Abstract
Objective
We sought to estimate what proportion of adults plan to stop cancer screening tests among adults who recently considered screening and to explore factors associated with these screening plans.
Design
Telephone Survey
Participants
A total of 1,237 participants aged 50 and older who reported having made one or more cancer screening decisions in the past 2 years completed 1,454 cancer screening modules for breast, prostate and colorectal screening.
Main Results
Of all module respondents, 9.8% reported plans to stop screening, 12.6% for breast, 6.0 % for prostate and 9.5% for colon cancer. We found no statistically significant differences in plans to stop for those ages ≥70 (8.2%) compared to those ages 50 to 69 (10.2%) (p = 0.14.) Black respondents were less likely to report plans to stop than white respondents (OR = 0.32, 95% CI 0.12, 0.87). Participation in the decision-making process was associated with plans to stop screening; those who reported they made the final decision about screening (OR 5.9, 95% CI 1.4, 24.7) or made the decision with the health care provider (OR 4.1, 95% CI 1.0, 16.8) were more likely to have plans to stop screening compared to respondents who reported that their health care provider made the final decision.
Conclusions
Plans to stop screening were uncommon among participants who had recently faced a screening decision. Given the recent US Preventive Services Task Force recommendations limiting routine cancer screening for older adults, additional efforts to educate adults about the potential risks and benefits of screening may be warranted.
KEY WORDS: cancer screening, decision making, aged
INTRODUCTION
Cancer screening tests for breast, colorectal and prostate cancers are used in an effort to decrease cancer specific mortality. However, the benefits from screening for these cancers may be delayed for 5 to 10 years 1–3. Given this delay, those at advanced age or with multiple co-morbid conditions are at risk of dying from other causes before they realize a survival benefit from screening 4,5. Furthermore, individuals who undergo screening tests put themselves at immediate risk of having complications, either from the screening tests or from subsequent evaluations because of a positive screening test. Consequently, some guidelines for cancer screening suggest stopping cancer screening in individuals with limited life expectancy 6,7. Recently, the USPSTF made specific age-related recommendations, recommending against prostate screening for men aged 75 and older 2, and recommending against routine screening for CRC screening after age 75 and any CRC screening after age 85 8.
Despite these recommendations to stop, relatively little attention has been directed to attitudes toward stopping or continuing cancer screening tests as people age 9–11. The US public’s expectations toward cancer screening have enormous implications for resource use in the US as the population ages. Prematurely stopping screening in those who could potentially benefit could lead to underutilization of screening and result in preventable deaths. On the other hand, continuing screening with little potential benefit could result in overutilization and the potential of net harm. Given the aging of the population in the US, understanding perceptions about continuing or stopping cancer screening tests is warranted to avoid misperceptions of ageism and to target resources at those most likely to benefit and least likely to be harmed.
In this study, we addressed the issue of stopping cancer screening in a sample of adults aged 50 and older who had previously undergone screening and reported having discussed cancer screening with their provider within the last 2 years or undergone screening within that time period. We sought to estimate what proportion of adults plan to stop cancer screening tests for colorectal, prostate and breast cancer among those who had recently faced decisions about cancer screening and to explore factors associated with these screening plans.
METHODS
The results reported in this paper are part of a larger study, the National Survey of Medical Decisions (the DECISIONS study) 12. The DECISIONS study used a list-assisted random-digit-dial (RDD) telephone survey methodology. Full details of the sampling, instrument development and data collection methodology have been reported by Zikmund-Fisher et al. 12. All procedures and instruments were approved by the University of Michigan and the Ann Arbor Veterans’ Affairs Medical Center institutional review boards.
PARTICIPANTS
The larger study included a national probability sample of 3,010 English-speaking US adults 40 years of age and older. Recruitment efforts achieved a weighted cooperation rate of 86.5% of all individuals screened and confirmed by interviewers as being eligible to participate and a weighted American Association of Public Opinion Research RR4 response rate 13 of 51.6%. These rates compare favorably to those obtained by many surveys that use random digit dialing methodology, such as the often cited Centers for Disease Control and Prevention’s Behavioral Risk Factor Surveillance System (2006 median response rate: 51.4%) 14–16 and reflect the growing difficulty of conducting telephone surveys in the general population 17.
