Abstract
BACKGROUND
Exacerbations in chronic respiratory diseases (CRDs) are sensitive to seasonal variations in exposure to respiratory infectious agents and allergens and patient factors such as non-adherence. Hence, regular general practitioner (GP) contact is likely to be important in order to recognise symptom escalation early and adjust treatment.
OBJECTIVE
To examine the association of regularity of GP visits with all-cause mortality and first CRD hospitalisation overall and within groups of pharmacotherapy level in older CRD patients.
DESIGN
A retrospective cohort design using linked hospital, mortality, Medicare and pharmaceutical data for participant, exposure and outcome ascertainment. GP visit pattern was measured during the first 3 years of the observation period. Patients were then followed for a maximum of 11.5 years for ascertainment of hospitalisations and deaths.
PARTICIPANTS
We studied 108,455 patients aged ≥65 years with CRD in Western Australia (WA) during 1992–2006.
MAIN MEASURES
A GP visit regularity score (range 0–1) was calculated and divided into quintiles. A clinician consensus panel classified levels of pharmacotherapy. Cox proportional hazards models, controlling for multiple factors including GP visit frequency, were used to calculate hazard ratios and confidence intervals.
KEY RESULTS
Differences in survival curves and hospital avoidance pattern between the GP visit regularity quintiles were statistically significant (p = 0.0279 and p < 0.0001, respectively). The protective association between GP visit regularity and death appeared to be confined to the highest pharmacotherapy level group (P for interaction = 0.0001). Higher GP visit regularity protected against first CRD hospitalisation compared with the least regular quintile regardless of pharmacotherapy level (medium regular: HR = 0.84, 95% CI = 0.77–0.92; 2nd most regular: HR = 0.74, 95% CI = 0.67–0.82; most regular HR = 0.77, 95% CI = 0.68–0.86).
CONCLUSIONS
The findings indicate that regular and proactive ‘maintenance’ primary care, as distinct from ‘reactive’ care, is beneficial to older CRD patients by reducing their risks of hospitalisation and death.
Electronic supplementary material
The online version of this article (doi:10.1007/s11606-010-1361-6) contains supplementary material, which is available to authorized users.
KEY WORDS: chronic respiratory disease, mortality, hospitalisation, primary care, record linkage
INTRODUCTION
Chronic respiratory diseases (CRDs) are relatively common in Australia with 10–12% of the population aged over 15 years suffering from asthma1 and 9–12% of those over 45 years suffering from symptomatic chronic obstructive pulmonary disease (COPD)2. These two conditions account for 80% of the total burden of CRDs in Australia3, and together they place a significant burden on the health care system1,4.
Primary medical care is delivered in Australia predominantly through general practice, where the Australian general practitioner (GP) is equivalent to a US family physician certified by the American Board of Family Medicine. The importance of general practice in the management of chronic diseases has been strongly promoted in Australia for the last decade5. Some evidence exists indicating that the pattern of GP services can influence the risk of adverse health events requiring provision of emergency and inpatient health care. For example, Tsai et al. found that patients with a relatively high frequency of emergency department visits for COPD exacerbations were less likely to have regular contact with a GP provider6, and Cree et al. found high continuity of care in asthma patients to be associated with a decreased risk of emergency department visits and hospitalisations7. GP accessibility was also found in several studies to protect against potentially preventable hospital admissions8–12.
Older CRD patients have a substantial excess of all-cause mortality compared with the general population13–16. Since treatment with medications is the mainstay of CRD management17–19, a sufficient frequency and, more salient still, a sufficient regularity of GP visits is likely to be an important marker of effective proactive disease management. Furthermore, since respiratory symptoms are sensitive to environmental variability such as seasonal variation, respiratory infections, air pollutants and allergens20,21, regular GP contact—as opposed to sporadic and frequent GP contact—is likely to be important in order to adjust treatment and recognise symptoms escalation early. Scheduled regular GP visits could prevent exacerbations as opposed to sporadic and frequent GP contact that is more likely to happen as a consequence of worsening disease symptoms. Hence, regular GP contact is likely to be important for prevention of adverse disease outcomes. Our aim was thus to estimate the association of regularity of GP visits with all-cause mortality and first CRD hospitalisations overall and according to levels of pharmacotherapy in older CRD patients.
METHODS
Data Sources
We based the study on administrative, whole-population health data that were linked and extracted in de-identified form using the Western Australian (WA) Data Linkage System (WADLS), which is maintained by the WA Department of Health. The record linking is based on full name and address, phonetic compression algorithms and other identifiers, and has been estimated to be 99.89% accurate22 (personal communication: Paul Stevens, data analyst, Hospital Morbidity Data Collection, WA Department of Health).
Linked data from 1 January 1992 to 31 December 2006 were extracted for individuals aged ≥65 years from the Hospital Morbidity Data System, the Death Registry, the Electoral Roll, the Medicare Benefits Scheme (MBS) and the Pharmaceutical Benefits Scheme (PBS). The MBS data fields included types of services provided by GPs that qualified for Medicare benefits23,24. The PBS data fields included type of medication prescribed. The Electoral Roll data were used to ascertain migration of the study population, since electoral registration is compulsory for all adult Australian citizens residing in the country.
