Abstract
Purpose
We previously found that c-FLIP, caspase 8 and TRAIL receptor 2 (DR5) are major regulators of cell viability and chemotherapy-induced apoptosis in colorectal cancer (CRC). In this study, we determined the prognostic significance of c-FLIP, caspase 8, TRAIL and DR5 expression in tissues from patients with stage II and III CRC.
Experimental design
Tissue Microarrays (TMAs) were constructed from matched normal and tumor tissue derived from patients (n=253) enrolled in a phase III trial of adjuvant 5-FU-based chemotherapy versus post-operative observation alone. TRAIL, DR5, caspase 8 and c-FLIP expression levels were determined by immunohistochemistry (IHC).
Results
Colorectal tumors displayed significantly higher expression levels of c-FLIP (p<0.001), caspase 8 (p=0.01) and DR5 (p<0.001), but lower levels of TRAIL (p<0.001) compared with matched normal tissue. In univariate analysis, higher TRAIL expression in the tumor was associated with worse overall survival (OS) (p=0.026), with a trend to decreased relapse-free survival (RFS) (p=0.06), and higher tumor c-FLIP expression was associated with a significantly decreased RFS (p=0.015). Using multivariate predictive modelling for RFS in all patients and including all biomarkers, age, treatment and stage, we found that the model was significant when the mean tumor c-FLIP expression score and disease stage were included (p<0.001). As regards OS, the overall model was predictive when both TRAIL expression and disease stage were included (p<0.001).
Conclusions
High c-FLIP and TRAIL expression may be independent adverse prognostic markers in stage II and III CRC and may identify patients most at risk of relapse.
Keywords: c-FLIP, caspase 8, TRAIL, DR5, colorectal cancer
Introduction
Colorectal cancer (CRC) is the second most common cause of cancer-related deaths. It is now well established that in patients with stage III disease undergoing curative surgical resection, adjuvant 5-FU-based chemotherapy reduces tumor recurrence rates and improves overall survival (1-3). However, as many as 65% of stage III CRC patients are cured by surgery alone (4). In stage II disease, the QUASAR trial comparing adjuvant chemotherapy versus observation alone concluded that although some patients benefited from adjuvant therapy, the improvement in 5-year survival was small (3.6%), and over 80% of stage II patients are cured by surgery alone (2). Thus, the identification and validation of prognostic biomarkers of relapse in stage II and III colorectal cancer are urgently needed to spare patients who would be cured by surgery alone from unnecessary treatment with chemotherapy. This has become even more important following the addition of oxaliplatin to a fluoropyrimidine as an option in adjuvant therapy, as there is now higher potential for longer term toxicity such as sensory neuropathy.
The aim of this study was to use IHC to determine the expression patterns of c-FLIP, caspase 8, tumor necrosis factor-related apoptosis inducing ligand (TRAIL) and TRAIL receptor 2 (DR5) in matched normal and tumor epithelium in stage II/III CRC patients enrolled in an adjuvant trial comparing post-operative 5-Fluorouracil (5-FU)-based chemotherapy versus observation alone and to correlate expression with clinicopathological variables and outcome. Deregulated apoptosis is a hallmark of cancer (5). Apoptosis can be divided into two main pathways: the death receptor (DR)-mediated (or extrinsic) pathway and the intrinsic mitochondrial-regulated pathway (6). The extrinsic apoptotic pathway is regulated via specialised cell surface receptors, which are members of the Tumor Necrosis Factor (TNF) receptor family, including TNF-RI, Fas, and the TRAIL receptors DR4 and DR5. Although Fas Ligand (FasL) and TNF-α are too toxic to be used as systemic therapies, TRAIL and agonistic antibodies targeting DR4 and DR5 have received much attention as potential therapeutic death ligands due to their high level of tumor specificity (7-10). Therefore, the relative expression of TRAIL signalling molecules in normal and tumor colorectal tissues is now a highly relevant issue.
A key inhibitor of DR-mediated apoptosis is c-FLIP (cellular FLICE inhibitory protein), which inhibits caspase 8 processing at the Death Inducing Signalling Complexes (DISCs) formed by these receptors (11). Differential splicing gives rise to long (c-FLIPL) and short (c-FLIPS) forms of c-FLIP (12). DISC-bound c-FLIP has also been reported to promote activation of multiple pro-survival signalling pathways (13, 14). We have previously found that c-FLIP is a major regulator of viability and chemotherapy-induced apoptosis in colorectal cancer (CRC) cells in vitro and in vivo due to its ability to block apoptosis mediated by caspase 8 and DR5 (15-17). These results and the advent of TRAIL-targeted therapeutics make it highly important to determine the relative expression of c-FLIP, caspase 8 and DR5 in normal and tumor colorectal tissues. In addition, two previously published studies identified c-FLIP expression as an independent adverse prognostic marker for disease outcome in CRC (18, 19). We sought to verify and extend these findings in the current study as we had access to paired normal and tumor specimens and equal numbers of untreated and chemotherapy treated stage II and III patients. Moreover, there have been conflicting reports on the prognostic significance of the expression of TRAIL and DR5 in CRC, which we sought to resolve (20, 21). In addition, although caspase 8 has been reported to be overexpressed in colorectal (22) and rectal cancer (23), to our knowledge, caspase 8 protein expression has not previously been correlated with disease outcome.