The sampling strategy for the larger study was designed to assess the prevalence of medical decisions for nine common medical decisions. Participants were recruited from a probability sample of households using random-digit-dial methods. Details of the sampling strategy have been described in detail elsewhere 18. Briefly, individual respondents, who had been selected at random from all eligible adults aged 40 and older in each household, were asked whether they had taken a particular action for the nine common medical issues within the last 2 years, and if not, whether they had discussed the action with a provider in that time frame.
To be eligible for the cancer screening modules, respondents had to have been screened in the past and therefore would have the opportunity to consider stopping. Those who had been previously diagnosed with the relevant cancer were excluded from these modules. In addition, respondents were eligible to complete a module if they reported having a screening test for these cancers within the preceding 2 years or having discussed doing so with a health care provider in that same time period. Consequently, we identified people who had previously been screened and had faced another decision about cancer screening within the past 2 years. This sampling strategy targets participants in the active decision-making stage and excludes those who had already decided to stop screening, and those who are up to date with screening for CRC with tests such as colonoscopy and therefore would not have had recent discussions.
For this paper, we restricted the sample to those 50 years old and older because these respondents are eligible for cancer screening. We report information from three targeted question modules related to decision making about colorectal, breast or prostate cancer screening. Because respondents were randomly assigned to respond to up to two modules for which they were eligible, some respondents answered two cancer screening models.
QUESTIONNAIRE
Each module included questions about whether or not the respondent had any plans to stop regular cancer screening. We asked, “Do you plan to stop getting screening tests for colon (breast or prostate) cancer at a certain age?” and (if yes) “At what age will (did you) stop getting tested?” We also asked participants questions about aspects of their discussions with health care providers within the last 2 years regarding cancer screening tests. In addition, all respondents answered a set of questions about socio-demographic characteristics and their perceptions of cancer risk.
ANALYSES
All analyses were adjusted for selection, non-response, post-stratification and module-randomization weights 17. Combined, these weights permit generalization to the population of adult patients aged 50 and older who had previously undergone each of the three types of cancer screening. We used SAS 9.13 survey procedures (PROC SURVEYFREQ and PROC SURVEYLOGISTIC) to estimate population means, 95% confidence intervals and the significance of differences in proportions while adjusting for this weighting and stratification structure. Missing data on selected demographic variables were imputed using sequential regression imputation, implemented in IVEWare. 19,20 Only four demographic variables (race, education, insurance status and income category) required imputation; missing data rates ranged from 0.14% for insurance status to 13.2% for income.
We initially report weighted frequencies for individual cancer types separately. We then combined the different cancer types and fit one multivariable model using logistic regression that included all the respondent characteristics (Table 2) and all aspects of discussions about cancer screening tests (Table 3), and adjusted for the clustering arising from some respondents answering more than one cancer screening module.
Table 2.