Study Population
Hospital, death, MBS and PBS data were combined to ascertain the study population. Individuals with a hospital or death record of asthma, COPD or emphysema (ICD-9-CM: 492-493.92, 496, 975.7, E945.7; ICD-10-AM: J43-J46, T48.6, Y55.6) or bronchitis (ICD-9-CM: 491-491.21, 491.8-491.9; ICD-10-AM: J41-J42) were identified. The MBS data were used to identify individuals undergoing a special asthma management plan in GP offices (asthma cycle of care) subsidised by the government (items 2546–2559 and 2664–2677). From the PBS data, a list of the most commonly used CRD medications in Australia was used to identify potential CRD patients. The list included beclomethasone, budesonide, fluticasone, eformoterol, salmeterol, terbutaline, salbutamol, ipratropium, tiotropium, nedocromil, theophylline and aminophylline.
We identified 116,983 individuals who had at least one record from any of the four databases. To exclude patients with acute bronchitis, we removed all individuals with only a bronchitis diagnosis or death record (n = 386). We then excluded individuals who were not registered on the WA Electoral Roll during the study period (n = 8,142). All other individuals were included in the study due to the high likelihood that they had CRD (n = 108,455).
A clinical consensus panel comprising seven general practitioners, two geriatricians and three clinical pharmacists was appointed to develop guidelines for the classification of CRD pharmacotherapy level (Table 1). If a patient had been dispensed a combination of medications from different levels during the observation period, the patient was assigned the level reflecting the highest pharmacotherapy.
Table 1.
Pharmacotherapy Levels for CRD Based on Medication Dose as Defined by a Clinical Consensus Panel
| SAB | Low dose ICSa | Medium dose ICS±LABa | High dose ICS±LAB±OSa |
|---|---|---|---|
| Terbutaline AND/OR | <250 μg Beclomethasone AND/OR | 250–500 μg Beclomethasone AND/OR | >500 μg Beclomethasone AND/OR |
| Salbutamol AND/OR | <400 μg Budesonide AND/OR | 400–800 μg Budesonide AND/OR | >800 μg Budesonide AND/OR |
| Ipratropium AND/OR | <250 μg Fluticasone | 250–500 μg Fluticasone | >500 μg Fluticasone |
| Tiotropium AND/OR | AND/OR | ||
| Nedocromil | Eformoterol AND/OR | Eformoterol AND/OR | |
| Salmeterol | Salmeterol | ||
| AND/OR | |||
| >1 prescription per year of hydrocortisone and/or prednisolone | |||
SAB = short-acting bronchodilators
ICS = inhaled corticosteroids
LAB = long-acting bronchodilators
OS = oral steriods
aDoses represent daily doses
Study Period
The period of observation began at the date of the first CRD record, start date of continuous Electoral Roll registration or 1 January 1992, whichever came last. Patients were censored at the date of the last database record, end date of continuous Electoral Roll registration, date of death or first CRD hospitalisation or 31 December 2006, whichever came first.
The exposure period, where pattern of GP service delivery was ascertained, represented the first 3 years of each patient’s observation period. GP visits were identified using 127 MBS service items for clinic attendance, out of surgery consultation and home visits23,24. We developed a regularity score (RS) to represent the regularity of GP visits during the exposure period, since entropy-related scores from other fields were unsuitable for our purpose25. This score was calculated as 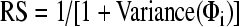 where Φi was the time interval between the (i-1)th and ith GP visits. The score ranged from 0 to 1—with 1 representing perfect regularity—and measured how regularly a person visited a GP, i.e. whether the time intervals in between GP visits were usually of the same length. The score was divided into quintiles for all analyses. Person-time of follow-up began 6 months after the end of the exposure period. This ‘wash-out’ period was applied to minimise the likelihood of immortal time bias26,27.
where Φi was the time interval between the (i-1)th and ith GP visits. The score ranged from 0 to 1—with 1 representing perfect regularity—and measured how regularly a person visited a GP, i.e. whether the time intervals in between GP visits were usually of the same length. The score was divided into quintiles for all analyses. Person-time of follow-up began 6 months after the end of the exposure period. This ‘wash-out’ period was applied to minimise the likelihood of immortal time bias26,27.
Statistical Analyses
Cox proportional hazards models were applied to calculate hazard ratios and confidence intervals for the effects of GP visit regularity on (1) all-cause death and (2) first CRD hospitalisation. Included in the analyses were those patients who were still alive at the end of the exposure and wash-out periods and who had not been hospitalised for CRD during those periods (hospitalisation analyses only). Interaction was assessed by including an interaction term between GP visit regularity and pharmacotherapy level in the models for the effect of (1) all-cause death and (2) first CRD hospitalisation. All models were adjusted for total number of GP visits during the exposure period (continuous), gender (dichotomous), age at start of follow-up (continuous), indigenous status (dichotomous), Charlson Index of Comorbidity (ordinal)28, area-based socioeconomic status (categorical) and residential remoteness (categorical) (obtained from the Australian Census conducted every 5 years). We assessed the proportional hazards assumption for each covariate in each Cox model using an interaction term between the covariate and the natural logarithm of the person-time and found no basis to reject proportionality. We also used Cox proportional hazards regression to estimate survival and hospitalisation-free probability in each quintile of GP visit regularity. The curves were adjusted for all variables mentioned above by using in the model the average value of each of the adjusting variables (i.e., the mean of covariates method). The overall p-value for the variable GP visit regularity in the Cox regression models represented the test for significance. All analyses were performed using the statistical software SAS version 9.1 (SAS Institute Inc., Cary, NC).
