Abstract
Estrogen–DNA adducts are potential biomarkers for assessing the risk and development of estrogen-associated cancers. 4-Hydroxyequilenin (4-OHEN) and 4-hydroxyequilin (4-OHEQ), the metabolites of equine estrogens present in common hormone replacement therapy (HRT) formulations, are capable of producing bulky 4-OHEN–DNA adducts. Although the formation of 4-OHEN–DNA adducts has been reported, their quantitative detection in mammalian cells has not been done. To quantify such DNA adducts, we generated a novel monoclonal antibody (4OHEN-1) specific for 4-OHEN–DNA adducts. The primary epitope recognized is one type of stereoisomers of 4-OHEN–dA adducts and of 4-OHEN–dC adducts in DNA. An immunoassay with 4OHEN-1 revealed a linear dose–response between known amounts of 4-OHEN–DNA adducts and the antibody binding to those adducts, with a detection limit of approximately five adducts/108 bases in 1 µg DNA sample. In human breast cancer cells, the quantitative immunoassay revealed that 4-OHEN produces five times more 4-OHEN–DNA adducts than does 4-OHEQ. Moreover, in a mouse model for HRT, oral administration of Premarin increased the levels of 4-OHEN–DNA adducts in various tissues, including the uterus and ovaries, in a time-dependent manner. Thus, we succeeded in establishing a novel immunoassay for quantitative detection of 4-OHEN–DNA adducts in mammalian cells.
INTRODUCTION
Hormone replacement therapy (HRT) is widely used to decrease menopausal symptoms and to protect against osteoporosis in post-menopausal women (1). However, long-term HRT increases the incidence of breast (2–4), ovarian (5,6) and endometrial cancers (7), and the risk of those cancers increases with increasing duration of HRT (3–5,8). Premarin (Wyeth–Ayerst) is the most common drug used for HRT and is composed of approximately 50% estrogens and 40% equine estrogens [equilenin (EN) and equilin (EQ)] (9).
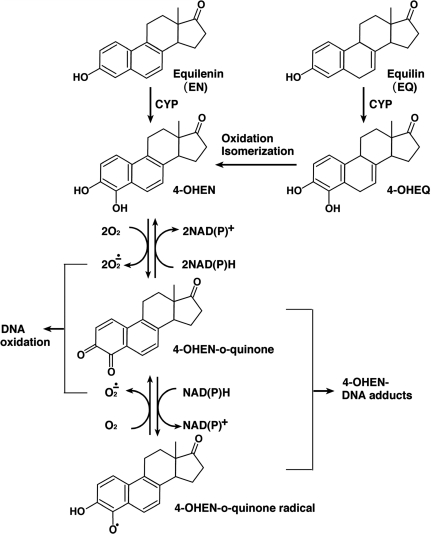
In vitro experiments have shown that equine estrogens are successively metabolized and are capable of forming various types of DNA damage (9–11) (Figure 1). Like estrogen, EN and EQ are metabolized by cytochrome P450 enzymes (CYP) to their 4-hydroxy and 2-hydroxy forms (9,10). 4-Hydroxyequilenin (4-OHEN) is rapidly auto-oxidized to an o-quinone (4-OHEN-o-quinone) which in turn readily reacts with DNA, resulting in the formation of unique dC, dA and dG adducts (4-OHEN–DNA adducts) with four possible stereoisomers for each base adduct (9,11,12) (Figure 2). 4-Hydroxyequilin (4-OHEQ) is also autoxidized to an o-quinone which isomerizes to 4-OHEN-o-quinone. As a result, 4-OHEQ and 4-OHEN produce the same 4-OHEN–DNA adduct (13). Simultaneously, oxidative DNA damage, such as 7,8-dihydro-8-oxodeoxyguanine (8-oxodG), is also generated by reactive oxygen species through redox cycling between the o-quinone of 4-OHEN and its semiquinone radicals (14).
Figure 1.
Metabolic pathway of equine estrogens.
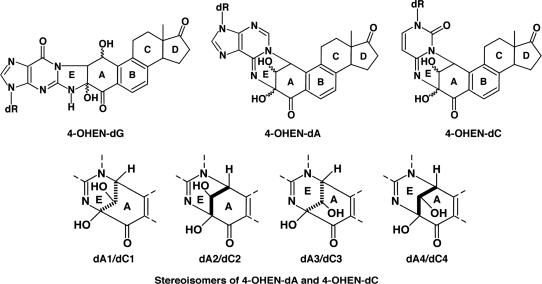
Figure 2.
Structures of 4-OHEN–DNA adducts and their stereoisomers. 4-OHEN–dA and 4-OHEN–dC form similar stereoisomers with four different types. dA1/dC1, 4-OHEN–dA1/4-OHEN–dC1; dA2/dC2, 4-OHEN–dA2/4-OHEN–dC2; dA3/dC3, 4-OHEN–dA3/4-OHEN–dC3; dA4/dC4, 4-OHEN–dA4/4-OHEN–dC4.
Indeed, when 4-OHEN was injected into mammary fat pads of rats, various types of DNA damage, including 4-OHEN-dG, 4-OHEN–dA and 8-oxodG were detected in the extracted mammary tissues (15). 4-OHEN–DNA adducts were also detected in two out of the seven DNA samples from five human breast tumors and two adjacent normal tissues of donors with a history of Premarin-based HRT (16). 4-OHEN-dC and 4-OHEN–dA adducts have been reported to be frequently miscoded during DNA synthesis catalyzed by human DNA polymerases that are highly expressed in reproductive organs (17–22). Moreover, the treatment of male Syrian hamsters for 9 months with Premarin and a mixture of EN and EQ resulted in 100% tumor incidence with many tumor foci in the kidneys (23). These results suggest that 4-OHEN–DNA adducts are specific biomarkers for assessing the risk and development of equine estrogen-associated cancers.