Unadjusted Percentages and Adjusted ORa for Associations Between Respondent Characteristics and Plans to Stop Cancer Screening for Any Cancer
| Characteristics | Plan to stop % | OR | 95% CI | p value |
|---|---|---|---|---|
| Age | p = 0.25 | |||
| 50–69 | 10.2% | |||
| ≥70 | 8.2% | 0.74 | 0.45–1.23 | |
| Education | p = 0.43 | |||
| High school graduate or less | 8.3% | |||
| Some college | 10.6% | 1.33 | 0.67–2.63 | |
| College graduate or more | 11.0% | 1.46 | 0.82–2.61 | |
| Gender | p = 0.15 | |||
| Women | 11.6% | |||
| Men | 7.4% | 0.61 | 0.31–1.19 | |
| Race | p = 0.008 | |||
| White | 10.2% | |||
| Black | 3.8% | 0.32 | 0.12–0.87 | |
| Other | 20.3% | 2.63 | 1.04–6.65 | |
| Household income | P = 0.65 | |||
| <$25 K | 7.4% | 1.00 | 0.35–2.85 | |
| $25 K–$49.9 K | 9.5% | 1.35 | 0.67–2.73 | |
| $50 K–$74.9 K | 10.8% | 1.25 | 0.62–2.53 | |
| $75 K–$99.9 K | 13.6% | 1.62 | 0.81–3.22 | |
| $100 K+ | 8.7% | |||
| Insured | p = 0.91 | |||
| Yes | 9.8% | |||
| No | 8.7% | 0.95 | 0.38–2.40 | |
| Health status | ||||
| Excellent-good | 9.9% | p = 0.74 | ||
| Fair/poor | 8.8% | 1.13 | 0.56–2.27 | |
| Cancer risk beliefs | p = 0.11 | |||
| Low risk | 12.1% | |||
| Medium risk | 8.6% | 0.69 | 0.43–1.09 | |
| High risk | 5.7% | 0.44 | 0.17–1.14 |
Table 3.
Unadjusted Percentages and Adjusted ORa for Associations Between Aspects of Discussions About Cancer Screening Tests and Plans to Stop Cancer Screening for Any Cancer
| Plan to stop % | OR* | 95% CI | p value | |
|---|---|---|---|---|
| Who first raised the idea of getting screening test? | p = 0.89 | |||
| Patient | 9.4% | 0.96 | 0.54–1.72 | |
| Health care provider | 9.9% | |||
| How much did health care provider discuss reasons to have a screening test? | p = 0.83 | |||
| Not at all | 12.2% | |||
| A little | 8.1% | 0.74 | 0.27–2.05 | |
| Some | 10.5% | 0.98 | 0.38–2.52 | |
| A lot | 8.6% | 0.95 | 0.37–2.43 | |
| How much did health care provider discuss reasons not to have a screening test? | p = 0.28 | |||
| Not at all | 9.3% | |||
| A little | 10.9% | 1.45 | 0.68–3.12 | |
| Some | 13.8% | 1.97 | 0.94–4.13 | |
| A lot | 8.6% | 1.06 | 0.37–3.02 | |
| Did health care provider express an opinion about whether or not you should have a screening test? | p = 0.18 | |||
| Yes | 9.9% | 1.51 | 0.82–2.78 | |
| No | 24.6% | |||
| Did the health care provider ask you what your preference was with regard to whether or not to have a screening test? | p = 0.48 | |||
| Yes | 9.4% | 0.84 | 0.53–1.36 | |
| No | 10.0% | |||
| Did the health care provider explain the reasons whether or not to have a screening test in a way that you could understand? | ||||
| Yes | 9.0% | 0.45 | 0.23–0.90 | P = 0.02 |
| No | 15.0% | |||
| Who made the final decision whether or not to have a screening test? | p = 0.03 | |||
| Mainly my decision | 12.5% | 5.89 | 1.41–24.66 | |
| We made the decision together | 8.7% | 4.07 | 0.99–16.75 | |
| Mainly health care provider’s decision | 2.2% |
RESULTS
A total of 1,237 participants completed 1,454 cancer screening modules (556 breast, 283 prostate and 615 colorectal). Two hundred sixty participants responded to more than one screening module; 156 women responded to both the colorectal and breast cancer modules, and 104 men responded to the colorectal and prostate cancer screening modules.
About one quarter (23%) of all the module respondents were age 70 and over (Table 1). The majority were women (57%), white (75%) and had health insurance (95%). Forty-three percent reported having a high school education or less, and 15% reported fair to poor health.
Table 1.