RESULTS
Characteristics of the study population overall and by pharmacotherapy level are shown in Table 2. The number and regularity of GP visits was lowest in the short-acting bronchodilator (SAB) group, but progressively increased with increasing pharmacotherapy level. We observed 4,841 deaths during the 11.5 years of follow-up, of which almost one half was in the highest pharmacotherapy level group. At the end of follow-up, 7,331 patients had been hospitalised for CRD. The hospitalisation rate was lowest for patients in the SAB group, but increased with increasing pharmacotherapy level.
Table 2.
Characteristics of Patients Aged ≥65 years with CRD in WA 1992–2006, Overall and by Pharmacotherapy Level
| Patient characteristic | All patients | SAB | Low dose ICS | Medium dose ICS ± LAB | High dose ICS ± LAB ± OS |
|---|---|---|---|---|---|
| Number of patients | 108,455 | 29,740 | 9,384 | 37,125 | 32,206 |
| Mean ag e ± SD at start of follow-up | 72.7 ± 7.0 | 74.7 ± 7.6 | 72.7 ± 6.8 | 71.4 ± 6.4 | 72.3 ± 6.7 |
| % Female patients | 53.1 | 54.0 | 56.3 | 53.3 | 51.0 |
| % Indigenous patients | 0.6 | 0.8 | 0.4 | 0.5 | 0.6 |
| Mean ± SD GP visit numbera | 30.2 ± 23.7 | 26.7 ± 22.3 | 28.8 ± 22.1 | 29.3 ± 22.1 | 34.9 ± 26.4 |
| Median GP visit regularity a,b | 45.1 | 29.0 | 43.2 | 46.0 | 62.2 |
| All-cause mortality analyses | |||||
| Number of patients included in analysesc | 66,390 | 14,159 | 5,985 | 23,849 | 22,397 |
| Average years ± SD of follow-up | 4.8 ± 3.2 | 4.2 ± 2.9 | 4.9 ± 3.3 | 4.6 ± 3.1 | 5.4 ± 3.3 |
| Person-years of follow-up | 319,138.2 | 59,641.7 | 29,285.3 | 109,150.4 | 121,060.8 |
| Number of deaths at the end of follow-up | 4,841 | 827 | 369 | 1,301 | 2,344 |
| Mortality rate (per 100,000 PY) | 1,516.9 | 1,386.6 | 1,260.0 | 1,191.9 | 1,936.2 |
| First CRD hospitalisation analyses | |||||
| Number of patients included in analysesd | 54,641 | 12,826 | 5,434 | 20,113 | 16,268 |
| Average± SD years of follow-up | 4.4 ± 3.1 | 4.2 ± 2.9 | 4.8 ± 3.3 | 4.1 ± 3.0 | 4.6 ± 3.2 |
| Person-years of follow-up | 237,839.6 | 54,101.4 | 25,924.6 | 83,435.4 | 74,378.3 |
| Number of first hospitalisations at the end of follow-up | 7,331 | 447 | 330 | 2,400 | 4,154 |
| Hospitalisation rate (per 100,000 PY) | 3,082.3 | 826.2 | 1272.9 | 2876.5 | 5585.0 |
aDuring the 3-year exposure period
bMultiplied by 100,000
cPatients who were alive at the end of the exposure and wash-out periods
dPatients who did not have a CRD hospitalisation during the exposure and wash-out periods and who were alive at the end of the two periods
SAB = short-acting bronchodilators
ICS = inhaled corticosteroids
LAB = long-acting bronchodilators
OS = oral steriods
PY = person-years
We show the characteristics of the 108,455 CRD patients by quintiles of GP visit regularity in Table 3. Number of GP visits and the percentage of females were lowest in the least regular group whilst the percentage of indigenous patients, percentage of patients from very remote locations and percentage of patients with over six comorbid diseases was highest in that group.
Table 3.
