Abstract
BACKGROUND
Many opioid-dependent patients do not receive care for addiction issues when hospitalized for other medical problems. Based on 3 years of clinical practice, we report the Transitional Opioid Program (TOP) experience using hospitalization as a “reachable moment” to identify and link opioid-dependent persons to addiction treatment from medical care.
METHODS
A program nurse identified, assessed, and enrolled hospitalized, out-of-treatment opioid-dependent drug users based on their receipt of methadone during hospitalization. At discharge, patients transitioned to an outpatient interim opioid agonist program providing 30-day stabilization followed by 60-day taper. The nurse provided case management emphasizing HIV risk reduction, health education, counseling, and medical follow-up. Treatment outcomes included opioid agonist stabilization then taper or transfer to long-term opioid agonist treatment.
RESULTS
From January 2002 to January 2005, 362 unique hospitalized, opioid-dependent drug users were screened; 56% (n = 203) met eligibility criteria and enrolled into the program. Subsequently, 82% (167/203) presented to the program clinic post-hospital discharge; for 59% (119/203) treatment was provided, for 26% (52/203) treatment was not provided, and for 16% (32/203) treatment was not possible (pursuit of TOP objectives precluded by medical problems, psychiatric issues, or incarceration). Program patients adhered to a spectrum of medical recommendations (e.g., obtaining prescription medications, medical follow-up).
CONCLUSIONS
The Transitional Opioid Program (TOP) identified at-risk hospitalized, out-of-treatment opioid-dependent drug users and, by offering a range of treatment intensity options, engaged a majority into addiction treatment. Hospitalization can be a “reachable moment” to engage and link drug users into addiction treatment.
KEY WORDS: harm reduction, opioid dependence, methadone, addiction treatment
INTRODUCTION
Opioid-dependent individuals are frequent users of hospital services for acute medical conditions.1–3 When hospitalized, they are often not engaged in addiction treatment.4 They avoid hospitalization for fear of withdrawal,5 and if hospitalized, resume drug use at discharge.6 However, hospitalization provides an opportunity to improve and coordinate addiction treatment. A hospital-based program could identify opioid-dependent patients, engage them in addiction treatment, and mitigate high-risk behaviors. Hospitalization is a “reachable moment”—to engage out-of-treatment individuals whose acute illness may render them willing to consider addiction treatment.7,8
Methadone is recommended for acute withdrawal in opioid-dependent patients to reduce early hospital departures and facilitate acute treatment.4,9,10 However, brief methadone exposure does not improve low abstinence rates (e.g., 80% of opioid-dependant individuals relapse within 1 year of detoxification).11,12 Moreover, opioid agonists administered during hospitalization do not result in adequate ongoing abstinence after discharge.8
Research programs targeting hospitalized opioid-dependent patients have combined engagement with intensive, structured substance use treatment.13–15 However, patients ambivalent about formal addiction treatment may be disinclined to enroll or remain in these types of programs. We created a clinical model to improve comprehensive health and lifestyle outcomes (e.g., linkage and adherence to treatment, reduction in unhealthy substance use behaviors) and promote low-threshold access that might engage reluctant patients. In 2001, the Transitional Opioid Program (TOP) was created for out-of-treatment, opioid-dependent patients hospitalized in an urban teaching hospital in coordination with but independent of an affiliated Opioid Treatment Program (OTP). TOP had three aims: (1) improve access to opioid addiction treatment; (2) provide risk reduction strategies to prevent HIV, hepatitis, and sexually transmitted diseases; (3) increase hospital discharge plan adherence. TOP identified hospitalized out-of-treatment, opioid-dependent patients and linked them to outpatient, interim opioid agonist addiction treatment, medical care, risk-reduction services, and individualized case management. Components of this clinical program and the initial 3-year experience are described.
METHODS
The program model was based on a conceptual framework that included the following important components: (1) interim opioid replacement therapy; (2) individualized case management; (3) group public health education; (4) principles of motivational interviewing and harm reduction.
