Abstract
Background
Orphanin FQ/Nociceptin (OFQ/N), the endogenous ligand of the opioid receptor-like (ORL1) receptor, blocks cocaine sensitization in rats. In the current study, we tested whether OFQ/N would block sensitization to the motor stimulatory and conditioned rewarding actions of cocaine in mice. We also examined whether OFQ/N, given to cocaine-sensitized mice, would reverse the sensitized response and whether it would prevent the amplified sensitized response induced by a second cocaine-sensitizing regimen in sensitized mice.
Methods
ORL1 knockout and wild-type mice were treated with saline or OFQ/N prior to saline or cocaine on days 1-3 and tested for sensitization on day 8. Additionally, wild-type mice were treated similarly but tested for the conditioned rewarding action of cocaine, in which mice were tested for place preference before and after single conditioning with cocaine. Furthermore, mice rendered sensitized, treated with saline or OFQ/N prior to saline or cocaine on days 13-15 and received cocaine on day 20 to test whether OFQ/N would reverse sensitization or block the amplified sensitized response induced by a second cocaine-sensitizing regimen in sensitized mice.
Results
OFQ/N blocked cocaine-induced psychomotor sensitization in wild-type but not knockout mice. It also blocked sensitization to the conditioned rewarding action of cocaine and reversed a pre-existing locomotor sensitized response. Furthermore, OFQ/N prevented the amplified sensitized response that developed following a second cocaine sensitizing regimen given to sensitized mice.
Conclusion
The current results illustrate that OFQ/N not only blocks but also reverses maladaptive behavioral changes induced by repeated cocaine treatment in mice.
Keywords: Cocaine, Locomotor sensitization, Orphanin FQ/Nociceptin, ORL1 receptor, Knockout mouse, Intracerebroventricular injection, Conditioned place preference (CPP)
Introduction
Cocaine abuse is recognized as a chronic relapsing brain disease that places a major burden on society. Chronic cocaine treatment induces neuronal adaptive changes along the mesocorticolimbic dopaminergic and corticolimbic glutamatergic neurons, leading to compulsive drug taking and drug seeking behaviors (for review, see (1-6). The transition from occasional drug use to compulsive drug intake or abuse involves instrumental and classical conditioning (for reviews, see (3, 6-10). Interestingly, the mesocorticolimbic dopaminergic and corticolimbic glutamatergic neurons play an important role in incentive learning and compulsive habit forming behaviors (3, 7, 11). Thus, drugs reducing the function of these neurons and reversing the adaptive changes induced by repeated cocaine treatment along these neurons may be proven useful as pharmacotherapy of cocaine addiction.
Orphanin FQ (also known as nociceptin; OFQ/N) and its cognate receptor, the opioid receptor-like (ORL1) receptor (also known as NOP), are widely distributed in brain regions involved in motivational behaviors (12, 13). Consistent with its localization, OFQ/N negatively regulates the function of the mesolimbic dopaminergic neurons (14-19). OFQ/N also inhibits neuronal plasticity (20-25), raising the possibility the OFQ/N/ORL1 receptor system may represent a potential target to block and/ or reverse neuronal and behavioral adaptive changes induced by drugs of abuse.
The phenomenon of psychomotor sensitization is referred to as an enhanced and enduring increase in locomotor activity induced by intermittent treatment with cocaine or other drugs of abuse (26-30). It involves neuronal adaptive changes along the mesolimbic dopaminergic and corticolimbic glutamatergic neurons (1, 3-6). Interestingly, similar neuronal adaptive changes observed in human addicts. Furthermore, this phenomenon is thought to model addicts’ motivational behavioral changes through a progressive increase in the salience of a drug and cues associated with its administration and/or predicting it (1, 2, 4, 5, 11, 31-33). Accordingly, using mice lacking the ORL1 receptor and their wild-type littermates, we determined whether OFQ/N would alter cocaine-induced motor stimulation and locomotor sensitization in mice and whether the regulatory actions of OFQ/N would be mediated via the ORL1 receptor. Given that OFQ/N and related drugs, if proven useful, would be given to drugs addicts but not cocaine-naïve subjects, we also examined whether OFQ/N would reverse a pre-existing sensitized response. Additionally, we examined whether OFQ/N would prevent the development of an amplified psychomotor sensitized response that results from treating cocaine-sensitized mice with a second cocaine-sensitizing regimen.
The conditioned place preference (CPP) has been used as an animal model of drug reward/incentive learning (34). Repeated intermittent cocaine treatment that induces locomotor sensitization also elicits sensitization to cocaine-induced CPP in rats (35). This phenomenon can be generalized to other drugs of abuse (36, 37). Therefore, in the present study, we also tested whether mice rendered sensitized to cocaine-induced hyperlocomotion would also express sensitization to the conditioned rewarding action of cocaine and whether OFQ/N would block this phenomenon.
Materials and Methods
Subjects
Male mice lacking the ORL1 receptor, generated by replacement of the first coding region with a lacZ-neo cassette (38), were the offspring of heterozygous breeding pairs crossed for 12 generations on C57BL/6J mouse strain. Pups were weaned between the ages of 21-24 days and genotyped. Mice (2-3 months old at the onset of experiments) were housed 2-4 per cage with free access to water and food in a temperature- and humidity-controlled room. All the experimental procedures were according to the National Institute of Health guideline for the proper use of laboratory animals in research and approved by the Institutional Animal Care and Use Committee at Western University of Health Sciences (Pomona, California, USA).
Drugs
OFQ/N was purchased from Bachem (Torrance, California, USA), dissolved in normal saline and injected intracerebroventriculalry (ICV). Cocaine hydrochloride was purchased from Sigma-Aldrich (St. Louis, MO, USA), dissolved in normal saline and injected intraperitoneally (i.p.) in a volume of 10 ml/kg of body weight.
Experimental Protocols
Experiment 1
We first studied whether OFQ/N would reduce cocaine-induced motor stimulation and block psychomotor sensitization in mice and whether the regulatory actions of OFQ/N would be mediated via the ORL1 receptor. To assess the effect of OFQ/N on cocaine-induced hyperlocomotion, mice lacking the ORL1 receptor and their wild-type littermates were implanted with a guide cannula (see below). Four days later, mice were habituated to the motor activity chamber (14 cm length × 14 cm width × 22 cm height) for 1 h and then injected with saline or OFQ/N (10 nmol in 3μL; ICV) immediately followed by saline or cocaine (15 mg/kg, i.p.). Motor activity was then recorded for 1 h. To determine the effect of OFQ/N on locomotor sensitization, mice were treated once daily for three consecutive days, as described above, and then tested for locomotor sensitization on day 8. On this day, mice were habituated to the motor activity chambers for 1 h, injected with cocaine (15 mg/kg; i.p.) and motor activity was recorded for 1 h.
Experiment 2
We evaluated whether OFQ/N would alter cocaine-induced motor stimulation in cocaine-sensitized mice and whether this effect would be mediated via the ORL1 receptor. Mice lacking the ORL1 receptor and their wild-type littermates were treated and tested for the development of locomotor sensitization on day 8, as described above. On day 9, mice were implanted with a guide cannula (see below). Four days later (day 13), mice were habituated to the motor activity chambers for 1 h, treated with saline or OFQ/N (10 nmol in 3μL; ICV) immediately followed by cocaine (15 mg/kg, i.p.) and motor activity was recorded for 1 h.
