Abstract
We have recently found that a zinc transporter, ZIP4, is overexpressed in human pancreatic cancer and contributes to pancreatic cancer pathogenesis and progression. However, the detailed mechanism that how ZIP4 regulates pancreatic cancer growth is not clear. In this study, we further investigated the key molecules regulated by ZIP4 in pancreatic cancer angiogenesis and metastasis. We found that overexpression of ZIP4 caused significantly increased expression of NRP-1, VEGF, MMP-2, and MMP-9 in both pancreatic cancer cell lines and xenografts. Conversely, silencing of ZIP4 by short hairpin RNA (shRNA) was associated with decreased expression of NRP-1 and VEGF in pancreatic cancer xenografts. The ZIP4 expression and NRP-1 level are also correlated in established human pancreatic cancer cell lines. These results indicate that ZIP4-mediated pancreatic cancer growth might involve angiogenesis, invasion, and metastasis pathways, and NRP-1, VEGF and MMPs are important intermediate molecules in transducing the ZIP4 initiated signal cascades in pancreatic cancer.
Keywords: ZIP4, NRP-1, VEGF, MMP, pancreatic cancer
Introduction
ZIP4 is a zinc transporter that plays an important role in maintaining the cellular zinc level 1-4. Our recent study has indicated that ZIP4 is overexpressed in human pancreatic cancer, and aberrant expression of ZIP4 contributes to pancreatic cancer pathogenesis and progression 5. Conversely, silencing of ZIP4 by shRNA significantly inhibits pancreatic cancer growth and increases survival rate of nude mice with pancreatic cancer xenografts 6, suggesting that ZIP4 might be a novel therapeutic target for pancreatic cancer treatment. Altered expression of zinc transporters is associated with many other cancers. ZIP6 (also known as LIV-1), a breast cancer-associated protein, has been indicated to play a critical role in estrogen-positive breast cancer and metastasis to lymph nodes 7. Similarly, Kagara et al found that zinc transporter ZIP10 were involved in invasive behavior of breast cancer cells 8. Those reports suggest a positive correlation between zinc importers and cancer progression. The overexpression of ZIP transporters in cancers might regulate the expression of many other genes or transcription factors by providing sufficient zinc ions to the fast growing tumor cells.
Neuropilins (NRPs) are type I transmembrane glycoproteins, originally identified in neuronal cells as receptors for class-3 semaphorin subfamily 9, 10. Recently they have also been characterized as receptors or co-receptors for specific isoforms of vascular endothelia growth factors (VEGFs) 11, 12. VEGF is a primary activator of angiogenesis, and also functions as a potent permeability-inducing agent and an endothelial cell-surviving factor. Several studies suggest that NRPs play an important role in tumor growth and angiogenesis, and might serve as markers for the progression of prostate cancer, breast cancer and pancreatic cancer 13, 14. Clinically, over expression of NRP-1 appears to be correlated with the metastatic potential of prostate carcinoma cells 15. Our previous study also found that pancreatic cancer cells overexpress NRP-1 and VEGF, but not VEGF receptors (VEGFRs) 16, indicating the importance of angiogenesis pathway in pancreatic cancer growth.
Currently, very limited data are available for the functions of NRP-1 in pancreatic cancer and other cancers. Whether there is any correlation and interaction between NRP-1 and ZIP4, two important molecules in pancreatic cancer, is not clear. In this study, we have examined the expression of NRP-1, VEGF, and other related molecules in tumor invasion and metastasis such as matrix metalloprotease 2 (MMP-2), and MMP-9 in ZIP4 overexpressing and silenced cells lines and xenografts by quantitative real time RT PCR. Comparison of the gene expression profiles in these cells and xenografts may provide additional important information in understanding the molecular mechanisms of ZIP4-mediated pancreatic cancer growth.
Materials and Methods
Chemicals and Reagents
The real time PCR reagents SYBR Green supermix and iScript cDNA synthesis kits were purchased from Bio-Rad (Hercules, CA). The Ambion “RNAqueous-4PCR” kit and DNA removing kits were obtained from Ambion (Austin, Texas). Other chemicals were obtained from Sigma (St. Louis, MO).
Cell culture and stable cell line selection
Human pancreatic cancer cell lines MIA PaCa-2 and ASPC-1 were purchased from the American Type Culture Collection (ATCC, Rockville, MD), and were cultured in DMEM and RPMI 1640 medium with 10% fetal bovine serum (FBS), respectively as previously described 16, 17. Stable ZIP4 overexpressing and shRNA silenced cells (MIA-ZIP4 and ASPC-shZIP4) were selected in MIA PaCa-2 and ASPC-1 cells with retrovirus vectors (Origene, Rockville, MD), as we described previously 5. The sequence of the ZIP4 shRNAs used in this study is as follows: 5’ ACGTAGCACTCTGCGACATGGTCAGGATG 3’. Other established human pancreatic cancer cell lines Panc-1, BxPC-3, Hs766T, Capan-1, and HPAF-II were purchased from ATCC. The human pancreatic ductal epithelium (HPDE) cells were provided as a generous gift from Dr. Ming-Sound Tsao 18, 19. All cells were cultured as previously described 16, 17.
Pancreatic cancer mouse model and xenograft tissue collection
Subconfluent MIA-V and MIA-ZIP4, or ASPC-shV and ASPC-shZIP4 cells (3×106) were inoculated either into the right flank (subcutaneous tumor model) or in the body of the pancreas (orthotopic tumor model) of 5- to 6-week-old male nude mice (NCI-Charles River) as described previously 5. All mice were cared for in accordance with the OPRR and Animal Welfare Act guidelines under an animal protocol approved by Baylor College of Medicine Institutional Animal Care and Use Committee (IACUC). After 4 weeks of tumor implantation, all surviving mice were euthanized by an overdose of CO2 exposure and evaluated macroscopically for the presence of primary tumors and the metastases in the abdominal cavity. For both subcutaneous and orthotopic experiments, the animals were euthanized when their tumor size reached 2 cm in diameter or the animals became moribund during the observation period, and the time of euthanization was recorded as the time of mortality. The subcutaneous tumors and primary orthotopic tumors were collected, and stored in RNAlater solution (Ambion, Austin, TX) for further analysis.