Materials and Methods
Patient Details
This study was based on a Phase III randomised trial involving 253 patients accrued between 1994-1997 with Stage II and III CRC, who following surgical resection, were randomised to either observation alone or adjuvant treatment with bolus 5-FU/Folinic acid (FA) (24). Matched normal and tumour tissue was obtained from the same patient. Prior to use, resected tissues were conserved as formalin-fixed tissue embedded in paraffin in accordance with routine diagnostic histopathology practice. At the time of study analysis, the median follow-up was 6.5 years. The clinical and pathological details of these patients are displayed in Table 1. The CONSORT diagram (Supplementary Figure 1) displays the number of patients involved in each arm. There was full approval from the local research ethics committee and all involved hospitals, and all patients gave consent for the use of their specimens in research, according to the Declaration of Helsinki.
Table 1.
Clinicopathological details of colorectal cancer patient cohort (n=253)
| 5-FU/Folinic Acid N=126 (%) |
Observation n=127 (%) |
||
|---|---|---|---|
| Age (years) | Median Range |
65 38-81 |
65 35-80 |
| Gender | Male Female |
71 (56) 55 (44) |
79 (62) 48 (38) |
| Stage | II III |
79 (63) 47 (37) |
81 (64) 46 (36) |
| Tumour Site | Right Colon Left Colon Rectum Synchronous |
37 (29) 50 (40) 35 (28) 4 (3) |
32 (25) 59 (47) 36 (28) 0 (0) |
| Grade of Differentiation | Low Grade High Grade Not specified |
107 (85) 15 (12) 4 (3) |
107 (84) 15 (12) 5 (4) |
| Vascular Invasion | No Yes Not specified |
67 (53) 27 (22) 32 (25) |
69 (54) 25 (20) 33 (26) |
Tissue Microarray Construction
Four 0.6mm cores were obtained from both the normal and tumor tissue of each patient using the Beecher Instruments™ arrayer and placed into a paraffin block. Sections 4μm in thickness were cut, floated onto adhesive slides and baked overnight at 55°C. Arrays were constructed at a density of 90-110 cores per array.
Immunohistochemical Detection Methods
The details of primary antibodies used are displayed in Supplementary Table 1. Initially, antibodies that were reported to stain formalin fixed paraffin embedded (FFPE) tissues were selected and tested using relevant positive control tissues (either recommended by the antibody manufacturer or selected from previous publications). For each antibody, a series of optimisation steps were performed to determine the most appropriate antigen retrieval protocol and antibody dilution to use. This optimisation was based on the staining pattern obtained in the positive control ensuring that the correct areas were stained intensely with background staining kept to a minimum. All staining was performed on a BondMax automated immunostainer (Vision BioSystems™). Briefly, for all markers except c-FLIP, following appropriate pre-treatment the diluted primary antibody was applied to the cores for 20 minutes, washed in buffered and then endogenous peroxidase was blocked via 3% hydrogen peroxide. Following post primary solution and Bond™ Polymer Refine solution application, with intervening wash cycle steps, peroxidase activity was localized by the enhanced diaminobenzidine (DAB) tetrachloride peroxidase reaction with Harris haematoxylin as a counterstain. For c-FLIP, the sections were dewaxed and antigen retrieval performed on the BondMax™ as above, with subsequent primary antibody incubation occurring overnight at 4°C. Antibody detection was then carried out as above using the Bond Polymer Refine kit, an enhanced DAB as chromagen and Harris haematoxylin as counterstain.
Scoring
Cores were evaluated by two independent observers (DMcL and HB) blinded to clinical data. The scoring field was X100 (X10 eyepiece with X10 objective), and the whole of each tissue was scored. Staining intensity was graded as 0 (no staining), 1 (weak staining), 2 (moderate staining) and 3 (strong staining). Staining extent was graded from 0 (0-5% positive epithelial cells), 1 (6-25% positive), 2 (26-50% positive), 3 (51-75% positive) and 4 (>75% positive). The intensity and extent scores were multiplied for each core and both the mean normal and mean tumor expression signature score for a particular IHC marker calculated. Any discordance between scores was agreed by consensus.
Statistics
Patient data was collected via a centralised trial co-ordinating office and stored electronically. Statistical analysis was performed via the SPSS™ 13-software package (SPSS Inc, Chicago, IL). Comparisons between matched normal and malignant tissues were performed via matched Student’s T-tests. Correlations were assessed via Pearson’s correlation coefficient method. Survival analysis was performed via Kaplan-Meier and Cox regression proportional analyses for overall survival (OS) and relapse-free survival (RFS). Multivariate predictive modelling was performed via the stepwise backward log rank method of Cox regression analysis. Follow up was from the date of surgery.
Results
Patient Cohort characteristics
A tissue microarray was constructed using tissue samples from patients entered into a phase III randomised controlled trial of treatment with 5-FU/FA chemotherapy versus observation alone in patients with surgically resected stage II and III tumors (24). Matched normal and tumor tissue samples were arrayed for each of the 254 patients recruited to the trial from hospitals throughout Northern Ireland between 1994 and 1997. The patients’ characteristics are shown in Table 1. Two-thirds of the patients were stage II. Age, gender, stage and grade of tumor were well matched between the two study arms. One patient was ineligible as a result of result of undiagnosed metastatic disease found to have been present at study entry.