Experiment 3
We next tested whether OFQ/N would block the development of cocaine-induced psychomotor sensitization in mice already sensitized to cocaine. Wild-type mice were treated, as described under Experiment 2. Four days after implantation of the guide cannula (day 13), mice were habituated to the motor activity chambers for 1 h, treated with saline or OFQ/N (10 nmol in 3μL; ICV) immediately followed by cocaine (15 mg/kg, i.p.) administration and motor activity was recorded for 1 h. Mice were treated with their respective treatment once daily for three consecutive days and then tested for locomotor sensitization five days later (day 20). On this day, mice were habituated to the test chambers for 1 h, injected with cocaine (15 mg/kg, i.p.) and motor activity was recorded for 1 h.
Experiment 4
We then examined whether OFQ/N administration to cocaine sensitized mice would reverse an established sensitized response. Wild-type mice were rendered sensitized and implanted with a guide cannula, as described above. Four days later (day 13), mice were treated with saline or OFQ/N (10 nmol in 3μL; ICV) and motor activity was recorded for 1 h. The same treatment was given for three consecutive days (days 13-15) and mice were then tested for locomotor sensitization five days later (day 20). On this day, mice were habituated to the test chamber for 1 h, injected with cocaine (15 mg/kg, i.p.) and motor activity was recorded for 1 h.
Experiment 5
We also assessed whether mice rendered sensitized to cocaine-induced motor stimulation would also express sensitization to the conditioned rewarding actions of cocaine. Mice were treated with cocaine (15 or 30 mg/kg) or saline once daily for three consecutive days and then tested for locomotor sensitization or CPP on day 8. For the assessment of locomotor sensitization, mice were habituated to locomotor activity chambers for 1 h, injected with cocaine (15 mg/kg) and their activity was recorded for 1 h. For the measurement of sensitization to the conditioned rewarding action of cocaine, mice were first tested for baseline place preference, in which each mouse was placed in the neutral central chamber of the CPP apparatus (consisting of a central smaller neutral grey chamber and two conditioning chambers distinguishable from each other by black and white horizontal or vertical stripes) and allowed to freely explore all the CPP chambers for 15 min. The amount of time that the mice spent in each conditioning chamber was recorded and used for analysis of the preconditioning data. The detailed description of the CPP apparatus is provided elsewhere (39). On the following day, mice received saline or cocaine in the morning and confined to vehicle- or drug-paired chamber for 15 min. In the afternoon, mice received the alternate treatment and confined to the opposite chamber for 15 min. The saline/cocaine conditioning was counterbalanced so that some mice received saline and some cocaine from each group in each conditioning session. On the following day, mice were tested for postconditioning place preference, as described above. The amount of time that the mice spent in each CPP chamber during the 15-min test period was recorded and used for analysis of the postconditioning data. We used a single cocaine conditioning protocol, as described previously (40), to be consistent with the locomotor sensitization paradigm.
Experiment 6
We finally examined whether OFQ/N would block the development of sensitization to the conditioned rewarding action of cocaine. On days 1-3, mice were daily habituated to the motor activity chambers for 1 h and then injected with saline or OFQ/N (10 nmol in 3μL) immediately followed by cocaine (15 mg/kg, i.p.), as described under Experiment 1. Mice were then left untreated until tested for baseline place preference on day 8. On the following day, mice received saline/cocaine (morning/afternoon) and confined to vehicle- or drug-paired chamber for 15 min. On the following day, mice were tested for postconditioning place preference, as described above.
Verification of the site of injection
All ICV injections were conducted according to our previously described method (39). Briefly, mice were anesthetized with isoflurane (5% for the induction and 1-2% for the maintenance of anesthesia) and implanted with a guide cannula in the right lateral ventricle and then tested for motor activity four days later. The injections were made unilaterally into the right lateral ventricle over 30 sec. The needle (DV = -4.0 mm depth) was left in place for an additional 20 sec. At the end of each experiment, 3 μl of the dye were injected through the guide cannula to verify the site of OFQ/N injection. Animals with incorrect injection site (n = 2) were excluded from data analysis.
Data Analysis
The data represent mean (±SEM) of distance traveled during the 1-h test period or the amount of time that mice spent in the conditioning chambers on the pre- and postconditioning test days. A one- or two-way analysis of variance (ANOVA) was used to analyze the data. The post-hoc Newman-Keuls test was used to reveal significant difference between various groups. A p<0.05 was considered statistically significant.
Results
OFQ/N blocked the development of cocaine-induced locomotor sensitization in wild-type but not in ORL1 receptor knockout mice
Sensitization developed in mice lacking the ORL1 receptor and their wild-type littermates (Fig. 1). OFQ/N blocked the development of the sensitized response only in wild-type mice (Fig. 1A). A one-way ANOVA of the data revealed a significant effect of treatments (F3,23 = 6.08; p<0.03). The post-hoc test showed that repeated cocaine treatment induced locomotor sensitization (Fig. 1A; compare Sal-Coc vs. Sal-Sal group) and this sensitized response was blunted in mice treated with OFQ/N prior to their daily cocaine administration on days 1-3 (Fig. 1A; compare OFQ/N-Coc vs. Sal-Coc group). On the other hand, OFQ/N did not alter cocaine-induced locomotor sensitization in mice lacking the ORL1 receptor (Fig. 1B). A one-way ANOVA revealed a significant effect of treatment (F3,23 = 5.92; p<0.005). Further analysis of the data showed that repeated cocaine treatment induced locomotor sensitization in ORL1 knockout mice but the magnitude of this response was not altered by OFQ/N (Fig. 1B; compare Sal-Sal vs. Sal-Coc and OFQ/N-Sal vs. OFQ/N-Coc group).
Fig. 1.