Interim Opioid Agonist Treatment
Opioid agonists facilitated patient engagement in the program by mitigating withdrawal, craving, and illicit drug procurement. The program worked to reduce risk of harm from injection drug use and permit participants to reflect on their circumstances, consider behavior change, and formulate goals.
Individualized Case Management
Individualized case management marshaled resources for vulnerable participants temporarily stabilized on opioid-agonist therapy. After hospitalization, patients face additional barriers to recovery (e.g., lack of long-term treatment availability, lack of insurance, homelessness). Case management provided a safe environment for both formal and informal addiction counseling. Unstructured interactions provided opportunities to address ambivalence to counseling.
Group Health Education
Group health education was an essential program component as patients face many risk-laden decisions.16,17 Drug users transmit information via “word-of-mouth” and may use knowledge gained through health education to reduce risky behaviors.18 Group education maximized staff efficiency and reinforced therapeutic messages.
Principles of Motivational Interviewing and Harm Reduction
The program used principles recommended by the Institute of Medicine including engaging patients in all states of readiness to change, setting intermediate goals, working collaboratively with patients towards them, and responding to individual needs.19 This approach was used to educate participants about prevention of sexually transmitted infections, increase linkage to mental health and primary medical care, and enhance adherence to medical treatment. Intermediate behavioral goals and harm reduction methods were employed to enhance readiness to change and decrease medical complications.20,21
The program nurse made frequent “check-in” visits during the hospitalization because experience suggested that repeated low-pressure engagement combined with motivational interviewing methods increased enrollment and enhanced outcomes for ambivalent participants. Incremental progress by achieving intermediate outcomes was considered productive and consistent with the transtheoretical model of behavior change.22 Any reduction of harmful behaviors or increased involvement in treatment or assistance services was positively emphasized.23
Transitional Opioid Program Phases
Phase 1. Inpatient—Identification, Screening, Assessment, and Enrollment
Phase 1 (during hospitalization) included identification, screening, comprehensive medical and psychosocial assessment, social service evaluation, daily visitation, substance abuse treatment education, and methadone induction and stabilization. The program nurse employed clinical judgment while determining program eligibility in interested individuals.
Phases 2. Outpatient Days 1 Through 30—Stabilization and Maintenance
Phase 2 (at the OTP during days 1 to 30 after hospital discharge) included methadone dose reassessment and titration, individualized case management, and comprehensive care planning. The program nurse transferred medication and medical information (last methadone dose, admission documentation, laboratory results, PPD status, and urine toxicology) to the OTP. Patients received daily observed methadone at the OTP up to a maximum daily dose (80 mg) to relieve withdrawal symptoms and opioid craving. The dose of 80 mg was the highest dose from which a 60-day detoxification was estimated to be tolerable. The first day of outpatient methadone administration, the program nurse oriented patients to the OTP and daily administration staff.
Participation in case management, education emphasizing risk reduction, health education, and formal addiction counseling was encouraged but not required. Supervised urine drug testing and medical follow-up were recommended. The program nurse monitored patients, offered support and informal treatment counseling, made referrals, and managed psycho-social crises. A physician met participants the first week at the OTP. The program nurse facilitated weekly public-health oriented educational group discussions at the OTP addressing common challenges (e.g., relapse prevention). Participants also met the program nurse weekly for individual 15-min “check-in” sessions. Methadone was administered within 15 min of the group session to encourage attendance. OTP nurses monitored participants daily during methadone administration and were reminded by an electronic alert to direct participants to keep their weekly “check-in” appointment with the program nurse.
Phase 3. Outpatient Days 31 Through 90—Taper or Titration and Transition to Long-Term Addiction Treatment
Phase 3 (days 31-90) included initiation of a 60-day methadone taper or preparation for transfer to another OTP. The program nurse provided both scheduled and drop-in counseling sessions during Phases 2 and 3. As case manager, the program nurse supported and guided participants, helping them clarify, define, and achieve personal treatment goals. Participants set measurable, achievable goals and identified action steps to meet them (e.g., decrease methadone dose 5 mg per week, keep next doctor appointment). The plan was reviewed weekly and revised based on progress, physical and mental health status, housing and legal status. The program nurse assisted and advocated for the participants to ensure that action steps were met as planned. Participants, in consultation with the program nurse, decided to taper or transfer to a long-term OTP based on individual preference, availability of treatment slots, insurance status, staff recommendations, and employment and family issues.