However, although the formation of 4-OHEN–DNA adducts has been reported, their quantitative detection in human or rodent samples has not been accomplished. Obstacles that hamper such experiments might come from the technically demanding nature of current methods for adduct detection (24). One popular current method is liquid chromatography electrospray ionization tandem mass spectrometry (LC–MS/MS) (25), which is potentially one of the most sensitive and accurate methods. However, it requires complete enzymatic digestion of the sample DNA into deoxynucleosides for accurate quantification of 4-OHEN base adducts (15,16,26). Moreover, the determination of each particular type of base adduct may force the technique close to the detection limit, because sample DNAs generally contain small amounts of 4-OHEN–DNA adducts which are theoretically composed of 12 different stereoisomers of dC, dA and dG adducts (9,11,12). In sharp contrast, most immunoassays using specific antibodies are capable of detecting DNA adducts without DNA hydrolysis (27–31). Therefore, immunoassays can be rapid, sensitive and reproducible, and can also be assessed by immunofluorescence to visualize DNA adducts within individual cells or a tissue. Moreover, they do not require radiolabeled compounds or expensive specialized instruments. However, since immunoassays using an antibody as a probe are indirect methods, the selective binding of the antibody to the specific DNA lesion is essential. For this reason, a monoclonal antibody specific for the DNA lesion appears to be the best choice, but as yet no monoclonal antibodies specific for 4-OHEN–DNA adducts have been established.
In the present study, we generated a novel monoclonal antibody that is highly specific for 4-OHEN–DNA adducts. An immunoassay with that antibody revealed a linear dose–response between known amounts of 4-OHEN–DNA adducts and the antibody binding to those adducts. In human breast cancer cells, the quantitative immunoassay revealed that 4-OHEN produces five times more 4-OHEN–DNA adducts than does 4-OHEQ. Moreover, in a mouse model for HRT, oral administration of Premarin increased the levels of 4-OHEN–DNA adducts in various tissues, including the uterus and ovaries, in a time-dependent manner.
MATERIALS AND METHODS
Chemicals
4-OHEN, 4-OHEQ and 4-OHEN-modified DNA containing 5.2 4-OHEN–dC adducts/104 bases and 1.2 4-OHEN–dA adducts/104 bases were prepared as described previously (12,19). 4-OHEN and 4-OHEQ were dissolved in acetone. An oligonucleotide containing a single 4-OHEN adduct (4-OHEN-oligo; 5′ -TTTGTXTTTT-3′) (where X is a stereoisomer of 4-OHEN-dA or 4-OHEN-dC) was prepared as described previously (17,19). An oligonucleotide containing a single deoxyguanosine-N2-6β-estradiol (dG-N2-6β-E2) (5′-GAGGTGCXTGTTTGT-3′) (where X is dG-N2-6β-E2) was also prepared as described previously (32). Unmodified oligonucleotides and methylated bovine serum albumin (mBSA) were purchased from Sigma Genosys Japan (Ishikari, Hokkaido, Japan) and from Calbiochem (San Diego, CA), respectively. N-Acetoxy-2-acetylaminofluorene-modified DNA (AAF-DNA) was prepared as described previously (31). UV-irradiated DNA (UV-DNA) was prepared by irradiation with 254-nm UV at 300 J/m2. Premarin was purchased from Wyeth–Ayerst (Philadelphia, PA).
Cell culture
Mouse myeloma cells (P3X63Ag8.653; Flow Laboratories, McLean, VA) and human breast cancer cells (MCF-7; a kind gift from Dr K. Shimoi, University of Shizuoka, Shizuoka, Japan) were cultured in Dulbecco’s modified Eagle’s medium (Nissui Seiyaku, Tokyo, Japan) supplemented with 10% fetal bovine serum and antibiotics. We designate this culture medium as growth medium.
Immunization
All animal experiments in this study were conducted according to the Guidelines for Animal Welfare and Experimentation at Nara Medical University. 4-OHEN-modified DNA described above was dissolved in phosphate-buffered saline (PBS; pH 7.4) and denatured by heating in boiled water for 10 min followed by rapid chilling in an ice bath. 4-OHEN-modified single-stranded DNA (4-OHEN-ssDNA; 500 µg/ml) was electrostatically conjugated with 500 µg/ml mBSA. The complex was then emulsified with an equal volume of complete Freund’s adjuvant (Difco Laboratories, Detroit, MI) and used as an immunogen. BALB/c mice (7-week-old female; Oriental BioService, Kyoto, Japan) were injected intraperitoneally with this immunogen (125 µg complex in 0.5 ml). Similar immunizations were carried out 2, 4 and 6 weeks later. One week after the fourth immunization, a booster injection of the immunogen without adjuvant was given into the tail vein.
Preparation of hybridoma cells
Three days after the booster injection, the spleen of the mouse was removed and dissociated by passage through 100-mesh steel gauze. The spleen cells (2×108) were fused with an equal number of myeloma cells in the presence of 50% polyethylene glycol 1500 (Boehringer Mannheim). The fused cells were suspended in selective growth medium (33) supplemented with 5% BriClone (Archport, Dublin, Ireland). The cell suspension (2×105 hybrid cells) was distributed into each well of 96-well culture plates and cultured with periodic changes of medium. Supernatants from wells containing hybridoma colonies were screened for the presence of specific antibodies using a direct enzyme-linked immunosorbent assay (ELISA) described below. The hybridoma cells from a positive well were cloned twice by limiting dilution. The clone was expanded in culture, and antibody-rich supernatants were concentrated by ammonium sulfate precipitation, dialyzed against PBS and stored at −80°C.