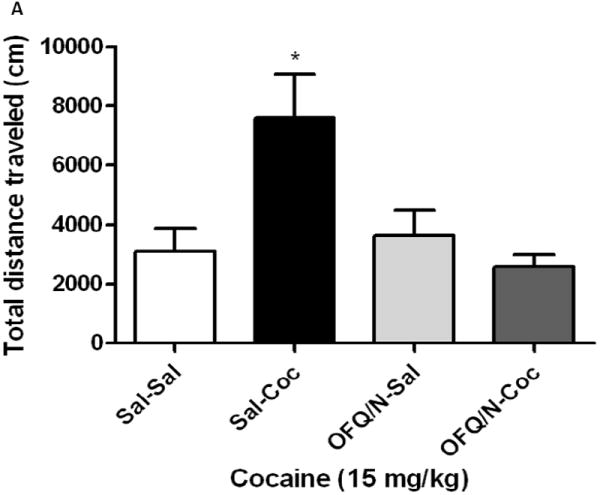
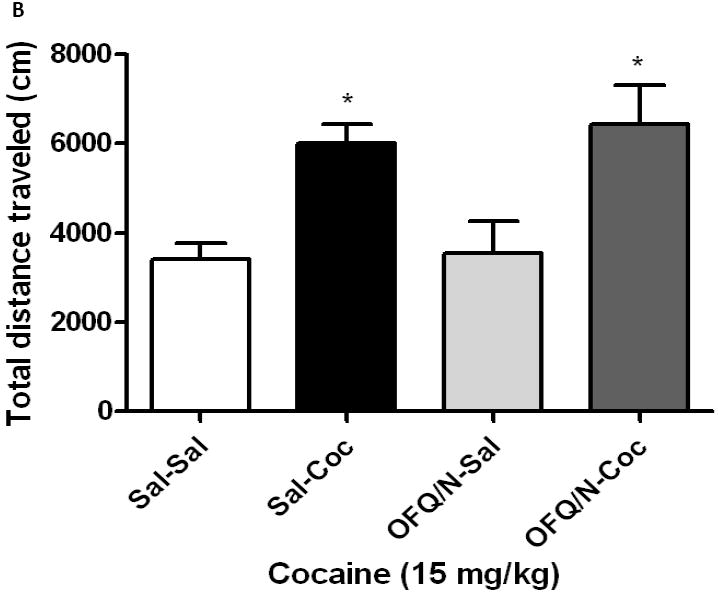
Intracerebroventricular OFQ/N administration blocked cocaine-induced locomotor sensitization in wild-type (1A) but not in ORL1 receptor knockout mice (IB). Mice were habituated to the motor activity chambers for 1 h, injected with saline or OFQ/N (10 nmol; ICV) immediately followed by saline or cocaine (15 mg/kg, i.p.) and motor activity was recorded for 1 h. Mice received their respective treatments for three consecutive days and tested on day 8. On this day, mice were habituated to the activity chambers for 1 h, injected with cocaine (15 mg/kg) and motor activity was measured for 1 h. Data are mean (±S.E.M.) of 6-8 mice per treatment for each genotype. *p<0.05 vs. their respective control group
OFQ/N attenuated cocaine-induced motor stimulation in cocaine-naïve but not in cocaine-sensitized wild-type mice
The effect of OFQ/N on cocaine-stimulated locomotor activity is shown in figure 2. Two-way ANOVA of the data in cocaine-naïve mice revealed a significant interaction between treatment and genotype (F1,26 = 4.71; p<0.04). The post-hoc analysis of the data showed that OFQ/N reduced the motor stimulatory action of cocaine in wild-type mice (Fig. 2A, compare saline vs. OFQ/N for the ORL1+/+ mice). In contrast, OFQ/N failed to alter the action of cocaine in mice lacking the ORL1 receptor (Fig. 2A; compare saline vs. OFQ/N for the ORL1-/- mice). Furthermore, OFQ/N failed to alter the motor stimulatory action of cocaine in sensitized mice (Fig. 2B). A two-way ANOVA of the data revealed no significant interaction between treatment and genotype (F1,24 = 0.01; p>0.05).
Fig. 2.
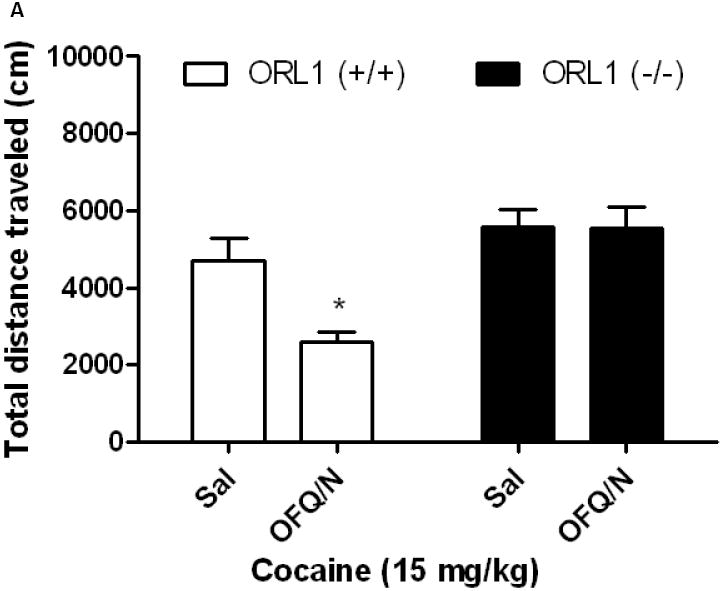
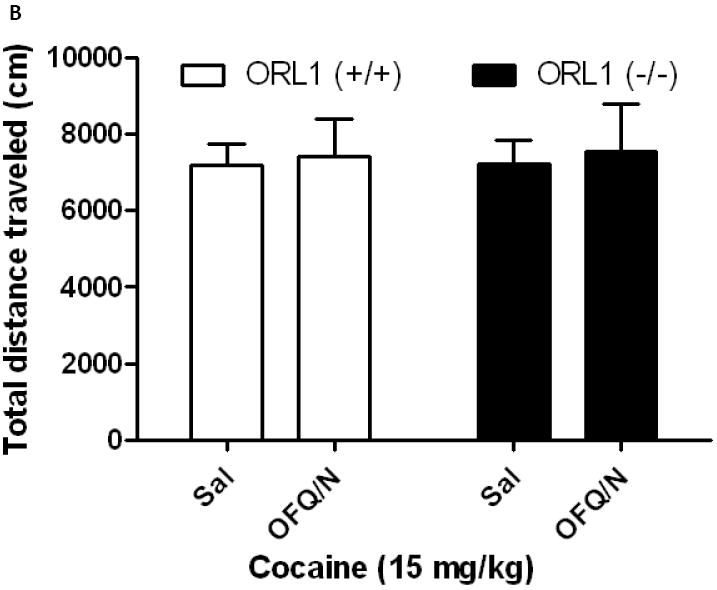
Intracerebroventricular OFQ/N administration attenuated cocaine-stimulated locomotor activity in cocaine-naïve wild-type but not ORL1 knockout mice (2A). However, OFQ/N failed to reduce the action of cocaine in cocaine-sensitized wild-type or knockout mice (2B). Naïve mice or mice sensitized to cocaine were habituated to the motor activity chambers for 1 h, injected with OFQ/N (10 nmol; ICV) immediately followed by cocaine (15 mg/kg, i.p.) and motor activity was recorded for 1 h. Data are mean (±S.E.M.) of 7-8 mice per treatment for each genotype. *p<0.05 vs. all other groups
OFQ/N attenuated the development of amplified psychomotor sensitization that developed in cocaine-sensitized wild-type mice exposed to a second cocaine-sensitizing regimen
As expected, sensitization developed to the motor stimulatory action of cocaine following the first sensitizing regimen and this response still persisted following implantation of a guide cannula in the lateral ventricle and a subsequent test for locomotor sensitization (Fig. 3; compare day 8 vs. day 13 for each group). Furthermore, a significantly greater sensitized response was observed in cocaine-sensitized mice receiving a second sensitizing regimen of cocaine (Fig. 3A; compare day 13 vs. day 20). On the other hand, ICV OFQ/N treatment given immediately prior to each daily cocaine injection on days 13-15 prevented the development of the amplified sensitized response (Fig. 3B; compare day 13 vs. day 20).
Fig. 3.