Outpatient Objectives During Phase 2 and 3
Phases 2 and 3 addressed educational issues, behaviors, and service utilization. Examples included discharge medical treatment adherence (obtaining prescriptions and medical follow-up); harm reduction (needle sharing avoidance, needle exchange program enrollment, vein care, overdose prevention); condom use; HIV counseling and testing; hepatitis C and HIV health education; and addiction treatment education (acupuncture, community resources, methadone, relapse prevention, smoking cessation, recovery tools, and 12-step groups).
Design and Implementation
The Program Nurse
The program nurse consistently engaged patients from hospital enrollment to program completion. Daily visits helped patients establish trust and rapport, address expectations and concerns, and optimize methadone dosing. The program nurse (DB), available to patients and all clinical staff by pager, was supervised by the program physician director (CWS). During Phase 2 and 3, the nurse reviewed treatment plans, assessed dosing, answered questions, and provided emotional support for participants at the OTP. OTP physicians oriented participants to opioid agonist treatment, performed assessments, and collaborated with the program nurse on the treatment plan. The program nurse provided ongoing education to the OTP clinic nursing staff about the philosophy and policies of the program to maintain support for TOP as a distinct program with specific goals within the larger OTP.
To determine program eligibility, hospitalized patients were screened for receipt of daily low-dose methadone (20–40 mg). Patients were identified through the inpatient computerized medication ordering system. The program nurse reviewed individual medical records and discussed the potential for enrollment with clinical staff. Eligibility criteria included: (1) active opioid use and dependence; (2) not currently enrolled in an opioid treatment program; (3) no chronic use of non-prescribed benzodiazepines; (4) no current alcohol-dependence; (5) no active psychosis or suicidal/homicidal ideation. Program participation did not guarantee the opportunity for long-term OTP enrollment. Methadone dosing in the OTP program was based on federal regulations authorizing up to 120 days of methadone without concurrent routine drug testing, counseling or rehabilitation services for individuals awaiting comprehensive treatment.24
Outcome Definitions
Important medical and psychosocial patient outcomes were determined after program design and implementation and were reported for descriptive purposes only. A comparison group was not available. Outcomes were defined in terms of whether or not treatment was provided to participants. All outcomes were classified as “Treatment Provided” (e.g., enrolled in long-term OTP), “Treatment Not Provided” (e.g., loss to follow-up), or “Treatment not Possible” because medical or psychiatric issues took precedence (e.g., too medically or mentally ill or incarcerated).
Data Collection
Data from the program case-management database and the hospital’s information system were extracted and transferred to a research database. Unique patients from the program’s first 3 years are reported. The Institutional Review Board of Boston Medical Center approved this study.
RESULTS
Patient Characteristics
Between January 2002—January 2005, 362 unique patients were screened from admissions to the medical service of Boston Medical Center that received methadone for opiate withdrawal treatment and were neither enrolled in an OTP nor prescribed methadone for pain control (Fig. 1). Average hospitalization was 5.7 days (SD 7.3; range 1-76 days). Average daily in-patient census (Phase 1) was 2, and average program census (Phase 1-3) was 17. The average length of participation in the entire program (Phases 1-3) was 60 days (SD 38; range, 1-154 days).
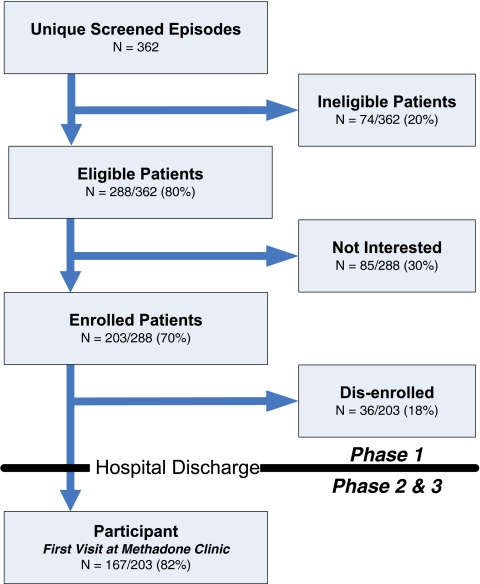
Figure 1.