RNA Extraction and real time RT PCR
Total RNA was extracted from the pancreatic cancer cell lines and homogenized xenograft tissues using an Ambion “RNAqueous-4PCR” kit following the manufacture's instructions. Briefly, cells or tissue samples were lysed by Ambion lysis buffer for 20 min and the cell lysates were mixed with equal volume of 64% ethanol. The lysates were transferred to an Ambion mini-column in a 2 ml collection tube, and centrifuged at 10,000 ×g for 1 min. The column was washed once with 700 μl of wash buffer 1, and twice with 500 μl of wash buffer 2/3. After incubation with 50 μl of pre-warmed elution buffer, the eluted fluid was collected using a new tube, and another 50 μl of elution buffer was added followed by a second centrifugation. The RNA solution was treated with DNase I to remove the trace amount of genomic DNA contamination by using an Ambion DNA removing kit (Austin, Texas). The mRNA levels for NRP-1, VEGF, MMP-2 and MMP-9 in pancreatic cancer cells and xenografts were analyzed by real time RT PCR using iCycler system (Bio-Rad, Hercules, CA) as described previously 16. PCR reaction included the following components: 100 nM each primer, diluted cDNA templates and iQ SYBR Green supermix, and running for 40 cycles at 95°C for 20 sec and 60°C for 1 min. PCR efficiency was examined by serially diluting the template cDNA and the melting curve data were collected to check PCR specificity. Each cDNA sample was run as triplicates and the corresponding no-reverse transcriptase (RT) mRNA sample was included as a negative control. The primer sequences for NRP-1, VEGF, MMP-2 and MMP-9 are listed as follows: NRP-1, forward: 5’ AAGGTTTCTCAGCAAACTACAGTG 3’, reverse: 5’ GGGAAGAAGCTGTGATCTGGTC 3’. VEGF, forward: 5’ AGATCGAGTACATCTTCAAGCCATC 3’, reverse: 5’ CGTCATTGCAGCAGCCC 3’. MMP-2, forward: 5’ CCCAGACAGGTGATCTTGACC 3’, reverse: 5’ CTTGCGAGGGAAGAAGTTGTAG 3’. MMP-9, forward: 5’ TGGCATCCGGCACCTCTATG 3’, reverse: 5’ CTCTGAGGGGTGGACAGTGG 3’. The β-actin primer was included in every plate to avoid sample variations. The mRNA level of each sample for each gene was normalized to that of the β-actin mRNA. The relative mRNA level was presented as 2^[(Ct/β-actin - Ct/gene of interest)].
Western Blot Analysis