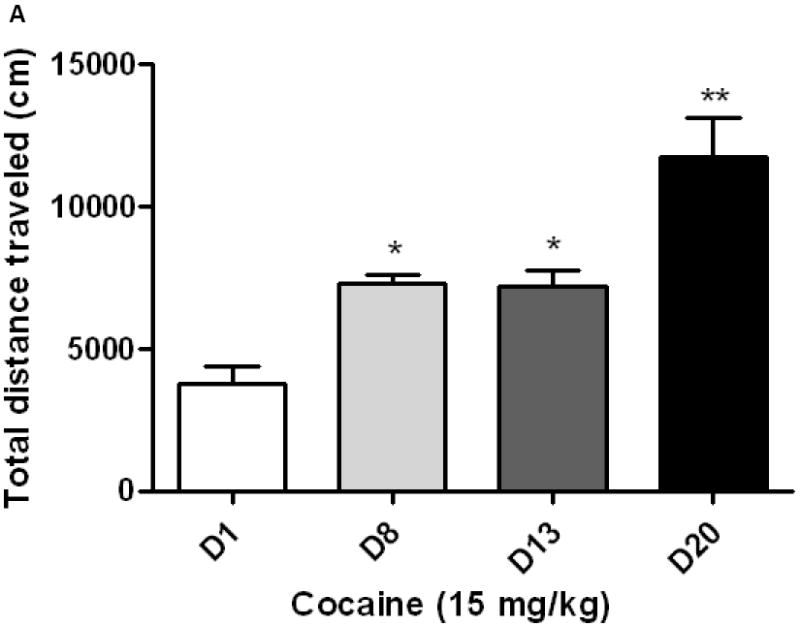
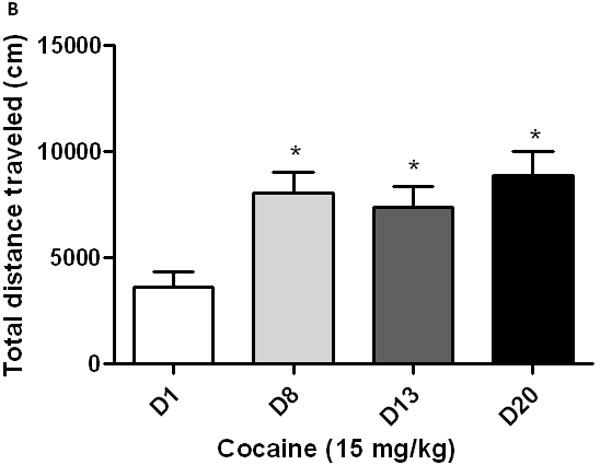
A second cocaine-sensitizing regimen administered in cocaine-sensitized mice induced significantly greater psychomotor sensitization (3A) and this amplified sensitized response was prevented by OFQ/N given prior to each cocaine administration during the induction of the amplified sensitized response (3B). Mice were treated with cocaine on days 1-3 and tested for sensitization on day 8. Mice were then implanted with a guide cannula and four days later treated with saline or OFQ/N (10 nmol; ICV) immediately followed by cocaine (15 mg/kg, i.p.) and motor activity was recorded for 1 h. Mice received their respective treatment on days 13-15 and tested for cocaine-induced motor stimulation on day 20. Data are mean (±S.E.M.) of 6 mice per group. *p<0.05 vs. cocaine response on day 1 (D1); **p<0.01 vs. all other groups
ICV OFQ/N reversed a pre-existing sensitized response
OFQ/N reduced basal locomotor activity in cocaine-naïve mice during the first 15 min of the 1 h test period. On the other hand, OFQ/N failed to suppress basal motor activity in mice sensitized to cocaine during this period (Table 1). The total distance traveled during the 1 h test period was significantly greater in cocaine-sensitized mice compared to their naïve controls, showing that OFQ/N increased basal motor activity in cocaine-sensitized compared to cocaine-naïve mice (Table 1). However, OFQ/N was still able to reverse the development of sensitization in these mice (Fig. 4). A two-way ANOVA of the data revealed a significant interaction between time and treatment (F3,36 = 3.19; p<0.05). Further analysis of the data revealed that cocaine did induce locomotor sensitization in both groups (Fig. 4; compare Dl vs. D8 for each group). Repeated ICV saline treatment given on days 13-15 did not alter the magnitude of the sensitized response (Fig. 4; compare D8 vs. D20 for open bars). On the other hand, ICV OFQ/N treatment prior to cocaine treatment on days 13-15 reversed the pre-existing sensitized response (Fig. 4; compare D8 vs. D20 for closed bars).
Table 1.
The effect of intracerebroventricular OFQ/N administration on basal locomotor activity in naïve and cocaine-sensitized mice. Data represent mean (±S.E.M.) of 7 mice per treatment.
| Group/Treatment | 15 min | 60 min |
|---|---|---|
| Naïve | ||
| Sal | 498.0 ± 111.5 | 843.2 ± 206.8 |
| OFQ/N | 257.7 ± 78.6* | 1482.2 ± 621.2 |
| Sensitized | ||
| Sal | 536.1 ± 171.9 | 1080.8 ± 323.4 |
| OFQ/N | 514.8 ± 259.1 | 3554.1 ± 718.2* |
p<0.05 versus its saline-treated controls
Fig. 4.
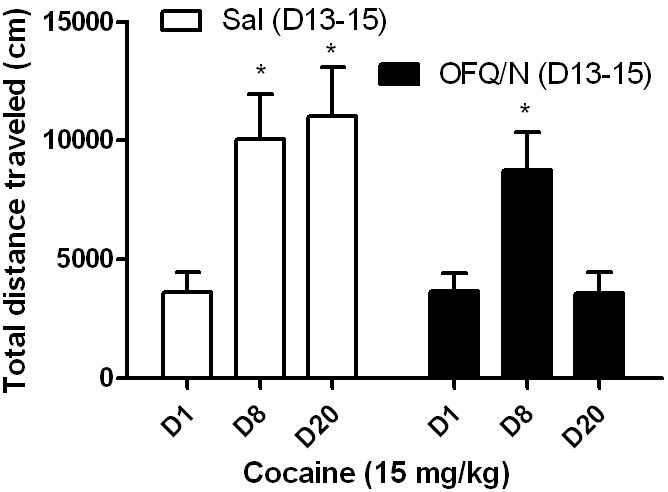
Repeated intracerebroventricular administration of OFQ/N compared to saline in cocaine-sensitized wild-type mice reversed the pre-existing sensitized response. Mice were treated with cocaine on days 1-3 and tested for sensitization on day 8. Mice were then implanted with a guide cannula and four days later treated with saline or OFQ/N (10 nmol; ICV) and motor activity was recorded for 1 h. Mice received their respective treatment on days 13-15 and tested for cocaine-induced motor stimulation on day 20. Data are mean (±S.E.M.) of 7 mice per treatment. *p<0.05 vs. cocaine response on day 1 (D1)
Repeated cocaine treatment that induced locomotor sensitization also enhanced the conditioned rewarding action of cocaine
Figure 5 depicts total distance traveled in response to cocaine (15 mg/kg) in mice treated with saline or cocaine (15 or 30 mg/kg) on days 1-3. A one-way ANOVA of the motor activity data revealed a significant effect of treatment (F2,17 = 8.32; p<0.003). The post-hoc test showed that cocaine stimulated locomotor activity to a greater extent in mice treated with cocaine (15 or 30 mg/kg) than their saline-treated controls (p<0.01), suggesting that both doses of cocaine induced psychomotor sensitization (Fig. 5A). Mice treated with cocaine on days 1-3 also displayed CPP following a single conditioning with cocaine (15 mg/kg). This conditioning paradigm however failed to induce CPP in saline-treated control mice (Fig. 5B). Analysis of the amount of time that mice spent in the CPP chambers during the postconditioning test day revealed a significant interaction between treatment and time in the CPP chambers (F2,42 = 3.27; p<0.05). The post-hoc analysis of the data showed that single cocaine conditioning failed to induce any CPP in control mice, as evidenced by no significant difference in the amount of time that mice spent in the drug-paired compared to vehicle-paired (Veh-paired) chamber (Fig. 5B). In contrast, the same conditioning protocol induced a significant CPP in mice receiving cocaine (15 or 30 mg/kg) during the development of locomotor sensitization (Fig. 5B).