ELISA
Binding of the monoclonal antibodies to antigens was measured using an ELISA (a direct ELISA). Polyvinylchloride flat-bottom 96-well microtiter plates (Thermo, Milford, MA; Part No. 2801), precoated with 0.003% protamine sulfate, were coated with 4-OHEN-ssDNA or ssDNA overnight at 37°C. After washing five times with PBS-T (0.05% Tween-20 in PBS), the plates were blocked with 2% fetal bovine serum in PBS for 30 min. After washing five times with PBS-T, the solid-phase antigens were detected by 30-min incubation with the monoclonal antibodies described below. The plates were washed five times with PBS-T, followed by 30-min incubation with goat anti-mouse IgG conjugated to peroxidase (diluted 1:3000 in PBS; Zymed, South San Fransisco, CA). After washing five times with PBS-T, the plates were finally incubated with the substrate solution consisting of 0.04% o-phenylene diamine and 0.007% H2O2 in citrate-phosphate buffer (pH 5.0) for 30 min. After stopping the reaction with 2 M H2SO4, the absorbance of colored products derived from o-phenylene diamine was measured at 492 nm. To determine very low levels of 4-OHEN–DNA adducts, a biotin–streptavidin system was adopted to a direct ELISA (a sensitive direct ELISA). The method was the same as that described above, except that goat anti-mouse IgG conjugated to biotin, F(ab′)2 fragment (diluted 1:2000 in PBS; Zymed) and then to streptavidin-multiple-peroxidase (diluted 1:2500 in PBS; AMDEX, GE Healthcare UK, Little Chalfont, UK) were used instead of goat anti-mouse IgG conjugated to peroxidase. For the competitive inhibition assay with ELISA (a competitive ELISA), the plates were coated with 4-OHEN-ssDNA. The method was the same as that used with the sensitive direct ELISA, except that in addition to utilizing streptavidin-peroxidase (diluted 1:10000 in PBS; Zymed) instead of streptavidin-multiple-peroxidase, the amount of monoclonal antibody which gives 50% of the maximum binding to the solid-phase antigen and various concentrations of the competitor were successively added as described previously (31).
Generation of a standard dose–response curve
DNA samples containing various known amounts of DNA adducts (5–50 adducts/108 bases) were prepared by mixing 4-OHEN-modified DNA described above and unmodified DNA in different ratios. After coating their heat-denatured samples on plates (1 µg/well), the sensitive direct ELISA with the antibody (diluted 1:1000 in PBS) was performed to generate a standard dose–response curve between the amounts of 4-OHEN–DNA adducts and the antibody binding to those adducts. A standard dose–response curve was generated in each ELISA experiment for determining the absolute amount of 4-OHEN–DNA adducts in sample DNA. For analysis of statistical significance, Student’s t-tests were performed between modified and unmodified DNA samples.
Measurement of induction of 4-OHEN–DNA adducts in human breast cancer cells
MCF-7 cells were cultured in 100-mm dishes and were exposed to various concentrations of 4-OHEN or 4-OHEQ for 3 h. After washing twice with Dulbecco’s PBS (DPBS), cells were harvested using a cell scraper and genomic DNAs of the cells were purified using a QIAamp Blood Kit (QIAGEN, Hilden, Germany). Each heat-denatured DNA sample (1 µg) was coated in quadruplicate in each well of a 96-well microtiter plate. 4-OHEN–DNA adducts were then detected using the sensitive direct ELISA and quantified using the standard dose–response curve obtained from the same plate.
Measurement of induction of 4-OHEN–DNA adducts in various mouse tissues
As a mouse model for HRT, female BALB/c mice (9 months old; Oriental BioService) were fed powdered food (CE-2, Clea Japan, Tokyo, Japan) mixed with or without powdered Premarin tablets (20.7 µg/g) for 4 or 12 weeks. Each group consisted of five mice. The intake of food and the body weights of mice were measured twice a week during the experiment. The average daily intake of Premarin for each mouse was 74 µg/32g body weight, which is roughly 100 times higher than the human daily dose (1.25 mg/60 kg body weight) used for HRT. After completing the drug treatment, the mice were sacrificed and five different tissues (liver, kidney, spleen, uterus and ovary) of each mouse were removed, quickly frozen in liquid nitrogen and stored at −80°C. After being thawed and minced, DNA was extracted from each tissue using the standard phenol–chloroform method. 4-OHEN–DNA adducts were then detected using the sensitive direct ELISA and quantified using the standard dose–response curve obtained from the same plate. For analysis of statistical significance, Student’s t-tests were performed between treated and untreated samples.
RESULTS
Generation of a novel monoclonal antibody, 4OHEN-1
Supernatants from 404 wells containing hybridoma cells were tested for production of antibodies that could bind 4-OHEN-ssDNA and/or ssDNA using the direct ELISA, and one was found to have preferential binding to 4-OHEN-ssDNA. After cloning those cells twice, we designated the monoclonal antibody as 4OHEN-1. Isotype analysis (Serotec, Oxford, UK) revealed that 4OHEN-1 is of the IgG1 (kappa) subclass.