ASPC-shV and ASPC-shZIP4 cells were lysed with ice-cold lysis buffer (20 mM Tris-HCl , pH 7.5, 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 μg/mL leupeptin and protease inhibitor cocktail) for 30 min in ice. Cell lysates were then collected and 100 μg of lysate protein was loaded and total cellular protein was separated with 15% SDS-polyacrylamide gel electrophoresis and then transblotted overnight at 4°C onto Hybond-P PVDF membrane (Amersham biosciences). The membrane was probed with anti-NRP-1 (1:200, Santa Cruz) or anti-β-actin (1:3000) antibody at 4°C over night and then washed three times with 0.1% Tween 20-TBS and incubated in a horseradish peroxidase-linked secondary antibody (1:2000) for 1 h at room temperature. The membrane was washed three times with 0.1% Tween 20-TBS and the immunoreactive bands were detected by using enhanced chemiluminescent (ECL) plus reagent kit.
Statistical analysis
Quantitative results are shown as means ± standard deviations. The statistical analysis was performed by the Student's t test between control and treatment groups. A p-value of < 0.05 was considered statistically significant.
Results
ZIP4 increases the expression of NRP-1 in pancreatic cancer cell lines
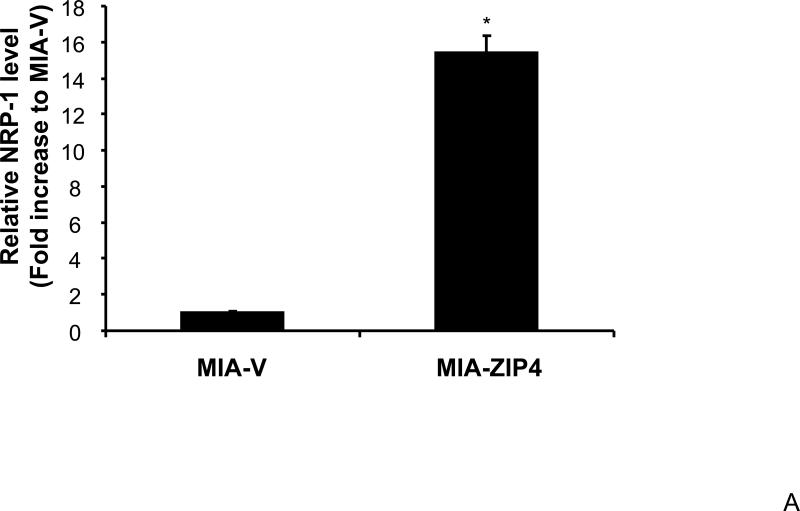
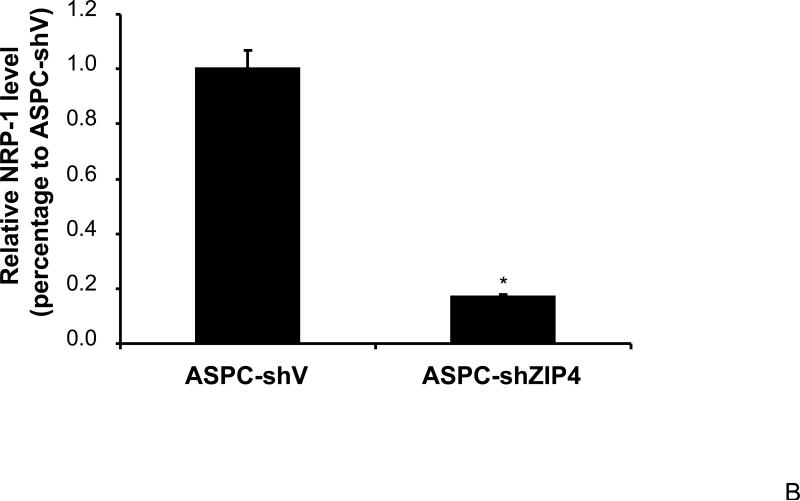
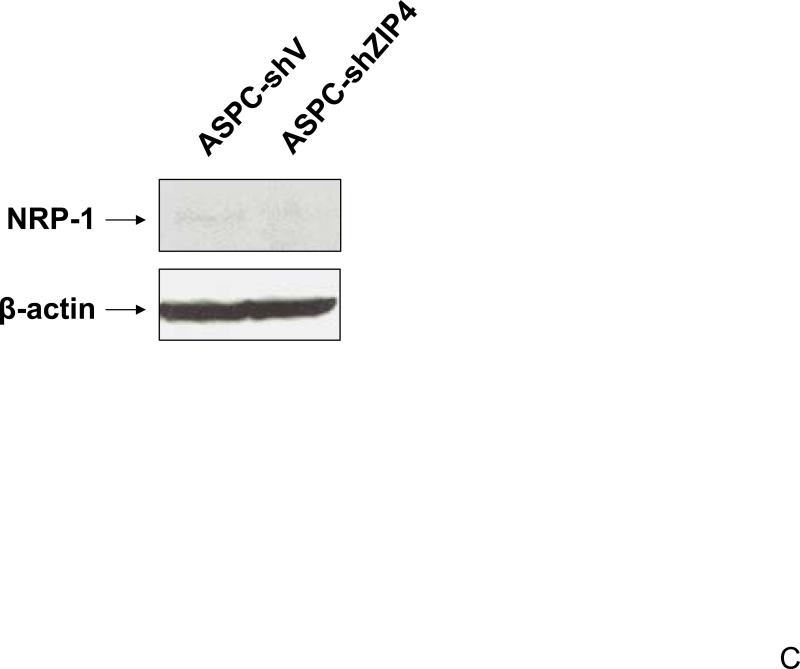
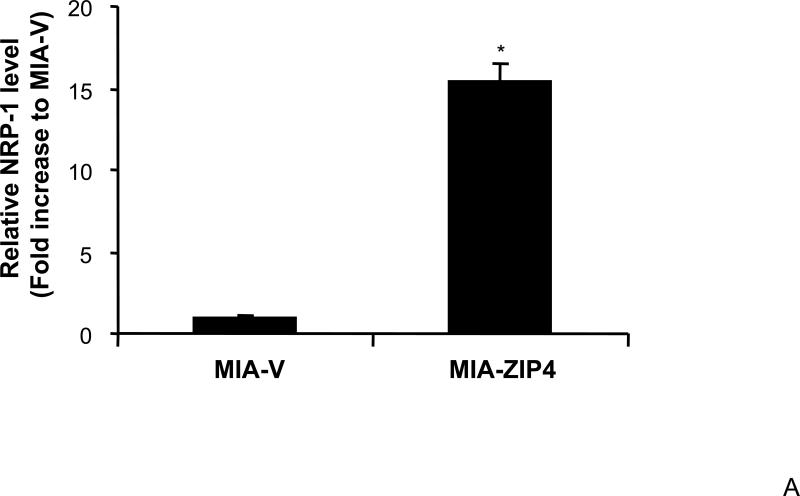
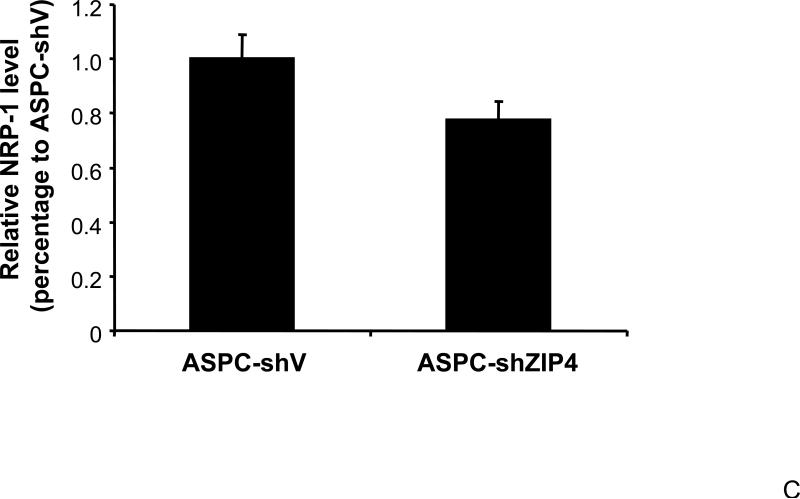
We have previously shown that ZIP4 is a malignant factor in human pancreatic cancer 5, 6. However, very little is known about whether ZIP4 affects the gene expression of other key molecules involved in the regulation of pancreatic cancer proliferation, invasion, and angiogenesis such as VEGF, VEGFRs, NRPs, and MMPs. We examined the expression of NRP-1 in stable ZIP4 overexpressing (MIA-ZIP4) and silenced (ASPC-shZIP4) cells, and also included the corresponding cells containing the empty vectors as controls. As shown in Fig. 1A, NRP-1 expression was significantly increased in MIA-ZIP4 cells (15.1-fold increase in mRNA level, P < 0.05) compared with that in MIA-V control cells. Conversely, NRP-1 expression was substantially decreased in ASPC-shZIP4 cells (83% decrease in mRNA level, P < 0.05) compared with that in ASPC-shV control cells (Fig. 1B). Similarly, NRP-1 protein level was also decreased in ASPC-shZIP4 cells compared with that in ASPC-shV cells (Fig. 1C), which is consistent with the mRNA data. Because the NRP-1 protein in MIA PaCa-2 cells is undetectable, we are unable to show the protein data in MIA PaCa-2 cells. These results suggest that ZIP4 is tightly associated with NRP-1 expression in pancreatic cancer.
Fig. 1. ZIP4 upregulates the expression of NRP-1 in pancreatic cancer cell lines.