Screening and enrollment schema of the transitional opioid program.
Unique Screened Patients
Of the 362 unique screened patients, there were 67% males: 50% White, 45% African-American, or 5% other; 24% self-reported Hispanic ethnicity. Mean age was 40 years. Medical conditions typical for this population were noted.1,2 Housing information available from a subset of patients assessed in the latter period of follow-up revealed 60% (69/115) “any homelessness in the previous 6 months,” 44% (51/115) “currently homeless,” and 65% (75/115) “anticipated homelessness in the next 6 months.”
Of the screened patients, 20% (74/362) were ineligible for the following reasons: active benzodiazepine abuse 24% (18/74); alcohol dependence with active alcohol use 24% (18/74); unstable psychiatric co-morbidity 14% (10/74); opioid use for less than 1 year 12% (9/74); medical illness severity 9% (7/74); non-daily opioid use 7% (5/74); other reasons 9% (7/74).
Eligible Patients
Of the 362 screened patients, 80% (288) were eligible for enrollment. However, 30% (85/288) declined program enrollment because they “desire residential treatment” 48% (41/85); “oppose methadone treatment” 24% (20/85); “have no interest in treatment at this time” 15% (13/85); “live too far away from the methadone clinic” 9% (8/85); or “want AA/NA only” 4% (3/85). Overall, 89% of those eligible (255/288) reported interest in addiction treatment.
Patients Enrolled in Hospital (Phase 1)
Of the remaining 203 patients that were eligible and accepted enrollment, 82% (167/203) became participants at the OTP clinic after hospitalization. Among the 18% (36/203) who “dropped-out” (failed to transition to the OTP—Phase 2), 44% (16/36) did not appear for the first dose, 22% (8/36) left the hospital “against medical advice,” 11% (4/36) became too ill (e.g., transferred to ICU or long-term nursing facility), 8% (3/36) were discharged to another facility, and 14% (5/36) dropped out for other reasons.
Overall Outcomes of Participants Enrolled in Hospital (Phase 1)
Of 203 participants initially enrolled during hospitalization, treatment was provided to 59% (119/203), treatment was not provided to 26% (52/203), or treatment was not possible 16% (32/203).
Short-Term Substance Abuse Treatment Outcomes of Program Participants (Phase 2 and 3)
Among the 203 enrolled participants who entered Phase 2 and had treatment provided, 35% (71/203) enrolled in a long-term OTP, 15% (31/203) completed methadone taper, 4% (9/203) entered outpatient or residential substance abuse treatment, and 2% (5/203) entered an inpatient detoxification facility.
Among 52 participants initially enrolled but who were not provided treatment, 46% (24/52) did not show at the methadone clinic or left the hospital against medical advice, and 54% (28/52) did not show at the OTP clinic for 14 consecutive days or were discharged for behavioral issues.
Other Short-Term Outcomes
Phase 2 participants (n = 167) attained other outcomes including: obtained discharge prescriptions, 56% (94/167); attended primary care appointment, 54% (90/167); attended 2 or more group counseling sessions, 50% (84/167); enrolled in a needle-exchange program, 17% (28/167); attended a 12-step program, 16% (27/167); became employed, 16% (27/167).
DISCUSSION
The Transitional Opioid Program identified, recruited, engaged, and linked hospitalized out-of-treatment opioid-dependent patients to addiction care using interim opioid agonist treatment, individualized case management, and both scheduled and drop-in counseling. Most enrolled participants, 82% (167/203), presented for treatment at the OTP clinic after hospital discharge suggesting that an unmet need was addressed. The program employed minimal enrollment standards that did not require commitment to long-term OTP.
