Abstract
The biology of spermatogonial stem cells is currently an area of intensive research, and contemporary studies in primates are emerging. Quantitative regulation of sperm output by the primate testis appears to be exerted primarily on the transition from undifferentiated to differentiating spermatogonia. This review examines recent advances in our understanding of the mechanisms governing spermatogonial renewal and early differentiation in male primates, with a focus on the monkey. Emerging revisions to the classic view of dark and pale type A spermatogonia as reserve and renewing spermatogonial stem cells, respectively, are critically evaluated, and essential features of endocrine control of undifferentiated spermatogonia throughout postnatal primate development are discussed. Obstacles in gaining a more complete understanding of primate spermatogonia are also identified.
Generic schemata for mammalian spermatogenesis
Spermatogenesis begins with division and differentiation of spermatogonial stem cells that are located in “niches” on the basement membrane of the seminiferous tubule and ends with release of testicular spermatozoa into the tubular lumen [1–3]. Differentiation of spermatogonial stem cells is balanced by self-renewing divisions enabling spermatogenesis to be maintained throughout adult life. The duration of mammalian spermatogenesis is species dependent, but generally takes 5 to 11 weeks [2]. This process is dependent on gonadotropin secretion; in the absence of LH and FSH only pre-meiotic germ cells are present in the primate testis [1]. The number of amplifying mitotic divisions that occur before the germ cell lineage enters meiosis is also species specific. Two major classes of spermatogonia are recognized: type A and type B [4, 5]. Type A spermatogonia fall into two categories: undifferentiated and differentiating. All type B spermatogonia are differentiating. At some stage in the lineage, the behavior of dividing A spermatogonia becomes coordinated and, as a result, cohorts of A spermatogonia enter S phase synchronously every 8–16 days depending on the species [2]. These cohorts subsequently divide to generate differentiating spermatogonia. Because cell cycle times of the differentiating cells in the lineage are invariant, as is the duration of spermiogenesis, a number of recurring specific germ cell associations termed stages (typically VI to XIV, depending on species) are generated, comprising the so-called cycle of the seminiferous epithelium [2]. The actual number of cellular associations identified for a given species is dependent, in part, on the skill of the observer. Synchronization of the activity of A spermatogonia (and therefore the subsequent stages of the cycle) spreads as a wave along the tubule and recurs cyclically with a rigid species dependent period of 8–16 days, i.e. the period of synchronized behavior of A spermatogonia.
Spermatogonial Stem Cells in Rodents
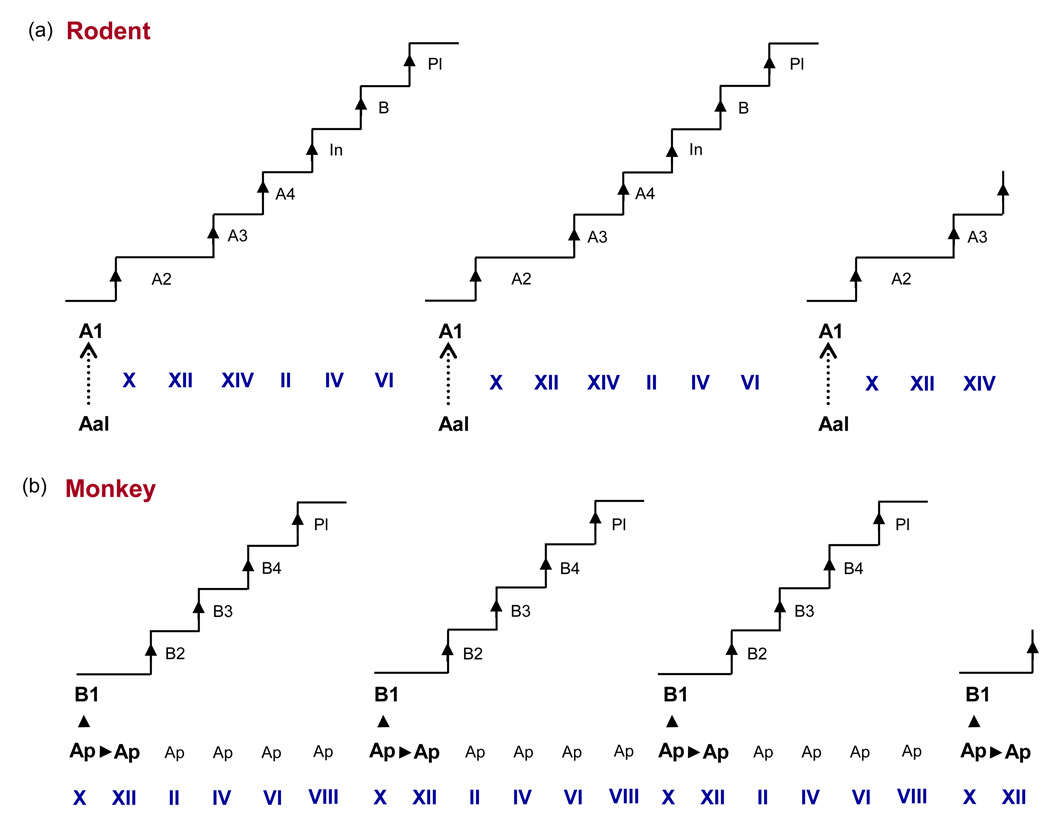
Before discussing spermatogonial stem cells in primates, it is helpful to present a brief synopsis of our current understanding of the situation in rodents, which has its foundation on the painstaking histological observations of germ cell morphology and kinetics in dissociated lengths of seminiferous tubule (whole mounts) from rats by Huckins [6] and in cross sections of testes from mice by Oakberg [7]. In addition to differentiating spermatogonia, a distinct population of undifferentiated type A spermatogonia was found. In whole mount, these existed either as single cells (As), as pairs of cells (Apr), or as chains of aligned cells (Aal). Whereas undifferentiated A spermatogonia were observed at all stages of the rat seminiferous epithelial cycle, a sharp increase in their number occurred during Stages I to V [6]. Their number was maintained during Stages VI to VIII, and then decreased precipitously in Stage IX to be replaced by the first generation of differentiating A spermatogonia (type A1 spermatogonia) [6]. It was concluded that the generation of A1 spermatogonia resulted from a synchronized non-mitotic “transformation” of Aal. Thus, in the rodent the duration of the seminiferous epithelial cycle is determined by periodic synchronized non-mitotic transformation of a cohort of undifferentiated spermatogonia (Aal) to the first generation of differentiating spermatogonia (A1) (Figure 1).