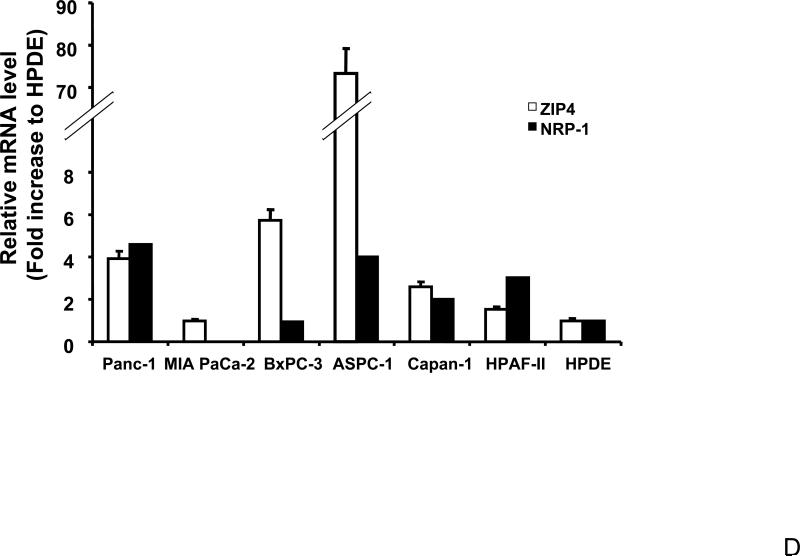
Total RNA was extracted from MIA-V, MIA-ZIP4, ASPC-shV, and ASPC-shZIP4 cells and the mRNA level for NRP-1 was analyzed by real time PCR, and normalized to that of the house keeping gene, β-actin. Relative mRNA level is presented as fold change compared with the vector control cells. A. Relative NRP-1 mRNA level in MIA-V and MIA-ZIP4 cells. B. Relative NRP-1 mRNA level in ASPC-shV and ASPC-shZIP4 cells. C. NRP-1 protein level in ASPC-shV and ASPC-shZIP4 cells. D. Relative ZIP4 and NRP-1 mRNA levels in human pancreatic cancer cell lines. All data shown are the mean±SD of three separate experiments. *p < 0.05.
To extend our findings, we compared the ZIP4 and NRP-1 levels in more established human pancreatic cancer cell lines. As shown in Fig. 1D, ZIP4 expression correlates with the NRP-1 level in human pancreatic cancer cells. In ZIP4-high cells, such as Panc-1 and ASPC-1, the NRP-1 level is also high. In ZIP4-intermediate cells, such as Capan-1 and HPAF-II, the NRP-1 level is lower than that in the above two cells. The ZIP4-low cells, MIA PaCa-2, express the lowest NRP-1. Those results indicate that ZIP4 might be involved in the regulation of NRP-1 expression in human pancreatic cancer cells, but the detailed mechanism is not clear.
ZIP4 upregulates the expression of NRP-1 in pancreatic cancer xenografts
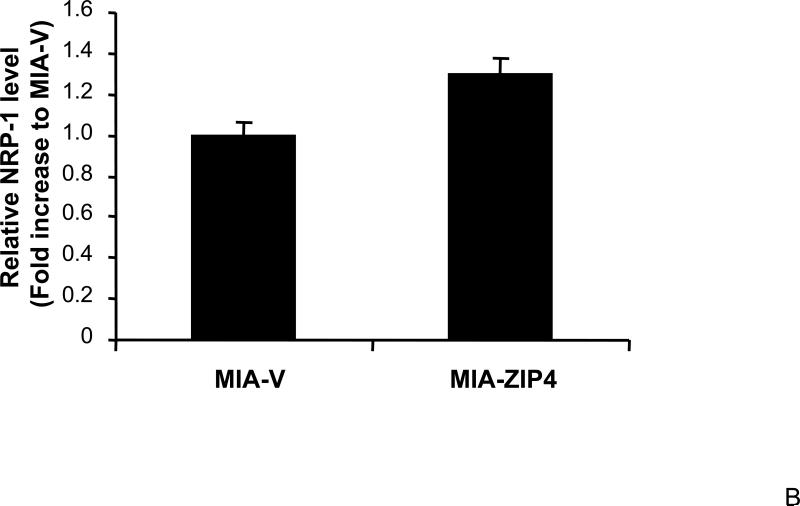
To further investigate the effect of ZIP4 on NRP-1 expression in a more physiologically related environment, we examined the NRP-1 mRNA levels in pancreatic cancer xenografts. MIA-ZIP4, ASPC-shZIP4, and corresponding vector control cells (MIA-V and ASPC-shV) were implanted into the immunodeficient nude mice through subcutaneous or orthotopic routes. After four weeks, the mice were euthanized, and the xenografts were collected for further analysis. As shown in Fig. 2A and 2B, NRP-1 expression was significantly increased in MIA-ZIP4 subcutaneous xenografts (15.4-fold increase in mRNA level), and orthotopic xenografts (1.3-fold increase in mRNA level), respectively, compared with that in the MIA-V control groups. Conversely, NRP-1 expression was decreased in ASPC-shZIP4 orthotopic xenografts (22% decrease in mRNA level) compared with that in ASPC-shV control group (Fig. 2C). Those results indicate that ZIP4 regulates the expression of NRP-1 not only in human pancreatic cancer cell lines, but also in the xenografts.
Fig. 2. ZIP4 upregulates the expression of NRP-1 in pancreatic cancer xenografts.
Subconfluent MIA-V and MIA-ZIP4, or ASPC-shV and ASPC-shZIP4 cells (3×106) were inoculated either into the right flank (subcutaneous tumor model) or the body of the pancreas (orthotopic tumor model) of nude mice. After 4 weeks of tumor implantation, all surviving mice were euthanized and the subcutaneous tumors and primary orthotopic tumors were collected, and the total RNA was extracted from the homogenized xenograft tissues. The mRNA level for NRP-1 was analyzed by real time PCR, and normalized to that of the house keeping gene, β-actin. Relative mRNA level is presented as fold change compared with the vector control cells. A. Relative NRP-1 mRNA level in MIA-V and MIA-ZIP4 subcutaneous xenografts. B. Relative NRP-1 mRNA level in MIA-V and MIA-ZIP4 orthotopic xenografts. C. Relative NRP-1 mRNA level in ASPC-shV and ASPC-shZIP4 orthotopic xenografts. All data shown are the mean±SD of three separate experiments. *p < 0.05.