Weighted Estimates of the Characteristics of Module Responses
| All cancers | Breast | Prostate | Colon | |
|---|---|---|---|---|
| n = 1,454 | n = 556 | n = 283 | n = 615 | |
| Age | ||||
| Mean (SD) | 62.4 (9.4) | 62.2 (9.3) | 61.7 (8.9) | 62.8 (9.8) |
| 50–69 % | 76.8% | 74.9% | 81.5% | 75.9% |
| 70 and over % | 23.2% | 24.1% | 18.5% | 24.1% |
| Gender % | ||||
| Women | 57.1% | 100% | 0% | 53.7% |
| Education % | ||||
| High school graduate or less | 42.9% | 52.4% | 37.3% | 38.8% |
| Some college | 20.9% | 18.2% | 21.2% | 22.5% |
| College graduate/post-graduate | 36.3% | 29.3% | 41.4% | 38.7% |
| Race % | ||||
| White | 73.1% | 74.6% | 78.9% | 69.5% |
| Black | 17.8% | 18.6% | 11.4% | 20.2% |
| Other | 9.1% | 6.9% | 9.7% | 10.3% |
| Household income | ||||
| <$25 K | 17.5% | 22.2% | 10.2% | 17.5% |
| $25 K–$49.9 K | 27.2% | 31.2% | 26.8% | 24.6% |
| $50 K–$74.9 K | 21.3% | 21.4% | 19.8% | 21.9% |
| $75 K–$99.9 K | 13.1% | 12.3% | 13.4% | 13.5% |
| $100 K+ | 21.0% | 12.9% | 29.9% | 22.5% |
| Insured % | ||||
| Yes | 95.3% | 95.0% | 95.4% | 95.4% |
| Self-reported health status % | ||||
| Excellent-good | 85.2% | 85.5% | 86.6% | 84.4% |
| Fair/poor | 14.8% | 14.5% | 13.4% | 15.6% |
| Self-perceived risk of cancer | ||||
| Low risk | 48.3% | 50.6% | 38.0% | 51.5% |
| Medium risk | 37.8% | 31.9% | 44.9% | 38.6% |
| High risk | 13.9% | 17.5% | 17.1% | 9.9% |
PLANS TO STOP CANCER SCREENING
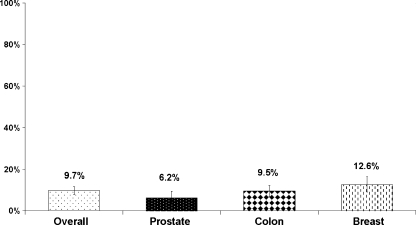
Overall 9.8% of module respondents reported having plans to stop screening (Fig. 1). The proportion of module respondents with plans to stop screening varied by cancer type. The smallest proportion reporting plans to stop was men considering prostate cancer screening, 6.0%. For breast cancer screening, 12.6% of women had plans to stop and for colorectal cancer screening, 9.5% of men and women reported plans to stop. For module respondents aged 70 and older, 8.2% reported plans to stop compared to 10.2% for those aged 50 to 69.
Figure 1.
Percent with plans to stop cancer screening among adults who recently considered screening.
The proportion planning to stop screening for each cancer differed by age when each cancer type was examined separately. For breast cancer, 5.7% of women aged 70 and older had plans to stop compared to 14.9% for women aged 50 to 69 (difference 9.2%, 95% CI 3.1%, 15.4%). For prostate cancer, 0% of men aged 70 and older compared to 7.4% of men aged 50 to 69 reported plans to stop (difference 7.4%, 95% CI 3.5%, 11.3%). For colorectal cancer, 8.2% of those 70 and older reported plans to stop compared to 10.2% of respondents aged 50 to 69 (difference 2.0%, 95% CI -2.1%, 6.1%).
Among the 153 respondents who reported plans to stop, 119 gave a specific stopping age. For breast cancer screening, the mean stopping age was 74.8 (SD 10.0), and for colorectal and prostate cancer screening, the mean stopping age was 76.8 (SD 11.6) and 82.9 (SD 8.4), respectively.