Other investigators have studied methods to engage opioid-dependent patients in addiction treatment. A randomized clinical trial targeting out-of-treatment opioid users identified and linked 126 selected individuals to methadone maintenance using four strategies: case management, free treatment voucher, case management plus voucher, or usual care for 6 months. At 3 months, long-term OTP enrollment was “usual care” (11%), case management alone (47%), treatment voucher (89%), and both case management and voucher (93%). Enhanced treatment access using a voucher was twice as effective as case management alone in linking hospitalized addicts to drug abuse treatment.25 Vouchers also enhanced 6-month methadone treatment enrollment rates for patients seeking addiction treatment.26 Schwartz et al. demonstrated that more than three-quarters of 319 heroin-dependent adults on an OTP waitlist randomly assigned to interim methadone remained engaged and entered a long-term OTP.27,28
TOP differs from previous reports in a few important ways. Populations identified by ours and other programs were similar in the range of need and readiness to change; however, TOP engaged non-treatment seeking, opioid-dependent patients ambivalent about substance abuse treatment. Moreover, the program approached ambivalence as a dynamic state and by use of interim opioid agonist therapy provided patients an opportunity to address indecision about addiction treatment and modify their readiness to change. Other “programs” described in the literature consisted of clinical trials that created linked but distinctly separate inpatient and outpatient services that were designed to test different methods and settings for engaging patients. These programs had different objectives, enforced stricter eligibility requirements, or used longer duration and standard OTP structure. In contrast to reports in the literature, our program encouraged but did not require monthly urine drug testing or formal counseling (i.e., no minimum engagement requirements). Similarly, urine testing, if obtained and positive, did not impact program participation. Moreover, duration of participation in the program was roughly half that of other interim opioid-agonist therapy programs.7–27 Similar to programs described by Aszalos and O’Toole, TOP participants were hospitalized; however, our program employed a single nurse who performed inpatient initial contact and screening through to final outpatient taper or referral to maintenance treatment in an OTP.13–15
The program provided the hospital-based clinical team an option for addressing opioid dependence. The TOP model appears sustainable and replicable using a modest staffing model (one nurse) and basic coordination within existing treatment services. The first 3 years of the program were supported by funding from the Massachusetts Department of Public Health. In year 4, the Boston Public Health Commission assumed program costs. Although data to examine health services implications of the program (e.g., hospital re-admissions) were not available, such analyses warrant further study.
Limitations
The program was not available for recruitment at all times but rather only 4 days/week, 8 h/day nor on all services (i.e., only medical service). The TOP model represents a single health care system’s experience and awaits replication. Long-term outcomes of the model could not be determined because subjects did not receive long-term follow-up. Use of electronic medication reports was important and enhanced efficiency, but was not essential to the TOP model, and other enrollment screening methods could be devised to facilitate replication. Quantification of the relative improvement in addiction treatment and other outcomes was not possible because a comparison group was not available. However, most participants were not seeking treatment when hospitalized, and few would have received addiction treatment had they not been enrolled in the program.
CONCLUSIONS
The Transitional Opioid Program (TOP) program model is based on collaboration between a traditional acute inpatient facility and an outpatient addiction treatment program. This model of a transitional opioid program (TOP) engaged and linked many opioid-dependent patients to appropriate addiction and medical care by providing interim opioid agonist treatment while offering a range of treatment intensity options along with exposure to case management and health education focused on personal risks. Given the descriptive nature of this report, its findings should be considered preliminary and hypothesis-generating, and further study will be required to evaluate model efficacy and long-term outcomes.
Acknowledgements
Preliminary study results were presented by CW Shanahan at the American Association for the Treatment of Opioid Dependence Conference, Washington DC (October 2003), and by JH Samet, in a symposium at the 2007 Addiction Health Services Research conference, Athens, Georgia (October 2007). We appreciate the support of the Massachusetts Department of Public Health—HIV/AIDS Bureau and the Bureau of Substance Abuse Services for funding this pilot project, with particular appreciation to Jean F. McGuire, PhD, former Director of the HIV/AIDS Bureau. We also appreciate the leadership of Mr. Tom Scott, former Director of the Boston Public Health Commission, Division of Substance Abuse Prevention and Treatment Services, and Barbara Dworetzky, MD, for her reviews of the manuscript.
