45149–45153. doi: 10.1074/jbc.M209123200. [DOI] [PubMed] [Google Scholar]
- 27.Meldrum RA, Botchway SW, Wharton CW, Hirst GJ. Nonoscale spatial induction of ultraviolet photoproducts in cellular DNA by three-photon near-infrared absorption. EMBO J. 2003;12:1144–1149. doi: 10.1038/sj.embor.7400028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dinant C, de Jager M, Essers J, van Cappellen WA, Kanaar R, Houtsmuller AB, Vermeulen W. Activation of multiple DNA repair pathways by subnuclear damage induction methods. J. Cell Sci. 2007;120:2731–2740. doi: 10.1242/jcs.004523. [DOI] [PubMed] [Google Scholar]
- 29.Kong X, Mohanty SK, Stephens J, Heale JT, Gomez-Godinez V, Shi LZ, Kim J.-S, Yokomori K, Brens MW. Comparative analysis of different laser systems to study cellular responses to DNA damage in mammalian cells. Nucleic Acids Res. 2009;37:e68. doi: 10.1093/nar/gkp221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Limoli CL, Ward JF. A new method for introducing double-strand breaks into cellular DNA. Radiat. Res. 1993;134:160–169. [PubMed] [Google Scholar]
- 31.Katsumi S, Kobayashi N, Imoto K, Nakagawa A, Yamashita Y, Miramatsu T, Shirai T, Miyagawa S, Sugiura S, Hanaoka F, et al. In Situ visualization of ultraviolet-light-induced DNA damage repair in locally irradiated human fibroblasts. J. Invest. Dermatol. 2001;117:1156–1161. doi: 10.1046/j.0022-202x.2001.01540.x. [DOI] [PubMed] [Google Scholar]
- 32.Mone MJ, Volker M, Nikaido O, Mullenders LHF, van Zeeland AA, Verschure PJ, Manders EMM, van Driel R. Local UV-induced DNA damage in cell nuclei results in local transcription inhibition. EMBO Rep. 2001;2:1013–1017. doi: 10.1093/embo-reports/kve224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Volker M, Mone MJ, Karmakar P, van Hoffen A, Schul W, Vermeulen W, Hoeijmakers JHJ, van Driel R, van Zeeland AA, Mullenders LHF. Sequential assembly of the nucleotide excision repair factors in vivo. Mol. Cell. 2001;8:213–224. doi: 10.1016/s1097-2765(01)00281-7. [DOI] [PubMed] [Google Scholar]
- 34.Krasin F, Hutchinson F. Double-strand breaks from single photochemical events in DNA containing 5-bromouracil. Biophys. J. 1978;24:645–656. doi: 10.1016/S0006-3495(78)85410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fernandez-Capetillo O, Lee A, Nussenzweig M, Nussenzweig A. H2AX: the histone guardian of the genome. DNA Repair. 2004;3:959–967. doi: 10.1016/j.dnarep.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 36.Olive PL. Detection of DNA damage in individual cells by analysis of histone H2AX phosphorylation. Methods Cell Biol. 2004;75:355–373. doi: 10.1016/s0091-679x(04)75014-1. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki K, Okada H, Yamauchi M, Oka Y, Kodama S, Watanabe M. Qualitative and quantitative analysis of phosphorylated ATM foci induced by low dose ionizing radiation. Radiat. Res. 2006;165:499–504. doi: 10.1667/RR3542.1. [DOI] [PubMed] [Google Scholar]
- 38.Marti TM, Hefner E, Feeney L, Natale V, Cleaver JE. H2AX phosphorylation within the G1 phase after UV irradiation depends on nucleotide excision repair and not DNA double-strand breaks. Proc. Natl Acad. Sci. USA. 2006;103:9891–9896. doi: 10.1073/pnas.0603779103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Setlow RB. Cyclobutane-type pyrimidine dimers in polynucleotides. Science. 1966;153:379–389. doi: 10.1126/science.153.3734.379. [DOI] [PubMed] [Google Scholar]
- 40.Mitchell DL, Nairn RS. The biology of the (6-4)photoproduct. Photochem. Photobiol. 1989;49:805–819. doi: 10.1111/j.1751-1097.1989.tb05578.x. [DOI] [PubMed] [Google Scholar]
- 41.Bootsma D, Heijmakers JH. The moleculra basis of nucleotide excision repair syndromes. Mutat. Res. 1994;307:15–23. doi: 10.1016/0027-5107(94)90273-9. [DOI] [PubMed] [Google Scholar]
- 42.Friedberg EC. DNA damage and repair. Nature. 2003;421:436–440. doi: 10.1038/nature01408. [DOI] [PubMed] [Google Scholar]
- 43.Cleaver JE. Cancer in xeroderma pigmentosum and related disorders of DNA repair. Nat. Rev. Cancer. 2005;5:564–573. doi: 10.1038/nrc1652. [DOI] [PubMed] [Google Scholar]
- 44.Reardon JT, Sancar A. Nucleotide excision repair. Prog. Nucleic Acid Res. Mol. Biol. 2005;79:183–235. doi: 10.1016/S0079-6603(04)79004-2. [DOI] [PubMed] [Google Scholar]
- 45.Beukers R, Eker AP, Lohman PH. 50 years thymine dimer. DNA Repair. 2008;7:530–543. doi: 10.1016/j.dnarep.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 46.Hanawalt PC, Spivak G. Transcription-coupled DNA repair: two decades of progress and surprise. Nat. Rev. Mol. Cell Biol. 2008;9:958–970. doi: 10.1038/nrm2549. [DOI] [PubMed] [Google Scholar]