CHARACTERISTICS ASSOCIATED WITH PLANS TO STOP CANCER SCREENING
We found that only one respondent characteristic was associated with having plans to stop cancer screening in the logistic regression modeling that included both participant characteristics (Table 2) and aspects of patient-provider discussions (Table 3). Compared to white respondents, black respondents were less likely to report plans to stop (OR = 0.32, 95% CI 0.12, 0.87, p = 0.03), while those of other non-Caucasian races were more likely to report plans to stop (OR = 2.63, 95% CI 1.04, 6.65, p = 0.04). The other respondent characteristics (age, gender, insurance status, education, self-reported health status, cancer risk and income) were not independently associated with plans to stop screening for the three cancers combined.
Among aspects of discussions with respondents’ health care providers, two were independently associated with plans to stop screening (Table 3) when we controlled for both respondent characteristics and aspects of discussions. Respondents who reported they made the final decision (OR 5.9, 95% CI 1.4, 24.7, p = 0.02) and those who reported that they made the decision with their health care providers (OR 4.1, 95% CI 1.0, 16.8, p=.05) were more likely to have plans to stop screening compared to respondents who reported that their health care providers made the final decision. Additionally, those who said their health care providers explained the reasons for and against screening “well” were less likely to have plans to stop screening (OR = 0.45, 95% CI 0.23, 0.90, p = 0.02).
DISCUSSION
Among a sample of adults aged 50 and older who had previously undergone screening for breast, colon or prostate cancer screening and had discussed screening with their providers or undergone testing within the past 2 years, we found that plans to stop screening were uncommon. A slightly higher proportion of respondents reported plans to stop breast cancer screening than prostate or colon cancer screening. A smaller proportion of respondents aged 70 years old and older reported plans to stop compared to those aged 50 to 69 years old. We also found that black respondents were less likely to report plans to stop screening than whites and that those of other races were more likely to have plans to stop than whites. When we examined aspects of discussions about cancer screening tests, we found that respondents who reported receiving information about stopping from providers or who did not participate in decision making about stopping were less likely to have plans to stop screening.
Our findings that a small proportion of people who have recently faced a decision about cancer screening report plans to stop cancer screening are consistent with other research in this area. One nationally representative survey found less than one-third of people surveyed reported that they would stop cancer screening 9. However, the question was posed hypothetically to younger adults and therefore may not reflect older adults’ actual perceptions. Another smaller study, from two continuing care retirement communities, found similar results in adults aged 70 and over. This study adds to these findings in a larger sample of adults in the US who have either recently been screened for breast, colon or prostate cancer or had recent discussions with their health care providers.
Our analyses were notable because we did not find expected associations between those with poorer health status or increased age and plans to stop screening. It is important to note that our sampling strategy excluded those who had stopped cancer screening prior to our 2-year eligibility requirement. Therefore, among older individuals in poorer health, those who may be reticent to stop screening were more likely to be participants in our study. The decision process about stopping cancer screening is particularly relevant for these individuals because those who are older or in poorer health status are those most likely to experience net harm 8. However, these negative results should be interpreted with caution given the small numbers, but suggest the need for further research about cancer screening in older adults with poorer health status.
As with respondent characteristics, we found few associations between aspects of discussions with providers and plans to stop screening. Our findings suggest that the provider input for people considering cancer screening, either by providing information or in the decision process, is inversely associated with plans to stop cancer screening. This suggests that providers’ attitudes toward stopping cancer screening and interactions between providers and patients could be an important target for future research.
Although our study does not address why a small proportion of respondents have plans to stop cancer screening, previous work suggests that people may be unaware of the delay in the benefit from screening and the need to consider life expectancy 10. Given the new recommendations by the USPSTF, it will be important to understand how patients perceive the benefits and downsides of screening in the context of limited life expectancy and determine whether further education is needed. Resnick found that educating older adults about the risks and benefits of screening resulted in more realistic expectations about screening 21. However, educating people about the complexities of screening may be challenging, as demonstrated by experience with prostate cancer screening 22–24.