Fig. 5.
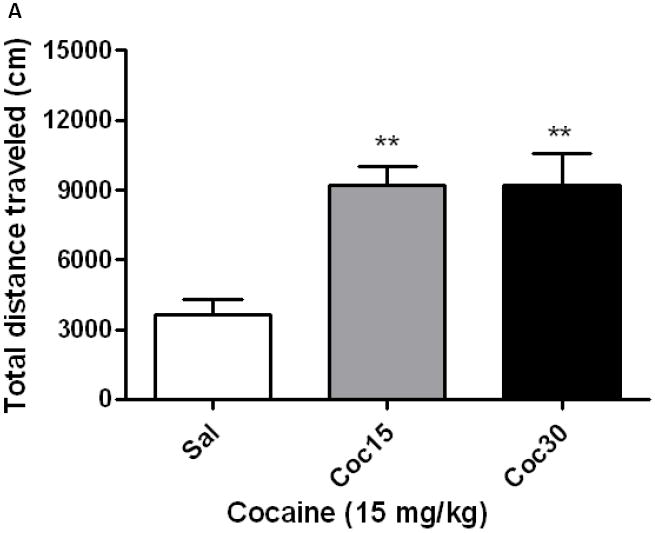
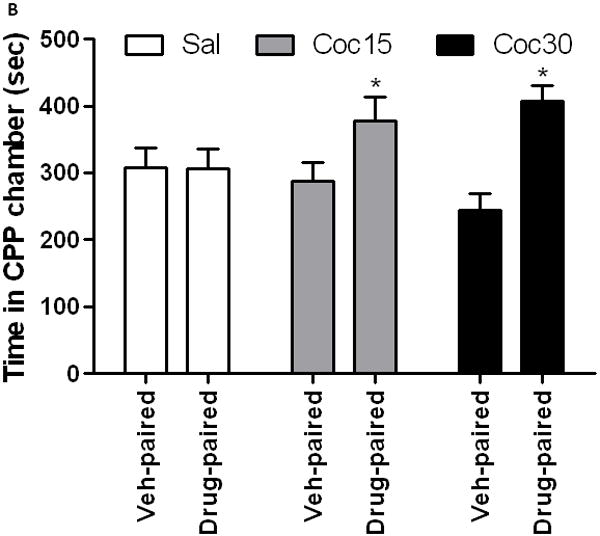
Repeated intermittent cocaine (15 or 30 mg/kg) compared to saline treatment induced sensitization to its motor stimulatory (5A) and conditioned rewarding (5B) actions. Mice were treated with saline or cocaine (15 or 30 mg/kg) on days 1-3 and tested on day 8 for locomotor sensitization, as described under legend to figure 1, or used for the CPP paradigm. For the CPP paradigm, mice were first tested for preconditioning place preference, in which each mouse was allowed to freely explore the CPP chambers for 15 min. The amount of time that mice spent in each conditioning chamber was recorded. On the following morning, mice received saline or cocaine and confined to the vehicle-paired or drug-paired chamber, respectively, for 15 min. In the afternoon, mice received the alternate treatment and confined to the opposite chamber for 15 min. Mice were then (24 h later) tested for postconditioning place preference, as described above. Data are mean (±S.E.M.) of 6-10 mice per treatment for each genotype. **p<0.01 vs. saline-treated controls group; *p<0.05 vs. vehicle-paired chamber
OFQ/N blocked the development of sensitization to the conditioned rewarding action of cocaine
Figure 6 illustrates the amount of time that mice spent in cocaine-paired vs. saline-paired chambers on the pre- and postconditioning days in mice treated with ICV saline (6A) or OFQ/N (6B) in conjunction with cocaine in the motor activity chambers on days 1-3. A two-way ANOVA of the data in mice treated with saline in conjunction with cocaine on days 1-3 revealed a significant interaction between test day and time in the conditioning chambers (F1,32 = 7.26; p<0.02). The post-hoc test showed that there was a significant difference between the amount of time that mice spent in the cocaine-paired compared to saline-paired chamber on postconditioning (p<0.05) but not preconditioning (p>0.05) day, demonstrating that a significant CPP was observed in these mice (Fig. 6A). On the other hand, mice treated with OFQ/N in conjunction with cocaine (15 mg/kg) in the motor activity chambers on days 1-3 did not display any CPP (p>0.05), as evidenced by no difference in the amount of time that the mice spent in the drug-paired as compared to vehicle-paired chamber (p>0.05), showing that OFQ/N blocked sensitization to the conditioned rewarding action of cocaine (Fig. 6B).
Fig. 6.