Survival analysis: impact of stage and treatment
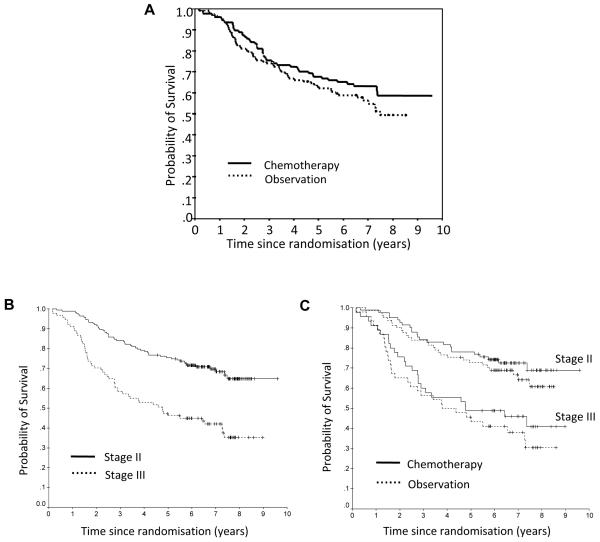
Analysis of survival was carried out when every patient had undergone at least five years of follow-up. Figure 1A depicts the overall survival (OS) for the two study arms. There was no statistically significant improvement in survival in the chemotherapy arm of the study (p=0.26; Figure 1A). As expected, there was a highly significant difference in OS when patients were analysed according to their tumor stage (p<0.001; Figure 1B). The trend towards improved survival with chemotherapy is present for both stage II and III patients, although neither result was statistically significant (p=0.396 and 0.384 respectively; Figure 1C). The lack of benefit for stage II patients is consistent with the QUASAR trial (2) and further supports the fact that the majority of these patients are cured by surgery alone. The lack of significant benefit from adjuvant chemotherapy for the stage III patients may reflect the relatively small numbers of patients in this group (n=47 in the treatment arm and n=46 in the observation arm).
Figure 1.
A, Overall survival in each treatment group. B, Overall survival based on tumor stage. C, Overall survival based on treatment group and stage.
Correlations between Individual Markers
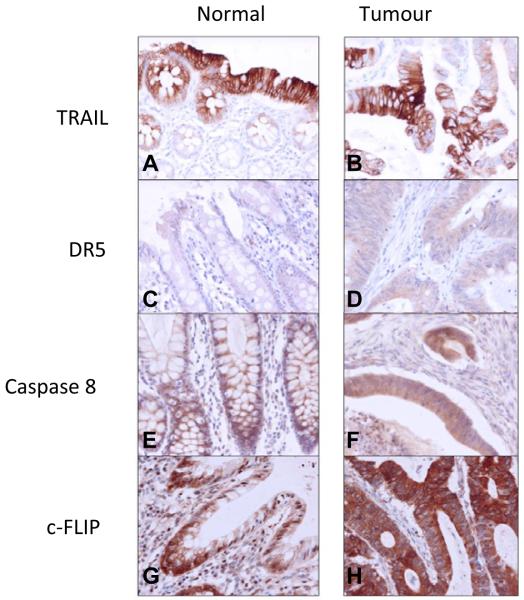
As we have previously found that c-FLIP regulates cell viability and chemotherapy-induced apoptosis in colorectal cancer (CRC) cells in vitro and in vivo by inhibiting DR5- and caspase 8-mediated apoptosis, the tissue microarrays were analysed for expression of DR5, TRAIL, c-FLIP and caspase 8 as described in Materials and Methods. Representative images for each individual marker are shown in Figure 2 for matched normal and tumor tissue. Within the adjacent normal tissue (Supplementary Table 2), there were positive correlations between the expression of c-FLIP and caspase 8 and DR5 (in all cases p<0.001). Significant correlations also existed between DR5 and TRAIL (p<0.001). In the tumor tissue, positive correlations existed between c-FLIP and both caspase 8 and DR5 expression (Supplementary Table 3; p=0.002). In addition, a significant correlation existed between DR5 and caspase 8 (p=0.003). Overall, the correlations between the apoptotic markers were stronger in the normal tissue than in the tumor tissue. This may reflect a greater degree of deregulated apoptosis in the tumor cells and/or a greater degree of cellular heterogeneity.
Figure 2.
Representative cores demonstrating the immunohistochemical expression of TRAIL (A and B), DR5 (C and D), caspase 8 (E and F) and c-FLIP (G and H) in matched normal colonic mucosa and tumour tissue. All images taken at x400 magnification.
Marker Expression and correlation with clinical outcome
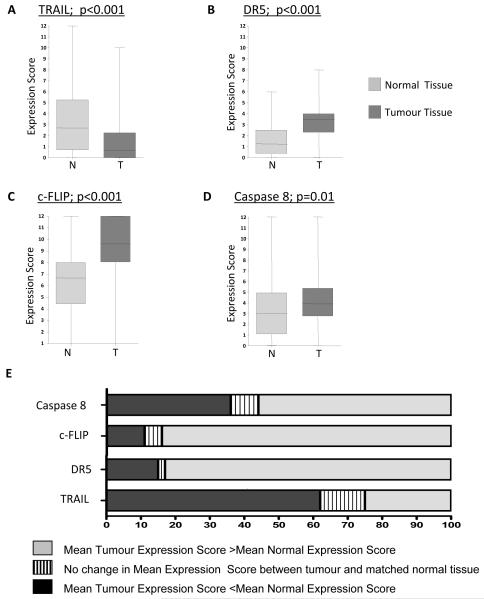
The overall mean expression scores of each individual marker in the normal and tumor tissues from the whole cohort are presented in Figure 3A-D. In supplementary figure 2, the expression of each marker between stage II and Stage III CRC is compared. In addition, we determined the percentage of patients for whom the marker was over-expressed, under-expressed or did not change between normal and tumor; this is presented in figure 3E. The results of these analyses are discussed below for each marker.
Figure 3.