Figure 1.
Schematic of the seminiferous epithelial cycle of the (a) rodent (rat) and (b)primate (rhesus monkey). The initiator of the rodent seminiferous epithelium cycle is provided by the synchronized and repetitive transformation (hashed arrow) of Aal to A1 spermatogonia, whereas, in the monkey, this function is provided by the synchronized and repetitive mitosis (arrowhead) of Ap to produce B1. The rat epithelial cycle repeats every 13 days, whereas the cycle of the monkey has a period of 10.5 days. Roman numerals indicate stages of the seminiferous epithelial cycle. In, intermediate spermatogonia; PL, preleptotene spermatocyte.
Huckins observed [3H] thymidine labeled S-phase As in most stages of the epithelial cycle, and following division these cells became either As or Apr [8]. Apr and Aal spermatogonia exhibited kinetics similar to As with proliferation generally between Stages I to V of the cycle. Together, the findings of Huckins led to the proposal that As comprised the stem cell population and that Apr and Aal were a proliferating spermatogonial compartment which, according to contemporary jargon, would be a component of what is referred to as a “transit-amplifying population” i.e. cells with high proliferative activity, a commitment to differentiate and low capacity for self-renewal [9]. Recent studies of mice indicate that As exhibit marked molecular heterogeneity [10,11] and the significance of this in the context of their stem cell properties remains to be established.
It is interesting to note that at the time Huckins proposed her schemata in 1971, she commented on two important aspects of the model that remain significant today [12,13]. The first issue is what dictates whether division of a spermatogonial stem cell leads to stem cell renewal or to cells committed to a path of differentiation. The second is whether any potential for “stemness” is retained by undifferentiated spermatogonia in the transit-amplifying population. With regard to the latter, Yoshida et al. [14] used an elegant genetic approach in mice to irreversibly pulse label a population of neurogenin 3 (Ngn3)-expressing undifferentiated A spermatogonia (i.e. As, Apr and Aal) with LacZ, and then follow the fate of the labeled cells in one of two paradigms – first in the transgenic mice themselves, and second after their transplantation to recipient testes of wild type mice that had been treated with Busulphan (a cytostatic drug) to eliminate endogenous germ cells in the recipient testes. Permanent LacZ-labeled spermatogenic colonies were observed in the transgenic mice indicating that some Ngn3-expressing spermatogonia labeled with LacZ were stem cells. Interestingly, when spermatogonial mitosis in the transgenic mice was interrupted with Busulphan shortly after LacZ labeling, the number of permanently labeled LacZ spermatogenic colonies was increased. Similarly, following transplantation of LacZ-labeled germ cells from the transgenic mice to recipient testes of host mice, the number of LacZ-labeled spermatogenic colonies was greater than that anticipated from the number of LacZ-expressing stem cells observed in untreated transgenic animals. Thus, an element of “stemness” appears to be retained by cells in the transit-amplifying population, and this potential is realized under conditions in which stem cells are lost and stem cell niches become available [14,15]. It has also been proposed that as the lineage As→Apr→Al4→Al8→Al16 progresses, the potential for self-renewal is decreased [16].
Spermatogonial Stem Cells in Primates
The Classical Schemata
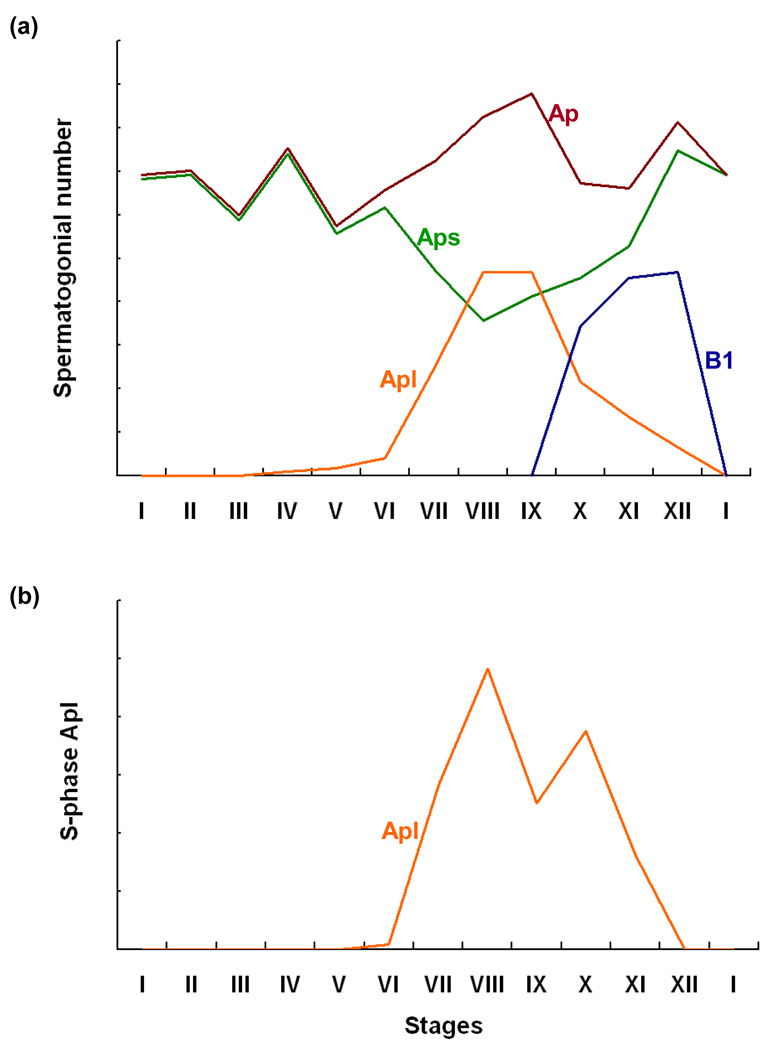
The classical schemata of spermatogenesis in primates is based largely on the work of Clermont and colleagues 40 to 50 years ago [4]. In the monkey, the seminiferous epithelial cycle is comprised of XII stages [1,4]. The first generation of differentiated spermatogonia (type B1 spermatogonia) are born in Stage X, and their numbers are maximal in Stages XI and XII [17] (Figure 2). The progenitor for B1 spermatogonia is recognized to be a type A spermatogonia, termed pale type A (Ap) because of the appearance of nuclear chromatin, after hematoxylin staining [4,17,18]. Proliferation of Ap, as indicated by S-phase labeling with either [3H]thymidine or BrdU, occurs during Stages VII to XI of the cycle and is temporally correlated with the appearance of B1 in Stage X [4,17; Figure 2]. When S-phase is approached or attained by the cohorts of Ap that divide during the later half of the epithelial cycle, the size of their nucleus increases, and for morphometric purposes we have referred to these cells as large Ap (Figure 2). The large Ap observed in Stages VIII to IX disappear in Stages X-XII as B1 are born: concomitantly, the number of Ap with a small nucleus (referred to as small Ap) are restored (Figure 2). Whereas it has generally been considered that all Ap divide each cycle [1,4], Simorangkir et al. [17] recently proposed that this is not the case; their argument is based on findings that the number of Ap that did not enter S phase in Stages VII to X (small Ap) accounted for more than 30% of the total population of this cell type, and that the ratio of the number of B1 per cross-section in Stages XI and XII to the total number of Ap per cross-section during the early part of the cycle before Ap approach S phase was <1:1. This proposal is consistent with the contemporaneous finding that cKit, a recognized marker of differentiating spermatogonia [19], was expressed by only 20% of Ap in the monkey testis [20].