Conflict of Interest None disclosed.
References
- 1.Samet JH, Shevitz A, Fowle J, Singer DE. Hospitalization decision in febrile intravenous drug users. Am J Med. 1990;89(1):53–7. doi: 10.1016/0002-9343(90)90098-X. [DOI] [PubMed] [Google Scholar]
- 2.Stein MD. Medical consequences of substance abuse. Psychiatr Clin North Am. 1999;22(2):351–70. doi: 10.1016/S0193-953X(05)70081-2. [DOI] [PubMed] [Google Scholar]
- 3.Burnam MA, Bing EG, Morton SC, et al. Use of mental health and substance abuse treatment services among adults with HIV in the United States. Arch Gen Psychiatry. 2001;58(8):729–36. doi: 10.1001/archpsyc.58.8.729. [DOI] [PubMed] [Google Scholar]
- 4.Hopper JA, Shafi T. Management of the hospitalized injection drug user. Infect Dis Clin North Am. 2002;16(3):571–87. doi: 10.1016/S0891-5520(02)00009-0. [DOI] [PubMed] [Google Scholar]
- 5.McCoy CB, Metsch LR, Chitwood DD, Miles C. Drug use and barriers to use of health care services. Subst Use Misuse. 2001;36(6–7):789–806. doi: 10.1081/JA-100104091. [DOI] [PubMed] [Google Scholar]
- 6.Chutuape MA, Jasinski DR, Fingerhood MI, Stitzer ML. One-, 3-, and 6-month outcomes after brief inpatient opioid detoxification. Am J Drug Alcohol Abuse. 2001;27(1):19–44. doi: 10.1081/ADA-100103117. [DOI] [PubMed] [Google Scholar]
- 7.Samet JH, Rollnick S, Barnes H. Beyond CAGE. A brief clinical approach after detection of substance abuse. Arch Intern Med. 1996;156(20):2287–93. doi: 10.1001/archinte.156.20.2287. [DOI] [PubMed] [Google Scholar]
- 8.O'Toole TP, Pollini RA, Ford D, Bigelow G. Physical health as a motivator for substance abuse treatment among medically ill adults: is it enough to keep them in treatment? J Subst Abuse Treat. 2006;31(2):143–50. doi: 10.1016/j.jsat.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 9.O'Connor PG, Samet JH, Stein MD. Management of hospitalized intravenous drug users: role of the internist. Am J Med. 1994;96(6):551–8. doi: 10.1016/0002-9343(94)90096-5. [DOI] [PubMed] [Google Scholar]
- 10.Chan AC, Palepu A, Guh DP, et al. HIV-positive injection drug users who leave the hospital against medical advice: the mitigating role of methadone and social support. J Acquir Immune Defic Syndr. 2004;35(1):56–9. doi: 10.1097/00126334-200401010-00008. [DOI] [PubMed] [Google Scholar]
- 11.Simpson DD, Savage LJ. Treatment re-entry and outcomes of opioid addicts during a 4-year follow-up after drug abuse treatment in the United States. Bull Narc. 1980;32(4):1–10. [PubMed] [Google Scholar]
- 12.Simpson DD. Treatment for drug abuse. Follow-up outcomes and length of time spent. Arch Gen Psychiatry. 1981;38(8):875–80. doi: 10.1001/archpsyc.1981.01780330033003. [DOI] [PubMed] [Google Scholar]
- 13.Aszalos R, McDuff D, Weintraub E, Montoya I, Schwartz R. Engaging hospitalized heroin-dependent patients into substance abuse treatment. J Subst Abuse Treat. 1999;17(1–2):149–158. doi: 10.1016/S0740-5472(98)00075-0. [DOI] [PubMed] [Google Scholar]
- 14.O'Toole TP, Pollini RA, Ford DE, Bigelow G. The effect of integrated medical-substance abuse treatment during an acute illness on subsequent health services utilization. Med Care. 2007;45(11):1110–5. doi: 10.1097/MLR.0b013e318127142b. [DOI] [PubMed] [Google Scholar]