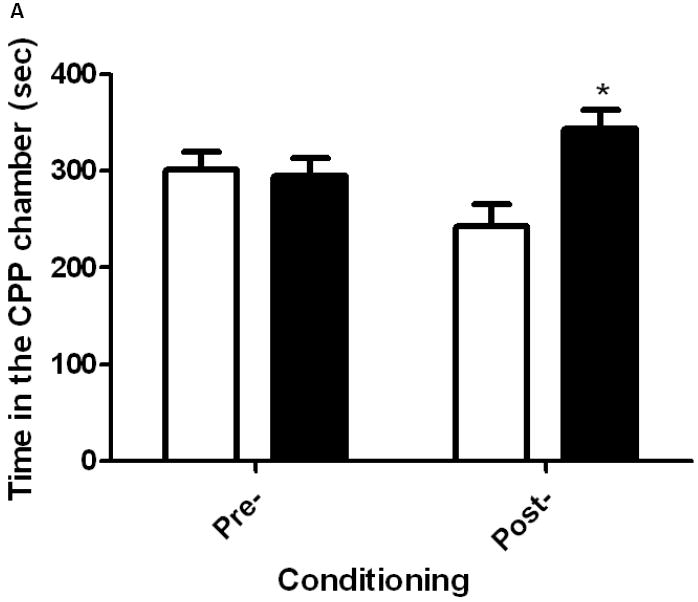
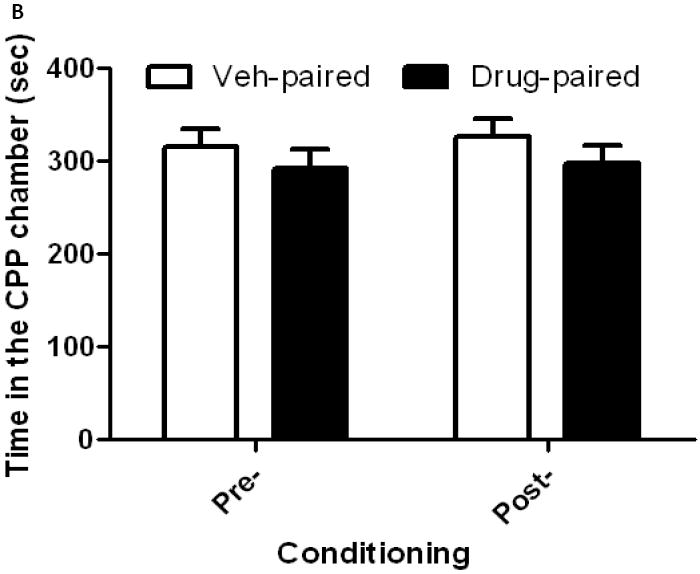
Mice rendered sensitized to the psychomotor action of cocaine also expressed sensitization to the conditioned rewarding action of the drug (6A) and this response was blocked in mice treated with OFQ/N in conjunction with cocaine (6B) during the development of locomotor sensitization. Mice were treated with saline or OFQ/N (10 nmol) immediately followed by cocaine (15 mg/kg) in the motor activity chambers on days 1-3 and tested in the CPP paradigm on day 8, as described under legend to figure 5. Data are mean (±S.E.M.) of 8-9 mice per treatment for each genotype. *p<0.05 vs. vehicle-paired chamber on the postconditioning test day
Discussion
The main findings of the present study are that OFQ/N reduced motor stimulation and blocked the development of psychomotor sensitization in wild-type but not ORL-1 receptor knockout mice, indicating that the ORL-1 receptor mediates the regulatory actions of OFQ/N. Mice rendered sensitized to the motor stimulatory action of cocaine also expressed a robust CPP response following single conditioning with a dose of cocaine that failed to induce CPP in cocaine-naïve mice, suggesting that sensitization develops to the rewarding and possibly other actions of cocaine. Interestingly, mice treated with OFQ/N in conjunction with cocaine during the development of cocaine-induced locomotor sensitization did not display CPP, indicating that OFQ/N treatment also blocked sensitization to the conditioned rewarding effect of cocaine. Furthermore, OFQ/N administration to cocaine-sensitized wild-type mice reversed the time course of the sensitized response. Additionally, OFQ/N treatment in conjunction with cocaine given to cocaine sensitized mice prevented the development of an amplified sensitized response in these mice. Together, these results suggest that OFQ/N interferes with the adaptive changes leading to the development of sensitization to the conditioned rewarding and motor stimulatory actions of cocaine.
OFQ/N has been shown to decrease basal as well as drug-elevated extracellular levels of dopamine in the nucleus accumbens (14, 16-18). Furthermore, OFQ/N inhibits neuronal plasticity in the hippocampus (20-23, 25, 41-43), raising the possibility that this system could prevent neuronal and behavioral adaptive changes induced by drugs of abuse. Interestingly, we have previously shown that intracerebroventricular OFQ/N administration blocked the development of locomotor sensitization in rats (44). In the current study, we initially determined whether OFQ/N would alter cocaine-induced hyperlocomotion and psychomotor sensitization in mice and whether the ORL1 receptor mediates the regulatory actions of OFQ/N. Our results revealed that intracerebroventricular OFQ/N administration reduced cocaine-stimulated motor activity and blocked cocaine-induced psychomotor sensitization in wild-type but not ORL1 knockout mice, indicating that the ORL1 receptor mediates the modulatory actions of OFQ/N on cocaine-induced motor stimulation and locomotor sensitization.
Repeated intermittent cocaine treatment has been shown to induce sensitization to its conditioned rewarding actions in rats (35). Thus, using CPP as an animal model of drug reward (34), we also assessed whether mice sensitized to the motor stimulatory action of cocaine would also express sensitization to the conditioned rewarding action of cocaine and whether OFQ/N would block this phenomenon. Our results corroborate these findings by illustrating that mice sensitized to the motor stimulatory action of cocaine also expressed a robust CPP response, suggesting that the phenomenon of sensitization can be generalized to other actions of cocaine. Notably, we discovered that OFQ/N treatment in conjunction with cocaine during the development of cocaine-induced locomotor sensitization prevented the acquisition of the CPP response (Fig. 6B), indicating that OFQ/N blocked the development of sensitization to the conditioned rewarding action of cocaine. Considering that mice were treated with OFQ/N in the motor activity chambers and received only cocaine during the conditioning, the current result suggests that OFQ/N exerted its inhibitory effect on neuronal plasticity leading to behavioral sensitization.
Given that OFQ/N or related drugs, if proven useful, would be given to cocaine addicts, we then examined whether OFQ/N would alter the actions of cocaine in mice with prior cocaine exposure. Accordingly, we rendered mice sensitized to cocaine and injected them with OFQ/N in the presence and absence of cocaine to assess whether OFQ/N would regulate cocaine-induced hyperlocomotion and psychomotor sensitization in sensitized mice. Our results demonstrated that OFQ/N reduced cocaine-induced hyperlocomotion in cocaine-naïve but not cocaine-sensitized mice, suggesting that repeated cocaine treatment reduced the ability of OFQ/N to attenuate the action of cocaine. These intriguing results imply that neuronal adaptive changes may occur along the OFQ/N/ORL-1 receptor system following repeated cocaine treatment. Consistent with this notion, we have previously shown that the level of OFQ/N was increased in the hippocampus of rats sensitized to cocaine (45). Thus, further research is needed to elucidate the underlying mechanisms of these neuronal and behavioral adaptive changes in the OFQ/N/ORL-1 receptor system following repeated cocaine treatment. Despite the fact that OFQ/N failed to reduce the motor stimulatory action of cocaine in sensitized mice, repeated OFQ/N administration in conjunction with cocaine in these mice blocked an amplified sensitized response that developed following administration of a second cocaine sensitizing regimen in sensitized mice. These results demonstrate that blockade of locomotor sensitization by OFQ/N is not exclusively due to the inhibitory action of OFQ/N on cocaine-induced hyperlocomotion.
One of the major problems in curbing drug addiction is the high prevalence of relapse, thereby leading to the reinstatement of addictive behaviors in former addicts (46, 47). Considering that OFQ/N blocked the development of cocaine sensitization, we tested whether OFQ/N given to cocaine-sensitized mice would reverse the development of the sensitized response. Our results revealed that mice sensitized to cocaine receiving saline on days 13-15 still displayed a robust sensitized response on day 20 (Fig. 4). On the other hand, mice treated with OFQ/N failed to express sensitization to the motor stimulatory action of cocaine (Fig. 4), indicating that OFQ/N overturned the behavioral adaptive changes elicited by repeated intermittent cocaine treatment probably via its inhibitory actions on neuronal plasticity but not simply by suppressing basal motor activity because OFQ/N paradoxically increased basal motor activity in cocaine-sensitized mice (Table 1). The observation that OFQ/N given in conjunction with cocaine blocked the amplified sensitized response but did not fully reverse sensitization (Fig. 3) appears contradictory with the notion that OFQ/N has the ability to even reverse sensitization. However, one needs to consider that OFQ/N was administered in conjunction with cocaine and the potency of OFQ/N was reduced in sensitized mice. Therefore, it may be possible to observe reversal of sensitization if a higher dose of OFQ/N is administered in conjunction with cocaine in sensitized mice. Future studies can be designed to test this possibility.