Figure 2.
Correlation of changes in the number of type A pale spermatogonia (total type A pale [Ap, red line], small Ap [Aps, green line], and large Ap [Apl, orange line]) with those of type B1 (B1, blue line) spermatogonia (a) in relationship to the mitotic labeling index of total type A pale (b) during the 12 stages of the seminiferous epithelium cycle of the rhesus monkey. Note the broad peak in labeling of Ap during Stages VII to XI and the temporal association between the disappearance of Ap and appearance of B1. Redrawn from ref [17].
Regardless of the size of the cohort of dividing Ap, recent data are consistent with the early proposal of Clermont [4], and one that has universally been accepted, namely, Ap not only synchronously differentiate to B1 but also renew themselves (see Discussion in ref. [17]). Thus, according to the classical schemata, the cornerstone of the seminiferous epithelial cycle in the primate appears fundamentally different from that in the rodent. This is because in rodents, the periodicity of the cycle is dictated by repetitive transformation of Aal into A1, whereas in primates the period of the seminiferous epithelial cycle is set by repetitive division of Ap to produce B1 (Figure 1).
Dark type A spermatogonia (Ad) are another class of undifferentiated A spermatogonia in the primate testes, exhibiting dense homogeneous chromatin as revealed by hematoxylin staining [4]. Ad are present in the monkey testis in approximately equal numbers to Ap [4,17]. Whereas studies of the mitotic activity of Ad in the adult testis are somewhat inconsistent, most groups have found it to be much less than that of Ap under physiological conditions [see 17], and Ad have generally been considered to be a distinct spermatogonial type described originally by Clermont as a reserve stem cell [4,17]. BrdU labeled Ad exhibiting a classical nuclear phenotype (dense and homogenous chromatin), however, have been observed in juvenile monkeys by this laboratory [18] but not by others [21,22] and in adult monkeys rendered chronically hypogonadotropic with a GnRH receptor antagonist [23]. Here, it is worth emphasizing that categorical classification of undifferentiated spermatogonia as either Ad or Ap is difficult even for the highly experienced observer [4] and, in the view of this laboratory, not always possible [17,18]. Thus much of the controversy surrounding the proliferative capacity of undifferentiated primate spermatogonia is likely related to how individual observers classify these cell types.
Emerging Challenges to the Classical Schemata
The kinetics and quantitative behavior of Ap during Stages VII to X of the seminiferous cycle of the monkey remain an area of conjecture. The debate is fuelled in part by the prolonged duration of Ap proliferation that spans more than a third of the seminiferous epithelial cycle [17]. Based on observations of whole mounts from two hormonally manipulated monkeys that were pulse labeled with BrdU 3 h earlier, Schlatt and colleagues [24] proposed that during the 10.5 day epithelial cycle, Ap divide twice – once in Stage VII to produce daughter Ap (doubling the Ap population), and again in Stage IX, with 75% of the dividing cells producing B1 and the remaining 25% producing daughter Ap. However, the data in this manuscript, namely that a total of 413 S-phase labeled Ap in Stage IX (recorded from 11 sites of Stage IX) were associated with a total of 952 S-phase labeled B1 in Stage XII (recorded from 12 sites in Stage X1I), indicates that the second division of Ap in Stage IX resulted only in differentiation to B. Thus, the model proposed does not fully account for the data. Additionally, the notion that Ap spermatogonial proliferation (renewal) might be achieved with such different cell cycle times is problematic because it is difficult to envisage a single spermatogonial type exhibiting such a plastic cell cycle.
From studies of normal monkeys similarly labeled with BrdU, in which examination of tubular cross sections was used to correlate the number and mitotic index of Ap throughout the cycle to the appearance of B1 in Stage X (Figure 2), Simorangkir et al. [17] proposed two alternate scenarios. For the first, it was posited that entry of Ap into S-phase is a poorly synchronized event, occurring across several stages of the cycle with the initial cohorts of Ap dividing to produce daughter Ap; therefore, as the cycle progresses and the remaining cohorts of Ap enter S-phase, these cells do not renew but instead differentiate and produce B1. It was further proposed that as the number of large Ap increases during Stages VII to IX, this expanding population generates a signal either directly or indirectly that inhibits, perhaps via the Sertoli cell, further proliferation of Ap and facilitates differentiation. In this manner, differentiation of Ap leading to the birth of B1 is more tightly synchronized than Ap renewal. In the second scenario, it was posited that 50% of the dividing Ap in Stage VII renew themselves whereas the other 50% produce a distinct, but morphologically similar, generation of undifferentiated A spermatogonia that are characterized by a short cell cycle such that they divide again in Stage X to produce the first generation of B spermatogonia. Both hypotheses provide an alternative explanation for the problematic short-lived population of Ap during Stages VII and IX that is required in the Schlatt model [24].
