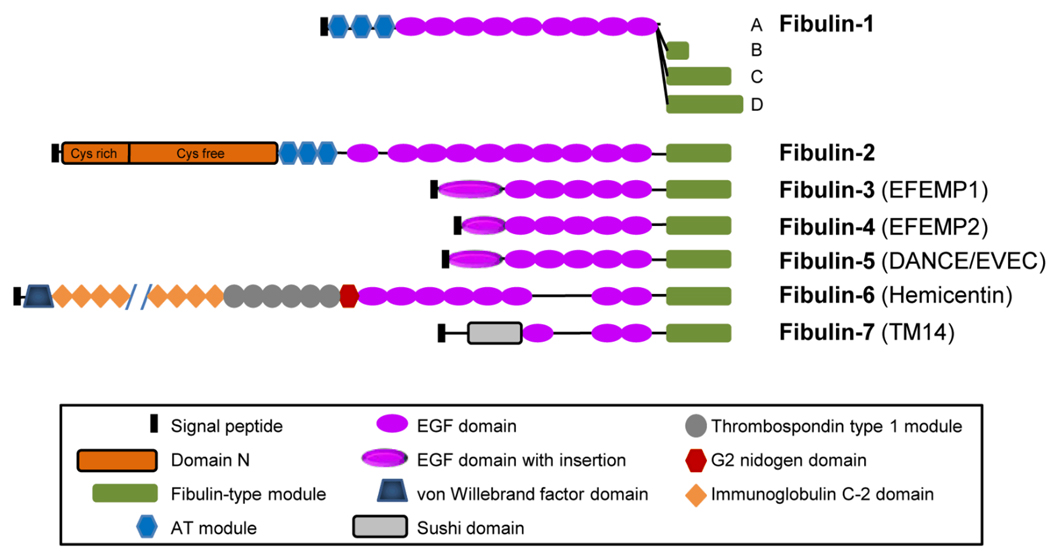
1. Structure
Fibulin-3 (also known as EFEMP1) is an extracellular glycoprotein broadly expressed throughout the body during development and in adult tissues. It is one of 7 members of the fibulin family of proteins. Fibulins are characterized by tandem arrays of calcium binding (cb) EGF domains and a C-terminal fibulin-type module (Fig. 1). Human fibulin-3 is encoded by the gene EFEMP1 (also known as S1-5; accession # NM_004105) located at chromosome 2p16. It contains 11 exons encoding a 493-amino acid protein with a molecular mass of 55 kDa. The protein sequence contains a signal peptide, five tandem arrays of cbEGF domains preceded by a modified cbEGF domain, and a C-terminal fibulin-type module. The modified cbEGF domain contains an 88-amino acid insert. Electron microscopy of the recombinant fibulin-3 after rotary shadowing demonstrates a short rod structure with a globule at one end. The globule is likely the modified cbEGF domain. Fibulin-3 exists as monomers under physiological conditions. It is highly conserved among different species with 92–94% of amino acids identical in human, rat and mouse (accession # AY251056 mouse).
Fig. 1.
The modular domain structures of fibulin family members. Fibulin-3, fibulin-4, and fibulin-5 share high homology with over 50% amino acid identity, and are nearly identical in their structural organization. They each have a modified cbEGF domain with an insertion of 28 to 88 amino acids. AT, anaphylatoxin.
2. Function
During development, fibulin-3 is expressed in condensing mesenchyme, giving rise to bone and cartilage structures, indicating that fibulin-3 is important in skeletal development. In adults, fibulin-3 is widely distributed in various tissues including eye. It is highly expressed by epithelial and endothelial cells and is localized in their basement membranes. In the eye, fibulin-3 is found in several locations including Bruch’s membrane, a double basement membrane structure consisting of retinal pigment epithelium (RPE) and choroidal endothelium basement membranes with collagen and fine elastic fibers in between. Fibulin-3 interacts with another basement membrane protein, extracellular matrix protein 1 (ECM1). It also interacts with tissue inhibitor of metalloproteinase-3 (TIMP-3), collagen XVIII/endostatin, hepatitis B virus-encoded X antigen, and elastin monomer tropoelastin. These interactions likely contribute to the integrity of basement membrane zones and anchor other ECM structures such as elastic fibers to basement membranes. Fibulin-3 stimulates the expression of TIMP-1 and TIMP-3 but inhibits the expression and activities of matrix metalloproteinase MMP-2, MMP-3, and MMP-9. It inhibits angiogenesis. Fibulin-3 is colocalized with fine elastic fibers but not with large elastic structures such as elastic lamina in the aorta. Efemp1 knockout mice exhibit early onset aging and develop multiple hernias including inguinal hernias, pelvic prolapse, and protrusions of the xiphoid process (McLaughlin et al., 2007). Fine elastic fibers in these mice are reduced and disrupted including those in fascia, adventitia, small blood vessel walls, and vaginal walls. Fascia is a thin layer of connective tissue present throughout the body, surrounding muscles, bones, joints and organs, providing support, protection and giving structure to the body. Adventitia is the outermost connective tissue covering of organs, vessels, or other structures. A disruption or reduction of elastic fibers in these connective tissues is likely responsible for the early aging and hernia phenotypes observed in Efemp1 knockout mice. In addition to its function in the ECM, fibulin-3 also has a signaling role. Expression of fibulin-3 is inversely correlated with cell growth. It interacts with DA41, a binding protein to tumor suppressor DAN, and it can stimulate DNA synthesis. The EFEMP1 locus is one of 20 loci identified in a genome-wide association analysis that influence adult height.