Characteristics of 108,455 WA CRD Patients ≥65 years 1992–2006 by Groups of GP Visit Regularity
| Least regular | 2nd least regular | Medium regular | 2nd most regular | Most regular | |
|---|---|---|---|---|---|
| Percent | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 |
| Mean age ± SD at start of follow-up | 74.4 ± 7.5 | 71.8 ± 6.9 | 71.1 ± 6.3 | 71.7 ± 6.5 | 73.0 ± 7.0 |
| % Female patients | 45.0 | 52.0 | 56.0 | 59.1 | 63.5 |
| % Indigenous patients | 0.79 | 0.74 | 0.43 | 0.48 | 0.43 |
| Mean ± SD GP visit numbera | 7.8 ± 6.3 | 18.8 ± 10.2 | 26.1 ± 10.8 | 36.6 ± 12.1 | 61.9 ± 26.7 |
| Socioeconomic status quintile (%) | |||||
| Least disadvantaged | 20.1 | 19.9 | 20.3 | 19.7 | 19.4 |
| 2nd least disadvantaged | 19.6 | 20.3 | 19.9 | 19.5 | 20.2 |
| Medium disadvantaged | 20.6 | 19.5 | 20.1 | 20.2 | 19.8 |
| 2nd most disadvantaged | 19.9 | 20.5 | 20.6 | 20.6 | 19.0 |
| Most disadvantaged | 19.8 | 19.8 | 19.2 | 20.0 | 21.6 |
| Residential remoteness (%) | |||||
| Major cities | 73.5 | 70.9 | 73.2 | 76.9 | 84.1 |
| Inner regional | 12.5 | 14.5 | 14.9 | 13.2 | 9.4 |
| Outer regional | 10.5 | 11.6 | 9.7 | 8.3 | 5.3 |
| Remote | 2.9 | 2.6 | 1.9 | 1.5 | 1.2 |
| Very remote | 0.6 | 0.4 | 0.2 | 0.1 | 0.1 |
| Charlson Index of Comorbidity (%) | |||||
| 0 | 20.9 | 29.6 | 30.0 | 25.5 | 17.2 |
| 1–2 | 26.8 | 29.7 | 31.3 | 31.7 | 28.4 |
| 3–5 | 26.1 | 22.8 | 22.8 | 25.6 | 31.1 |
| 6+ | 26.2 | 17.9 | 15.9 | 17.3 | 23.4 |
*During the 3-year exposure period
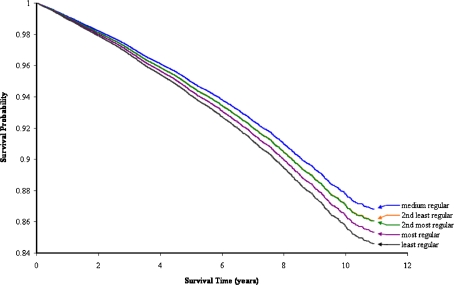
Figure 1 shows the adjusted survival functions for CRD patients within each quintile of GP visit regularity. Patients with the least regular GP visit pattern experienced relatively poor survival with 84.6% still alive at the end of follow-up, whereas the other groups of patients experienced slightly better survivals (2nd least regular 86.1%; medium regular 86.8%; 2nd most regular 86.0%; most regular 85.3%). The difference in survival patterns between the GP visit regularity quintiles was significant (global p = 0.0279).
Figure 1.
Estimated survival functions for chronic respiratory disease patients in strata of GP visit regularity quintile with all-cause death as outcome. The functions are adjusted for number of GP visits, gender, age at start of follow-up, ethnicity, area-based socioeconomic status, residential remoteness, the Charlson Index of Comorbidity and indigenous status. The survival curves for 2nd least regular and medium regular merge together.
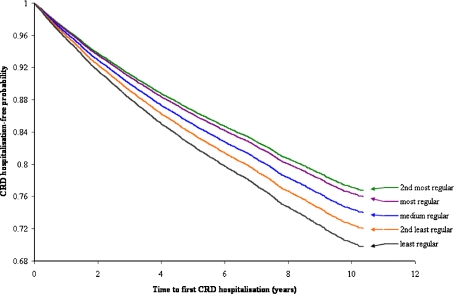
Presented in Figure 2 are survival functions representing the probability of avoiding first CRD hospitalisation within each quintile of GP visit regularity. Survival increased with greater regularity of GP visits, with 69.8% of patients with the least regular GP visits still hospitalisation-free at the end of observation, compared with 72.0% (2nd least regular), 74.0% (medium regular), 76.7% (2nd most regular) and 76.0% (most regular). These differences in hospital avoidance between the GP visit regularity quintiles were statistically significant (global p < 0.0001).
Figure 2.
Estimated survival functions for CRD patients in strata of GP visit regularity quintile with first CRD hospitalisation as outcome. The functions are adjusted for number of GP visits, gender, age at start of follow-up, ethnicity, area-based socioeconomic status, residential remoteness, the Charlson Index of Comorbidity and indigenous status. CRD = chronic respiratory disease.
The likelihood of all-cause death or first CRD hospitalisation for increased GP visit regularity compared with the least regular quintile, overall and by pharmacotherapy level, is shown in Table 4. Increased GP visit regularity appeared to have a weak protective association against death, which was modified by pharmacotherapy level, where it was confined to the highest pharmacotherapy level group (P for interaction between GP visit regularity and pharmacotherapy level for the effect on all-cause death = 0.0001). Higher GP visit regularity also showed a protective relationship against first CRD hospitalisation, with the statistically significant hazard ratios mostly decreasing with increasing regularity. This relationship was not modified by pharmacotherapy level (P for interaction between GP visit regularity and pharmacotherapy level for the effect on first CRD hospitalisation = 0.9243). The unadjusted results for the relationship between GP visit regularity and all-cause death and first hospitalisation can be found in the online additional material.