A-D, IHC expression signature scores were derived from the multiplication product of (staining intensity) x (extent) for each core. Significant differences in mean expression scores between matched normal and tumour samples were noted for TRAIL, DR5, Caspase 8 and c-FLIP. p values are two tailed and represent paired t test analysis. E, The percentage of patients displaying differences between matched normal and tumour samples; up-regulation, down-regulation and no change for each individual marker is illustrated.
TRAIL
Expression of TRAIL was detected in 60% of the tumor samples and 78% of the matched normal samples. There were significantly higher expression scores in the normal tissue compared with the tumors (p<0.001; Figure 3A). When comparing matched normal and tumor tissue for each patient, 62% of patients had higher TRAIL expression in the normal tissue compared to 25% of patients who had higher TRAIL expression in their tumors (Figure 3E). In univariate survival analysis of all patients (Table 2), inclusive of all stages and treatment arms, higher tumor TRAIL expression was significantly associated with decreased OS (p=0.026) and a near significant trend towards impaired RFS (p=0.062) (Table 3). However, there were no significant correlations between tumor TRAIL expression and either RFS or OS when the cohort was divided by stage or by stage then treatment. TRAIL expression in the normal tissue had no impact on RFS or OS (Table 2 and 3).
Table 2.
Impact of individual marker expression scores on Overall Survival (OS) for all Patients (n=253) as determined by Cox regression Proportional analysis
| Marker | Regression Coefficient estimate |
SE | Significance | Risk Ratio (Exp (B)) |
95% CI for Exp (B) | ||
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| TRAIL | N T |
0.025 0.101 |
0.031 0.046 |
0.417 0.026* |
1.026 1.107 |
0.965 1.012 |
1.091 1.210 |
| DR5 | N T |
0.078 −0.045 |
0.066 0.056 |
0.237 0.426 |
1.081 0.956 |
0.950 0.857 |
1.231 1.067 |
| c-FLIP | N T |
−0.003 0.050 |
0.037 0.043 |
0.944 0.238 |
0.997 1.052 |
0.928 0.967 |
1.07 1.143 |
| Caspase 8 | N T |
0.008 −0.008 |
0.031 0.042 |
0.791 0.839 |
1.008 0.992 |
0.949 0.914 |
1.072 1.076 |
p<0.05
Table 3.
Impact of individual marker expression scores on Recurrence Free Survival (RFS) for all Patients (n=253) as determined by Cox regression Proportional analysis
| Marker | Regression Coefficient estimate |
SE | Significance | Risk Ratio (Exp (B)) |
95% CI for Exp (B) | ||
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| TRAIL | N T |
0.022 0.098 |
0.036 0.053 |
0.545 0.062 |
1.022 1.103 |
0.953 0.955 |
1.096 1.222 |
| DR5 | N T |
0.53 −0.15 |
0.076 0.064 |
0.484 0.811 |
1.054 0.985 |
0.909 0.868 |
1.224 1.117 |
| c-FLIP | N T |
0.004 0.128 |
0.042 0.053 |
0.916 0.015* |
1.004 1.136 |
0.925 1.025 |
1.090 1.260 |
| Caspase 8 | N T |
0.027 −0.002 |
0.034 0.047 |
0.440 0.973 |
1.027 0.998 |
0.960 0.911 |
1.099 1.094 |
p<0.05.
DR5
Expression of DR5 was detected in 96% of the tumor samples and 80% of the adjacent normal tissue. Overall, there was significantly higher DR5 expression in the tumor tissues compared with matched normal tissues (p<0.001) (Figure 3B), and this was reflected in the high percentage of patients (83%) with higher DR5 expression in their tumors compared with matched normal tissue (Figure 3E). In univariate survival analysis inclusive of all patients, or on subgroup analysis divided by stage/treatment, there were no significant associations between DR5 expression and either RFS or OS in normal or tumor tissues (Tables 2 and 3).
c-FLIP
Expression of c-FLIP was detected in 99.5% of the tumor tissues and 99% of the normal tissues. Overall, there were significantly higher expression scores in the tumor tissue compared with the matched normal tissue (p<0.001) (Figure 3C). Moreover, when the matched normal and tumor tissues for each patient were compared, it was found that the tumors of 84% of individuals over-expressed c-FLIP, whereas only 11% had reduced expression of c-FLIP (Figure 3E). Notably, in univariate Cox regression analysis, inclusive of all patients, higher c-FLIP expression was associated with decreased RFS (p=0.015), but failed to have a significant effect on OS (p=0.24) (Tables 2 and 3). High c-FLIP expression also had a significant adverse effect on RFS (p=0.026) when the Stage III individuals were considered alone.
Caspase 8
Expression of caspase 8 was cytoplasmic in nature and evident in 99.5 % of tumor tissue and 90% of normal tissue. In agreement with previous studies (22, 23), there were significantly higher expression scores in the tumor tissue compared with the matched normal tissue (Figure 3D, p=0.01). Expression was markedly heterogeneous, both inter-individually and also within cores from the same individual. Comparing matched normal and tumor samples, 56% of individuals displayed over expression of caspase 8 in their tumors, 36% under expression and 8% no change (Figure 3E). Following univariate analysis of the entire cohort, and following subsequent subgroup analysis according to stage and treatment, there was no association between the degree of caspase 8 expression and survival endpoints.