- 47.Cortez D, Guntuku S, Qin J, Elledge SJ. ATR and ATRIP: partners in checkpoint signaling. Science. 2001;294:1713–1716. doi: 10.1126/science.1065521. [DOI] [PubMed] [Google Scholar]
- 48.Ward IM, Chen J. Histone H2AX is phosphorylated in an ATR-dependent manner in response to replicational stress. J. Biol. Chem. 2001;276:47759–47762. doi: 10.1074/jbc.C100569200. [DOI] [PubMed] [Google Scholar]
- 49.Stiff T, Walker SA, Cerosaletti K, Goodarzi AA, Petermann E, Concannon P, O'Driscoll M, Jeggo PA. ATR-dependent phosphorylation and activation of ATM in response to UV treatment or replication fork stalling. EMBO J. 2006;25:5775–5782. doi: 10.1038/sj.emboj.7601446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature. 2003;421:499–506. doi: 10.1038/nature01368. [DOI] [PubMed] [Google Scholar]
- 51.Aten JA, Stap J, Krawczyk PM, van Oven CH, Hoebe RA, Essers J, Kanaar R. Dynamics of DNA double-strand breaks revealed by clustering of damaged chromosome domains. Science. 2004;303:92–95. doi: 10.1126/science.1088845. [DOI] [PubMed] [Google Scholar]
- 52.Karlsson KH, Stenerlow B. Focus formation of DNA repair proteins in normal and repair-deficient cells irradiated with high-LET ions. Radiat. Res. 2004;161:517–527. doi: 10.1667/rr3171. [DOI] [PubMed] [Google Scholar]
- 53.Desai N, Davis E, O'Neill P, Durante M, Cucinotta FA, Wu H. Immunofluorescence detection of clustered gamma-H2AX foci induced by HZE-particle radiation. Radiat. Res. 2005;164:518–522. doi: 10.1667/rr3431.1. [DOI] [PubMed] [Google Scholar]
- 54.Asithamby A, Uematsu N, Chatterjee A, Story MD, Burma S, Chen DJ. Repair of HZE-particle-induced DNA double-strand breaks in normal human fibroblasts. Radiat. Res. 2008;169:437–446. doi: 10.1667/RR1165.1. [DOI] [PubMed] [Google Scholar]
- 55.Nagy Z, Soutoglou E. DNA repair: easy to visualize, difficult to elucidate. Trends Cell Biol. 2009;19:617–629. doi: 10.1016/j.tcb.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 56.Tartier L, Spenlehauer C, Newman HC, Folkard M, Prise KM, Michael BD, Menissier-de Murcia J, de Murcia G. Local DNA damage by proton microbeam irradiation induces poly(ADP-ribose) synthesis in mammalian cells. Mutagenesis. 2003;18:411–416. doi: 10.1093/mutage/geg015. [DOI] [PubMed] [Google Scholar]
- 57.Hauptner A, Krucken R, Grenbel C, Hable V, Dollinger G, Drexler GA, Deutsch M, Lowe R, Friedl AA, Dietzel S, et al. DNA-repair protein distribution along the tracks of energetic ions. Radiat. Prot. Diodosimetry. 2006;122:147–149. doi: 10.1093/rpd/ncl420. [DOI] [PubMed] [Google Scholar]
- 58.Jakob B, Splinter J, Durante M, Taucher-Scholz G. Live cell microscopy analysis of radiation-induced DNA double-strand break motion. Proc. Natl Acad. Sci. USA. 2009;106:3172–3177. doi: 10.1073/pnas.0810987106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van Oven C, Krawczyk PM, Stap J, Melo AM, Piazzetta MHO, Gobbi AL, van Veen HA, Verhoeven J, Aten JA. An ultrasoft X-ray multi-microbeam irradiation system for studies of DNA damage responses by fixed-and live-cell fluorescence microscopy. Eur. Biophys. J. 2009;38:721–728. doi: 10.1007/s00249-009-0472-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cremer C, Cremer T, Fukuda M, Nakanishi K. Detection of lase-UV microirradiation-induced DNA photolesions by immunofluorescent staining. Hum. Genet. 1980;54:107–110. doi: 10.1007/BF00279058. [DOI] [PubMed] [Google Scholar]
- 61.Lan L, Nakajima S, Oohata Y, Takao M, Okano S, Masutani M, Wilson SH, Yaui A. In situ analysis of repair processes for oxidative DNA damage in mammalian cells. Proc. Natl Acad. Sci. USA. 2004;101:13738–13743. doi: 10.1073/pnas.0406048101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hashiguchi K, Matsumoto Y, Yasui A. Recruitment of DNA repair synthesis machinery to sites of DNA damage/repair in living human cells. Nucleic Acids Res. 2007;35:2913–2923. doi: 10.1093/nar/gkm115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mortusewicz O, Ame J-C, Schreiber V, Leonhardt H. Feedback-regulated poly(ADP-ribosyl)ation by PARP-1 is required for rapid response to DNA damage in living cells. Nucleic Acids Res. 2007;35:7665–7675. doi: 10.1093/nar/gkm933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Matsumoto M, Yaginuma K, Imura M, Hasegawa M, Iwabuchi K, Sate T, Mori Y, Ishizaki K, Yamashita K, Inobe M, et al. Perturbed gap-filling synthesis in nucleotide excision repair causes histone H2AX phosphorylation in human quiescent cells. J. Cell Sci. 2007;120:1104–1112. doi: 10.1242/jcs.03391. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.