The ventral tegmental area (VTA) has been identified as the neuronal substrate for the development of locomotor sensitization (48, 49) and a neuroanatomical site of OFQ/N’s action to regulate mesolimbic dopaminergic neurons (19, 50, 51). Interestingly, we have previously shown that OFQ/N exerts its inhibitory actions on cocaine-induced psychomotor sensitization in the rat VTA (44). Moreover, we have demonstrated that OFQ/N suppressed basal motor activity via a selective action along the mesoaccumbens axis (52). Given that OFQ/N blocked the development of sensitization in cocaine-naïve as well as in cocaine-sensitized mice and overturned the time-course of the sensitized response in mice sensitize to cocaine, we hypothesize that OFQ/N exerts its actions in the VTA to prevent the neuronal plasticity elicited by repeated cocaine treatment. However, further research is needed to fully identify the neuroanatomical site of regulatory actions of OFQ/N on cocaine-induced locomotor sensitization.
In summary, the current results illustrate that OFQ/N blocked the development of cocaine-induced locomotor sensitization via the ORL1 receptor. OFQ/N also blocked sensitization to the conditioned rewarding action of cocaine and reversed the time-course of locomotor sensitization in mice sensitized to cocaine. Accordingly, the present preclinical data suggest that the OFQ/N/ORL1 receptor system represents a potential target for the development of pharmacotherapy to curb maladaptive behavioral changes elicited by cocaine and other drugs of abuse.
Acknowledgments
The authors wish to express their gratitude to Drs. Arbi Nazarian and Fadi Khasawneh for review of the article. The current study was supported in part by the NIDA grant R01DA016682 to KL and in part by the NIDA Grant R24DA017298-03A1. AH was supported by the NIDA Grant R24DA017298-03A1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kalivas PW, Lalumiere RT, Knackstedt L, Shen H. Glutamate transmission in addiction. Neuropharmacology. 2009;56(Suppl 1):169–173. doi: 10.1016/j.neuropharm.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalivas PW, Pierce RC, Cornish J, Sorg BA. A role for sensitization in craving and relapse in cocaine addiction. J Psychopharmacol. 1998;12:49–53. doi: 10.1177/026988119801200107. [DOI] [PubMed] [Google Scholar]
- 3.Kelley AE. Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron. 2004;44:161–179. doi: 10.1016/j.neuron.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 4.Thomas MJ, Kalivas PW, Shaham Y. Neuroplasticity in the mesolimbic dopamine system and cocaine addiction. Br J Pharmacol. 2008;154:327–342. doi: 10.1038/bjp.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology (Berl) 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- 6.Wolf ME. Addiction: making the connection between behavioral changes and neuronal plasticity in specific pathways. Mol Interv. 2002;2:146–157. doi: 10.1124/mi.2.3.146. [DOI] [PubMed] [Google Scholar]
- 7.Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- 8.Koob G. Drug addiction. Neurobiol Dis. 2000;7:543–545. doi: 10.1006/nbdi.2000.0351. [DOI] [PubMed] [Google Scholar]
- 9.Koob GF. Neurobiology of addiction. Toward the development of new therapies. Ann N Y Acad Sci. 2000;909:170–185. doi: 10.1111/j.1749-6632.2000.tb06682.x. [DOI] [PubMed] [Google Scholar]
- 10.Nestler EJ, Landsman D. Learning about addiction from the genome. Nature. 2001;409:834–835. doi: 10.1038/35057015. [DOI] [PubMed] [Google Scholar]
- 11.Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci. 2001;2:119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- 12.Neal CR, Jr, Mansour A, Reinscheid R, Nothacker HP, Civelli O, Akil H, et al. Opioid receptor-like (ORL1) receptor distribution in the rat central nervous system: comparison of ORL1 receptor mRNA expression with (125)l-[(14)Tyr]-orphanin FQ binding. J Comp Neurol. 1999;412:563–605. [PubMed] [Google Scholar]
- 13.Neal CR, Jr, Mansour A, Reinscheid R, Nothacker HP, Civelli O, Watson SJ., Jr Localization of orphanin FQ (nociceptin) peptide and messenger RNA in the central nervous system of the rat. J Comp Neurol. 1999;406:503–547. [PubMed] [Google Scholar]
- 14.Di Giannuario A, Pieretti S, Catalani A, Loizzo A. Orphanin FQ reduces morphine-induced dopamine release in the nucleus accumbens: a microdialysis study in rats. Neurosci Lett. 1999;272:183–186. doi: 10.1016/s0304-3940(99)00579-0. [DOI] [PubMed] [Google Scholar]
- 15.Koizumi M, Midorikawa N, Takeshima H, Murphy NP. Exogenous, but not dogenous nociceptin modulates mesolimbic dopamine release in mice. J Neurochem. 2004;89:257–263. doi: 10.1111/j.1471-4159.2003.02322.x. [DOI] [PubMed] [Google Scholar]
- 16.Lutfy K, Do T, Maidment NT. Orphanin FQ/nociceptin attenuates motor stimulation and changes in nucleus accumbens extracellular dopamine induced by cocaine in rats. Psychopharmacology (Berl) 2001;154:1–7. doi: 10.1007/s002130000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murphy NP, Ly HT, Maidment NT. Intracerebroventricular orphanin FQ/nociceptin suppresses dopamine release in the nucleus accumbens of anaesthetized rats. Neuroscience. 1996;75:1–4. doi: 10.1016/0306-4522(96)00322-3. [DOI] [PubMed] [Google Scholar]
- 18.Murphy NP, Maidment NT. Orphanin FQ/nociceptin modulation of mesolimbic dopamine transmission determined by microdialysis. J Neurochem. 1999;73:179–186. doi: 10.1046/j.1471-4159.1999.0730179.x. [DOI] [PubMed] [Google Scholar]
- 19.Zheng F, Grandy DK, Johnson SW. Actions of orphanin FQ/nociceptin on rat ventral tegmental area neurons in vitro. Br J Pharmacol. 2002;136:1065–1071. doi: 10.1038/sj.bjp.0704806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manabe T, Noda Y, Mamiya T, Katagiri H, Houtani T, Nishi M, et al. Facilitation of long-term potentiation and memory in mice lacking nociceptin receptors. Nature. 1998;394:577–581. doi: 10.1038/29073. [DOI] [PubMed] [Google Scholar]
- 21.Sandin J, Georgieva J, Schott PA, Ogren SO, Terenius L. Nociceptin/orphanin FQ microinjected into hippocampus impairs spatial learning in rats. Eur J Neurosci. 1997;9:194–197. doi: 10.1111/j.1460-9568.1997.tb01367.x. [DOI] [PubMed] [Google Scholar]
- 22.Sandin J, Ogren SO, Terenius L. Nociceptin/orphanin FQ modulates spatial learning via ORL-1 receptors in the dorsal hippocampus of the rat. Brain Res. 2004;997:222–233. doi: 10.1016/j.brainres.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 23.Wei WZ, Xie CW. Orphanin FQ suppresses NMDA receptor-dependent long-term depression and depotentiation in hippocampal dentate gyrus. Learn Mem. 1999;6:467–477. doi: 10.1101/lm.6.5.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu J, Wolda SL, Frazier AL, Florio VA, Martins TJ, Snyder PB, et al. Identification and characterisation of a human calmodulin-stimulated phosphodiesterase PDE1B1. Cell Signal. 1997;9:519–529. doi: 10.1016/s0898-6568(97)00046-6. [DOI] [PubMed] [Google Scholar]