Assessment of biomarker-treatment interactions
In each of the individual biomarker expression analyses, a treatment interaction effect was sought to determine whether these potential biomarkers had predictive value in terms of the impact of chemotherapy treatment post surgical resection. None of these biomarkers significantly predicted response to chemotherapy treatment post surgical resection. However, on subdivision of stage III individuals into chemotherapy treatment versus observation alone groups, high tumor c-FLIP expression conveyed a non-significant trend for adverse outcome that was more significant in the chemotherapy treatment arm (p=0.07 for treatment arm and p=0.1 in observation arm). This finding has relevance to our previous studies, in which we have shown that c-FLIP is a critical inhibitor of chemotherapy-induced apoptosis in colorectal cancer cells in vitro and in vivo (17).
Multivariate analysis: exploratory predictive modelling
Using the backward log rank method of Cox regression analysis, whereby candidate variables are considered together and non-significant variables to the overall model are removed in a stepwise fashion, we attempted to create a predictive model for both RFS and OS for all patients in the cohort. We included age, stage, treatment and all the normal and tumor expression marker scores in the model. With respect to RFS, two variables remained in the overall model after 12 elimination steps; these were disease stage (II versus III) and tumor c-FLIP expression (p<0.001). When we examined OS, two variables were left in the model after 12 elimination steps: tumor TRAIL score and disease stage (p<0.001). Therefore in multivariate models, tumor c-FLIP and TRAIL expression hold adverse prognostic significance with respect to RFS and OS respectively.
Discussion
We have previously found that c-FLIP is a key regulator of colorectal cancer cell survival, both constitutively and in the context of chemotherapy and death ligand treatment through its ability to regulate caspase 8 activation by the TRAIL death receptor DR5 (15-17). In this study we have assessed the expression and prognostic relevance of c-FLIP, caspase 8, DR5 and TRAIL in tissues from patients with stage II and III CRC using TMAs constructed from matched normal and tumor tissue from CRC patients. These patients (n=253) were enrolled in a phase III trial of adjuvant 5-FU-based chemotherapy versus post-operative observation alone. There was a higher proportion of stage II (64%) than stage III patients (36%) in this trial. This was a reflection of the growing consensus during the time period of the trial that most stage III patients should be considered for adjuvant 5-FU based chemotherapy. In fact, this change resulted in early closure of the trial with only 50% of the planned sample size reached. Consequently, the trial was not sufficiently powered to demonstrate that the use of adjuvant chemotherapy results in significantly increased survival for stage III patients.
In agreement with previous studies, the colorectal tumor tissues displayed marked up-regulated expression of DR5 but lower TRAIL expression when compared with matched normal tissue (20, 25, 26). The relative over-expression of DR5 highlights the potential exploitation of TRAIL receptors as therapeutic targets in colorectal cancer. TRAIL receptor-targeted therapeutics are in clinical development (27), and we and others have shown that CRC cells are susceptible to TRAIL-induced apoptosis, both as a single agent and in combination with chemotherapy and targeted therapies (15, 28-31). However, we and others have also shown that the over-expression of c-FLIP in colorectal cancer cells limits the effectiveness of TRAIL receptor-targeted therapeutics and that down-regulating c-FLIP synergistically enhances TRAIL-induced apoptosis (15, 29). Therefore, the overexpression of c-FLIP that we observed in the matched colorectal cancer tissues is likely to be a key resistance factor to TRAIL receptor-targeted therapeutics. Moreover, inhibiting c-FLIP expression or function is therefore likely to be important for maximizing the clinical activity of these agents.
In two previous studies in colorectal cancer, no prognostic association was found between TRAIL expression and clinical outcome (20, 21). However, in univariate analysis, we found that high tumor TRAIL expression in the entire cohort was associated with a significant 11% increase in the risk of death and had a near-significant trend to adversely affect RFS (p=0.062). Tumor expression of FasL has been linked to the so-called ‘Fas counterattack’ in which tumor FasL induces apoptosis of Fas sensitive immune cells (32, 33), although this is somewhat controversial (34). It has also been proposed that tumor expressed TRAIL can act in a similar way to suppress tumor-specific T cell responses (35); this may provide an explanation for the adverse prognosis associated with TRAIL overexpression. Moreover, it is becoming increasingly apparent that TRAIL can activate anti-apoptotic signalling pathways in certain cellular contexts. For example, TRAIL has been shown to induce activation of Nuclear Factor kappa B (NFκB) in a range of cell lines (13, 14, 36-38). TRAIL-induced NFκB induction is dependent on c-FLIP blocking caspase 8 activation and recruiting TNF receptor associated factor-2 (TRAF-2) (39). Thus, in the presence of c-FLIP overexpression, TRAIL may activate NFκB and thereby induce transcriptional up regulation of anti-apoptotic genes rather than apoptosis. This may also partly explain the adverse clinical outcome in those individuals overexpressing TRAIL. In agreement with previous studies (20, 21), we found that expression of DR5 was not prognostic.
We have previously found that caspase 8 is an important mediator of chemotherapy-induced apoptosis in colorectal cancer (17). Despite playing a pivotal role in death receptor-mediated apoptosis, to the best of our knowledge, the prognostic role of caspase 8 protein expression in CRC has not previously been reported. In the tumor tissue examined in this study, caspase 8 expression was significantly higher than in matched normal tissue. This is in agreement with another study in colorectal (22) and rectal cancer (23) and has important implications for the effectiveness of TRAIL receptor-targeted agents in CRC. Furthermore, these results suggest that caspase 8 has non-apoptotic functions that may contribute to tumorigenesis. Indeed, such non-apoptotic functions have been identified (13, 40, 41), and it appears that the loss of caspase 8 expression that is observed in certain cancers (for example, small cell lung cancer (42)) is the exception rather than the rule. There was also a positive correlation between caspase 8 and c-FLIP tumor expression, however, caspase 8 expression was not found to be prognostic.