Hermann et al. [25] recently pointed out that relative changes in the total number of Ap during the seminiferous epithelial cycle of the cynomolgus monkey (M. fasicularis), as reported earlier by Fouquet and Dadonne [22] using semithin sections stained with toluidine, appear qualitatively similar to that for Aal in rodents. Whereas changes in total number of Ap during the epithelial cycle of the rhesus monkey (Figure 2) are less remarkable than those described for cynomolgus, in the former species the distribution of Ap in or approaching S-phase throughout the cycle (i.e. large Ap) track in a qualitative sense those in total Ap described for the cynomolgus monkey. However, because the large Ap are about to enter mitosis, it is unlikely that Ap in primates are analogous to the transforming Aal in rodents.
Information regarding the clonal behavior of proliferating Ap is limited. Schlatt and his colleagues [24] have observed clones of Ap during stages of the epithelial cycle when these cells are known to divide (VII to XI), and clones of these undifferentiated spermatogonia are easily discerned during repopulation of the monkey seminiferous tubule following testicular irradiation [26,27].
The classical view of Ad as a reserve stem cell is also being subjected to reexamination. One idea that has been proposed is that Ad and Ap spermatogonia are the same cell and that the distinct difference in their nuclear phenotype corresponds to whether the cell is in the “growth fraction” or in G0, respectively [25]. The latter view, however, is not readily reconciled with the finding of BrdU-labeled Ad in juvenile monkeys and in adult monkeys rendered chronically hypogonadotropic with a GnRH receptor antagonist [18,23]. Similarly, it is also difficult to reconcile with the notion that Ap comprise both a cycling and non-cycling (G0) cohort, yet based on nuclear hematoxylin staining appear to be a homogeneous population. However the case might be, following radiation-induced catastrophic damage to the germinal epithelium in the monkey, Ad “activate” to replenish the population of Ap, either by transformation or proliferation [27].
The extent to which undifferentiated spermatogonia in the primate testis exhibit stemness is a critical question, but one that has not been empirically answered. Currently, spermatogonia are assessed for stemness by the so-called “transplantation” assay pioneered by Brinster and colleagues [28]. Whereas this bioassay has been successfully applied to quantify spermatogonial stem cell populations in rodents and some other non-primate species, the value of its application to studies of primates is less clear because although primate testicular germ cells colonize the seminiferous tubules of recipient mice, spermatogenesis has not been achieved [20,29–33]. Without the maintenance of spermatogenic colonies in the recipient testis, conclusions concerning the stem cell properties of the transplanted cells are equivocal.
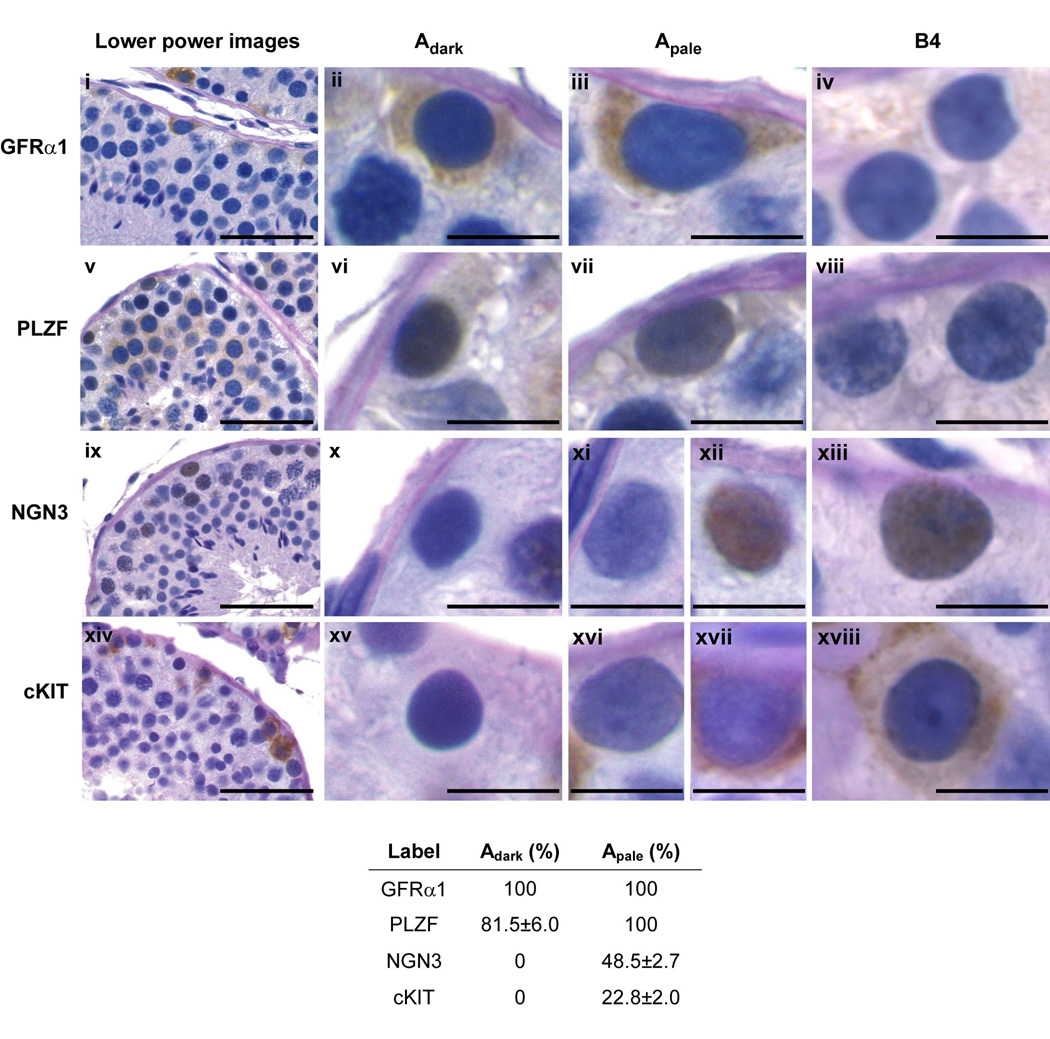
Therefore, indirect approaches to investigate the biology of these germ cells in primates are beginning to be taken. One of these, based on the notion that the molecular biology underlying spermatogonial stem cell behavior is conserved across mammalian species, was recently applied by Hermann et al. [20], who examined in the monkey testis the expression of proteins considered to be markers of spermatogonial stem cells in rodents, namely Gfrα1, Plzf and Ngn3. Interestingly, Gfrα1 was expressed by all Ad and Ap, and Plzf was expressed in all Ap and approximately 80% of Ad (Figure 3). That Ngn3 was not detected in Ad and present in only 50% of Ap was initially puzzling. As described earlier, elegant studies of transgenic mice by Yoshida and colleagues [14,16] had demonstrated unequivocally that Ngn3-expressing cells, in which immortal expression of LacZ was genetically engineered, formed LacZ-positive spermatogonial colonies that were maintained for several months in the testis of transgenic mice, thus providing unequivocal evidence of Ngn3 expression at least by some mouse spermatogonial stem cells. The number of LacZ-labeled spermatogenic colonies, however, was very low, but the significance of this metric was unclear because the efficiency of LacZ labeling was not provided. More recently, detailed anatomical studies of mice using whole mounts revealed that the majority of As are Ngn3− [10], indicating that the low contribution of LacZ-labeled Ngn3 cells to spermatogenic colonies in the earlier study was probably attributable to the majority of spermatogonial stem cells being Ngn3−. Thus, the finding that only about 20% of undifferentiated A spermatogonia in the monkey were labeled with Ngn3 is consistent with the situation in the mouse. Whereas our understanding of the biology of primate spermatogonial stem cells will remain relatively imprecise and vague until the stemness of Ad and Ap can be functionally quantified, the studies of Herman et al. [20] indicate that the phenotype of most Ad and Ap, as characterized to date (i.e. Plzf+, Gfrα+, cKit− and Ngn−), is similar to that of As. Moreover, only 25% of all undifferentiated A spermatogonia in the monkey were characterized by a phenotype (Gfrα1+, Plzf+, Ngn3+ and cKit+/−) that marks the transit-amplifying population of rodents [20] leading Hermann et al. to propose that spermatogonial stem cells in the primate testis potentially comprise 75% of the population of undifferentiated A spermatogonia (400 to 600 million), i.e. approximately 375 million in a testis that typically weighs 25 gm [17,34] or 15 million per gm testis. This compares to an As population in mice of approximately 0.35 million per gm testis [35]. Herman et al. [20] further suggested that, because sperm production rate per unit weight of testis is similar in the rodent and monkey [2], different cellular strategies have evolved to achieve comparable sperm production rates: specifically, in primates the ratio of stem cells to transit-amplifying cells is relatively large, whereas in rodents this ratio is reversed. Dogma holds that spermatogonial stem cells are rare [35], and therefore it is unlikely that the foregoing view of undifferentiated spermatogonia in the primate testis will be wholeheartedly embraced without considerably more empirical documentation.