- 25.Yu TP, Xie CW. Orphanin FQ/nociceptin inhibits synaptic transmission and long-term potentiation in rat dentate gyrus through postsynaptic mechanisms. J Neurophysiol. 1998;80:1277–1284. doi: 10.1152/jn.1998.80.3.1277. [DOI] [PubMed] [Google Scholar]
- 26.Kalivas PW, Weber B. Amphetamine injection into the ventral mesencephalon sensitizes rats to peripheral amphetamine and cocaine. J Pharmacol Exp Ther. 1988;245:1095–1102. [PubMed] [Google Scholar]
- 27.Post RM, Rose H. Increasing effects of repetitive cocaine administration in the rat. Nature. 1976;260:731–732. doi: 10.1038/260731a0. [DOI] [PubMed] [Google Scholar]
- 28.Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res. 1986;396:157–198. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- 29.Stripling JS, Ellinwood EH., Jr Potentiation of the behavioral and convulsant effects of cocaine by chronic administration in the rat. Pharmacol Biochem Behav. 1977;6:571–579. doi: 10.1016/0091-3057(77)90119-8. [DOI] [PubMed] [Google Scholar]
- 30.Stripling JS, Ellinwood EH., Jr Augmentation of the behavioral and electrophysiologic response to cocaine by chronic administration in the rat. Exp Neurol. 1977;54:546–564. doi: 10.1016/0014-4886(77)90256-4. [DOI] [PubMed] [Google Scholar]
- 31.De Vries TJ, Schoffelmeer AN, Binnekade R, Vanderschuren LJ. Dopaminergic mechanisms mediating the incentive to seek cocaine and heroin following long-term withdrawal of IV drug self-administration. Psychopharmacology (Berl) 1999;143:254–260. doi: 10.1007/s002130050944. [DOI] [PubMed] [Google Scholar]
- 32.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 33.Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction. 2000;95(Suppl 2):S91–117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- 34.Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl) 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- 35.Shippenberg TS, Heidbreder C. Sensitization to the conditioned rewarding effects of cocaine: pharmacological and temporal characteristics. J Pharmacol Exp Ther. 1995;273:808–815. [PubMed] [Google Scholar]
- 36.Shippenberg TS, Chefer VI, Thompson AC. Delta-opioid receptor antagonists prevent sensitization to the conditioned rewarding effects of morphine. Biol Psychiatry. 2009;65:169–174. doi: 10.1016/j.biopsych.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shippenberg TS, Heidbreder C, Lefevour A. Sensitization to the conditioned rewarding effects of morphine: pharmacology and temporal characteristics. Eur J Pharmacol. 1996;299:33–39. doi: 10.1016/0014-2999(95)00852-7. [DOI] [PubMed] [Google Scholar]
- 38.Nishi M, Houtani T, Noda Y, Mamiya T, Sato K, Doi T, et al. Unrestrained nociceptive response and disregulation of hearing ability in mice lacking the nociceptin/orphaninFQ receptor. EMBO J. 1997;16:1858–1864. doi: 10.1093/emboj/16.8.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marquez P, Bebawy D, Lelievre V, Coute AC, Evans CJ, Waschek JA, et al. The role of endogenous PACAP in motor stimulation and conditioned place preference induced by morphine in mice. Psychopharmacology (Berl) 2009;204:457–463. doi: 10.1007/s00213-009-1476-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marquez P, Baliram R, Dabaja I, Gajawada N, Lutfy K. The role of beta-endorphin in the acute motor stimulatory and rewarding actions of cocaine in mice. Psychopharmacology (Berl) 2008;197:443–448. doi: 10.1007/s00213-007-1053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bongsebandhu-phubhakdi S, Manabe T. The neuropeptide nociceptin is a synaptically released endogenous inhibitor of hippocampal long-term potentiation. J Neurosci. 2007;27:4850–4858. doi: 10.1523/JNEUROSCI.0876-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Higgins GA, Kew JN, Richards JG, Takeshima H, Jenck F, Adam G, et al. A combined pharmacological and genetic approach to investigate the role of orphanin FQ in learning and memory. Eur J Neurosci. 2002;15:911–922. doi: 10.1046/j.1460-9568.2002.01926.x. [DOI] [PubMed] [Google Scholar]
- 43.Mamiya T, Yamada K, Miyamoto Y, Konig N, Watanabe Y, Noda Y, et al. Neuronal mechanism of nociceptin-induced modulation of learning and memory: involvement of N-methyl-D-aspartate receptors. Mol Psychiatry. 2003;8:752–765. doi: 10.1038/sj.mp.4001313. [DOI] [PubMed] [Google Scholar]
- 44.Lutfy K, Khaliq I, Carroll Fl, Maidment NT. Orphanin FQ/nociceptin blocks cocaine-induced behavioral sensitization in rats. Psychopharmacology (Berl) 2002;164:168–176. doi: 10.1007/s00213-002-1192-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lutfy K, Lam H, Narayanan S. Alterations in the level of OFQ/N-IR in rat brain regions by cocaine. Neuropharmacology. 2008;55:198–203. doi: 10.1016/j.neuropharm.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O’Brien CP. Anticraving medications for relapse prevention: a possible new class of psychoactive medications. Am J Psychiatry. 2005;162:1423–1431. doi: 10.1176/appi.ajp.162.8.1423. [DOI] [PubMed] [Google Scholar]
- 47.O’Brien CP, Gardner EL. Critical assessment of how to study addiction and its treatment: human and non-human animal models. Pharmacol Ther. 2005;108:18–58. doi: 10.1016/j.pharmthera.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 48.Cornish JL, Kalivas PW. Repeated cocaine administration into the rat ventral tegmental area produces behavioral sensitization to a systemic cocaine challenge. Behav Brain Res. 2001;126:205–209. doi: 10.1016/s0166-4328(01)00239-x. [DOI] [PubMed] [Google Scholar]
- 49.Vezina P. Amphetamine injected into the ventral tegmental area sensitizes the nucleus accumbens dopaminergic response to systemic amphetamine: an in vivo microdialysis study in the rat. Brain Res. 1993;605:332–337. doi: 10.1016/0006-8993(93)91761-g. [DOI] [PubMed] [Google Scholar]
- 50.Maidment NT, Chen Y, Tan AM, Murphy NP, Leslie FM. Rat ventral midbrain dopamine neurons express the orphanin FQ/nociceptin receptor ORL-1. Neuroreport. 2002;13:1137–1140. doi: 10.1097/00001756-200207020-00013. [DOI] [PubMed] [Google Scholar]
- 51.Norton CS, Neal CR, Kumar S, Akil H, Watson SJ. Nociceptin/orphanin FQ and opioid receptor-like receptor mRNA expression in dopamine systems. J Comp Neurol. 2002;444:358–368. doi: 10.1002/cne.10154. [DOI] [PubMed] [Google Scholar]
- 52.Narayanan S, Lam H, Carroll Fl, Lutfy K. Orphanin FQ/nociceptin suppresses motor activity through an action along the mesoaccumbens axis in rats. J Psychiatry Neurosci. 2004;29:116–123. [PMC free article] [PubMed] [Google Scholar]