A variety of methods and antibodies have been evaluated for detecting c-FLIP by IHC. We initially tested a range of c-FLIP antibodies including those used in other publications (18, 19), and of several antibodies tested, only the Santa Cruz™ c-FLIPS/L mouse IgG1 antibody (G-11) gave the immunohistochemical pattern of staining that we expected when tested on cell pellets derived from stable c-FLIPL and c-FLIPS over-expressing HCT116 CRC cell lines (data not shown). In our hands, none of the other published antibodies and protocols gave the expected pattern of staining in these c-FLIP overexpressing control samples. Despite the different antibodies used, our study agrees with a previous study that also found significantly higher expression of c-FLIP in colorectal tumor tissue compared with matched normal tissue (43). Indeed, in their smaller series of 52 matched normal and tumor specimens, Ryu et al found that a very similar percentage of tumors overexpressed c-FLIP (79% compared with 84% in our study). Based on our previous in vitro and in vivo studies (17), this over-expression of c-FLIP in colorectal tumors is a potentially important mechanism of resistance to chemotherapy. In support of this, high tumor c-FLIP expression conveyed a non-significant trend for adverse outcome in stage III patients that was more significant in the chemotherapy treatment arm than in the observation arm.
A key finding of our study is that high c-FLIP expression was associated with a significant 14% increase in the relative risk of CRC recurrence for all patients combined and a 17% increase in the relative risk of recurrence when individuals with stage III disease were considered separately. Importantly, a multivariate predictive model for recurrence was found to be significant when stage and c-FLIP tumor scores were included. Two other studies have previously investigated the prognostic relevance of c-FLIP expression in CRC, and in addition to the different antibodies and scoring systems used, it is important to highlight the differences and similarities between those studies and our study. Ullenhag et al. analysed c-FLIP expression in 396 stage I-IV CRC tumors by IHC (no normal tissue analysed) and defined expression as negative, weak, moderate or strong as defined by intensity only (18). They found that ‘strong’ c-FLIPL expression was associated with adverse disease-specific survival as determined by Kaplan-Meier analysis; c-FLIPS expression was not found to have a prognostic effect. However, only a very small percentage of tumors (19/396, <5%) were defined as having ‘strong’ c-FLIPL expression; in the tumors that were classified as either weak (60%) or moderate (30%), c-FLIPL expression was not prognostic. In a smaller study, (n=90), c-FLIP expression was determined more quantitatively as the percentage of colorectal tumor cells with clear cytoplasmic positivity (19). Tumor immunoreactivity for c-FLIP was relatively low (69%) in this study compared with our study and other studies (18, 43). However, in agreement with our study, higher than median c-FLIP expression (defined as positivity in >10% of cancer cells) was associated with impaired survival in univariate and multivariate analyses. Overall, despite the differences in antibodies and scoring systems used, our work and that of others suggest that c-FLIP overexpression may be a potential adverse prognostic biomarker in colorectal cancer. However, there is clearly a need for more extensive studies and standardisation of the protocols used to determine c-FLIP expression by IHC.
In conclusion, we have found significant alterations in c-FLIP, caspase 8, DR5 and TRAIL expression between matched normal and tumor colorectal tissue, which may have important clinical implications for the effectiveness of chemotherapy and novel TRAIL-targeted therapies in this disease. Moreover, we have found that c-FLIP and TRAIL expression may convey significant prognostic information for stage II and III CRC, suggesting that this signalling pathway is relevant for the pathogenesis of this disease. Further studies are warranted in larger patient cohorts to confirm the prognostic significance of c-FLIP and TRAIL in colorectal cancer. Preferably, these studies should be conducted in a blinded, prospective manner.
Statement of Translational Relevance.
Deregulated apoptotic signaling is a hallmark of cancer. In previous studies, we identified c-FLIP, caspase 8 and the TRAIL death receptor DR5 as important regulators of colorectal cancer cell survival, both constitutively and in the context of chemotherapy treatment. In this study, we have analyzed expression of c-FLIP, caspase 8, TRAIL and DR5 in matched normal and tumor tissues from a cohort of colorectal cancer patients enrolled in a phase III trial of adjuvant 5-FU-based chemotherapy versus observation alone. Colorectal tumors expressed significantly higher levels of c-FLIP, caspase 8 and DR5, but lower levels of TRAIL compared with matched normal tissue, suggesting that altered expression of these proteins may be important in the pathogenesis of this disease. Importantly, we found that high c-FLIP and TRAIL expression were independent adverse prognostic markers that may identify patients most at risk of relapse and who therefore would most benefit from adjuvant chemotherapy treatment.