Figure 3.
Molecular phenotypes of monkey spermatogonia. (a) Correlation of nuclear staining patterns (PAS-hematoxylin) of Ad (Adark), Ap (Apale) and B4 spermatogonia in testis of adult rhesus monkeys with immunostaining for molecular markers (GFRα1 [i-iv], PLZF [v-viii], NGN3 [ix-xiii] and cKIT [xiv-xviii]) of rodent spermatogonia. The left hand column of photomicrographs (lower power images) shows arcs of seminiferous tubule (scale bar, 50 µm). High power images of selected spermatogonia are shown to the right (scale bar, 10 µm). (b) The table presents the percentage (mean±SEM) of Adark and Apale labeled with each of the 4 markers. Reprinted from ref 20.
Regardless of the validity of the concept regarding the relative fraction of spermatogonial stem cells in the population of undifferentiated spermatogonia in the monkey, it seems reasonable to conclude that molecular markers of these cells show considerable conservation across mammalian species. GFRα1 stained cells on the basement membrane of the seminiferous tubule of adult rhesus monkeys have also been reported by others, and the majority of the GFRα1 cells (80%) co-stained for CD49f (α6-integrin), another marker of undifferentiated spermatogonia in rodent [29]. Additionally, a small proportion of spermatogonia also stained for SSEA-4 [29,36], a marker of pluripotent stem cells. Recent studies of human testis also describe expression of GFRα1, PLZF and CD49f by germ cells on the basement membrane [33,37,38], and microarray analysis of spermatogonia isolated from testicular biopsies prior to puberty indicate conservation of expression of genes associated with self-renewal [33].
The discovery of additional molecular markers for undifferentiated spermatogonia in primates is likely to proceed in the wake of research on mice and rat. In this regard, it should be noted that very recent studies of mice indicate that, like Ngn3, Nanos 2-expressing cells also permanently labeled with LacZ are able to form long lived spermatogenic colonies [39] but that in contrast to Ngn3, Nanos 2 marks the majority of As [11]. Thus, studies of Nanos expression in the primate testis are likely to be very informative.
Endocrine Regulation of Spermatogonial Proliferation and Differentiation
The endocrine milieu to which the primate testis is exposed changes dramatically throughout postnatal development [40]. During infancy, gonadotropin secretion is robust and testicular testosterone release is elevated in response to LH stimulation. Juvenile development (and childhood in man), on the other hand, is a relatively hypogonadotropic state and testicular testosterone secretion is minimal. At puberty, which in the monkey occurs at approximately 4 years of age [40], gonadotropin secretion is reactivated and testicular testosterone release is again elevated. During the protracted phase of prepubertal development, changes within the seminiferous tubule of the monkey testis appear to be unremarkable and limited primarily to proliferation of Sertoli cells and undifferentiated spermatogonia [40]. The situation in man, however, might be different because the appearance of significant B spermatogonia has been observed in testes of boys as early as 4–7 years of age [41]. In the case of the monkey testis, the number of Ad and Ap increase from around 40 million at birth to approximately 200–300 million in the adult [18,40]. Whereas S-phase labeling studies indicate that Ap proliferation is robust during prepubertal development [18], the biology underlying the expansion of Ad prior to puberty is less clear. Studies from our laboratory indicate that Ad undergo mitosis with a similar frequency to that of Ap [18] leading to the conclusion that the prepubertal expansion of both Ad and Ap is achieved by proliferation. Because juvenile development in primates represents a hypogonadotropic state, these labeling results support the notion that proliferation of A spermatogonia is relatively gonadotropin-independent [18,40]. Studies of the juvenile monkey by other laboratories, however, did not find Ad labeling [21,22]. As discussed above, the reasons for this discrepancy is unclear but might be related to difficulties in classifying spermatogonial types, particular when the nucleus is labeled. Although proliferation of A spermatogonia is markedly increased at puberty in association with increased gonadotropin secretion, it seems reasonable to propose that division of these cells at this stage of development follows in the wake of increased numbers of spermatogonial niches produced as a result of gonadotropin-dependent Sertoli cell expansion [40] rather than as a direct action of elevated gonadotropin levels on germ cells.