The antibody is highly specific for 4-OHEN–DNA adducts such as 4-OHEN-dC and 4-OHEN-dA
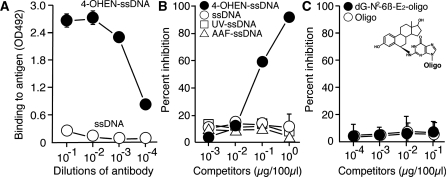
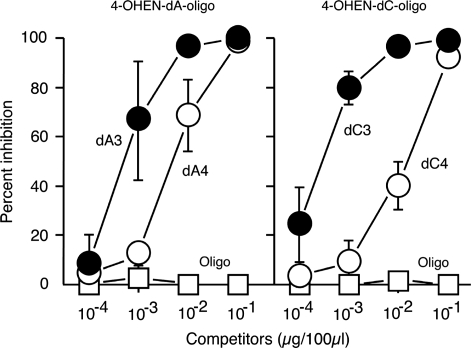
To characterize the binding specificity of 4OHEN-1, different dilutions of it were tested for binding to 4-OHEN-ssDNA and to ssDNA using the direct ELISA (Figure 3A). 4OHEN-1 showed high binding to 4-OHEN-ssDNA but had undetectable or minimal binding to undamaged ssDNA. We next examined whether 4OHEN-1 specifically binds to 4-OHEN–DNA adducts but not to other types of DNA damage using the competitive ELISA (Figure 3B). 4-OHEN-ssDNA efficiently inhibited antibody binding to 4-OHEN-ssDNA, but UV-ssDNA, AAF-ssDNA or undamaged ssDNA did not, indicating that 4OHEN-1 specifically binds to 4-OHEN–DNA adducts but does not bind to general DNA helix distortions induced by various types of DNA damage. It has been suggested that 4-OHEN–DNA adducts include 4-OHEN–dC, 4-OHEN–dA and 4-OHEN–dG, with four different stereoisomers for each base adduct (4-OHEN–dC1–4, 4-OHEN–dA1–4 and 4-OHEN–dG1–4) (34–39) (Figure 2). Among those adducts, the 4-OHEN–dC3, –dC4, –dA3 and –dA4 isomers are thought to be the major types (12,24,40). We examined whether those isomers comprised the antigenic determinant in the 4-OHEN–DNA adducts recognized by 4OHEN-1 (Figure 4). Indeed, oligonucleotides containing 4-OHEN–dC3, –dC4, –dA3 or –dA4 inhibited the antibody binding to 4-OHEN-ssDNA in a concentration-dependent manner. The 4-OHEN–dC3 and –dA3 isomers showed higher inhibition of antibody binding than did the 4-OHEN–dC4 and –dA4 isomers, indicating that the 4-OHEN–dC3 and –dA3 isomers of DNA are the primary epitope recognized by 4OHEN-1. We confirmed that 4OHEN-1 does not bind to a DNA adduct derived from endogenous estrogen (17ß-estradiol); deoxyguanosine-N2-6β-estradiol (dG-N2-6β-E2) (Figure 3C). Taken together, these results demonstrate that 4OHEN-1 is highly specific for 4-OHEN–DNA adducts in DNA such as 4-OHEN–dC and –dA isomers.
Figure 3.
The novel monoclonal antibody is specific for 4-OHEN-DNA adducts. (A) The antibody shows high binding to 4-OHEN-ssDNA but undetectable or minimal binding to undamaged ssDNA. Different dilutions of the antibody were tested for binding to immobilized denatured antigens (50 ng/well) using a direct ELISA. (B) The antibody binds to 4-OHEN–DNA adducts but not to other types of DNA damage. Competitive inhibition of the antibody (1/10 000) binding to immobilized 4-OHEN-ssDNA (40 ng/well) by various competitors was performed using a competitive ELISA. (C) The antibody does not bind to a DNA adduct derived from endogenous estrogen (17β-estradiol). Competitive inhibition of the antibody (1/3000) binding to immobilized 4-OHEN-ssDNA (5 ng/well) by various competitors was performed using a competitive ELISA. oligo, oligonucleotide; dG-N2-6β-E2-oligo, oligonucleotide containing a deoxyguanosine-N2-6β-estradiol. Each point shows the mean (±SD) of three independent experiments.
Figure 4.
The primary epitope recognized is contained in 4-OHEN–dA3 and –dC3 adducts in DNA. Competitive inhibition of antibody (1/3000) binding to immobilized 4-OHEN-ssDNA (5 ng/well) by various competitors was performed using a competitive ELISA. oligo, oligonucleotide; dA3, 4-OHEN–dA3-oligo; dA4, 4-OHEN–dA4-oligo; dC3, 4-OHEN–dC3-oligo; dC4, 4-OHEN-dC4-oligo. Each point shows the mean (±SD) of three independent experiments.
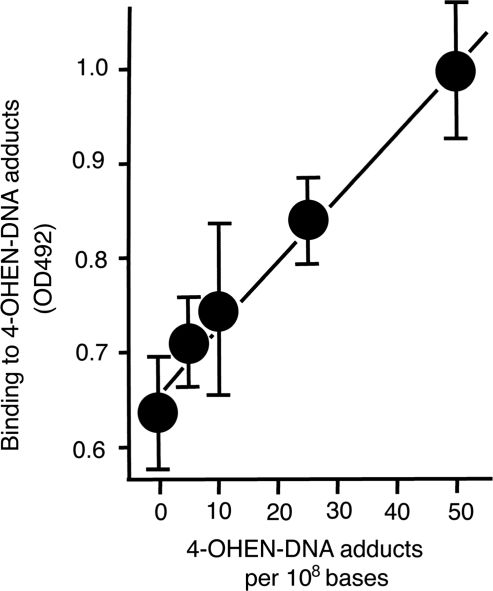
A linear standard dose–response curve was obtained
4OHEN-1 was used in the sensitive direct ELISA to detect 4-OHEN-derived adducts in DNA. A linear standard dose–response curve between amounts of 4-OHEN–DNA adducts coated in the wells (5–50 adducts/108 bases) and antibody binding was obtained (Figure 5). A similar linear dose–response was also obtained at a higher dose range (25–300 adducts/108 bases) (data not shown). Student’s t-tests indicated significant differences (P < 0.05) in OD values between DNA containing zero adduct and five adducts per 108 bases.
Figure 5.
The sensitive direct ELISA reveals the linear dose–response between the amounts of 4-OHEN-DNA adducts and the antibody binding to those adducts. After coating denatured DNA samples containing various known amounts of DNA adducts on plates (1 µg/well), the sensitive direct ELISA with the antibody (1/1000) was performed to generate a standard dose–response curve. Each point shows the mean (± SD) of four independent experiments.
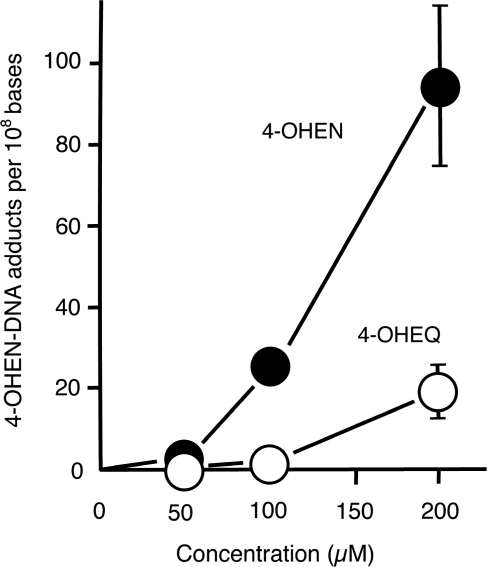
4-OHEN and 4-OHEQ produce 4-OHEN–DNA adducts in a dose-dependent manner in human breast cancer cells
Premarin includes two types of equine estrogens (EN and EQ), thus we examined whether either of their metabolites (4-OHEN and 4-OHEQ) induce 4-OHEN–DNA adducts in MCF-7 cells using the sensitive direct ELISA (Figure 6). We found that 3-h exposure to either chemical produces 4-OHEN–DNA adducts in a concentration-dependent manner, and that 4-OHEN forms five times more 4-OHEN–DNA adducts than does 4-OHEQ.