Supplementary Material
Acknowledgments
Funding: This work was supported by grants from the Medical Research Council (DPM, DBL), Cancer Research UK (VMC, SVS, RW, DBL, PGJ), Digestive Disorders Foundation (UMcD)
References
- 1.Moertel CG, Fleming TR, Macdonald JS, et al. Fluorouracil plus levamisole as effective adjuvant therapy after resection of stage III colon carcinoma: a final report. Ann Intern Med. 1995;122:321–6. doi: 10.7326/0003-4819-122-5-199503010-00001. [DOI] [PubMed] [Google Scholar]
- 2.Quasar Collaborative G. Gray R, Barnwell J, et al. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet. 2007;370:2020–9. doi: 10.1016/S0140-6736(07)61866-2. [DOI] [PubMed] [Google Scholar]
- 3.Andre T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27:3109–16. doi: 10.1200/JCO.2008.20.6771. [DOI] [PubMed] [Google Scholar]
- 4.O’Connell JB, Maggard MA, Ko CY. Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J Natl Cancer Inst. 2004;96:1420–5. doi: 10.1093/jnci/djh275. [DOI] [PubMed] [Google Scholar]
- 5.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 6.Wilson TR, Johnston PG, Longley DB. Anti-apoptotic mechanisms of drug resistance in cancer. Curr Cancer Drug Targets. 2009;9:307–19. doi: 10.2174/156800909788166547. [DOI] [PubMed] [Google Scholar]
- 7.Burton ER, Libutti SK. Targeting TNF-alpha for cancer therapy. J Biol. 2009;8:85. doi: 10.1186/jbiol189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ogasawara J, Watanabe-Fukunaga R, Adachi M, et al. Lethal effect of the anti-Fas antibody in mice. Nature. 1993;364:806–9. doi: 10.1038/364806a0. [DOI] [PubMed] [Google Scholar]
- 9.Fesik SW. Promoting apoptosis as a strategy for cancer drug discovery. Nat Rev Cancer. 2005;5:876–85. doi: 10.1038/nrc1736. [DOI] [PubMed] [Google Scholar]
- 10.Ashkenazi A. Targeting death and decoy receptors of the tumour-necrosis factor superfamily. Nat Rev Cancer. 2002;2:420–30. doi: 10.1038/nrc821. [DOI] [PubMed] [Google Scholar]
- 11.Krueger A, Baumann S, Krammer PH, Kirchhoff S. FLICE-inhibitory proteins: regulators of death receptor-mediated apoptosis. Mol Cell Biol. 2001;21:8247–54. doi: 10.1128/MCB.21.24.8247-8254.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Irmler M, Thome M, Hahne M, et al. Inhibition of death receptor signals by cellular FLIP. Nature. 1997;388:190–5. doi: 10.1038/40657. [DOI] [PubMed] [Google Scholar]
- 13.Hu WH, Johnson H, Shu HB. Activation of NF-kappaB by FADD, Casper, and caspase-8. J Biol Chem. 2000;275:10838–44. doi: 10.1074/jbc.275.15.10838. [DOI] [PubMed] [Google Scholar]
- 14.Kataoka T, Budd RC, Holler N, et al. The caspase-8 inhibitor FLIP promotes activation of NF-kappaB and Erk signaling pathways. Curr Biol. 2000;10:640–8. doi: 10.1016/s0960-9822(00)00512-1. [DOI] [PubMed] [Google Scholar]
- 15.Galligan L, Longley DB, McEwan M, et al. Chemotherapy and TRAIL-mediated colon cancer cell death: the roles of p53, TRAIL receptors, and c-FLIP. Mol Cancer Ther. 2005;4:2026–36. doi: 10.1158/1535-7163.MCT-05-0262. [DOI] [PubMed] [Google Scholar]
- 16.Wilson TR, McLaughlin KM, McEwan M, et al. c-FLIP: a key regulator of colorectal cancer cell death. Cancer Res. 2007;67:5754–62. doi: 10.1158/0008-5472.CAN-06-3585. [DOI] [PubMed] [Google Scholar]
- 17.Longley DB, Wilson TR, McEwan M, et al. c-FLIP inhibits chemotherapy-induced colorectal cancer cell death. Oncogene. 2006;25:838–48. doi: 10.1038/sj.onc.1209122. [DOI] [PubMed] [Google Scholar]
- 18.Ullenhag GJ, Mukherjee A, Watson NF, et al. Overexpression of FLIPL is an independent marker of poor prognosis in colorectal cancer patients. Clin Cancer Res. 2007;13:5070–5. doi: 10.1158/1078-0432.CCR-06-2547. [DOI] [PubMed] [Google Scholar]
- 19.Korkolopoulou P, Saetta AA, Levidou G, et al. c-FLIP expression in colorectal carcinomas: association with Fas/FasL expression and prognostic implications. Histopathology. 2007;51:150–6. doi: 10.1111/j.1365-2559.2007.02723.x. [DOI] [PubMed] [Google Scholar]
- 20.van Geelen CM, Westra JL, de Vries EG, et al. Prognostic significance of tumor necrosis factor-related apoptosis-inducing ligand and its receptors in adjuvantly treated stage III colon cancer patients. J Clin Oncol. 2006;24:4998–5004. doi: 10.1200/JCO.2006.06.8809. [DOI] [PubMed] [Google Scholar]
- 21.Strater J, Hinz U, Walczak H, et al. Expression of TRAIL and TRAIL receptors in colon carcinoma: TRAIL-R1 is an independent prognostic parameter. Clin Cancer Res. 2002;8:3734–40. [PubMed] [Google Scholar]
- 22.Heijink DM, Kleibeuker JH, Jalving M, et al. Independent induction of caspase-8 and cFLIP expression during colorectal carcinogenesis in sporadic and HNPCC adenomas and carcinomas. Cell Oncol. 2007;29:409–19. doi: 10.1155/2007/564605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu B, Zhou ZG, Li Y, et al. Clinicopathological significance of caspase-8 and caspase-10 expression in rectal cancer. Oncology. 2008;74:229–36. doi: 10.1159/000151392. [DOI] [PubMed] [Google Scholar]