In contrast to proliferation of A spermatogonia, differentiation of Ap to B1 and survival of the progeny, which represents the first step in the initiation of spermatogenesis at puberty, is dependent on gonadotropin secretion. Thus, during infancy when androgen receptor (AR) and FSH receptor (FSHR) signaling in the Sertoli cell is not fully developed [40,42,43], only the occasional differentiating spermatogonia is observed. That AR and FSHR signaling pathways are extant in Sertoli cells of the juvenile testis and that they are able to regulate expression of appropriate growth factor genes that govern the balance between proliferation and differentiation of spermatogonia is indicated by the finding that leptotene-zygotene spermatocytes were found in the juvenile monkey testis after combined treatment with FSH and LH for as little as 11 days [40]. Although the Sertoli cell acquires the capacity to respond to androgen and FSH during the infantile–juvenile transition, initiation of spermatogonial differentiation continues to be limited prior to puberty because of the hypogonadotropic state that is characteristic of juvenile development [40].
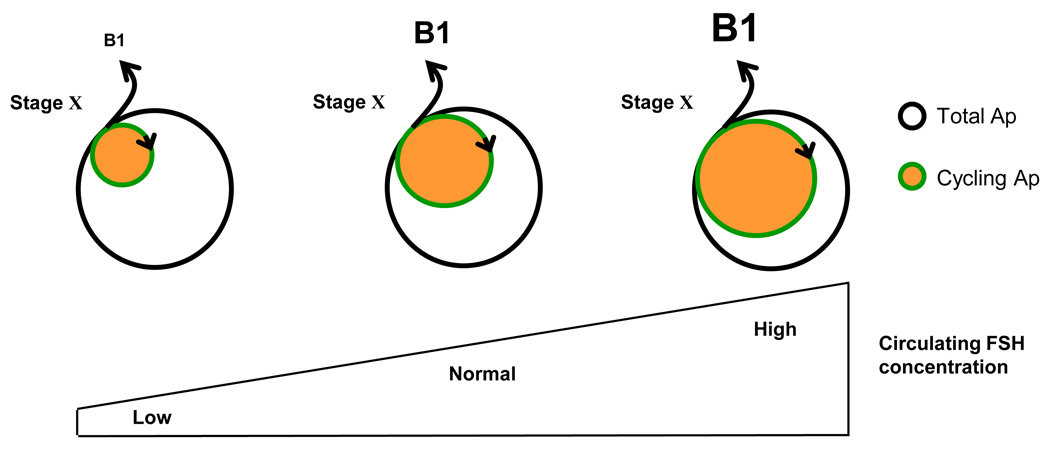
Spermatogonial proliferation and differentiation in the testis of the adult monkey is maintained by actions of testosterone (driven by LH) and FSH that are exerted indirectly, primarily on the Sertoli cell [1]. Testosterone also initiates and maintains spermatogenesis in the primate testis in the absence of FSH secretion or FSHR signaling [1]. Thus, it might be concluded that AR signaling alone is sufficient to activate the cellular mechanisms that govern the balance between spermatogonial proliferation and differentiation and permit meiosis to occur and spermiogenesis to unfold. Interestingly, however, under physiological conditions the level of intratesticular testosterone does not appear to control the quantitative aspects of spermatogonial proliferation. Using the testicular “clamp” (Box 1), a selective increase in FSH drive to the adult testis dramatically stimulated spermatogenesis as reflected by increased testicular volume and enhanced numbers of differentiating spermatogonia and meiotic cells [44]. On the other hand, a selective increase in LH that doubled testosterone secretion did not affect the seminiferous tubules [44]. The earliest step of spermatogenesis stimulated by elevated FSH signaling was Ap differentiation, as manifest by a significant increase in the number of B1 in Stages X-XII in the FSH stimulated group – an effect most likely attributable to the recruitment of a greater fraction of the Ap population into the mitotic pool (Figure 4), although an action of FSH on spermatogonial survival cannot be ruled out. Because it is well-established that FSHR is expressed only by the Sertoli cell [45], this somatic cell must be the site of FSH action that leads to Ap expansion resulting in an increased number of differentiating spermatogonia entering the cycle. Although a selective increase in FSH results in a small elevation in circulating testosterone concentrations, it is highly unlikely that enhanced AR signaling mediated FSH-induced proliferation of Ap because the more marked increase in testicular testosterone secretion induced by selectively elevating LH was without effect on this parameter.
Box 1. The testicular clamp
The testicular clamp is an experimental model that has been developed in the monkey to enable the testis to be exposed to a selective monotropic and sustained physiological increase in either FSH or LH [44]. In this model, endogenous gonadotropin secretion in the adult is abolished by daily injections of a GnRH receptor antagonist (Acyline) and immediately replaced with recombinant human FSH and LH, administered iv and in a pulsatile manner to mimic the physiological mode of release. During the control period, the doses per pulse of the recombinant gonadotropins are adjusted to maintain testicular volume and circulating testosterone (an endocrine marker of LH action) and inhibin (an endocrine marker of FSH action) concentrations at pre-clamp levels. Having established a physiologic pattern of gonadotropin replacement, the impact of a selective monotropic increase (or decrease) in either FSH or LH, whereas maintaining invariant replacement with the other gonadotropin, may be studied.
Figure 4.
A model for the action of FSH to amplify spermatogenic output in the monkey. Circulating FSH concentrations govern the fraction of A pale spermatogonia in the cycling pool. As levels of this gonadotropin increase, the pool of cycling Ap is expanded resulting in an increased number of B1 spermatogonia that are produced in stage X of the seminiferous epithelial cycle as a result of the differentiating division of Ap at this time. The total number of A pale spermatogonia is not FSH dependent. Under normal conditions, the monkey testis is not operating at its spermatogenic ceiling and sperm output is regulated by the blood level of FSH [1].