Table 4.
Association between GP Visit Regularity of Patients with CRD and Risk of Death and First CRD Hospitalisation, Overall and by Pharmacotherapy Level
| GP visit regularity quintile | All patients | SAB | Low dose ICS | Medium dose ICS ± LAB | High dose ICS ± LAB ± OS |
|---|---|---|---|---|---|
| HR (95% CI)a | HR (95% CI)a | HR (95% CI)a | HR (95% CI)a | HR (95% CI)a | |
| All-cause mortality | |||||
| Least regular | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 2nd least regular | 0.90 (0.79–1.01) | 0.85 (0.62–1.16) | 1.05 (0.68–1.61) | 1.03 (0.82–1.30) | 0.84 (0.71–1.00) |
| Medium regular | 0.84 (0.75–0.95) | 0.95 (0.70–1.29) | 0.81 (0.53–1.23) | 0.93 (0.74–1.17) | 0.79 (0.67–0.93) |
| 2nd most regular | 0.90 (0.80–1.01) | 1.17 (0.87–1.57) | 0.81 (0.53–1.23) | 0.95 (0.75–1.20) | 0.81 (0.69–0.96) |
| Most regular | 0.95 (0.83–1.08) | 1.34 (0.97–1.86) | 0.91 (0.57–1.44) | 1.13 (0.86–1.50) | 0.75 (0.62–0.91) |
| First CRD hospitalisation | |||||
| Least regular | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 2nd least regular | 0.92 (0.83–1.00) | 0.82 (0.58–1.17) | 1.29 (0.81–2.06) | 0.93 (0.79–1.09) | 0.97 (0.86–1.10) |
| Medium regular | 0.84 (0.77–0.92) | 0.66 (0.46–0.94) | 1.20 (0.76–1.91) | 0.87 (0.74–1.02) | 0.90 (0.80–1.02) |
| 2nd most regular | 0.74 (0.67–0.82) | 0.51 (0.34–0.76) | 1.04 (0.64–1.69) | 0.72 (0.60–0.85) | 0.82 (0.72–0.94) |
| Most regular | 0.77 (0.68–0.86) | 0.72 (0.46–1.12) | 0.90 (0.50–1.62) | 0.72 (0.58–0.89) | 0.84 (0.71–0.99) |
aAdjusted for number of GP visits, gender, ethnicity, age, socioeconomic status, residential remoteness and Charlson Index using Cox multivariate regression.
HR = hazard ratio; 95% CI = 95% confidence interval; SAB = short-acting bronchodilators; ICS = inhaled corticosteroids; LAB = long-acting bronchodilators; OS = oral steriods
DISCUSSION
Our results indicate that a pattern of more regular GP visits rather than a sporadic pattern decreases the likelihood of all-cause mortality for older CRD patients in the highest pharmacotherapy level group and first CRD hospitalisation in patients overall.
This whole-population-based, retrospective study used linked administrative medical data for exposure and outcome ascertainment. The methodology possessed advantages in terms of minimising loss to follow-up and decreasing various forms of information bias. Furthermore, being able to collect data from a whole population meant that prima facie the study results have strong external validity. Nevertheless, a few concerns need to be addressed. Firstly, a potential reason for caution in interpreting our results was the method used to ascertain patients with CRDs. Around 70% of the patients were identified based solely on medication use without confirmation by hospital, death or MBS records. This case ascertainment strategy may have overestimated the cases of CRD in this population. However, based on data analysed by our group from 12 general practices in WA that included diagnostic information, out of 23,850 CRD prescriptions prescribed by GPs, 92% had CRD as reason for visit or prescription (unpublished data). Also, we re-performed all of our analyses separately for these 70% of patients and for the remaining 30% and found no difference between the results of analyses of the two separate groups and the aggregate analysis. Thus, by using this selection strategy we can be reasonably confident that with considerable specificity we have included all CRD cases in the older WA population who have ever seen a doctor for breathing problems. This is not surprising given that the majority of medications prescribed for CRDs do not have alternative therapeutic indications.
Secondly, immortal time bias can occur in health services research when the increased amount of health services that are needed to deal with the early symptoms of the study outcome create a false association between the services and the outcome26,27. We endeavoured to reduce this bias by applying a 6-month ‘wash out’ period between the exposure period and the follow-up and thus excluding from the study those GP visits directly related to the early symptoms of the outcome. Applying a ‘wash out’ period of 6 months has been considered adequate to control immortal time bias in other analogous research26. However, the possibility remains that a 6-month or even longer period may not be sufficient to remove all immortal time bias in circumstances akin to the study. Residual immortal time bias could explain the tendency for our results to show a loss of apparent advantage of high regular GP contact against mortality.