- 24.McDermott U, Boyd RE, Houston RF, et al. A phase III trial of short duration adjuvant chemotherapy with bolus/infusional 5-Fluorouracil and Folinic Acid versus surgery alone in Dukes’ B and C colorectal cancer. Proc Am Soc Clin Oncol. 2003;22 Abstract#339. [Google Scholar]
- 25.Koornstra JJ, Kleibeuker JH, van Geelen CM, et al. Expression of TRAIL (TNF-related apoptosis-inducing ligand) and its receptors in normal colonic mucosa, adenomas, and carcinomas. J Pathol. 2003;200:327–35. doi: 10.1002/path.1364. [DOI] [PubMed] [Google Scholar]
- 26.Daniels RA, Turley H, Kimberley FC, et al. Expression of TRAIL and TRAIL receptors in normal and malignant tissues. Cell Res. 2005;15:430–8. doi: 10.1038/sj.cr.7290311. [DOI] [PubMed] [Google Scholar]
- 27.Johnstone RW, Frew AJ, Smyth MJ. The TRAIL apoptotic pathway in cancer onset, progression and therapy. Nat Rev Cancer. 2008;8:782–98. doi: 10.1038/nrc2465. [DOI] [PubMed] [Google Scholar]
- 28.Vasilevskaya IA, O’Dwyer PJ. 17-Allylamino-17-demethoxygeldanamycin overcomes TRAIL resistance in colon cancer cell lines. Biochem Pharmacol. 2005;70:580–9. doi: 10.1016/j.bcp.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 29.Lippa MS, Strockbine LD, Le TT, et al. Expression of anti-apoptotic factors modulates Apo2L/TRAIL resistance in colon carcinoma cells. Apoptosis. 2007;12:1465–78. doi: 10.1007/s10495-007-0076-6. [DOI] [PubMed] [Google Scholar]
- 30.Yoo J, Lee YJ. Effect of hyperthermia and chemotherapeutic agents on TRAIL-induced cell death in human colon cancer cells. J Cell Biochem. 2008;103:98–109. doi: 10.1002/jcb.21389. [DOI] [PubMed] [Google Scholar]
- 31.Zhu H, Zhang L, Huang X, et al. Overcoming acquired resistance to TRAIL by chemotherapeutic agents and calpain inhibitor I through distinct mechanisms. Mol Ther. 2004;9:666–73. doi: 10.1016/j.ymthe.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 32.O’Connell J, Bennett MW, O’Sullivan GC, Collins JK, Shanahan F. Fas counter-attack--the best form of tumor defense? Nat Med. 1999;5:267–8. doi: 10.1038/6477. [DOI] [PubMed] [Google Scholar]
- 33.Ryan AE, Shanahan F, O’Connell J, Houston AM. Addressing the “Fas counterattack” controversy: blocking fas ligand expression suppresses tumor immune evasion of colon cancer in vivo. Cancer Res. 2005;65:9817–23. doi: 10.1158/0008-5472.CAN-05-1462. [DOI] [PubMed] [Google Scholar]
- 34.Igney FH, Krammer PH. Immune escape of tumors: apoptosis resistance and tumor counterattack. J Leukoc Biol. 2002;71:907–20. [PubMed] [Google Scholar]
- 35.Giovarelli M, Musiani P, Garotta G, et al. A “stealth effect”: adenocarcinoma cells engineered to express TRAIL elude tumor-specific and allogeneic T cell reactions. J Immunol. 1999;163:4886–93. [PubMed] [Google Scholar]
- 36.Ishimura N, Isomoto H, Bronk SF, Gores GJ. Trail induces cell migration and invasion in apoptosis-resistant cholangiocarcinoma cells. Am J Physiol Gastrointest Liver Physiol. 2006;290:G129–36. doi: 10.1152/ajpgi.00242.2005. [DOI] [PubMed] [Google Scholar]
- 37.Ehrhardt H, Fulda S, Schmid I, et al. TRAIL induced survival and proliferation in cancer cells resistant towards TRAIL-induced apoptosis mediated by NF-kappaB. Oncogene. 2003;22:3842–52. doi: 10.1038/sj.onc.1206520. [DOI] [PubMed] [Google Scholar]
- 38.Wang S. The promise of cancer therapeutics targeting the TNF-related apoptosis-inducing ligand and TRAIL receptor pathway. Oncogene. 2008;27:6207–15. doi: 10.1038/onc.2008.298. [DOI] [PubMed] [Google Scholar]
- 39.Kataoka T, Tschopp J. N-terminal fragment of c-FLIP(L) processed by caspase 8 specifically interacts with TRAF2 and induces activation of the NF-kappaB signaling pathway. Mol Cell Biol. 2004;24:2627–36. doi: 10.1128/MCB.24.7.2627-2636.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frisch SM. Caspase-8: fly or die. Cancer Res. 2008;68:4491–3. doi: 10.1158/0008-5472.CAN-08-0952. [DOI] [PubMed] [Google Scholar]
- 41.Bell BD, Leverrier S, Weist BM, et al. FADD and caspase-8 control the outcome of autophagic signaling in proliferating T cells. Proc Natl Acad Sci U S A. 2008;105:16677–82. doi: 10.1073/pnas.0808597105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hopkins-Donaldson S, Ziegler A, Kurtz S, et al. Silencing of death receptor and caspase-8 expression in small cell lung carcinoma cell lines and tumors by DNA methylation. Cell Death Differ. 2003;10:356–64. doi: 10.1038/sj.cdd.4401157. [DOI] [PubMed] [Google Scholar]
- 43.Ryu BK, Lee MG, Chi SG, Kim YW, Park JH. Increased expression of cFLIP(L) in colonic adenocarcinoma. J Pathol. 2001;194:15–9. doi: 10.1002/path.835. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.