The important question that remains in regard to the pubertal activation of Ap differentiation at the time of puberty and the regulation of this critical step of spermatogenesis is the identity of the downstream products of signaling pathways triggered by AR and FSH receptor activation, and how these hormonal signals are transduced by the Sertoli cell and communicated to undifferentiated spermatogonia.
Concluding Remarks
The degree of stemness exhibited by different types of undifferentiated spermatogonia, the significance of transit-amplification and the identity of intratesticular growth factors that govern spermatogonial renewal, proliferation and differentiation throughout postnatal primate development remain to be established. This state of affairs is due, in part, to the absence of systematic studies of spermatogonial behavior in whole mounted primate seminiferous tubules analogous to those conducted in rats and mice. Whereas contemporary studies indicate that the molecular signatures of undifferentiated and differentiating spermatogonia are probably conserved across species, the level of anatomic expertise required to relate these findings to the classical descriptions of primate spermatogonial types is significant. This hurdle, coupled with the relative limited availability of primate tissue and the fact that cutting edge genetic approaches now being employed to study the biology of rodent spermatogonia are simply not available to those investigating cognate problems in primates, suggests that progress will proceed at a modest pace and will follow in the wake of knowledge gained from studies of mice.
Acknowledgements
Work from the author’s laboratory was supported by NIH grants (HD012354 and HD008160), which are gratefully acknowledged. Many of the ideas developed in this review evolved over several years during discussions with many colleagues in Pittsburgh, most notably Drs. Gary Marshall, Kyle Orwig, Brian Hermann, Mahmoud Huleheil, David Simorangkir and Stefan Schlatt. The author is also most grateful to Drs. Dirk de Rooij and Anthony Zeleznik for their helpful comments/suggestions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Plant TM, Marshall GR. The functional significance of FSH in spermatogenesis and the control of its secretion in male primates. Endo. Rev. 2001;22:764–786. doi: 10.1210/edrv.22.6.0446. [DOI] [PubMed] [Google Scholar]
- 2.Sharpe RM. Regulation of Spermatogenesis. In: Knobil E, Neill JD, editors. The Physiology of Reproduction, Second Edition. Raven Press, Ltd.; 1994. pp. 1363–1434. [Google Scholar]
- 3.de Rooij D. The spermatogonial stem cell niche. Microscopy Res. Tech. 2009;72:580–585. doi: 10.1002/jemt.20699. [DOI] [PubMed] [Google Scholar]
- 4.Clermont Y. Kinetics of spermatogenesis in mammals: Seminiferous epithelium cycle and spermatogonial renewal. Physiological Reviews. 1972;52:198–236. doi: 10.1152/physrev.1972.52.1.198. [DOI] [PubMed] [Google Scholar]
- 5.de Rooij DG, Russell LD. All you wanted to know about spermatogonia but were afraid to ask. J. Androl. 2000;21:776–798. [PubMed] [Google Scholar]
- 6.Huckins C. The spermatogonial stem cell population in adult rats. I. Their morphology, proliferation and maturation. Anat. Rec. 1971;169:533–557. doi: 10.1002/ar.1091690306. [DOI] [PubMed] [Google Scholar]
- 7.Oakberg EF. Spermatogonial stem-cell renewal in the mouse. Anat. Rec. 1971;169:515–532. doi: 10.1002/ar.1091690305. [DOI] [PubMed] [Google Scholar]
- 8.Huckins C. The spermatogonial stem cell population in adult rats. II. A radiographic analysis of their cell cycle properties. Cell Tissue Kinet. 1971;4:313–334. doi: 10.1111/j.1365-2184.1971.tb01543.x. [DOI] [PubMed] [Google Scholar]
- 9.Watt FM. Stem cell fate and patterning in mammalian epidermis. Curr. Opinion Gene. & Dev. 2001;11:410–417. doi: 10.1016/s0959-437x(00)00211-2. [DOI] [PubMed] [Google Scholar]
- 10.Zheng K, et al. The pluripotency factor LIN28 marks undifferentiated spermatogonia in mouse. BMC Devel. Biology. 2009;9:38–48. doi: 10.1186/1471-213X-9-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzuki H, et al. The heterogeneity of spermatogonia is revealed by their topology and expression of marker proteins including the germ cell-specific proteins Nanos2 and Nanos3. Dev. Biol. 2009;336:222–231. doi: 10.1016/j.ydbio.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Wu Z, et al. Capacity for stochastic self-renewal and differentiation in mammalian spermatogonial stem cells. J. Cell Biol. 2009;187:513–524. doi: 10.1083/jcb.200907047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoshida S. Casting back to stem cells. Nature Cell Biol. 2009;11:118–120. doi: 10.1038/ncb0209-118. [DOI] [PubMed] [Google Scholar]
- 14.Nakagawa T, et al. Functional identification of the actual and potential stem cell compartments in mouse spermatogenesis. Dev. Cell. 2007;12:195–206. doi: 10.1016/j.devcel.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Barroca V, et al. Mouse differentiating spermatogonia can generate germinal stem cells in vivo. Nature Cell Biol. 2009;11:190–196. doi: 10.1038/ncb1826. [DOI] [PubMed] [Google Scholar]
- 16.Yoshida S, et al. Actual and potential stem cell compartments in mouse spermatogenesis. Ann. N.Y. Acad. Sci. 2007;1120:47–58. doi: 10.1196/annals.1411.003. [DOI] [PubMed] [Google Scholar]
- 17.Simorangkir DR, et al. A re-examination of proliferation and differentiation of type A spermatogonia in the adult rhesus monkey (Macaca mulatta) Hum. Reprod. 2009;24:1596–1604. doi: 10.1093/humrep/dep051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simorangkir DR, et al. Prepubertal expansion of dark and pale type A spermatogonia in the rhesus monkey (Macaca mulatta) results from proliferation during infantile and juvenile development in a relatively gonadotropin independent manner. Biol. Reprod. 2005;73:1109–1115. doi: 10.1095/biolreprod.105.044404. [DOI] [PubMed] [Google Scholar]