Figure 6.
4-OHEN produces five times more 4-OHEN–DNA adducts than does 4-OHEQ. MCF-7 cells were exposed to either 4-OHEN or 4-OHEQ in various concentrations for 3 h. The induction of 4-OHEN–DNA adducts was then quantified using the sensitive direct ELISA as shown in Figure 5. Each point shows the mean (±SD) of three independent experiments.
Oral administration of Premarin induces 4-OHEN–DNA adducts in tissues of aged female mice
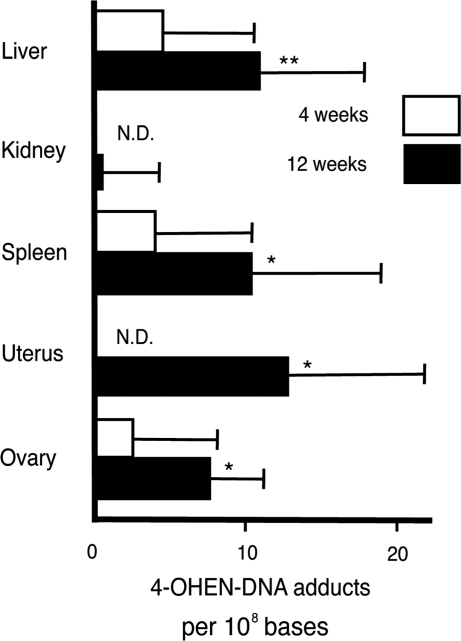
To verify that oral administration of Premarin results in the formation of 4-OHEN–DNA adducts in tissues of aged female mice, quantitative detection of the DNA adducts was performed using the sensitive direct ELISA (Figure 7). In mice treated with Premarin for 4 weeks, 4-OHEN–DNA adducts were detected in the liver, spleen and ovary (with 4.5, 3.9 and 2.5 adducts per 108 bases, respectively) but were not detected in the kidney or uterus, though these amounts are close to the detection limit. The levels of DNA adducts increased in all tissues examined in mice treated with Premarin for 12 weeks. We detected 10.9, 0.48, 10.3, 13.1 and 7.6 adducts per 108 bases, respectively, in the liver, kidney, spleen, uterus and ovary. Those amounts of DNA adducts were statistically significant except in the kidney. These results indicate that oral administration of Premarin induces 4-OHEN–DNA adducts in a time-dependent manner, and that relatively similar amounts of DNA adducts are produced in the tissues examined except for the kidney.
Figure 7.
Oral administration of Premarin induces 4-OHEN–DNA adducts in tissues of aged female mice. As a mouse model for HRT, 9-month-old female mice were orally treated with Premarin for 4 or 12 weeks. 4-OHEN–DNA adducts in tissues of each mouse were then quantified using the sensitive direct ELISA as shown in Figure 5. Each bar shows the mean (±SD) of five independent experiments. Significant differences between Premarin-treated and untreated samples are noted (*P < 0.05, **P < 0.01). N.D.; not detected.
DISCUSSION
A novel monoclonal antibody (4OHEN-1) has been generated that is specific for 4-OHEN-DNA adducts, and the primary epitope recognized is contained in the 4-OHEN–dC3 and –dA3 adducts (Figure 2). The presence of the 4–OHEN-derived structure common to 4-OHEN–dC and –dA adducts accounts for this. We have not determined the binding activity to 4-OHEN–dG adducts because of the unavailability of oligonucleotides containing them. As the 4-OHEN–dC and –dA adducts are known to be the major products generated in DNA treated with 4-OHEN (12,24,40), this binding specificity is advantageous to increase the sensitivity for the detection of 4-OHEN–DNA adducts. We have previously prepared a monoclonal antibody specific for AAF-DNA adduct (31). Although two types of AAF adducts [N-(deoxyguanosin-8-yl)-2-acetylaminofluorene (dG-C8-AAF) and 3-(deoxyguanosin-N2-yl)-2-acetylaminofluorene (dG-N2-AAF)] are produced at guanine sites of DNA, the antibody can discriminate between the adducts, binds specifically to the former but not to the latter. Moreover, when dG-C8-AAF is slightly changed into dG-C8-AF by deacetylation, the binding affinity is reduced by more than 10-fold, showing its highly specific binding. Similarly, in the present study, the binding affinity to another stereoisomer of 4-OHEN–dC and –dA adducts is reduced by 10-fold or more (Figure 4), indicating the excellent binding specificity of 4OHEN-1. Indeed, the antibody did not cross-react with a DNA adduct derived from endogenous estrogen; deoxyguanosine-N2-6β-estradiol that lacks an OH moiety from the DNA adduct derived from 2-OHE2 (Figure 3C). Regarding the cross-reactivity with 4-OHEN–RNA adducts, it is unable to test because no identified standards are available. However, this issue seems not to much affect the practical detection using a sensitive direct ELISA, since RNA is carefully removed during extracting DNA. The 4OHEN-1 antibody binds to 4-OHEN–DNA adducts with high affinity, and the 50% antibody-binding inhibition values for 4-OHEN–dC3 and –dA3 adducts were calculated as 8.83 × 10−10 M and 1.57 × 10−9 M, respectively (Figure 4). Those values are superior or comparable to those of other useful polyclonal and monoclonal antibodies that recognize carcinogen–DNA adducts (31,41). Moreover, the 4OHEN-1 antibody is capable of binding to 4-OHEN adducts without requiring the hydrolysis of the DNA to nucleosides. This is a very important property of an antibody for damage detection, because this enables it to be applied in immunofluorescence to visualize DNA adducts in cells or tissues, to save time for experiments and to avoid a possible decrease in detection sensitivity due to incomplete DNA hydrolysis (24). Indeed, 4-OHEN–DNA adducts were visualized in the nuclei of 4-OHEN-treated MCF-7 cells by using immunofluorescence with 4OHEN-1 (data not shown). It is worth noting that all other competitive methods require DNA hydrolysis for damage detection (12,15,16,26).