Our study has several limitations. First, our sample was limited to those who had recent contact with a health care provider and may bias our results in favor of screening. Although it is reassuring that our results are consistent with previous studies 9–11, these studies may have similar biases. On the other hand, our intent was to estimate the proportion of people who have plans to stop screening in a population where the decision-making process was active, as this population would be a target to improve decision making. A different study design would be needed to estimate the proportion of the population who had already decided to stop cancer screening. Given the exclusion of those who may have already stopped, these results should not be construed to represent the overall stopping rate among the US population. Another limitation was that our survey questions have not been formally validated. However, we did perform cognitive interviews to help ensure if respondents were interpreting the questions in the manner we intended. Finally, the perceptions of the non-respondents may differ significantly from those reported here.
Plans to stop screening were uncommon among participants who had recently faced a screening decision. Further study is required to better understand how patients perceive the benefits and downsides of screening in the context of limited life expectancy. Given the recent US Preventive Services Task Force recommendations either against or limiting routine cancer screening for adults aged 75 and older, additional efforts to educate adults about the importance of considering the potential risks and benefits of screening may be warranted.
Acknowledgements
The DECISIONS study was supported by the Foundation for Informed Medical Decision Making. Dr. Lewis was supported by a K07 Mentored Career Development Award (5K07CA104128) from the National Cancer Institute. Dr. Zikmund-Fisher was supported by a Mentored Research Scholar Grant from the American Cancer Society (MRSG-06-130-01-CPPB).
Potential Financial Conflicts of Interest The Foundation for Informed Medical Decision Making (FIMDM) provided the funding for this research. While the research involved substantial collaboration among Dr. Lewis, the University of Michigan (UM) research team and FIMDM representatives, the research grant was awarded in compliance with UM’s policies, which bar funder interference in scholarly work. Design of the survey and control of the research data rested with the UM investigator team, and Dr. Lewis had final authority regarding the content of the paper. During this research, Dr. Levin was Director of Research at FIMDM. She provided input on the research design, feedback on analyses and constructive comments on manuscript drafts consistent with her listed co-authorship role.
References
- 1.Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med. 1993;328(19):1365–1371. doi: 10.1056/NEJM199305133281901. [DOI] [PubMed] [Google Scholar]
- 2.Unites States Preventive Services Task Force, Agency for Healthcare Research and Quality. Screening for Prostate Cancer. August 2008; http://www.ahrq.gov/clinic/uspstf/uspsprca.htm. Accessed March 18, 2010.
- 3.Unites States Preventive Services Task Force, Agency for Healthcare Research and Quality. Screening for Breast Cancer. November 2003; http://www.ahrq.gov/clinic/uspstf/uspsbrca.htm. Accessed March 18, 2010.
- 4.Walter LC, Covinsky KE. Cancer screening in elderly patients: a framework for individualized decision making. JAMA. 2001;285(21):2750–2756. doi: 10.1001/jama.285.21.2750. [DOI] [PubMed] [Google Scholar]
- 5.Welch HG, Albertsen PC, Nease RF, Bubolz TA, Wasson JH. Estimating treatment benefits for the elderly: the effect of competing risks. Ann. Intern. Med. 1996;124(6):577–584. doi: 10.7326/0003-4819-124-6-199603150-00007. [DOI] [PubMed] [Google Scholar]
- 6.American Geriatrics Society Ethics Committee. AGS position paper: Health screening decisions for older adults. 2002; http://www.americangeriatrics.org/products/positionpapers/stopscreening.shtml. Accessed March 18, 2010. [DOI] [PubMed]
- 7.American Cancer Society. ACS Cancer Detection Guidelines. http://www.cancer.org/docroot/PED/content/PED_2_3X_ACS_Cancer_Detection_Guidelines_36.asp Accessed March 18, 2010.