ZIP4 upregulates the expression of VEGF
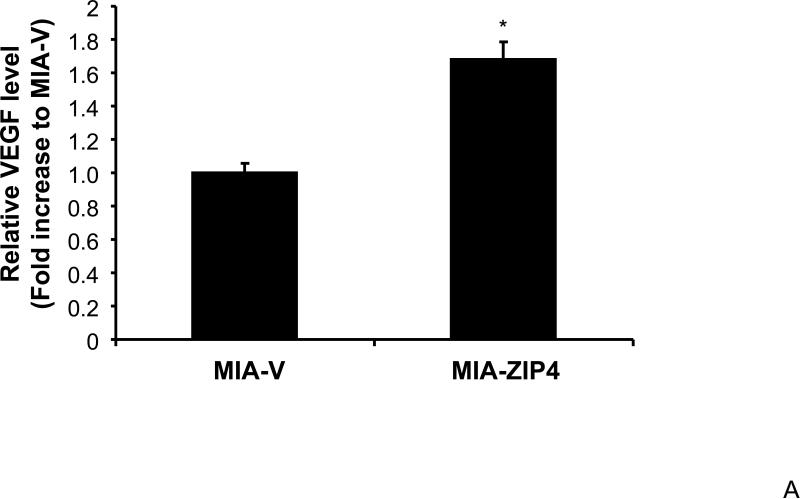
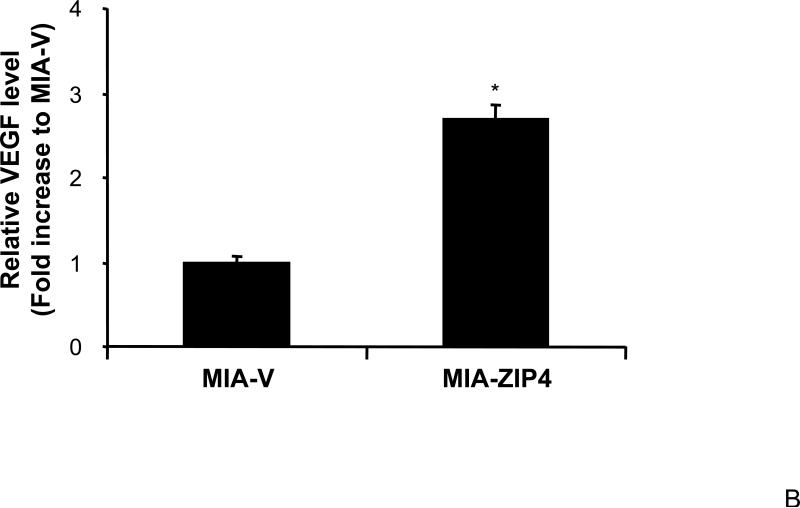
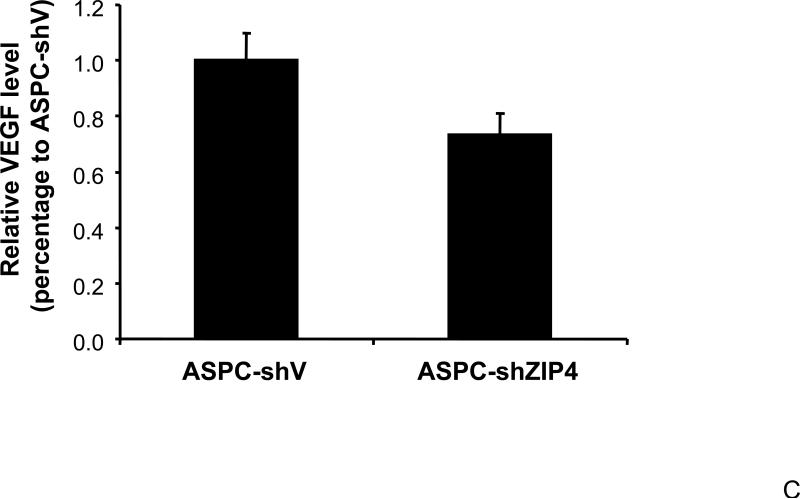
Our previous study has shown that VEGF and NRP-1 are overexpressed in pancreatic cancer, but not VEGF receptors. NRP-1 may serve as the major receptor for VEGF in pancreatic cancer 16. Therefore, we also examined the expression of VEGF in ZIP4 overexpressing and silenced pancreatic cancer xenografts, to see if the ligand of NRP-1 is also regulated by ZIP4. As shown in Fig. 3A and 3B, VEGF expression was significantly increased in MIA-ZIP4 subcutaneous xenografts (1.68-fold increase in mRNA level), and orthotopic xenografts (2.69-fold increase in mRNA level), respectively, compared with that in MIA-V control groups. On the contrary, VEGF expression was decreased in ASPC-shZIP4 orthotopic xenografts (26% decrease in mRNA level) compared with that in ASPC-shV control group (Fig. 3C). Those results indicate that ZIP4 also correlates with the expression of VEGF in pancreatic cancer xenografts.
Fig. 3. ZIP4 regulates the expression of VEGF.
Total RNA was extracted from MIA-V, MIA-ZIP4, ASPC-shV, and ASPC-shZIP4 xenografts and the mRNA level for VEGF was analyzed by real time PCR, and normalized to that of the house keeping gene, β-actin. Relative mRNA level is presented as fold change compared with the vector control. A. Relative VEGF mRNA level in MIA-V and MIA-ZIP4 subcutaneous xenografts. B. Relative VEGF mRNA level in MIA-V and MIA-ZIP4 orthotopic xenografts. C. Relative VEGF mRNA level in ASPC-shV and ASPC-shZIP4 orthotopic xenografts. All data shown are the mean±SD of three separate experiments. *p < 0.05.