Thirdly, the PBS database was limited to dispensed medications that were subsidised by the Australian Government. However, this was unlikely to have adversely affected the study because all inhaled corticosteroid and long-acting bronchodilator medications were subsidised by the PBS, and short-acting bronchodilator medication and oral corticosteroids were consistently subsidised for pensioners29. The latter point is especially relevant to our study as it was restricted to patients aged 65 or more years. Short-acting bronchodilator medication may have been purchased ‘over the counter’, but it was cheaper in Australia for pensioners to purchase them with a doctor’s prescription due to the PBS subsidy.
Lastly, smoking is a potential confounder in this study since it is strongly related to mortality and hospitalisation, and there is a reason to believe it is also related to the regularity of GP visits. We did not have information on smoking in our study, but our results were adjusted for a number of factors associated with smoking, such as socioeconomic status, residential remoteness, gender, indigenous status and comorbidity. Hence, although smoking status was not measured directly, its potential to cause a confounding effect was limited. Given this partial control mechanism, we do not consider that confounding by smoking is an important source of systematic error in the results.
Asthma was designated as a National Health Priority Area by the Australian health ministers in 1999. This resulted in a national Asthma Management Program commencing in 2001–2002 with an aim to improve the quality of care provided by GPs to people with moderate to severe asthma5. Many state governments, including in Western Australia, have since then implemented strategies to encourage health professionals to improve asthma care1. The initiative was justified given the fact that GPs play a pivotal role in the management of CRDs within the Australian health care system30. GP visits for CRDs rarely result in referrals to secondary/tertiary care in Australia, with previous reports indicating that only 1% of patients in 2004–2007 were referred to hospitals or emergency departments1. The importance of primary medical care in the management of CRDs has been supported by previous studies indicating that access to and patterns of GP contact can influence risk of adverse health events6,8–12. For example, higher primary care physician density has been associated with a lower risk of potentially preventable hospital admissions12, whilst individuals without a primary care physician8, individuals reporting fewer physician visits9, individuals living in primary medical care shortage areas10, or individuals with less access to primary care11 have been found to experience more preventable hospitalisations. In addition, Tsai et al. found that patients without a primary medical care provider were more likely to visit emergency departments for COPD exacerbations6.
Contained within the CRD management role of the GP are the functions of disease assessment, patient education, management of acute exacerbations and prescription of regular medications1. Since medications are the mainstay of CRD management17–19, the planning of therapies and education of the patient in their use represent two of the most important roles of the GP. In spite of this, CRDs have been found to be under-treated in the elderly31–34, which has been associated with elevated risks of all-cause mortality and hospitalisation35–38. Moreover, poor patient knowledge about asthma39,40 and the absence of an asthma management plan41,42 have been found to increase hospital emergency department visits for asthma. In light of these previous findings, the results of our study underline the importance of at least a minimum level of regular ‘maintenance’ primary medical care, as distinct from sporadic and ‘reactive’ medical care, in older patients with CRDs to manage their disease adequately and reduce the likelihood of hospitalisation and death.
CONCLUSIONS
In this study of 108,455 older patients with CRD in WA, we found that regular GP visits—as opposed to sporadic visits—appeared to decrease the likelihood of all-cause mortality for patients in the highest pharmacotherapy level and first CRD hospitalisation in all patients. The findings suggest that regular primary medical care is important for the effective treatment of CRDs. Not only does it appear to be important for patients to visit a GP, but it also seems to be imperative that they do so regularly on a proactive basis and not merely at times when their symptoms are at their worst.
Electronic supplementary materials
Below is the link to the electronic supplementary material.
Unadjusted association between GP visit regularity of patients with CRD and risk of death and first CRD hospitalisation, overall and by pharmacotherapy level. (DOC 36 kb)
Acknowledgements
We thank the Australian Department of Health and Ageing, Medicare Australia, the Australian Electoral Commission, the WA Department of Health and the Registrar Generals Office of WA for providing the data used for this investigation. We are furthermore grateful to the Data Linkage Branch of the WA Department of Health for extracting and linking the data. We also thank the clinical consensus panel for designing guidelines for the pharmacotherapy level classification. The research was supported by a project grant from Australia’s National Health and Medical Research Council.
Conflict of Interest: None disclosed.
Footnotes
We thank the Australian Department of Health and Ageing, Medicare Australia, the Australian Electoral Commission, the WA Department of Health and the Registrar Generals Office of WA for providing the data used for this investigation. We are furthermore grateful to the Data Linkage Branch of the WA Department of Health for extracting and linking the data. We also thank the clinical consensus panel for designing guidelines for the pharmacotherapy level classification. The research was supported by a project grant from Australia’s National Health and Medical Research Council.
References
- 1.Asthma in Australia 2008. Canberra: Australian centre for asthma monitoring and Australian Institute of Health and Welfare; 2008. Report No.: AIHW cat. no. ACM 14.
- 2.Asthma Management Handbook 2006. Melbourne: National Asthma Council Australia; 2006.
- 3.Begg S, Vos T, Barker B, Stevenson C, Stanley L, Lopez A. The burden of disease and injury in Australia 2003. Australian Institute of Health and Ageing. Canberra; 2007. Report No.: AIHW cat. no. PHE 82.