Based on these promising results, 4OHEN-1 was applied in ELISA to determine 4-OHEN-derived adducts in various DNA samples. It is worth noting that only monoclonal antibodies with high specificity (not polyclonal antibodies) are practically applicable to a direct binding ELISA. The sensitive direct ELISA revealed a linear dose–response between known amounts of 4-OHEN–DNA adducts (5–50 adducts/108 bases) and the antibody binding to those adducts (Figure 5). There are significant differences (P < 0.05) in OD values between DNA containing zero adduct and five adducts per 108 bases, indicating that the sensitive ELISA can detect five adducts/108 bases in 1 µg DNA sample at least. A similar linear dose–response was also obtained at a higher dose range (25–300 adducts/108 bases). These results suggest the validity of the present DNA sample preparation for calibration, which was done by mixing 4-OHEN-modified DNA and unmodified DNA in different ratios. This is supported by our previous report demonstrating that the detection of UV-induced DNA lesions by the direct binding ELISA is not much influenced by their distribution (dense or sparse) within DNA when equal amounts of photolesions are induced (42). The standard curve generated in each ELISA experiment is essential for determining the absolute amount of 4-OHEN–DNA adducts in sample DNA. Two types of equine estrogens (EN and EQ) are contained in Premarin, and their metabolites (4-OHEN and 4OHEQ, respectively) were found to induce 4-OHEN–DNA adducts in MCF-7 cells (Figure 6). About 90 DNA adducts per 108 bases were induced after 3-h exposure to 200 µM 4-OHEN. Interestingly, 4-OHEN produced five times more 4-OHEN–DNA adducts than did 4-OHEQ. This suggests that although EN represents about one-third of the content of EQ in Premarin, EN is still responsible for more induction of 4-OHEN–DNA adducts than is EQ. In a mouse model for HRT, oral administration of Premarin increased the levels of 4-OHEN-DNA adducts in various tissues, including the uterus and ovaries, in a time-dependent manner (Figure 7). This suggests that 4-OHEN–DNA adducts may be induced in women receiving HRT for long periods (16), and that those adducts could be determined by increasing the sensitivity of the ELISA.
Many evidence suggests that 4-OHEN–DNA adducts are specific biomarkers for assessing the risk and development of equine estrogen-associated cancers. The present new approach utilizing monoclonal antibodies that are highly specific for such DNA adducts provides a valuable and sensitive detection method. This method could prove very useful in identifying individuals harboring 4-OHEN–DNA adducts; they could then be counseled to avoid hormone replacement drugs such as Premarin whose formulations include equine estrogens that are capable of producing these kinds of DNA lesions.
FUNDING
The Ministry of Education, Culture, Sports, Science and Technology of Japan (17510048 to T.M.); US National Institute of Environmental Health Sciences (ES012408 to S.S.). Funding for open access charge: The Ministry of Education, Culture, Sports, Science and Technology of Japan.
Conflict of interest statement. None declared.
ACKNOWLEDGEMENTS
The authors thank Dr K. Shimoi (University of Shizuoka) for the human breast cancer cell line (MCF-7).
REFERENCES
- 1.Grodstein F, Stampfer MJ, Colditz GA, Willett WC, Manson JE, Joffe M, Rosner B, Fuchs C, Hankinson SE, Hunter DJ, et al. Postmenopausal hormone therapy and mortality. N. Engl. J. Med. 1997;336:1769–1775. doi: 10.1056/NEJM199706193362501. [DOI] [PubMed] [Google Scholar]
- 2.Colditz GA, Hankinson SE, Hunter DJ, Willett WC, Manson JE, Stampfer MJ, Hennekens C, Rosner B, Speizer FE. The use of estrogens and progestins and the risk of breast cancer in postmenopausal women. N. Engl. J. Med. 1995;332:1589–1593. doi: 10.1056/NEJM199506153322401. [DOI] [PubMed] [Google Scholar]
- 3.Chen CL, Weiss NS, Newcomb P, Barlow W, White E. Hormone replacement therapy in relation to breast cancer. JAMA. 2002;287:734–741. doi: 10.1001/jama.287.6.734. [DOI] [PubMed] [Google Scholar]
- 4.Beral V. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362:419–427. doi: 10.1016/s0140-6736(03)14065-2. [DOI] [PubMed] [Google Scholar]
- 5.Lacey JV, Jr, Mink PJ, Lubin JH, Sherman ME, Troisi R, Hartge P, Schatzkin A, Schairer C. Menopausal hormone replacement therapy and risk of ovarian cancer. JAMA. 2002;288:334–341. doi: 10.1001/jama.288.3.334. [DOI] [PubMed] [Google Scholar]
- 6.Morch LS, Lokkegaard E, Andreasen AH, Kruger-Kjaer S, Lidegaard O. Hormone therapy and ovarian cancer. JAMA. 2009;302:298–305. doi: 10.1001/jama.2009.1052. [DOI] [PubMed] [Google Scholar]
- 7.Grady D, Gebretsadik T, Kerlikowske K, Ernster V, Petitti D. Hormone replacement therapy and endometrial cancer risk: a meta-analysis. Obstet. Gynecol. 1995;85:304–313. doi: 10.1016/0029-7844(94)00383-O. [DOI] [PubMed] [Google Scholar]