ZIP4 increases the expression of MMP-2 and MMP-9
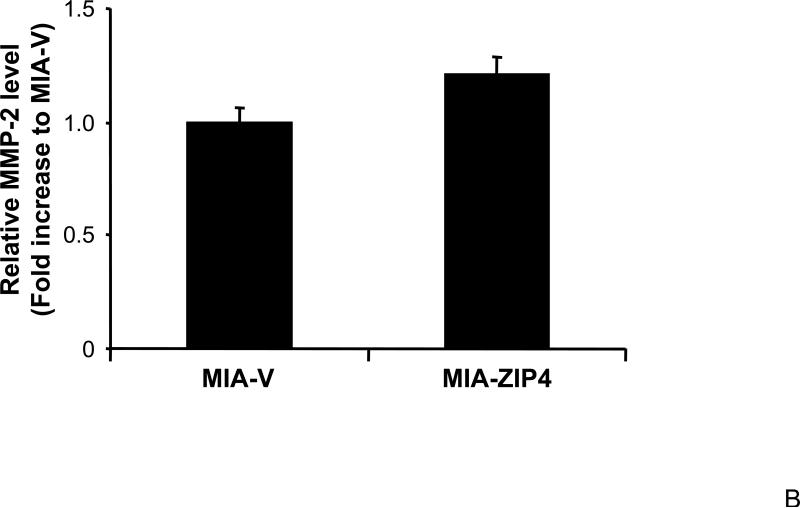
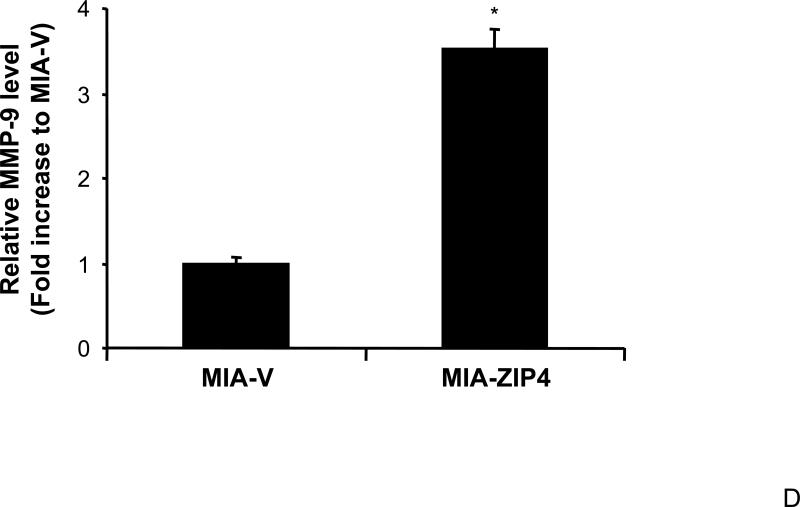
We have shown that ZIP4 is involved in pancreatic cancer cell invasion, and the conditioned medium of MIA-ZIP4 cells stimulates the cell proliferation of human umbilical vein endothelial cells (HUVEC), suggesting that ZIP4 plays an important role in cell invasion, angiogenesis, and tumor metastasis. We examined the expression of MMPs, the key molecules in invasion and tumor metastasis, in ZIP4 overexpressing pancreatic cancer xenografts, to see if the MMP levels correlate with ZIP4 expression. As shown in Fig. 4A and 4B, MMP-2 expression was increased in MIA-ZIP4 subcutaneous xenografts (1.25-fold increase in mRNA level), and orthotopic xenografts (1.21-fold increase in mRNA level), respectively, compared with that in the MIA-V control groups. MMP-9 expression was also significantly increased in MIA-ZIP4 subcutaneous xenografts (8.46-fold increase in mRNA level), and orthotopic xenografts (3.51-fold increase in mRNA level), respectively, compared with that in the MIA-V control groups (Fig. 4C, and 4D). Those results indicate that ZIP4 might be also involved in the regulation of MMP expression in pancreatic cancer.
Fig. 4. ZIP4 increases the expression of MMP-2 and MMP-9.
The mRNA level for MMP-2 and MMP-9 were analyzed by real time PCR. Relative mRNA level is presented as fold change compared with the vector control. A. Relative MMP-2 mRNA level in MIA-V and MIA-ZIP4 subcutaneous xenografts. B. Relative MMP-2 mRNA level in MIA-V and MIA-ZIP4 orthotopic xenografts. C. Relative MMP-9 mRNA level in MIA-V and MIA-ZIP4 subcutaneous xenografts. D. Relative MMP-9 mRNA level in MIA-V and MIA-ZIP4 orthotopic xenografts. All data shown are the mean±SD of three separate experiments. *p < 0.05.
Discussion
This study represents a detailed analysis of the expression profiles of NRP-1, VEGF and MMPs in ZIP4 overexpressing and silenced pancreatic cancer cell lines and xenografts. We found that overexpression of ZIP4 caused increase of the NRP-1 level in pancreatic cancer cells and xenograft, and silencing of ZIP4 decreased the NRP-1 level. Similarly, ZIP4 also upregulates the expression of VEGF, MMP-2 and MMP-9 in pancreatic cancer xenografts. The ZIP4 expression and NRP-1 level are also correlated in established human pancreatic cancer cell lines. These results indicate that ZIP4 mediated pancreatic cancer growth might involve angiogenesis, invasion, and metastasis pathways.
Pancreatic cancer cell growth is regulated by many factors and whether there are interactions between these factors and pathways has not been elucidated 5, 16, 20. Our previous studies have indicated the importance of ZIP4 in pancreatic cancer cell proliferation and tumor progression 5. We have also shown that NRP-1 and VEGF, but not VEGFRs, are overexpressed in pancreatic cancer cells, and VEGF promotes Panc-1 cell proliferation by interacting with NRP-1 16. To further investigate whether ZIP4-mediated pancreatic cancer cell growth involve other factors and pathways, in this current study, we examined the effect of ZIP4 overexpression or silencing on the gene expression of several key molecules in pancreatic cancer cell growth including NRP-1 and VEGF. In ZIP4 overexpressing cells (MIA-ZIP4) and xenografts, the expression of NRP-1 was upregulated compared with that in the vector control cells and xenografts. Conversely, in ZIP4 silenced cells (ASPC-shZIP4) and xenografts, the expression of NRP-1 was downregulated compared with that in the vector control cells and xenografts. In other established human pancreatic cancer cell lines, we also found a correlation of the expression of ZIP4 and NRP-1, which suggest that NRP-1 might be a mediator of ZIP4-induced pancreatic cancer growth.
Similarly, in ZIP4 overexpressing xenografts (MIA-ZIP4), the expression of VEGF was upregulated compared with that in the vector control group. While in ZIP4 silenced xenografts (ASPC-shZIP4), the VEGF level was downregulated. The VEGF family and VEGFRs have been involved in the formation of new blood vessels in both malignant and nonmalignant conditions 21-23. VEGF is a multifunctional cytokine that is secreted by a wide variety of tissues including premalignant lesions, invasive tumors and cell lines in breast cancer, colon cancer, melanoma, lung cancer and pancreatic carcinoma 24-29. VEGF overexpression is thought to be associated with increased vascularization, poor prognosis, and high grade tumor metastasis in several cancer types including lung cancer and pancreatic cancer 24, 30. Our previous study have shown that VEGF stimulates pancreatic cancer cell proliferation through NRP-1, but not VEGFRs 16. Pancreatic cancer cells expressed higher levels of VEGF and NRP-1, however, many of the pancreatic cancer cells do not express VEGFRs. Our study provides evidence supporting the existence of an autocrine pathway in pancreatic cancer cells through ZIP4-VEGF-NRP-1 interaction.