- 19.Shinohara T, et al. Spermatogonial stem cell enrichment by multiparameter selection of mouse testis cells. Proc. Natl. Acad. Sci. 2000;97:8346–8351. doi: 10.1073/pnas.97.15.8346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hermann BP, et al. Molecular dissection of the male germ cell lineage identifies putative spermatogonial stem cells in rhesus macaques. Hum. Reprod. 2009;24:1704–1716. doi: 10.1093/humrep/dep073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kluin PhM, et al. Testicular development in Macaca irus after birth. Int. J. Androl. 1983;6:25–43. doi: 10.1111/j.1365-2605.1983.tb00321.x. [DOI] [PubMed] [Google Scholar]
- 22.Fouquet JP, Dadoune JP. Renewal of spermatogonia in the monkey (Macaca fascicularis) Biol. Reprod. 1986;35:199–207. doi: 10.1095/biolreprod35.1.199. [DOI] [PubMed] [Google Scholar]
- 23.Marshall GR, et al. Gonadotropin independent proliferation of the pale type A spermatogonia in the adult rhesus monkey (Macaca mulatta) Biol. Reprod. 2005;73:222–229. doi: 10.1095/biolreprod.104.038968. [DOI] [PubMed] [Google Scholar]
- 24.Ehmcke J, et al. Clonal organization of proliferating spermatogonial stem cells in adult males of two species of non-human primates, Macaca mulatta and Callithrix. Biol. Reprod. 2005;72:293–300. doi: 10.1095/biolreprod.104.033092. [DOI] [PubMed] [Google Scholar]
- 25.Hermann BP, et al. Spermatogonial stem cells in higher primates: are there differences to those in rodents? Reproduction. 2010;139:479–493. doi: 10.1530/REP-09-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Alphen MMA, et al. Repopulation of the seminiferous epithelium of the rhesus monkey after X irradiation. Rad. Res. 1988;113:487–500. [PubMed] [Google Scholar]
- 27.van Alphen MMA, et al. Depletion of the spermatogonia from the seminiferous epithelium of the rhesus monkey after X irradiation. Rad. Res. 1988;113:473–486. [PubMed] [Google Scholar]
- 28.Brinster RL. Germline stem cell transplantation and transgenesis. Science. 2002;296:2174–2176. doi: 10.1126/science.1071607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maki CB, et al. Phenotypic and molecular characterization of spermatogonial stem cells in adult primate testes. Hum. Reprod. 2009;24:1480–1491. doi: 10.1093/humrep/dep033. [DOI] [PubMed] [Google Scholar]
- 30.Nagano M, et al. Primate spermatogonial stem cells colonize mouse testes. Biol. Reprod. 2001;64:2409–2416. doi: 10.1095/biolreprod64.5.1409. [DOI] [PubMed] [Google Scholar]
- 31.Nagano M, et al. Long-term survival of human spermatogonial stem cells in mouse testes. Fertil. Steril. 2002;78:1225–1233. doi: 10.1016/s0015-0282(02)04345-5. [DOI] [PubMed] [Google Scholar]
- 32.Hermann BP, et al. Characterization, cryopreservation, and ablation of spermatogonial stem cells in adult rhesus macaques. Stem Cells. 2007;25:2330–2338. doi: 10.1634/stemcells.2007-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu X, et al. Prepubertal human spermatogonia and mouse genocytes share conserved gene expression of germline stem cell regulatory molecules. Proc. Natl. Acad. Sci. 2009;106:21672–21677. doi: 10.1073/pnas.0912432106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marshall GR, Plant TM. Puberty occurring either spontaneously or induced precociously in rhesus monkey (Macaca mulatta) is associated with a marked proliferation of Sertoli cells. Biol. Reprod. 1996;54:1192–1199. doi: 10.1095/biolreprod54.6.1192. [DOI] [PubMed] [Google Scholar]
- 35.Tegelenbosch RA, de Rooij DG. A quantitative study of spermatogonial multiplication and stem cell renewal in the C3H/101F hybrid mouse. Mut. Res. 1993;290:193–200. doi: 10.1016/0027-5107(93)90159-d. [DOI] [PubMed] [Google Scholar]
- 36.Müller T, et al. Glycan stem-cell markers are specifically expressed by spermatogonia in the adult non-human primate testis. Hum. Reprod. 23:2292–2298. doi: 10.1093/humrep/den253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.He Z, et al. Isolation, characterization, and culture of human spermatogonia. Biol. Reprod. 2009;82:363–372. doi: 10.1095/biolreprod.109.078550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grisanti L, et al. Identification of spermatogonial stem cell subsets by morphological analysis and prospective isolation. Stem Cells. 2009;27:3043–3052. doi: 10.1002/stem.206. [DOI] [PubMed] [Google Scholar]
- 39.Sada A, et al. The RNA-binding protein NANOS2 is required to maintain murine spermatogonial stem cells. Science. 2009;325:1394–1398. doi: 10.1126/science.1172645. [DOI] [PubMed] [Google Scholar]
- 40.Plant TM, et al. Postnatal and pubertal development of the rhesus monkey (Macaca mulatta) testis. Ann. N.Y. Acad. Sci. 2005;1061:149–162. doi: 10.1196/annals.1336.016. [DOI] [PubMed] [Google Scholar]
- 41.Paniagua R, Nistal M. Morphological and histometric study of human spermatogonia from birth to the onset of puberty. J. Anat. 1984;139:535–552. [PMC free article] [PubMed] [Google Scholar]
- 42.Berensztein EB, et al. Expression of aromatase, estrogen receptor alpha and beta, androgen receptor, and cytochrome P-450scc in the human early prepubertal testis. Pediatr. Res. 2006;60:740–744. doi: 10.1203/01.pdr.0000246072.04663.bb. [DOI] [PubMed] [Google Scholar]
- 43.Rathi R, et al. Maturation of testicular tissue from infant monkeys after xenografting into mice. Endocrinology. 2008;149:5288–5296. doi: 10.1210/en.2008-0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simorangkir DR, et al. A selective monotropic elevation of FSH, but not that of LH, amplifies the proliferation and differentiation of spermatogonia in the adult rhesus monkey (Macaca mulatta) Hum. Reprod. 2009;24:1584–1595. doi: 10.1093/humrep/dep052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simoni M, et al. The follicle-stimulating hormone receptor: biochemistry, molecular biology, physiology, and pathophysiology. Endocr. Rev. 1997;18:739–733. doi: 10.1210/edrv.18.6.0320. [DOI] [PubMed] [Google Scholar]