- 4.Frith PA, Cafarella PA, Duffy JM. Chronic obstructive pulmonary disease (COPD) is a major personal and public health burden in Australia. Aust N Z J Public Health. 2008;32:139–41. doi: 10.1111/j.1753-6405.2008.00190.x. [DOI] [PubMed] [Google Scholar]
- 5.Glasgow N. Systems for the management of respiratory disease in primary care—an international series: Australia. Prim Care Respir J. 2008;17:19–25. doi: 10.3132/pcrj.2008.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsai CL, Griswold SK, Clark S, Camargo CA., Jr Factors associated with frequency of emergency department visits for chronic obstructive pulmonary disease exacerbation. J Gen Intern Med. 2007;22:799–804. doi: 10.1007/s11606-007-0191-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cree M, Bell NR, Johnson D, Carriere KC. Increased continuity of care associated with decreased hospital care and emergency department visits for patients with asthma. Dis Manag. 2006;9:63–71. doi: 10.1089/dis.2006.9.63. [DOI] [PubMed] [Google Scholar]
- 8.Shi L, Samuels ME, Pease M, Bailey WP, Corley EH. Patient characteristics associated with hospitalizations for ambulatory care sensitive conditions in South Carolina. South Med J. 1999;92:989–98. doi: 10.1097/00007611-199910000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Rizza P, Bianco A, Pavia M, Angelillo IF. Preventable hospitalization and access to primary health care in an area of Southern Italy. BMC Health Serv Res. 2007;7:134. doi: 10.1186/1472-6963-7-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parchman ML, Culler SD. Preventable hospitalizations in primary care shortage areas. An analysis of vulnerable Medicare beneficiaries. Arch Fam Med. 1999;8:487–91. doi: 10.1001/archfami.8.6.487. [DOI] [PubMed] [Google Scholar]
- 11.Bindman AB, Grumbach K, Osmond D, et al. Preventable hospitalizations and access to health care. JAMA. 1995;274:305–11. doi: 10.1001/jama.274.4.305. [DOI] [PubMed] [Google Scholar]
- 12.Basu J, Friedman B, Burstin H. Primary care, HMO enrollment, and hospitalization for ambulatory care sensitive conditions: a new approach. Med Care. 2002;40:1260–9. doi: 10.1097/00005650-200212000-00013. [DOI] [PubMed] [Google Scholar]
- 13.Sunyer J, Anto JM, McFarlane D, et al. Sex differences in mortality of people who visited emergency rooms for asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;158:851–6. doi: 10.1164/ajrccm.158.3.9801093. [DOI] [PubMed] [Google Scholar]
- 14.Huiart L, Ernst P, Suissa S. Cardiovascular morbidity and mortality in COPD. Chest. 2005;128:2640–6. doi: 10.1378/chest.128.4.2640. [DOI] [PubMed] [Google Scholar]
- 15.Dantzer C, Tessier JF, Nejjari C, Barberger-Gateau P, Dartigues JF. Mortality of elderly subjects with self-reported asthma in a French cohort, 1991–1996. Eur J Epidemiol. 2001;17:57–63. doi: 10.1023/A:1010996718008. [DOI] [PubMed] [Google Scholar]
- 16.Bellia V, Pedone C, Catalano F, et al. Asthma in the elderly: mortality rate and associated risk factors for mortality. Chest. 2007;132:1175–82. doi: 10.1378/chest.06-2824. [DOI] [PubMed] [Google Scholar]
- 17.Restrepo RD. Use of inhaled anticholinergic agents in obstructive airway disease. Respir Care. 2007;52:833–51. [PubMed] [Google Scholar]
- 18.Phua GC, Macintyre NR. Inhaled corticosteroids in obstructive airway disease. Respir Care. 2007;52:852–8. [PubMed] [Google Scholar]
- 19.Miller-Larsson A, Selroos O. Advances in asthma and COPD treatment: combination therapy with inhaled corticosteroids and long-acting beta 2-agonists. Curr Pharm Des. 2006;12:3261–79. doi: 10.2174/138161206778194187. [DOI] [PubMed] [Google Scholar]
- 20.Yawn BP. Factors accounting for asthma variability: achieving optimal symptom control for individual patients. Prim Care Respir J. 2008;17:138–47. doi: 10.3132/pcrj.2008.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walter MJ, Castro M, Kunselman SJ, et al. Predicting worsening asthma control following the common cold. Eur Respir J. 2008;32:1548–54. doi: 10.1183/09031936.00026808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holman CD, Bass AJ, Rouse IL, Hobbs MS. Population-based linkage of health records in Western Australia: development of a health services research linked database. Aust N Z J Public Health. 1999;23:453–9. doi: 10.1111/j.1467-842X.1999.tb01297.x. [DOI] [PubMed] [Google Scholar]
- 23.Harris MG, Harris RD. The Australian health system: continuity and change. J Health Hum Serv Adm. 1998;20:442–67. [PubMed] [Google Scholar]
- 24.Medicare Benefits Schedule Book. Canberra: Department of Health and Ageing, Australian government; 2008.