- 8.Steinberg KK, Smith SJ, Thacker SB, Stroup DF. Breast cancer risk and duration of estrogen use: the role of study design in meta-analysis. Epidemiology. 1994;5:415–421. doi: 10.1097/00001648-199407000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Bolton JL, Pisha E, Zhang F, Qiu S. Role of quinoids in estrogen carcinogenesis. Chem. Res. Toxicol. 1998;11:1113–1127. doi: 10.1021/tx9801007. [DOI] [PubMed] [Google Scholar]
- 10.Bolton JL, Thatcher GR. Potential mechanisms of estrogen quinone carcinogenesis. Chem. Res. Toxicol. 2008;21:93–101. doi: 10.1021/tx700191p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen L, Qiu S, Chen Y, Zhang F, van Breemen RB, Nikolic D, Bolton JL. Alkylation of 2′-deoxynucleosides and DNA by the Premarin metabolite 4-hydroxyequilenin semiquinone radical. Chem. Res. Toxicol. 1998;11:94–101. doi: 10.1021/tx970181r. [DOI] [PubMed] [Google Scholar]
- 12.Terashima I, Suzuki N, Shibutani S. 32P-Postlabeling/polyacrylamide gel electrophoresis analysis: application to the detection of DNA adducts. Chem. Res. Toxicol. 2002;15:305–311. doi: 10.1021/tx010083c. [DOI] [PubMed] [Google Scholar]
- 13.Zhang F, Chen Y, Pisha E, Shen L, Xiong Y, van Breemen RB, Bolton JL. The major metabolite of equilin, 4-hydroxyequilin, autoxidizes to an o-quinone which isomerizes to the potent cytotoxin 4-hydroxyequilenin-o-quinone. Chem. Res. Toxicol. 1999;12:204–213. doi: 10.1021/tx980217v. [DOI] [PubMed] [Google Scholar]
- 14.Chen Y, Shen L, Zhang F, Lau SS, van Breemen RB, Nikolic D, Bolton JL. The equine estrogen metabolite 4-hydroxyequilenin causes DNA single-strand breaks and oxidation of DNA bases in vitro. Chem. Res. Toxicol. 1998;11:1105–1111. doi: 10.1021/tx980083l. [DOI] [PubMed] [Google Scholar]
- 15.Zhang F, Swanson SM, van Breemen RB, Liu X, Yang Y, Gu C, Bolton JL. Equine estrogen metabolite 4-hydroxyequilenin induces DNA damage in the rat mammary tissues: formation of single-strand breaks, apurinic sites, stable adducts, and oxidized bases. Chem. Res. Toxicol. 2001;14:1654–1659. doi: 10.1021/tx010158c. [DOI] [PubMed] [Google Scholar]
- 16.Embrechts J, Lemiere F, Van Dongen W, Esmans EL, Buytaert P, Van Marck E, Kockx M, Makar A. Detection of estrogen DNA-adducts in human breast tumor tissue and healthy tissue by combined nano LC-nano ES tandem mass spectrometry. J. Am. Soc. Mass Spectrom. 2003;14:482–491. doi: 10.1016/S1044-0305(03)00130-2. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki N, Yasui M, Santosh Laxmi YR, Ohmori H, Hanaoka F, Shibutani S. Translesion synthesis past equine estrogen-derived 2′-deoxycytidine DNA adducts by human DNA polymerases eta and kappa. Biochemistry (Mosc). 2004;43:11312–11320. doi: 10.1021/bi049273n. [DOI] [PubMed] [Google Scholar]
- 18.Yasui M, Matsui S, Laxmi YR, Suzuki N, Kim SY, Shibutani S, Matsuda T. Mutagenic events induced by 4-hydroxyequilin in supF shuttle vector plasmid propagated in human cells. Carcinogenesis. 2003;24:911–917. doi: 10.1093/carcin/bgg029. [DOI] [PubMed] [Google Scholar]
- 19.Yasui M, Laxmi YR, Ananthoju SR, Suzuki N, Kim SY, Shibutani S. Translesion synthesis past equine estrogen-derived 2′-deoxyadenosine DNA adducts by human DNA polymerases eta and kappa. Biochemistry (Mosc). 2006;45:6187–6194. doi: 10.1021/bi0525324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yasui M, Suzuki N, Liu X, Okamoto Y, Kim SY, Laxmi YR, Shibutani S. Mechanism of translesion synthesis past an equine estrogen-DNA adduct by Y-family DNA polymerases. J. Mol. Biol. 2007;371:1151–1162. doi: 10.1016/j.jmb.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gerlach VL, Aravind L, Gotway G, Schultz RA, Koonin EV, Friedberg EC. Human and mouse homologs of Escherichia coli DinB (DNA polymerase IV), members of the UmuC/DinB superfamily. Proc. Natl Acad. Sci. USA. 1999;96:11922–11927. doi: 10.1073/pnas.96.21.11922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogi T, Kato T, Jr, Kato T, Ohmori H. Mutation enhancement by DINB1, a mammalian homologue of the Escherichia coli mutagenesis protein dinB. Genes Cells. 1999;4:607–618. doi: 10.1046/j.1365-2443.1999.00289.x. [DOI] [PubMed] [Google Scholar]
- 23.Li JJ, Li SA, Oberley TD, Parsons JA. Carcinogenic activities of various steroidal and nonsteroidal estrogens in the hamster kidney: relation to hormonal activity and cell proliferation. Cancer Res. 1995;55:4347–4351. [PubMed] [Google Scholar]
- 24.Wang Z, Edirisinghe P, Sohn J, Qin Z, Geacintov NE, Thatcher GR, Bolton JL. Development of a liquid chromatography electrospray ionization tandem mass spectrometry method for analysis of stable 4-hydroxyequilenin-DNA adducts in human breast cancer cells. Chem. Res. Toxicol. 2009;22:1129–1136. doi: 10.1021/tx900063g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh R, Farmer PB. Liquid chromatography-electrospray ionization-mass spectrometry: the future of DNA adduct detection. Carcinogenesis. 2006;27:178–196. doi: 10.1093/carcin/bgi260. [DOI] [PubMed] [Google Scholar]