MMPs play critical roles in cancer cell invasion and metastasis. Upregulated MMPs are often observed in many cancers including pancreatic cancer 31-34. In this study, we found that in ZIP4 overexpressing xenografts (MIA-ZIP4), the expression of MMP-2 and MMP-9, the two predominant MMPs, were significantly increased compared with that in the vector control xenografts. Those results indicate that ZIP4-mediated pancreatic cancer cell growth may involve MMP pathway. Recent studies also indicate that MMP consist of a family of zinc-containing enzymes, and their activities can be activated by zinc. MMP expression has been linked with an increased risk of metastasis. There was no significant difference of MMP-2 and MMP-9 expression observed between ZIP4 silenced (ASPC-shZIP4) and control (ASPC-shV) xenografts (data not shown), suggesting that different pancreatic cancer cells respond to the alteration of ZIP4 level in different mechanisms. Whether ZIP4 affects the gene expression of other signal intermediates in pancreatic cancer cells warrants further investigations.
Taken together, this study demonstrates that zinc transporter ZIP4 upregulates the expression of NRP-1, VEGF, MMP-2, and MMP-9 in pancreatic cancer cells and xenografts, and different pancreatic cancer cells respond to the alteration of ZIP4 level in different mechanisms. Comparison of the gene expression profiles in pancreatic cancer cells with ZIP4 overexpression or silencing may help us to elucidate the mechanism of ZIP4-mediated cell growth, providing new insights for better understanding of pancreatic cancer pathogenesis and for developing effective therapeutic strategies against pancreatic cancer.
Acknowledgement
This work was supported in part by the National Institutes of Health (NIH) grant R21CA133604, American Cancer Society Grant #IRG-93-034-09, the MacDonald Research Fund, and the Dan L. Duncan Cancer Center pilot grant (M Li).
References
- 1.Liuzzi JP, Cousins RJ. Mammalian zinc transporters. Annu Rev Nutr. 2004;24:151–72. doi: 10.1146/annurev.nutr.24.012003.132402. [DOI] [PubMed] [Google Scholar]
- 2.Liuzzi JP, Bobo JA, Lichten LA, Samuelson DA, Cousins RJ. Responsive transporter genes within the murine intestinal-pancreatic axis form a basis of zinc homeostasis. Proc Natl Acad Sci U S A. 2004;101:14355–60. doi: 10.1073/pnas.0406216101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mao X, Kim BE, Wang F, Eide DJ, Petris MJ. A histidine-rich cluster mediates the ubiquitination and degradation of the human zinc transporter, hZIP4, and protects against zinc cytotoxicity. J Biol Chem. 2007;282:6992–7000. doi: 10.1074/jbc.M610552200. [DOI] [PubMed] [Google Scholar]
- 4.Kim BE, Wang F, Dufner-Beattie J, Andrews GK, Eide DJ, Petris MJ. Zn2+-stimulated endocytosis of the mZIP4 zinc transporter regulates its location at the plasma membrane. J Biol Chem. 2004;279:4523–30. doi: 10.1074/jbc.M310799200. [DOI] [PubMed] [Google Scholar]
- 5.Li M, Zhang Y, Liu Z, Bharadwaj U, Wang H, Wang X, et al. Aberrant expression of zinc transporter ZIP4 (SLC39A4) significantly contributes to human pancreatic cancer pathogenesis and progression. Proc Natl Acad Sci U S A. 2007;104:18636–41. doi: 10.1073/pnas.0709307104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li M, Zhang Y, Bharadwaj U, Zhai Q, Ahern C, Fisher WE, et al. Downregulation of ZIP4 by RNA interference inhibits pancreatic cancer growth and increases survival of nude mice with pancreatic cancer xenografts. Clin Cancer Res. 2009 doi: 10.1158/1078-0432.CCR-09-0557. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor KM, Morgan HE, Johnson A, Hadley LJ, Nicholson RI. Structure-function analysis of LIV-1, the breast cancer-associated protein that belongs to a new subfamily of zinc transporters. Biochem J. 2003;375:51–9. doi: 10.1042/BJ20030478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kagara N, Tanaka N, Noguchi S, Hirano T. Zinc and its transporter ZIP10 are involved in invasive behavior of breast cancer cells. Cancer Sci. 2007;98:692–7. doi: 10.1111/j.1349-7006.2007.00446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luo Y, Raible D, Raper JA. Collapsin: a protein in brain that induces the collapse and paralysis of neuronal growth cones. Cell. 1993;75:217–27. doi: 10.1016/0092-8674(93)80064-l. [DOI] [PubMed] [Google Scholar]
- 10.He Z, Tessier-Lavigne M. Neuropilin is a receptor for the axonal chemorepellent Semaphorin III. Cell. 1997;90:739–51. doi: 10.1016/s0092-8674(00)80534-6. [DOI] [PubMed] [Google Scholar]
- 11.Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92:735–45. doi: 10.1016/s0092-8674(00)81402-6. [DOI] [PubMed] [Google Scholar]
- 12.Soker S, Fidder H, Neufeld G, Klagsbrun M. Characterization of novel vascular endothelial growth factor (VEGF) receptors on tumor cells that bind VEGF165 via its exon 7-encoded domain. J Biol Chem. 1996;271:5761–7. doi: 10.1074/jbc.271.10.5761. [DOI] [PubMed] [Google Scholar]
- 13.Stephenson JM, Banerjee S, Saxena NK, Cherian R, Banerjee SK. Neuropilin-1 is differentially expressed in myoepithelial cells and vascular smooth muscle cells in preneoplastic and neoplastic human breast: a possible marker for the progression of breast cancer. Int J Cancer. 2002;101:409–14. doi: 10.1002/ijc.10611. [DOI] [PubMed] [Google Scholar]