3. Disease involvement
A missense mutation in fibulin-3, R345W, causes Malattia Leventinese (ML), also known as Doyne’s honeycomb retinal dystrophy, an autosomal dominant inherited macular degenerative disease (Stone et al., 1999). The first clinically identifiable pathology of ML is drusen. Early visual symptoms include decreased visual acuity, dyschromatopsia, relative scotomas, photophobia, or metamorphopsia. In the later stages of the condition, central vision deteriorates and absolute scotomas develop following geographic atrophy and/or choroidal neovascularization. With the exception of an early age of onset, this phenotype of ML patients is highly compatible with the clinical diagnosis of age-related macular degeneration (AMD).
In a knock-in mouse model carrying the R345W mutation in the murine Efemp1 gene, basal laminar deposits (BLDs) are histologically identified as the earliest change in the eyes (Fu et al., 2007; Marmorstein et al., 2007). BLD is not detectable by currently available clinical tests. In older knock-in mice membranous debris is present within BLDs and within Bruch’s membrane to form basal linear deposits. In human patients, large quantities of debris build up focally in Bruch’s membrane to form drusen. In contrast to Efemp1 knockout mice, the knock-in mice do not have other obvious phenotypes. On the other hand, no apparent macular degeneration associated defects are found in Efemp1 knockout mice. This suggests that loss of fibulin-3 function is not the mechanism by which the R345W mutation causes macular degeneration.
In addition to macular degeneration, inactivation of EFEMP1 by promoter methylation is associated with lung and breast cancers. The methylation frequency of EFEMP1 in lung cancer is similar to those of commonly used methylation markers. EFEMP1 is down-regulated in 60% of sporadic breast cancer tissues. Promoter methylation is the major cause of this down-regulation. Analysis of clinically well characterized primary breast cancers reveals a significant correlation of reduced EFEMP1 expression with poor disease-free and overall survival. EFEMP1 might be useful as molecular markers of lung or breast cancers.
4. Future studies
Our understanding of fibulin-3’s function is still at an early stage. We now know that fibulin-3 is an important component of basement membranes. Dysfunction of basement membranes results in a wide variety of disorders such as inflammation and cancer metastasis. It remains to be determined what fibulin-3’s role is in basement membranes and how inactivation or disruption of that role leads to disease.
The difference in the phenotypes of mice lacking fibulin-3 or carrying the R345W mutation indicates that R345W does not disrupt fibulin-3’s normal function. So it is likely that the mutation has an added detrimental effect that alters Bruch’s membrane to a greater extent than other structures. Our previous study shows that mutant fibulin-3 containing R345W is misfolded, secreted less efficiently than normal fibulin-3, and accumulates within cells. In both ML and AMD eyes, fibulin-3 accumulates along drusen or other basal deposits. In Efemp1 knock-in mice fibulin-3 also accumulates in the basal deposits. This indicates that the R345W mutation causes protein misfolding and aggregation. It is important to understand how this is related to the formation of drusen or other basal deposits and ultimately macular degeneration.
Acknowledgements
This work was supported by the National Eye Institute and Research to Prevent Blindness.
References
- McLaughlin PJ, Bakall B, Choi J, Liu Z, Sasaki T, Davis EC, Marmorstein AD, Marmorstein LY. Lack of fibulin-3 causes early aging and herniation, but not macular degeneration in mice. Hum. Mol. Genet. 2007;16:3059–3070. doi: 10.1093/hmg/ddm264. [DOI] [PubMed] [Google Scholar]
- Stone EM, Lotery AJ, Munier FL, Heon E, Piguet B, Guymer RH, Vandenburgh K, Cousin P, Nishimura D, Swiderski RE, Silvestri G, Mackey DA, Hageman GS, Bird AC, Sheffield VC, Schorderet DF. A single EFEMP1 mutation associated with both Malattia Leventinese and Doyne honeycomb retinal dystrophy. Nat Genet. 1999;22:199–202. doi: 10.1038/9722. [DOI] [PubMed] [Google Scholar]
- Fu L, Garland D, Yang Z, Shukla D, Rajendran A, Pearson E, Stone EM, Zhang K, Pierce EA. The R345W mutation in EFEMP1 is pathogenic and causes AMD-like deposits in mice. Hum. Mol. Genet. 2007;16:2411–2422. doi: 10.1093/hmg/ddm198. [DOI] [PubMed] [Google Scholar]
- Marmorstein LY, McLaughlin PJ, Peachey NS, Sasaki T, Marmorstein AD. Formation and Progression of Sub-Retinal Pigment Epithelium Deposits in Efemp1 Mutation Knock-in Mice: A Model for the Early Pathogenic Course of Macular Degeneration. Hum. Mol. Genet. 2007;16:2423–2432. doi: 10.1093/hmg/ddm199. [DOI] [PubMed] [Google Scholar]