- 26.Embrechts J, Lemiere F, Van Dongen W, Esmans EL. Equilenin-2′-deoxynucleoside adducts: analysis with nano-liquid chromatography coupled to nano-electrospray tandem mass spectrometry. J. Mass Spectrom. 2001;36:317–328. doi: 10.1002/jms.136. [DOI] [PubMed] [Google Scholar]
- 27.Haugen A, Groopman JD, Hsu IC, Goodrich GR, Wogan GN, Harris CC. Monoclonal antibody to aflatoxin B1-modified DNA detected by enzyme immunoassay. Proc. Natl Acad. Sci. USA. 1981;78:4124–4127. doi: 10.1073/pnas.78.7.4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leadon SA, Hanawalt PC. Monoclonal antibody to DNA containing thymine glycol. Mutat. Res. 1983;112:191–200. doi: 10.1016/0167-8817(83)90006-8. [DOI] [PubMed] [Google Scholar]
- 29.Santella RM, Lin CD, Cleveland WL, Weinstein IB. Monoclonal antibodies to DNA modified by a benzo[a]pyrene diol epoxide. Carcinogenesis. 1984;5:373–377. doi: 10.1093/carcin/5.3.373. [DOI] [PubMed] [Google Scholar]
- 30.Mori T, Nakane M, Hattori T, Matsunaga T, Ihara M, Nikaido O. Simultaneous establishment of monoclonal antibodies specific for either cyclobutane pyrimidine dimer or (6-4)photoproduct from the same mouse immunized with ultraviolet-irradiated DNA. Photochem. Photobiol. 1991;54:225–232. doi: 10.1111/j.1751-1097.1991.tb02010.x. [DOI] [PubMed] [Google Scholar]
- 31.Iwamoto TA, Kobayashi N, Imoto K, Yamamoto A, Nakamura Y, Yamauchi Y, Okumura H, Tanaka A, Hanaoka F, Shibutani S, et al. In situ detection of acetylaminofluorene-DNA adducts in human cells using monoclonal antibodies. DNA Repair. 2004;3:1475–1482. doi: 10.1016/j.dnarep.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 32.Itoh S, Shibutani S, Ikegami M, Watanabe S, Laxmi YR, Suzuki N, Kohda K, Takanashi K, Yoshizawa I. Synthesis of oligodeoxynucleotides containing a single 6alpha- or 6beta-diastereoisomer of N2-(estradiol-6-yl)-2′-deoxyguanosine. Chem. Res. Toxicol. 2006;19:450–456. doi: 10.1021/tx0503294. [DOI] [PubMed] [Google Scholar]
- 33.Littlefield JW. Selection of Hybrids from Matings of Fibroblasts in Vitro and Their Presumed Recombinants. Science. 1964;145:709–710. doi: 10.1126/science.145.3633.709. [DOI] [PubMed] [Google Scholar]
- 34.Ding S, Shapiro R, Geacintov NE, Broyde S. Conformations of stereoisomeric base adducts to 4-hydroxyequilenin. Chem. Res. Toxicol. 2003;16:695–707. doi: 10.1021/tx0340246. [DOI] [PubMed] [Google Scholar]
- 35.Ding S, Wang Y, Kolbanovskiy A, Durandin A, Bolton JL, van Breemen RB, Broyde S, Geacintov NE. Determination of absolute configurations of 4-hydroxyequilenin-cytosine and -adenine adducts by optical rotatory dispersion, electronic circular dichroism, density functional theory calculations, and mass spectrometry. Chem. Res. Toxicol. 2008;21:1739–1748. doi: 10.1021/tx800095f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ding S, Shapiro R, Cai Y, Geacintov NE, Broyde S. Conformational properties of equilenin-DNA adducts: stereoisomer and base effects. Chem. Res. Toxicol. 2008;21:1064–1073. doi: 10.1021/tx800010u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ding S, Shapiro R, Geacintov NE, Broyde S. Equilenin-derived DNA adducts to cytosine in DNA duplexes: structures and thermodynamics. Biochemistry (Mosc). 2005;44:14565–14576. doi: 10.1021/bi051090t. [DOI] [PubMed] [Google Scholar]
- 38.Ding S, Shapiro R, Geacintov NE, Broyde S. 4-hydroxyequilenin-adenine lesions in DNA duplexes: stereochemistry, damage site, and structure. Biochemistry (Mosc). 2007;46:182–191. doi: 10.1021/bi061652o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang N, Ding S, Kolbanovskiy A, Shastry A, Kuzmin VA, Bolton JL, Patel DJ, Broyde S, Geacintov NE. NMR and computational studies of stereoisomeric equine estrogen-derived DNA cytidine adducts in oligonucleotide duplexes: opposite orientations of diastereomeric forms. Biochemistry (Mosc). 2009;48:7098–7109. doi: 10.1021/bi9006429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kolbanovskiy A, Kuzmin V, Shastry A, Kolbanovskaya M, Chen D, Chang M, Bolton JL, Geacintov NE. Base selectivity and effects of sequence and DNA secondary structure on the formation of covalent adducts derived from the equine estrogen metabolite 4-hydroxyequilenin. Chem. Res. Toxicol. 2005;18:1737–1747. doi: 10.1021/tx050190x. [DOI] [PubMed] [Google Scholar]
- 41.Poirier MC. Antibodies to carcinogen-DNA adducts. J. Natl. Cancer Inst. 1981;67:515–519. [PubMed] [Google Scholar]
- 42.Imoto K, Kobayashi N, Katsumi S, Nishiwaki Y, Iwamoto TA, Yamamoto A, Yamashina Y, Shirai T, Miyagawa S, Dohi Y, et al. The total amount of DNA damage determines ultraviolet-radiation-induced cytotoxicity after uniform or localized irradiation of human cells. J. Invest. Dermatol. 2002;119:1177–1182. doi: 10.1046/j.1523-1747.2002.19514.x. [DOI] [PubMed] [Google Scholar]