- 14.Parikh AA, Liu WB, Fan F, Stoeltzing O, Reinmuth N, Bruns CJ, et al. Expression and regulation of the novel vascular endothelial growth factor receptor neuropilin-1 by epidermal growth factor in human pancreatic carcinoma. Cancer. 2003;98:720–9. doi: 10.1002/cncr.11560. [DOI] [PubMed] [Google Scholar]
- 15.Latil A, Bieche I, Pesche S, Valeri A, Fournier G, Cussenot O, et al. VEGF overexpression in clinically localized prostate tumors and neuropilin-1 overexpression in metastatic forms. Int J Cancer. 2000;89:167–71. doi: 10.1002/(sici)1097-0215(20000320)89:2<167::aid-ijc11>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 16.Li M, Yang H, Chai H, Fisher WE, Wang X, Brunicardi FC, et al. Pancreatic carcinoma cells express neuropilins and vascular endothelial growth factor, but not vascular endothelial growth factor receptors. Cancer. 2004;101:2341–50. doi: 10.1002/cncr.20634. [DOI] [PubMed] [Google Scholar]
- 17.Li M, Zhai Q, Bharadwaj U, Wang H, Li F, Fisher WE, et al. Cyclophilin A is overexpressed in human pancreatic cancer cells and stimulates cell proliferation through CD147. Cancer. 2006;106:2284–94. doi: 10.1002/cncr.21862. [DOI] [PubMed] [Google Scholar]
- 18.Furukawa T, Duguid WP, Rosenberg L, Viallet J, Galloway DA, Tsao MS. Long-term culture and immortalization of epithelial cells from normal adult human pancreatic ducts transfected by the E6E7 gene of human papilloma virus 16. Am J Pathol. 1996;148:1763–70. [PMC free article] [PubMed] [Google Scholar]
- 19.Ouyang H, Mou L, Luk C, Liu N, Karaskova J, Squire J, et al. Immortal human pancreatic duct epithelial cell lines with near normal genotype and phenotype. Am J Pathol. 2000;157:1623–31. doi: 10.1016/S0002-9440(10)64800-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korc M. Pathways for aberrant angiogenesis in pancreatic cancer. Mol Cancer. 2003;2:8. doi: 10.1186/1476-4598-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. Faseb J. 1999;13:9–22. [PubMed] [Google Scholar]
- 22.Karkkainen MJ, Makinen T, Alitalo K. Lymphatic endothelium: a new frontier of metastasis research. Nat Cell Biol. 2002;4:E2–5. doi: 10.1038/ncb0102-e2. [DOI] [PubMed] [Google Scholar]
- 23.Lymboussaki A, Olofsson B, Eriksson U, Alitalo K. Vascular endothelial growth factor (VEGF) and VEGF-C show overlapping binding sites in embryonic endothelia and distinct sites in differentiated adult endothelia. Circ Res. 1999;85:992–9. doi: 10.1161/01.res.85.11.992. [DOI] [PubMed] [Google Scholar]
- 24.Lantuejoul S, Constantin B, Drabkin H, Brambilla C, Roche J, Brambilla E. Expression of VEGF, semaphorin SEMA3F, and their common receptors neuropilins NP1 and NP2 in preinvasive bronchial lesions, lung tumours, and cell lines. J Pathol. 2003;200:336–47. doi: 10.1002/path.1367. [DOI] [PubMed] [Google Scholar]
- 25.Kerbel RS, Viloria-Petit A, Okada F, Rak J. Establishing a link between oncogenes and tumor angiogenesis. Mol Med. 1998;4:286–95. [PMC free article] [PubMed] [Google Scholar]
- 26.Veikkola T, Alitalo K. VEGFs, receptors and angiogenesis. Semin Cancer Biol. 1999;9:211–20. doi: 10.1006/scbi.1998.0091. [DOI] [PubMed] [Google Scholar]
- 27.Veikkola T, Karkkainen M, Claesson-Welsh L, Alitalo K. Regulation of angiogenesis via vascular endothelial growth factor receptors. Cancer Res. 2000;60:203–12. [PubMed] [Google Scholar]
- 28.Tian X, Song S, Wu J, Meng L, Dong Z, Shou C. Vascular endothelial growth factor: acting as an autocrine growth factor for human gastric adenocarcinoma cell MGC803. Biochem Biophys Res Commun. 2001;286:505–12. doi: 10.1006/bbrc.2001.5409. [DOI] [PubMed] [Google Scholar]
- 29.Decaussin M, Sartelet H, Robert C, Moro D, Claraz C, Brambilla C, et al. Expression of vascular endothelial growth factor (VEGF) and its two receptors (VEGF-R1-Flt1 and VEGF-R2-Flk1/KDR) in non-small cell lung carcinomas (NSCLCs): correlation with angiogenesis and survival. J Pathol. 1999;188:369–77. doi: 10.1002/(SICI)1096-9896(199908)188:4<369::AID-PATH381>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 30.Fujimoto K, Hosotani R, Wada M, Lee JU, Koshiba T, Miyamoto Y, et al. Expression of two angiogenic factors, vascular endothelial growth factor and platelet-derived endothelial cell growth factor in human pancreatic cancer, and its relationship to angiogenesis. Eur J Cancer. 1998;34:1439–47. doi: 10.1016/s0959-8049(98)00069-0. [DOI] [PubMed] [Google Scholar]
- 31.Mahadevan D, Von Hoff DD. Tumor-stroma interactions in pancreatic ductal adenocarcinoma. Mol Cancer Ther. 2007;6:1186–97. doi: 10.1158/1535-7163.MCT-06-0686. [DOI] [PubMed] [Google Scholar]
- 32.Bloomston M, Zervos EE, Rosemurgy AS., 2nd Matrix metalloproteinases and their role in pancreatic cancer: a review of preclinical studies and clinical trials. Ann Surg Oncol. 2002;9:668–74. doi: 10.1007/BF02574483. [DOI] [PubMed] [Google Scholar]
- 33.Orlichenko LS, Radisky DC. Matrix metalloproteinases stimulate epithelial-mesenchymal transition during tumor development. Clin Exp Metastasis. 2008;25:593–600. doi: 10.1007/s10585-008-9143-9. [DOI] [PubMed] [Google Scholar]
- 34.Stetler-Stevenson WG. The tumor microenvironment: regulation by MMP-independent effects of tissue inhibitor of metalloproteinases-2. Cancer Metastasis Rev. 2008;27:57–66. doi: 10.1007/s10555-007-9105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]