- 15.O'Toole TP, Conde-Martel A, Young JH, Price J, Bigelow G, Ford DE. Managing acutely ill substance-abusing patients in an integrated day hospital outpatient program: medical therapies, complications, and overall treatment outcomes. J Gen Intern Med. 2006;21(6):570–6. doi: 10.1111/j.1525-1497.2006.00398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sears C, Guydish JR, Weltzien EK, Lum PJ. Investigation of a secondary syringe exchange program for homeless young adult injection drug users in San Francisco, California, U.S.A. J Acquir Immune Defic Syndr. 2001;27(2):193–201. doi: 10.1097/00126334-200106010-00015. [DOI] [PubMed] [Google Scholar]
- 17.Bortolotti F, Stivanello A, Dall'Armi A, Rinaldi R, Grasta F. AIDS information campaign has significantly reduced risk factors for HIV infection in Italian drug abusers. J Acquir Immune Defic Syndr. 1988;1(4):412–3. [PubMed] [Google Scholar]
- 18.Friedman SR, Des Jarlais DC, Sotheran JL. AIDS health education for intravenous drug users. Health Educ Q. 1986;13(4):383–93. doi: 10.1177/109019818601300409. [DOI] [PubMed] [Google Scholar]
- 19.Improving the Quality of Health Care for Mental and Substance-use Conditions, editor. Quality Chasm Series. Washington: National Academy Press; 2006. A frame work for improving quality; pp. 56–76. [PubMed] [Google Scholar]
- 20.Miller WR, Rollnick S. Phase 2: Strengthening commitment to change. In: Motivational Interviewing, editor. Preparing People for Change. New York: Guilford Press; 2002. p. 428. [Google Scholar]
- 21.Marlett GA. Harm reduction around the world: a brief history. In: Marlett GA, editor. Harm Reduction: Pragmatic Strategies for Managing High-Risk Behaviors. New York: Guilford Press; 1998. p. 390. [Google Scholar]
- 22.Prochaska JO, DiClemente CC, Norcross JC. In search of how people change. Applications to addictive behaviors. Am Psychol. 1992;47(9):1102–14. doi: 10.1037/0003-066X.47.9.1102. [DOI] [PubMed] [Google Scholar]
- 23.Marlatt GA. Basic principles and strategies of harm reduction. In: Marlatt GA, editor. Harm Reduction: Pragmatic Strategies for Managing High-Risk Behaviors. New York: The Guilford Press; 1998. pp. 50–52. [Google Scholar]
- 24.Federal Register, title 21, 1993. Codified at 58 CFR 496, pt 291.
- 25.Sorensen JL, Masson CL, Delucchi K, et al. Randomized trial of drug abuse treatment-linkage strategies. J Consult Clin Psychol. 2005;73(6):1026–35. doi: 10.1037/0022-006X.73.6.1026. [DOI] [PubMed] [Google Scholar]
- 26.Barnett PG, Masson CL, Sorensen JL, Wong W, Hall S. Linking opioid-dependent hospital patients to drug treatment: health care use and costs 6 months after randomization. Addiction. 2006;101(12):1797–1804. doi: 10.1111/j.1360-0443.2006.01636.x. [DOI] [PubMed] [Google Scholar]
- 27.Schwartz RP, Highfield DA, Jaffe JH, et al. A randomized controlled trial of interim methadone maintenance. Arch Gen Psychiatry. 2006;63(1):102–9. doi: 10.1001/archpsyc.63.1.102. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz RP, Jaffe JH, Highfield DA, Callaman JM, O'Grady KE. A randomized controlled trial of interim methadone maintenance: 10-month follow-up. Drug Alcohol Depend. 2007;86(1):30–6. doi: 10.1016/j.drugalcdep.2006.04.017. [DOI] [PubMed] [Google Scholar]