- 8.Unites States Preventive Services Task Force, Agency for Healthcare Research and Quality. Screening for Colorectal Cancer. 2009; http://www.ahrq.gov/clinic/uspstf/uspscolo.htm. Accessed March 18, 2010.
- 9.Schwartz LM, Woloshin S, Fowler FJ, Jr, Welch HG. Enthusiasm for cancer screening in the United States. JAMA. 2004;291(1):71–78. doi: 10.1001/jama.291.1.71. [DOI] [PubMed] [Google Scholar]
- 10.Lewis CL, Kistler CE, Amick HR, et al. Older adults’ attitudes about continuing cancer screening later in life: a pilot study interviewing residents of two continuing care communities. BMC Geriatr. 2006;6:10. doi: 10.1186/1471-2318-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schonberg MA, Ramanan RA, McCarthy EP, Marcantonio ER. Decision making and counseling around mammography screening for women aged 80 or older. J. Gen. Intern. Med. 2006;21(9):979–985. doi: 10.1007/BF02743148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zikmund-Fisher BJ, Couper MP, Singer E, et al. The DECISIONS Study: A Nationwide Survey of US Adults Regarding Nine Common Medical Decisions Med Decis Making. In Press. [DOI] [PubMed]
- 13.American Association for Public Opinion Research. Standard Definitions: Final Dispositions of Case Codes and Outcome Rates for Surveys http://www.aapor.org/AM/Template.cfm?Section=Standard_Definitions&Template=/CM/ContentDisplay.cfm&ContentID=1273. Accessed March 18, 2010.
- 14.Centers for Disease Control and Prevention. 2006 Behavioral Risk Factor Surveillance System Summary Data Quality Report. May 3, 2007; http://ftp.cdc.gov/pub/Data/Brfss/2006SummaryDataQualityReport.pdf. Accessed March 18, 2010.
- 15.Use of mammograms among women aged ≥40 years—United States, 2000-2005. JAMA. 2007;297(9):942–943. [PubMed]
- 16.Influenza and pneumococcal vaccination coverage among persons aged ≥65 Years—United States, 2004–2005. JAMA. 2006;296(18):2196–2198. [PubMed]
- 17.Lepkowski JM, Tucker NC, Brick JM, et al., editors. Advances in Telephone Survey Methodology. New York: Wiley; 2007. [Google Scholar]
- 18.Couper MP, Zikmund-Fisher BJ, Singer E, et al. Summary of methods for the National Survey of Medical Decisions (the DECISIONS study). http://www.cbdsm.org/downloads/decisionsmethods.pdf. Accessed March 16, 2009.
- 19.Raghunathan TE, Lepkowski JM, Van Hoewyk J, Solenberger PW. A multivariate technique for multiply imputing missing values using a sequence of regression models. Survey Methodology. June 2001 2001;27(1):85–95.
- 20.Raghunathan TE, Trivellore E, Lepkowski JM, Van Hoewyk J, Solenberger PW. IVEware, A Software for the Analysis of Complex Survey Data with or Without Multiple Imputations. www.isr.umich.edu/src/smp/ive. Accessed March 18, 2010.
- 21.Resnick B. Health promotion practices of older adults: testing an individualized approach. J Clin Nurs. Jan 2003;12(1):46–55; discussion 56. [DOI] [PubMed]
- 22.Ransohoff DF. Screening colonoscopy in balance: Issues of implementation. Gastroenterol Clin North Am. 2002;31(4):1031–1044, vii. [DOI] [PubMed]
- 23.Chan EC, Vernon SW, O’Donnell FT, Ahn C, Greisinger A, Aga DW. Informed consent for cancer screening with prostate-specific antigen: how well are men getting the message? Am. J. Public Health. 2003;93(5):779–785. doi: 10.2105/AJPH.93.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gwede CK, McDermott RJ. Prostate cancer screening decision making under controversy: implications for health promotion practice. Health Promot Pract. 2006;7(1):134–146. doi: 10.1177/1524839904263682. [DOI] [PubMed] [Google Scholar]