- 25.Liu H, Wong L. Data mining tools for biological sequences. J Bioinform Comput Biol. 2003;1:139–67. doi: 10.1142/S0219720003000216. [DOI] [PubMed] [Google Scholar]
- 26.Tamim H, Monfared AA, LeLorier J. Application of lag-time into exposure definitions to control for protopathic bias. Pharmacoepidemiol Drug Saf. 2007;16:250–8. doi: 10.1002/pds.1360. [DOI] [PubMed] [Google Scholar]
- 27.Strom BL. Pharmacoepidemiology. 4. West Sussex: Wiley; 2005. [Google Scholar]
- 28.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 29.Patterns of asthma medication use in Australia. Canberra: Australian Centre for Asthma Monitoring and Australian Institute of health and welfare; 2007. Report No.: AIHW cat. no. ACM 11.
- 30.Cranston JM, Crockett AJ, Moss JR, Pegram RW, Stocks NP. Models of chronic disease management in primary care for patients with mild-to-moderate asthma or COPD: a narrative review. Med J Aust. 2008;188:S50–2. doi: 10.5694/j.1326-5377.2008.tb01744.x. [DOI] [PubMed] [Google Scholar]
- 31.Sin DD, Tu JV. Underuse of inhaled steroid therapy in elderly patients with asthma. Chest. 2001;119:720–5. doi: 10.1378/chest.119.3.720. [DOI] [PubMed] [Google Scholar]
- 32.Schmier JK, Halpern MT, Jones ML. Effects of inhaled corticosteroids on mortality and hospitalisation in elderly asthma and chronic obstructive pulmonary disease patients: appraising the evidence. Drugs Aging. 2005;22:717–29. doi: 10.2165/00002512-200522090-00001. [DOI] [PubMed] [Google Scholar]
- 33.Krigsman K, Moen J, Nilsson JL, Ring L. Refill adherence by the elderly for asthma/chronic obstructive pulmonary disease drugs dispensed over a 10-year period. J Clin Pharm Ther. 2007;32:603–11. doi: 10.1111/j.1365-2710.2007.00866.x. [DOI] [PubMed] [Google Scholar]
- 34.Enright PL, McClelland RL, Newman AB, Gottlieb DJ, Lebowitz MD. Underdiagnosis and undertreatment of asthma in the elderly. Cardiovascular Health Study Research Group. Chest. 1999;116:603–13. doi: 10.1378/chest.116.3.603. [DOI] [PubMed] [Google Scholar]
- 35.Vollmer WM, Peters D, Crane B, Kelleher C, Buist AS. Impact of regular inhaled corticosteroid use on chronic obstructive pulmonary disease outcomes. COPD. 2007;4:135–42. doi: 10.1080/15412550701341186. [DOI] [PubMed] [Google Scholar]
- 36.Sin DD, Tu JV. Inhaled corticosteroids and the risk of mortality and readmission in elderly patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164:580–4. doi: 10.1164/ajrccm.164.4.2009033. [DOI] [PubMed] [Google Scholar]
- 37.Sin DD, Tu JV. Inhaled corticosteroid therapy reduces the risk of rehospitalization and all-cause mortality in elderly asthmatics. Eur Respir J. 2001;17:380–5. doi: 10.1183/09031936.01.17303800. [DOI] [PubMed] [Google Scholar]
- 38.Balkrishnan R, Christensen DB. Inhaled corticosteroid use and associated outcomes in elderly patients with moderate to severe chronic pulmonary disease. Clin Ther. 2000;22:452–69. doi: 10.1016/S0149-2918(00)89013-X. [DOI] [PubMed] [Google Scholar]
- 39.Radeos MS, Leak LV, Lugo BP, Hanrahan JP, Clark S, Camargo CA., Jr Risk factors for lack of asthma self-management knowledge among ED patients not on inhaled steroids. Am J Emerg Med. 2001;19:253–9. doi: 10.1053/ajem.2001.21712. [DOI] [PubMed] [Google Scholar]
- 40.Goeman DP, Aroni RA, Sawyer SM, et al. Back for more: a qualitative study of emergency department reattendance for asthma. Med J Aust. 2004;180:113–7. doi: 10.5694/j.1326-5377.2004.tb05831.x. [DOI] [PubMed] [Google Scholar]
- 41.Fernandes AK, Mallmann F, Steinhorst AM, et al. Characteristics of acute asthma patients attended frequently compared with those attended only occasionally in an emergency department. J Asthma. 2003;40:683–90. doi: 10.1081/JAS-120023487. [DOI] [PubMed] [Google Scholar]
- 42.Adams RJ, Smith BJ, Ruffin RE. Factors associated with hospital admissions and repeat emergency department visits for adults with asthma. Thorax. 2000;55:566–73. doi: 10.1136/thorax.55.7.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Below is the link to the electronic supplementary material.
Unadjusted association between GP visit regularity of patients with CRD and risk of death and first CRD hospitalisation, overall and by pharmacotherapy level. (DOC 36 kb)